Abstract
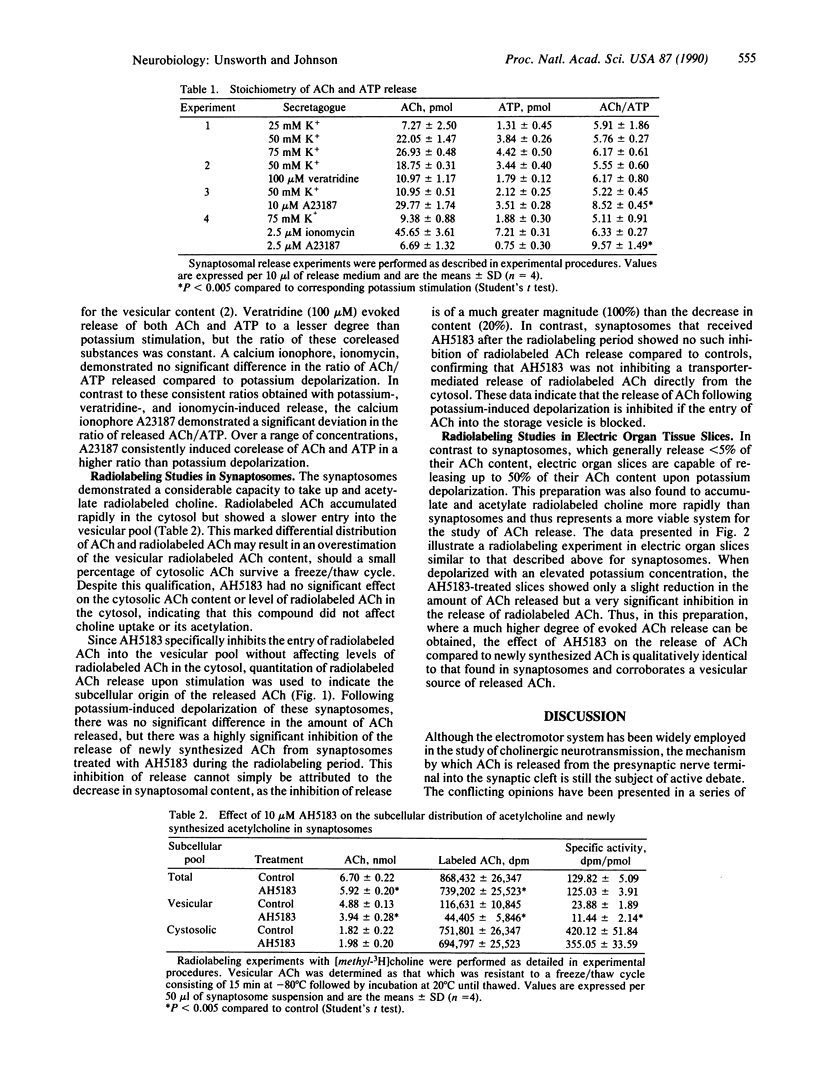
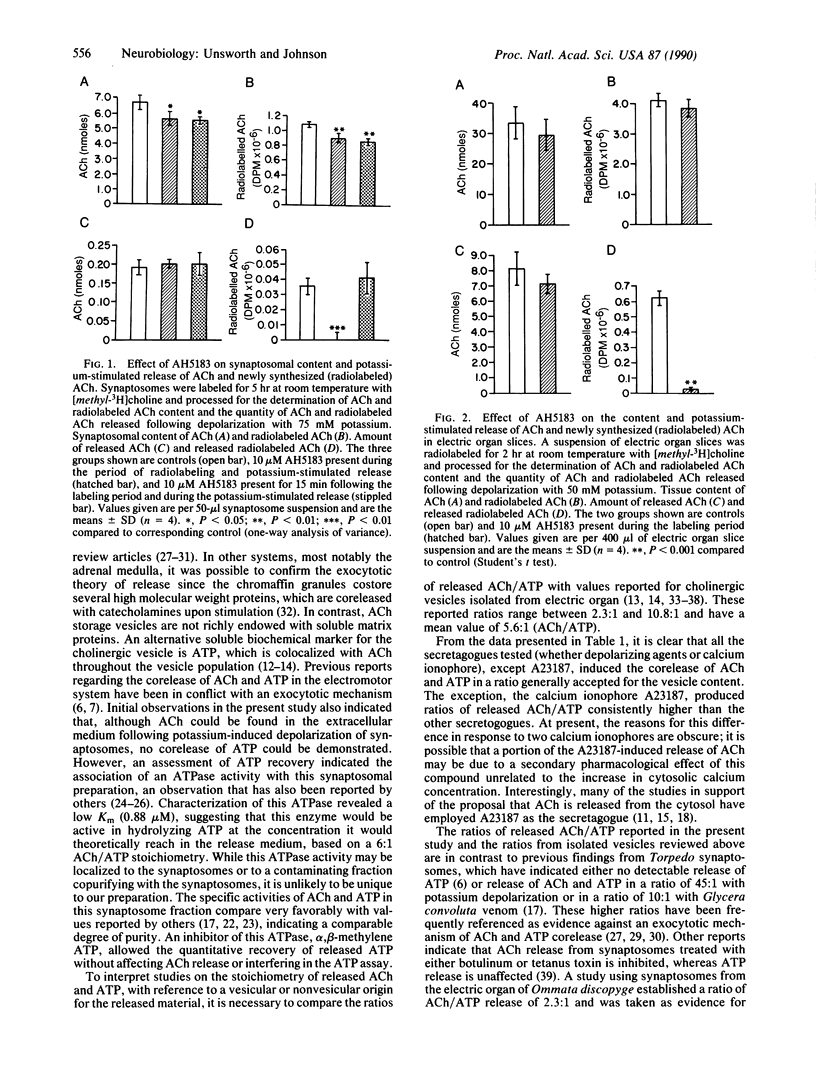
Although the exocytotic mechanism for quantal acetylcholine (ACh) release has been widely accepted for many years, it has repeatedly been challenged by reports that ACh released upon stimulation originates from the cytosol rather than synaptic vesicles. In this report, two independent experimental approaches were taken to establish the source of ACh released from the electromotor system of Narcine brasiliensis. Since ATP is colocalized with ACh in the cholinergic vesicle, the exocytotic theory predicts the corelease of these two components with a stoichiometry identical to that of the vesicle contents. The stimulated release of ATP from isolated synaptosomes could be accurately quantitated in the presence of the ATPase inhibitor adenosine 5'-[alpha, beta-methylene]triphosphate (500 microM), which prevented degradation of the released ATP. Various concentrations of elevated extracellular potassium (25-75 mM), veratridine (100 microM), and the calcium ionophore ionomycin (5 microM) all induced the corelease of ACh and ATP in a constant molar ratio of 5-6:1 (ACh/ATP), a stoichiometry consistent with that established for the vesicle content. In parallel to these stoichiometry studies, the compound 2-(4-phenylpiperidino)cyclohexanol (AH5183) was used to inhibit specifically the vesicular accumulation of newly synthesized (radiolabeled) ACh without affecting cytosolic levels of newly synthesized ACh in cholinergic nerve terminals. Treatment with AH5183 (10 microM) was shown to inhibit the release of newly synthesized ACh without markedly affecting total ACh release; thus, the entry of newly synthesized ACh into the synaptic vesicle is essential for its release. We conclude that ACh released upon stimulation originates exclusively from the vesicular pool and is coreleased stoichiometrically with other soluble vesicle contents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., King S. C., Parsons S. M. Pharmacological characterization of the acetylcholine transport system in purified Torpedo electric organ synaptic vesicles. Mol Pharmacol. 1983 Jul;24(1):48–54. [PubMed] [Google Scholar]
- Birman S., Israël M., Lesbats B., Morel N. Solubilization and partial purification of a presynaptic membrane protein ensuring calcium-dependent acetylcholine release from proteoliposomes. J Neurochem. 1986 Aug;47(2):433–444. doi: 10.1111/j.1471-4159.1986.tb04520.x. [DOI] [PubMed] [Google Scholar]
- Boyne A. F. Isolation of synaptic vesicles from Narcine brasiliensis electric organ: some influences on release of vesicular acetylcholine and ATP. Brain Res. 1976 Sep 24;114(3):481–491. doi: 10.1016/0006-8993(76)90969-0. [DOI] [PubMed] [Google Scholar]
- Carlson S. S., Wagner J. A., Kelly R. B. Purification of synaptic vesicles from elasmobranch electric organ and the use of biophysical criteria to demonstrate purity. Biochemistry. 1978 Apr 4;17(7):1188–1199. doi: 10.1021/bi00600a009. [DOI] [PubMed] [Google Scholar]
- Carroll P. T. The effect of the acetylcholine transport blocker 2-(4-phenylpiperidino) cyclohexanol (AH5183) on the subcellular storage and release of acetylcholine in mouse brain. Brain Res. 1985 Dec 9;358(1-2):200–209. doi: 10.1016/0006-8993(85)90964-3. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol Rev. 1980 Apr;60(2):396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- Collier B., Welner S. A., Rícný J., Araujo D. M. Acetylcholine synthesis and release by a sympathetic ganglion in the presence of 2-(4-phenylpiperidino) cyclohexanol (AH5183). J Neurochem. 1986 Mar;46(3):822–830. doi: 10.1111/j.1471-4159.1986.tb13046.x. [DOI] [PubMed] [Google Scholar]
- Dowdall M. J., Boyne A. F., Whittaker V. P. Adenosine triphosphate. A constituent of cholinergic synaptic vesicles. Biochem J. 1974 Apr;140(1):1–12. doi: 10.1042/bj1400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunant Y. On the mechanism of acetylcholine release. Prog Neurobiol. 1986;26(1):55–92. doi: 10.1016/0301-0082(86)90020-1. [DOI] [PubMed] [Google Scholar]
- Feldberg W., Fessard A. The cholinergic nature of the nerves to the electric organ of the Torpedo (Torpedo marmorata). J Physiol. 1942 Aug 18;101(2):200–216. doi: 10.1113/jphysiol.1942.sp003975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F. Isolation of choline esters from aqueous solutions by extraction with sodium tetraphenylboron in organic solvents. Biochem J. 1969 Jun;113(2):291–298. doi: 10.1042/bj1130291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondal E. J., Zimmermann H. Ectonucleotidase activities associated with cholinergic synaptosomes isolated from Torpedo electric organ. J Neurochem. 1986 Sep;47(3):871–881. doi: 10.1111/j.1471-4159.1986.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Israel M., Dunant Y., Manaranche R. The present status of the vesicular hypothesis. Prog Neurobiol. 1979;13(3):237–275. doi: 10.1016/0301-0082(79)90017-0. [DOI] [PubMed] [Google Scholar]
- Israël M., Lazereg S., Lesbats B., Manaranche R., Morel N. Large-scale purification of Torpedo electric organ synaptosomes. J Neurochem. 1985 Apr;44(4):1107–1110. doi: 10.1111/j.1471-4159.1985.tb08731.x. [DOI] [PubMed] [Google Scholar]
- Israël M., Lesbats B. Continuous determination by a chemiluminescent method of acetylcholine release and compartmentation in Torpedo electric organ synaptosomes. J Neurochem. 1981 Dec;37(6):1475–1483. doi: 10.1111/j.1471-4159.1981.tb06317.x. [DOI] [PubMed] [Google Scholar]
- Israël M., Lesbats B., Manaranche R. ACh release from osmotically shocked synaptosomes refilled with transmitter. Nature. 1981 Dec 3;294(5840):474–475. doi: 10.1038/294474a0. [DOI] [PubMed] [Google Scholar]
- Israël M., Lesbats B., Morel N., Manaranche R., Gulik-Krzywicki T., Dedieu J. C. Reconstitution of a functional synaptosomal membrane possessing the protein constituents involved in acetylcholine translocation. Proc Natl Acad Sci U S A. 1984 Jan;81(1):277–281. doi: 10.1073/pnas.81.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël M., Manaranche R., Marsal J., Meunier F. M., Morel N., Frachon P., Lesbats B. ATP-dependent calcium uptake by cholinergic synaptic vesicles isolated from Torpedo electric organ. J Membr Biol. 1980 May 23;54(2):115–126. doi: 10.1007/BF01940565. [DOI] [PubMed] [Google Scholar]
- Israël M., Manaranche R., Mastour-Frachon P., Morel N. Isolation of pure cholinergic nerve endings from the electric organ of Torpedo marmorata. Biochem J. 1976 Oct 15;160(1):113–115. doi: 10.1042/bj1600113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël M., Morel N., Lesbats B., Birman S., Manaranche R. Purification of a presynaptic membrane protein that mediates a calcium-dependent translocation of acetylcholine. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9226–9230. doi: 10.1073/pnas.83.23.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope R. S., Johnson G. V. Quinacrine and 2-(4-phenylpiperidino)cyclohexanol (AH5183) inhibit acetylcholine release and synthesis in rat brain slices. Mol Pharmacol. 1986 Jan;29(1):45–51. [PubMed] [Google Scholar]
- Keller F., Zimmermann H. Ecto-adenosine triphosphatase activity at the cholinergic nerve endings of the Torpedo electric organ. Life Sci. 1983 Dec 26;33(26):2635–2641. doi: 10.1016/0024-3205(83)90347-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lundin A., Richardsson A., Thore A. Continous monitoring of ATP-converting reactions by purified firefly luciferase. Anal Biochem. 1976 Oct;75(2):611–620. doi: 10.1016/0003-2697(76)90116-0. [DOI] [PubMed] [Google Scholar]
- Luqmani Y. A., Sudlow G., Whittaker V. P. Homocholine and acetylhomocholine: false transmitters in the cholinergic electromotor system of Torpedo. Neuroscience. 1980;5(1):153–160. doi: 10.1016/0306-4522(80)90081-0. [DOI] [PubMed] [Google Scholar]
- Michaelson D. M., Burstein M. Biochemical evidence that acetylcholine release from cholinergic nerve terminals is mostly vesicular. FEBS Lett. 1985 Sep 2;188(2):389–393. doi: 10.1016/0014-5793(85)80408-7. [DOI] [PubMed] [Google Scholar]
- Michaelson D. M. Is presynaptic acetylcholine release accompanied by the secretion of the synaptic vesicles contents? FEBS Lett. 1978 May 1;89(1):51–53. doi: 10.1016/0014-5793(78)80520-1. [DOI] [PubMed] [Google Scholar]
- Michaelson D. M., Ophir I. Purification and characterization of synaptic vesicles from the electric organ of Torpedo ocellata. Monogr Neural Sci. 1980;7:19–29. doi: 10.1159/000388811. [DOI] [PubMed] [Google Scholar]
- Morel N., Israel M., Manaranche R., Mastour-Frachon P. Isolation of pure cholinergic nerve endings from Torpedo electric organ. Evaluation of their metabolic properties. J Cell Biol. 1977 Oct;75(1):43–55. doi: 10.1083/jcb.75.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel N., Meunier F. M. Simultaneous release of acetylcholine and ATP from stimulated cholinergic synaptosomes. J Neurochem. 1981 May;36(5):1766–1773. doi: 10.1111/j.1471-4159.1981.tb00429.x. [DOI] [PubMed] [Google Scholar]
- Nagy A. K., Shuster T. A., Delgado-Escueta A. V. Ecto-ATPase of mammalian synaptosomes: identification and enzymic characterization. J Neurochem. 1986 Sep;47(3):976–986. doi: 10.1111/j.1471-4159.1986.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Ohsawa K., Dowe G. H., Morris S. J., Whittaker V. P. The lipid and protein content of cholinergic synaptic vesicles from the electric organ of Torpedo marmorata purified to constant composition: implications for vesicle structure. Brain Res. 1979 Feb 9;161(3):447–457. doi: 10.1016/0006-8993(79)90674-7. [DOI] [PubMed] [Google Scholar]
- Rabasseda X., Solsona C., Marsal J., Egea G., Bizzini B. ATP release from pure cholinergic synaptosomes is not blocked by tetanus toxin. FEBS Lett. 1987 Mar 23;213(2):337–340. doi: 10.1016/0014-5793(87)81518-1. [DOI] [PubMed] [Google Scholar]
- Richardson P. J., Whittaker V. P. The Na+ and K+ content of isolated Torpedo synaptosomes and its effect on choline uptake. J Neurochem. 1981 Apr;36(4):1536–1542. doi: 10.1111/j.1471-4159.1981.tb00597.x. [DOI] [PubMed] [Google Scholar]
- Schweitzer E. Coordinated release of ATP and ACh from cholinergic synaptosomes and its inhibition by calmodulin antagonists. J Neurosci. 1987 Sep;7(9):2948–2956. doi: 10.1523/JNEUROSCI.07-09-02948.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro T., Stadler H. Chemical composition of cholinergic synaptic vesicles from Torpedo marmorata based on improved purification. Eur J Biochem. 1978 Oct 16;90(3):479–487. doi: 10.1111/j.1432-1033.1978.tb12627.x. [DOI] [PubMed] [Google Scholar]
- Tauc L. Non vesicular release of neurotransmitter. Physiol Rev. 1982 Jul;62(3):857–893. doi: 10.1152/physrev.1982.62.3.857. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. Acetylcholine quanta are released from vesicles by exocytosis (and why some think not). Neuroscience. 1988 Jan;24(1):1–7. doi: 10.1016/0306-4522(88)90305-3. [DOI] [PubMed] [Google Scholar]
- Viveros O. H., Arqueros L., Kirshner N. Quantal secretion from adrenal medulla: all-or-none release of storage vesicle content. Science. 1969 Aug 29;165(3896):911–913. doi: 10.1126/science.165.3896.911. [DOI] [PubMed] [Google Scholar]
- Wagner J. A., Carlson S. S., Kelly R. B. Chemical and physical characterization of cholinergic synaptic vesicles. Biochemistry. 1978 Apr 4;17(7):1199–1206. doi: 10.1021/bi00600a010. [DOI] [PubMed] [Google Scholar]
- Whittaker V. P. Cholinergic synaptic vesicles from the electromotor nerve terminals of Torpedo. Composition and life cycle. Ann N Y Acad Sci. 1987;493:77–91. doi: 10.1111/j.1749-6632.1987.tb27185.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman H., Denston C. R. Recycling of synaptic vesicles in the cholinergic synapses of the Torpedo electric organ during induced transmitter release. Neuroscience. 1977;2(5):695–714. doi: 10.1016/0306-4522(77)90024-0. [DOI] [PubMed] [Google Scholar]
- Zimmermann H., Denston C. R. Adenosine triphosphate in cholinergic vesicles isolated from the electric organ of Electrophorus electricus. Brain Res. 1976 Jul 30;111(2):365–376. doi: 10.1016/0006-8993(76)90780-0. [DOI] [PubMed] [Google Scholar]
- Zimmermann H., Denston C. R. Separation of synaptic vesicles of different functional states from the cholinergic synapses of the Torpedo electric organ. Neuroscience. 1977;2(5):715–730. doi: 10.1016/0306-4522(77)90025-2. [DOI] [PubMed] [Google Scholar]


