Abstract
PKCζ is required for nuclear factor κ-B (NF-κB) activation in several cell systems. NF-κB is a suppressor of liver apoptosis during development and in concanavalin A (ConA)-induced T-cell-mediated hepatitis. Here we show that PKCζ−/− mice display inhibited ConA-induced NF-κB activation and reduced damage in liver. As the IL-4/Stat6 pathway is necessary for ConA-induced hepatitis, we addressed here the potential role of PKCζ in this cascade. Interestingly, the loss of PKCζ severely attenuated serum IL-5 and liver eotaxin-1 levels, two critical mediators of liver damage. Stat6 tyrosine phosphorylation and Jak1 activation were ablated in the liver of ConA-injected PKCζ−/− mice and in IL-4-stimulated PKCζ−/− fibroblasts. PKCζ interacts with and phosphorylates Jak1 and PKCζ activity is required for Jak1 function. In contrast, Par-4−/− mice have increased sensitivity to ConA-induced liver damage and IL-4 signaling. This unveils a novel and critical involvement of PKCζ in the IL-4/Stat6 signaling pathway in vitro and in vivo.
Keywords: inflammation, Jak1, NF-κB, PKCζ, Stat6
Introduction
Inflammation is a pathological condition in which different signaling mechanisms control a complex network of cellular and molecular interactions involving the crosstalk between apparently independent biochemical cascades that end up in the activation of gene expression programs for cytokines and chemokines. Nuclear factor κ-B (NF-κB) transcription factor complexes are critically involved in the control of a number of cellular responses during inflammation, as well as in the innate and adaptive immunity, and in repression of apoptosis (Karin, 1998; Karin and Lin, 2002; Li and Verma, 2002). In the canonical pathway, NF-κB is retained in the cytosol of unstimulated cells by the inhibitor proteins IκB, which are degraded upon cell activation by a number of stimuli, including TNFα, IL-1, and bacterial lipopolysaccharide (LPS), in fibroblasts and macrophages, as well as during the activation of the T-cell receptor and the B-cell receptor in lymphocytes (Li and Verma, 2002; Chen and Greene, 2004). This leads to the release and subsequent translocation of NF-κB to the nucleus. The degradation of IκB takes place after its ubiquitination and is carried out by the proteasome system (Ghosh and Karin, 2002). The triggering event in this pathway is the phosphorylation of IκB by the IKK complex, which is composed of two catalytic subunits (IKKα and IKKβ) and a scaffold protein named NEMO, IKKγ, or IKKAP. Genetic evidence demonstrates that IKKβ and IKKγ are ubiquitously required for IκB phosphorylation (Ghosh and Karin, 2002), whereas IKKα seems to be necessary only in mammary gland epithelial cells (Cao et al, 2001). However, IKKα can also play additional roles in the NF-κB pathways through its ability to control histone H3 phosphorylation (Anest et al, 2003; Yamamoto et al, 2003), and through the activation of an alternative noncanonical NF-κB cascade initiated by the processing of NF-κB2/p100, which is critical for the BAFF and lymphotoxin-β receptor signaling pathways that control B-cell maturation and the development of secondary lymphoid organs (Senftleben et al, 2001; Xiao et al, 2001; Claudio et al, 2002; Dejardin et al, 2002; Kayagaki et al, 2002).
The atypical PKCs (aPKCs; PKCλ/ι and PKCζ) have been implicated as important mediators in the control of cell survival through the activation of NF-κB (Diaz-Meco et al, 1993; Moscat and Diaz-Meco, 2000; Moscat et al, 2003). Particularly, the genetic inactivation of PKCζ in mice provokes a severe impairment in NF-κB activation at two levels (Leitges et al, 2001). In lung, in which PKCζ is particularly abundant, this kinase is required for the activation of IKK in vivo, whereas, in other systems like embryo fibroblasts (EFs) and B cells (Martin et al, 2002), PKCζ controls the phosphorylation of the RelA subunit of the NF-κB complex, enabling its interaction with the transcriptional co-activator CBP and the subsequent gene expression (Duran et al, 2003). The canonical NF-κB pathway is essential in the control of fetal liver survival as IKKβ and RelA knockout (KO) mice die of liver apoptosis during gestation in a TNFα-dependent manner (Karin, 1998).
Surprisingly, recent results using a liver-specific IKKβ conditional KO mice demonstrated that the loss of NF-κB does not sensitize hepatocytes to apoptosis induced by circulating TNFα in LPS-challenged mice in vivo, whereas injection of concanavalin A (ConA) produces massive hepatocyte apoptosis through a cell-bound TNFα-mediated mechanism involving both TNF receptor 1 and 2, as well as the sustained activation of JNK (Maeda et al, 2003). Interestingly, ConA-induced liver injury is also an excellent model of T-cell-mediated hepatitis (Tiegs et al, 1992), which by the release of cytokines impact on liver cells through the STAT signaling cascades. In this regard, recent evidence using mice in which IL-4 or Stat6 have been genetically inactivated demonstrates that ConA injection induces hepatitis through an IL-4/Stat6 pathway that upregulates IL-5 and eotaxin levels that trigger the recruitment of leukocytes provoking hepatitis (Jaruga et al, 2003). IL-4 is a Th2 cytokine that activates the tyrosine phosphorylation of Stat6 through a Jak1/Jak3-dependent mechanism, promoting its homodimerization and nuclear translocation (Ho and Glimcher, 2002; O'Shea et al, 2002; Shuai and Liu, 2003). Therefore, it seems that whereas the IL-4/Stat6 cascade plays a pro-inflammatory role in ConA-induced hepatitis, NF-κB exerts a protective function. This is also of interest from the point of view of PKCζ signaling, as T cells from Par-4-deficient mice, an inhibitor of the aPKCs, overproduce IL-4 when chronically challenged through the TCR (Lafuente et al, 2003). Therefore, conceivably, PKCζ, in addition to regulating NF-κB, could also play an important role in the IL-4 signaling pathway. Since both cascades are critical for liver damage, we sought to investigate here the role of PKCζ in the model of T-cell-mediated liver injury induced by injection of ConA as a paradigmatic example of complex interconnecting signaling pathways.
Results
Inhibition of ConA-induced hepatitis in PKCζ−/− mice
NF-κB inactivation sensitizes cells to liver injury in ConA-injected mice (Maeda et al, 2003). As PKCζ is important for the activation of NF-κB, we first determined whether the loss of this kinase makes livers more susceptible to ConA-induced damage. Therefore, ConA was intravenously injected in mice, either wild type (WT) or PKCζ KO, after which they were killed and serum levels of alanine aminotransferase (ALT) were determined as a parameter of liver injury. ConA injection leads to a reproducible and significant induction of liver damage in WT mice (Figure 1A). Surprisingly, instead of increased serum ALT levels, as it would be expected when NF-κB is inhibited, the PKCζ KO mice displayed a clear reduction in ALT levels (Figure 1A), suggesting that the loss of PKCζ, instead of enhancing liver injury, actually protects from ConA-induced liver damage. Consistent with this observation, liver apoptosis, determined as caspase-3 activation in liver homogenates, is induced in WT mice but not in the PKCζ KOs (Figure 1B). Histological examination of livers confirms that apoptosis (Figure 1C) and liver damage (Figure 1D) induced by ConA injection are attenuated in the PKCζ KO mice as compared to the WT controls. In addition, the number of eosinophils, that in the liver of WT ConA-injected mice amounts to 17±2 per field (× 20), is undetectable in the liver of identically treated KO mice. Collectively, these results indicate that PKCζ plays an active role in the induction of liver injury in response to ConA injection.
Figure 1.
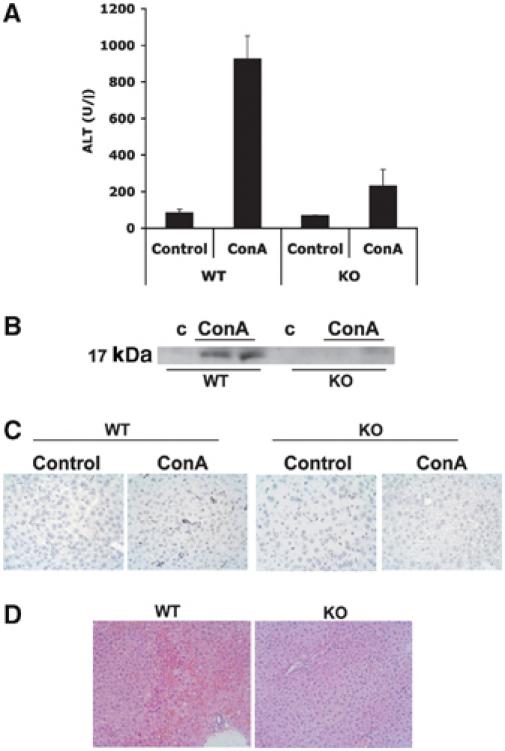
PKCζ−/− mice display reduced sensitivity to ConA-induced liver injury. (A) Serum levels of ALT were determined 8 h after injection with 12 μg/g of ConA. Results are the mean±s.d. of n=5 for each genotype. (B) Liver extracts from the same experiment were analyzed by immunoblotting to determine the activation of caspase-3. Representative autoradiographs are displayed of one mouse untreated and two mice injected with 12 μg/g of ConA, either WT or PKCζ KO, of a total of n=5 for each genotype. Histological analysis of representative livers of the same type of experiment in which tissue sections were subjected to TUNEL (× 40) (C) or H&E (× 20) (D) staining.
PKCζ is required for liver NF-κB activation in ConA-injected mice
TNFα is central to the induction of liver apoptosis. Interestingly, ConA injection produces a robust increase in serum TNFα levels both in WT and in PKCζ-deficient mice (Figure 2A), indicating that the diminished liver injury detected in the PKCζ KO mice cannot be accounted for by a reduction in TNFα generation. Consistent with this notion, the synthesis of TNFα in ConA-stimulated T cells in vitro is not inhibited in PKCζ−/− cells (Figure 2B). Interestingly, injection of ConA caused a significant increase in CD69+ CD4+ T cells both in WT and PKCζ−/− mice (Figure 2C). This suggests that the loss of PKCζ does not produce a general defect in T-cell activation. Since NF-κB blockade exacerbates liver damage induced by ConA injection, whereas the loss of PKCζ prevents liver injury in this model, and this kinase plays a role in NF-κB activation in other cell systems, we next determined whether NF-κB is impaired or not in PKCζ−/− livers. Thus, mice, either WT or PKCζ-deficient, were intravenously injected with ConA, after which they were killed and the synthesis of IκB and MCP-1 mRNAs, as representative NF-κB target genes, was determined by RT–PCR. Results of Figure 2D and E demonstrate that the loss of PKCζ significantly impairs the synthesis of liver IκB (P<0.003) and MCP-1 (P<0.01) mRNAs in response to ConA injection, indicating that, consistently with previously published data in EFs, PKCζ is required for the transcription of NF-κB-dependent genes. In addition, the synthesis of iNOs, another well-established marker of NF-κB activation, is severely inhibited in the livers of KO mice as compared to the WT controls in response to the ConA challenge (Figure 3A). Next we determined whether NF-κB nuclear activity measured by electrophoretic mobility shift assays (EMSAs) was impaired in the liver of PKCζ-deficient mice. WT and KO mice were injected as above, liver nuclear extracts were prepared, and NF-κB activity was determined by EMSA. Interestingly, ConA injection potently activates NF-κB in the liver of WT mice (Figure 3B). However, the induction of this parameter in the livers of PKCζ KO mice was dramatically inhibited (Figure 3B). Consistent with this evidence, the activation of liver IKK in response to the ConA challenge was also severely attenuated in the PKCζ KO mice (Figure 3C).
Figure 2.
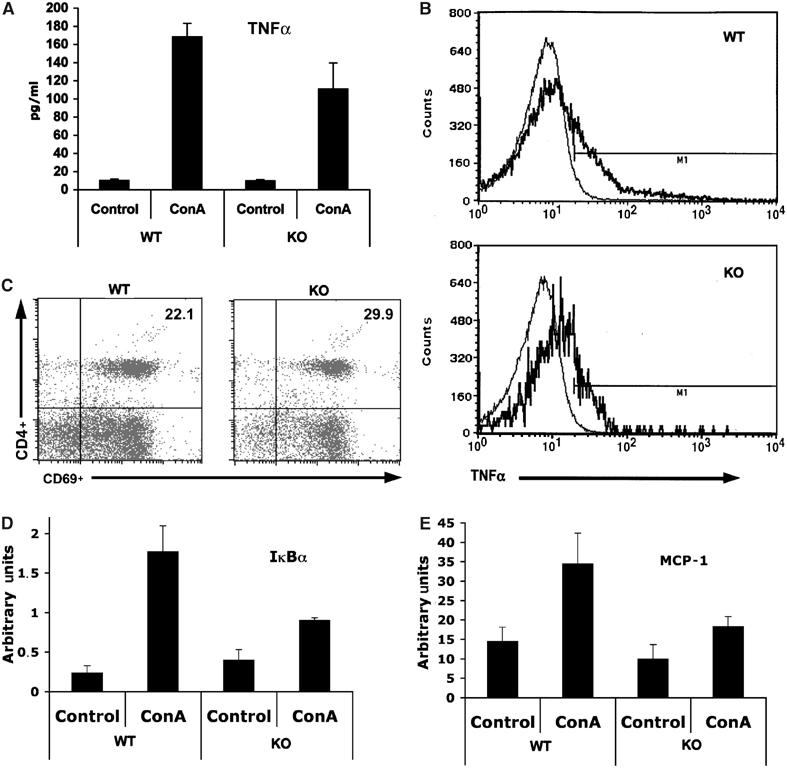
Impaired transcription of κB-dependent genes in ConA-induced livers from PKCζ−/− mice. (A) Serum TNFα levels were determined 2 h after injection of 12 μg/g of ConA. Results are the mean±s.d. of n=5 for each genotype. (B) TNFα synthesis was determined by FACS in lymph node T cells from WT or PKCζ KO mice activated (thick line) or not (thin line) with ConA (10 μg/ml). (C) Hepatic CD4+ T cells were isolated from WT or KO mice that have been injected with 12 μg/g of ConA for 4 h, and subsequently analyzed for surface expression of CD69 by FACS. In another set of experiments, liver mRNA levels of IκBα (D) and MCP-1 (E) were determined by real-time RT–PCR 8 h after injection with ConA. Results are the mean±s.d. of n=5 for each genotype in duplicate.
Figure 3.
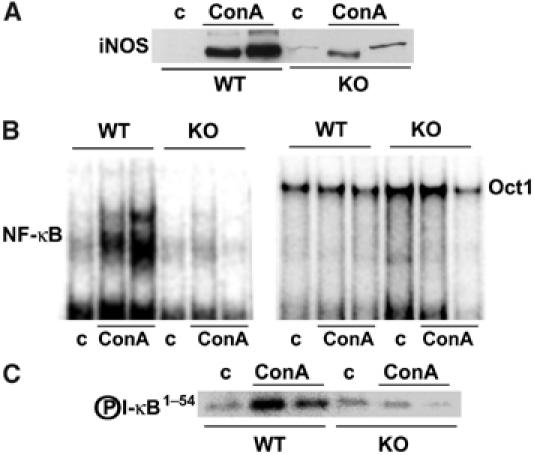
Impaired liver NF-κB signaling in ConA-injected PKCζ−/− mice. (A) Levels of iNOS were determined by immunoblot analysis of extracts from mice injected with 12 μg/g of ConA for 8 h. Results show representative (n=5) autoradiographs of one mouse untreated and two mice injected with ConA either WT or PKCζ KO. (B) Nuclear extracts from the above experiment were analyzed by EMSA using a κB probe (left panel) or an Oct1 probe (right panel), as a negative control. (C) Liver IKK activity was also determined in extracts from the above experiment.
PKCζ and the IL-4/Stat6 pathway
Collectively, these results indicate that the loss of PKCζ reduces liver NF-κB activation in response to ConA injection but that, in contrast to the data from mice in which IKKβ was selectively knocked out in the liver, this does not correlate with increased susceptibility to liver damage. These unexpected observations could be interpreted as that the loss of NF-κB by itself is not sufficient to sensitize the liver to ConA-induced injury and that IKKβ may control pathways other than NF-κB that may also be required to provide liver protection in this system. Alternatively, it is possible that PKCζ may play a dual role in liver. On the one hand, it could promote liver protection through NF-κB but, in addition, it may be simultaneously required for the induction of liver injury in response to the ConA challenge. If this model is correct, the loss of PKCζ could prevent liver damage induced by ConA. Under these circumstances, the potential sensitization to liver injury in the PKCζ−/− mice will not be apparent, as there will not be an appreciable induction of liver damage in the PKCζ-deficient mice. A potential mechanism whereby PKCζ may be necessary for ConA-induced liver damage may be through the IL-4/Stat6 system (see above). In order to determine if this hypothesis is correct, we initially investigated serum IL-4 levels in ConA-injected WT and PKCζ KO mice. Results of Figure 4A show that, although IL-4 levels are slightly reduced in the KO mice after the ConA challenge, a substantial amount of this cytokine is produced in the mutant mice as compared to the WT controls. T cell-released IL-4 targets NKT cells and hepatocytes, leading to the production of IL-5 and eotaxin-1, respectively, both important mediators in the control of eosinophil infiltration and liver injury in ConA-induced hepatitis (Jaruga et al, 2003). Interestingly, ConA injection produces a robust elevation in serum IL-5 levels in WT, but not in PKCζ-deficient mice (Figure 4B). Similarly, liver eotaxin-1 mRNA levels are dramatically induced in the WT but not in the PKCζ-deficient mice (Figure 4C). These results indicate that PKCζ may be required for IL-4 signaling to IL-5 and eotaxin-1 synthesis, and explains the reduced liver injury observed in the PKCζ KO mice in ConA-induced hepatitis.
Figure 4.
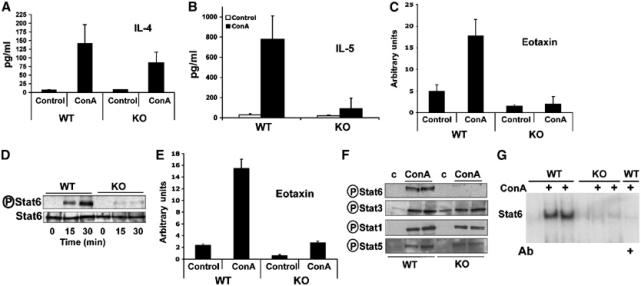
Impaired Stat6 signaling in PKCζ−/− mice. Serum IL-4 (A) and IL-5 (B) levels were determined 2 h (A) or 8 h (B) after injection of ConA (12 μg/g). Results are the mean±s.d. of n=5 for each genotype. In another set of experiments, liver mRNA levels of eotaxin-1 (C) were determined by real-time RT–PCR 8 h after injection with ConA. Results are the mean±s.d. of n=5 for each genotype. Primary EFs, either WT or PKCζ−/−, were stimulated with IL-4 for 15 and 30 min (D) or 48 h (E), after which Stat6 phosphorylation and Stat6 levels were determined by immunoblotting (D), and eotaxin-1 mRNA levels were determined by real-time RT–PCR (E). Tyrosine phosphorylation of liver Stat proteins was determined 2 h after injection of 12 μg/g of ConA (F). Nuclear extracts from the above experiment were analyzed by EMSA using a Stat6 probe (G). Incubation with a neutralizing anti-Stat6 antibody demonstrates the presence of Stat6 in the shifted band. Representative autoradiographs are displayed of one mouse untreated and two mice injected with ConA, either WT or PKCζ KO, of a total of n=5 for each genotype.
Essential role of PKCζ in Stat6 activation in response to IL-4
As PKCζ is required for IL-5 and eotaxin-1 synthesis in ConA-injected mice, and the production of these mediators is regulated by IL-4, we next determined in mouse primary EFs, either WT or PKCζ-deficient, if the activation of Stat6, a hallmark of IL-4 signaling, is affected by the loss of PKCζ as determined by the induced phosphorylation of its Tyr-641. Thus, EFs, either WT or PKCζ−/−, were incubated with IL-4 for different times, after which cell extracts were prepared and analyzed by immunoblotting with an anti-phospho-Y641-Stat6 antibody. Results of Figure 4D demonstrate that, whereas in WT cells IL-4 provokes a clear induction of Stat6 phosphorylation, this is dramatically impaired in the PKCζ−/− EFs. Therefore, from these results we can conclude that PKCζ is required for the efficient tyrosine phosphorylation of Stat6. Consistent with this notion, eotaxin-1 mRNA levels induced by IL-4 were dramatically reduced in EFs from PKCζ−/− mice as compared to WT controls (Figure 4E), which is in good agreement with the results shown in Figure 4C. In order to determine whether the requirement of PKCζ for Stat6 phosphorylation can be confirmed in vivo, livers from WT and KO mice that had been untreated or injected with ConA as above were extracted and analyzed by immunoblotting with the anti-phospho-Y641 antibody. Consistent with the in vitro data, the phosphorylation of Stat6 induced by ConA in liver was severely reduced in the PKCζ−/− mice as compared to the WT controls (Figure 4F). In contrast, tyrosine phosphorylation of Stat1, Stat3 or Stat5 was little or not affected (Figure 4F), as were the total levels of Stat6 both in in vitro (Figure 4D) and in vivo (not shown) experiments. To further confirm the role of PKCζ in Stat6 activation, liver nuclear extracts from mice, either WT or KO, that have been either sham treated or injected with ConA were analyzed by EMSA with a Stat6-specific DNA probe. The data of Figure 4G demonstrate that the induction of nuclear Stat6 levels detected in ConA-treated WT mice was severely reduced in the KO mice. Incubation of the EMSA WT sample with a Stat6-neutralizing antibody severely inhibits the shifted band (Figure 4G). It is very unlikely that the slight reduction in IL-4 serum levels in the ConA-injected KO mice could account for the dramatic inhibition of Stat6 activation described in Figure 4F. To further rule out this possibility, WT and KO mice were injected with 100 ng of IL-4, which gives after 1 h serum levels comparable to those found in ConA-injected mice (Supplementary Figure 1A). Interestingly, although serum IL-4 levels were comparable in WT and KO mice under these conditions (Supplementary Figure 1A), liver Stat6 phosphorylation was severely inhibited in the KO as compared to the WT mice (Supplementary Figure 1B). This demonstrates that PKCζ is required for optimal IL-4 signaling in liver and EFs.
PKCζ is essential for Jak1 activation
IL-4 triggers the heterodimerization of the IL-4Rα chain and the common γ chain (γC) in lymphoid cells. This activates the nonreceptor tyrosine kinases (Tyk) Janus kinase (Jak)1 and Jak3, which are constitutively associated with IL-4Rα and γC, respectively (Nelms et al, 1999; O'Shea et al, 2002; Kelly-Welch et al, 2003). Once activated, the Jaks phosphorylate specific residues in the intracellular domain of IL-4Rα, creating docking sites for PTB and SH2 domain-containing proteins (Nelms et al, 1999). The recruitment of Stat6 to the receptor facilitates its tyrosine phosphorylation by Jak1. The other arm of the IL-4 signaling pathway involves the insulin receptor substrate (IRS) family of PTB-containing adapters that orchestrate the signaling pathways, culminating in the activation of PI 3-kinase/Akt and Ras/ERK cascades (Nelms et al, 1999; Kelly-Welch et al, 2003). As our data demonstrate that PKCζ is necessary for an efficient phosphorylation of Stat6 in IL-4-stimulated EFs and ConA-injected mice, we next sought to determine whether PKCζ could control the activity of Jak1 (Kelly-Welch et al, 2003). To address this possibility, we analyzed by immunoblotting the level of tyrosine phosphorylation of Jak1 in extracts from IL-4-treated EFs and in liver extracts from ConA-injected mice. Interestingly, the loss of PKCζ provokes a dramatic inhibition of the phosphorylation of Jak1 in livers (Figure 5A) and EFs (Figure 5B), as compared with their respective WT controls. However, IL-4 induced ERK and Akt activation in PKCζ−/− EFs to an extent comparable to that detected in WT cells, indicating that the requirement of PKCζ for efficient Jak1 stimulation is specific and cannot be accounted for by the hypothetical general disruption of the IL-4 receptor complex by the loss of PKCζ (Figure 5C). The fact that ERK and Akt activation are not affected in the PKCζ−/− cells despite the inhibition in Jak1 phosphorylation suggests that the residual Jak1 activity of the KO cells may be sufficient to activate the ERK and Akt arm of the pathway but not Stat6.
Figure 5.
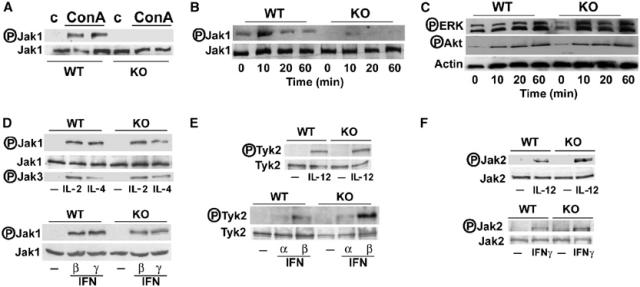
Selective impairment of Jak1 activation by the loss of PKCζ. (A) Jak1 tyrosine phosphorylation was determined by immunoblotting of liver extracts from mice 2 h after injection with 12 μg/g of ConA. The levels of Jak1 are also shown as loading controls. Representative autoradiographs are displayed of one mouse untreated and two mice injected with ConA, either WT or PKCζ KO, of a total of n=5 for each genotype. Primary EFs, either WT or PKCζ−/−, were stimulated with IL-4 for different times, after which phosho-Jak1 and Jak1 levels (B) and phosho-ERK, phospho-Akt and actin levels (C) were determined by immunoblotting. Splenocytes (D–F; upper panels) or EFs (D–F; lower panels) from WT and PKCζ−/− mice were stimulated or not with IL-2 (D), IL-4 (D), IL-12 (E, F), IFNα (E), IFNβ (D, E), and IFNγ (D, F), and the phosphorylation of Jak1, Jak2, Jak3, and Tyk2 was determined by immunoblotting with the corresponding phospho-specific antibodies. Representative autoradiographs are displayed of two other experiments with similar results.
We next determined the cytokine specificity of this potentially important observation. Therefore, splenocytes or EFs from WT and PKCζ−/− mice were stimulated or not with IL-2 (Figure 5D), IL-4 (Figure 5D), IL-12 (Figure 5E and F), IFNα (Figure 5E), IFNβ (Figure 5D and E), and IFNγ (Figure 5D and F), and the phosphorylation of Jak1, Jak2, Jak3, and Tyk2 was determined by immunoblotting with the corresponding phospho-specific antibodies. Jak1 activation by IL-2 was not inhibited in splenocytes of PKCζ−/− mice (Figure 5D). IL-4-induced activation of Jak1 was only partially inhibited in splenocytes from PKCζ KO mice (Figure 5D), which is in contrast to the more robust inhibition detected in EFs (Figure 5B) and the even more dramatic reduction observed in livers (Figure 5A) from PKCζ-deficient mice. Stat6 phosphorylation in response to IL-4 in splenocytes was likewise partially inhibited (not shown). The activation of Jak3 was not inhibited at all in this system (Figure 5D), suggesting the specificity of PKCζ action on Jak1. The activation of Jak1 by IFNβ and IFNγ is not inhibited in the PKCζ−/− EFs (Figure 5D). Together, these results indicate that PKCζ is selectively implicated in the activation of Jak1 by IL-4 signaling in liver and EFs, and that there is no general defect in Jak1 activation in the PKCζ-deficient mice (Figure 5D). The activation of Jak2 or Tyk2 by different cytokines is not affected by the loss of PKCζ (Figure 5E and F) indicating, again, the specificity of PKCζ actions. Of note, IL-4Rα and γc levels in splenocytes were not reduced in the KO (Supplementary Figure 2A and B). In addition, IL-4Rα levels in PKCζ−/− EFs were not affected either (Supplementary Figure 2C). When EFs from WT and KO mice were challenged with IL-13, which uses IL-4Rα and IL-13Rα1 to signal, the activation of Jak1 was impaired, but that of Jak2, which depends on IL-13Rα1, was not affected (Supplementary Figure 2D). These results indicate not only that the expression of IL-4Rα is intact but also that the expression and function of the IL-13Rα1 component of the receptor complex are not affected by the loss of PKCζ. The reason why PKCζ is more important for IL-4 signaling in EFs and liver than in splenocytes cannot be accounted for by a potential redundant role of PKCλ/ι based on the expression levels of these PKC isotypes in the different tissues (Supplementary Figure 2E).
PKCζ interacts with and phosphorylates Jak1
Once the role of PKCζ in Jak1 activation was established, we next performed a series of experiments aimed at determining the mechanistic details of this novel PKCζ action. Stat6 phosphorylation in response to IL-4 was significantly restored when EFs from KO mice were transduced with WT HA-tagged PKCζ, but not with a mutant construct lacking enzymatic activity (Figure 6A). Furthermore, to confirm that the enzymatic activity of PKCζ is necessary for Stat6 activation, HeLa cells were transfected with either a control plasmid or expression vectors for HA-PKCζ WT, or a kinase-deficient version of this enzyme. The data of Figure 6B (upper panel) demonstrate that the ectopic expression of WT PKCζ enhances the nuclear translocation of Stat6 in response to IL-4, but that the expression of the kinase-deficient PKCζ mutant severely impairs this parameter. This correlates with Stat6 phosphorylation in parallel identically treated cultures (Figure 6B, lower panel). Collectively, these results indicate that the enzymatic activity of PKCζ is necessary for IL-4 signaling. One potential mechanism whereby PKCζ could regulate this pathway is by a direct interaction with Jak1. To address this possibility, we first transfected HA-tagged PKCζ into 293 cells, after which they were stimulated or not with IL-4 for 10 min. Extracts were prepared and the transfected PKCζ was immunoprecipitated with an anti-HA antibody, and the associated Jak1 was determined by immunoblotting. Results of Figure 6C demonstrate that there is little PKCζ–Jak1 interaction in unstimulated cells. However, this interaction is dramatically induced upon IL-4 stimulation, indicating that IL-4 treatment promotes the interaction of PKCζ with Jak1 in vivo. Importantly, a similar inducible interaction was observed when both endogenous proteins were analyzed in IL-4-treated EFs (Figure 6D). As a specificity control, no PKCζ–Jak1 association was detected (not shown) when EFs were stimulated with IFNγ, which potently activates Jak1 (Figure 5D). The results of Figure 6E are consistent with the notion that PKCζ forms part of the IL-4Rα complex in EFs, since this kinase is readily detectable in receptor immunoprecipitates from IL-4-stimulated cells as compared to untreated controls. To determine the in vitro PKCζ–Jak1 interaction, in vitro translated HA-PKCζ was incubated with in vitro translated Jak1 and their association was analyzed in anti-HA immunoprecipitates. Our data (Figure 6F) show a reproducible interaction between both proteins in vitro, irrespective of the kinase activity of PKCζ. This interaction requires the regulatory domain of PKCζ as demonstrated in the results of Figure 6G, in which in vitro translated HA-tagged PKCζ fragments corresponding to the catalytic and the regulatory domain of this kinase that had previously been incubated with in vitro translated Jak1 were immunoprecipitated with the anti-HA antibody. These results demonstrate that PKCζ uses the regulatory domain to promote a stable interaction with Jak1. In order to determine whether Jak1 could be a direct substrate of PKCζ, we prepared recombinant bacterially expressed Jak1, which was incubated with recombinant baculovirus-expressed PKCζ in a kinase reaction. The data of Figure 6H demonstrate that PKCζ directly phosphorylates Jak1 in vitro. Overexpression of WT PKCζ but not its kinase-inactive mutant promotes the Ser/Thr phosphorylation of Jak1 in vivo (Figure 6I). Together, these results indicate that PKCζ directly interacts and phosphorylates Jak1, which is important for the activation of the Jak1/Stat6 pathway in vitro and in vivo.
Figure 6.
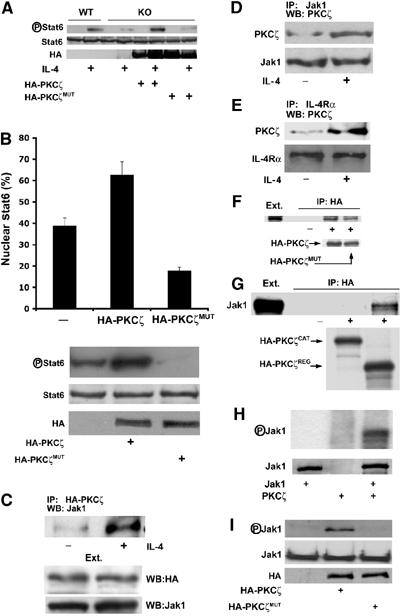
PKCζ interacts and phosphorylates Jak1. (A) PKCζ−/− EFs were transduced with an empty plasmid or with WT or kinase-inactive HA-PKCζ, after which they were stimulated with IL-4 (50 ng/ml) for 10 min and the level of Stat6 tyrosine phosphorylation was determined as above. As a control, the stimulation of WT EFs was also shown. (B) HeLa cells were transfected with empty plasmid, HA-PKCζ or HA-PKCζMUT, after which they were stimulated with IL-4 (50 ng/ml) for 60 min and the nuclear translocation of Stat6 (upper panel) or Stat6 phosphorylation (lower panel) was determined. Results of upper panel are the mean±s.d. of the percentage of cells with nuclear Stat6 in three independent experiments. (C) 293 cells transfected with HA-PKCζ were stimulated or not with IL-4 (50 ng/ml) for 10 min, after which transfected PKCζ was immunoprecipitated with an anti-HA antibody and the associated Jak1 determined by immunoblotting. This is a representative experiment of two with similar results. EFs were stimulated as above and the associated endogenous PKCζ was determined in immunoprecipitates of endogenous Jak1 (D) or IL-4Rα (E). This is a representative experiment of two with similar results. HA-tagged versions of in vitro translated full-length WT or kinase-inactive PKCζ mutant (F) or the regulatory or catalytic fragments of the WT kinase (G) were incubated with in vitro translated Jak1, after which the associated Jak1 was determined in the anti-HA immunoprecipitates. This is a representative experiment of two with similar results. (H) Recombinant baculovirus-expressed PKCζ phosphorylates recombinant bacterially expressed Jak1. This is a representative experiment of two with similar results. (I) Subconfluent 293 cells were transfected with WT or kinase-inactive HA-PKCζ constructs, after which Jak1 was immunoprecipitated and the Ser/Thr phosphorylation levels of Jak1 were determined by immunoblotting with a mix of anti-phospho-serine and anti-phospho-threonine antibodies.
Par-4 negatively regulates IL-4 signaling
PKCζ is negatively regulated by Par-4, a protein that interacts with the zinc-finger domain of both aPKCs and is induced in cells undergoing apoptosis in response to different forms of stress, including UV irradiation, but also as a negative feedback mechanism to control and excessive activation of the aPKCs in, for example, B and T lymphocytes (Diaz-Meco et al, 1996; Lafuente et al, 2003). We have recently generated Par-4−/− mice and the study of different cell types from these animals, including EFs, demonstrate that Par-4 can be considered a bona fide physiological inhibitor of PKCζ (Garcia-Cao et al, 2003; Lafuente et al, 2003). If our model is correct, we could predict that ConA-induced liver damage should be more prominent in Par-4 KO mice as compared to the WT controls. Interestingly, the histochemical analysis of livers from WT or Par-4 KO mice injected with ConA reveals that the loss of Par-4 exacerbates the appearance of liver necrosis (Figure 7A) and of caspase-3 activation in liver homogenates (Figure 7B), as well as ALT serum levels (Figure 7C). Consistent with the notion that PKCζ is critical for IL-4 signaling and that Par-4 antagonizes PKCζ actions, serum levels of IL-5 and liver eotaxin-1 mRNA content of Par-4−/− mice injected with ConA were significantly higher than in the WT mice (Figure 7D and E). Consistently, activation of eotaxin-1, as well as that of Stat6 and Jak1, was also significantly enhanced in Par-4−/− EFs as compared to the WT controls in response to IL-4 (Figure 7F, G and H), but not to IFNγ (not shown).
Figure 7.
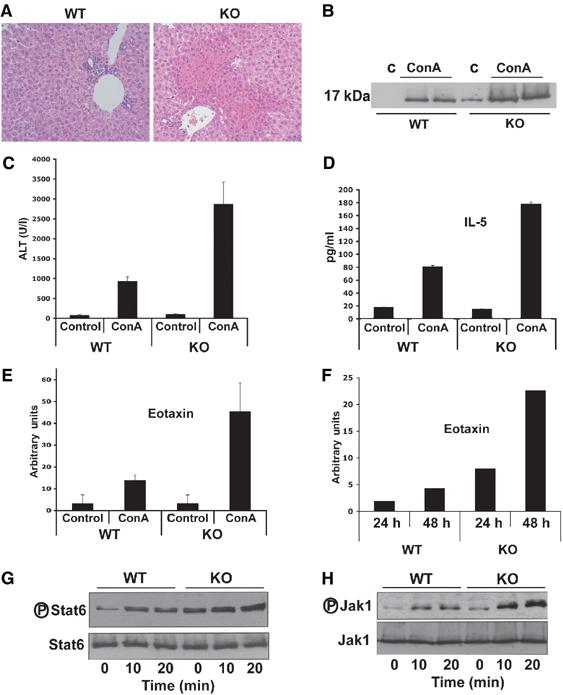
Increased IL-4 signaling in Par-4 KO mice. (A) Histological (H&E) analysis of representative livers from mice of both genotypes 8 h after injection with 12 μg/g of ConA (× 40). (B) Liver extracts from the same experiment were analyzed by immunoblotting to determine the activation of caspase-3. Representative autoradiographs are displayed of one mouse untreated and two mice injected with ConA, either WT or Par-4 KO, of a total of n=5 for each genotype. (C) Serum levels of ALT were determined 8 h after injection with ConA. Results are the mean±s.d. of n=5 for each genotype. (D) Serum IL-5 levels were determined 8 h after injection of ConA. Results are the mean±s.d. of n=5 for each genotype. In another set of experiments, liver mRNA levels of eotaxin-1 (E) were determined by real-time RT–PCR 8 h after injection with ConA. Results are the mean±s.d. of n=5 for each genotype. Primary EFs, either WT or Par-4−/−, were stimulated with IL-4 for 24 or 48 h (F), or 10 and 20 min (G, H), after which eotaxin-1 mRNA levels were determined by real-time RT–PCR (F), Stat6 phosphorylation and Stat6 levels (G) and Jak1 phosphorylation and Jak1 levels (H) were determined by immunoblotting.
Discussion
Emerging evidence demonstrates the existence of complex crosstalks between different signal transduction cascades during intricate biological processes such as development, differentiation or inflammation. Thus, for example, different members of the NF-κB pathway, such as NF-κB2 or IKK, can modulate under certain circumstances the activation of ERK and the forkhead protein FOXO3a, respectively (Xiao et al, 2001; Hu et al, 2004). This is most surprising, as ERK had previously been shown to be the end point of the Ras/Raf/MEK pathway and the forkhead proteins are targets of the PI 3-kinase/Akt cascade. Therefore, it seems that, under complex biological situations, there is a substantial degree of interrelation between once-considered independent pathways. In this regard, we show here the unexpected observation that PKCζ, which has been shown to control NF-κB activation at different levels (Leitges et al, 2001; Moscat et al, 2003), also critically regulates the IL-4 signaling cascade in EFs in vitro, and in vivo in a model of T-cell-mediated hepatitis. Therefore, PKCζ, like other components of the NF-κB pathway, impinges different signaling cascades when complex cellular networks need to be activated like in inflammation. Thus, the fact that PKCζ is necessary for the proper activation of the IL-4 pathway, which plays an essential role in the recruitment of eosinophils and the subsequent induction of hepatitis (Jaruga et al, 2003), accounts for the lower degree of liver injury observed in PKCζ mutant mice injected with ConA.
Therefore, PKCζ must be considered a novel and important player in the IL-4 signaling cascade that activates Stat6. The mechanism whereby PKCζ controls IL-4-triggered Stat6 tyrosine phosphorylation involves the direct interaction with Jak1, which leads to its phosphorylation. The precise mechanism whereby the phosphorylation of Jak1 by PKCζ impacts the tyrosine phosphorylation and activation of Jak1 is still unclear, but definitely is important for Stat6 activation, as its nuclear translocation is enhanced by the ectopic expression of WT PKCζ and inhibited in cells expressing a kinase-dead version of this enzyme, which is in keeping with the notion that Jak1 is a critical player in the activation of Stat6 in response to IL-4 (Ho and Glimcher, 2002; O'Shea et al, 2002; Shuai and Liu, 2003). On the other hand, it is clear that the IL-4 pathway is subjected to a finely tuned negative feedback by the SOCS proteins that downmodulate cell signaling by IL-4 and other cytokines, preventing an excessive immune response (Chen et al, 2000; Alexander, 2002b; Kubo et al, 2003). Therefore, it could be possible at least theoretically that PKCζ may also negatively modulate members of the SOCS proteins and that the loss of PKCζ could lead to their hyperactivation, leading to the subsequent blockade of the pathway. However, our data show that the induction of SOCS1, 2, and 3 is not affected in PKCζ−/− livers from ConA-injected mice (Supplementary Figure 3) or in PKCζ-deficient EFs activated by IL-4 (not shown). Other negative regulators of this pathway, such as the tyrosine phosphatase SHP-1, have been reported to functionally interact with both Jak1 and Stat6, promoting their inactivation (Scharenberg and Kinet, 1996; Alexander, 2002a; Kelly-Welch et al, 2003). It is also possible that Jak1 phosphorylation by PKCζ may prevent SHP-1 actions on Jak1, inhibiting its potentially premature inactivation. Whatever the mechanism, it is evident that PKCζ is important for Jak1 activation by IL-4 and that this is specific for this pathway. Thus, the activation of Jak1 by other cytokines is not affected by the loss of PKCζ; neither is the activation of Jak2 or Tyk2 by different stimuli. Consistent with the notion that PKCζ actions are restricted to IL-4 pathway is the fact that, although IL-4 activation triggers the PKCζ-Jak1 interaction, this is not promoted by an IFNγ challenge, despite the fact that Jak1 is as potently activated by IFNγ as by IL-4. The precise mechanism whereby the IL-4 receptor confers this specificity to PKCζ actions is not clear yet and deserves further investigation.
The evidence that PKCζ is not generally affecting Jak1 explains why the phenotype of the PKCζ−/− mice differs from that of the Jak1 KO mice. Thus, whereas the Jak1-deficient mice display perinatal lethality with reduced numbers of thymocytes, pre-B cells, and mature T and B lymphocytes (Rodig et al, 1998), the PKCζ-deficient mice do not (Leitges et al, 2001). In this regard, the PKCζ KO phenotype is more similar to that of the Stat6−/− mice. Thus, like the Stat6 KO, the PKCζ−/− mice display reduced liver damage in response to ConA injection (Jaruga et al, 2003). However, there are two aspects of our study that deserve further consideration. First, PKCζ−/− mice, like the Stat6−/− mice, have reduced serum IL-5 and liver eotaxin levels. As eotaxin is synthesized by hepatocytes and liver sinusoidal endothelial cells, whereas IL-5 is produced by NKT cells, our results imply that the loss of PKCζ affects the function of both liver cells and NKT cells. In this regard, the adoptive transfer of liver mononuclear cells (MNC) from PKCζ−/− mice into PKCζ−/− mice was unable to restore ConA-induced liver injury, whereas the adoptive transfer of WT MNC into PKCζ−/− mice did restore that effect (Supplementary Figure 4). This is similar, although more dramatic in the PKCζ−/− mice, to what has been reported for the Stat6−/− mice (Jaruga et al, 2003), and confirms that, even though PKCζ plays a major role in liver cells through the control of eotaxin synthesis, its ability to regulate IL-5 serum levels most likely in NKT cells is functionally relevant.
Another interesting aspect of this study is the double effect of PKCζ in Jak1/Stat6 and NF-κB. When ConA is injected at 12 μg/g, the Jak1/Stat6 and the NF-κB pathways are severely inhibited in the PKCζ−/− mice, which show reduced liver damage. Since NF-κB is not activated under these conditions, the liver will be deprived of damage protection, but, as Stat6 is not activated, liver injury is not induced and the fact that NF-κB is not triggered in the KO mice is irrelevant. However, when mice were injected with a higher dose of ConA, we observed that, although IL-5 serum levels were still at least partially inhibited in the PKCζ−/− mice (Supplementary Figure 5A), liver eotaxin and phospho-Stat6 levels were not reduced (Supplementary Figure 5B and C), indicating that PKCζ becomes completely redundant in the liver but not in the IL-5-producing cells under these conditions. Interestingly, NF-κB is still inhibited in the liver of PKCζ−/− mice (Supplementary Figure 5D) and, consistently, liver damage is increased in the KO mice as compared to the WT controls (Supplementary Figure 5E). Therefore, although at a high ConA dose IL-5 production is still impaired, liver damage occurs because eotaxin is produced. This indicates that, although IL-5 synthesis is important for ConA-induced liver damage in the adoptive transfer experiment (Supplementary Figure 4), the synthesis of eotaxin is the critical parameter for liver damage at least at high ConA doses. Nevertheless, it should be emphasized that, although IL-5 levels are still reduced in the KO mice injected with a high dose of ConA, some IL-5 is produced, which together with the normal synthesis of eotaxin found under these conditions may be sufficient to trigger liver damage. In general terms, this is consistent with the evidence published recently (Jaruga et al, 2003), according to which the adoptive transfer of WT MNC into Stat6−/− mice restores, albeit only partially, ConA-induced liver injury. However, we observed more liver damage in the PKCζ−/− mice than in the WT controls (Supplementary Figure 5E) due to the fact that not only is eotaxin being produced under these conditions but also NF-κB is still inhibited, depriving the liver of κB-dependent protecting signals. Therefore, PKCζ emerges as a finely tuned switch that may determine the final physiological outcome of a biological complex process such as liver inflammation, depending on the intensity of the insult, and due to its ability to interact with two independent and antagonistic pathways.
In summary, the data presented here clearly unveil a novel and physiologically relevant in vivo connection between PKCζ and the IL-4/Jak1/Stat6 pathway, which has implications for T-cell-mediated hepatitis and probably asthma, a pathological situation in which IL-4 also plays an essential role.
Materials and methods
Reagents and antibodies
Reagents were purchased as follows: recombinant IL-4 was from R&D, concanavalin A from Amersham Biosciences, IFNα and IFNβ from PBL Biomedical Laboratories, and IFNγ from Preprotech. Polyclonal anti-phospho-Stat6, anti-phospho-Stat1, anti-phospho-Stat3, anti-phospho-Stat5, anti-phospho-JAK1, anti-phospho-AKT, anti-phospho-Jak2, anti-phospho-Tyk2, and anti-JAK1 antibodies were from Cell Signalling. Anti-actin, anti-Stat6, anti-iNOS, anti-PKCζ, anti-SOCS1, anti-Jak2, anti-Tyk2, anti-phospho-Jak3, anti-IL-4Rα, anti-γc, anti-HA, anti-TNFα/CD69/CD4, and anti-phospho-ERK antibodies were purchased from Santa Cruz. Anti-CD4-FITC, anti-CD69-PE, anti-PKCλ/ι, and anti-caspase-3 antibodies were from Becton Dickinson. Selective anti-PKCζ antibody was generated against amino acids 1–12 of PKCζ and does not crossreact with PKCλ/ι. Anti-phospho-serine and anti-phospho-threonine antibodies were from Qiagen.
Mice
The ζPKC KO and Par-4 KO mice were described previously (Leitges et al, 2001; Garcia-Cao et al, 2003). All mice were born and kept under pathogen-free conditions. Animal-handling protocols conform to the NIH guidelines. Sex-matched 7–8-week-old mice were injected i.v. with ConA at 12 μg/g, or 20 μg/g, or 100 ng of murine IL-4. At different times, depending on the experiment, blood was collected via retro orbital puncture and serum was separated and analyzed for ALT (Labipath). Plasma levels of cytokines (TNFα, IL-4, IL-5) were measured by ELISA (Becton Dickinson). Isolation of liver MNCs and adoptive transfer were performed as described previously (Jaruga et al, 2003). Activation of CD4+ T cells was determined by anti-CD4 plus anti-CD69 through a FACS (FACSCalibur, Becton Dickinson). Splenocytes from WT and PKCζ−/− mice were prepared as described (Karaghiosoff et al, 2000). TNFα synthesis was determined in T-cells isolated from lymph nodes by FACS, using the Mo-TNFα/CD69/CD4 in an intracellular staining according to the manufacturer's instructions (Santa Cruz).
Cell culture, immunoprecipitations, and Western blot analysis
EFs were isolated from 13.5 d.p.c. embryos and cultured in DMEM supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine. Cell culture reagents were from Invitrogen. Transfection of cells was performed by Lipofectamine 2000 (Invitrogen). PKCζ−/− EFs were transduced with pBabe-HA-ζPKC or pBabe-HA-ζPKCMUT as described (Duran et al, 2003). Cell cultures were growth arrested by incubation in medium without serum for 30 min and stimulated with IL-4 (50 ng/ml) for different times. Cells were lysed with cell extraction buffer (Cell Signaling), separated by SDS–PAGE and transferred to Nitrocellulose-ECL membranes (Amersham Biosciences), and the immune complex was detected by chemiluminescence (Amersham Biosciences). For co-immunoprecipitations, cells extracts were prepared in PD buffer and immunoprecipitations were carried out as described (Duran et al, 2004). For in vitro interactions assays, HA-ζPKC, HA-ζPKCMUT, HA-ζPKCCAT, HA-ζPKCREG or JAK1 were in vitro translated in rabbit reticulocyte lysates using the TNT System (Promega). Confocal immunofluorescence was performed as described (Sanz et al, 2000).
Electrophoretic mobility shift and in vitro kinase assays
EMSA experiments were performed as described previously (Diaz-Meco et al, 1993). The duplex oligonucleotide probes used are as follows: for NF-κB: 5′-AGTTGAGGGGAATTTCCCAGGC-3′ and 5′-GCCTGGGAAATTCCCCTCAACT-3′; for Stat6: 5′-GATCGCTCTTCTTCCCAGGAACTCAATG-3′ and 5′-CATTGAGTTCCTGGGAAGAAGAGCGATC-3′; and for Oct1: 5′-TGTCGAATGCAAATCACTAGAA-3′ and 5′-TTCTAGTGATTTGCATTCGACA-3′. IKK activity was determined in anti-IKKγ immunoprecipitates using GST-IκB (1–54) as substrate as described (Lallena et al, 1999). In vitro phosphorylation of GST-JAK1 by baculovirus-expressed ζPKC was performed as described (Duran et al, 2003).
Gene expression analysis
Total RNA was extracted and DNase treated using the Nucleospin RNA purification kit from Becton Dickinson. The relative levels of IκBα, eotaxin-1, MCP-1, SOCS2 and 3 mRNAs were determined by real-time quantitative RT–PCR (LightCycler RNA Amplification Sybr Green, Roche) using actin mRNA for normalization. Primer sequences used are as follows: 5′-GCCTTCCTCAACTTCCAGAACAAC-3′ and 5′-CAGACGCTGGCCTCCAAACACACAG-3′ (IκBα); 5′-AGAGCCAGACGGGAGGAAG-3′ and 5′-CCAGCCTACTCATTGGGATC-3′ (MCP-1); 5′-CACTTCATGATGGAATTGAATGTAGTT-3′ and 5′-AGGTCATCACTATTGGCAACGA-3′ (actin); 5′-TCCACAGCGCTTCTATTCCT-3′ and 5′-CTATGGCTTTCAGGGTGCAT-3′ (eotaxin-1); 5′-GGTTGCCGGAGGAACAGTC-3′ and 5′-GAGCCTCTTTTAATTTCTCTTTGGC-3′ (SOCS2); 5′-AAGACCTTCAGCTCCAAGAGC-3′ and 5′-CTTGAGTACACAGTCGAAGCGC-3′ (SOCS3).
Immunohistochemistry
Livers were fixed in 4% formaldehyde, dehydrated, embedded in paraffin, and sectioned (5 μm). Sections were stained with hematoxylin and eosin (H&E). TUNEL staining was performed according to manufacturers' instructions (ApopDETEK, DAKO).
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Acknowledgments
This work was supported by grants SAF2003-02613 (to MTD-M) and SAF2002-0187 (to JM) from MCYT and by an institutional grant from Fundación Ramón Areces to the CBMSO. JM is recipient of the Ayuda Investigación Juan March 2001.
References
- Alexander DR (2002a) Kinase regulation: competing phosphatases in JAK dephosphorylation. Curr Biol 12: R288–R290 [DOI] [PubMed] [Google Scholar]
- Alexander WS (2002b) Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol 2: 410–416 [DOI] [PubMed] [Google Scholar]
- Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS (2003) A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature 423: 659–663 [DOI] [PubMed] [Google Scholar]
- Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M (2001) IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 107: 763–775 [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC (2004) Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 5: 392–401 [DOI] [PubMed] [Google Scholar]
- Chen XP, Losman JA, Rothman P (2000) SOCS proteins, regulators of intracellular signaling. Immunity 13: 287–290 [DOI] [PubMed] [Google Scholar]
- Claudio E, Brown K, Park S, Wang H, Siebenlist U (2002) BAFF-induced NEMO-independent processing of NF-kappaB2 in maturing B cells. Nat Immunol 3: 958–965 [DOI] [PubMed] [Google Scholar]
- Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR (2002) The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity 17: 525–535 [DOI] [PubMed] [Google Scholar]
- Diaz-Meco MT, Berra E, Municio MM, Sanz L, Lozano J, Dominguez I, Diaz-Golpe V, Lain de Lera MT, Alcami J, Paya CV, Arenzana-Seisdedos F, Virelizier JL, Moscat M (1993) A dominant negative protein kinase C zeta subspecies blocks NF-kappa B activation. Mol Cell Biol 13: 4770–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Meco MT, Municio MM, Frutos S, Sanchez P, Lozano J, Sanz L, Moscat J (1996) The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell 86: 777–786 [DOI] [PubMed] [Google Scholar]
- Duran A, Diaz-Meco MT, Moscat J (2003) Essential role of RelA Ser311 phosphorylation by zetaPKC in NF-kappaB transcriptional activation. EMBO J 22: 3910–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Serrano M, Leitges M, Flores JM, Picard S, Brown JP, Moscat J, Diaz-Meco MT (2004) The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev Cell 6: 303–309 [DOI] [PubMed] [Google Scholar]
- Garcia-Cao I, Lafuente M, Criado L, Diaz-Meco M, Serrano M, Moscat J (2003) Genetic inactivation of Par4 results in hyperactivation of NF-κB and impairment of JNK and p38. EMBO Rep 4: 307–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Karin M (2002) Missing pieces in the NF-kappaB puzzle. Cell 109 (Suppl): S81–S96 [DOI] [PubMed] [Google Scholar]
- Ho IC, Glimcher LH (2002) Transcription: tantalizing times for T cells. Cell 109 (Suppl): S109–S120 [DOI] [PubMed] [Google Scholar]
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC (2004) IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117: 225–237 [DOI] [PubMed] [Google Scholar]
- Jaruga B, Hong F, Sun R, Radaeva S, Gao B (2003) Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: up-regulating eotaxins and IL-5 and recruiting leukocytes. J Immunol 171: 3233–3244 [DOI] [PubMed] [Google Scholar]
- Karaghiosoff M, Neubauer H, Lassnig C, Kovarik P, Schindler H, Pircher H, McCoy B, Bogdan C, Decker T, Brem G, Pfeffer K, Müller M (2000) Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity 13: 549–560 [DOI] [PubMed] [Google Scholar]
- Karin M (1998) The NF-kappa B activation pathway: its regulation and role in inflammation and cell survival. Cancer J Sci Am 4 (Suppl 1): S92–S99 [PubMed] [Google Scholar]
- Karin M, Lin A (2002) NF-kappaB at the crossroads of life and death. Nat Immunol 3: 221–227 [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Yan M, Seshasayee D, Wang H, Lee W, French DM, Grewal IS, Cochran AG, Gordon NC, Yin J, Starovasnik MA, Dixit VM (2002) BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity 17: 515–524 [DOI] [PubMed] [Google Scholar]
- Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD (2003) Interleukin-4 and interleukin-13 signaling connections maps. Science 300: 1527–1528 [DOI] [PubMed] [Google Scholar]
- Kubo M, Hanada T, Yoshimura A (2003) Suppressors of cytokine signaling and immunity. Nat Immunol 4: 1169–1176 [DOI] [PubMed] [Google Scholar]
- Lafuente MJ, Martin P, Garcia-Cao I, Diaz-Meco MT, Serrano M, Moscat J (2003) Regulation of mature T lymphocyte proliferation and differentiation by Par-4. EMBO J 22: 4689–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallena MJ, Diaz-Meco MT, Bren G, Pay CV, Moscat J (1999) Activation of IkappaB kinase beta by protein kinase C isoforms. Mol Cell Biol 19: 2180–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J (2001) Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol Cell 8: 771–780 [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM (2002) NF-kappaB regulation in the immune system. Nat Rev Immunol 2: 725–734 [DOI] [PubMed] [Google Scholar]
- Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M (2003) IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity 19: 725–737 [DOI] [PubMed] [Google Scholar]
- Martin P, Duran A, Minguet S, Gaspar ML, Diaz-Meco MT, Rennert P, Leitges M, Moscat J (2002) Role of zeta PKC in B-cell signaling and function. EMBO J 21: 4049–4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT (2000) The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep 1: 399–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT, Rennert P (2003) NF-kappaB activation by protein kinase C isoforms and B-cell function. EMBO Rep 4: 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE (1999) The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol 17: 701–738 [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Gadina M, Schreiber RD (2002) Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell 109 (Suppl): S121–S131 [DOI] [PubMed] [Google Scholar]
- Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, King KL, Sheehan KC, Yin L, Pennica D, Johnson EM, Schreiber RD (1998) Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 93: 373–383 [DOI] [PubMed] [Google Scholar]
- Sanz L, Diaz-Meco MT, Nakano H, Moscat J (2000) The atypical PKC-interacting protein p62 channels NF-kappaB activation by the IL-1-TRAF6 pathway. EMBO J 19: 1576–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharenberg AM, Kinet JP (1996) The emerging field of receptor-mediated inhibitory signaling: SHP or SHIP? Cell 87: 961–964 [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M (2001) Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293: 1495–1499 [DOI] [PubMed] [Google Scholar]
- Shuai K, Liu B (2003) Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol 3: 900–911 [DOI] [PubMed] [Google Scholar]
- Tiegs G, Hentschel J, Wendel A (1992) A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest 90: 196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Harhaj EW, Sun SC (2001) NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell 7: 401–409 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB (2003) Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature 423: 655–659 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5


