Abstract
Tumor heterogeneity is a major obstacle to the development of effective therapies and is thus an important focus of cancer research. Genetic and epigenetic alterations, as well as altered tumor microenvironments, result in tumors made up of diverse subclones with different genetic and phenotypic characteristics. Intratumor heterogeneity enables competition, but also supports clonal cooperation via cell-cell contact or secretion of factors, resulting in enhanced tumor progression. Here, we summarize recent findings related to interclonal interactions within a tumor and the therapeutic implications of such interactions, with an emphasis on how different subclones collaborate with each other to promote proliferation, metastasis and therapy-resistance. Furthermore, we propose that disruption of clonal cooperation by targeting key factors (such as Wnt and Hedgehog, amongst others) can be an alternative approach to improving clinical outcomes.
Introduction
The fact that tumors are non-uniform has been well accepted for decades, as cells with distinct morphologies and/or aneuploidy within a tumor have long been described by pathologists1. More recently, advances in next-generation sequencing and digital pathology have provided abundant evidence for intratumor heterogeneity, which is observed not only by distinct cellular morphologies, but also by diverse mutation and expression profiles, metabolic profiles, and invasive potentials2,3. Such heterogeneity is thought to play a crucial role in tumor growth, resistance, recurrence and metastasis3–7.
Two well accepted models that explain the rise of tumor heterogeneity are the clonal evolution model8,9 and the cancer stem cell model10–12. These two models are not mutually exclusive. Moreover, both models appreciate the impact of the tumor microenvironment (TME) on tumor cell fate13–15. After all, only cells that have a competitive advantage (high cellular fitness) are selected within a given environment.
The presence of diverse genetic and phenotypic subclones within a tumor provides a substrate for Darwinian-like evolution, potentiating clonal cooperation and clonal competition. As a result, “unsuccessful” subclones can be outcompeted, whereas subclones possessing the greatest fitness will survive, accelerating the process of tumor progression9,16,17. Despite the important role of clonal competition in shaping tumor composition, clonal cooperation can also substantially impact the cancer evolutionary trajectory18,19, particularly in the context of metastasis and therapy resistance20–25. Therefore, disruption of clonal cooperation may be critical to improve clinical outcomes.
In this review we discuss recent discoveries that have led to a more comprehensive landscape of the tumor environment, encompassing heterogeneous subclones and microenvironmental niches, with a focus on how clonal cooperation promotes tumor progression. Such knowledge should provide insight into developing therapies that target reciprocal interactions that occur amongst different tumor subpopulations.
The rise of intratumor heterogeneity
Genetic heterogeneity
Tumor cells often possess high genomic instability26. Such instability results from dysfunction of pathways such as base and nucleotide excision repair, mismatch repair, double-strand break repair, DNA replication, telomere maintenance and chromosome segregation; allowing for rapid acquisition of genetic alterations5,27.
The classic view of tumor evolution is that tumor initiating cells acquire driver mutations which endow them with a fitness advantage in their given microenvironment 28–30, after which various passenger mutations are sequentially accumulated as the tumor progresses5,8,9,27. One theory as to why tumors accumulate passenger mutations proposes that in expanding tumor populations, the effects of genetic drift (random loss or fixation of genotypes) may be magnified, thus neutral and even deleterious mutants can be preserved during expansion, resulting in genetic diversity16. Although individual passenger mutations can be neutral or even slightly deleterious, combinatorial effects of multiple passenger mutations could confer a selective fitness advantage (through epistatic interactions)18. Eventually, different subclones likely undergo branched evolution rather than linear evolution2,9,16,27, allowing tumor cells to follow diverse evolutionary paths. Branched evolution is a more likely path because selective sweep (a series of clonal expansions that dominate the whole neoplasm31,32) can only occur if one subclone can sweep through the neoplasm before the next driver mutation emerges, which is unlikely given the high mutation rate of tumor cells9. Indeed, acute leukemias33, breast carcinomas34, colon carcinomas and adenomas35, clear-cell renal carcinomas2, pancreatic carcinomas36 and prostate cancers37 have all been reported to follow branched evolutionary trajectories.
The aforementioned “gradual” model of genetic alteration acquisition is challenged by catastrophic events such as chromosome rearrangements38. Multiple studies suggest that genomic alterations can be achieved not only by incremental steps but also in a single leap. For example, chromothripsis is a process in which hundreds of chromosomal rearrangements can take place across only a few chromosomes39. Similarly, transient telomere dysfunction itself can result in drastic chromosomal instability allowing catastrophic genomic alterations to occur40. Moreover, genome doubling, a potential intermediate phase to aneuploidy41, is yet another type of dramatic genomic change42. Intriguingly, although many cancer cells will not survive these types of catastrophic events, such events can rapidly lead to high genetic diversity, supplying large amounts of substrates for evolution to act upon.
Importantly, genomic alterations conferred by either gradual or catastrophic events are heritable. Thus, tumors become increasingly genetically heterogeneous as they progress.
Non-genetic heterogeneity
There is a high degree of phenotypic plasticity in tumor cells sharing the same genotype; governed by non-genetic mechanisms. Two types of non-genetic heterogeneity have been described: deterministic and stochastic 6. In deterministic heterogeneity, cues from the microenvironment impinge on the tumor cell expression profile, thereby defining phenotypes. According to the cancer stem cell model, which is deterministic, progeny cells that originate from cancer stem cells can adjust their differentiation status based on the given tumor microenvironment10,43. For instance, clonally related CD24+ and CD44+ breast cancer cells can exhibit distinct properties, where CD44+ cells with active TGF-β signaling express high levels of stem-cell markers and are associated with decreased patient survival, while CD24+ cells are much less aggressive44. Nonetheless, even in the deterministic model, cellular phenotypes are dynamic.
In contrast, stochastic heterogeneity is a result of random fluctuation within the cells45. Moreover, random “noise” within the cell, when surpassing a certain threshold, can change genetic circuits and epigenetic landscapes, and can even facilitate evolutionary transitions 6,46,47. For example, biochemical processes within cells often involve low-frequency events. Therefore, stochastic fluctuations can occur in the cell. Importantly, these fluctuations affect cellular processes such as signal transduction, gene expression and cell fate decision. For instance, in the early mouse embryo, both epiblast and primitive endoderm cells are derived from the inner cell mass. This lineage segregation is determined by random “salt and pepper” (mutually exclusive) expression patterns of Nanog and Gata6 in the inner cell mass. The epiblast cells are derived from the Nanog positive inner cell mass, whereas the primitive endoderm cells originate from the Gata6 positive inner cell mass48,49. Another intriguing example is that sister cells exhibit different responsiveness to TRAIL-mediated apoptosis, and this cell-to-cell variability is caused by endogenous variation in the levels of apoptotic regulators (BID, BAX, BCL2, XIAP and caspase 3)50. Thus, noise can result in phenotypic spectrums of tumor cells with given genotypes, thereby facilitating tumor evolution.
Although non-genetic heterogeneity does not involve genetic alterations, it can bestow upon cells a transient and/or stable advantageous phenotype. If such cells can maintain their phenotypes over several cell divisions, it is likely that new genetic mutations will emerge 11,51. Thus, non-genetic heterogeneity can be temporarily heritable and provide substrates for selection as well.
Microenvironment and intratumor heterogeneity
The microenvironment, regarded as dynamic and heterogeneous, can serve as a double edged sword during tumor development, as it can shift from anti to tumor promotional as tumors progress15,52. Three major sources of microenvironmental heterogeneity include the extracellular matrix (ECM), the vascular network and the immune cell infiltrate53. Varying microhabitats confer diverse commanding signals and selective forces on tumor cells, contributing to intratumor heterogeneity54. For example, high ECM stiffness releases Twist1 from its cytoplasmic binding partner G3BP2 [GTPase activating protein (SH3 domain) binding protein 2]55,56, leading to Twist1 nuclear translocation, which results in epithelial-mesenchymal transition (EMT). Such an induced EMT then drives tumor invasion and metastasis57. In addition, aberrant ECM remodeling can stimulate tumor growth, CSCs phenotypes, and has been experimentally shown to play a significant role in the progression of tumors (review 55). Insufficient vasculature can lead to hypoxia in tumors, which is associated with alteration of multiple signaling pathways and the induction of aggressive phenotypes58, such as abnormal angiogenesis (e.g. VEGF and Ang2), inflammation (e.g. IL-6), CSC phenotypes (e.g. β-catenin and Oct4), EMT (e.g. Snail), secretion of growth factors (e.g. IGF1 and TGF-α) and resistance to radiotherapy, chemotherapy and immunotherapy (e.g. entering quiescence) (review 57). Finally, infiltration of immune cells into tumors, which was described by Rudolf Virchow in the 19th century, is now known to lead to considerable crosstalk between these cells and tumor cells. Ironically, immune cells can be altered by tumor cells as tumors progress, changing them from suppressive to promotional, and thus tumor infiltrating immune cells play crucial roles in eliminating or fostering tumor cells at different stages of tumor development59,60. For example, macrophage phenotypes largely depend on microenvironmental cues, and thus, distinct macrophages can be educated by tumor cells to serve different roles in tumor progression. In early stage tumors, macrophages can stimulate tumor growth by generating an inflammatory niche and by inducing angiogenesis to supply nutrients for tumor cells. Then, as tumors progress, subpopulations of macrophages may facilitate primary tumor cell dissemination by enhancing their migration and invasion, while different subpopulations of macrophages can prepare the pre-metastatic niche in secondary sites to allow tumor cells to colonize (review 58, 59).
Notably, in addition to effects of the microenvironment on tumor cells, spatial constraints restrict competition to the immediately neighboring subpopulations. Thus, selective sweep may be unachievable even by highly fit subpopulations, contributing to the generation and maintenance of intratumor heterogeneity61. Therefore, creating a relatively homogeneous and steady tumor microenvironment in early stage tumors may be an alternative approach to constrain tumor heterogeneity and decelerate tumor evolution.
Clonal cooperation and tumor malignancy
Given high intratumor heterogeneity, the tumor mass is thought to function in a similar manner as an evolving ecosystem involving intense interactions among various cellular components62–64. Even though most interactions among subclones are neutral, detectable interactions should be positive or negative, resulting in a phenotypic switch65. Recently, a multi-agent computational model postulated that cooperative cells tend to be spatially segregated from exploitive cells, and that the cooperators can increase their abundance faster than defectors, potentiating the maintenance of this collaborative interaction18,66. Below we outline how positive subclonal interactions promote tumor growth, metastasis and therapeutic resistance via cell-cell physical communication, paracrine effects or remodeling of the tumor microenvironment.
Clonal cooperation induces tumor growth
Cell proliferation is regulated by intrinsic signaling, nutrient availability, growth factors, ECM composition and many other factors. Cooperation between tumor subpopulations can significantly contribute to tumor growth via increasing growth factor abundance and activating pro-proliferative signaling pathways. For example, in Drosophila, Ras activation, in cooperation with mitochondrial defects in imaginal epithelium, cooperatively induced the production of reactive oxygen species (ROS), leading to the activation of JNK signaling and cellular senescence. The cellular senescence that arose exhibited a senescence-associated secretory phenotype, which includes secretion of interleukins, chemokines, growth factors, proteases and ECM components67). Activated JNK coupled with oncogenic Ras could then inactivate the hippo pathway in senescent cells, stimulating secretion of Unpaired (an Interleukin-6 homologue) and Wingless (a Wnt homologue). Eventually, these secreted factors could cooperate with Ras signaling in neighboring cells (which have normal mitochondrial function), resulting in overgrowth of neighboring tissue68,69.
Interclonal cooperation has also been extensively described in mouse models. For instance, in glioblastoma multiforme (GBM), amplification of wildtype (wt) EGFR and EGFR mutations (not amplified) are frequently observed in a heterogeneous manner. Using human glioma cells (U87) and immortalized mouse astrocytes (mAstr-Ink4a/Arf −/−), it was demonstrated that glioma cells with mutant EGFR express IL-6 and/or LIF cytokines, thus activating amplified wt EGFR in neighboring cells and resulting in heightened proliferation70. Similarly, in a breast cancer context, aberrant expression of Wnt1 can generate mammary tumors consisting of both basal Hrasmut Wnt1low and luminal Hraswt Wnt1high cell subtypes. Importantly, both subclones were shown to be necessary for full tumor expansion, as the basal cells relied on Wnt1 secreted by luminal subclones for growth25.
Intriguingly, apoptosis can also induce proliferation in neighboring cells. “Cell competition” has been intensively studied in Drosophila: a process in which apoptosis of cells with low fitness (loser) is induced by neighboring cells with high fitness (winner). This mechanism is thought to be a surveillance mechanism to maintain tissue homeostasis by removing mutated cells with reduced fitness71. Moreover, cell competition is not limited to flies, as it has been observed in the early mouse embryo. During normal development, Myc is heterogeneously expressed in epiblasts, and epiblast cells with high Myc levels eliminate the ones with lower Myc levels to refine the epiblast cell population72. However, increasing evidence has shown that cell competition can also be utilized by tumor cells to promote tumor progression23,73,74. For instance, in Drosophila, growth of Rab5 mutant tumors is dependent on the cell-competition-induced apoptosis inside the tumor. In this case, competition between cells acts in a tumor promotional manner by up-regulating JNK and Wg (a Wnt homologue) signaling in the viable tumor cells, thereby enhancing their proliferation74. Similarly, dying “oncogenic niche cells” (ONCs, cells which promote tumor progression non-cell autonomously via interaction with surrounding cells) have been shown to stimulate growth in neighboring tissues23. Cell death is often induced (potentially by cell competition) in ONCs, however, because ONCs express high levels of caspase inhibitors, cell death can’t be executed in these cells. Eventually, ONCs go on to exhibit SASP, which stimulates neighboring cell growth via Dpp (a BMP/TGFβ homologue) and Wg signaling23,75,76. This phenomena has also been observed in human patients, where apoptotic tumor cells can activate a “phoenix rising” pathway to stimulate wound healing and tissue regeneration77. Specifically, activated caspase 3/7 in apoptotic tumor cells caused downstream prostaglandin (PGE2) secretion, promoting growth of neighboring tumor cells77,78.
Using mathematical models, it has been proposed that non-cell-autonomous-induced tumor growth (such as that described above), combined with clonal interference, can stabilize intratumor heterogeneity thereby maintaining interclonal interactions19. This stabilization occurs because a positive growth effect on subclones can confer a fitness advantage to them, and because multiple subclones with high fitness will then interfere with each other, inhibiting expansion of individual subclones. Despite the advantages conferred by cooperation between subpopulations, clonal cooperation can also lead to tumor collapse if the driver subclone, that stimulates other subclones to grow non-cell autonomously, gets outcompeted by fast-growing exploitive subclones19,79. This may lead to tumor collapse because it leads to homogeneity within the tumor, and thus may result in the tumor being incapable of responding to adverse conditions such as hypoxia if “incompetent communicators” become dominant80.
Clonal cooperation induces metastasis
It has long been acknowledged that subpopulations that originate from the same tumor can possess different metastatic potentials81,82. Moreover, a positive correlation between tumor heterogeneity and metastasis has been established53,83. Despite the clear importance of diverse intrinsic characteristics of different subpopulations, collaborative interactions present among subpopulations can additionally facilitate the metastatic cascade. Subpopulations may gain a selective advantage during the metastatic process, if inter-clonal cooperation is present84.
Decades ago, several studies demonstrated that metastatic subclones can enhance the metastatic potential of non-metastatic subclones82,85–87. O’Grady and colleagues found that the rat mammary carcinoma cell line, B1, consists of two stable subtypes which were characterized as epithelioid cells (E-cells) and myoepithelioid (M-cells) cells. Interestingly, collagenase could only be sufficiently secreted when both cellular types were present. In brief, a soluble factor released by M-cells was able to induce collagenase secretion by E-cells, indicating that inter-clonal cooperation may promote at least local invasiveness87. Another study conducted by Miller and colleagues directly demonstrated that, in a syngeneic mouse mammary tumor model, presence of a metastatic subpopulation (410.4 cells) in the circulation was able to enhance the metastatic potential of non-metastatic subpopulations located in either subcutaneous sites (168 cells) or in circulation (67 cells)82. Thus, both studies demonstrated clear cooperativity between metastatic and non-metastatic cells, although neither elucidated the molecular mechanisms behind the clonal-cooperation-induced metastasis.
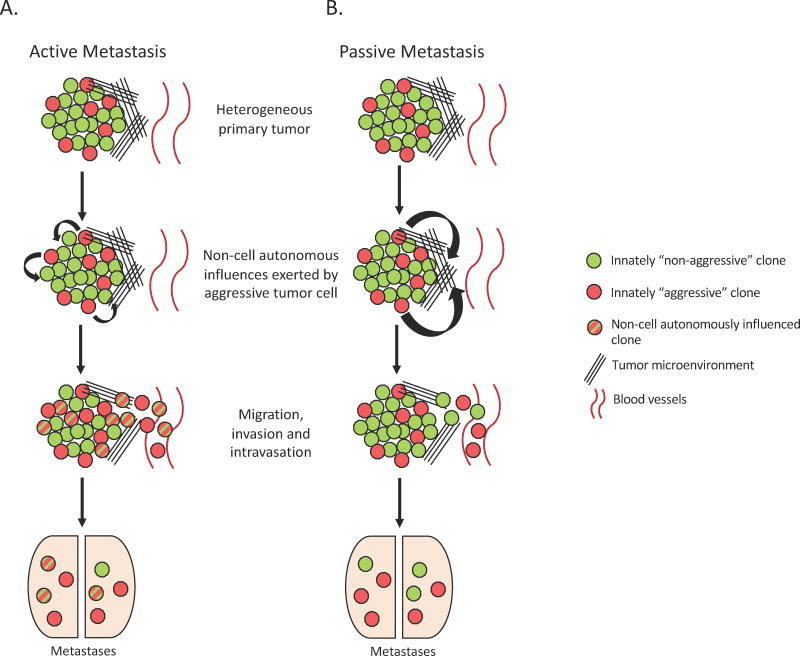
Several recent studies have revisited how clonal cooperation mechanistically influences metastasis. Clonal cooperation may facilitate metastasis in different ways; either by causing a phenotypic switch (active escape) or through microenvironment remodeling (passive escape). Importantly, these two mechanisms are not mutually exclusive. As an example of phenotypic switch, poorly metastatic F1 melanoma cells could uptake Met 72 tumor antigen containing exosomes, released by the BL6–10 highly metastatic melanoma cells, thereby increasing their own expression of Met 72 tumor antigen and their metastatic ability88. Similarly, Lieberman and colleagues presented that, in the context of breast cancer, murine (4T1E) and human (BPLER) breast cancer cell lines could transfer their metastatic potential via secretion of miR-200-containing extracellular vesicles, leading to mesenchymal-to-epithelial transition in non-metastatic cells (4TO7 cells and MB-231 cells), which increases their ability to colonize secondary sites. Strikingly, these extracellular vesicles were not only found in the primary site, but they could penetrate basement membrane and enter the circulation, thus influencing tumor cells at a distance89. Phenotypic switch can also be simply induced by paracrine factors. For example, using the PC-3 prostate cancer cell model it was demonstrated that non-CSC subpopulations (PC-3S) could secrete the matricellular protein SPARC, which then induced the invasiveness of a CSC-enriched subpopulation (PC-3M), leading to heightened metastasis in lungs90. (Fig. 1 active/passive models)
Fig. 1. Mechanisms of metastasis of heterogeneous tumors.
(A) Active Metastasis- Heterogeneous tumors contain a small percentage of innately aggressive subclones that secrete exosomes and/or release paracrine factors to enhance the aggressive properties of nearby cells. Aggressive and “transformed” subclones then actively perform the various steps of metastasis together. (B) Alternatively, innately aggressive subclones within the heterogeneous tumor remodel the tumor microenvironment, without acting directly on neighboring tumor cells. These microenvironmental alterations, such as degradation of the ECM, pave the path for innately “non-aggressive” tumor subclones to passively escape the primary tumor site and to enter the bloodstream (intravasate) and eventually metastasize. These two models for metastasis are not mutually exclusive and allow for overall increase in metastatic incidence of heterogeneous primary tumors.
As outlined above, a second means through which clonal cooperativity may mediate metastasis is via microenvironment remodeling. The process of epithelial to mesenchymal transition (EMT) is a well-described mediator of metastasis, and has been shown to increase invasiveness of tumor cells that also have enhanced ECM remodeling ability. Thus, studies focusing on how EMT cells and non-EMT cells interact during metastasis may provide intriguing mechanistic insights. For example, Hu and colleagues conducted an elegant study using a hamster cheek pouch carcinoma model in which the authors demonstrated that mice inoculated subcutaneously with EMT (expressing high p12CDK2-AP1) cells had these cells in the bloodstream, but metastases did not form. In contrast, when they subcutaneously injected the non-EMT cells, these cells could not be found in the bloodstream, nor were they able to form metastases at secondary sites. However, the non-EMT cells could form lung metastasis by intravenous inoculation, whereas the EMT cells could not. Importantly, subcutaneous co-injection of EMT and non-EMT cells led to the presence of both cell types in the circulation, and to the presence of overt metastases that were composed entirely of the non-EMT cells. These data suggest that EMT cells are likely responsible for degrading the ECM and breaching the basement membrane, allowing the non-EMT cells to intravasate91. Once these cells have made it into the bloodstream, they are then capable of extravasating and colonizing the secondary site.
Interestingly, a similar phenomenon was uncovered in a zebrafish melanoma xenograft model, where inherently invasive cells exhibiting high protease activity were able to deposit ECM leading to co-invasion of poorly invasive cells. The poorly invasive cells did not undergo a phenotype switch, but instead, the inherently invasive cells switched their invasion pattern from protease-independent to MT1-MMP-dependent, mediating ECM degradation and aiding the invasion of poorly invasive cells. Thus, the subpopulation with low invasiveness passively benefited from the microenvironmental remodeling ability of the highly invasive subpopulation92.
One can imagine that much of the time clonal-cooperation-induced metastasis is a combination of both active and passive mechanisms. Indeed, using a PC-3 prostate cancer model, Thomson and colleagues demonstrated that microenvironmental cues can induce some tumor initiating cells (TICs) to undergo a permanent EMT, thus losing their CSCs properties. These first generation “mesenchymalized” cells can then non-cell autonomously induce other TICs to undergo permanent or transient EMT, resulting in increased invasiveness of the tumor entity on a more global scale, ultimately leading to the breach of local barriers. Such a breach of local barriers then allows not only the EMT cells (active escape) but also the non-EMT cells (passive escape) to leave the primary site. Eventually, non-EMT and transient EMT cells will then colonize secondary sites, since they retain CSCs properties. In short, some metastatic cells actively participate in enhancing tumor invasiveness under the stimulation of “mesenchymalized” cells by undergoing transient EMT, while others can passively follow the paved path to disseminate93.
Collectively, tumor subpopulations are able to initiate their cooperation within the primary site, leading to enhanced invasiveness of the whole tumor mass via active and/or passive mechanisms. Hence, disruption of clonal cooperation present in primary tumors may be an effective approach to suppress metastasis.
Clonal cooperation induces therapeutic resistance
In addition to influencing metastasis, an association between intratumor heterogeneity and therapeutic resistance, relapse and poor prognosis has been established across cancers, as a result of both genome plasticity and cooperation among subclones94–97.
Recently, several studies have demonstrated that cancer cells promote therapy resistance and relapse at least in part through clonal cooperation20,78,98. For instance, it was demonstrated that cetuximab-resistant, KRASmut colorectal cancer cells were not only resistant to EGFR blockade, but were also able to increase resistance of surrounding KRASwt sensitive cells in a paracrine manner, via their ability to secrete TGFα and amphiregulin 98. In addition to influencing neighboring cells via secretion of key factors, tumor cells can boost their resistance through direct cell-cell interactions. A recent study by Winkler and colleagues demonstrated that astrocytoma cells could interconnect by extending ultra-long protrusions, referred to as microtubes. Importantly, Connexin 43 was critical for the formation and function of these microtubes. These microtubes allowed for multicellular communication through gap junctions and were critical for invasion and proliferation in the brain. Furthermore, these microtubes could be used for repair once this multicellular network was damaged. After exposing astrocytoma cells to a fatal laser dose, new microtubes were extended to the dead cells if the ablated cell was a member of the interconnected network, and a new-formed nucleus migrated to the original location of the ablated cell through the microtubes. Therefore, interconnected astrocytoma cells exhibit much higher radiotherapy resistance than unconnected ones20. In addition, many other cooperative mechanisms may contribute to therapy resistance. For example, one can imagine that subclones expressing high levels of immune-inhibitory molecules (e.g. PD-L1) may facilitate other subclones to escape from immune therapies such as chimeric antigen receptor (CAR) T-cell therapy, by creating an immune-suppressive TME99–102.
Altogether, cooperation occurring among subpopulations can profoundly influence sensitivity of tumor cells to therapeutics via secreted factors or direct cell-cell interactions. Thus, reducing intratumor heterogeneity or disrupting existing clonal cooperation may be critical to overcoming resistance and postponing relapse.
Therapeutic implications of intratumor heterogeneity
As outlined above, intratumor heterogeneity positively correlates with shorter time to relapse and increased multidrug resistance in different types of cancers. Decades ago, Dexter and colleagues had already identified a correlation between intraneoplastic diversity and therapy failure103, which has since been supported in numerous studies104–106. For example, it was reported that mutant-allele tumor heterogeneity (MATH), which measures clonal diversity by quantifying mutant-allele fractions in tumors, is associated with adverse outcomes in head and neck squamous cell carcinoma (HNSCC)106. Similarly, in chronic lymphocytic leukemia, Landau and colleagues showed that the presence of subclonal driver mutations was not only an independent risk factor for cancer progression but also an indicator of relapse104. However, mechanistic underpinnings of how heterogeneity drives therapy resistance have only more recently been uncovered.
Poor penetrance of therapeutic agents can be the first obstacle to achieving a high therapeutic index53,107. As a result of heterogeneous distribution of drugs, as well as leakiness of abnormal vascular networks108, tumor cells will be exposed to differing concentrations of therapeutic agents109. Therefore, individual tumor cells will adapt to different drug concentrations by utilizing diverse mechanisms, in part dependent on the level of drug to which they are exposed. Such mechanisms include increasing drug efflux110, suppressing apoptotic signaling111, entering quiescence112, activating alternative signaling pathways113 and collaborating with the tumor microenvironment114. Nonetheless, many tumor cells are unable to survive a given therapeutic insult. Thus, it is of interest to explore whether tumor resistance is predominantly driven by expansion of pre-existing resistant subclones, or by de novo alterations of surviving cells.
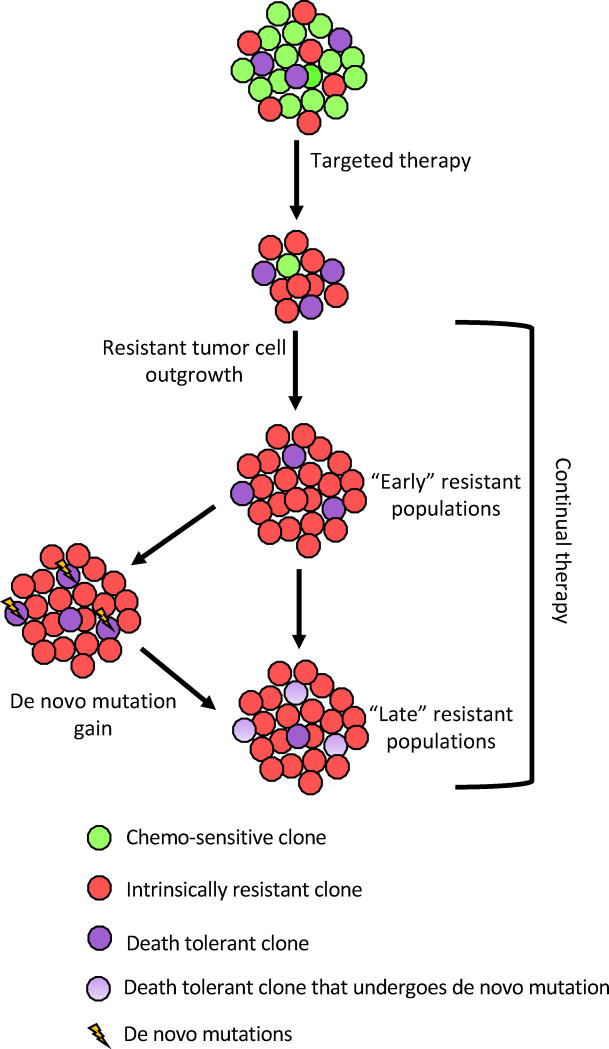
Vogelstein and colleagues recently demonstrated that after EGFR blockade treatment (panitumumab), circulating KRASmut DNA was detectable in the sera of 38% patients bearing KRASwt colorectal tumors. Given their mathematical modeling, the authors suggest that those mutations must have been present prior to the panitumumab treatment, indicating that acquired resistance can arise from undetectable pre-existing subclones115. Similarly, it has been demonstrated that MET amplification, which contributes to resistance to EGFR inhibition, is present in non-small cell lung cancer (NSCLC) subpopulations before EGFR inhibitor treatment116. Recently, an impressive study by Stegmeier and colleagues, in which a high-complexity barcode library was used to perform high-resolution tracking of tumor cells [both in NSCLC and chronic myeloid leukemia (CML)], clearly demonstrated that the majority of resistant subclones originated from rare pre-existing subpopulations, which gained a fitness advantage upon treatment challenge (EGFR inhibitor and c-Met inhibitor for NSCLC; ABL inhibitor for CML). This study not only validated that acquired resistance can be derived from rare pre-existing resistant subclones, but also suggested that expansion of pre-existing resistant subclones outweighs de novo alterations in mediating tumor resistance117. Moreover, Engelman and colleagues further dissected which evolutionary path(s) NSCLC cells can follow to generate their resistance to EGFR inhibition, and suggested that two routes can lead to a 2nd generation EGFR inhibitor gatekeeper mutation (EGFRT790M) in the tumor mass. One route is through the pre-existence of a small number of EGFRT790M positive cells, which can drive the growth of early-resistant subclones by selection. The second route is through rare apoptosis-suppressive EGFRT790M negative cells which can also tolerate EGFR inhibition, and then slowly acquire the EGFRT790M mutation through evolution, giving rise to late-resistant subclones118. Interestingly, the late-resistant subclones are also resistant to 3rd generation EGFR inhibitors, which target the EGFRT790M mutation, due to their ability to suppress apoptosis. This study provides temporal clues of how different resistant subclones arise during resistance development, and further confirms the probability that pre-existing resistant subclones will dominate resistant tumors after treatments. Taken together, a simplified tumor resistance development model (after initial treatment) can be proposed: upon initial treatment challenge, both intrinsic resistant subclones and death-tolerant subclones will survive and get released from clonal interference from drug-sensitive subclones. Then, intrinsic resistant subclones will expand rapidly and form early-resistant populations, taking up a large portion of the tumor, while death-tolerant subclones can gain resistance through de novo alterations and form the late-resistant populations, taking up a small proportion of the tumor. (Fig. 2)
Fig. 2. Therapy resistance in heterogeneous tumors.
Treatment of heterogeneous tumors with targeted therapies leads to the elimination of therapy-sensitive subclones, leaving behind intrinsically drug-resistant as well as death-resistant subclones. Drug-resistant subclones, which represent the “early” resistant tumor cell subpopulations, will then repopulate the majority of the tumor. Continual treatment challenges or stochastic events can cause de novo mutations in the death-resistant subclones, rendering them therapy-resistant and allowing them to occupy the tumor as “late” resistant subpopulations.
Most therapies induce massive cytotoxic effects, which function as double-edged swords. Even though cytotoxic effects can contribute to eliminating tumor cells, they can also cause a series of adverse responses such as local genomic instability119,120, SASP in surviving cells67,121,122, chronic inflammation123,124, heightened regional hypoxia125, wound healing responses126, accelerated tumor evolution127 and destruction of tissue homeostasis. Noteworthy, SASP, inflammation, hypoxia and wound healing responses can all facilitate the emergence of cells with CSC properties128–132, which often possess high therapy resistance. Furthermore, inflammation, combined with mutagens released by dead cells, can further promote genomic instability of surviving cells119,120,133,134, contributing to intratumor heterogeneity. Additionally, cytotoxic effects induce intense selection pressures which rapidly drive tumor evolution, thus potentiating the appearance of resistant subclones127. Inflammation plays a critical role in these intertwined adverse responses, rendering anti-inflammatory agents a tempting adjuvant treatment to diminish tumor resistance and intratumor heterogeneity. Alternatively, due to aforementioned disadvantages of cytotoxic effects, cytostatic agents may provide better clinical outcome for certain patients135, based on the idea of trying to contain cancer as a chronic disease.
Clinical strategies to overcome intratumor heterogeneity
Characterization of intratumor heterogeneity
Accurate characterization of tumor heterogeneity will be required if we are to optimize therapy regimes. With the substantial progress that has been made in non-invasive imaging and high throughput sequencing, which can be integrated with an increasingly sophisticated computational workflow, it is becoming more and more feasible to characterize genetic and phenotypic heterogeneity of specific tumors, even before treatment begins136–141. For example, Lambin and colleagues have shown that, by performing radiomic analysis on computed tomography (CT) data, one can quantify tumor shape, as well as intensity and texture, and these radiomics features can be compared with clinical data and gene-expression patterns to allow for prognostic radiomic signatures to be obtained140. Intriguingly, Rosenfeld and colleagues successfully tracked genomic evolution of advanced breast, ovarian and lung cancers in response to therapy using serial sequencing of plasma DNA. The authors detected increased mutant alleles in plasma with the emergence of therapy resistance, indicating genomic alteration in solid tumors can be measured by sequencing circulating DNA released from tumor cells137. Thus, advanced non-invasive analyses may enable researchers to overcome biased sampling issues that were previously an issue with conventional sequencing approaches, thus gaining a more quantitative view of intratumor heterogeneity. Moreover, accurate measures of intratumor heterogeneity can help estimate the risk of mortality across cancers142.
Targeting clonal cooperation
Although ablation of dominant tumor subclones using targeted therapy has improved clinical outcome substantially143, taking clonal cooperation into consideration may be an effective therapeutic avenue, particularly when targets identified may affect multiple aggressive phenotypes. Wnt1, matrix metalloproteinases (MMPs), miR-200, TGF α/β, amphiregulin, PGE2 and deregulated Hedgehog (Hh) signaling144–146 could all promote tumor progression non-cell autonomously, making them potential candidates for disruption of clonal cooperation. In addition, inhibition of secreted factors may suppress multiple exploitive subclones, reducing evolved drug resistance. Such effects have been reported in modeling of drugs targeting pathogens147. Below we outline recent progress in Wnt and Hh signaling inhibition, and some obstacles to targeting factors such as MMPs and miR-200.
Wnt inhibition
Wnt signaling may be an attractive pathway to target to inhibit clonal cooperativity. The canonical pathway is activated in response to Wnt secretion, which occurs following extensive post-translational modification of the Wnt proteins. Secreted Wnts then bind to Frizzled receptors on recipient cells, resulting in a signaling cascade that ultimately releases βcatenin from degradation and enhances nuclear entry of βcatenin, leading to TCF-LEF/β-catenin mediated transcription148. In addition to the critical non-cell autonomous role of Wnt1 in supporting multiple subpopulations in tumors, Wnt signaling has been reported to be associated with tumor growth149, migration, invasion150 and CSC phenotypes151 in a cell-autonomous manner. Therefore, inhibition of Wnt signaling may limit tumor progression from multiple aspects. However, targeting essential developmental signaling pathways is challenging, because somatic stem cells often utilize the above pathways for tissue repair and homeostasis of the stem cell niche148. In particular, targeting Wnt signaling would be anticipated to have significant adverse effects in the gut, where this pathway is critical for stem cell maintenance148,152,153. Because a precise balance has to be achieved between the healing and harmful effects of Wnt pathway inhibition, to date, no inhibitors targeting the Wnt pathway have been approved148,152,154. However, exciting new research from Virshup and colleagues has shown that pharmacological inhibition of Porcupine, which is a membrane-bound O-acyltransferase in charge of palmitoylation of all Wnts to enable their normal secretion and activity, has promising efficacy in MMTV-WNT1 mouse mammary tumors, without apparent toxicities155,156. Indeed, these inhibitors are currently in clinical trials, and are a promising approach to inhibiting Wnt signaling perhaps without associated side effects148.
Hedgehog inhibition
Like Wnt signaling, Hedgehog (Hh) signaling is another intriguing pathway to target because of its cell-autonomous and non cell-autonomous role in tumor progression. In brief, the Hh pathway is activated when Hh ligands (Sonic Hedgehog, Indian Hedgehog and Desert Hedgehog) bind to their receptors, Patched 1 or 2 (Ptch1/2), releasing Smoothened (Smo) from Ptch suppression, and allowing Smo to activate Gli transcription factors. Heightened Hh signaling is known to stimulate growth in a variety of cancers including basal cell carcinomas (BCCs), medulloblastomas, pancreatic, breast and prostate cancers146. In addition, cells carrying deregulated Hh activity were demonstrated to induce overgrowth and resistance to apoptosis in wildtype neighboring cells144,145. Interestingly, Tuveson and colleagues have demonstrated that inhibition of Hh signaling using the Smo inhibitor, IPI-926, led to an increase in gemcitabine delivery (as the inhibitor increased angiogenesis) in pancreatic ductal adenocarcinoma (PDA) using a KPC (K-Ras+/LSLG12D; p53R172H/+) mouse model157.
Currently, vismodegib, the first FDA-approved Smo inhibitor, has shown promising effects in tumors where Hh signaling is altered via mutations, such as basal cell carcinoma158. In a BCC study, vismodegib reduced both the size of existing BCCs and per-patient rate of new surgically eligible BCCs. Moreover, all BCCs regressed in some patients159. However, acquired resistance rapidly develops after Smo inhibitor treatment, through the development of secondary mutations which disrupt binding of the drug to Smo160, or activation of Gli through compensatory pathways (e.g. mTOR) bypassing Smo161. Furthermore, it was reported that the inflammatory cytokine, osteopontin (OPN), activates Gli in a non-canonical manner (bypassing Smo), resulting in EMT and enhanced drug resistance162. Therefore, direct targeting of the downstream Gli transcription factors using small molecules such as GANT58 and GANT61 may be a better approach to suppress Hh signaling163.
Limitations of MMP and miR-200 inhibition
It has been well established that, due to their ability to remodel ECM, MMPs can significantly contribute to tumor invasion and metastasis by increasing cellular invasiveness and liberating growth factors stored in the ECM92,164,165. Furthermore, as mentioned above, MMPs can non cell-autonomously induce invasion. These data suggest that MMP intervention should be a promising therapeutic strategy. Unfortunately, more than 50 MMP inhibitors have failed in clinical trials due to low specificity and resultant severe toxicities166,167. Even though targeting broad-spectrum MMPs may be unwise, it may still be beneficial to selectively inhibit membrane-anchored MMPs using antibody-based agents168,169. For example, it was recently shown that DX-2400, an antibody selectively inhibits MMP-14 (a membrane-bound zinc endopeptidase), suppresses angiogenesis and tumor progression in xenograft models170
Although miR-200-containing vesicles can promote non-metastatic breast cancer cells to metastasize, even at a distance, by inducing MET89, inhibition of the miR-200 family should be carefully evaluated. miR-200 family members suppress the E-cadherin repressors ZEB1 and ZEB2, thereby increasing E-cadherin levels and inhibiting EMT and stem cell like phenotypes171–175. Because the EMT-MET axis plays a multifaceted role during the metastatic cascade, in which EMT is thought to play an important role in early metastatic dissemination176–178, whereas MET is thought to be required for metastatic outgrowth at the secondary site176–182, inhibition of a key regulator of MET may not be a wise choice. Of note, the plastic nature of tumor cells, allowing them to switch between epithelial and mesenchymal states, may be the key to successful metastasis178,183, and novel approaches to inhibit this plasticity in the primary tumor may be a worthwhile approach.
The above examples are only a few of many pathways/molecules that could be targeted to limit tumor cell cooperatively and inhibit tumor progression. A comprehensive characterization of tumor heterogeneity will allow us to uncover many more avenues through which to inhibit clonal cooperation in addition to those mentioned above. As always, the balance between efficacy and toxicity will need to be considered.
Conclusions
In summary, due to genomic instability and the heterogeneous tumor microenvironment, various selection pressures cause tumor cells to undergo branched evolution forming a heterogeneous and dynamic tumor. Diverse subclones can establish their cooperation through paracrine, cell-cell contact and microenvironment remodeling, which allows them to exhibit a fitness advantage during tumor progression. Thus, disruption of these clonal cooperations may disturb the balance in the tumor ecosystem, leading to the repression of tumor growth, metastasis, therapy resistance, and hopefully, tumor collapse.
Acknowledgments
The authors would like to thanks Drs. James DeGregori and Andriy Marusyk for providing helpful suggestions after critical reading of our manuscript. HLF is funded by a grant from The National Cancer Institute (R01-CA095277) that supports her work on EMT and tumor heterogeneity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frierson HF. Grade and flow cytometric analysis of ploidy for infiltrating ductal carcinomas. Hum Pathol. 1993;24:24–29. doi: 10.1016/0046-8177(93)90058-o. [DOI] [PubMed] [Google Scholar]
- 2.Gerlinger M, et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patch AM, et al. Whole–genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 4.Marusyk A, Polyak K. Tumor heterogeneity: Causes and consequences. Biochim Biophys Acta BBA - Rev Cancer. 2010;1805:105–117. doi: 10.1016/j.bbcan.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelstein B, et al. Cancer Genome Landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- 7.Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217:998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- 8.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 9.Greaves M, Maley CC. CLONAL EVOLUTION IN CANCER. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22:457–472. doi: 10.1038/cr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brock A, Chang H, Huang S. Non-genetic heterogeneity — a mutation-independent driving force for the somatic evolution of tumours. Nat Rev Genet. 2009;10:336–342. doi: 10.1038/nrg2556. [DOI] [PubMed] [Google Scholar]
- 12.Weissman IL. Stem cells are units of natural selection for tissue formation, for germline development, and in cancer development. Proc Natl Acad Sci. 2015;112:8922–8928. doi: 10.1073/pnas.1505464112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heindl A, Nawaz S, Yuan Y. Mapping spatial heterogeneity in the tumor microenvironment: a new era for digital pathology. Lab Invest. 2015;95:377–384. doi: 10.1038/labinvest.2014.155. [DOI] [PubMed] [Google Scholar]
- 14.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerlinger M, et al. Cancer: Evolution Within a Lifetime. Annu Rev Genet. 2014;48:215–236. doi: 10.1146/annurev-genet-120213-092314. [DOI] [PubMed] [Google Scholar]
- 17.de Visser JAGM, Krug J. Empirical fitness landscapes and the predictability of evolution. Nat Rev Genet. 2014;15:480–490. doi: 10.1038/nrg3744. [DOI] [PubMed] [Google Scholar]
- 18.Nadell CD, Foster KR, Xavier JB. Emergence of Spatial Structure in Cell Groups and the Evolution of Cooperation. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marusyk A, et al. Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity. Nature. 2014;514:54–58. doi: 10.1038/nature13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osswald M, et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015 doi: 10.1038/nature16071. advance online publication. [DOI] [PubMed]
- 21.Neelakantan D, Drasin DJ, Ford HL. Intratumoral heterogeneity: Clonal cooperation in epithelial-to-mesenchymal transition and metastasis. Cell Adhes Migr. 2014;9:265–276. doi: 10.4161/19336918.2014.972761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le MTN, et al. miR-200–containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest. 2014;124:5109–5128. doi: 10.1172/JCI75695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enomoto M, Vaughen J, Igaki T. Non-autonomous overgrowth by oncogenic niche cells: Cellular cooperation and competition in tumorigenesis. Cancer Sci. 2015;106:1651–1658. doi: 10.1111/cas.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. 2012;18:1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cleary AS, Leonard TL, Gestl SA, Gunther EJ. Tumour cell heterogeneity maintained by cooperating subclones in Wnt-driven mammary cancers. Nature. 2014;508:113–117. doi: 10.1038/nature13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338–345. doi: 10.1038/nature12625. [DOI] [PubMed] [Google Scholar]
- 28.Martins VC, et al. Cell competition is a tumour suppressor mechanism in the thymus. Nature. 2014;509:465–470. doi: 10.1038/nature13317. [DOI] [PubMed] [Google Scholar]
- 29.Rangarajan A, Hong SJ, Gifford A, Weinberg RA. Species- and cell type-specific requirements for cellular transformation. Cancer Cell. 2004;6:171–183. doi: 10.1016/j.ccr.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 31.Bozic I, et al. Accumulation of driver and passenger mutations during tumor progression. Proc Natl Acad Sci. 2010;107:18545–18550. doi: 10.1073/pnas.1010978107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beerenwinkel N, et al. Genetic Progression and the Waiting Time to Cancer. PLoS Comput Biol. 2007;3 doi: 10.1371/journal.pcbi.0030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Notta F, et al. Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature. 2011;469:362–367. doi: 10.1038/nature09733. [DOI] [PubMed] [Google Scholar]
- 34.Navin N, et al. Tumor Evolution Inferred by Single Cell Sequencing. Nature. 2011;472:90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thirlwell C, et al. Clonality Assessment and Clonal Ordering of Individual Neoplastic Crypts Shows Polyclonality of Colorectal Adenomas. Gastroenterology. 2010;138:1441–1454.e7. doi: 10.1053/j.gastro.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Campbell PJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haffner MC, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest. 2013;123:4918–4922. doi: 10.1172/JCI70354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang CZ, Leibowitz ML, Pellman D. Chromothripsis and beyond: rapid genome evolution from complex chromosomal rearrangements. Genes Dev. 2013;27:2513–2530. doi: 10.1101/gad.229559.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephens PJ, et al. Massive Genomic Rearrangement Acquired in a Single Catastrophic Event during Cancer Development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007;17:157–162. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Carter SL, et al. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol. 2012;30:413–421. doi: 10.1038/nbt.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaffer CL, et al. Poised chromatin at the ZEB1 promoter enables cell plasticity and enhances tumorigenicity. Cell. 2013;154:61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shipitsin M, et al. Molecular Definition of Breast Tumor Heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Raj A, van Oudenaarden A. Nature, Nurture, or Chance: Stochastic Gene Expression and Its Consequences. Cell. 2008;135:216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eldar A, Elowitz MB. Functional roles for noise in genetic circuits. Nature. 2010;467:167–173. doi: 10.1038/nature09326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Altschuler SJ, Wu LF. Cellular Heterogeneity: Do Differences Make a Difference? Cell. 2010;141:559–563. doi: 10.1016/j.cell.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early Lineage Segregation between Epiblast and Primitive Endoderm in Mouse Blastocysts through the Grb2-MAPK Pathway. Dev Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 49.Dietrich JE, Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo. Development. 2007;134:4219–4231. doi: 10.1242/dev.003798. [DOI] [PubMed] [Google Scholar]
- 50.Spencer SL, Gaudet S, Albeck JG, Burke JM, Sorger PK. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature. 2009;459:428–432. doi: 10.1038/nature08012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asatryan AD, Komarova NL. Evolution of genetic instability in heterogeneous tumors. J Theor Biol. 2016;396:1–12. doi: 10.1016/j.jtbi.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basanta D, et al. Investigating prostate cancer tumour–stroma interactions: clinical and biological insights from an evolutionary game. Br J Cancer. 2012;106:174–181. doi: 10.1038/bjc.2011.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheele CLGJ, Maynard C, van Rheenen J. Intravital Insights into Heterogeneity, Metastasis, and Therapy Responses. Trends Cancer. 2016;2:205–216. doi: 10.1016/j.trecan.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim MM, Wiederschain D, Kennedy D, Hansen E, Yuan ZM. Modulation of p53 and MDM2 activity by novel interaction with Ras-GAP binding proteins (G3BP) Oncogene. 2007;26:4209–4215. doi: 10.1038/sj.onc.1210212. [DOI] [PubMed] [Google Scholar]
- 56.Prigent M, Barlat I, Langen H, Dargemont C. IkappaBalpha and IkappaBalpha/NF-kappa B complexes are retained in the cytoplasm through interaction with a novel partner, RasGAP SH3-binding protein 2. J Biol Chem. 2000;275:36441–36449. doi: 10.1074/jbc.M004751200. [DOI] [PubMed] [Google Scholar]
- 57.Wei SC, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17:678–688. doi: 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain RK. Antiangiogenesis Strategies Revisited: From Starving Tumors to Alleviating Hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qian BZ, Pollard JW. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lipinski KA, et al. Cancer Evolution and the Limits of Predictability in Precision Cancer Medicine. Trends Cancer. 2016;2:49–63. doi: 10.1016/j.trecan.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korolev KS, Xavier JB, Gore J. Turning ecology and evolution against cancer. Nat Rev Cancer. 2014;14:371–380. doi: 10.1038/nrc3712. [DOI] [PubMed] [Google Scholar]
- 63.Merlo LMF, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6:924–935. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- 64.Basanta D, Anderson ARA. Exploiting ecological principles to better understand cancer progression and treatment. Interface Focus. 2013;3 doi: 10.1098/rsfs.2013.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tabassum DP, Polyak K. Tumorigenesis: it takes a village. Nat Rev Cancer. 2015;15:473–483. doi: 10.1038/nrc3971. [DOI] [PubMed] [Google Scholar]
- 66.Korolev KS. The Fate of Cooperation during Range Expansions. PLoS Comput Biol. 2013;9 doi: 10.1371/journal.pcbi.1002994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coppé JP, Desprez PY, Krtolica A, Campisi J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohsawa S, et al. Mitochondrial defect drives non-autonomous tumour progression through Hippo signalling in Drosophila. Nature. 2012;490:547–551. doi: 10.1038/nature11452. [DOI] [PubMed] [Google Scholar]
- 69.Nakamura M, Ohsawa S, Igaki T. Mitochondrial defects trigger proliferation of neighbouring cells via a senescence-associated secretory phenotype in Drosophila. Nat Commun. 2014;5:5264. doi: 10.1038/ncomms6264. [DOI] [PubMed] [Google Scholar]
- 70.Inda MM, et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 2010;24:1731–1745. doi: 10.1101/gad.1890510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Menéndez J, Pérez-Garijo A, Calleja M, Morata G. A tumor-suppressing mechanism in Drosophila involving cell competition and the Hippo pathway. Proc Natl Acad Sci U S A. 2010;107:14651–14656. doi: 10.1073/pnas.1009376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clavería C, Giovinazzo G, Sierra R, Torres M. Myc-driven endogenous cell competition in the early mammalian embryo. Nature. 2013;500:39–44. doi: 10.1038/nature12389. [DOI] [PubMed] [Google Scholar]
- 73.Ohsawa S, Takemoto D, Igaki T. Dissecting tumour heterogeneity in flies: genetic basis of interclonal oncogenic cooperation. J Biochem (Tokyo) 2014;156:129–136. doi: 10.1093/jb/mvu045. [DOI] [PubMed] [Google Scholar]
- 74.Ballesteros-Arias L, Saavedra V, Morata G. Cell competition may function either as tumour-suppressing or as tumour-stimulating factor in Drosophila. Oncogene. 2014;33:4377–4384. doi: 10.1038/onc.2013.407. [DOI] [PubMed] [Google Scholar]
- 75.Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends Cell Biol. 2008;18:467–473. doi: 10.1016/j.tcb.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Enomoto M, Igaki T. Src controls tumorigenesis via JNK-dependent regulation of the Hippo pathway in Drosophila. EMBO Rep. 2013;14:65–72. doi: 10.1038/embor.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li F, et al. Apoptotic Cells Activate the ‘Phoenix Rising’ Pathway to Promote Wound Healing and Tissue Regeneration. Sci Signal. 2010;3:ra13–ra13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang Q, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McGranahan N, Swanton C. Biological and Therapeutic Impact of Intratumor Heterogeneity in Cancer Evolution. Cancer Cell. 2015;27:15–26. doi: 10.1016/j.ccell.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 80.Nagy JD. Competition and natural selection in a mathematical model of cancer. Bull Math Biol. 2004;66:663–687. doi: 10.1016/j.bulm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 81.Fidler IJ. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978;38:2651–2660. [PubMed] [Google Scholar]
- 82.Miller FR. Tumor subpopulation interactions in metastasis. Invasion Metastasis. 1983;3:234–242. [PubMed] [Google Scholar]
- 83.Koren S, Bentires-Alj M. Breast Tumor Heterogeneity: Source of Fitness, Hurdle for Therapy. Mol Cell. 2015;60:537–546. doi: 10.1016/j.molcel.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 84.Rinner B, et al. Molecular evidence for the bi-clonal origin of neuroendocrine tumor derived metastases. BMC Genomics. 2012;13:594. doi: 10.1186/1471-2164-13-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller FR, Heppner GH. Cellular interactions in metastasis. Cancer Metastasis Rev. 1990;9:21–34. doi: 10.1007/BF00047586. [DOI] [PubMed] [Google Scholar]
- 86.Baban D, Matsumura Y, Kocialkowski S, Tarin D. Studies on relationships between metastatic and non-metastatic tumor cell populations using lineages labeled with dominant selectable genetic markers. Int J Dev Biol. 1993;37:237–243. [PubMed] [Google Scholar]
- 87.Lyons JG, Siew K, O’Grady RL. Cellular interactions determining the production of collagenase by a rat mammary carcinoma cell line. Int J Cancer. 1989;43:119–125. doi: 10.1002/ijc.2910430123. [DOI] [PubMed] [Google Scholar]
- 88.Hao S, et al. Epigenetic transfer of metastatic activity by uptake of highly metastatic B16 melanoma cell-released exosomes. Exp Oncol. 2006;28:126–131. [PubMed] [Google Scholar]
- 89.Le MTN, et al. miR-200–containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest. 2014;124:5109–5128. doi: 10.1172/JCI75695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mateo F, et al. SPARC mediates metastatic cooperation between CSC and non-CSC prostate cancer cell subpopulations. Mol Cancer. 2014;13:237. doi: 10.1186/1476-4598-13-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsuji T, et al. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 2008;68:10377–10386. doi: 10.1158/0008-5472.CAN-08-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chapman A, et al. Heterogeneous Tumor Subpopulations Cooperate to Drive Invasion. Cell Rep. 2014;8:688–695. doi: 10.1016/j.celrep.2014.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Celià-Terrassa T, et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Invest. 2012;122:1849–1868. doi: 10.1172/JCI59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McGranahan N, Burrell RA, Endesfelder D, Novelli MR, Swanton C. Cancer chromosomal instability: therapeutic and diagnostic challenges. EMBO Rep. 2012;13:528–538. doi: 10.1038/embor.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mullighan CG, et al. GENOMIC ANALYSIS OF THE CLONAL ORIGINS OF RELAPSED ACUTE LYMPHOBLASTIC LEUKEMIA. Science. 2008;322:1377–1380. doi: 10.1126/science.1164266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang J, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science. 2014;346:256–259. doi: 10.1126/science.1256930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patel AP, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hobor S, et al. TGFα and Amphiregulin Paracrine Network Promotes Resistance to EGFR Blockade in Colorectal Cancer Cells. Clin Cancer Res. 2014;20:6429–6438. doi: 10.1158/1078-0432.CCR-14-0774. [DOI] [PubMed] [Google Scholar]
- 99.Taube JM, et al. Colocalization of Inflammatory Response with B7-H1 Expression in Human Melanocytic Lesions Supports an Adaptive Resistance Mechanism of Immune Escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kelderman S, Schumacher TNM, Haanen JBAG. Acquired and intrinsic resistance in cancer immunotherapy. Mol Oncol. 2014;8:1132–1139. doi: 10.1016/j.molonc.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.John LB, Kershaw MH, Darcy PK. Blockade of PD-1 immunosuppression boosts CAR T-cell therapy. Oncoimmunology. 2013;2 doi: 10.4161/onci.26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Newick K, Moon E, Albelda SM. Chimeric antigen receptor T-cell therapy for solid tumors. Mol Ther — Oncolytics. 2016;3:16006. doi: 10.1038/mto.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dexter DL, Leith JT. Tumor heterogeneity and drug resistance. J Clin Oncol. 1986;4:244–257. doi: 10.1200/JCO.1986.4.2.244. [DOI] [PubMed] [Google Scholar]
- 104.Landau DA, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152:714–726. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fisher R, Pusztai L, Swanton C. Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer. 2013;108:479–485. doi: 10.1038/bjc.2012.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mroz EA, Rocco JW. MATH, a novel measure of intratumor genetic heterogeneity, is high in poor-outcome classes of head and neck squamous cell carcinoma. Oral Oncol. 2013;49:211–215. doi: 10.1016/j.oraloncology.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 108.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 109.Thurber GM, et al. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat Commun. 2013;4:1504. doi: 10.1038/ncomms2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 111.Pommier Y, Sordet O, Antony S, Hayward RL, Kohn KW. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene. 2004;23:2934–2949. doi: 10.1038/sj.onc.1207515. [DOI] [PubMed] [Google Scholar]
- 112.Li L, Bhatia R. Molecular Pathways: Stem Cell Quiescence. Clin Cancer Res Off J Am Assoc Cancer Res. 2011;17:4936–4941. doi: 10.1158/1078-0432.CCR-10-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bivona TG, Doebele RC. A framework for understanding and targeting residual disease in oncogene-driven solid cancers. Nat Med. 2016;22:472–478. doi: 10.1038/nm.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 115.Diaz LA, Jr, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537–540. doi: 10.1038/nature11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Turke AB, et al. Pre-existence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bhang HC, et al. Studying clonal dynamics in response to cancer therapy using high-complexity barcoding. Nat Med. 2015;21:440–448. doi: 10.1038/nm.3841. [DOI] [PubMed] [Google Scholar]
- 118.Hata AN, et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med. 2016;22:262–269. doi: 10.1038/nm.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shimizu T, Marusawa H, Endo Y, Chiba T. Inflammation-mediated genomic instability: roles of activation-induced cytidine deaminase in carcinogenesis. Cancer Sci. 2012;103:1201–1206. doi: 10.1111/j.1349-7006.2012.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 121.Lasry A, Ben-Neriah Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 2015;36:217–228. doi: 10.1016/j.it.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 122.Ewald JA, Desotelle JA, Wilding G, Jarrard DF. Therapy-Induced Senescence in Cancer. JNCI J Natl Cancer Inst. 2010;102:1536–1546. doi: 10.1093/jnci/djq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Modiano JF, et al. Inflammation, Apoptosis, and Necrosis Induced by Neoadjuvant Fas Ligand Gene Therapy Improves Survival of Dogs With Spontaneous Bone Cancer. Mol Ther. 2012;20:2234–2243. doi: 10.1038/mt.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kuraishy A, Karin M, Grivennikov SI. Tumor Promotion via Injury- and Death-induced Inflammation. Immunity. 2011;35:467–477. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 126.Haubner F, Ohmann E, Pohl F, Strutz J, Gassner HG. Wound healing after radiation therapy: Review of the literature. Radiat Oncol Lond Engl. 2012;7:162. doi: 10.1186/1748-717X-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gillies RJ, Verduzco D, Gatenby RA. Evolutionary Dynamics Unifies Carcinogenesis and Cancer Therapy. Nat Rev Cancer. 2012;12:487–493. doi: 10.1038/nrc3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cahu J, Bustany S, Sola B. Senescence-associated secretory phenotype favors the emergence of cancer stem-like cells. Cell Death Dis. 2012;3:e446. doi: 10.1038/cddis.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peng G, Liu Y. Hypoxia-inducible factors in cancer stem cells and inflammation. Trends Pharmacol Sci. 2015;36:374–383. doi: 10.1016/j.tips.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shigdar S, et al. Inflammation and cancer stem cells. Cancer Lett. 2014;345:271–278. doi: 10.1016/j.canlet.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 131.Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer. 2012;12:170–180. doi: 10.1038/nrc3217. [DOI] [PubMed] [Google Scholar]
- 132.Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 133.Nagar S, Smith LE, Morgan WF. Mechanisms of cell death associated with death-inducing factors from genomically unstable cell lines. Mutagenesis. 2003;18:549–560. doi: 10.1093/mutage/geg033. [DOI] [PubMed] [Google Scholar]
- 134.Sanada M, Takagi Y, Ito R, Sekiguchi M. Killing and mutagenic actions of dacarbazine, a chemotherapeutic alkylating agent, on human and mouse cells: effects of Mgmt and Mlh1 mutations. DNA Repair. 2004;3:413–420. doi: 10.1016/j.dnarep.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 135.Millar AW, Lynch KP. Rethinking clinical trials for cytostatic drugs. Nat Rev Cancer. 2003;3:540–545. doi: 10.1038/nrc1124. [DOI] [PubMed] [Google Scholar]
- 136.Forshew T, et al. Noninvasive Identification and Monitoring of Cancer Mutations by Targeted Deep Sequencing of Plasma DNA. Sci Transl Med. 2012;4:136ra68–136ra68. doi: 10.1126/scitranslmed.3003726. [DOI] [PubMed] [Google Scholar]
- 137.Murtaza M, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497:108–112. doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- 138.O’Connor JPB, et al. Imaging Intratumor Heterogeneity: Role in Therapy Response, Resistance, and Clinical Outcome. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21:249–257. doi: 10.1158/1078-0432.CCR-14-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhong Q, et al. Image-based computational quantification and visualization of genetic alterations and tumour heterogeneity. Sci Rep. 2016;6 doi: 10.1038/srep24146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aerts HJWL, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5 doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mayr NA, et al. Characterizing Tumor Heterogeneity With Functional Imaging and Quantifying High-Risk Tumor Volume for Early Prediction of Treatment Outcome: Cervical Cancer as a Model. Int J Radiat Oncol. 2012;83:972–979. doi: 10.1016/j.ijrobp.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Andor N, et al. Pan-cancer analysis of the extent and consequences of intra-tumor heterogeneity. Nat Med. 2016;22:105–113. doi: 10.1038/nm.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang M, Shen A, Ding J, Geng M. Molecularly targeted cancer therapy: some lessons from the past decade. Trends Pharmacol Sci. 2014;35:41–50. doi: 10.1016/j.tips.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 144.Christiansen AE, et al. Non-cell autonomous control of apoptosis by ligand-independent Hedgehog signaling in Drosophila. Cell Death Differ. 2013;20:302–311. doi: 10.1038/cdd.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Christiansen AE, Ding T, Bergmann A. Ligand-independent activation of the Hedgehog pathway displays non-cell autonomous proliferation during eye development in Drosophila. Mech Dev. 2012;129:98–108. doi: 10.1016/j.mod.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Atwood SX, Chang ALS, Oro AE. Hedgehog pathway inhibition and the race against tumor evolution. J Cell Biol. 2012;199:193–197. doi: 10.1083/jcb.201207140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pepper JW. Drugs that target pathogen public goods are robust against evolved drug resistance. Evol Appl. 2012;5:757–761. doi: 10.1111/j.1752-4571.2012.00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu J, et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci U S A. 2013;110:20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang JM, et al. Inhibition of Cancer Cell Migration and Invasion through Suppressing the Wnt1-mediating Signal Pathway by G-quadruplex Structure Stabilizers. J Biol Chem. 2014;289:14612–14623. doi: 10.1074/jbc.M114.548230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Choi AR, et al. Inhibition of Wnt1 expression reduces the enrichment of cancer stem cells in a mouse model of breast cancer. Biochem Biophys Res Commun. 2012;425:436–442. doi: 10.1016/j.bbrc.2012.07.120. [DOI] [PubMed] [Google Scholar]
- 152.Blagodatski A, Poteryaev D, Katanaev VL. Targeting the Wnt pathways for therapies. Mol Cell Ther. 2014;2:28. doi: 10.1186/2052-8426-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Madan B, Virshup DM. Targeting Wnts at the Source—New Mechanisms, New Biomarkers, New Drugs. Am Assoc Cancer Res. 2015;14:1087–1094. doi: 10.1158/1535-7163.MCT-14-1038. [DOI] [PubMed] [Google Scholar]
- 154.Voronkov A, Krauss S. Wnt/beta-Catenin Signaling and Small Molecule Inhibitors. Curr Pharm Des. 2012;19:634–664. doi: 10.2174/138161213804581837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Proffitt KD, et al. Pharmacological Inhibition of the Wnt Acyltransferase PORCN Prevents Growth of WNT-Driven Mammary Cancer. Cancer Res. 2013;73:502–507. doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- 156.Proffitt KD, Virshup DM. Precise Regulation of Porcupine Activity Is Required for Physiological Wnt Signaling. J Biol Chem. 2012;287:34167–34178. doi: 10.1074/jbc.M112.381970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Olive KP, et al. Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tang JY, et al. Inhibiting the Hedgehog Pathway in Patients with the Basal-Cell Nevus Syndrome. N Engl J Med. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yauch RL, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang Y, et al. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 2012;21:374–387. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Das S, Samant RS, Shevde LA. Nonclassical Activation of Hedgehog Signaling Enhances Multidrug Resistance and Makes Cancer Cells Refractory to Smoothened-targeting Hedgehog Inhibition. J Biol Chem. 2013;288:11824–11833. doi: 10.1074/jbc.M112.432302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lauth M, Bergström Å, Shimokawa T, Toftgård R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci U S A. 2007;104:8455–8460. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- 165.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 166.Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov. 2014;13:904–927. doi: 10.1038/nrd4390. [DOI] [PubMed] [Google Scholar]
- 167.Fingleton B. MMPs as therapeutic targets – still a viable option? Semin Cell Dev Biol. 2008;19:61–68. doi: 10.1016/j.semcdb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cathcart J, Pulkoski-Gross A, Cao J. Targeting matrix metalloproteinases in cancer: Bringing new life to old ideas. Genes Dis. 2015;2:26–34. doi: 10.1016/j.gendis.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Devy L, Dransfield DT, Devy L, Dransfield DT. New Strategies for the Next Generation of Matrix-Metalloproteinase Inhibitors: Selectively Targeting Membrane-Anchored MMPs with Therapeutic Antibodies, New Strategies for the Next Generation of Matrix-Metalloproteinase Inhibitors: Selectively Targeting Membrane-Anchored MMPs with Therapeutic Antibodies. Biochem Res Int Biochem Res Int 2011. 2010;2011:e191670. doi: 10.1155/2011/191670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Devy L, et al. Selective Inhibition of Matrix Metalloproteinase-14 Blocks Tumor Growth, Invasion, and Angiogenesis. Cancer Res. 2009;69:1517–1526. doi: 10.1158/0008-5472.CAN-08-3255. [DOI] [PubMed] [Google Scholar]
- 171.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gregory PA, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 173.Korpal M, Kang Y. The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol. 2008;5:115–119. doi: 10.4161/rna.5.3.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lim YY, et al. Epigenetic modulation of the miR-200 family is associated with transition to a breast cancer stem-cell-like state. J Cell Sci. 2013;126:2256–2266. doi: 10.1242/jcs.122275. [DOI] [PubMed] [Google Scholar]
- 175.Feng X, Wang Z, Fillmore R, Xi Y. MiR-200, a new star miRNA in human cancer. Cancer Lett. 2014;344:166–173. doi: 10.1016/j.canlet.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhao D, et al. Cytoplasmic p27 promotes epithelial–mesenchymal transition and tumor metastasis via STAT3-mediated Twist1 upregulation. Oncogene. 2015;34:5447–5459. doi: 10.1038/onc.2014.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Somarelli JA, et al. Distinct routes to metastasis: plasticity-dependent and plasticity-independent pathways. Oncogene. 2016 doi: 10.1038/onc.2015.497. [DOI] [PMC free article] [PubMed]
- 178.Beerling E, et al. Plasticity between Epithelial and Mesenchymal States Unlinks EMT from Metastasis-Enhancing Stem Cell Capacity. Cell Rep. 2016;14:2281–2288. doi: 10.1016/j.celrep.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zheng X, et al. EMT Program is Dispensable for Metastasis but Induces Chemoresistance in Pancreatic Cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yao D, Dai C, Peng S. Mechanism of the Mesenchymal–Epithelial Transition and Its Relationship with Metastatic Tumor Formation. Am Assoc Cancer Res. 2011;9:1608–1620. doi: 10.1158/1541-7786.MCR-10-0568. [DOI] [PubMed] [Google Scholar]
- 181.Ocaña OH, et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22:709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 182.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tsai JH, Yang J. Epithelial–mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]