Abstract
Toward the development of photoresponsive anion receptors, a stiff-stilbene photoswitch has been equipped with two urea anion-binding motifs. Photoinduced E/Z isomerization has been studied in detail by UV–vis and NMR spectroscopy. Titration experiments (1H NMR) reveal strong binding of acetate and phosphate to the (Z)-isomer, in which the urea groups are closely together. Isomerization to the (E)-form separates the urea motifs, resulting in much weaker binding. Additionally, geometry optimizations by density functional theory (DFT) illustrate that oxo-anion binding to the (Z)-form involves four hydrogen bonds.
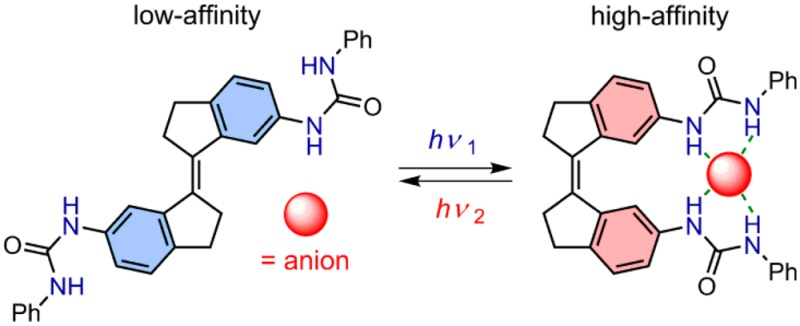
In natural systems, transmembrane anion transport is mediated by protein carriers that are able to regulate their substrate affinity in response to environmental stimuli.1 Because of the important regulatory role of anions in many biological processes (e.g., osmosis, metabolism, signaling), a huge number of artificial anion receptors has been developed over the past decades.2 Importantly, dysregulation of anion transport has been linked to various diseases3 (cystic fibrosis, among others), and hence, synthetic systems that imitate the function of natural protein carriers have therapeutic potential.4 Nevertheless, where proteins are able to switch between distinct affinity modes, integrating responsive behavior into artificial anion receptors is enormously challenging.5,6
Light has proven to be a particularly promising tool to control the binding properties of receptors.7,8 For example, the groups of Flood6b and Jiang6c successfully controlled the halide affinity for light-responsive azobenzene-based foldamers. In addition, Jeong and co-workers demonstrated photocontrol of chloride transport across membranes using a bis-urea-functionalized azobenzene.6g Recently, our group developed an overcrowded alkene-based bis-urea receptor that can be switched uniquely between three states that possess different anion binding affinity and selectivity.9 Although the binding properties were controlled successfully, this receptor required activation by high energy UV light (λirr = 312 nm), which restricts its application in biological systems.
In our search for alternative photoswitchable scaffolds, we considered stiff-stilbene10 (Scheme 1) to be especially suitable for developing responsive receptors. Due to their rigid core structure and the pronounced geometrical change upon isomerization, good binding selectivities and large differences in the binding properties between the photoaddressable states are expected. Furthermore, stiff-stilbenes are known to have a high activation barrier for thermal isomerization and a high quantum yield for the photoisomerization process. In the past, different functional groups have been installed onto the benzene rings of these switches in order to construct a molecular force probe,11 supramolecular polymers,12 molecular hosts,6a,13 and an enantioselective catalyst.14 Nevertheless, the utilization of stiff-stilbenes in supramolecular systems remains highly underexplored.
Scheme 1. Photoswitching of Anion Binding to Bis-urea 1.
Previously, the group of Shinmyozu linked two binaphthol (BINOL) moieties, serving as anion-binding motifs, to a stiff-stilbene core.6a Although an 8-fold difference in the affinity for chloride was measured, unexpectedly, the (E)-isomer turned out to be the strongest binding form. To favor binding to the more selective (Z)-form as well as to enhance the binding strength, we have designed a synthetic method to equip stiff-stilbene with two urea moieties. Bis-urea compounds are among the most effective organic anion receptors reported to date and hold excellent selectivity toward acetate (CH3CO2–) and dihydrogen phosphate (H2PO4–) oxo-anions.15
Herein, we describe the synthesis and properties of the stiff-stilbene-based bis-urea receptor 1 (see Scheme 1). This receptor selectively binds CH3CO2– and H2PO4–, of which the affinity is controlled successfully via photoinduced E/Z isomerization using 365/385 nm light. We envision that this class of compounds will play an important role as light-gated anion carriers in the dynamic regulation of membrane transport.
Both (E)-1 and (Z)-1 were synthesized by following a similar route (Scheme 2). McMurry coupling of the commercially available 6-bromo-1-indanone gave dibromide 2 as a mixture of (E)- and (Z)-isomers that could be separated by precipitation (see the Supporting Information for details). Subsequent Buchwald–Hartwig amination, followed by hydrolysis, afforded the bis-amine precursors (E)-3 and (Z)-3. These bis-amines were then reacted with phenyl isocyanate to provide the corresponding bis-ureas. The reduced yield of (E)-1 is due to some loss in the purification by crystallization.
Scheme 2. Synthesis of Bis-ureas (E)-1 and (Z)-1.
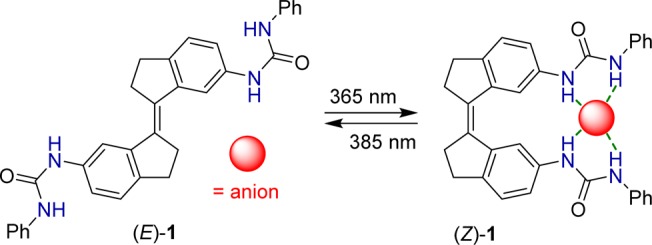
The photoswitching behavior of 1 was studied in dimethyl sulfoxide (DMSO) solution by UV–vis and NMR spectroscopy. Irradiation of the (E)-isomer with λmax = 365 nm light at 20 °C resulted in a decrease of the absorption maxima at 347 and 364 nm, in addition to a slight bathochromic shift, indicative of transition to the (Z)-isomer (Figure 1A).11−14 When starting from the (Z)-isomer, irradiation with λmax = 385 nm light gave the opposite spectral changes, i.e., increase of the absorption maxima at 354 and 367 nm as well as a hypsochromic shift (Figure 1B). In both cases, irradiation was continued until no further changes were noted, meaning that the photostationary states (PSS) had been reached. During the course of irradiation, clear isosbestic points were observed, which reveals that E/Z isomerization is a unimolecular process. Furthermore, 365/385 nm irradiation could be alternated several times without major signs of fatigue (Figure 1C).
Figure 1.
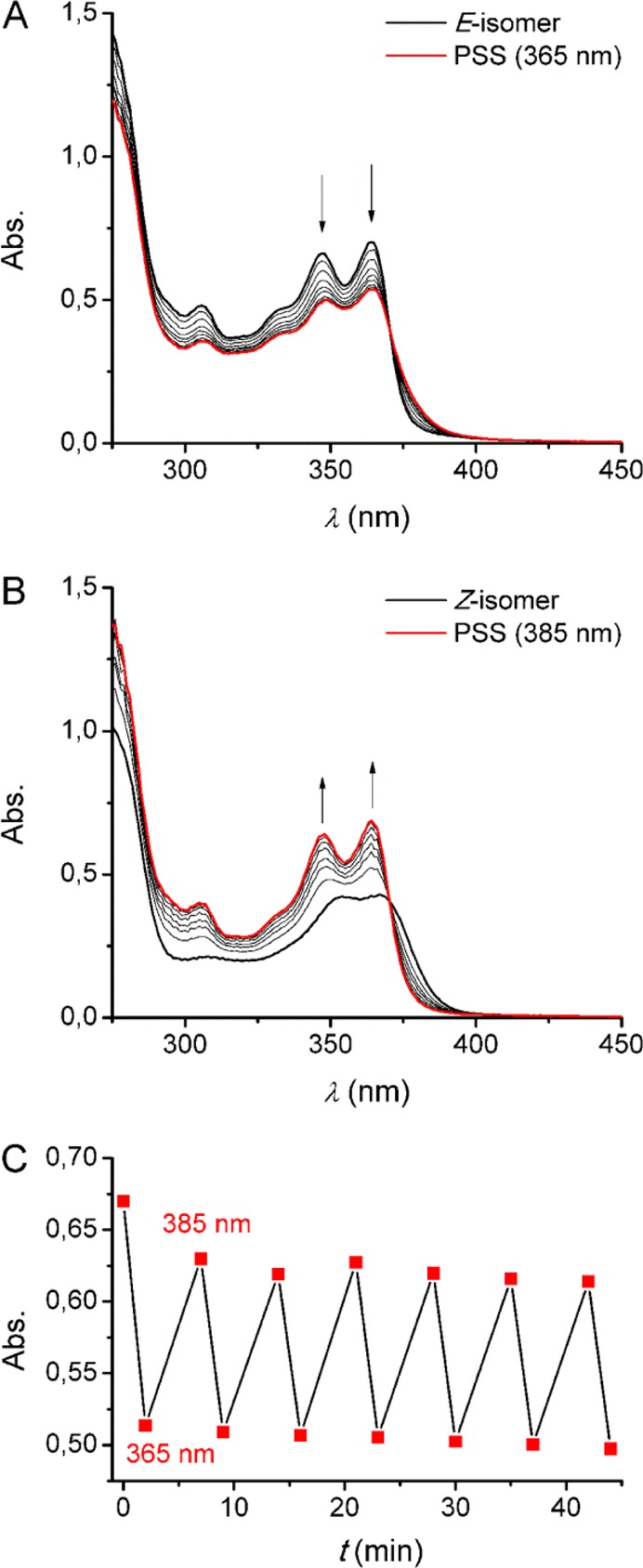
UV–vis spectral changes of (A) (E)-1 (B) and (Z)-1 (2.5 × 10–5 M in degassed DMSO) upon 365 nm irradiation for 60 s and 385 nm irradiation for 220 s, respectively. (C) Plot of the change in absorption at λ = 365 nm during 365/385 nm irradiation cycles starting with (E)-1 (2.5 × 10–4 M in degassed DMSO).
In the 1H NMR spectrum, 365 nm irradiation of (E)-1 led to the appearance of a new set of signals that can be attributed to (Z)-1 (see Figure S12). Subsequent irradiation with 385 nm light led to nearly full recovery of the initial 1H NMR spectrum of the (E)-isomer. The PSS365 and PSS385 ratios were determined by integration of the NMR signals as (E/Z) 49:51 and 93:7, respectively. Similar results have been observed for related stiff-stilbene switches.12,13
The quantum yield (Φ) for the 365 nm induced E → Z isomerization process was estimated by monitoring the absorption increase at λ = 380 nm at a concentration high enough to absorb all incident light (see Figure S14 for details). Then, the rate of formation of (Z)-1 was compared to the rate of formation of Fe2+ ions from potassium ferrioxalate under identical conditions giving: ΦE→Z = 5.0% ± 0.15%. By using the PSS365 ratio, the quantum yield for the “backward” photoisomerization process at the same irradiation wavelength was calculated as ΦZ→E = 8.0% ± 0.23%.
Anion binding was studied in a DMSO-d6/0.5%H2O mixture using 1H NMR titrations, starting with (Z)-1, for which the strongest anion–receptor interaction was expected. In line with structurally related bis-urea receptors,9,15 the stepwise addition of tetrabutylammonium acetate ([NBu4]+[CH3CO2]−) and dihydrogen phosphate ([NBu4]+[H2PO4]−), among various other tetrabutylammonium anions, caused the largest downfield shifts of the urea-NH signals (see Figures S15–S20). However, in the case of phosphate addition, these signals also broadened significantly, which suggests urea deprotonation.16 Job plot analysis pointed to the anticipated 1:1 complexation mode for these anions, and hence, the titration data were fitted to a 1:1 binding model using HypNMR software17 (see Figures S26 and S27). The association constants (Ka) thus obtained (given in Table 1) reveal that acetate and phosphate binding to (Z)-1 are substantial. Furthermore, the Ka values for these anions are similar to those reported for other effective bis-urea receptors.9,15
Table 1. Anion-Binding Constants of Bis-urea 1 (M–1)a,b.
| anion | (Z)-1c | (E)-1d |
|---|---|---|
| Cl | 33 | 17 |
| Br | <10 | |
| NO3 | <10 | |
| CH3CO2 | 1.40 × 103 | 1.04 × 102 |
| H2PO4 | 2.02 × 103 | 77 |
| HSO4 | <10 |
Tetrabutylammonium anions were added to 1 (5.0 × 10–3 M) in DMSO-d6/0.5%H2O.
Microscopic constants for the first binding step are provided; errors are estimated to be no more than ±15%.
Spectral changes upon the addition of Br–, NO3–, and HSO4– to (Z)-1 were too small to accurately determine a binding constant.
Not determined for Br–, NO3– and HSO4–.
Titration of tetrabutylammonium chloride ([NBu4]+[Cl]−) to (Z)-1 led to similar, though less pronounced, changes in the position of the urea-NH signals. Interestingly, for this anion Job plot analysis indicated a 2:1 chloride/(Z)-1 binding stoichiometry (see Figure S24). In this case, the data were analyzed using either a 1:1 or 2:1 binding model (see Scheme S1 and Figure S25). Based on analysis of residuals, the 2:1 binding model is more likely to be correct. Apparently, the urea moieties are too far apart from each other to favor cooperative binding of the chloride anion via four hydrogen bonds, as is the case for CH3CO2– and H2PO4–. Instead, each urea group is expected to bind to one anion involving two hydrogen bonds. Data analysis, considering the presence of 2:1 as well as 1:1 species, afforded a much lower association constant than those obtained for CH3CO2– and H2PO4– (see Figure S25 and Table 1).
The addition of [NBu4]+[CH3CO2]−, [NBu4]+[H2PO4]−, and [NBu4]+[Cl]− to (E)-1 likewise resulted in downfield shifts of the urea-NH signals (see Figures S21–S23). As expected for the (E)-isomer, for the reason that the relative distance between the urea units is too large to bind an anion cooperatively, Job plot analysis revealed 2:1 complexation. For CH3CO2– and H2PO4– the distinct binding modes are reflected in considerably lower Ka values (see Table 1 and Figures S29 and S30). Remarkably, the binding of Cl– to (E)-1 is slightly weaker than to (Z)-1 (see Table 1 and Figure S28), which might be tentatively explained by an increased acidity of the urea protons in the (Z)-form.
To investigate whether photoswitching is still feasible in the presence of the anion, we prepared an NMR sample of (E)-1 containing 10 equiv of [NBu4]+[CH3CO2]− in DMSO-d6/0.5%H2O (see Figure S13). Consecutive irradiation of this sample with 365 and 385 nm light afforded PSS365 and PSS385 ratios of (E/Z) 48:52 and 88:12. Although these ratios are very similar to those determined for the “free” receptor (vide supra), it seems that the (Z)-form becomes slightly more favored in the photoequilibrium established by 385 nm irradiation. It is not clear at the moment whether this difference originates from an electronic or a mechanochemical11b effect. Importantly, no sign of degradation was detected during these photoswitching steps.18
Energy minimization of the complexes of (Z)-1 with either CH3CO2– and H2PO4– was carried out by DFT on the B3LYP/6-31G++(d,p) level of theory, using an IEFPCM, DMSO solvation model (see Tables S1 and S2 for details). The optimized structures (Figure 2) for CH3CO2– and H2PO4– illustrate that the binding of these oxo-anions via four hydrogen bonds is feasible (Figure 2). The bond distances (N···O) are all in the same range and vary between 2.84 and 2.91 Å. Furthermore, the dihedral angle over the central double bond is virtually the same in both structures [i.e., 11.0° for (Z)-1⊃CH3CO2– and 12.1° for (Z)-1⊃H2PO4–].
Figure 2.
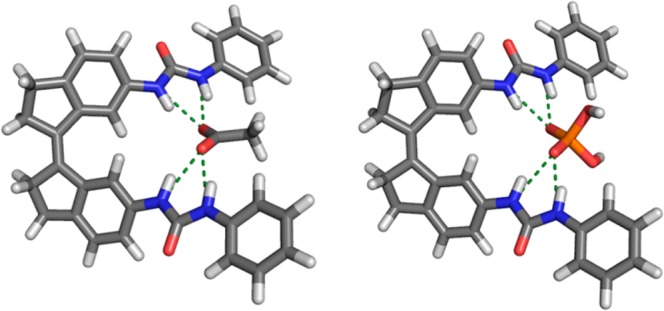
DFT-optimized molecular geometries [B3LYP/6-31G++(d,p)] of (Z)-1⊃CH3CO2– (left) and (Z)-1⊃H2PO4– (right).
In summary, we have equipped a stiff-stilbene scaffold with two bis-urea moieties to obtain a photoresponsive anion receptor. This receptor selectively binds CH3CO2– and H2PO4–, for which the affinity can be tuned via light-induced E/Z isomerization. Importantly, the receptor can be operated by 365/385 nm light and is tolerant to the presence of water, which is an essential improvement with respect to our previously reported overcrowded alkene-based bis-urea receptor.9,18 As a consequence, this new system can be placed among the most successful photoswitchable anion receptors reported to date.6 Our current work is directed toward the application of this type of receptor as a light-gated anion carrier in membrane transport, in addition to further optimization of the photoswitching and anion-binding properties.
Acknowledgments
This work was financially supported by The Netherlands Organization for Scientific Research (NWO-CW, Veni Grant No. 722.014.006 to S.J.W.), the Ministry of Education, Culture and Science (Gravitation Program No. 024.001.035), and the European Research Council (Advanced Investigator Grant No. 227897 to B.L.F). We thank Pieter van der Meulen (University of Groningen) for assistance with NMR irradiation experiments.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.6b03423.
Synthetic methods and characterization of new compounds; quantum yield determination; 1H NMR photoisomerization and anion-binding studies; theoretical calculations (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Cooper G. M.; Hausman R. E.. The Cell: A Molecular Approach, 7th ed.; Sinauer Associates, Inc.: Sunderland, MA, 2016. [Google Scholar]
- a Sessler J. L.; Gale P. A.; Cho W. S. In Anion Receptor Chemistry; Stoddart J. F., Ed.; RSC: London, 2006. [Google Scholar]; b Anion Coordination Chemistry; Bowman-James K., Bianchi A., García-España E., Eds.; Wiley-VCH: Weinheim, 2012. [Google Scholar]; c Gale P. A.; Howe E. N. W.; Wu X. Chem. 2016, 1, 351–422. 10.1016/j.chempr.2016.08.004. [DOI] [Google Scholar]
- Ashcroft F. M.Ion Channels and Disease; Academic Press: San Diego, 2000. [Google Scholar]
- a Davis J. T.; Okunola O.; Quesada R. Chem. Soc. Rev. 2010, 39, 3843–3862. 10.1039/b926164h. [DOI] [PubMed] [Google Scholar]; b Gale P. A.; Pérez-Tomás R.; Quesada R. Acc. Chem. Res. 2013, 46, 2801–2813. 10.1021/ar400019p. [DOI] [PubMed] [Google Scholar]; c Valkenier H.; Davis A. P. Acc. Chem. Res. 2013, 46, 2898–2909. 10.1021/ar4000345. [DOI] [PubMed] [Google Scholar]
- For selected nonlight-related examples, see:; a Beer P. D.; Stokes S. E. Polyhedron 1995, 14, 2631–2635. 10.1016/0277-5387(95)00129-G. [DOI] [Google Scholar]; b Rashdan S.; Light M. E.; Kilburn J. D. Chem. Commun. 2006, 4578–4580. 10.1039/b611138f. [DOI] [PubMed] [Google Scholar]; c Kuwabara J.; Yoon H. J.; Mirkin C. A.; DiPasquale A. G.; Rheingold A. L. Chem. Commun. 2009, 4557–4559. 10.1039/b905150c. [DOI] [PubMed] [Google Scholar]; d Bazzicalupi C.; Bencini A.; Puccioni S.; Valtancoli B.; Gratteri P.; Garau A.; Lippolis V. Chem. Commun. 2012, 48, 139–141. 10.1039/C1CC15934H. [DOI] [PubMed] [Google Scholar]; e Beck C. L.; Winter A. H. J. Org. Chem. 2014, 79, 3152–3158. 10.1021/jo500276h. [DOI] [PubMed] [Google Scholar]; f Zhao W.; Wang Y.; Shang J.; Che Y.; Jiang H. Chem. - Eur. J. 2015, 21, 7731–7735. 10.1002/chem.201500899. [DOI] [PubMed] [Google Scholar]
- For light-responsive anion receptors, see:; a Shimasaki T.; Kato S.; Ideta K.; Goto K.; Shinmyozu T. J. Org. Chem. 2007, 72, 1073–1087. 10.1021/jo061127v. [DOI] [PubMed] [Google Scholar]; b Hua Y.; Flood A. H. J. Am. Chem. Soc. 2010, 132, 12838–12840. 10.1021/ja105793c. [DOI] [PubMed] [Google Scholar]; c Wang Y.; Bie F.; Jiang H. Org. Lett. 2010, 12, 3630–3633. 10.1021/ol1014043. [DOI] [PubMed] [Google Scholar]; d Li Z.; Zhang C.; Ren Y.; Yin J.; Liu S. H. Org. Lett. 2011, 13, 6022–6025. 10.1021/ol202491e. [DOI] [PubMed] [Google Scholar]; e Han M.; Michel R.; He B.; Chen Y.-S.; Stalke D.; John M.; Clever G. H. Angew. Chem., Int. Ed. 2013, 52, 1319–1323. 10.1002/anie.201207373. [DOI] [PubMed] [Google Scholar]; f Lee S.; Hua Y.; Flood A. H. J. Org. Chem. 2014, 79, 8383–8396. 10.1021/jo501595k. [DOI] [PubMed] [Google Scholar]; g Choi Y. R.; Kim G. C.; Jeon H.-G.; Park J.; Namkung W.; Jeong K.-S. Chem. Commun. 2014, 50, 15305–15308. 10.1039/C4CC07560A. [DOI] [PubMed] [Google Scholar]; h Dąbrowa K.; Niedbała P.; Jurczak J. Chem. Commun. 2014, 50, 15748–15751. 10.1039/C4CC07798A. [DOI] [PubMed] [Google Scholar]; i Dąbrowa K.; Niedbała P.; Jurczak J. J. Org. Chem. 2016, 81, 3576–3584. 10.1021/acs.joc.6b00200. [DOI] [PubMed] [Google Scholar]
- For reviews, see:; a Natali M.; Giordani S. Chem. Soc. Rev. 2012, 41, 4010–4029. 10.1039/c2cs35015g. [DOI] [PubMed] [Google Scholar]; b Lee S.; Flood A. H. J. Phys. Org. Chem. 2013, 26, 79–86. 10.1002/poc.2973. [DOI] [Google Scholar]; c Qu D.-H.; Wang Q.-C.; Zhang Q.-W.; Ma X.; Tian H. Chem. Rev. 2015, 115, 7543–7588. 10.1021/cr5006342. [DOI] [PubMed] [Google Scholar]
- For illustrative examples, see:; a Shinkai S.; Ogawa T.; Kusano Y.; Manabe O. Chem. Lett. 1980, 9, 283–286. 10.1246/cl.1980.283. [DOI] [Google Scholar]; b Takeshita M.; Irie M. J. Org. Chem. 1998, 63, 6643–6649. 10.1021/jo980290q. [DOI] [Google Scholar]; c Mulder A.; Jukovic A.; Lucas L. N.; van Esch J.; Feringa B. L.; Huskens J.; Reinhoudt D. N. Chem. Commun. 2002, 2734–2735. 10.1039/B208692A. [DOI] [PubMed] [Google Scholar]; d Liu M.; Yan X.; Hu M.; Chen X.; Zhang M.; Zheng B.; Hu X.; Shao S.; Huang F. Org. Lett. 2010, 12, 2558–2561. 10.1021/ol100770j. [DOI] [PubMed] [Google Scholar]; e Berryman O. B.; Sather A. C.; Rebek J. Jr. Chem. Commun. 2011, 47, 656–658. 10.1039/C0CC03865B. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Arroyave F. A.; Ballester P. J. Org. Chem. 2015, 80, 10866–10873. 10.1021/acs.joc.5b02008. [DOI] [PubMed] [Google Scholar]; g Guentner M.; Uhl E.; Mayer P.; Dube H. Chem. - Eur. J. 2016, 22, 16433–16436. 10.1002/chem.201604237. [DOI] [PubMed] [Google Scholar]
- a Wezenberg S. J.; Vlatković M.; Kistemaker J. C. M.; Feringa B. L. J. J. Am. Chem. Soc. 2014, 136, 16784–16787. 10.1021/ja510700j. [DOI] [PubMed] [Google Scholar]; b Vlatković M.; Feringa B. L.; Wezenberg S. J. Angew. Chem., Int. Ed. 2016, 55, 1001–1004. 10.1002/anie.201509479. [DOI] [PubMed] [Google Scholar]
- a Feringa B.; Wynberg H. J. Am. Chem. Soc. 1977, 99, 602–603. 10.1021/ja00444a046. [DOI] [Google Scholar]; b Shimasaki T.; Kato S.; Shinmyozu T. J. Org. Chem. 2007, 72, 6251–6254. 10.1021/jo0701233. [DOI] [PubMed] [Google Scholar]; c Quick M.; Berndt F.; Dobryakov A. L.; Ioffe I. N.; Granovsky A. A.; Knie C.; Mahrwald R.; Lenoir D.; Ernsting N. P.; Kovalenko S. A. J. Phys. Chem. B 2014, 118, 1389–1402. 10.1021/jp411656x. [DOI] [PubMed] [Google Scholar]
- a Yang Q.-Z.; Huang Z.; Kucharski T. J.; Khvostichenko D.; Chen J.; Boulatov R. Nat. Nanotechnol. 2009, 4, 302–306. 10.1038/nnano.2009.55. [DOI] [PubMed] [Google Scholar]; b Huang Z.; Yang Q.-Z.; Khvostichenko D.; Kucharski T. J.; Chen J.; Boulatov R. J. Am. Chem. Soc. 2009, 131, 1407–1409. 10.1021/ja807113m. [DOI] [PubMed] [Google Scholar]; c Huang Z.; Boulatov R. Chem. Soc. Rev. 2011, 40, 2359–2384. 10.1039/c0cs00148a. [DOI] [PubMed] [Google Scholar]
- a Xu J.-F.; Chen Y.-Z.; Wu D.; Wu L.-Z.; Tung C.-H.; Yang Q.-Z. Angew. Chem., Int. Ed. 2013, 52, 9738–9742. 10.1002/anie.201303496. [DOI] [PubMed] [Google Scholar]; b Yan X.; Xu J.-F.; Cook T. R.; Huang F.; Yang Q.-Z.; Tung C.-H.; Stang P. J. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 8717–8722. 10.1073/pnas.1408620111. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wang Y.; Xu J.-F.; Chen Y.-Z.; Niu L.-Y.; Wu L.-Z.; Tung C. H.; Yang Q.-Z. Chem. Commun. 2014, 50, 7001–7003. 10.1039/c4cc02760d. [DOI] [PubMed] [Google Scholar]
- Xu J.-F.; Chen Y.-Z.; Wu L.-Z.; Tung C.-H.; Yang Q.-Z. Org. Lett. 2014, 16, 684–687. 10.1021/ol403343s. [DOI] [PubMed] [Google Scholar]
- Kean Z. S.; Akbulatov S.; Tian Y.; Widenhoefer R. A.; Boulatov R.; Craig S. L. Angew. Chem., Int. Ed. 2014, 53, 14508–14511. 10.1002/anie.201407494. [DOI] [PubMed] [Google Scholar]
- a Nishizawa S.; Bühlmann P.; Iwao M.; Umezawa Y. Tetrahedron Lett. 1995, 36, 6483–6486. 10.1016/0040-4039(95)01296-T. [DOI] [Google Scholar]; b Snellink-Ruël B. H. M.; Antonisse M. M. G.; Engbersen J. F. J.; Timmerman P.; Reinhoudt D. N. Eur. J. Org. Chem. 2000, 2000, 165–170. . [DOI] [Google Scholar]; c Brooks S. J.; Gale P. A.; Light M. E. Chem. Commun. 2005, 4696–4698. 10.1039/b508144k. [DOI] [PubMed] [Google Scholar]
- a Boiocchi M.; Del Boca L.; Esteban-Gómez D.; Fabbrizzi L.; Licchelli M.; Monzani E. Chem. - Eur. J. 2005, 11, 3097–3104. 10.1002/chem.200401049. [DOI] [PubMed] [Google Scholar]; b Amendola V.; Esteban-Gómez D.; Fabbrizzi L.; Licchelli M. Acc. Chem. Res. 2006, 39, 343–353. 10.1021/ar050195l. [DOI] [PubMed] [Google Scholar]
- Frassineti C.; Ghelli S.; Gans P.; Sabatini A.; Moruzzi M. S.; Vacca A. Anal. Biochem. 1995, 231, 374–382. 10.1006/abio.1995.9984. [DOI] [PubMed] [Google Scholar]
- Some degradation was noted previously upon photoswitching of an overcrowded alkene-based bis-urea analogue in the presence of 0.5% water and anion (see ref (9a)).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.