Abstract
Flagellin, the primary structural component of bacterial flagella, is recognized by Toll-like receptor 5 (TLR5) present on the basolateral surface of intestinal epithelial cells. Utilizing biochemical assays of proinflammatory signaling pathways and mRNA expression profiling, we found that purified flagellin could recapitulate the human epithelial cell proinflammatory responses activated by flagellated pathogenic bacteria. Flagellin-induced proinflammatory activation showed similar kinetics and gene specificity as that induced by the classical endogenous proinflammatory cytokine TNF-α, although both responses were more rapid than that elicited by viable flagellated bacteria. Flagellin, like TNF-α, activated a number of antiapoptotic mediators, and pretreatment of epithelial cells with this bacterial protein could protect cells from subsequent bacterially mediated apoptotic challenge. However, when NF-κB-mediated or phosphatidylinositol 3-kinase/Akt proinflammatory signaling was blocked, flagellin could induce programmed cell death. Consistently, we demonstrate that flagellin and viable flagellate Salmonella induces both the extrinsic and intrinsic caspase activation pathways, with the extrinsic pathway (caspase 8) activated by purified flagellin in a TLR5-dependant fashion. We conclude that interaction of flagellin with epithelial cells induces caspase activation in parallel with proinflammatory responses. Such intertwining of proinflammatory and apoptotic signaling mediated by bacterial products suggests roles for host programmed cell death in the pathogenesis of enteric infections.
Keywords: Salmonella, inflammation, Toll-like receptor, cDNA microarray
GRAM-NEGATIVE BACTERIA of the Salmonella enteritidis group are common human pathogens often isolated from cases of acute food-borne gastroenteritis in the United States and developing countries (49). S. enteritidis interaction with the intestinal epithelia provokes secretion of chemokines and subsequent luminal translocation of neutrophils (51). The result of this acute inflammatory response is the disruption of barrier integrity permitting transepithelial fluxes of ions and water as well as neutrophils. These events correlate clinically with an acute inflammatory diarrhea. Other enteric bacterial infections, such as those caused by Shigella, enteropathogenic Escherichia coli, and Yersinia, elicit similar responses (10).
Many of the epithelial responses to intestinal infections involve the transcriptional activation of inflammatory mediators such as chemokines, adhesion molecules, and antibacterial peptides. These gene products are controlled by the action of proinflammatory signaling cascades such as the NF-κB (Rel) and MAPK pathways that culminate in the activation of nuclear transcription factors and the initiation of de novo mRNA transcription. Indeed, work in our and other laboratories has identified NF-κB activation as a central event in the proinflammatory gene expression that mediates infectious enterocolitis (13, 16). Enterocytes (and most other cells) can perceive bacterial threats and activate the NF-κB pathway via a battery of transmembrane Toll-like receptors (TLRs) (35). These sentinel pattern recognition receptors are potently activated by pathogen-associated molecular patterns (PAMPs), complex molecules with a macromolecular structure limited to and characteristic of prokaryotic life, permitting stable and efficient recognition by eukaryotic TLRs (48).
Flagellin is a bacterial product that is generally considered a PAMP, with TLR5 as its physiological receptor in vertebrates (22). This pattern recognition receptor is present on the basolateral aspect of intact intestinal epithelial cells (15) and binds a 13-amino acid motif present in the flagellin protofilament but not accessible in polymerized flagella (46). In S. typhimurium, the flagellar protofilaments are 55-kDa monomers encoded by the two similar but not identical genes FliC and FljB (34). Approximately 20,000 subunits of flagellin assemble to form the extracellular filament structure that is necessary for bacterial motility. Flagellin is found on a wide variety of bacteria, and it is assumed that structural constraints necessary for motor function permit TLR5 recognition of flagellin encoded in a variety of organisms (47).
Purified flagellin can activate transcription and secretion of the proinflammatory chemokine IL-8 in in vitro cell culture systems (45). Flagellin is also a potent activator of systemic inflammation in murine models (12), and, in humans, serum levels of the protein correlate with clinical severity of bacteremic shock syndromes (31). Interestingly, studies of circulating antibodies in the serum of human Crohn’s disease patients and in murine colitis models identified flagellin as a dominant antigen, suggesting a role for this bacterial protein in the immunopathogenesis of inflammatory bowel disease (32).
In our laboratory, we have shown that infection of model epithelia with live S. typhimurium elicited a classic proinflammatory transcriptional response involving NF-κB (16). Strikingly, mutation of both Salmonella genes encoding flagellin (FliC and FljB) could totally abolish this response (17, 53), indicating that this PAMP is necessary for the proinflammatory responses to this pathogen. Because of the vital role flagellin plays in Salmonella pathogenesis (and probably other bacterial infections) as well as the ability of this protein to activate known proinflammatory genes, we sought to characterize the signaling pathways and transcriptional responses elicited by this exogenous prokaryotic protein. We report herein that flagellin is sufficient to recapitulate epithelial proinflammatory responses to S. typhimurium. Furthermore, we demonstrate that flagellin also activates apoptotic signaling pathways. We also observe transcriptional upregulation of antiapoptotic factors that serve to arrest caspase activation in a NF-κB-dependant manner; however, when the NF-κB pathway is blocked, caspase activation proceeds to cell death. We speculate that this parallel activation may be a general feature of activators of innate immunity and that flagellin may play a previously under appreciated role in host monitoring of, and response to, microbes.
MATERIALS AND METHODS
Reagents and constructs
Flagellin (FliC) from wild-type S. typhimurium (SL3201) was purified through sequential cation and anion-exchange chromatography and used at the concentrations indicated in the figures (16). Purity was verified as previously described (15). Cycloheximide and wortmannin were obtained from Calbiochem (La Jolla, CA), MG-262 was from BioMol (Plymouth Meeting, PA), staurosporine was from Sigma (St. Louis, MO), 4′,6-diamidino-2-phenylindole dihydrochloride was from Molecular Probes (Eugene, OR), and TNF-α was from R&D Systems (Minneapolis, MN). Antibodies used included caspase 3, cleaved caspase 3, caspase 8, caspase 9, phospho-p38, phospho-Akt, phospho-IκBα, phospho-JNK, phospho-ERK, p38 (all from Cell Signaling; Beverly, MA), poly(ADP) ribose polymerase (PARP; BD Pharmingen; San Diego, CA), IκBα (Santa Cruz Biotechnology; Santa Cruz, CA), cIAP-2 (R&D Systems), and β-actin (Sigma). Epitope-tagged antibodies included T7 (EMD Biosciences; Madison, WI) and V5 (Invitrogen; Carlsbad, CA). The pDsRed2 construct was obtained from BD Biosciences. The mutant IκBα pCMV4 T7-IκB-α S32/36A construct, containing Ser→Ala replacements at Ser32 and Ser36, was kindly provided by Dr. Dean W. Ballard (Vanderbilt University School of Medicine). The cellular inhibitor of apoptosis-2 (cIAP-2) expression vector pcDNA4.1-cIAP2 was constructed by cloning RT-PCR products into the pcDNA4.1A vector (Invitrogen).
Cell culture and bacterial infection
Model human intestinal epithelial cells (T84) were prepared on 0.33- or 5-cm2 permeable filters and used 9–14 days after they had been plated and achieved a stable transepithelial resistance of >1,000 Ω · cm2. Monolayers were washed twice with Hanks’ balanced salt solution at 37°C and equilibrated for 15 min before treatment. Primary rat intestinal IEC-6 epithelial cells were maintained in DMEM, 10% fetal bovine serum, and 4 μg/ml insulin (Invitrogen). For bacterial treatments, prepared bacterial cultures were washed, concentrated, and applied to the apical aspects of cells (both T84 and IEC-6 cells) at a multiplicity of infection of 30 organisms/cell as described (53). HeLa/TLR5 cells were generated by stable transfection of HeLa cells with a vector encoding a V5-TLR5 and were maintained in DMEM supplemented with 10% fetal bovine serum and 5 μg/ml blasticidin (52).
Transfection
IEC-6 cells were transiently transfected by FuGENE 6 reagent (Roche Diagnostics; Indianapolis, IN) according to the manufacturer’s instruction. HeLa and 293T cells were transfected with Lipofectamine 2000 (Invitrogen).
SDS-PAGE immunoblot analysis
Differently treated cells, as described in the figures, were collected and lysed in ice-cold buffer containing 50 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitor cocktail (Sigma). Protein concentration was determined with the Bradford reagent (Bio-Rad; Hercules, CA). Equal amounts of proteins were loaded on SDS-PAGE and blotted with different antibodies as indicated, following standard protocols.
Microarray analysis
Details of cDNA microarray fabrication, hybridization, scanning, and labeling of RNA samples are described in Ref. 53. Briefly, total RNA from treated T84 cells (sample) and untreated T84 cells (reference) was prepared using TRIzol reagent (Invitrogen). The integrity of RNA transcripts was verified by gel electrophoresis. With the use of an Oligo-dT primer and Superscript II Reverse Transcriptase (Invitrogen), labeled cDNA was synthesized from 40 μg of total RNA. Reference and experimental RNA samples were labeled with Cy3- and Cy5-coupled dCTP (Amersham Biosciences; Piscataway, NJ), respectively, hybridized to microarrays, washed, and scanned. Three repeats for each experimental condition were performed. All experiments were compared with the same reference to allow the relative expression level of each gene to be compared across all experiments. After normalization, genes with intensity over four times of background mean were selected. To minimize variability, the mean of three independent experiments for each gene was calculated and used for final data clustering. Only genes that showed significant change (over 2-fold difference) were selected for further characterization. Cluster/Tree view (Michael Eisen, Stanford University; Stanford, CA) analytic software packages were used for hierarchical clustering.
Real-time quantitative PCR
Total RNA (1.0 μg) from sample and reference T84 cells was reverse transcribed with a Taqman RT kit (Applied Biosystems; Foster City, CA). One microliter of the product was subjected to SYBR green Real-Time PCR assay (Applied Bio-systems). Reactions were performed in triplicate and normalized to 18s rRNA. The level of expression for a given gene was first normalized by subtracting the mean value of the cycle threshold (Ct) with that of 18s rRNA (ΔCt). Relative levels of gene expression were then determined by subtracting the individual ΔCt values of samples with those of reference (ΔΔCt) and expressing the final quantification value as 2−ΔΔCt. Primers for the genes of interest were designed with PrimerExpress (Applied Biosystems), and their sequences are available upon request.
Caspase staining and quantification
T84 cells on permeable supports or IEC-6 cells grown on glass coverslips to 95% confluence were stimulated with purified flagellin. After experimental incubation, caspase 8 and caspase 9 activation was detected using APO LOGIX carboxyfluorescein Caspase Detection Kits (Cell Technology; Minneapolis MN) through irreversible binding of substrate to enzymatically active caspases in living cells. Cells were visualized by confocal microscopy (Zeiss LSM 510) at 505 nm. The numbers of caspase positive cells from 5 representative viewing fields including at least 500 cells were counted. Data are presented as one of three representative experiments. Caspase 8 and caspase 9 activities were also determined with luminescent substrates, using Caspase-Glo 8 and 9 Assays (Promega; Madison, WI) according to the manufacturer’s protocol, and quantified by a TD-20/20 luminometer (Turner Designs; Sunnyvale, CA).
Programmed cell death assay
Two different assays were applied to IEC-6 cells (both floating and attached cells) to detect apoptotic cells after treatment at various times. In the propidium iodide staining assay, cells were fixed in 70% ethanol and analyzed for DNA content by flow cytometry. With this method, the percentage of apoptotic cells was determined by quantification of the sub-G1 fraction of cells. For TdT-mediated dUTP nick-end labeling (TUNEL) staining, the In Situ Cell Death Detection Kit (Roche) was used according to the manufacturer’s instructions. Briefly, IEC-6 cells were harvested, fixed in 2% paraformaldehyde, permeabilized in 0.1% Triton X-100–0.1% sodium citrate, and labeled with TUNEL reaction mixture. In both assays, 10,000 fluorescent events were measured by a FACScalibur flow cytometer (Becton Dickinson Immunocytometry Systems; San Jose, CA) for each sample. Flow cytometric data were analyzed using FlowJo software.
RESULTS
Flagellin elicits proinflammatory signaling in epithelial cells
We have previously utilized genetic “loss of function” experiments using Salmonella flagellin mutants to show that this protein was necessary for proinflammatory cellular responses (53). In a complementary “gain of function” approach, we sought to determine whether purified flagellin was sufficient to mediate the proinflammatory effects of the viable pathogen. We evaluated kinetics of established proinflammatory signal response pathways in T84 model epithelia activated by flagellin (applied basolaterally) and directly compared responses with those elicited by the canonical endogenous proinflammatory agonist TNF-α (also applied basolaterally). When fully polarized T84 model epithelia were stimulated with purified flagellin (100 ng/ml, a concentration comparable with that seen during coculture with live S. typhimurium), we observed rapid (within 5 min) activation of the NF-κB pathway, as measured by the appearance of phosphorylated IκBα and subsequent degradation of the unmodified form (Fig. 1). We also noted robust activation of p38 and JNK kinases of the MAPK signaling group as well as ERK and Akt kinase activation within 5 min of treatment with both flagellin and TNF-α. Notably, flagellin elicited a more prolonged phosphorylation of these effectors relative to TNF-α, particularly JNK and p38, but overall cellular responses to these proinflammatory agonists appeared highly similar. These results demonstrated the ability of purified flagellin, a prokaryotic protein, to activate cellular proinflammatory response pathways highly similar to those activated by an endogenous proinflammatory agonist.
Fig. 1.
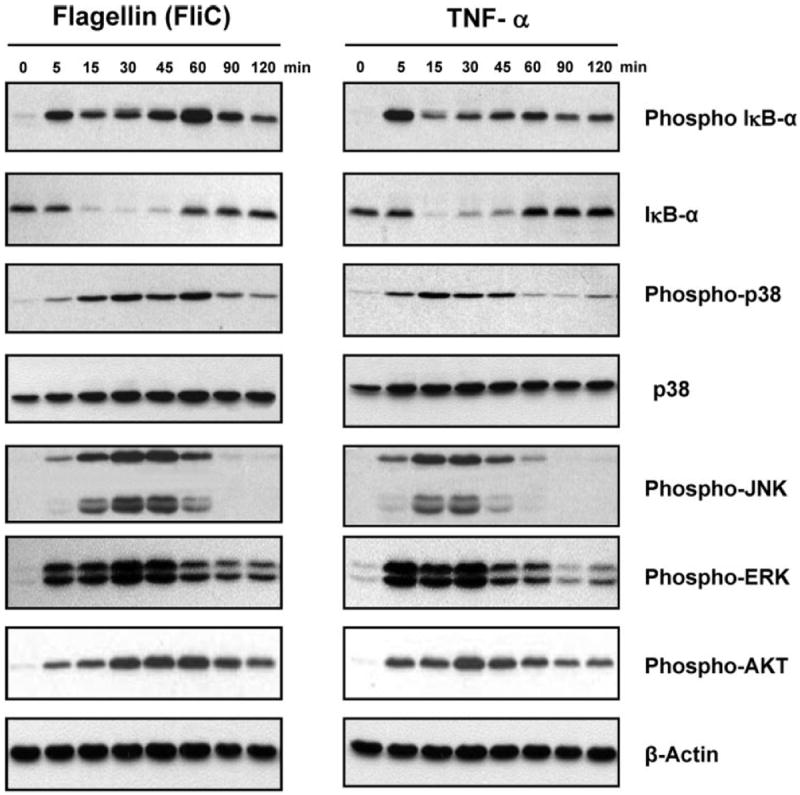
Flagellin (FliC) and TNF-α induce similar patterns of proinflammatory signaling activation. Polarized T84 cells were stimulated with TNF-α (10 ng/ml) or purified flagellin (100 ng/ml) applied basolaterally for the indicated time points. Whole cell lysates were subjected to immunoblots with the indicated antibodies. β-Actin is shown as a loading control. Data shown are derived from a single set of cell lysates and are representative of 3 independent experiments.
Flagellin elicits a proinflammatory transcriptional response in epithelial cells
To study the transcriptional program elicited by bacterial flagellin, we utilized the same epithelial model stimulated with proinflammatory agonists over 1–6 h and measured mRNA abundance by cDNA microarray. This time frame was chosen to evaluate primary, largely transcriptional changes in epithelial gene expression. The microarray system and data management methodology have been reported previously (53). Model epithelia were treated with 1) purified flagellin, applied basolaterally; 2) TNF-α, also applied basolaterally; and 3) the wild-type pathogen S. typhimurium (strain SL3201, from which we purified flagellin), cocultured apically with the monolayer.
Model epithelia were stimulated for 1, 2, 3, 4, 5, and 6 h. After experimental manipulation, RNA was prepared and subjected to microarray analysis. From the collected data, 263 genes reproducibly exhibited a greater than doubling differential expression; for display purposes, 103 genes showing significantly greater than twofold change relative to control are shown. The genes comprising the data set are represented in matrix form with the expression patterns of individual genes organized by hierarchical clustering (Fig. 2), and also listed as supplementary Table 1. This clustering method calculates the mathematical degree of relatedness between the expression patterns of each gene in an iterative fashion and arranges similar expression patterns adjacent to each other along the y-axis. A striking aspect of this series of experiments was the degree to which cellular responses to S. typhimurium could be recapitulated by endogenous (TNF-α) or exogenous (flagellin) proinflammatory protein agonists. Most of these genes have recognized roles in innate immune and inflammatory reactions, and many of them are regulated by the NF-κB transcription factor pathway (37). The characteristic differences between early induced genes [often acute inflammatory mediators such as IL-8 and chemokine, CXC motif, ligand 3 (CXCL3)] and later induced genes [typically chronic mediators such as inter-feron regulatory factor 1 (IRF-1) and IFN-γ receptors 1 and 2] were clearly apparent. Overall, TNF-α- and flagellin-mediated upregulation was more rapid than induction elicited by S. typhimurium, consistent with the kinetics of bacteria-mediated activation of NF-κB reported earlier (16).
Fig. 2.

Transcriptional response of polarized model epithelia to proinflammatory stimuli. Cells were treated with TNF-α (3 ng/ml, applied basolaterally), wild-type (WT) Salmonella typhimurium (SL3201, applied apically), and purified flagellin (5 ng/ml, applied basolaterally) for 1, 2, 3, 4, 5, and 6 h. Flagellin (FliC) was purified from the SL3201 strain. Data are a time course of expression profiles of 103 genes differentially regulated by proinflammatory stimuli with >2-fold induction. The expression patterns of individual genes were organized by uncentered correlation complete linkage hierarchical clustering. Data are presented as a matrix; each row represents an individual array element (gene), and each column represents an experimental condition. Red designates relative upregulation, whereas green designates downregulation, in a semicontinuous fashion (see scale, bottom left). The intensity of the color corresponds to the mean ratio of transcript abundance of the experimental condition relative to the transcript abundance of the untreated cell line (T84). The green vertical bar delineates the “bacterially enhanced” group of genes, whereas the red bar denotes “flagellin-enhanced” genes. Antiapoptotic effector genes are shown in red.
Flagellin mediates antiapoptotic effects in epithelial cells
In addition to transcriptional activation of genes with well-known roles in cellular inflammation, flagellin also activated a panel of antiapoptotic mediator genes including cIAP-1, cIAP-2, and A20 (indicated in red in Fig. 2). These genes are rapidly induced in a NF-κB-dependent manner (7) and can inhibit caspase activation and subsequent proapoptotic signaling (9, 27). For example, the IAPs are ubiquitin ligases that can bind to and induce degradation of activated caspases (9), whereas A20 is a zinc finger protein that disrupts assembly of proapoptotic adaptor molecules to the TNF receptor (23). The unexpected appearance of antiapoptotic mediator genes induced by an exogenous stimulus led us to confirm this regulation by real-time PCR. cIAP-1, cIAP-2, and A20 antiapoptotic proteins are well known TNF-α-responsive genes; as shown in Fig. 3A, flagellin is also a potent inducer. Given the very high levels of cIAP-2 transcript induced by flagellin, we also verified expression of this antiapoptotic protein by Western blot analysis (Fig. 3B). Purified flagellin (FliC) induced this antiapoptotic protein with kinetics consistent with the microarray analysis.
Fig. 3.
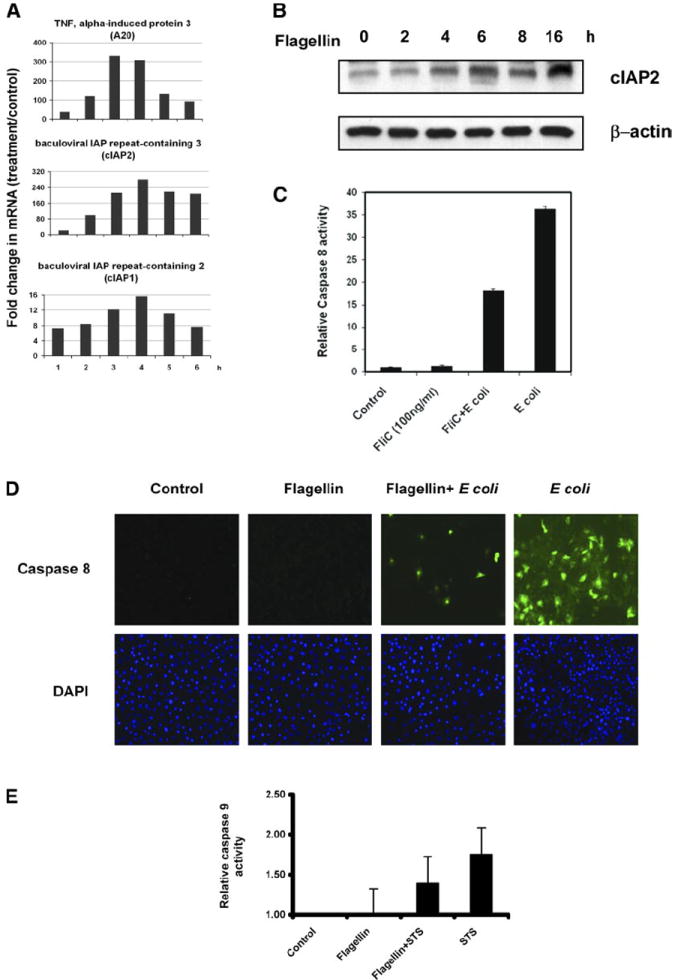
Flagellin mediates antiapoptotic effects in epithelial cells. A: real-time PCR quantification of 3 selected antiapoptotic effector genes: cellular inhibitor of apoptosis (cIAP)1, cIAP2, and A20. Cells were stimulated with flagellin as described in Fig. 2 for the indicated time points. Data are shown as fold upregulation relative to basal (unstimulated) levels. B: immunoblot for cIAP-2. T84 cells were stimulated with flagellin for the times indicated. Actin is included as a loading control. C: IEC-6 cells were stimulated with flagellin (100 ng/ml) for 4 h before challenge by the addition of viable Escherichia coli for an additional 6 h at a multiplicity of infection (MOI) of 30. Caspase activation was detected and quantitated with a luminescent caspase 8 substrate. Data are from a single experiment and representative of 3 experiments that showed an identical pattern of results.D: IEC-6 cells were treated as in C. Caspase 8 activation in viable cells was detected with an APO LOGIX carboxyfluorescein (FAM) caspase detection kit, and nuclei were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI). Data are from a single experiment and representative of 3 experiments that showed an identical pattern of results. E: IEC-6 cells were treated with flagellin as in C and then stimulated with 1 μM staurosporine (STS) for 4 h. Caspase activation was detected and quantitated with a luminescent caspase 9 substrate. Data are from a single experiment and representative of 3 experiments that showed an identical pattern of results.
To test whether flagellin-induced gene expression could protect cells from subsequent apoptotic stimuli, we used a nontransformed epithelial model, rat IEC-6 cells. These cells are commonly used in studies of epithelial cell apoptosis (6). IEC-6 epithelial cells were pretreated for 4 h with flagellin (this duration elicited the maximum induction of antiapoptotic genes; Fig. 2) and subsequently challenged them by incubation with the intestinal commensal bacteria E. coli for 6 h. Non-pathogenic E. coli was used to stimulate multiple TLR/PAMP receptor ligand pairs simultaneously without the presence of type III secretion system (TTSS) effectors that might be present in pathogens. Under conditions where cells were not treated with flagellin, this coculture with E. coli induced potent caspase 8 activation within 6 h, as measured quantitatively with luminescent substrates and morphologically with fluorescent caspases substrates (Fig. 3, C and D). However, cells pretreated with flagellin before identical E. coli coincubation showed a marked reduction in caspase-positive cells in both assays. Flagellin treatment alone for a total of 10 h showed no activation. To determine flagellin-mediated cytoprotective effects against agents that stimulate the intrinsic, mitochondrial pathway, we performed similar experiments utilizing the apoptotic activator staurosporine (11). Pretreatment of IEC-6 cells with flagellin reduced caspase 9 stimulated by staurosporine (as expected, staurosporine had no effect on caspase 8 activity; data not shown), although the effects were not statistically significant (Fig. 3E). Collectively, these data indicate that the flagellin-induced epithelial transcriptional program shown in Fig. 2 involves an antiapoptotic component.
Flagellin can induce caspase activation and apoptosis in cells under blockade of NF-κB
Given the similarity of proinflammatory signaling between TNF and flagellin, we attempted to determine whether flagellin could also positively influence apoptotic processes in the manner of the TNF receptor. TNF-α signaling has a dualistic nature: although it is potently proinflammatory and strongly induces antiapoptotic genes, TNF-α is also an activator of the extrinsic pathway of apoptotic activation through caspase 8 activation (50). Usually, the concurrent activation of proinflammatory pathways, especially NF-κB, results in upregulation of antiapoptotic effector genes, which aborts caspase action and resultant cell death (27). Activation of apoptotic pathways by ligands/TLRs is poorly defined, although many studies have evaluated the TNF/TNF receptor (death receptor) interaction (50).
To investigate a potential role of flagellin in proapoptotic signaling, we examined T84 cells stimulated with basolateral flagellin over a time course for evidence of caspase activation. We utilized specific antibodies to evaluate activation of initiator caspases of the extrinsic pathway (caspase 8) and intrinsic pathway (caspase 9) as well as executioner caspases (caspase 3) and substrates (PARP) in Western blots. These antibodies react with the processed forms of the specific caspases, a proteolytic event necessary for activation of enzymatic function, whereas PARP cleavage is another marker of the execution phase of apoptosis. With treatment with flagellin alone, trace appearances of the cleaved/activated forms of caspases were seen over 4–8 h (Fig. 4A). In cells under blockade of the NF-κB pathway with proteasomal inhibitors (MG-262), activation of caspases (capase 8, caspase 9, and caspase 3) was markedly more intense than that with flagellin or MG-262 alone, most noticeable with caspase 8. In cells treated with wortmannin (an inhibitor of P13K/Akt kinase) flagellin potently induced caspase-3 cleavage, suggesting a role for this pathway in flagellin-mediated caspase activation (Fig. 4B).
Fig. 4.
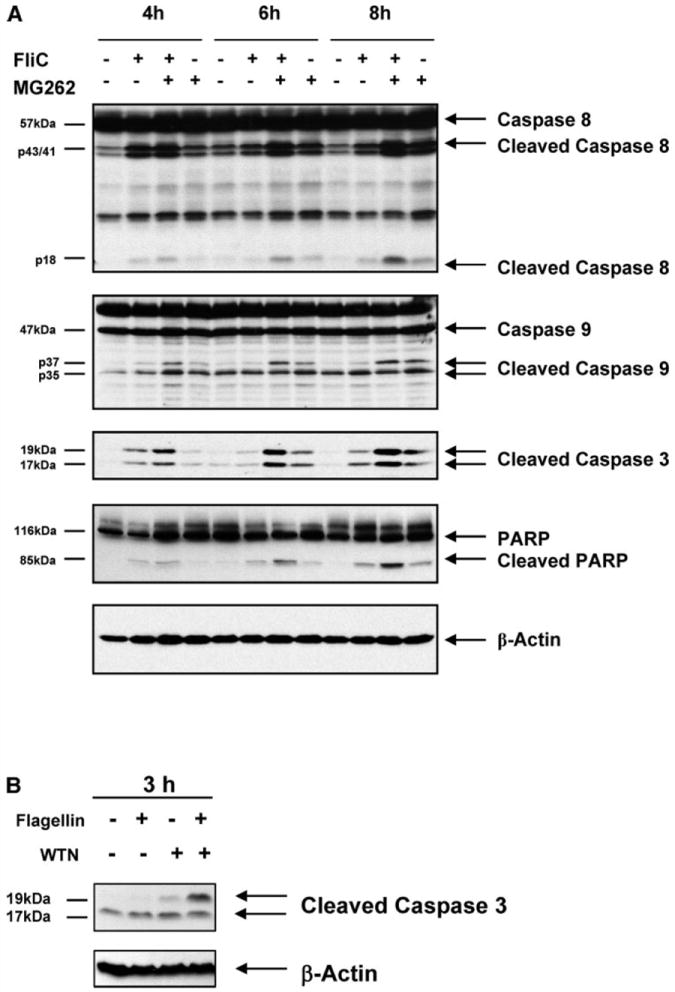
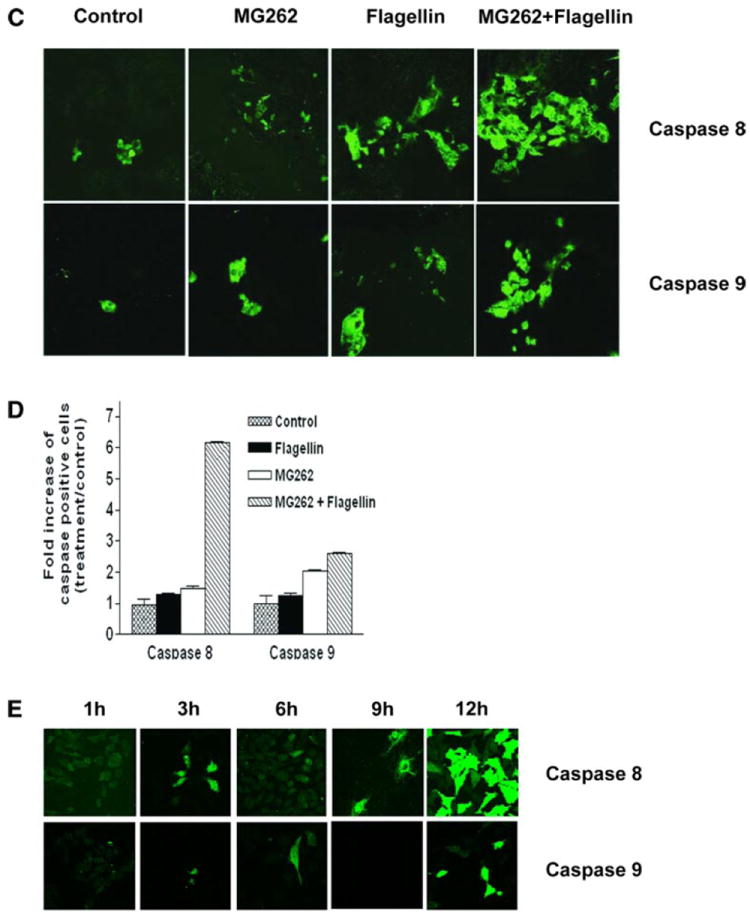
Flagellin induces caspase activation. A: polarized T84 cells were stimulated with flagellin (100 ng/ml), MG-262 (1 μM), or a combination for the indicated durations. Whole cell lysates were subjected to immunoblots with the indicated antibodies. Cleaved fragments are designated with arrows. β-Actin is shown as a loading control. Data shown are derived from a single set of cell lysates and are representative of 5 independent experiments. B: T84 cells were pretreated with 100 nM wortmannin (WTN) for 30 min and then stimulated with 100 ng/ml flagellin for 3 h. Cells were lysed and immunoblotted with antibody to cleaved caspase 3. β-Actin is shown as a loading control. C: polarized T84 cells were stimulated with flagellin (100 ng/ml), MG-262 (250 ng/ml), or a combination for 6 h. Monolayers were treated with fluorescent caspase substrates (caspase 8: FAM-LETD-FMK and caspase 9: FAM-LEHD-FMK) and visualized by confocal microscopy. D: quantification of images described in C. Results are displayed as fold increase of caspase positive cells over the unstimulated baseline. E: IEC-6 cells were colonized with flagellated viable S. typhimurium for the indicated times, and cells were treated with fluorescent caspase substrates as in C.
To further study caspase activation, carboxyfluorescein (FAM) caspase substrates were employed. Polarized T84 cells were stimulated with flagellin and/or MG-262 as above, treated with the fluorescent substrates, and evaluated by fluorescence microscopy. Occasional fluorescent cells were seen within 6 h of flagellin treatment alone with substrates specific for both caspase 8 and caspase 9 (Fig. 4, C and D). Similar activation was observed in cells treated with MG-262. However, the combination of flagellin stimulation and NF-κB blockade revealed a marked quantitative upregulation of caspase 8 and caspase 9 activation, a pattern highly consistent to the data obtained with anti-active caspase antibodies.
To demonstrate that viable Salmonella could activate apoptotic signaling in cultured model epithelia, we cocultured live wild-type Salmonella with IEC-6 cells and evaluated caspase 8 and caspase 9 activation with fluorescent substrates (Fig. 4E). High levels of caspase 8 activation was seen within 12 h postinoculation with the wild-type strain, whereas activation of caspase 9 was less significant. These data are consistent with prior evaluation of Salmonella-mediated apoptotic activation (28) and suggests that Salmonella-mediated apoptotic stimulation is predominately via the extrinsic pathway.
Collectively, these data indicate that flagellin and flagellated bacteria can initiate caspase activation, a process that is markedly enhanced when proinflammatory (survival) pathways are blocked. Further experiments were undertaken to determine whether this caspase activation proceeded to cell death. We observed that at 6 h of flagellin stimulation, no cell death occurred in IEC-6 epithelial cells, as measured by an increase in the sub-G1 DNA fraction (Fig. 5A), as would be expected with the simultaneous and unimpeded activation of the NF-κB pathway and secondary arrest of activated caspases. However, during flagellin stimulation under conditions of NF-κB blockade (with the proteasome inhibitor MG-262), IEC-6 cells showed evidence of programmed cell death. The sub-G1 fraction was comparable with that induced by staurosporine. Proteasome inhibitors are commonly used to augment apoptosis experimentally (21). MG-262 alone did show some apoptotic activation, presumably due to inhibition of cyclin turnover.
Fig. 5.
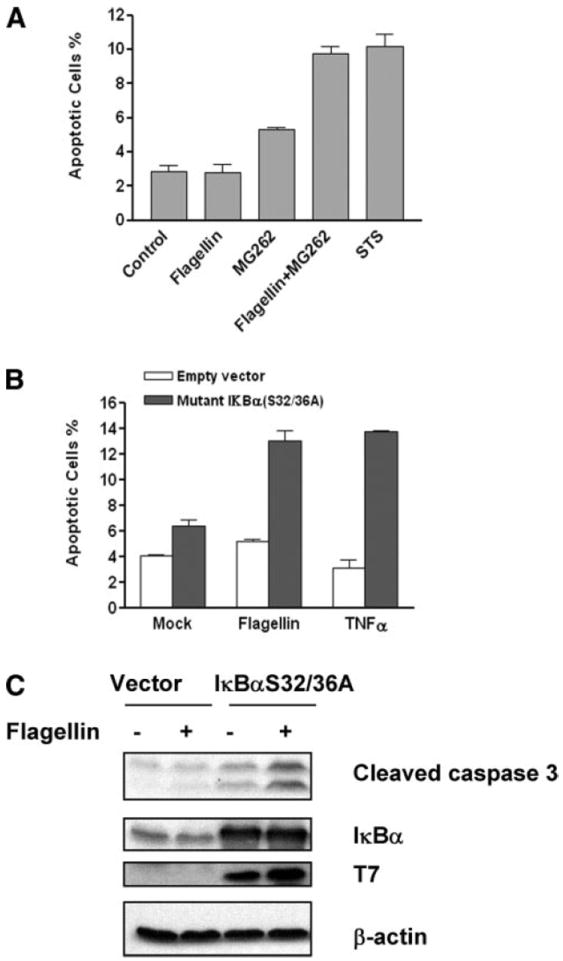
Flagellin can elicit apoptosis under conditions of NF-κB blockade. A: IEC-6 cells were treated with or without 100 ng/ml of flagellin and/or 1 μM MG-262 or 2 μM STS for 6 h. Cells were fixed, stained with propidium iodide, and analyzed by flow cytometry to assay DNA cleavage (sub-G1 fraction); 10,000 fluorescent events were measured for each sample. The percentages of positive cells are graphed on the y-axis. B: IEC-6 cells were transiently cotransfected with expression vector pDsRed2, encoding red fluorescent protein; mutant IκBα, pCMV4 T7-IκBα S32/36A; or empty control vector by FuGENE 6 reagent according to the manufacturer’s instruction. Twenty hours after transfection, cultures were treated with flagellin (100 ng/ml) or TNF-α (10 ng/ml). Cells were harvested 24 h later, a TdT-mediated dUTP nick-end labeling (TUNEL) assay was performed, and cells were analyzed by flow cytometry; 10,000 fluorescent events were measured for each sample. C: 293T cells were treated as in B. Lysates were prepared and probed for indicated antibodies.
To support the notion that specific inhibition of the NF-κB pathway would allow flagellin-induced caspase activation to proceed to irreversible cell death, we transfected cells with an IκBα mutant bearing Ser to Ala transitions (S32/36A) on the phosphorylation motif. This mutant cannot be degraded by proinflammatory stimuli and also acts as a dominant negative substrate for inhibitor of κB, kinase (IKK), effectively and selectively inhibiting the NF-κB pathway. IEC-6 cells were transfected (with expression confirmed by Western blot) and assayed for apoptosis by TUNEL staining or immunoblot for activated caspase 3 with and without stimulation of the cells with flagellin (Fig. 5, B and C). Whereas the mutant IκBα construct alone had relatively little affect on TUNEL positively, transfected cells exposed to flagellin for 12–24 h showed a more than doubling of the numbers of apoptotic cells. TNF-α was also tested as a positive control; this cytokine was equally effective in activating apoptosis under conditions of NF-κB blockade. Collectively, these data indicate that flagellin-induced signals can augment apoptosis during specific inhibition of the proinflammatory/antiapoptotic NF-κB signaling pathway.
Flagellin activates the extrinsic pathway in a TLR5-dependant fashion
Proinflammatory signaling elicited by flagellin requires TLR5. To investigate whether flagellin-elicited apoptotic responses are also TLR5 dependent, we utilized a cell line stably transfected with TLR5 that can confer the ability to secrete IL-8 in response to flagellin (the parent cell line does not react to flagellin) (52). These cells were stimulated with flagellin with and without cycloheximide (a protein synthesis inhibitor) for 4–6 h and evaluated with immunoblots for activated caspases. As expected, strong caspase 3 and caspase 8 activation was only seen in the presence of both flagellin and cycloheximide, at 4–6 h (Fig. 6, A–C). Caspase 9 activation was not consistently observed with these reagents. The control HeLa cell line, without the transfected TLR5, did not show any caspase activation under identical conditions. Taken together, these results indicate that flagellin can activate the extrinsic pathway of caspase processing through its physiological TLR5 receptor.
Fig. 6.
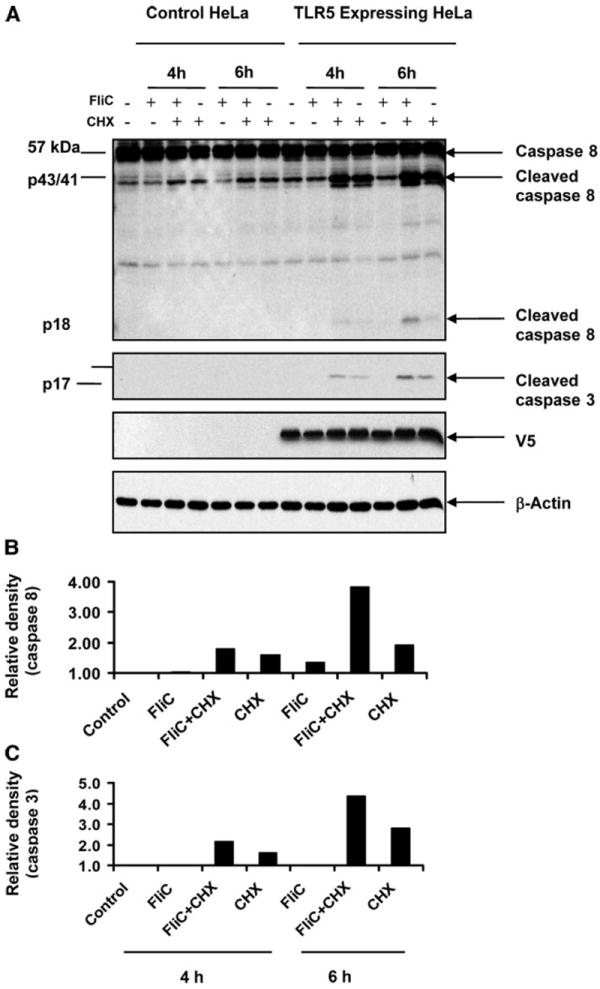
Flagellin activates caspase 8 (extrinsic pathway) in a Toll-like receptor (TLR)5-dependant manner. A: HeLa cells with and without a stably transfected TLR5 expression plasmid were stimulated with flagellin (100 ng/ml), cycloheximide (CHX; 5 μg/ml), or a combination for the indicated durations. Whole cell lysates were subjected to immunoblots with the indicated activated caspase antibodies. V5 is an epitope tag of the transfected TLR5. B and C: densitometric analysis of caspase 8 (B) and caspase 3 (C) immunoreactivity. Cleaved caspase 8 and caspase 3 bands were scanned with Bio-Rad Imaging Densitometer GS-700 and quantitated with Bio-Rad Quantity One acquisition software.
We have shown that flagellin induces antiapoptotic proteins as part of its transcriptional program, with cIAP-2 being the most highly induced example. We have also shown that when survival pathways are inhibited, TLR5-mediated signaling can activate caspases. We hypothesize that in most flagellin-TLR5 interactions, these antiapoptotic proteins inhibit caspase activation and permit inflammation. To obtain mechanistic data, we overexpressed cIAP-2 under conditions where flagellin induces apoptotic activation, by blockade of the NF-κB pathway with proteasome inhibitors, as demonstrated in Fig. 5C. As shown in Fig. 7, cIAP-2 reduced executioner caspase 3 cleavage by ~50% under these conditions.
Fig. 7.
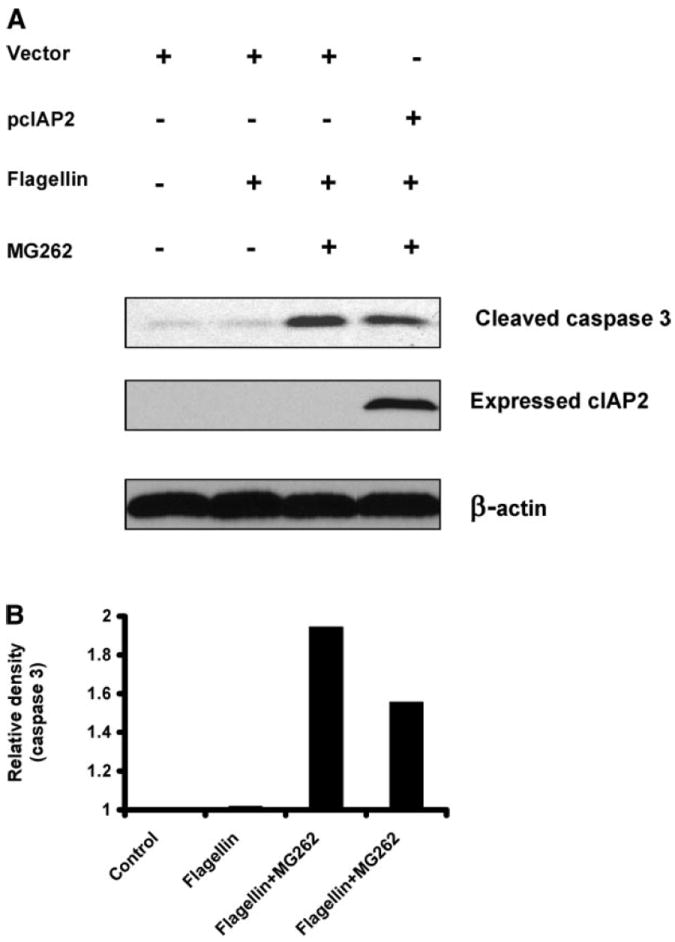
cIAP-2 can inhibit flagellin-mediated caspase activation. A: cells were transfected with vector for cIAP-2 and subsequently treated with flagellin and MG-262 as in Fig. 4C. B: densitometric analysis of caspase 3 immunoreactivity was performed as in Fig. 6C.
DISCUSSION
Flagellin is increasingly recognized as a bacterial inducer of innate and adaptive immunity in mammals. It also serves to stimulate cellular defensive responses in plants [acting through a specific pattern recognition receptors (FLS2)] (18, 19, 55) and invertebrates (43), suggesting a wide range of eukaryotic life has evolved mechanisms to perceive this protein and react to it as a threat. In our experiments, flagellin largely recapitulated the transcriptional response induced by viable S. typhimurium. The responses are also highly similar to those responses induced by TNF-α, a classical endogenous promoter of innate immunity/inflammation. The differences that were observed, such as the preferential activation of inducible nitric oxide synthase and more prolonged MAPK (both JNK and p38) phosphorylation by flagellin, presumably stems from different receptor utilization (TNF receptor vs. TLR5) and distinct receptor specific secondary signaling intermediates (i.e., MyD88) that ultimately control downstream transcriptional responses (35). Our current data showing the essentially similar transcriptional responses of flagellin and TNF-α underscore the importance of acute neutrophilic inflammation in control of infection by flagellated organisms.
Although signaling cascades such as NF-κB are generally understood as components of the proinflammatory response mediating upregulation of cytokines, chemokines, adhesion molecules, etc., it is becoming increasingly apparent that integral components of this response are protein modifiers of cellular apoptotic pathways such as the IAP family and A20. This observation is highly supportive of the notion that apoptotic activation proceeds in parallel with proinflammatory activation (7, 27). Certain inducers of inflammation, such as TNF-α, can initiate apoptotic signaling via the extrinsic (caspase 8) pathway (2). The TNF receptor proteins employ differential utilization of adaptor proteins to control the bifurcation of proapoptotic and proinflammatory signaling. Receptor-bound TNF receptor-associated death domain protein (TRADD) may interact with TNF receptor-associated factor (TRAF2) to activate downstream IKK and NF-κB, or it may bind the death effector domain-containing adaptor FAS-associated death domain protein (FADD) to initiate caspase 8 processing (50). Evidence is beginning to emerge that indicates that the TLRs can also mediate both proinflammatory and proapoptotic signaling in a similar fashion. TLR4/LPS interactions have been demonstrated to induce apoptotic pathways in macrophages under conditions of proteasomal blockade, and TLR4-deficient macrophages were shown to be resistant to Yersinia-induced apoptosis (21). Aliprantis et al. (3) reported that TLR2/bacterial lipoprotein interactions activated the extrinsic pathway (caspase 8) via signaling involving MyD88 and subsequent recruitment of FADD, indicating that TLRs can act as “death receptors.” Proapoptotic signaling via TLR4 has been described in macrophages (25, 41), involving the TLR interacting adaptor protein inducing IFN-β (TRIF). TRIF-null macrophages show reduced TLR4-dependant apoptosis, and overexpressed TRIF itself is proapoptotic (42). Our data indicate that flagellin-induced signaling can also mediate activation of the proapoptotic cascades. The potential roles of MyD88, FADD, and TRIF in transducing caspase activation via TLR5 are under investigation.
It is evident that epithelial cells exposed to a microbial challenge can eventuate in cellular proinflammatory responses or in programmed cell death. Natural infection can vary in numbers of microbes, duration of interaction, associated organisms, concurrent state of innate or adaptive immune activation, and undoubtedly many other variables. It is probably accurate to say that proinflammatory and proapoptotic activation both occur rapidly and reliably during the initial interactions of bacterial organisms with a potential host. The cellular end point of these biochemical events depends whether proinflammatory or proapoptotic signaling “gains the upper hand.” We propose that epithelial cells, once they perceive bacterial flagellin in an inappropriate location (i.e., intracellular or basolateral) via the TLR5 receptor, initiate in parallel tandem pathways, both proinflammatory, conducted along sequential transfer of covalent modifications, and apoptotic, conducted along proteolytic cascades (Fig. 8). In most cases, proinflammatory upregulation of antiapoptotic effectors, such as the IAP molecules, serves to arrest apoptotic pathways before activation of executioner caspases and irreversible cell damage occurs. In the event of inhibition of proinflammatory signaling and consequent transcription, caspase activation could occur unimpeded and terminate in the dismantling of the cell. Such inhibition is now recognized to play an important role in the pathogenesis of certain bacterial infections. Yersinia possess a translocated effector protein, YopJ, with demonstrated effects against NF-κB and MAPK survival pathways, which allows them to induce macrophage apoptosis in a TLR4-dependent manner, efficiently eliminating an immunomodulatory cell (54). Salmonella are well known inducers of apoptosis in macrophages (28, 29) and can induce apoptosis in HT-29 epithelial cell cultures after at least 24 h of coculture with wild-type Salmonella (36). These bacteria also possess a YopJ homolog, AvrA, which has been demonstrated to inhibit NF-κB and promote apoptosis in epithelial cells (8). While it is possible that such bacterial effectors may be directly activating caspases, prokaryotic proteins and small molecules that specifically inhibit proinflammatory pathways would not be proapoptotic unless accompanied by a concurrent signal that set caspase processing in motion. Because flagellin and other PAMP induced signaling through the TLRs fulfill this requirement, it will be interesting to determine whether the presence of these molecules is required for bacteria-mediated apoptosis.
Fig. 8.
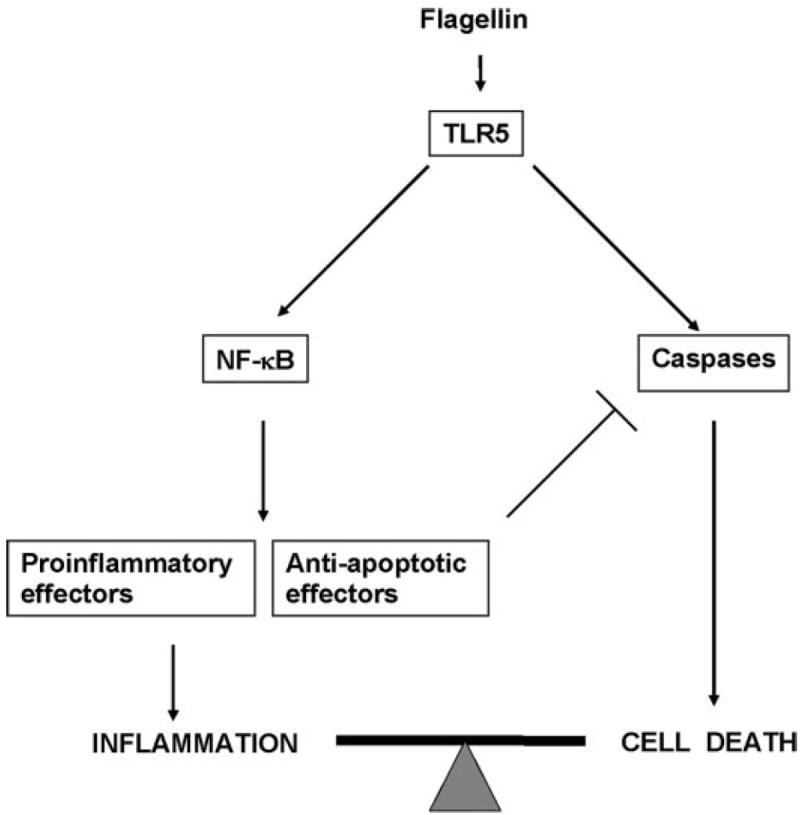
Model of pro- and antiapoptotic signaling from flagellin/TLR5. Flagellin activates the proinflammatory NF-κB pathway in parallel with caspase cascades. Usually, transcriptional activation of antiapoptotic effectors arrests caspase activation, and reversible inflammation occurs. If proinflammatory pathways are blocked, caspase activation can proceed to irreversible cell death.
Flagellin is emerging as a key regulator of innate and adaptive immunity against flagellated pathogens (40). Why are cells wired to respond to flagellin (and probably most proinflammatory agonists) in part by activation of the apoptotic program? It has been suggested that apoptosis itself may represent the earliest form of “immunity,” whereas the responses we now consider “innate immunity (i.e., inflammation)” are a later evolutionary development (4, 26). Colonial, multicellular eukaryotes may have utilized a form of programmed cell death to eliminate infected cells without lethal effects to the whole organism. Indeed, such a strategy is common in plant immunity and in lower metazoans such as nematodes (1), and it is well known that PAMPs including flagellin, peptidoglycan (PGN), and LPS are potent inducers of apoptosis in these organisms (30, 44). Pattern recognition receptors may have evolved to altruistically eliminate a threatened cell via activation of apoptotic proteolytic cascades or, if such drastic steps are unwarranted, induce a parallel biochemical signaling pathway the results in synthesis and release of soluble mediators including chemotactic chemokines and antimicrobial peptides.
How flagellin (an extracellular agonist) stimulates the intrinsic/mitochondrial pathway is not clear, although potential mechanisms can be envisioned. Bid is a proapoptotic member of the Bcl-2 family of apoptotic regulators (33). These proteins, defined by the “BH3” domain, can heterodimerize with each other; a relative excess of pro- over antiapoptotic members is thought to permit formation of a mitochondrial “pore,” allowing the escape of cytochrome c (20) and subsequent initiation of the intrinsic pathway. Bid is regulated by cleavage and activation by caspase 8, thus providing an amplifying link from extrinsic proapoptotic signals to intrinsic pathways. Transient Bid cleavage was observed maximally in T84 cells at 3 h of flagellin treatment, but, again, the exposure of cells to flagellin under conditions where survival genes could not be upregulated (MG-262) resulted in a strong appearance of the cleaved, 15-kDa active form, tBid (data not shown). Further experiments to characterize this process are underway.
Our data demonstrating that flagellin pretreatment and cIAP-2 overexpression can reduce caspase activation (in normal cells with intact proinflammatory pathways) elicited by a separate potentially injurious stimulus are consistent with our current understanding of the role of antiapoptotic effectors (27). Recent work has also suggested that constitutive TLR stimulation by PAMPs normally present in the intestinal lumen is cytoprotective to immunochemical epithelial injury (39). Although flagellin per se was not identified as a cytoprotective PAMP in that study, our results suggest that abundant lumenal flagellin may mediate a similar effect. Consistently, other studies have shown flagellin-mediated proinflammatory signaling in vivo induced by flagellated commensals or purified flagellin (5, 24).
Acknowledgments
GRANTS
This study was supported by National Institutes of Health Grants RO1 AI-51282 and AI-49741 (to A. S. Neish) and DK-062851 (to P. Lin), Emory Digestive Disease Research Center Grant R24 DK-064399, and the Woodruff Research Foundation.
References
- 1.Aballay A, Ausubel FM. Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proc Natl Acad Sci USA. 2001;98:2735–2739. doi: 10.1073/pnas.041613098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. doi: 10.1101/gad.1126903. [DOI] [PubMed] [Google Scholar]
- 3.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 2000;19:3325–3336. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ameisen JC. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Differ. 2002;9:367–393. doi: 10.1038/sj.cdd.4400950. [DOI] [PubMed] [Google Scholar]
- 5.Bambou JC, Giraud A, Menard S, Begue B, Rakotobe S, Heyman M, Taddei F, Cerf-Bensussan N, Gaboriau-Routhiau V. In vitro and ex vivo activation of the TLR5 signaling pathway in intestinal epithelial cells by a commensal Escherichia coli strain. J Biol Chem. 2004;279:42984–42992. doi: 10.1074/jbc.M405410200. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya S, Ray RM, Viar MJ, Johnson LR. Polyamines are required for activation of c-Jun NH2-terminal kinase and apoptosis in response to TNF-α in IEC-6 cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G980–G991. doi: 10.1152/ajpgi.00206.2003. [DOI] [PubMed] [Google Scholar]
- 7.Burstein E, Duckett CS. Dying for NF-kappaB? Control of cell death by transcriptional regulation of the apoptotic machinery. Curr Opin Cell Biol. 2003;15:732–737. doi: 10.1016/j.ceb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Collier-Hyams LS, Zeng H, Sun J, Tomlinson AD, Bao ZQ, Chen H, Madara JL, Orth K, Neish AS. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-kappa B pathway. J Immunol. 2002;169:2846–2850. doi: 10.4049/jimmunol.169.6.2846. [DOI] [PubMed] [Google Scholar]
- 9.Deveraux QL, Reed JC. IAP family proteins–suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 10.Donnenberg MS. Pathogenic strategies of enteric bacteria. Nature. 2000;406:768–774. doi: 10.1038/35021212. [DOI] [PubMed] [Google Scholar]
- 11.Duan S, Hajek P, Lin C, Shin SK, Attardi G, Chomyn A. Mitochondrial outer membrane permeability change and hypersensitivity to digitonin early in staurosporine-induced apoptosis. J Biol Chem. 2003;278:1346–1353. doi: 10.1074/jbc.M209269200. [DOI] [PubMed] [Google Scholar]
- 12.Eaves-Pyles T, Murthy K, Liaudet L, Virag L, Ross G, Soriano FG, Szabo C, Salzman AL. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: I kappa B alpha degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J Immunol. 2001;166:1248–1260. doi: 10.4049/jimmunol.166.2.1248. [DOI] [PubMed] [Google Scholar]
- 13.Elewaut D, DiDonato JA, Kim JM, Truong F, Eckmann L, Kagnoff MF. NF-kappa B is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J Immunol. 1999;163:1457–1466. [PubMed] [Google Scholar]
- 14.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 15.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 16.Gewirtz AT, Rao AS, Simon PO, Jr, Merlin D, Carnes D, Madara JL, Neish AS. Salmonella typhimurium induces epithelial IL-8 expression via Ca2+-mediated activation of the NF-kappaB pathway. J Clin Invest. 2000;105:79–92. doi: 10.1172/JCI8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gewirtz AT, Simon PO, Jr, Schmitt CK, Taylor LJ, Hagedorn CH, O’Brien AD, Neish AS, Madara JL. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Invest. 2001;107:99–109. doi: 10.1172/JCI10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez-Gomez L, Boller T. Flagellin perception: a paradigm for innate immunity. Trends Plant Sci. 2002;7:251–256. doi: 10.1016/s1360-1385(02)02261-6. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Gomez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5:1003–1011. doi: 10.1016/s1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 20.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 21.Haase R, Kirschning CJ, Sing A, Schrottner P, Fukase K, Kusumoto S, Wagner H, Heesemann J, Ruckdeschel K. A dominant role of Toll-like receptor 4 in the signaling of apoptosis in bacteria-faced macrophages. J Immunol. 2003;171:4294–4303. doi: 10.4049/jimmunol.171.8.4294. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 23.He KL, Ting AT. A20 inhibits tumor necrosis factor (TNF) alpha-induced apoptosis by disrupting recruitment of TRADD and RIP to the TNF receptor 1 complex in Jurkat T cells. Mol Cell Biol. 2002;22:6034–6045. doi: 10.1128/MCB.22.17.6034-6045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honko AN, Mizel SB. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect Immun. 2004;72:6676–6679. doi: 10.1128/IAI.72.11.6676-6679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu LC, Park JM, Zhang K, Luo JL, Maeda S, Kaufman RJ, Eckmann L, Guiney DG, Karin M. The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature. 2004;428:341–345. doi: 10.1038/nature02405. [DOI] [PubMed] [Google Scholar]
- 26.James ER, Green DR. Infection and the origins of apoptosis. Cell Death Differ. 2002;9:355–357. doi: 10.1038/sj.cdd.4400986. [DOI] [PubMed] [Google Scholar]
- 27.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immun. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 28.Kim JM, Eckmann L, Savidge TC, Lowe DC, Witthoft T, Kagnoff MF. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Invest. 1998;102:1815–1823. doi: 10.1172/JCI2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knodler LA, Finlay BB. Salmonella and apoptosis: to live or let die? Microbes Infect. 2001;3:1321–1326. doi: 10.1016/s1286-4579(01)01493-9. [DOI] [PubMed] [Google Scholar]
- 30.Koropatnick TA, Engle JT, Apicella MA, Stabb EV, Goldman WE, McFall-Ngai MJ. Microbial factor-mediated development in a host-bacterial mutualism. Science. 2004;306:1186–1188. doi: 10.1126/science.1102218. [DOI] [PubMed] [Google Scholar]
- 31.Liaudet L, Szabo C, Evgenov OV, Murthy KG, Pacher P, Virag L, Mabley JG, Marton A, Soriano FG, Kirov MY, Bjertnaes LJ, Salzman AL. Flagellin from gram-negative bacteria is a potent mediator of acute pulmonary inflammation in sepsis. Shock. 2003;19:131–137. doi: 10.1097/00024382-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 34.Macnab RM. Escherichia coli and Salmonella typhimurium. Washington, DC: American Society for Microbiology; 1999. Flagella; pp. 123–145. [Google Scholar]
- 35.O’Neill LA. Signal transduction pathways activated by the IL-1 receptor/toll-like receptor superfamily. Curr Top Microbiol Immunol. 2002;270:47–61. [PubMed] [Google Scholar]
- 36.Paesold G, Guiney DG, Eckmann L, Kagnoff MF. Genes in the Salmonella pathogenicity island 2 and the Salmonella virulence plasmid are essential for Salmonella-induced apoptosis in intestinal epithelial cells. Cell Microbiol. 2002;4:771–781. doi: 10.1046/j.1462-5822.2002.00233.x. [DOI] [PubMed] [Google Scholar]
- 37.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 38.Park JM, Greten FR, Li ZW, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science. 2002;297:2048–2051. doi: 10.1126/science.1073163. [DOI] [PubMed] [Google Scholar]
- 39.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Ramos HC, Rumbo M, Sirard JC. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 2004;12:509–517. doi: 10.1016/j.tim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Ruckdeschel K, Mannel O, Schrottner P. Divergence of apoptosis-inducing and preventing signals in bacteria-faced macrophages through myeloid differentiation factor 88 and IL-1 receptor-associated kinase members. J Immunol. 2002;168:4601–4611. doi: 10.4049/jimmunol.168.9.4601. [DOI] [PubMed] [Google Scholar]
- 42.Ruckdeschel K, Pfaffinger G, Haase R, Sing A, Weighardt H, Hacker G, Holzmann B, Heesemann J. Signaling of apoptosis through TLRs critically involves toll/IL-1 receptor domain-containing adapter inducing IFN-beta, but not MyD88, in bacteria-infected murine macrophages. J Immunol. 2004;173:3320–3328. doi: 10.4049/jimmunol.173.5.3320. [DOI] [PubMed] [Google Scholar]
- 43.Samakovlis C, Asling B, Boman HG, Gateff E, Hultmark D. In vitro induction of cecropin genes–an immune response in a Drosophila blood cell line. Biochem Biophys Res Commun. 1992;188:1169–1175. doi: 10.1016/0006-291x(92)91354-s. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu R, Taguchi F, Marutani M, Mukaihara T, Inagaki Y, Toyoda K, Shiraishi T, Ichinose Y. The DeltafliD mutant of Pseudomonas syringae pv. tabaci, which secretes flagellin monomers, induces a strong hypersensitive reaction (HR) in non-host tomato cells. Mol Genet Genomics. 2003;269:21–30. doi: 10.1007/s00438-003-0817-3. [DOI] [PubMed] [Google Scholar]
- 45.Sierro F, Dubois B, Coste A, Kaiserlian D, Kraehenbuhl JP, Sirard JC. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc Natl Acad Sci USA. 2001;98:13722–13727. doi: 10.1073/pnas.241308598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SL, Cookson BT, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immun. 2003;4:1247–1253. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 47.Smith KD, Ozinsky A. Toll-like receptor-5 and the innate immune response to bacterial flagellin. Curr Top Microbiol Immunol. 2002;270:93–108. doi: 10.1007/978-3-642-59430-4_6. [DOI] [PubMed] [Google Scholar]
- 48.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 49.Tauxe R, Pavia A. Bacterial infections in humans: epidemiology and control. New York: Plenum; 1998. Salmonellosis: nontyphoidal; pp. 613–630. [Google Scholar]
- 50.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 51.Wick MJ. Living in the danger zone: innate immunity to Salmonella. Curr Opin Microbiol. 2004;7:51–57. doi: 10.1016/j.mib.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Yu Y, Zeng H, Lyons S, Carlson A, Merlin D, Neish AS, Gewirtz AT. TLR5-mediated activation of p38 MAPK regulates epithelial IL-8 expression via posttranscriptional mechanism. Am J Physiol Gastrointest Liver Physiol. 2003;285:G282–G290. doi: 10.1152/ajpgi.00503.2002. [DOI] [PubMed] [Google Scholar]
- 53.Zeng H, Carlson AQ, Guo Y, Yu Y, Collier-Hyams LS, Madara JL, Gewirtz AT, Neish AS. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J Immunol. 2003;171:3668–3674. doi: 10.4049/jimmunol.171.7.3668. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Bliska JB. Role of Toll-like receptor signaling in the apoptotic response of macrophages to Yersinia infection. Infect Immun. 2003;71:1513–1519. doi: 10.1128/IAI.71.3.1513-1519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]


