Abstract
WNK (With-No-Lysine (K)) kinases are serine-threonine kinases characterized by an atypical placement of a catalytic lysine within the kinase domain. Mutations in human WNK1 or WNK4 cause an autosomal dominant syndrome of hypertension and hyperkalemia, reflecting the fact that WNK kinases are critical regulators of renal ion transport processes. Here, the role of WNKs in the regulation of ion transport processes in vertebrate and invertebrate renal function, cellular and organismal osmoregulation, cell migration and cerebral edema will be reviewed, along with emerging literature demonstrating roles for WNKs in cardiovascular and neural development, Wnt signaling, and cancer. Conserved roles for these kinases across phyla are emphasized.
Keywords: kidney, blood pressure, SPAK/OSR1, Mo25/Cab39, Lhx8, Wnt signaling, excretory canal, Malpighian tubule, Drosophila, C. elegans, Zebrafish
1. Introduction
The With-No-Lysine (K) (WNK) kinases are a family of serine/threonine kinases, first identified in 2000 by Cobb and colleagues in a screen for novel mitogen-activated protein kinase (MAPK) kinases (Xu et al., 2000). The characteristic feature of the “With No Lysine” kinases is the absence of the catalytic lysine found in subdomain II in most other kinases; instead, this lysine is found in subdomain I (Fig. 1A, B) (Xu et al., 2000). Another unique feature of WNKs is their regulation by chloride, which directly binds to the kinase active site and inhibits autophosphorylation and kinase activation (Fig. 1C) (Bazua-Valenti et al., 2015; Piala et al., 2014; Terker et al., 2016). WNKs are evolutionarily ancient: in the initial description, homologs from the nematode Caenorhabditis elegans, the plants Oryza and Arabidopsis, and the fungus Phycomyces were noted (Xu et al., 2000). Here, aspects of the roles of WNKs in physiology, development, and disease, with an emphasis on recent discoveries in model organisms, will be reviewed.
Figure 1. Unique features of WNK kinases.
(A) Alignment of the kinase domains of human WNK1 (Hs WNK1), zebrafish Wnk1a (Dr Wnk1a), and fruit fly (Dm Wnk) and worm (Ce WNK) WNKs with human protein kinase A (Hs PKA). Atypical placement of the subdomain II lysine in subdomain I is indicated by magenta. Chloride-binding residues are indicated in red. The site of autophosphorylation, required for kinase activation, is indicated in blue. (B) Crystal structure of the kinase domain of rat WNK1. The atypically-placed catalytic lysine, Lys233 in rat WNK1, is shown in comparison to Cys250, the usual lysine position. From (Min et al., 2004). (C) Kinase domain of WNK1, showing Cl− binding (green ball). Enlargement shows hydrogen-bonding distances to Leu369 and Leu371. Note that Leu369 is a substitution of Phe in the “DFG” motif that is characteristic of most protein kinases in subdomain VII, including PKA (see A). Modified from (Piala et al., 2014).
2. The WNK-SPAK/OSR1 kinase cascade: roles in physiology and disease
2.1 Overview of the WNK-SPAK/OSR1 kinase cascade
WNK homologs are found throughout the animal kingdom. Mammalian genomes encode four WNK paralogs, some of which are duplicated in the zebrafish Danio rerio (discussed in 3.2 below). There is a single WNK homolog in the genomes of the invertebrates Drosophila melanogaster and C. elegans. The WNK kinase domain is highly conserved (Figs. 1A and 2A). In contrast, the C-terminus, which is of varying length, has lower sequence homology. Common features include predicted coiled-coil domains, PXXP motifs, and an RFX(V/I) motif required for binding to the downstream kinases Ste20/SPS1-related proline/alanine-rich kinase (SPAK, also known as PASK) and oxidative stress responsive-1 (OSR1) (Figs. 2A and 2B; reviewed in (McCormick and Ellison, 2011)). SPAK and OSR1 are closely related Sterile 20 (Ste20)-related kinases that arose from gene duplication (Delpire and Gagnon, 2008) and have highly conserved orthologs in D. melanogaster and C. elegans (Fig. 2B).
Figure 2. Schematic representation of selected WNK.
(A) and OSR1 (B) kinase family members. Sequence identities of kinase domains to human WNK1 and OSR1, respectively, are given in percent based on BLASTP scores (Johnson et al., 2008). Note that other protein splice-isoforms also exist (reviewed in (McCormick and Ellison, 2011)) and that some of the indicated motifs are predictions (Pred.) and have not been functionally verified. PF1/2: PASK/Fray homology domains 1/2; Hs: Homo sapiens; Dr: Danio rerio (zebrafish); Dm: Drosophila melanogaster; Ce: Caenorhabditis elegans.
In 2001, Lifton and colleagues published their finding that two of the four human WNK paralogs, WNK1 and WNK4, are mutated in a syndrome variously known as pseudohypoaldosteronism type II, familial hyperkalemic hypertension (PHAII/FHHt), or Gordon’s syndrome. The WNK mutations are transmitted in an autosomal dominant fashion and result in high blood pressure and high serum potassium concentrations in affected individuals (Wilson et al., 2001). This phenotype suggested that WNKs may play a role in renal physiology, and this has been substantiated in extensive subsequent research, as recently reviewed (Dbouk et al., 2014; Hadchouel et al., 2016). Consistent with this, mutations in the E3 ubiquitin ligase complex components, Kelch-like 3 and Cullin 3, were also found to cause PHAII/FHHt (Boyden et al., 2012; Louis-Dit-Picard et al., 2012), likely due to their role in WNK degradation (reviewed in (Ferdaus and McCormick, 2016)).
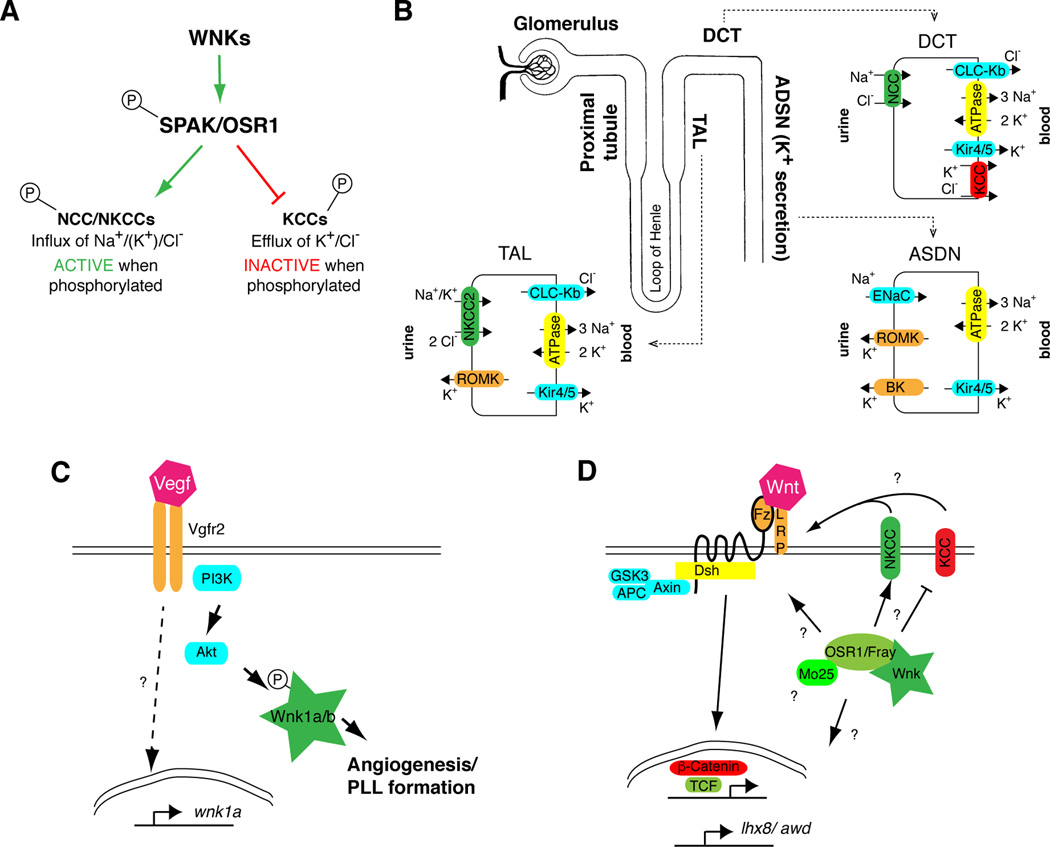
The best-understood function of WNKs is their ability to phosphorylate SPAK and OSR1 (Anselmo et al., 2006; Moriguchi et al., 2005; Vitari et al., 2005). Phosphorylation of the SPAK/OSR1 T-loop threonine, T243 (SPAK) or T185 (OSR1), is required for SPAK/OSR1 activation, while the function of phosphorylation on a C-terminal serine, Ser373 (SPAK) or Ser 325 (OSR1), in the PF1 domain (PASK and Fray; Fig. 2B), is less clear (Gagnon and Delpire, 2010; Gagnon et al., 2006; Moriguchi et al., 2005; Vitari et al., 2005). Activated SPAK and OSR1 phosphorylate members of the SLC12 family of cation-chloride cotransporters. These include the three related sodium-coupled chloride cotransporters, NCC (sodium chloride cotransporter), and the sodium-potassium-2-chloride cotransporters NKCC1 and NKCC2 (Anselmo et al., 2006; Dowd and Forbush, 2003; Gagnon and Delpire, 2010; Gagnon et al., 2006; Moriguchi et al., 2005; Richardson et al., 2008; Richardson et al., 2011), as well as the potassium-coupled chloride cotransporters, KCC1–4 (potassium chloride cotransporters) (de Los Heros et al., 2014; Melo et al., 2013). Phosphorylation of NCC, NKCC1 and NKCC2 results in transporter activation, whereas phosphorylation of KCCs results in transporter inactivation (Fig. 3A). Regulation of these transporters by WNKs are important for cell volume control, transepithelial ion transport, and the regulation of intracellular chloride concentration (Kahle et al., 2006). In neurons, for example, intracellular chloride concentration determines whether activation of ligand-gated chloride channels, such as the GABAA or glycine receptors, results in a hyperpolarizing or depolarizing effect. When intracellular Cl− concentration is low, GABA or glycine binding to their receptors results in Cl− influx, resulting in hyperpolarization. Conversely, when intracellular Cl− concentration is high, GABA or glycine binding to their receptors results in Cl− efflux and neuronal depolarization. In cell volume control, activation of NKCCs results in inward ion flux, due to the low intracellular sodium concentration generated by the activity of the Na+/K+-ATPase, which pumps 3 Na+ ions out of the cell in exchange for 2 K+ ions in. Similarly, the high intracellular K+ concentration generated by the Na+/K+-ATPase generates an outward driving force for K+ and Cl− through KCCs, and inhibition of KCCs by WNK-SPAK/OSR1 signaling decreases this outward ion flux. WNK-SPAK/OSR1 signaling also plays important roles in the regulation of transepithelial ion transport through SLC12 transporters.
Figure 3. WNK Pathway.
(A) WNKs phosphorylate the two related Ste20 kinases, SPAK and OSR1, on a T-loop threonine in the active site, which is required for SPAK/OSR1 activation, and on a serine in the PF1 domain in the C-terminus of the protein. SPAK and OSR1 phosphorylate conserved serines and threonines in the sodium-coupled SLC12 chloride cotransporters, NCC, NKCC1 and NKCC2 in mammals, increasing transport activity. Phosphorylation of the potassium-coupled SLC12 chloride cotransporters, KCCs 1–4 in mammals, results in transporter inactivation. (B) Schematic of the nephron showing sites of WNK action. WNK-SPAK/OSR1 signaling positively regulates NKCC2 in the thick ascending limb (TAL) of the loop of Henle, and NCC in the distal convoluted tubule (DCT), promoting sodium chloride reabsorption. Decreased sodium delivery to the K+-secretory principal cell of the aldosterone-sensitive distal nephron (ASDN), where K+ secretion depends on a lumen-negative charge generated by Na+ reabsorption through the epithelial Na channel (ENaC), likely contributes to the hyperkalemia observed in patients with PHAII. WNKs also regulate ENaC and the K+-secretory channels renal outer medullary potassium channel (ROMK) and big potassium channel (BK, also known as maxi-K) (Carrisoza-Gaytan et al., 2016; Welling, 2013). Also pictured are the Na+/K+-ATPase, which generates the driving force for sodium reabsorption in the TAL, DCT and the principal cell of the ASDN; Clc-Kb, a chloride channel allowing basolateral exit of Cl−, and the heterodimeric Kir4.1/5.1 potassium channel, which is important for recycling K+ entering through the Na+/K+-ATPase and setting the basolateral membrane potential. KCC4 could also play a role in basolateral KCl exit (see text). (C) Summary of Wnk1 function in in zebrafish. During angiogenesis, Wnk1 is regulated by Vegfr signaling via Akt phosphorylation and is also a transcriptional target of Vegf signaling. Vgfr2 and PI3K are encoded by the flk1 and pi3kc2α genes, respectively. (D)Drosophila Wnk regulates Wnt signaling and the expression of Awh/ Lhx8 via Fray/OSR1.
In PHAII/FHHt, overexpression of WNK4 or WNK1, either due to gain-of-function alleles of those genes, or loss-of-function of Kelch-like 3 or Cullin 3, results in increased phosphorylation of NCC in the kidney (Ferdaus and McCormick, 2016; Hadchouel et al., 2016; Huang and Cheng, 2015). Phosphorylation of NCC by the WNK-SPAK/OSR1 kinase cascade results in increased NCC activity (Richardson et al., 2008). Overactivation of NCC results in increased NaCl reabsorption, causing hypertension. Concomitant decreased sodium delivery to the downstream aldosterone-sensitive distal nephron, where potassium secretion is dependent on sodium delivery, results in hyperkalemia (Fig. 3B); direct effects of WNKs on potassium channels may also contribute. Furthermore, activation of WNK-SPAK/OSR1 signaling in the vasculature results in vasoconstriction through effects on NKCC1 (Bergaya et al., 2011; Susa et al., 2012; Yang et al., 2010; Zeniya et al., 2013), which may contribute to the hypertensive phenotype, particularly in individuals with WNK1 mutations. Additional effects of WNK signaling on other transport processes in the nephron, such as regulation of the epithelial sodium channel, the chloride/bicarbonate exchanger pendrin, and paracellular chloride reabsorption through claudins, may also contribute to the PHAII/FHHt phenotype, but this is less well established (reviewed in (Hadchouel et al., 2016)).
Polymorphisms in serine threonine kinase 39 (STK39), which encodes the human SPAK ortholog, have been associated with essential hypertension (Xi et al., 2013). One of these, rs375477, was shown to increase STK39 mRNA and SPAK protein expression when introduced into human embryonic kidney cells using CRISPR technology (Mandai et al., 2015), again suggesting a connection between increased WNK-SPAK/OSR1 signaling and elevated blood pressure.
2.2 WNK-SPAK/OSR1 signaling in invertebrates
2.2.1 WNK-SPAK/OSR1 signaling regulates NKCC in Drosophila renal tubule function
D. melanogaster and C. elegans each have a single wnk ortholog, called wnk in Drosophila and wnk-1 in C. elegans, and a single SPAK/OSR1 ortholog, called frayed (fray, CG7693) in Drosophila and gck-3 in C. elegans (Fig. 2). Using bacterially expressed, purified components, Sato et al. and Serysheva et al. demonstrated that Drosophila Wnk phosphorylates Fray in vitro (Sato and Shibuya, 2013; Serysheva et al., 2013). Similarly, Fray phosphorylates the N-terminus of Ncc69 (Wu et al., 2014), a fly NKCC (Leiserson et al., 2011; Sun et al., 2010). As discussed in further detail in section 3.3, examination of developmental phenotypes placed fray downstream of wnk (Sato and Shibuya, 2013; Serysheva et al., 2013). In addition, loss-of-function mutations in both fray and Ncc69 result in similar axon bulging phenotypes in the Drosophila larval nervous system, suggesting that they may act in the same pathway.
The Malpighian (renal) tubule is part of the iono- and osmo-regulatory system of the fly. Unlike the mammalian nephron, the Malpighian tubule is aglomerular and blind-ended. Urine generation therefore occurs through the isosmotic secretion of KCl-rich fluid across the main segment of the tubule, from the hemolymph to the tubule lumen. Transepithelial cation flux occurs through the principal cell of the main segment, whereas chloride flux occurs through the neighboring stellate cells (Fig. 4A) (Cabrero et al., 2014; Linton and O’Donnell, 1999; O’Donnell et al., 1996; Rheault and O’Donnell, 2001).
Figure 4. Wnk function in the Malpighian (renal) tubule in Drosophila.
(A) The Malpighian tubule main segment secretes a KCl-rich fluid into the lumen (urine). Transepithelial cation transport in the Drosophila Malpighian tubule occurs through the principal cell, whereas transepithelial chloride transport occurs through the neighboring stellate cells. The apical vacuolar H+-ATPase drives fluid secretion (Dow et al., 1994), and generates a lumen-positive transepithelial potential difference (O’Donnell et al., 1996). This drives exchange of protons for cations (K+ or Na+, primarily K+ in Drosophila renal tubules). Chloride secretion is also driven by the lumen-positive charge. The fly NKCC, Ncc69, is required for normal transepithelial K+ flux. Na+ entering through the NKCC is recycled by the basolateral Na+/K+-ATPase (Rodan et al., 2012). Cl may also be recycled through Cl− channels, or through basolateral Cl−/HCO3− exchangers (not shown) (Romero et al., 2000; Sciortino et al., 2001). The inwardly rectifying potassium channels Irk1 and Irk2 are also required for normal transepithelial K+ flux (Wu et al., 2015). (B) The WNK-SPAK/OSR1 (=Fray in flies) pathway regulates transepithelial K+ flux in the Drosophila renal tubule principal cell. Hypotonic bathing medium stimulates transepithelial K+ flux in a WNK/Fray/NKCC-dependent manner.
It was proposed that WNK-SPAK/OSR1 signaling regulates transepithelial ion flux through NKCC2 and NCC in the mammalian kidney, although there are few studies directly demonstrating this, due to the technical difficulty of directly assaying transepithelial ion flux in the mammalian nephron (Cheng et al., 2012; Cheng et al., 2015). The fly thus affords the opportunity to study the molecular physiology of WNK-SPAK/OSR1 signaling in a genetically manipulable transporting epithelium. Indeed, it was demonstrated that the fly NKCC, Ncc69, is required in the cation-conducting principal cell for normal transepithelial fluid and potassium secretion in the fly renal tubule, where it functions as a secretory NKCC (as compared to the absorptive NKCCs in the mammalian kidney) (Rodan et al., 2012). Tubule Ncc69 is regulated by the WNK-SPAK/OSR1 pathway (Fig. 4B). Knocking down either wnk or fray in the tubule principal cell decreases transepithelial potassium flux, similar to the Ncc69 null phenotype. As in developmental processes, fray operates downstream of wnk. Mutation of the predicted Wnk phosphorylation site in Fray, Thr 206, to a phospho-mimicking Asp, results in constitutive kinase activity towards Ncc69 in vitro and restores normal transepithelial potassium flux to wnk knockdown tubules. Importantly, wnk or fray knockdown do not reduce potassium flux in Ncc69 null tubules, indicating that the NKCC transporter is the target of Wnk and Fray regulation (Wu et al., 2014). Thus, WNK and Fray regulate transepithelial ion flux through the regulation of the fly renal tubule NKCC.
The WNK-Fray signaling pathway has also been shown to regulate transport processes in the Drosophila prepupal salivary gland. Farkaš et al. made the surprising observation that the salivary gland at this developmental stage secretes a calcium oxalate-rich fluid, which may form part of the secretory “glue” that allows puparia to fix themselves to a substrate during metamorphosis (Farkas et al., 2016). Based on prior work from the Romero laboratory showing that the Slc26a5/6 transporter, Prestin, functions as a chloride/oxalate exchanger and is involved in calcium oxalate secretion by the Drosophila Malpighian tubule (Hirata et al., 2012a; Hirata et al., 2012b; Landry et al., 2016), the investigators tested the hypothesis that Prestin was involved in salivary gland calcium oxalate excretion. Indeed, prestin knockdown in the salivary gland decreased calcium oxalate excretion (Farkas et al., 2016). The Romero laboratory had also previously demonstrated that Prestin is positively regulated by Fray (Hirata et al., 2012b), and Farkas et al. demonstrated that knocking down either wnk or fray in the salivary gland decreased calcium oxalate excretion (Farkas et al., 2016). The salivary gland secretory process, like that of the Malpighian tubule (Dow et al., 1994), is dependent on the vacuolar H+-ATPase (Farkas et al., 2016). Thus, salivary gland transport has elements that are conserved with the renal tubule, including the requirement for the H+-ATPase and regulation by WNK-SPAK/OSR1 signaling.
2.2.2 WNK-SPAK/OSR1 signaling in C. elegans and chloride channel regulation
Elegant studies in C. elegans have uncovered roles for WNK-SPAK/OSR1 signaling in multiple physiological processes. As is the case with the mammalian and Drosophila proteins, C. elegans Wnk-1 phosphorylates the worm SPAK/OSR1 ortholog, GCK-3, in vitro (Hisamoto et al., 2008). C. elegans with mutations in wnk-1 or gck-3 prematurely terminate the excretory canal, which forms part of the nematode renal system (Hisamoto et al., 2008; Kupinski et al., 2010). The defect in wnk-1 mutant worms is rescued by re-expression of wild-type wnk-1, but not by wnk-1 that is kinase-dead, or that carries a mutation in the RFXV motifs that are required for GCK-3 binding. GCK-3 carrying a phosphomimicking mutation in the Wnk target T-loop threonine, T280E, which has increased kinase activity in vitro, also rescues the wnk-1 mutant phenotype, again indicating that gck-3 lies downstream of wnk-1. In the gck-3 mutants, expression of wild-type gck-3, but not kinase-dead or T280A mutant gck-3, rescues the excretory canal phenotype. In contrast, gck-3 with a mutation in the Wnk-1-phosphorylated serine (S419A) in the PF1-domain is able to rescue, suggesting that phosphorylation of this residue by Wnk-1 is not necessary in this in vivo context (Hisamoto et al., 2008).
Interestingly, extension of the C. elegans excretory canal during development is modulated by osmolarity: placing worms on a hyper-osmolar medium (e.g. 500 mM NaCl), and then returning them to an isotonic medium (50 mM NaCl), promotes the fusion of vesicles with the apical surface and canal extension. These processes fail to occur in gck-3 mutant worms. This suggests that during development, excretory canal extension depends on the ability to sense extracellular osmolarity, and gck-3 mutant worms are unable to either sense or respond to changes in osmolarity, resulting in stalled excretory canal extension (Kolotuev et al., 2013).
Because Strange and colleagues had previously identified the ClC chloride channel, CLH-3, as a target of GCK-3 in worm oocytes (Denton et al., 2005), Hisamoto et al. examined whether the shortened excretory canal phenotype in gck-3 mutant worms was due to dysregulation of CLH-3. Indeed, a clh-3 mutation partially suppressed the mutant phenotype of gck-3 mutant worms, indicating that clh-3 is downstream of gck-3 and is negatively regulated by the SPAK/OSR1 ortholog (Hisamoto et al., 2008). Similar suppression was also observed for decreased fertility observed in gck-3 mutant worms, but not for early larval lethality or additional developmental phenotypes resulting from loss of gck-3 function, indicating that gck-3 likely has additional targets (Hisamoto et al., 2008; Kupinski et al., 2010). NKCC1 does not appear to be such a target, at least for excretory canal extension, as nkcc-1 mutant worms have normal excretory canal morphology (Hisamoto et al., 2008). Whether WNK-SPAK/OSR1 signaling in the excretory canal regulates NKCC-1 in non-developmental contexts, for example in excretory canal function, has not been determined.
CLH-3b is a splice variant of the clh-3 gene in C. elegans. It is expressed in worm oocytes and is activated by serine/threonine dephosphorylation during oocyte meiotic maturation, or in response to cell swelling. GCK-3 negatively regulates CLH-3b by phosphorylating the channel on Ser 742 and Ser 747 and inducing conformational changes that decrease channel activity (Denton et al., 2005; Falin et al., 2009; Miyazaki and Strange, 2012; Miyazaki et al., 2012; Yamada et al., 2013). Interestingly, GCK-3 activity towards CLH-3b appears to be Wnk-independent, and rather is downstream of the C. elegans ERK (extracellular signal-regulated) MAPK, MPK-1 (Falin et al., 2011).
SPAK/OSR1 regulation of chloride channels may also have relevance to the mammalian kidney, where chloride channels play important roles in renal physiology, such as the transepithelial reabsorption of sodium chloride in the thick ascending limb (TAL) of the loop of Henle and the distal convoluted tubule (DCT; see schematic in Fig. 3B) (Zaika et al., 2016). C. elegans CLH-3b is a member of the CLC-1/2/Ka/Kb chloride channel family. In the mammalian kidney, CLC-Ka and CLC-Kb are expressed in the loop of Henle and distal nephron, and mice lacking the CLC-Ka ortholog (CLC-K1 in mice) have nephrogenic diabetes insipidus. In humans, simultaneous mutations in the genes encoding CLC-Ka and CLC-Kb, or mutations in CLC-Kb alone, cause Bartter’s syndrome, a salt-losing tubulopathy characterized by hypokalemic metabolic alkalosis and secondary hyperaldosteronism. Bartter’s syndrome can be also be caused by mutations in the gene encoding Barrtin, which is a CLC-K channel regulatory subunit (reviewed in (Andrini et al., 2015; Zaika et al., 2016)).
Recent work in mice has suggested that Clc-Kb plays a role in potassium sensing by the distal convoluted tubule (DCT). Dietary potassium intake has natriuretic effects (Barker, 1932; Keith, 1935; Krishna et al., 1989; Womersley and Darragh, 1955), likely contributing to the antihypertensive effect of a high-potassium diet (Aburto et al., 2013; Mente et al., 2014). Potassium infusion or ingestion results in decreased sodium reabsorption in the proximal tubule and the thick ascending limb (Battilana et al., 1978; Brandis et al., 1972; Cheng et al., 2012; Higashihara and Kokko, 1985; Stokes, 1982), which promotes increased distal delivery of sodium and, therefore, potassium secretion (Fig. 3B). More recently, the effect of potassium on the sodium chloride cotransporter (NCC), which reabsorbs sodium chloride in the distal convoluted tubule, has been examined. Like the proximal tubule and the thick ascending limb, the distal convoluted tubule lies upstream of the potassium-secretory portion of the nephron. Therefore, changes in NaCl reabsorption by NCC influence potassium secretion by affecting sodium delivery to the potassium-secretory segment, where potassium is secreted in exchange for sodium. As described above, NCC phosphorylation by SPAK/OSR1 results in increased NCC transport activity. High dietary potassium results in decreased expression and reduced phosphorylation of NCC, which is predicted to decrease NCC activity (Castaneda-Bueno et al., 2014; Rengarajan et al., 2014; Sorensen et al., 2013; van der Lubbe et al., 2013; Wade et al., 2011), while low dietary potassium increases NCC expression and phosphorylation (Castaneda-Bueno et al., 2014; Frindt et al., 2011; Terker et al., 2015; Vallon et al., 2009; Wade et al., 2015). Consistent with the role of WNK-SPAK/OSR1 signaling in NCC phosphorylation, a high potassium diet alters the subcellular distribution of phosphorylated (activated) SPAK in the DCT (van der Lubbe et al., 2013), and a low potassium diet increases WNK4 levels (Terker et al., 2015), SPAK abundance and phosphorylation (Castaneda-Bueno et al., 2014; Terker et al., 2015; Wade et al., 2015), and the apical abundance of OSR1 in the DCT (Wade et al., 2015). The effect of dietary potassium on NCC phosphorylation is blunted in SPAK knockout or SPAK/OSR1 knockout mice (Terker et al., 2015; Wade et al., 2015), and abolished in a mouse with SPAK knockout and inducible renal OSR1 knockout (Ferdaus et al., 2016). These data indicate that a low potassium diet activates WNK-SPAK/OSR1 signaling in the DCT, increasing NCC phosphorylation and activity. While this decreases potassium secretion by decreasing sodium delivery to the potassium-secretory portion of the nephron (Fig. 3B), renal salt reabsorption is increased and can result in increased blood pressure, particularly in individuals consuming the high salt/low potassium diet typical of the modern diet (Cogswell et al., 2012; Mente et al., 2014).
How is low dietary potassium sensed by the DCT? Ellison and colleagues have proposed a model in which a low potassium diet hyperpolarizes the basolateral membrane of DCT epithelial cells by increasing the driving force for potassium efflux from DCT cells through the basolateral inwardly rectifying potassium channel, Kir4.1/5.1 (Fig. 3B). This in turn is expected to increase chloride efflux through CLC-Kb, lowering intracellular chloride and activating WNK (Terker et al., 2015). WNK4, which is the predominant regulator of NCC in the DCT, is particularly sensitive to changes in chloride (Terker et al., 2016). This model is supported by experiments in cultured HEK cells expressing wild-type or mutant variants of Kir4.1 and CLC-Kb, as well as by mathematical modeling (Terker et al., 2015). The functional coupling of Kir4.1 with CLC-Kb is also supported by studies in Kir4.1 knockout mice, in which the basolateral chloride conductance of the DCT was strongly diminished. Interestingly, SPAK and NCC expression was very low in these mice (Zhang et al., 2014).
An as-yet unexplored topic is whether SPAK/OSR1 could be regulating CLC-Kb, since, as mentioned above, GCK-3 negatively regulates CLH-3b in C. elegans. If this were the case, activation of SPAK/OSR1 under low potassium/low intracellular chloride conditions could inhibit CLC-Kb, putting a brake on further chloride efflux from the DCT. Alternatively, if SPAK/OSR1 acts downstream of MAPK signaling rather than WNK, as is the case for GCK-3/CLH-3b, this would afford additional opportunities for regulation of CLC-Kb independent of WNK. Another unknown is whether KCC4, which has been localized to the basolateral membrane of DCT in the rabbit kidney (Fig. 3B) (Velazquez and Silva, 2003), plays a role in chloride efflux in low dietary potassium conditions. Since WNK-SPAK/OSR1 signaling negatively regulates KCCs, this could serve as another negative feedback mechanism to avoid ongoing activation of the WNK pathway. Finally, WNK-SPAK/OSR1 signaling also modulates sodium chloride reabsorption through NKCC2 in the thick ascending limb (Fig. 3B) (Cheng et al., 2012; Cheng et al., 2015; Rafiqi et al., 2010). Whether the pathway also regulates CLC-Kb in this segment is unknown. Mammalian CLC-2 appears to be negatively regulated by SPAK and OSR1, based on decreased chloride conductance, as measured by two-electrode voltage clamp in Xenopus oocytes co-expressing CLC-2 with SPAK or OSR1 (Warsi et al., 2014). CLC-Ka and CLC-Kb have predicted SPAK/OSR1-binding RFXI motifs, but their regulation by SPAK/OSR1 has not been studied.
2.3 WNK-SPAK/OSR1 signaling in osmoregulation
Activation of the WNK-SPAK/OSR1 pathway has been observed in cells under both hypertonic and hypotonic conditions (Chen et al., 2004; Dowd and Forbush, 2003; Lenertz et al., 2005; Moriguchi et al., 2005; Naito et al., 2011; Richardson et al., 2008; Zagorska et al., 2007). Hypertonicity also results in redistribution of WNK1 and WNK4 in cells (Sengupta et al., 2012; Shaharabany et al., 2008; Zagorska et al., 2007), although the functional significance of this is unknown. In hypotonic conditions, intracellular chloride initially falls due to the dilutional effect of water moving into cells. Subsequently, during the process of regulatory volume decrease, intracellular chloride falls further as K+ and Cl− efflux from the cell, followed by osmotically obliged water, to allow the cell volume to return towards normal (Hoffmann et al., 2009). Piala et al. demonstrated, surprisingly, that chloride binds directly to the kinase domain of WNK1, and stabilizes the kinase in an inactive conformation that prevents autophosphorylation and kinase activation (Fig. 1C) (Piala et al., 2014). The fall in intracellular chloride that occurs during hypotonicity and the subsequent regulatory volume decrease response likely explains at least part of the mechanism for WNK activation in hypotonic conditions (Bazua-Valenti et al., 2015; Ponce-Coria et al., 2008); the physiological significance of WNK activation under these conditions is under investigation. The mechanism by which hypertonicity, causing cell shrinkage, activates the WNK-SPAK/OSR1 pathway is unknown. However, activation of the WNK-SPAK/OSR1-NKCC1 pathway after a hypertonic challenge stimulates ion influx into cells, allowing recovery of cell volume (Cruz-Rangel et al., 2012; Roy et al., 2015).
Fluid secretion from the main segment of the Drosophila renal tubule decreases in hypertonic conditions, and increases in hypotonic conditions (Blumenthal, 2005). Consistent with this, transepithelial potassium flux in the main segment also decreases in hypertonic conditions, and increases in hypotonic conditions (Wu et al., 2014). The decrease in hypertonic conditions occurs in fray knockdown tubules, suggesting that this effect is independent of WNK-SPAK/OSR1 signaling in principal cells (Wu et al., 2014). Blumenthal demonstrated that the hypertonic effect on fluid secretion is due to decreased tubule sensitivity to the diuretic effects of tyramine (Blumenthal, 2005). In contrast, the hypotonic stimulation of transepithelial potassium flux is abolished in tubules in which wnk or fray is knocked down in the principal cells, or in tubules carrying a null mutation in the NKCC, Ncc69 (Wu et al., 2014), indicating that hypotonicity stimulates transepithelial potassium flux in a WNK-SPAK/OSR1-NKCC-dependent manner (Fig. 4B). Because urine generation occurs through the transepithelial secretion of a KCl-rich fluid in the main segment of the aglomerular fly renal tubule, the hypotonic stimulation of urine generation in the main segment may allow for more efficient excretion of a water load following ingestion of a hypotonic meal, if ions are reabsorbed in subsequent segments that the urine passes through (tubule lower segment and hindgut) to allow generation of a hypotonic excreta (Larsen et al., 2014).
Roles for WNK-SPAK/OSR1 signaling in osmoregulation have also been described in C. elegans. Worms in which wnk-1 or gck-3 are knocked down have impaired survival during hypertonic stress; interestingly, survival on sorbitol is less impaired than survival on an iso-osmolar concentration of sodium chloride. Wild-type worms shrink and then recover volume after exposure to hypertonic stress, whereas recovery was impaired in wnk-1 and gck-3 knockdown worms. The survival and volume regulatory defects of gck-3 knockdown worms were rescued by preventing knockdown in the intestine or hypodermis, the worm epidermis, suggesting that the skin or gut are critical for the response to ionic stress (Choe and Strange, 2007). Presumably, the WNK-SPAK/OSR1 pathway is regulating ion channels or transporters in these organs to mediate the response to hypertonic stress, but the identity of these channels/transporters has not been determined.
A subsequent study from the Strange laboratory demonstrated that in worms exposed to hypertonic sodium chloride stress, protein translation is inhibited by GCN1/2 (general control nonderepressible) kinase complex mediated phosphorylation of the eukaryotic translation initiation factor eIF2α. Through unknown mechanisms, decreased protein translation activates WNK1-GCK3 signaling, which then results in increased expression of the glycerol synthesis enzyme glycerol-3-phosphate dehydrogenase-1, which allows the accumulation of the organic osmolyte glycerol (Lee and Strange, 2012). Interestingly, in mouse inner medullary collecting duct cells, hyperosmolar urea stress results in a similar increase in eIF2α-phosphorylation by GCN2, which is protective for cell survival under the osmotic stress faced by cells in the renal medulla (Cai and Brooks, 2011). Whether WNK is activated in this circumstance has not been examined.
In mammals, several studies have connected cellular osmoregulation, WNK-SPAK/OSR1 signaling, and disease. ASK3 (apoptosis signal-regulating kinase 3) is a mammalian MAPK kinase kinase that is activated under hypotonic conditions and repressed under hypertonic conditions. ASK3 is a negative regulator of WNK-SPAK/OSR1 signaling, and therefore is expected to increase WNK-SPAK/OSR1 activation under hypertonic conditions. Consistent with its role as a negative regulator of WNK-SPAK/OSR1 signaling, ASK3 knockouts have increased SPAK/OSR1 phosphorylation and hypertension (Naguro et al., 2012). In addition, an ASK3 phosphorylation site on WNK4, Ser 575, has been identified, although the functional consequence of this phosphorylation event has not been described. (Maruyama et al., 2016).
Two studies have linked activation of WNK-SPAK/OSR1 signaling and cell volume regulation to the migration of glioma cells. Gliomas are locally invasive glial cell tumors, and the malignant glial cells undergo dynamic cell volume changes during migration. Glioma cells express WNKs 1, 3, and 4, SPAK and OSR1, and NKCC1. Inhibiting NKCC1 with bumetanide, or knocking down WNK1 or WNK3, inhibits cell volume recovery after a hypertonic challenge (Haas et al., 2011; Zhu et al., 2014). Bumetanide or WNK3 knockdown decreased glioma cell migration in a Transwell assay (Haas et al., 2011). The chemotherapeutic agent temozolomide, which is used to treat gliomas, stimulated migration and serum-induced microchemotaxis by activating the WNK1-OSR1-NKCC1 pathway in some glioma cell lines, an effect which could diminish temozolomide’s anti-neoplastic properties (Zhu et al., 2014). Bumetanide treatment, or knockdown of WNK1 or OSR1, abolished this effect, suggesting that inhibition of the WNK1-OSR1-NKCC1 pathway could be beneficial as adjunctive treatment with temozolomide for some patients with glioma (Zhu et al., 2014).
A role for the WNK1-SPAK/OSR1-NKCC1 pathway has also been demonstrated in T cell migration. Knocking down WNK1, SPAK, OSR1 or NKCC1, or treatment with bumetanide, decreased T cell migration in multiple assays. Furthermore, Wnk1-deficient T cells have decreased homing and migration in lymph nodes in vivo in mice (Kochl et al., 2016). Whether this is due to alterations in cell volume was not examined, but is a possible explanation for the altered migration. WNK1 is also a negative regulator of T cell adhesion, but this effect was independent of SPAK/OSR1 or NKCC1 (Kochl et al., 2016), suggesting multiple roles for WNK1 in T cell biology and immune system function.
In stroke, activation of WNK3-SPAK/OSR1 signaling is deleterious. Cerebral edema accompanies severe strokes and is associated with mortality rate of up to 80%, leading to increased interest in treating this complication (Bardutzky and Schwab, 2007). An early component of cerebral edema is cytotoxic edema (cell swelling) (Stokum et al., 2016). After middle cerebral artery occlusion in mice, the WNK3-SPAK/OSR1-NKCC1 pathway is activated in neurons and glial oligodendrocytes by unknown mechanisms, potentially increasing cerebral edema. Indeed, mice in which WNK3 or SPAK are knocked out have decreased edema, as well as decreased infarct size and axonal demyelination. Importantly, functional neurological outcomes after stroke are also improved, suggesting that the WNK3-SPAK/OSR1-NKCC1 pathway could be a therapeutic target in stroke (Begum et al., 2015; Zhao et al., 2016). The currently available NKCC1 inhibitor, bumetanide, has low blood-brain barrier permeability, and a poor side effect profile due to inhibition of renal NKCC2 (Donovan et al., 2016; Pressler et al., 2015). Attempts to develop compounds that distinguish between NKCC1 and NKCC2 have been complicated by the structural similarity of the transporters (Lykke et al., 2016). Despite the presence of WNK3 in the kidney, WNK3 knockout has minimal effect on renal function, probably because of compensation by WNK4 and WNK1 (Mederle et al., 2013; Oi et al., 2012). Thus, WNK3 may be an attractive target for inhibition in the setting of stroke.
2.4 The role of Mo25 in WNK-SPAK/OSR1 signaling
Mouse protein-25 (Mo25, also called calcium binding protein 39 or Cab39), is a scaffold protein that binds to the pseudokinase STE20-related adaptor (STRAD) and liver kinase B1 (LKB1) to activate LKB1 (Boudeau et al., 2003; Zeqiraj et al., 2009). SPAK/OSR1/Fray are additional members of the STE20 kinase family (Delpire and Gagnon, 2008) and a 2008 study in Drosophila revealed that Mo25 and fray work together in the process of asymmetric cell division (reviewed in greater detail in section 3.3.3) (Yamamoto et al., 2008). A subsequent study demonstrated that Mo25α increases the in vitro kinase activity of SPAK and OSR1 by 70- to 90-fold (Filippi et al., 2011). The related Mo25α also stimulated SPAK and OSR1 in vitro, though to a somewhat lesser degree. These experiments utilized SPAK and OSR1 mutants in which the T-loop threonine targeted by WNKs was mutated to a phospho-mimicking glutamic acid. Mo25 did not stimulate the activity of wild-type OSR1, unless WNK1 was co-incubated in the reaction. These experiments suggested that Mo25 and WNKs synergistically increase SPAK/OSR1 kinase activity. In cultured human embryonic kidney cells, NKCC1 activity was decreased in both baseline and stimulated (hypotonic low-chloride) conditions when Mo25α was knocked down (Filippi et al., 2011).
The crystal structure of dimerized OSR1 demonstrated domain swapping of the activation loop in OSR1 (Lee et al., 2009). Domain swapping allows exchange of identical structural elements between monomers within a protein dimer, without disrupting chemical interactions present in monomeric forms. Lee et al. proposed that OSR1 domain swapping may allow for trans-autophosphorylation. Based on structural and mutational analysis of MST4, another STE20 kinase that complexes with Mo25, Shi and coworkers proposed that Mo25 may facilitate the trans-autophosphorylation of MST4 dimers in order to fully activate MST4 (Shi et al., 2013). Indeed, the crystal structure of a SPAK mutant in which the WNK target T-loop threonine is mutated to a phospho-mimicking aspartic acid (T243D) demonstrated a partially active conformation, supporting the hypothesis that Mo25 binding allows for full activation of SPAK/OSR1 kinases after partial activation by WNK phosphorylation (Taylor et al., 2015). Further support for the hypothesis that Mo25 facilitates domain swapping in SPAK/OSR1 dimers was provided by an elegant series of experiments by Delpire and colleagues. They examined NKCC1 activation in Xenopus oocytes injected with cRNAs for NKCC1, Mo25, and wild-type or mutated SPAK monomers or concatemerized dimers. In the presence of Mo25, a wild-type Thr in the swap domain of SPAK could substitute for a mutated Thr (to Ala) in the SPAK in the other half of the concatamerized dimer, allowing for NKCC1 activation. This did not occur if wild-type SPAK and the Thr-to-Ala mutant SPAK were introduced as separate monomers, indicating that prior dimerization (experimentally recapitulated by concatamerization) is required to observe the Mo25 effect. The authors proposed that WNK phosphorylation of SPAK allows it to assume a domain swapping-competent conformation, which is further facilitated by Mo25 (Ponce-Coria et al., 2012), consistent with the results of the structural studies of SPAK T243D described above (Taylor et al., 2015). An additional study from the Delpire group, examining mouse and sea urchin OSR1 with or without Mo25, adds additional insights into OSR1 activation mechanisms (Gagnon et al., 2011).
The Delpire group also observed that WNK4 contains a domain that resembles the PF2 WNK binding domain of SPAK and OSR1. They therefore wondered whether WNK4 could bind to NKCCs directly, independently of SPAK/OSR1, and phosphorylate and activate the transporters. Indeed, while WNK4 alone did not stimulate NKCC1 or NKCC2 activity when cRNAs were co-injected into oocytes, the combination of WNK4 and Mo25 was able to stimulate both NKCC1 and NKCC2. Xenopus oocytes express an endogenous OSR1 (Pacheco-Alvarez et al., 2012), but the WNK4/Mo25 stimulation of NKCC1 was not inhibited by co-injection of kinase-dead SPAK, nor was it abolished by mutating the WNK4 RFXV motif required for SPAK/OSR1 binding, suggesting independence from SPAK/OSR1 activity. Similarly, Mo25 mutants lacking the ability to bind to SPAK/OSR1 were still able to stimulate NKCC1 activity when co-expressed with WNK4. However, a WNK4 mutant lacking NKCC1 binding was not able to stimulate NKCC1 activity, even in the presence of Mo25. Together, these results suggest that WNK4 could directly activate NKCC1 independently of SPAK/OSR1, in the presence of Mo25 (Ponce-Coria et al., 2014). The role of Mo25 in transepithelial ion transport has not been elucidated, but Mo25 is expressed in both the thick ascending limb and the distal convoluted tubule (Grimm et al., 2012), suggesting that it could play a modulatory role in regulation of NKCC2 and NCC by WNK-SPAK/OSR1 signaling in the mammalian nephron.
3. Emerging functions of the WNK signaling axis in development
3.1 Mammalian WNKs
As discussed in the previous sections, a substantial amount of knowledge about the function of the WNK-SPAK/OSR1 kinase axis in the regulation of ion transport has been discovered. Potential additional roles of WNKs important for the development of vertebrates and invertebrates have started to emerge only recently, and surprisingly little is known about embryonic functions of WNKs.
Human WNK1 is widely expressed in most tissues, including in the embryonic heart, skin, spleen, and the small intestine (Verissimo and Jordan, 2001). WNK2 is expressed in the fetal brain and heart, WNK3 in fetal brain, while WNK4 appears more restricted to the embryonic liver and skin (Verissimo and Jordan, 2001). While the phenotype of Wnk2 knock-out mice is unknown, Wnk3 mutant mice are homozygous viable and show no gross abnormalities (Mederle et al., 2013; Oi et al., 2012). Similarly, mice lacking WNK4 are born at Mendelian ratios and show no overt developmental or behavioral defects (Castaneda-Bueno et al., 2012; Takahashi et al., 2014). WNK3 and WNK4 are thus either not required for early development or their functions may be redundant with other WNK family members.
In contrast, a gene trap allele of Wnk1 in mice is embryonic lethal prior to day E13 with heterozygotes showing no developmental phenotype (but a reduced blood pressure as adults) (Zambrowicz et al., 2003). A more detailed time-course analysis by Xie et al. showed that homozygous Wnk1 mutant embryos start to show growth retardation at E9.5 and are all abnormal by E10.5, displaying pericardial edema and hemorrhage (Xie et al., 2009). Importantly, the lack of detectable blood flow suggested cardiovascular developmental defects. Indeed, while the four cardiac chambers and the dorsal aortae and cardinal veins form, the heart chambers are hypoplastic and show significantly reduced trabeculation and thinner outer myocardial walls (Fig. 5A,B) (Xie et al., 2009). The dorsal aortae and cardinal veins are smaller or collapsed, the latter likely caused by secondary blood circulation defects. Furthermore, Wnk1 mutant vessels of the yolk sac do not properly remodel and embryonic arteries and veins show defective angiogenesis including co-expression of the arterial and venous markers Neuropilin-1 and EphB4, which are usually expressed in a mutually exclusive manner. WNK1 is thus either involved in venous versus arterial fate specification or maintenance of those fates (Xie et al., 2009).
Figure 5. Phenotypes of loss of WNKs in mouse, zebrafish, and Drosophila.
(A, B) H&E staining of transverse sections of an E10.5 wild-type (A) and Wnk1 mutant embryo (B). Compared to WT (A), Wnk1 mutant embryos show reduced ventricular trabeculation (yellow arrows) and dilatation of pericardial sac (black arrows). RA/ LA: right/ left atrium; BC: bulbus cordis; CV: common ventricle. Modified from (Xie et al., 2009). (C, D) Lateral views of the trunk of uninjected zebrafish control embryos (C) and wnk1b morphants (D) at 33 hpf (hours post fertilization). Growth of the intersegmental vessels (ISVs) and formation of the dorsal longitudinal anastomotic vessel (DLAV) are inhibited in wnk1 morphants. Vessels formed by the vasculogenesis process, including the dorsal aorta (DA) and the posterior cardinal vein (PCV), are unaffected. After (Lai et al., 2014). (E, F) Compared to WT zebrafish (E), embryos specifically lacking the Wnk1/HSN2 isoform at 72 hpf (F) show posterior lateral line defects (neuromasts stained with vital dye 4-di-2-ASP are indicated with yellow arrows in lower panels) After (Bercier, 2013). (G–L) Drosophila wnk phenotypes. (G–J) Compared to the abdomen of a WT fly covered with cuticle and bristles (G), homozygous wnk mutant tissue (identified by the absence of pigment due to concomitant lack of the yellow gene; H) is unable to form cuticle and bristles. (I) Re-expression of Awh in clones mutant for wnk largely restores cuticle formation and partially suppresses bristle defects. (J) Coexpression of constitutively active Fray restores the cuticle and bristles on abdomina expressing dominant-negative Wnk, which lack abdominal cuticle and bristles (not shown). Yellow arrowheads indicate mutant tissue in H, I. Modified from (Sato and Shibuya, 2013). (K, L) Loss of wnk leads to a reduction in expression of the Wnt target gene Sens in 3rd instar wing imaginal discs. (K) WT wing discs express Wg in a line along the dorso-ventral boundary (red; single channel shown in K’) where it induces the expression of its target gene Sens in abutting cells (green; green arrowheads in single channel image K’’). (L) Homozygous wnk mutant cells marked by the absence of GFP (green) in mosaic discs cell autonomously express reduced levels of Sens (blue; single channel in L’’; yellow arrowheads indicate mutant areas). Note that there is no effect on Wg expression (red; single channel in L’). After (Serysheva et al., 2013). Scale bars are 100 µm in A-E.
Even though Wnk1 is expressed in all layers of the developing heart, endothelial-specific knock-out of Wnk1 using a conditional allele recapitulates all phenotypes of the global knock-out, suggesting that the observed phenotypes are due to an endothelial-specific requirement of WNK1 (Xie et al., 2009). Consistent with this, the heart and vascular phenotypes of the global Wnk1 knock-out were rescued by a Wnk1 transgene specifically expressed in endothelial cells, but not by reexpressing Wnk1 in somatic embryonic cells only. However, these animals are smaller at birth and die perinatally for uncharacterized reasons, suggesting additional roles for WNK1 beyond the cardiovascular system.
WNK1 functions either via OSR1/SPAK, or through kinase-independent mechanisms, such as the activation of SGK kinase or by modulating GPCR signaling (An et al., 2011; Xu et al., 2005a; Xu et al., 2005b). It was thus important to determine the mechanism by which WNK1 regulates cardiovascular development. Intriguingly, homozygous Osr1 mutant mice in which the catalytic domain is truncated show indistinguishable phenotypes to the Wnk1 mutants (Xie et al., 2013). Moreover, expression of a constitutively active form of OSR1 in endothelial cells is sufficient to suppress the heart and angiogenesis defects of global Wnk1 mutant embryos, showing that WNK1 acts via OSR1 during mouse embryonic development (Xie et al., 2013). The mechanistic cause of the heart and angiogenesis defects downstream of OSR1 remains to be determined. In particular, SLC12 cation chloride cotransporter knockouts do not show similar phenotypes, although redundant roles cannot be excluded (reviewed in (Arroyo et al., 2013; Delpire and Mount, 2002; Gamba, 2005)).
3.2 Zebrafish Wnk1a/b have roles in angiogenesis and neural development
The zebrafish genome encodes two paralogs each of wnk1 and wnk4, and a single wnk2 gene (Howe et al., 2013), with only wnk1a and wnk1b expression being detectable during early embryogenesis (prior to 48 hours post fertilization) (Lai et al., 2014). Knockdown of either wnk1a or wnk1b causes significant defects in angiogenesis of head and trunk blood vessels. In particular, intersegmental vessels (ISVs) that sprout and elongate dorsally from the dorsal aorta and the posterior cardinal vein (PCV) fail to form or do not properly extend (Fig. 5C,D) (Lai et al., 2014). This phenotype can be significantly rescued by re-expression of wnk1, suggesting that the morpholino effect is specific (Kok et al., 2015). In contrast to the angiogenesis defect, vasculogenesis, the de novo formation of blood vessels, is normal as judged by normal expression of the vasculogenesis marker etv2 (Sumanas and Lin, 2006) and the presence of the dorsal aorta or the caudal and posterior cardinal veins (Fig. 5D) (Lai et al., 2014). Intriguingly, the knockdown phenotype of wnk1a or wnk1b is similar to the knockdown of flk1, the gene encoding Vegfr2 (Vascular endothelial growth factor receptor 2). Vegfr2 mediates most of the angiogenic effects of Vegf via the activation of phosphoinositide-dependent protein kinase PI3K and Akt/Protein kinase B (PKB), and knockdown of pi3kc2α causes similar angiogenesis defects as reduction of flk1 or wnk1 (Lai et al., 2014). Human WNK1 has been shown to be an Akt substrate (Vitari et al., 2004), and zebrafish Wnk1 contains a putative Akt phosphorylation site, suggesting that Wnk1 could be downstream of Akt in the VEGF signaling pathway (Fig. 3C). Interestingly, the vascular phenotype of flk1 knockdown is partially rescued by injection of mRNA encoding wild-type Wnk1a, but not by kinase-dead Wnk1a or Wnk1a with a mutation in the putative Akt site. This is consistent with a role for Wnk1 kinase activity downstream of Vegfr2 during angiogenesis in zebrafish (Fig. 3C). In addition, VEGF signaling also appears to play a role in transcriptional regulation of wnk1, as wnk1 mRNA is downregulated upon flk1 knockdown (Lai et al., 2014). As in mice, the downstream effectors of Wnk1 in angiogenesis remain to be determined. Although it is not known if WNK1 acts downstream of Vegfr in mice, based on the fish and mouse data Wnk1 may have a conserved role in vascular development in humans (Lai et al., 2014; Xie et al., 2009; Xie et al., 2013). Such a function would also be consistent with recent data showing a requirement for WNK1 in human umbilical vein endothelial cell (HUVECs) and human dermal microvascular endothelial cell (HDMECs) models of in vitro angiogenesis (Dbouk et al., 2014).
WNK kinases may also have a function in the nervous system (see also 3.3. for Drosophila Wnk). For example, although no loss of function data is available, WNK2 is strongly expressed in the mouse brain, where it is found in a phospho-protein complex with SPAK and may regulate GABAergic signaling ((Rinehart et al., 2011); see also (Alessi et al., 2014) for a review of potential involvement of ion transporters). Whole genome exome sequencing also identified rare variants in WNK1 in patients affected by Charcot-Marie-Tooth (CMT), a form of peripheral neuropathy (Gonzaga-Jauregui et al., 2015). Most interestingly though, stop codon mutations in an extra neuron-specific exon between exons 8 and 9 in WNK1 have been identified in hereditary sensory and autonomic neuropathy type II (HSANII) patients (WNK1/HSN2 isoform; note that WNK1/HSN2 mutations can occur in trans to an allele truncating WNK1) (Lafreniere et al., 2004; Shekarabi et al., 2008). HSANII is a recessive disease characterized by an early onset of lack of peripheral sensory functions (Auer-Grumbach et al., 2006). As the disease is non-progressive and nerves of affected individuals show fewer fibers without signs of degeneration, HSANII is thought to have developmental roots, which is supported by an elegant zebrafish model of HSANII developed by Bercier et al. (Bercier, 2013; Bercier et al., 2013). Sequence comparison showed that only zebrafish wnk1b has the ability to encode a HSN2 exon and antibody stainings confirmed its expression in the neuromasts of the posterior lateral line (PLL), a peripheral sensory organ responsive to water pressure. Knockdown of the HSN2 isoform of wnk1b using MOs targeting corresponding splice sites causes a strong reduction of neuromasts (Fig. 5E,F) and their hair cells that is partially rescued by injecting mRNA coding for the HSNII-type of wnk1b (Bercier et al., 2013). Interestingly, loss of neuromast hair cells coincides with a transcriptional upregulation of kcc2 (distinct from regulating KCCs through phosphorylation). Indeed, overexpression of human KCC2 mRNA mimics the PLL defects and the reduced size of neuromast precursor area of wnk1b knockdown. Consistent with this, combined knockdown of kcc2 with wnk1b partially suppresses the defects caused by loss of wnk1b. Unexpectedly, although the phenotype was weaker than with overexpression of wild-type human KCC2, expression of a KCl transport- incompetent mutant of KCC2, KCC2C568A (Reynolds et al., 2008), also prevented proper PLL formation, suggesting that loss of Wnk1b/HSN2 causes a transcriptional upregulation of KCC2, in turn preventing correct development of this sensory neuronal system in a potentially (at least partially) transport-independent manner (Bercier et al., 2013). Whether the effects of Wnk1 on KCC2 are mediated by SPAK/OSR1 was not examined in this study.
HSANII patients lose peripheral nerve fibers concomitant with reduced pain sensation (Lafreniere et al., 2004). Wnk1 mutant mice specifically lacking the HSN2 exon (Wnk1ΔHsn2) have a somewhat different phenotype, with normal peripheral sensory neuron morphology and distribution (Kahle et al., 2016). Nevertheless, these mice were less susceptible to pain hypersensitivity resulting from peripheral nerve injury. Interestingly, in mice, loss of Wnk1ΔHsn2 led to a reduced phosphorylation of KCC2 and thus a more active transporter (Kahle et al., 2016). Thus, the WNK1 HSN2 isoform may be a target for treatment of pain syndromes resulting from peripheral nerve injury. Future experiments will have to address the mechanistic differences between WNK1 and KCC2 in mice and fish in the peripheral sensory nervous system, and how these relate to the human HSANII phenotype.
The WNK1-KCC2 axis also plays a role in the developmental maturation of neurons. Neuronal intracellular chloride concentration in many cases decreases with postnatal development, due to an increase in KCC2 expression and activity (reviewed in (Kaila et al., 2014)). One potential role for maintaining lower KCC2 activity at earlier developmental timepoints is to allow normal neuronal migration (Inoue et al., 2012), while later increases in KCC2 activity allow the lowering of intraneuronal chloride concentration that allows GABA and glycine neurotransmission to result in a hyperpolarizing or inhibitory effect by opening ligand-gated chloride channels (Kaila et al., 2014). In cultured hippocampal neurons, the developmental shift in KCC2 activity is mimicked by WNK1 knockdown. Expression of a dominant-negative, kinase-dead WNK1 in immature neurons has a similar effect that is reversed by simultaneous knockdown of KCC2. These results were also recapitulated with chemical inhibition of WNK1 (Friedel et al., 2015) suggesting that WNK1 inhibition of KCC2 in immature neurons maintains a higher intracellular chloride concentration. Indeed, WNK1 inhibits the activity of all mammalian KCCs when co-expressed in Xenopus oocytes (Fig. 3A) (Mercado et al., 2016). In mouse brain (Rinehart et al., 2009), or in cultured hippocampal and cortical neurons (Friedel et al., 2015), KCC2 phosphorylation, which results in transporter inactivation (Rinehart et al., 2009), decreases with maturation. In cells in which KCC2 is inactivated by introducing phospho-mimicking threonine-to-glutamate mutations, WNK1 inhibition has no effect on intracellular chloride. Together, the results suggest that increased WNK1 activity in immature neurons maintains KCC in a phosphorylated, inactive state that allows for higher intracellular chloride at that developmental timepoint (Friedel et al., 2015). How is higher WNK1 activity maintained in immature neurons despite the higher intracellular chloride, which is inhibitory toward WNK1? One possible mechanism is through activation of WNK1 by intracellular taurine, which is high in the fetal brain, though additional mechanisms may also play a role (Inoue et al., 2012). Additional roles for WNK signaling in nervous system physiology and disease are reviewed in (Alessi et al., 2014) and (Tang, 2016).
3.3 Insights from the Drosophila Wnk-Frayed axis
Over recent years, unexpected WNK functions critical for organismal development have been discovered using the fruitfly Drosophila melanogaster model, and at least some of these are conserved in mice or human cells. As discussed above, the genome of D. melanogaster encodes one wnk gene and one homolog of OSR1/SPAK, fray (Fig. 2). Drosophila wnk was first identified in a genetic mosaic screen for axon pathfinding in the eye, a classical model system used by geneticists as the eye is dispensable for viability under lab conditions. Based on the identification of mutations within the kinase domain, it was suggested that the axon targeting function of Wnk required kinase activity, but no further functional studies were performed (Berger et al., 2008).
More recently, it was shown that Drosophila Wnk has additional important functions regulating Wnt signaling during wing development and regulating the LIM-homeobox transcription factor Arrowhead (Curtiss and Heilig, 1995; Curtiss and Heilig, 1997) during development of the adult cuticle and likely the embryonic nervous system (Sato and Shibuya, 2013; Serysheva et al., 2013; Serysheva et al., 2014).
3.3.1 A conserved role of Wnk in the activation of arrowhead/ Lhx8
Like fray mutations, wnk mutations are embryonic or larval homozygous lethal (Leiserson et al., 2000; Sato and Shibuya, 2013; Serysheva et al., 2013). Homozygous mutant abdominal tissue in mosaic animals or abdominal tissue overexpressing a kinase dead, dominant negative (DN) form of Wnk (WnkD420A) fails to form cuticle (Fig. 5G, H and not shown), a phenotype that can be suppressed by co-overexpression of constitutively active Fray (FrayS347D; Fig. 5J) (Sato and Shibuya, 2013). Similarly, the peripheral axon growth phenotype caused by dominant negative Wnk in Drosophila embryos can be suppressed by FrayS347D. In addition, the formation of ectopic wing vein tissue in the posterior wing compartment caused by overexpression of Wnk is dominantly suppressed by the removal of one gene dose of fray, while Fray or human OSR1 overexpression causes similar wing vein defects (Sato and Shibuya, 2013). Thus, as is the case in the control of transepithelial ion flux in the Drosophila renal tubule (Wu et al., 2014), these data indicate that Wnk can act through Fray in Drosophila.
The adult Drosophila abdominal epidermal cuticle develops from histoblast cells set aside as nests in the embryo that only divide and migrate over the forming abdomen after metamorphosis (Madhavan and Madhavan, 1980). The mutant abdomen phenotype of wnk is highly reminiscent of the one caused by the loss of the LIM-Homeobox gene arrowhead (Awh), the homolog of vertebrate Lhx8 (Curtiss and Heilig, 1995; Curtiss and Heilig, 1997). Indeed, Sato et al. showed that histoblast nest-specific expression of Awh is lost in wnk mutant embryos and that overexpression of Awh in wnk mutant mosaics (Fig. 5I), or in the background of dominant-negative WnkD420A, can suppress the cuticle phenotype due to loss of wnk function (Sato and Shibuya, 2013).
Intriguingly, the functional axis from WNK to LHX8 is conserved in mice: expression of Lhx8 mRNA is strongly reduced in E9.5 Wnk1 mutant mouse embryos. Additionally, overexpression of Wnk1 and Wnk4 in NIH3T3 cells induces Lhx8 expression in the presence of cycloheximide and in an Osr1-dependent manner. Lhx8 mRNA and protein are also induced upon hypertonic stimulation, a known activator of WNK1 (Lenertz et al., 2005; Moriguchi et al., 2005), which is prevented by siRNA-mediated knockdown of Wnk1 or Wnk4. LHX8 was known to be involved in the determination of cholinergic neurons in the forebrain (Zhao et al., 2003). Therefore, Sato et al. further tested if differentiation of Neuro2A cells in culture was mediated by WNK. Indeed, induction of differentiation by retinoic acid treatment stimulated OSR1 phosphorylation and neurite outgrowth. Additionally, WNK1 and WNK4 were required in a redundant manner for the induction of the neural differentiation marker Choline Acetyl Transferase (ChAT) in Neuro2A cells. However, while constitutively active OSR1S325D expression was sufficient to suppress the knockdown of Wnk1 and Wnk4 with respect to neurite outgrowth and ChAT induction, overexpression of Lhx8 was not (Sato and Shibuya, 2013). This parallels Wnk function in the fly nervous system, where only overexpression of constitutively active Fray (see above), but not Awh was able to suppress the dominant-negative WnkD420A phenotype. This suggests that in addition to LHX8, there are other pathways downstream of Wnk-Fray/OSR1 required for neurite outgrowth. Clearly, these experiments demonstrate a strong conservation between flies and vertebrates of a novel function of the Wnk-Fray/OSR1 axis in regulating the transcription factor Awh/LHX8. It will be critical to identify missing pathway components that link OSR1/Fray to the transcriptional induction of Lhx8/Awh and the additional factors under control of WNK required in addition to LHX8/Awh for neurite outgrowth.
It is worth noting that while Wnk is required for axon growth of photoreceptor neurons in the fly eye (Berger et al., 2008) and dominant-negative Wnk prevents correct axon outgrowth in the embryo, homozygous mutant wnk embryos don’t show a clear axon outgrowth phenotype in the embryonic peripheral nervous system. Neither have axon outgrowth problems been reported for fray mutants. A very likely explanation for this discrepancy is that wnk and fray mRNAs are maternally deposited (Attrill et al., 2016) and identification of a peripheral axon outgrowth phenotype thus will require analysis of maternal-zygotic mutants (see also below). As mentioned, fray mutants show a defasciculation and axon bulging phenotype in embryos due to a function of Fray in ensheathing glia cells (Leiserson et al., 2000). In fray mutant embryos, subperineural glia cells (SPG) that form the paracellular nerve blood barrier fail to completely wrap axons and cause fluid-filled bulges between axons and glia (Leiserson et al., 2000). These phenotypes can be rescued by glial specific re-expression of Fray or its rat homolog SPAK. Further characterization of the mechanism showed that this function of Fray is mediated by Ncc69, the fly homolog of NKCC1 (Leiserson et al., 2011). ncc69 null mutants are homozygous viable and give rise to apparently normal adults, but mutant embryos show similar nerve bulging as fray mutants without affecting action potentials propagated by the nerves (Leiserson et al., 2011). Therefore, failure by SPGs to remove KCl from the space between neuron and glia likely draws H2O into the intercellular space via osmosis, causing nerve bulging (Leiserson et al., 2011).
WNK kinases may also influence the etiology of spinocerebellar ataxia type 1 (SCA1), a neurodegenerative disease caused by the extension of a polyglutamine repeat in ataxin 1, as human WNK4 and Drosophila Wnk were identified in kinome-wide screens for SCA1 (Park et al., 2013). siRNA-mediated reduction of WNK4 destabilized ATXN1(82Q) in culture, while knockdown of Drosophila wnk in vivo suppressed the photoreceptor neuron degeneration induced by ATXN1(82Q) overexpression. Both screens also identified several components of MAP kinase cascades, suggesting that Wnks may affect ATXN1 stability via their effect on MAPK signaling, reduction of which in turn was shown to ameliorate phenotypes of a mouse SCA1 model (Park et al., 2013).
3.3.2 Wnk in Wnt signaling
An additional function for WNK in Drosophila has been identified as a regulator of canonical Wnt signaling during wing development (Serysheva et al., 2013; Serysheva et al., 2014). The Wnt pathway (Fig. 3D) is a major and conserved signaling pathway regulating embryonic axis establishment in vertebrates and segmentation and patterning in Drosophila. Aberrant Wnt signaling not only causes strong developmental defects, but also various diseases including cancer (see also section 4 below) (Clevers, 2006; Clevers and Nusse, 2012; Swarup and Verheyen, 2012). In the absence of Wnt signaling, its central transcriptional cofactor, β-Catenin, is targeted for degradation by a destruction complex consisting of Axin, GSK3 and APC. GSK3 phosphorylation of β-Catenin marks it for ubiquitination and subsequent degradation by the proteasome. Signaling is activated by binding of a Wnt ligand (Wingless [Wg] in Drosophila) to a seven-pass transmembrane receptor of the Frizzled (Fz) family and a LRP5/6 coreceptor (Arrow [Arr] in Drosophila; Fig. 3D). Wnt binding induces recruitment of the Dishevelled (Dsh) adapter protein and formation of the LRP signalosome consisting of a complex of Fz, LRP5/6, Dsh, Axin and GSK3 (Bilic et al., 2007). This ultimately leads to the inactivation of the destruction complex and concomitant stabilization of β-Catenin (Kim et al., 2013; Li et al., 2012), allowing it to translocate to the nucleus to activate Wnt target genes by binding the transcription factor TCF/LEF (Pangolin in Drosophila) and additional cofactors (Brunner et al., 1997; Kramps et al., 2002).
In Drosophila in vivo, Wnt signaling can be assessed by its function during wing formation. Adult fly wings develop from epithelial cells, the so-called wing imaginal discs, that are set aside in the embryo and proliferate and differentiate during larval and pupal stages (reviewed in (Swarup and Verheyen, 2012)). In 3rd instar imaginal discs, Wg is expressed in a line of cells along the dorso-ventral boundary in the wing pouch, the part of the wing disc that will give rise to the adult wing blade (Fig. 5K) (Swarup and Verheyen, 2012). There, Wg induces the bHLH transcription factor Senseless (Sens) and Distalless (Dll), the fly member of the Dlx family of homeobox genes, both of which are required for patterning of the wing. In particular, Sens specifies margin bristles, which are thus structures that depend on a high level of Wnt signaling (Jafar-Nejad et al., 2006). A loss of Wnt signaling can be detected as a loss of Sens or Dll during development and wing margin defects in adults. In vitro, Wnt signaling is commonly assessed using transcriptional reporters (TOPFLASH assays) (Korinek et al., 1997) or by monitoring the phosphorylation state of Dishevelled in gel shift assays, which correlates with Wnt pathway activation (Lee et al., 1999; Matsubayashi et al., 2004; Yanagawa et al., 1995; Yanfeng et al., 2011).
Wnk kinase has recently been identified in two kinome-wide screens as a regulator of Wnt signaling. First, in a cell culture based screen, knockdown of wnk was found to reduce the level of Dsh phosphorylation (Serysheva et al., 2013). The second screen was an in vivo knockdown screen monitoring Wnt targets by immunohistochemistry (Swarup et al., 2015). Phenotypically, overexpression of dominant-negative Wnk, in vivo RNAi mediated knockdown of wnk in the wing, or loss of wnk function in mutant clones (patches of homozygous mutant wnk cells in a heterozygous background) cause lack of sensory bristles and margin defects in the adult wing (Sato and Shibuya, 2013; Serysheva et al., 2013). On the molecular level, staining of 3rd instar wing imaginal discs showed that Sens and Dll expression are lost after RNAi-mediated knockdown of wnk (Serysheva et al., 2013; Swarup et al., 2015) and Sens is lost in a cell autonomous manner in wnk mutant clones (Fig. 5L) (Serysheva et al., 2013). As the expression of the Wg ligand itself is not affected (Fig. 5L’), Wnk likely acts downstream of the Wnt ligand, but upstream or at the level of Dsh (the phosphorylation of which it affects). This is further supported by genetic interaction experiments: the cell death induced by overexpression of Dsh in the fly eye is dominantly suppressed by removal of one gene dose of wnk. Analogously, the ectopic Sens expression and margin bristles formed upon overexpression of the major Wnt receptor, dFz2, are dominantly suppressed by removal of one gene dose of wnk (the latter is also enhanced by concomitant overexpression of Wnk) (Serysheva et al., 2013). Consistent with this, knockdown of GSK3β to inhibit the destruction complex or expression of stable β-Catenin in wnk mutant cells does not prevent constitutive Wnt pathway activation.
Significantly, the function of Wnk in regulating Wnt signaling is conserved in human HEK293T cells. siRNA-mediated knockdown of WNK1 or WNK2, the two WNKs expressed in the HEK293T cells tested, reduces Wnt3a induced Wnt signaling as measured by a decrease in soluble (active) β-Catenin or by using a transcriptional luciferase reporter (TOPFLASH) (Serysheva et al., 2013). In contrast, overexpression of WNK2, but not a catalytically inactive version (WNK2K207A) promotes Wnt3a-induced stabilization of β-Catenin and TOPFLASH reporter activity, suggesting that the kinase activity is required for WNK to promote Wnt signaling. In line with these data, human WNK2/4 kinases were also identified in a genome-wide screen as candidate positive regulators of Wnt/β-catenin signaling in A375 melanoma cells (Biechele et al., 2012). However, for reasons not assessed, WNK1 in the same screen and Wnk in Drosophila Clone-8 cells antagonized Wnt signaling (Biechele et al., 2012; DasGupta et al., 2007).
Using purified Drosophila fusion proteins, direct phosphorylation of upstream Wnt signaling components, including Dsh, was not observed. As expected, however, Drosophila Wnk directly phosphorylates Fray (Sato and Shibuya, 2013; Serysheva et al., 2013). Consistent with this, knockdown of OSR1 and SPAK in HEK293T cells or RNAi-mediated knockdown of fray in Drosophila wing discs inhibits Wnt signaling, as assessed by reduction in TOPFLASH reporter activity and reduced Sens staining, respectively (Serysheva et al., 2013). This suggests that the conserved role of Wnk in Wnt signaling is mediated by OSR1/SPAK/Fray.
3.3.3 Intriguing issues to be addressed
As reviewed above, the mechanistic link(s) of the WNK-Fray/OSR1/SPAK signaling axis to various downstream effectors in various developmental pathways is unknown, but may well be conserved between flies and vertebrates. In particular, it is unknown whether OSR1/SPAK/Fray can phosphorylate Wnt pathway components such as Dsh or the Fz receptors or co-receptors, or whether Wnt signaling is influenced by CCCs (cation chloride cotransporters) via an unknown mechanism. The D. melanogaster genome encodes one KCC (kcc/CG5594) and two putative NKCCs (Ncc69/CG4357 and Ncc83/CG31547; NKCC transport activity has only been demonstrated for Ncc69) (Leiserson et al., 2011; Sun et al., 2010). ncc69 null mutants are viable and show no obvious developmental defects (such as of wing margins). Cold-sensitive, hypomorphic kcc alleles are bang-sensitive and show seizures likely due to a function of KCC in neurons of the mushroom and ellipsoid bodies in the brain, but also appear externally normal (Hekmat-Scafe et al., 2006; Hekmat-Scafe et al., 2010). Because strong loss-of-function kcc alleles are homozygous lethal, mosaics will have to be analyzed for Wnt related defects, likely also in combination with ncc69 and/or ncc83.
To this end, it is worth noting that there is no indication that Wnt signaling regulates Awh function in flies, and Wnk may thus independently affect Wnt signaling and the Awh expression required for cuticle formation and axon growth (Sato and Shibuya, 2013). In mice, however, the enhancer of Lhx8, which is required for craniofacial development, contains a conserved binding site for TCF/LEF that is critical for direct control of Lhx8 expression in primary maxillary arch cells by Wnt signaling (Landin Malt et al., 2014). Whether activation of this enhancer is mediated by WNKs via effects on Wnt signaling remains to be determined.
An additional protein that may have functional relevance with respect to WNK signaling in development is Mouse protein 25 (MO25), two paralogs of which exist in mice (see also section 2.4 above). MO25 proteins in various species from yeast to mammals have been shown to interact with Ste20-like kinases, stimulating their activity either directly, as for OSR1 and SPAK, or indirectly, via the induction of a trimeric complex with the pseudokinase STRAD (STE20-related kinase adaptor) in the case of the tumor suppressor LKB1 (Boudeau et al., 2003; Filippi et al., 2011; Mendoza et al., 2005; Nozaki et al., 1996). In Drosophila, Mo25 is implicated in asymmetric division of embryonic neuroblasts (Yamamoto et al., 2008). Neuroblasts are stem cells in the embryo that asymmetrically divide to give rise to a neuroblast (self-renewal) and a ganglion mother cell (GMC) that later divides and differentiates into neurons or glia cells (reviewed in (Gonczy, 2008)). During neuroblast division, the cell fate determinants Prospero, a homeobox transcription factor, the phosphotyrosine binding protein Numb and their respective adaptor proteins Miranda and Pons (Partner of Numb) localize basally and are thus asymmetrically inherited, specifying the basal daughter cell as the GMC. Loss of maternal and zygotic mo25 or fray in germline clones causes identical phenotypes: localization of Miranda in the cytoplasm and at the mitotic spindle instead of at the basal cortex (Yamamoto et al., 2008). The originally described requirement of Lkb1 for asymmetric neuroblast division is controversial, as it was later reported that lkb1 maternal-zygotic mutants show phenotypes during cellularization preventing the embryos to reach the stage of neurogenesis (Bonaccorsi et al., 2007; Yamamoto et al., 2008). On the other hand, Lkb1 overexpression causes a similar phenotype as loss of fray or mo25 and recruits Fray and Mo25 that are normally diffusely localized to the neuroblast cortex. Overexpression of both Mo25 and Fray are required to revert this effect, suggesting that levels of cytoplasmic Fray and Mo25 are critical for proper function in this context (Yamamoto et al., 2008). While Lkb1 can interact with Mo25 (in vivo co-IP) and Fray (in culture), it is not entirely clear whether the role of Lkb1 in neuroblast division is physiological. In particular, Lkb1 may not form a trimer with Fray and Mo25, as a Fray dimer formed via domain exchange will likely occur in a complex with Mo25 as discussed above (Ponce-Coria et al., 2012). Whether Drosophila Wnk has a role in regulation of Mo25/Fray in asymmetric neuroblast division has not been determined.
4. Functions of WNKs in cancer
WNK kinases have been linked to various cancers in recent years, although the mechanism by which they act is not well understood (for reviews see also (McCormick and Ellison, 2011; Moniz and Jordan, 2010; Tang, 2016)). Since WNKs can positively regulate Wnt signaling in Drosophila and cultured human cells, it is conceivable that WNKs affect Wnt related cancers such as the ones of the colon that are frequently caused by increased Wnt signaling (Clevers and Nusse, 2012). To this end, it was recently shown that β-Catenin is an (indirect) transcriptional target of WNK1 and that the proliferation of certain cancer cell lines with high β-Catenin activity is dependent on WNK1 (Dbouk et al., 2014; Rosenbluh et al., 2012). Furthermore, reduction of WNK1 also lowers levels of the EMT (epithelial mesenchymal transition) transcription factor Slug, thereby possibly having the potential to favor metastasis formation (Dbouk et al., 2014). In addition, WNK1 and WNK4 can phosphorylate Smad2, and silencing of WNK1 reduces Smad2 protein levels in HeLa cells, suggesting that WNKs have complex effects on TGFβ signaling (Lee et al., 2007), which itself can promote cancer or act in a tumor suppressing manner (Derynck et al., 2001). To delineate the mechanism of tumor- promoting and -antagonizing effects of WNKs will be a demanding process, as it has also been shown that RNAi-mediated knockdown of wnk in cultured Drosophila cells reduces the level of mTORC1 activity (as measured by S6-kinase phosphorylation), and Wnks thus likely also can alter cellular metabolism and growth (Lindquist et al., 2011).
Recently, it was shown that overexpression of FoxF1 upregulates WNK1 and its target ERK5-MAPKinase in a mouse transgenic adenocarcinoma prostate cancer model (TRAMP) (Fulford et al., 2016). Furthermore, FOXF1 expression correlated positively with that of MAP3K2 and WNK1 in human tumors. Wnk1 is a direct target of FoxF1 and its knockdown in Myc-CaP cells, a prostate carcinoma cell line from Myc overexpressing mice (Watson et al., 2005), reduced primary tumor size and metastasis formation in an orthotopic transplantation model. This effect was enhanced by simultaneous knockdown of MAP3K2 and is recapitulated by knockdown of their common downstream component ERK5, suggesting a role of WNK1 in the formation of prostate cancer (Fulford et al., 2016).
The situation for WNK2 is surprisingly different. In HeLa cells, WNK2 depletion reduces RhoA activation but increases Rac-GTP levels, thus causing stimulation of the p21-Cdc42-Rac1 activated kinase PAK1 and subsequent activation of MEK1 and ERK1/2 (Moniz et al., 2008), explaining WNK2’s anti-proliferative roles (Moniz et al., 2007). Promoter methylation studies showed that WNK2 is often silenced in gliomas and meningiomas (tumors originating from glia or the membranous layers surrounding the CNS, respectively) (Hong et al., 2007; Jun et al., 2009; Moniz et al., 2013). Knockdown of WNK2 in SW1088 cells, a ‘non-silenced’ glioblastoma cell line, promoted soft agar colony formation and increased cell migration in wound-healing (scratch) assays and invasion in Matrigel/membrane assays (Moniz et al., 2013). Conversely, re-expression of WNK2 in ‘silenced’ A172H glioblastoma cells inhibited their ability to form colonies in soft agar and tumor growth in a chick chorioallantoic membrane tumor model. Nevertheless, although WNK2 promoter methylation correlated with protein expression in cell lines, a statistically significant association between methylation-dependent silencing of WNK2 and disease progression has not been found (Moniz et al., 2013). However, lower WNK2 mRNA levels correlated with shorter survival time, suggesting that additional mechanisms other than promoter methylation must exist to lower WNK2 levels in gliomas (Moniz et al., 2013). Taken together, these studies thus provide strong evidence for WNK2 acting as a tumor suppressor.
5. Conclusions
WNK kinases have multiple and expanding roles in numerous physiological and patho-physiological processes. Best characterized to date is the role of WNKs in the kidney in the regulation of ion transport and the maintenance of blood pressure and electrolyte homeostasis. This role of WNKs, as well as a role in osmoregulation, is evolutionary conserved in invertebrate organisms. Intriguingly, in recent years, WNKs also have been shown to function during development, including vascular and neuronal development, and in the Wnt signaling pathway. Additionally, studies support roles for WNKs in cancer and the immune system. Ongoing study of these fascinating kinases is sure to uncover more.
Acknowledgments
The authors would like to thank Betsy Goldsmith, Xiaoshan Min, Chou-Long Huang, Chiou-Hwa (Cathy) Yuh, Pierre Drapeau, Atsushi Sato, and Jian Xie for sharing original figures, and Kevin Strange for helpful discussion. ARR is supported by NIDDK (DK091316 and DK106350) and the AHA (16CSA28530002). AJ is supported by AHA (13GRNT14680002) and NIGMS (R01GM115646).
References
- Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ. 2013;346:f1378. doi: 10.1136/bmj.f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Zhang J, Khanna A, Hochdorfer T, Shang Y, Kahle KT. The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci Signal. 2014;7:re3. doi: 10.1126/scisignal.2005365. [DOI] [PubMed] [Google Scholar]
- An SW, Cha SK, Yoon J, Chang S, Ross EM, Huang CL. WNK1 promotes PIP(2) synthesis to coordinate growth factor and GPCR-Gq signaling. Curr Biol. 2011;21:1979–1987. doi: 10.1016/j.cub.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrini O, Keck M, Briones R, Lourdel S, Vargas-Poussou R, Teulon J. ClC-K chloride channels: emerging pathophysiology of Bartter syndrome type 3. Am J Physiol Renal Physiol. 2015;308:F1324–F1334. doi: 10.1152/ajprenal.00004.2015. [DOI] [PubMed] [Google Scholar]
- Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH. WNK1 and OSR1 regulate the Na+, K+, 2Cl- cotransporter in HeLa cells. Proc Natl Acad Sci U S A. 2006;103:10883–10888. doi: 10.1073/pnas.0604607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo JP, Kahle KT, Gamba G. The SLC12 family of electroneutral cation-coupled chloride cotransporters. Mol Aspects Med. 2013;34:288–298. doi: 10.1016/j.mam.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Attrill H, Falls K, Goodman JL, Millburn GH, Antonazzo G, Rey AJ, Marygold SJ, FlyBase C. FlyBase: establishing a Gene Group resource for Drosophila melanogaster. Nucleic Acids Res. 2016;44:D786–D792. doi: 10.1093/nar/gkv1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer-Grumbach M, Mauko B, Auer-Grumbach P, Pieber TR. Molecular genetics of hereditary sensory neuropathies. Neuromolecular Med. 2006;8:147–158. doi: 10.1385/nmm:8:1-2:147. [DOI] [PubMed] [Google Scholar]
- Bardutzky J, Schwab S. Antiedema therapy in ischemic stroke. Stroke. 2007;38:3084–3094. doi: 10.1161/STROKEAHA.107.490193. [DOI] [PubMed] [Google Scholar]
- Barker M. Edema as influenced by a low ratio of sodium to potassium intake: clinical observations. J Amer Med Assoc. 1932;98:2193–2197. [Google Scholar]
- Battilana CA, Dobyan DC, Lacy FB, Bhattacharya J, Johnston PA, Jamison RL. Effect of chronic potassium loading on potassium secretion by the pars recta or descending limb of the juxtamedullary nephron in the rat. J Clin Invest. 1978;62:1093–1103. doi: 10.1172/JCI109215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazua-Valenti S, Chavez-Canales M, Rojas-Vega L, Gonzalez-Rodriguez X, Vazquez N, Rodriguez-Gama A, Argaiz ER, Melo Z, Plata C, Ellison DH, Garcia-Valdes J, Hadchouel J, Gamba G. The Effect of WNK4 on the Na+-Cl- Cotransporter Is Modulated by Intracellular Chloride. J Am Soc Nephrol. 2015;26:1781–1786. doi: 10.1681/ASN.2014050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum G, Yuan H, Kahle KT, Li L, Wang S, Shi Y, Shmukler BE, Yang SS, Lin SH, Alper SL, Sun D. Inhibition of WNK3 Kinase Signaling Reduces Brain Damage and Accelerates Neurological Recovery After Stroke. Stroke. 2015;46:1956–1965. doi: 10.1161/STROKEAHA.115.008939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercier V. WNK1/HSN2 isoform and the regulation of KCC2 activity. Rare Dis. 2013;1:e26537. doi: 10.4161/rdis.26537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercier V, Brustein E, Liao M, Dion PA, Lafreniere RG, Rouleau GA, Drapeau P. WNK1/HSN2 mutation in human peripheral neuropathy deregulates KCC2 expression and posterior lateral line development in zebrafish (Danio rerio) PLoS Genet. 2013;9:e1003124. doi: 10.1371/journal.pgen.1003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergaya S, Faure S, Baudrie V, Rio M, Escoubet B, Bonnin P, Henrion D, Loirand G, Achard JM, Jeunemaitre X, Hadchouel J. WNK1 regulates vasoconstriction and blood pressure response to alpha 1-adrenergic stimulation in mice. Hypertension. 2011;58:439–445. doi: 10.1161/HYPERTENSIONAHA.111.172429. [DOI] [PubMed] [Google Scholar]
- Berger J, Senti KA, Senti G, Newsome TP, Asling B, Dickson BJ, Suzuki T. Systematic identification of genes that regulate neuronal wiring in the Drosophila visual system. PLoS genetics. 2008;4:e1000085. doi: 10.1371/journal.pgen.1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biechele TL, Kulikauskas RM, Toroni RA, Lucero OM, Swift RD, James RG, Robin NC, Dawson DW, Moon RT, Chien AJ. Wnt/beta-catenin signaling and AXIN1 regulate apoptosis triggered by inhibition of the mutant kinase BRAFV600E in human melanoma. Sci Signal. 2012;5:ra3. doi: 10.1126/scisignal.2002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Blumenthal EM. Modulation of tyramine signaling by osmolality in an insect secretory epithelium. Am J Physiol Cell Physiol. 2005;289:C1261–C1267. doi: 10.1152/ajpcell.00026.2005. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi S, Mottier V, Giansanti MG, Bolkan BJ, Williams B, Goldberg ML, Gatti M. The Drosophila Lkb1 kinase is required for spindle formation and asymmetric neuroblast division. Development. 2007;134:2183–2193. doi: 10.1242/dev.02848. [DOI] [PubMed] [Google Scholar]
- Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, Prescott AR, Clevers HC, Alessi DR. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;22:5102–5114. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482:98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandis M, Keyes J, Windhager EE. Potassium-induced inhibition of proximal tubular fluid reabsorption in rats. Am J Physiol. 1972;222:421–427. doi: 10.1152/ajplegacy.1972.222.2.421. [DOI] [PubMed] [Google Scholar]
- Brunner E, Peter O, Schweizer L, Basler K. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila . Nature. 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- Cabrero P, Terhzaz S, Romero MF, Davies SA, Blumenthal EM, Dow JA. Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proc Natl Acad Sci U S A. 2014;111:14301–14306. doi: 10.1073/pnas.1412706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Brooks HL. Phosphorylation of eIF2alpha via the general control kinase, GCN2, modulates the ability of renal medullary cells to survive high urea stress. Am J Physiol Renal Physiol. 2011;301:F1202–F1207. doi: 10.1152/ajprenal.00272.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrisoza-Gaytan R, Carattino MD, Kleyman TR, Satlin LM. An unexpected journey: conceptual evolution of mechanoregulated potassium transport in the distal nephron. Am J Physiol Cell Physiol. 2016;310:C243–C259. doi: 10.1152/ajpcell.00328.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda-Bueno M, Cervantes-Perez LG, Rojas-Vega L, Arroyo-Garza I, Vazquez N, Moreno E, Gamba G. Modulation of NCC activity by low and high K(+) intake: insights into the signaling pathways involved. Am J Physiol Renal Physiol. 2014;306:F1507–F1519. doi: 10.1152/ajprenal.00255.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda-Bueno M, Cervantes-Perez LG, Vazquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci U S A. 2012;109:7929–7934. doi: 10.1073/pnas.1200947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Yazicioglu M, Cobb MH. Characterization of OSR1, a member of the mammalian Ste20p/germinal center kinase subfamily. J Biol Chem. 2004;279:11129–11136. doi: 10.1074/jbc.M313562200. [DOI] [PubMed] [Google Scholar]
- Cheng CJ, Truong T, Baum M, Huang CL. Kidney-specific WNK1 inhibits sodium reabsorption in the cortical thick ascending limb. Am J Physiol Renal Physiol. 2012;303:F667–F673. doi: 10.1152/ajprenal.00290.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CJ, Yoon J, Baum M, Huang CL. STE20/SPS1-related proline/alanine-rich kinase (SPAK) is critical for sodium reabsorption in isolated, perfused thick ascending limb. Am J Physiol Renal Physiol. 2015;308:F437–F443. doi: 10.1152/ajprenal.00493.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KP, Strange K. Evolutionarily conserved WNK and Ste20 kinases are essential for acute volume recovery and survival after hypertonic shrinkage in Caenorhabditis elegans. Am J Physiol Cell Physiol. 2007;293:C915–C927. doi: 10.1152/ajpcell.00126.2007. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-Catenin Signaling and Disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Cogswell ME, Zhang Z, Carriquiry AL, Gunn JP, Kuklina EV, Saydah SH, Yang Q, Moshfegh AJ. Sodium and potassium intakes among US adults: NHANES 2003–2008. Am J Clin Nutr. 2012;96:647–657. doi: 10.3945/ajcn.112.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Rangel S, Gamba G, Ramos-Mandujano G, Pasantes-Morales H. Influence of WNK3 on intracellular chloride concentration and volume regulation in HEK293 cells. Pflugers Arch. 2012;464:317–330. doi: 10.1007/s00424-012-1137-4. [DOI] [PubMed] [Google Scholar]
- Curtiss J, Heilig JS. Establishment of Drosophila imaginal precursor cells is controlled by the Arrowhead gene. Development. 1995;121:3819–3828. doi: 10.1242/dev.121.11.3819. [DOI] [PubMed] [Google Scholar]
- Curtiss J, Heilig JS. Arrowhead encodes a LIM homeodomain protein that distinguishes subsets of Drosophila imaginal cells. Dev Biol. 1997;190:129–141. doi: 10.1006/dbio.1997.8659. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Nybakken K, Booker M, Mathey-Prevot B, Gonsalves F, Changkakoty B, Perrimon N. A case study of the reproducibility of transcriptional reporter cell-based RNAi screens in Drosophila. Genome Biol. 2007;8:R203. doi: 10.1186/gb-2007-8-9-r203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbouk HA, Weil LM, Perera GK, Dellinger MT, Pearson G, Brekken RA, Cobb MH. Actions of the protein kinase WNK1 on endothelial cells are differentially mediated by its substrate kinases OSR1 and SPAK. Proc Natl Acad Sci U S A. 2014;111:15999–16004. doi: 10.1073/pnas.1419057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Los Heros P, Alessi DR, Gourlay R, Campbell DG, Deak M, Macartney TJ, Kahle KT, Zhang J. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+-Cl- co-transporters. Biochem J. 2014;458:559–573. doi: 10.1042/BJ20131478. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J. 2008;409:321–331. doi: 10.1042/BJ20071324. [DOI] [PubMed] [Google Scholar]
- Delpire E, Mount DB. Human and murine phenotypes associated with defects in cation-chloride cotransport. Annu Rev Physiol. 2002;64:803–843. doi: 10.1146/annurev.physiol.64.081501.155847. [DOI] [PubMed] [Google Scholar]
- Denton J, Nehrke K, Yin X, Morrison R, Strange K. GCK-3, a newly identified Ste20 kinase, binds to and regulates the activity of a cell cycle-dependent ClC anion channel. J Gen Physiol. 2005;125:113–125. doi: 10.1085/jgp.200409215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- Donovan MD, Schellekens H, Boylan GB, Cryan JF, Griffin BT. In vitro bidirectional permeability studies identify pharmacokinetic limitations of NKCC1 inhibitor bumetanide. Eur J Pharmacol. 2016;770:117–125. doi: 10.1016/j.ejphar.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Dow JA, Maddrell SH, Gortz A, Skaer NJ, Brogan S, Kaiser K. The malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J Exp Biol. 1994;197:421–428. doi: 10.1242/jeb.197.1.421. [DOI] [PubMed] [Google Scholar]
- Dowd BF, Forbush B. PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1) J Biol Chem. 2003;278:27347–27353. doi: 10.1074/jbc.M301899200. [DOI] [PubMed] [Google Scholar]
- Falin RA, Miyazaki H, Strange K. C. elegans STK39/SPAK ortholog-mediated inhibition of ClC anion channel activity is regulated by WNK-independent ERK kinase signaling. Am J Physiol Cell Physiol. 2011;300:C624–C635. doi: 10.1152/ajpcell.00343.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falin RA, Morrison R, Ham AJ, Strange K. Identification of regulatory phosphorylation sites in a cell volume- and Ste20 kinase-dependent ClC anion channel. J Gen Physiol. 2009;133:29–42. doi: 10.1085/jgp.200810080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas R, Pecenova L, Mentelova L, Beno M, Benova-Liszekova D, Mahmoodova S, Tejnecky V, Raska O, Juda P, Svidenska S, Hornacek M, Chase BA, Raska I. Massive excretion of calcium oxalate from late prepupal salivary glands of Drosophila melanogaster demonstrates active nephridial-like anion transport. Dev Growth Differentiation. 2016;58:562–574. doi: 10.1111/dgd.12300. [DOI] [PubMed] [Google Scholar]
- Ferdaus MZ, Barber KW, Lopez-Cayuqueo KI, Terker AS, Argaiz ER, Gassaway BM, Chambrey R, Gamba G, Rinehart J, McCormick JA. SPAK and OSR1 play essential roles in potassium homeostasis through actions on the distal convoluted tubule. Journal of Physiology. 2016;594:4945–4966. doi: 10.1113/JP272311. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdaus MZ, McCormick JA. The CUL3/KLHL3-WNK-SPAK/OSR1 pathway as a target for antihypertensive therapy. Am J Physiol Renal Physiol. 2016;310:F1389–F1396. doi: 10.1152/ajprenal.00132.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi BM, de los Heros P, Mehellou Y, Navratilova I, Gourlay R, Deak M, Plater L, Toth R, Zeqiraj E, Alessi DR. MO25 is a master regulator of SPAK/OSR1 and MST3/MST4/YSK1 protein kinases. EMBO J. 2011;30:1730–1741. doi: 10.1038/emboj.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel P, Kahle KT, Zhang J, Hertz N, Pisella LI, Buhler E, Schaller F, Duan J, Khanna AR, Bishop PN, Shokat KM, Medina I. WNK1-regulated inhibitory phosphorylation of the KCC2 cotransporter maintains the depolarizing action of GABA in immature neurons. Sci Signal. 2015;8 doi: 10.1126/scisignal.aaa0354. ra65. [DOI] [PubMed] [Google Scholar]
- Frindt G, Houde V, Palmer LG. Conservation of Na+ vs. K+ by the rat cortical collecting duct. Am J Physiol Renal Physiol. 2011;301:F14–F20. doi: 10.1152/ajprenal.00705.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford L, Milewski D, Ustiyan V, Ravishankar N, Cai Y, Le T, Masineni S, Kasper S, Aronow B, Kalinichenko VV, Kalin TV. The transcription factor FOXF1 promotes prostate cancer by stimulating the mitogen-activated protein kinase ERK5. Sci Signal. 2016;9 doi: 10.1126/scisignal.aad5582. ra48. [DOI] [PubMed] [Google Scholar]
- Gagnon KB, Delpire E. On the substrate recognition and negative regulation of SPAK, a kinase modulating Na+-K+-2Cl- cotransport activity. Am J Physiol Cell Physiol. 2010;299:C614–C620. doi: 10.1152/ajpcell.00074.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon KB, England R, Delpire E. Characterization of SPAK and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol Cell Biol. 2006;26:689–698. doi: 10.1128/MCB.26.2.689-698.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon KB, Rios K, Delpire E. Functional insights into the activation mechanism of Ste20-related kinases. Cell Physiol Biochem. 2011;28:1219–1230. doi: 10.1159/000335854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev. 2005;85:423–493. doi: 10.1152/physrev.00011.2004. [DOI] [PubMed] [Google Scholar]
- Gonczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nature reviews. Molecular cell biology. 2008;9:355–366. doi: 10.1038/nrm2388. [DOI] [PubMed] [Google Scholar]
- Gonzaga-Jauregui C, Harel T, Gambin T, Kousi M, Griffin LB, Francescatto L, Ozes B, Karaca E, Jhangiani SN, Bainbridge MN, Lawson KS, Pehlivan D, Okamoto Y, Withers M, Mancias P, Slavotinek A, Reitnauer PJ, Goksungur MT, Shy M, Crawford TO, Koenig M, Willer J, Flores BN, Pediaditrakis I, Us O, Wiszniewski W, Parman Y, Antonellis A, Muzny DM, Baylor-Hopkins Center for Mendelian G, Katsanis N, Battaloglu E, Boerwinkle E, Gibbs RA, Lupski JR. Exome Sequence Analysis Suggests that Genetic Burden Contributes to Phenotypic Variability and Complex Neuropathy. Cell Rep. 2015;12:1169–1183. doi: 10.1016/j.celrep.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, Wade JB, Welling PA. SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem. 2012;287:37673–37690. doi: 10.1074/jbc.M112.402800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BR, Cuddapah VA, Watkins S, Rohn KJ, Dy TE, Sontheimer H. With-No-Lysine Kinase 3 (WNK3) stimulates glioma invasion by regulating cell volume. Am J Physiol Cell Physiol. 2011;301:C1150–C1160. doi: 10.1152/ajpcell.00203.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadchouel J, Ellison DH, Gamba G. Regulation of Renal Electrolyte Transport by WNK and SPAK-OSR1 Kinases. Annu Rev Physiol. 2016;78:367–389. doi: 10.1146/annurev-physiol-021115-105431. [DOI] [PubMed] [Google Scholar]
- Hekmat-Scafe DS, Lundy MY, Ranga R, Tanouye MA. Mutations in the K+/Cl- cotransporter gene kazachoc (kcc) increase seizure susceptibility in Drosophila. J Neurosci. 2006;26:8943–8954. doi: 10.1523/JNEUROSCI.4998-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekmat-Scafe DS, Mercado A, Fajilan AA, Lee AW, Hsu R, Mount DB, Tanouye MA. Seizure sensitivity is ameliorated by targeted expression of K+-Cl- cotransporter function in the mushroom body of the Drosophila brain. Genetics. 2010;184:171–183. doi: 10.1534/genetics.109.109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashihara E, Kokko JP. Effects of aldosterone on potassium recycling in the kidney of adrenalectomized rats. Am J Physiol. 1985;248:F219–F227. doi: 10.1152/ajprenal.1985.248.2.F219. [DOI] [PubMed] [Google Scholar]
- Hirata T, Cabrero P, Berkholz DS, Bondeson DP, Ritman EL, Thompson JR, Dow JA, Romero MF. In vivo Drosophilia genetic model for calcium oxalate nephrolithiasis. Am J Physiol Renal Physiol. 2012a;303:F1555–F1562. doi: 10.1152/ajprenal.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T, Czapar A, Brin L, Haritonova A, Bondeson DP, Linser P, Cabrero P, Thompson J, Dow JA, Romero MF. Ion and solute transport by Prestin in Drosophila and Anopheles. J Insect Physiol. 2012b;58:563–569. doi: 10.1016/j.jinsphys.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamoto N, Moriguchi T, Urushiyama S, Mitani S, Shibuya H, Matsumoto K. Caenorhabditis elegans WNK-STE20 pathway regulates tube formation by modulating ClC channel activity. EMBO Rep. 2008;9:70–75. doi: 10.1038/sj.embor.7401128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- Hong C, Moorefield KS, Jun P, Aldape KD, Kharbanda S, Phillips HS, Costello JF. Epigenome scans and cancer genome sequencing converge on WNK2, a kinase-independent suppressor of cell growth. Proc Natl Acad Sci U S A. 2007;104:10974–10979. doi: 10.1073/pnas.0700683104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe DG, Bradford YM, Conlin T, Eagle AE, Fashena D, Frazer K, Knight J, Mani P, Martin R, Moxon SA, Paddock H, Pich C, Ramachandran S, Ruef BJ, Ruzicka L, Schaper K, Shao X, Singer A, Sprunger B, Van Slyke CE, Westerfield M. ZFIN, the Zebrafish Model Organism Database: increased support for mutants and transgenics. Nucleic Acids Res. 2013;41:D854–D860. doi: 10.1093/nar/gks938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Cheng CJ. A unifying mechanism for WNK kinase regulation of sodium-chloride cotransporter. Pflugers Arch. 2015;467:2235–2241. doi: 10.1007/s00424-015-1708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Furukawa T, Kumada T, Yamada J, Wang T, Inoue R, Fukuda A. Taurine inhibits K+-Cl- cotransporter KCC2 to regulate embryonic Cl- homeostasis via with-no-lysine (WNK) protein kinase signaling pathway. J Biol Chem. 2012;287:20839–20850. doi: 10.1074/jbc.M111.319418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafar-Nejad H, Tien AC, Acar M, Bellen HJ. Senseless and Daughterless confer neuronal identity to epithelial cells in the Drosophila wing margin. Development. 2006;133:1683–1692. doi: 10.1242/dev.02338. [DOI] [PubMed] [Google Scholar]
- Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. NCBI BLAST: a better web interface. Nucleic Acids Res. 2008;36:W5–W9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun P, Hong C, Lal A, Wong JM, McDermott MW, Bollen AW, Plass C, Held WA, Smiraglia DJ, Costello JF. Epigenetic silencing of the kinase tumor suppressor WNK2 is tumor-type and tumor-grade specific. Neuro Oncol. 2009;11:414–422. doi: 10.1215/15228517-2008-096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Rinehart J, Ring A, Gimenez I, Gamba G, Hebert SC, Lifton RP. WNK protein kinases modulate cellular Cl- flux by altering the phosphorylation state of the Na-K-Cl and K-Cl cotransporters. Physiology (Bethesda) 2006;21:326–335. doi: 10.1152/physiol.00015.2006. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Schmouth JF, Lavastre V, Latremoliere A, Zhang J, Andrews N, Omura T, Laganiere J, Rochefort D, Hince P, Castonguay G, Gaudet R, Mapplebeck JC, Sotocinal SG, Duan J, Ward C, Khanna AR, Mogil JS, Dion PA, Woolf CJ, Inquimbert P, Rouleau GA. Inhibition of the kinase WNK1/HSN2 ameliorates neuropathic pain by restoring GABA inhibition. Sci Signal. 2016;9 doi: 10.1126/scisignal.aad0163. ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci. 2014;15:637–654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith NMa, Binger MW. Diuretic action of potassium. Journal of American Medical Association. 1935;105:1584–1591. 1935. [Google Scholar]
- Kim SE, Huang H, Zhao M, Zhang X, Zhang A, Semonov MV, MacDonald BT, Zhang X, Garcia Abreu J, Peng L, He X. Wnt stabilization of beta-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. 2013;340:867–870. doi: 10.1126/science.1232389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochl R, Thelen F, Vanes L, Brazao TF, Fountain K, Xie J, Huang CL, Lyck R, Stein JV, Tybulewicz VL. WNK1 kinase balances T cell adhesion versus migration in vivo. Nat Immunol. 2016 doi: 10.1038/ni.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, DeSantis DF, Sheppard-Tindell S, Ebarasi L, Betsholtz C, Schulte-Merker S, Wolfe SA, Lawson ND. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev Cell. 2015;32:97–108. doi: 10.1016/j.devcel.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolotuev I, Hyenne V, Schwab Y, Rodriguez D, Labouesse M. A pathway for unicellular tube extension depending on the lymphatic vessel determinant Prox1 and on osmoregulation. Nat Cell Biol. 2013;15:157–168. doi: 10.1038/ncb2662. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002;109:47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- Krishna GG, Miller E, Kapoor S. Increased blood pressure during potassium depletion in normotensive men. N Engl J Med. 1989;320:1177–1182. doi: 10.1056/NEJM198905043201804. [DOI] [PubMed] [Google Scholar]
- Kupinski AP, Muller-Reichert T, Eckmann CR. The Caenorhabditis elegans Ste20 kinase, GCK-3, is essential for postembryonic developmental timing and regulates meiotic chromosome segregation. Dev Biol. 2010;344:758–771. doi: 10.1016/j.ydbio.2010.05.505. [DOI] [PubMed] [Google Scholar]
- Lafreniere RG, MacDonald ML, Dube MP, MacFarlane J, O’Driscoll M, Brais B, Meilleur S, Brinkman RR, Dadivas O, Pape T, Platon C, Radomski C, Risler J, Thompson J, Guerra-Escobio AM, Davar G, Breakefield XO, Pimstone SN, Green R, Pryse-Phillips W, Goldberg YP, Younghusband HB, Hayden MR, Sherrington R, Rouleau GA, Samuels ME. Identification of a novel gene (HSN2) causing hereditary sensory and autonomic neuropathy type II through the Study of Canadian Genetic Isolates. Am J Hum Genet. 2004;74:1064–1073. doi: 10.1086/420795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JG, Tsai SM, Tu HC, Chen WC, Kou FJ, Lu JW, Wang HD, Huang CL, Yuh CH. Zebrafish WNK lysine deficient protein kinase 1 (wnk1) affects angiogenesis associated with VEGF signaling. PLoS One. 2014;9:e106129. doi: 10.1371/journal.pone.0106129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landin Malt A, Cesario JM, Tang Z, Brown S, Jeong J. Identification of a face enhancer reveals direct regulation of LIM homeobox 8 (Lhx8) by wingless-int (WNT)/beta-catenin signaling. J Biol Chem. 2014;289:30289–30301. doi: 10.1074/jbc.M114.592014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry GM, Hirata T, Anderson JB, Cabrero P, Gallo CJ, Dow JA, Romero MF. Sulfate and thiosulfate inhibit oxalate transport via a dPrestin (Slc26a6)-dependent mechanism in an insect model of calcium oxalate nephrolithiasis. Am J Physiol Renal Physiol. 2016;310:F152–F159. doi: 10.1152/ajprenal.00406.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen EH, Deaton LE, Onken H, O’Donnell M, Grosell M, Dantzler WH, Weihrauch D. Osmoregulation and excretion. Compr Physiol. 2014;4:405–573. doi: 10.1002/cphy.c130004. [DOI] [PubMed] [Google Scholar]
- Lee BH, Chen W, Stippec S, Cobb MH. Biological cross-talk between WNK1 and the transforming growth factor beta-Smad signaling pathway. The Journal of biological chemistry. 2007;282:17985–17996. doi: 10.1074/jbc.M702664200. [DOI] [PubMed] [Google Scholar]
- Lee EC, Strange K. GCN-2 dependent inhibition of protein synthesis activates osmosensitive gene transcription via WNK and Ste20 kinase signaling. Am J Physiol Cell Physiol. 2012;303:C1269–C1277. doi: 10.1152/ajpcell.00294.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Ishimoto A, Yanagawa S. Characterization of mouse dishevelled (Dvl) proteins in Wnt/Wingless signaling pathway. The Journal of biological chemistry. 1999;274:21464–21470. doi: 10.1074/jbc.274.30.21464. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Cobb MH, Goldsmith EJ. Crystal structure of domain-swapped STE20 OSR1 kinase domain. Protein Sci. 2009;18:304–313. doi: 10.1002/pro.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiserson WM, Forbush B, Keshishian H. Drosophila glia use a conserved cotransporter mechanism to regulate extracellular volume. Glia. 2011;59:320–332. doi: 10.1002/glia.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiserson WM, Harkins EW, Keshishian H. Fray, a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron. 2000;28:793–806. doi: 10.1016/s0896-6273(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Lenertz LY, Lee BH, Min X, Xu BE, Wedin K, Earnest S, Goldsmith EJ, Cobb MH. Properties of WNK1 and implications for other family members. J Biol Chem. 2005;280:26653–26658. doi: 10.1074/jbc.M502598200. [DOI] [PubMed] [Google Scholar]
- Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, Mohammed S, Heck AJ, Maurice MM, Mahmoudi T, Clevers H. Wnt Signaling through Inhibition of beta-Catenin Degradation in an Intact Axin1 Complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Lindquist RA, Ottina KA, Wheeler DB, Hsu PP, Thoreen CC, Guertin DA, Ali SM, Sengupta S, Shaul YD, Lamprecht MR, Madden KL, Papallo AR, Jones TR, Sabatini DM, Carpenter AE. Genome-scale RNAi on living-cell microarrays identifies novel regulators of Drosophila melanogaster TORC1-S6K pathway signaling. Genome Res. 2011;21:433–446. doi: 10.1101/gr.111492.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton SM, O’Donnell MJ. Contributions of K+:Cl- cotransport and Na+/K+-ATPase to basolateral ion transport in malpighian tubules of Drosophila melanogaster. J Exp Biol. 1999;202:1561–1570. doi: 10.1242/jeb.202.11.1561. [DOI] [PubMed] [Google Scholar]
- Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, International Consortium for Blood P, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet. 2012;44:456–460. doi: 10.1038/ng.2218. S1–3. [DOI] [PubMed] [Google Scholar]
- Lykke K, Tollner K, Feit PW, Erker T, MacAulay N, Loscher W. The search for NKCC1-selective drugs for the treatment of epilepsy: Structure-function relationship of bumetanide and various bumetanide derivatives in inhibiting the human cation-chloride cotransporter NKCC1A. Epilepsy Behav. 2016;59:42–49. doi: 10.1016/j.yebeh.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Madhavan MM, Madhavan K. Morphogenesis of the epidermis of adult abdomen of Drosophila. J Embryol Exp Morphol. 1980;60:1–31. [PubMed] [Google Scholar]
- Mandai S, Mori T, Sohara E, Rai T, Uchida S. Generation of Hypertension-Associated STK39 Polymorphism Knockin Cell Lines With the Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 System. Hypertension. 2015;66:1199–1206. doi: 10.1161/HYPERTENSIONAHA.115.05872. [DOI] [PubMed] [Google Scholar]
- Maruyama J, Kobayashi Y, Umeda T, Vandewalle A, Takeda K, Ichijo H, Naguro I. Osmotic stress induces the phosphorylation of WNK4 Ser575 via the p38MAPK-MK pathway. Sci Rep. 2016;6:18710. doi: 10.1038/srep18710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi H, Sese S, Lee JS, Shirakawa T, Iwatsubo T, Tomita T, Yanagawa S. Biochemical characterization of the Drosophila wingless signaling pathway based on RNA interference. Mol Cell Biol. 2004;24:2012–2024. doi: 10.1128/MCB.24.5.2012-2024.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev. 2011;91:177–219. doi: 10.1152/physrev.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederle K, Mutig K, Paliege A, Carota I, Bachmann S, Castrop H, Oppermann M. Loss of WNK3 is compensated for by the WNK1/SPAK axis in the kidney of the mouse. Am J Physiol Renal Physiol. 2013;304:F1198–F1209. doi: 10.1152/ajprenal.00288.2012. [DOI] [PubMed] [Google Scholar]
- Melo Z, de los Heros P, Cruz-Rangel S, Vazquez N, Bobadilla NA, Pasantes-Morales H, Alessi DR, Mercado A, Gamba G. N-terminal serine dephosphorylation is required for KCC3 cotransporter full activation by cell swelling. J Biol Chem. 2013;288:31468–31476. doi: 10.1074/jbc.M113.475574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza M, Redemann S, Brunner D. The fission yeast MO25 protein functions in polar growth and cell separation. Eur J Cell Biol. 2005;84:915–926. doi: 10.1016/j.ejcb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, Mony P, Devanath A, Rosengren A, Oguz A, Zatonska K, Yusufali AH, Lopez-Jaramillo P, Avezum A, Ismail N, Lanas F, Puoane T, Diaz R, Kelishadi R, Iqbal R, Yusuf R, Chifamba J, Khatib R, Teo K, Yusuf S, Investigators P. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371:601–611. doi: 10.1056/NEJMoa1311989. [DOI] [PubMed] [Google Scholar]
- Mercado A, de Los Heros P, Melo Z, Chavez-Canales M, Murillo-de-Ozores AR, Moreno E, Bazua-Valenti S, Vazquez N, Hadchouel J, Gamba G. With no lysine L-WNK1 isoforms are negative regulators of the K+:Cl- cotransporters. Am J Physiol Cell Physiol. 2016 doi: 10.1152/ajpcell.00193.2015. ajpcell 00193 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min X, Lee BH, Cobb MH, Goldsmith EJ. Crystal structure of the kinase domain of WNK1, a kinase that causes a hereditary form of hypertension. Structure. 2004;12:1303–1311. doi: 10.1016/j.str.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Miyazaki H, Strange K. Differential regulation of a CLC anion channel by SPAK kinase ortholog-mediated multisite phosphorylation. Am J Physiol Cell Physiol. 2012;302:C1702–C1712. doi: 10.1152/ajpcell.00419.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki H, Yamada T, Parton A, Morrison R, Kim S, Beth AH, Strange K. CLC anion channel regulatory phosphorylation and conserved signal transduction domains. Biophys J. 2012;103:1706–1718. doi: 10.1016/j.bpj.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniz S, Jordan P. Emerging roles for WNK kinases in cancer. Cell Mol Life Sci. 2010;67:1265–1276. doi: 10.1007/s00018-010-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniz S, Martinho O, Pinto F, Sousa B, Loureiro C, Oliveira MJ, Moita LF, Honavar M, Pinheiro C, Pires M, Lopes JM, Jones C, Costello JF, Paredes J, Reis RM, Jordan P. Loss of WNK2 expression by promoter gene methylation occurs in adult gliomas and triggers Rac1-mediated tumour cell invasiveness. Hum Mol Genet. 2013;22:84–95. doi: 10.1093/hmg/dds405. [DOI] [PubMed] [Google Scholar]
- Moniz S, Matos P, Jordan P. WNK2 modulates MEK1 activity through the Rho GTPase pathway. Cell Signal. 2008;20:1762–1768. doi: 10.1016/j.cellsig.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Moniz S, Verissimo F, Matos P, Brazao R, Silva E, Kotelevets L, Chastre E, Gespach C, Jordan P. Protein kinase WNK2 inhibits cell proliferation by negatively modulating the activation of MEK1/ERK1/2. Oncogene. 2007;26:6071–6081. doi: 10.1038/sj.onc.1210706. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- Naguro I, Umeda T, Kobayashi Y, Maruyama J, Hattori K, Shimizu Y, Kataoka K, Kim-Mitsuyama S, Uchida S, Vandewalle A, Noguchi T, Nishitoh H, Matsuzawa A, Takeda K, Ichijo H. ASK3 responds to osmotic stress and regulates blood pressure by suppressing WNK1-SPAK/OSR1 signaling in the kidney. Nat Commun. 2012;3:1285. doi: 10.1038/ncomms2283. [DOI] [PubMed] [Google Scholar]
- Naito S, Ohta A, Sohara E, Ohta E, Rai T, Sasaki S, Uchida S. Regulation of WNK1 kinase by extracellular potassium. Clin Exp Nephrol. 2011;15:195–202. doi: 10.1007/s10157-010-0378-9. [DOI] [PubMed] [Google Scholar]
- Nozaki M, Onishi Y, Togashi S, Miyamoto H. Molecular characterization of the Drosophila Mo25 gene, which is conserved among Drosophila, mouse, and yeast. DNA Cell Biol. 1996;15:505–509. doi: 10.1089/dna.1996.15.505. [DOI] [PubMed] [Google Scholar]
- O’Donnell MJ, Dow JA, Huesmann GR, Tublitz NJ, Maddrell SH. Separate control of anion and cation transport in malpighian tubules of Drosophila Melanogaster. J Exp Biol. 1996;199:1163–1175. doi: 10.1242/jeb.199.5.1163. [DOI] [PubMed] [Google Scholar]
- Oi K, Sohara E, Rai T, Misawa M, Chiga M, Alessi DR, Sasaki S, Uchida S. A minor role of WNK3 in regulating phosphorylation of renal NKCC2 and NCC co-transporters in vivo. Biol Open. 2012;1:120–127. doi: 10.1242/bio.2011048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Alvarez D, Vazquez N, Castaneda-Bueno M, de-Los-Heros P, Cortes-Gonzalez C, Moreno E, Meade P, Bobadilla NA, Gamba G. WNK3-SPAK interaction is required for the modulation of NCC and other members of the SLC12 family. Cell Physiol Biochem. 2012;29:291–302. doi: 10.1159/000337610. [DOI] [PubMed] [Google Scholar]
- Park J, Al-Ramahi I, Tan Q, Mollema N, Diaz-Garcia JR, Gallego-Flores T, Lu HC, Lagalwar S, Duvick L, Kang H, Lee Y, Jafar-Nejad P, Sayegh LS, Richman R, Liu X, Gao Y, Shaw CA, Arthur JS, Orr HT, Westbrook TF, Botas J, Zoghbi HY. RAS-MAPK-MSK1 pathway modulates ataxin 1 protein levels and toxicity in SCA1. Nature. 2013;498:325–331. doi: 10.1038/nature12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014;7 doi: 10.1126/scisignal.2005050. ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce-Coria J, Gagnon KB, Delpire E. Calcium-binding protein 39 facilitates molecular interaction between Ste20p proline alanine-rich kinase and oxidative stress response 1 monomers. Am J Physiol Cell Physiol. 2012;303:C1198–C1205. doi: 10.1152/ajpcell.00284.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce-Coria J, Markadieu N, Austin TM, Flammang L, Rios K, Welling PA, Delpire E. A novel Ste20-related proline/alanine-rich kinase (SPAK)-independent pathway involving calcium-binding protein 39 (Cab39) and serine threonine kinase with no lysine member 4 (WNK4) in the activation of Na-K-Cl cotransporters. J Biol Chem. 2014;289:17680–17688. doi: 10.1074/jbc.M113.540518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce-Coria J, San-Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, de Los Heros P, Juarez P, Munoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci U S A. 2008;105:8458–8463. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler RM, Boylan GB, Marlow N, Blennow M, Chiron C, Cross JH, de Vries LS, Hallberg B, Hellstrom-Westas L, Jullien V, Livingstone V, Mangum B, Murphy B, Murray D, Pons G, Rennie J, Swarte R, Toet MC, Vanhatalo S, Zohar S, consortium, NEs. t. w. M. O.-p Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. Lancet Neurol. 2015;14:469–477. doi: 10.1016/S1474-4422(14)70303-5. [DOI] [PubMed] [Google Scholar]
- Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanovic S, Jovanovic A, O’Shaughnessy KM, Alessi DR. Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med. 2010;2:63–75. doi: 10.1002/emmm.200900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA. Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol. 2014;306:F1059–F1068. doi: 10.1152/ajprenal.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A, Brustein E, Liao M, Mercado A, Babilonia E, Mount DB, Drapeau P. Neurogenic role of the depolarizing chloride gradient revealed by global overexpression of KCC2 from the onset of development. J Neurosci. 2008;28:1588–1597. doi: 10.1523/JNEUROSCI.3791-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheault MR, O’Donnell MJ. Analysis of epithelial K(+) transport in Malpighian tubules of Drosophila melanogaster: evidence for spatial and temporal heterogeneity. J Exp Biol. 2001;204:2289–2299. doi: 10.1242/jeb.204.13.2289. [DOI] [PubMed] [Google Scholar]
- Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+-Cl- cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- Richardson C, Sakamoto K, de los Heros P, Deak M, Campbell DG, Prescott AR, Alessi DR. Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and -independent pathways. J Cell Sci. 2011;124:789–800. doi: 10.1242/jcs.077230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart J, Maksimova YD, Tanis JE, Stone KL, Hodson CA, Zhang J, Risinger M, Pan W, Wu D, Colangelo CM, Forbush B, Joiner CH, Gulcicek EE, Gallagher PG, Lifton RP. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell. 2009;138:525–536. doi: 10.1016/j.cell.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart J, Vazquez N, Kahle KT, Hodson CA, Ring AM, Gulcicek EE, Louvi A, Bobadilla NA, Gamba G, Lifton RP. WNK2 is a novel regulator of essential neuronal cation-chloride cotransporters. The Journal of biological chemistry. 2011 doi: 10.1074/jbc.M111.222893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan AR, Baum M, Huang CL. The Drosophila NKCC Ncc69 is required for normal renal tubule function. Am J Physiol Cell Physiol. 2012;303:C883–C894. doi: 10.1152/ajpcell.00201.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MF, Henry D, Nelson S, Harte PJ, Dillon AK, Sciortino CM. Cloning and characterization of a Na+-driven anion exchanger (NDAE1). A new bicarbonate transporter. J Biol Chem. 2000;275:24552–24559. doi: 10.1074/jbc.M003476200. [DOI] [PubMed] [Google Scholar]
- Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, Shao DD, Schumacher SE, Weir BA, Vazquez F, Cowley GS, Root DE, Mesirov JP, Beroukhim R, Kuo CJ, Goessling W, Hahn WC. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Goodman JH, Begum G, Donnelly BF, Pittman G, Weinman EJ, Sun D, Subramanya AR. Generation of WNK1 knockout cell lines by CRISPR/Cas-mediated genome editing. Am J Physiol Renal Physiol. 2015;308:F366–F376. doi: 10.1152/ajprenal.00612.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Shibuya H. WNK signaling is involved in neural development via Lhx8/Awh expression. PLoS One. 2013;8:e55301. doi: 10.1371/journal.pone.0055301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciortino CM, Shrode LD, Fletcher BR, Harte PJ, Romero MF. Localization of endogenous and recombinant Na(+)-driven anion exchanger protein NDAE1 from Drosophila melanogaster. Am J Physiol Cell Physiol. 2001;281:C449–C463. doi: 10.1152/ajpcell.2001.281.2.C449. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Tu SW, Wedin K, Earnest S, Stippec S, Luby-Phelps K, Cobb MH. Interactions with WNK (with no lysine) family members regulate oxidative stress response 1 and ion co-transporter activity. J Biol Chem. 2012;287:37868–37879. doi: 10.1074/jbc.M112.398750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serysheva E, Berhane H, Grumolato L, Demir K, Balmer S, Bodak M, Boutros M, Aaronson S, Mlodzik M, Jenny A. Wnk kinases are positive regulators of canonical Wnt/beta-catenin signalling. EMBO Rep. 2013;14:718–725. doi: 10.1038/embor.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serysheva E, Mlodzik M, Jenny A. WNKs in Wnt/beta-catenin signaling. Cell Cycle. 2014;13:173–174. doi: 10.4161/cc.27038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaharabany M, Holtzman EJ, Mayan H, Hirschberg K, Seger R, Farfel Z. Distinct pathways for the involvement of WNK4 in the signaling of hypertonicity and EGF. FEBS J. 2008;275:1631–1642. doi: 10.1111/j.1742-4658.2008.06318.x. [DOI] [PubMed] [Google Scholar]
- Shekarabi M, Girard N, Riviere JB, Dion P, Houle M, Toulouse A, Lafreniere RG, Vercauteren F, Hince P, Laganiere J, Rochefort D, Faivre L, Samuels M, Rouleau GA. Mutations in the nervous system--specific HSN2 exon of WNK1 cause hereditary sensory neuropathy type II. J Clin Invest. 2008;118:2496–2505. doi: 10.1172/JCI34088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Jiao S, Zhang Z, Ma M, Zhang Z, Chen C, Wang K, Wang H, Wang W, Zhang L, Zhao Y, Zhou Z. Structure of the MST4 in complex with MO25 provides insights into its activation mechanism. Structure. 2013;21:449–461. doi: 10.1016/j.str.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83:811–824. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- Stokes JB. Consequences of potassium recycling in the renal medulla. Effects of ion transport by the medullary thick ascending limb of Henle’s loop. J Clin Invest. 1982;70:219–229. doi: 10.1172/JCI110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokum JA, Gerzanich V, Simard JM. Molecular pathophysiology of cerebral edema. J Cereb Blood Flow Metab. 2016;36:513–538. doi: 10.1177/0271678X15617172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Tian E, Turner RJ, Ten Hagen KG. Developmental and functional studies of the SLC12 gene family members from Drosophila melanogaster. Am J Physiol Cell Physiol. 2010;298:C26–C37. doi: 10.1152/ajpcell.00376.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susa K, Kita S, Iwamoto T, Yang SS, Lin SH, Ohta A, Sohara E, Rai T, Sasaki S, Alessi DR, Uchida S. Effect of heterozygous deletion of WNK1 on the WNK-OSR1/ SPAK-NCC/NKCC1/NKCC2 signal cascade in the kidney and blood vessels. Clin Exp Nephrol. 2012;16:530–538. doi: 10.1007/s10157-012-0590-x. [DOI] [PubMed] [Google Scholar]
- Swarup S, Pradhan-Sundd T, Verheyen EM. Genome-wide identification of phospho-regulators of Wnt signaling in Drosophila. Development. 2015;142:1502–1515. doi: 10.1242/dev.116715. [DOI] [PubMed] [Google Scholar]
- Swarup S, Verheyen EM. Wnt/Wingless signaling in Drosophila. Cold Spring Harbor perspectives in biology. 2012;4:a007930. doi: 10.1101/cshperspect.a007930. http://dx.doi.org/10.1101/cshperspect.a007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi D, Mori T, Nomura N, Khan MZ, Araki Y, Zeniya M, Sohara E, Rai T, Sasaki S, Uchida S. WNK4 is the major WNK positively regulating NCC in the mouse kidney. Biosci Rep. 2014;34:e00107. doi: 10.1042/BSR20140047. http://dx.doi.org/10.1042/BSR20140047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL. (WNK)ing at death: With-no-lysine (Wnk) kinases in neuropathies and neuronal survival. Brain Res Bull. 2016;125:92–98. doi: 10.1016/j.brainresbull.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Taylor CAt, Juang YC, Earnest S, Sengupta S, Goldsmith EJ, Cobb MH. Domain-Swapping Switch Point in Ste20 Protein Kinase SPAK. Biochemistry. 2015;54:5063–5071. doi: 10.1021/acs.biochem.5b00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int. 2016;89:127–134. doi: 10.1038/ki.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21:39–50. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl- cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol. 2009;297:F704–F712. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl- cotransporter. Am J Physiol Renal Physiol. 2013;305:F1177–F1188. doi: 10.1152/ajprenal.00201.2013. [DOI] [PubMed] [Google Scholar]
- Velazquez H, Silva T. Cloning and localization of KCC4 in rabbit kidney: expression in distal convoluted tubule. Am J Physiol Renal Physiol. 2003;285:F49–F58. doi: 10.1152/ajprenal.00389.2002. [DOI] [PubMed] [Google Scholar]
- Verissimo F, Jordan P. WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene. 2001;20:5562–5569. doi: 10.1038/sj.onc.1204726. [DOI] [PubMed] [Google Scholar]
- Vitari AC, Deak M, Collins BJ, Morrice N, Prescott AR, Phelan A, Humphreys S, Alessi DR. WNK1, the kinase mutated in an inherited high-blood-pressure syndrome, is a novel PKB (protein kinase B)/Akt substrate. Biochem J. 2004;378:257–268. doi: 10.1042/BJ20031692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade JB, Fang L, Coleman RA, Liu J, Grimm PR, Wang T, Welling PA. Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol. 2011;300:F1385–F1393. doi: 10.1152/ajprenal.00592.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade JB, Liu J, Coleman R, Grimm PR, Delpire E, Welling PA. SPAK-mediated NCC regulation in response to low-K+ diet. Am J Physiol Renal Physiol. 2015;308:F923–F931. doi: 10.1152/ajprenal.00388.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warsi J, Hosseinzadeh Z, Elvira B, Bissinger R, Shumilina E, Lang F. Regulation of ClC-2 activity by SPAK and OSR1. Kidney Blood Press Res. 2014;39:378–387. doi: 10.1159/000355816. [DOI] [PubMed] [Google Scholar]
- Watson PA, Ellwood-Yen K, King JC, Wongvipat J, Lebeau MM, Sawyers CL. Context-dependent hormone-refractory progression revealed through characterization of a novel murine prostate cancer cell line. Cancer Res. 2005;65:11565–11571. doi: 10.1158/0008-5472.CAN-05-3441. [DOI] [PubMed] [Google Scholar]
- Welling PA. Regulation of potassium channel trafficking in the distal nephron. Curr Opin Nephrol Hypertens. 2013;22:559–565. doi: 10.1097/MNH.0b013e328363ff76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- Womersley RA, Darragh JH. Potassium and sodium restriction in the normal human. J Clin Invest. 1955;34:456–461. doi: 10.1172/JCI103094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Baum M, Huang CL, Rodan AR. Two inwardly rectifying potassium channels, Irk1 and Irk2, play redundant roles in Drosophila renal tubule function. Am J Physiol Regul Integr Comp Physiol. 2015;309:R747–R756. doi: 10.1152/ajpregu.00148.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Schellinger JN, Huang CL, Rodan AR. Hypotonicity stimulates potassium flux through the WNK-SPAK/OSR1 kinase cascade and the Ncc69 sodium-potassium-2-chloride cotransporter in the Drosophila renal tubule. J Biol Chem. 2014;289:26131–26142. doi: 10.1074/jbc.M114.577767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi B, Chen M, Chandak GR, Shen Y, Yan L, He J, Mou SH. STK39 polymorphism is associated with essential hypertension: a systematic review and meta-analysis. PLoS One. 2013;8:e59584. doi: 10.1371/journal.pone.0059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Wu T, Xu K, Huang IK, Cleaver O, Huang CL. Endothelial-specific expression of WNK1 kinase is essential for angiogenesis and heart development in mice. Am J Pathol. 2009;175:1315–1327. doi: 10.2353/ajpath.2009.090094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Yoon J, Yang SS, Lin SH, Huang CL. WNK1 protein kinase regulates embryonic cardiovascular development through the OSR1 signaling cascade. J Biol Chem. 2013;288:8566–8574. doi: 10.1074/jbc.M113.451575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem. 2000;275:16795–16801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- Xu BE, Stippec S, Chu PY, Lazrak A, Li XJ, Lee BH, English JM, Ortega B, Huang CL, Cobb MH. WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci U S A. 2005a;102:10315–10320. doi: 10.1073/pnas.0504422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu BE, Stippec S, Lazrak A, Huang CL, Cobb MH. WNK1 activates SGK1 by a phosphatidylinositol 3-kinase-dependent and non-catalytic mechanism. J Biol Chem. 2005b;280:34218–34223. doi: 10.1074/jbc.M505735200. [DOI] [PubMed] [Google Scholar]
- Yamada T, Bhate MP, Strange K. Regulatory phosphorylation induces extracellular conformational changes in a CLC anion channel. Biophys J. 2013;104:1893–1904. doi: 10.1016/j.bpj.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Izumi Y, Matsuzaki F. The GC kinase Fray and Mo25 regulate Drosophila asymmetric divisions. Biochem Biophys Res Commun. 2008;366:212–218. doi: 10.1016/j.bbrc.2007.11.128. [DOI] [PubMed] [Google Scholar]
- Yanagawa S, van Leeuwen F, Wodarz A, Klingensmith J, Nusse R. The Dishevelled protein is modified by Wingless signalling in Drosophila . Genes Dev. 1995;9:1087–1097. doi: 10.1101/gad.9.9.1087. [DOI] [PubMed] [Google Scholar]
- Yanfeng WA, Berhane H, Mola M, Singh J, Jenny A, Mlodzik M. Functional dissection of phosphorylation of Disheveled in Drosophila. Developmental biology. 2011;360:132–142. doi: 10.1016/j.ydbio.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol. 2010;21:1868–1877. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorska A, Pozo-Guisado E, Boudeau J, Vitari AC, Rafiqi FH, Thastrup J, Deak M, Campbell DG, Morrice NA, Prescott AR, Alessi DR. Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J Cell Biol. 2007;176:89–100. doi: 10.1083/jcb.200605093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaika O, Tomilin V, Mamenko M, Bhalla V, Pochynyuk O. New perspective of ClC-Kb/2 Cl- channel physiology in the distal renal tubule. Am J Physiol Renal Physiol. 2016;310:F923–F930. doi: 10.1152/ajprenal.00577.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrowicz BP, Abuin A, Ramirez-Solis R, Richter LJ, Piggott J, BeltrandelRio H, Buxton EC, Edwards J, Finch RA, Friddle CJ, Gupta A, Hansen G, Hu Y, Huang W, Jaing C, Key BW, Jr, Kipp P, Kohlhauff B, Ma ZQ, Markesich D, Payne R, Potter DG, Qian N, Shaw J, Schrick J, Shi ZZ, Sparks MJ, Van Sligtenhorst I, Vogel P, Walke W, Xu N, Zhu Q, Person C, Sands AT. Wnk1 kinase deficiency lowers blood pressure in mice: a gene-trap screen to identify potential targets for therapeutic intervention. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14109–14114. doi: 10.1073/pnas.2336103100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeniya M, Sohara E, Kita S, Iwamoto T, Susa K, Mori T, Oi K, Chiga M, Takahashi D, Yang SS, Lin SH, Rai T, Sasaki S, Uchida S. Dietary salt intake regulates WNK3-SPAK-NKCC1 phosphorylation cascade in mouse aorta through angiotensin II. Hypertension. 2013;62:872–878. doi: 10.1161/HYPERTENSIONAHA.113.01543. [DOI] [PubMed] [Google Scholar]
- Zeqiraj E, Filippi BM, Goldie S, Navratilova I, Boudeau J, Deak M, Alessi DR, van Aalten DM. ATP and MO25alpha regulate the conformational state of the STRADalpha pseudokinase and activation of the LKB1 tumour suppressor. PLoS Biol. 2009;7:e1000126. doi: 10.1371/journal.pbio.1000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1) Proc Natl Acad Sci U S A. 2014;111:11864–11869. doi: 10.1073/pnas.1411705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Nepomuceno R, Gao X, Foley LM, Wang S, Begum G, Zhu W, Pigott VM, Falgoust LM, Kahle KT, Yang SS, Lin SH, Alper SL, Hitchens TK, Hu S, Zhang Z, Sun D. Deletion of the WNK3-SPAK kinase complex in mice improves radiographic and clinical outcomes in malignant cerebral edema after ischemic stroke. J Cereb Blood Flow Metab. 2016;2016 doi: 10.1177/0271678X16631561. pii:0271678×16631561 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Marin O, Hermesz E, Powell A, Flames N, Palkovits M, Rubenstein JL, Westphal H. The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc Natl Acad Sci U S A. 2003;100:9005–9010. doi: 10.1073/pnas.1537759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Begum G, Pointer K, Clark PA, Yang SS, Lin SH, Kahle KT, Kuo JS, Sun D. WNK1-OSR1 kinase-mediated phospho-activation of Na+-K+-2Cl- cotransporter facilitates glioma migration. Mol Cancer. 2014;13:31. doi: 10.1186/1476-4598-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]