Abstract
Three‐dimensional (3D) stem cell differentiation cultures recently emerged as a novel model system for investigating human embryonic development and disease progression in vitro, complementing existing animal and two‐dimensional (2D) cell culture models. Organoids, the 3D self‐organizing structures derived from pluripotent or somatic stem cells, can recapitulate many aspects of structural organization and functionality of their in vivo organ counterparts, thus holding great promise for biomedical research and translational applications. Importantly, faithful recapitulation of disease and development processes relies on the ability to modify the genomic contents in organoid cells. The revolutionary genome engineering technologies, CRISPR/Cas9 in particular, enable investigators to generate various reporter cell lines for prompt validation of specific cell lineages as well as to introduce disease‐associated mutations for disease modeling. In this review, we provide historical overviews, and discuss technical considerations, and potential future applications of genome engineering in 3D organoid models.
Keywords: CRISPR, disease modeling, homology‐directed repair, organoids, stem cells
Subject Categories: Development & Differentiation, Methods & Resources, Stem Cells
Glossary
- 2D
two‐dimensional
- 3D
three‐dimensional
- APC
adenomatosis polyposis coli
- Atoh1
atonal bHLH transcription factor 1
- Bf1
brain factor 1
- C2c1
Class 2 candidate 1
- Cas9
CRISPR‐associated protein 9
- Cas9n
Cas9 nickase
- CFTR
cystic fibrosis transmembrane conductance regulator
- Cpf1
CRISPR from Prevotella and Francisella 1
- CRISPR
clustered regularly interspaced short palindromic repeats
- CRX
cone‐rod homeobox
- dCas9
nuclease‐deactivated Cas9
- DKC1
dyskerin pseudouridine synthase 1
- DMD
Duchenne muscular dystrophy
- DSB
double‐stranded break
- ESC
embryonic stem cell
- FACS
fluorescence‐activated cell sorting
- FOXG1
forkhead box G1
- GFP
green fluorescent protein
- GRHL2
grainyhead‐like transcription factor 2
- gRNA
guide RNA
- h (e.g., in hESC)
human
- HA
homology arm
- HDR
homology‐directed repair
- HR
homologous recombination
- indel
insertion and/or deletion
- iPSC
induced pluripotent stem cell
- Lgr5
leucine‐rich repeat containing G protein coupled receptor 5
- m (e.g., in mESC)
mouse
- MARCKS
myristoylated alanine‐rich protein kinase C substrate
- MIXL1
Mix1 homeobox‐like 1
- NES
nuclear export signal
- NHEJ
non‐homologous end‐joining
- NLS
nuclear localization signal
- PAX6
paired box 6
- PSC
pluripotent stem cell
- RFP
red fluorescent protein
- RNF43
ring finger protein 43
- ROCK
Rho‐associated coiled‐coil containing protein kinase
- Rx
retinal homeobox
- SMAD4
SMAD family member 4
- SSC
somatic stem cell
- ssDNA
single‐stranded DNA
- TALEN
transcription activator‐like effector nuclease
- Tbx19
T‐box 19
- TP53
tumor protein p53
- WT
wild type
- ZFN
zinc finger nuclease
Introduction
Built upon knowledge accumulated through decades of research in developmental biology, miniorgans can now be grown in petri dishes from aggregates of stem cells by stepwise manipulation of molecular signals, mimicking the innate signaling cues during in vivo organ development. These stem cell‐derived three‐dimensional (3D) structures, designated “organoids”, recapitulate many aspects of bona fide organs in the body in terms of cell fate, cellular organization, and function 1, 2. Over the past few years, a number of organoid models have been established, resembling tissues from the eye 3, 4, brain 5, 6, 7, intestine 8, 9, 10, 11, kidney 12, 13, 14, 15, liver 16, 17, 18, lung 19, 20, 21, and inner ear 22, 23, among others. These organoids can be derived from human pluripotent stem cells (PSCs) and thus may serve as human model systems for disease modeling, drug screening, and drug safety testing, as well as providing replaceable tissues in regenerative therapeutics. However, success in these applications would be limited without the ability to modify the genomic contents. For example, although induced pluripotent stem cells (iPSCs) established from patients’ fibroblasts can be used for disease modeling, comparison of organoids derived from patient and control iPS cell lines may not reveal disease‐relevant phenotypes, but rather may reflect the variation in the genetic backgrounds and the reprogramming history of individual cell lines 24. Genome editing, on the other hand, can be used to induce specific changes in an otherwise identical genetic background to overcome this limitation. Such an isogenic pair of disease‐specific and control cell lines can be generated through genome editing by either introducing mutations in wild‐type (WT) cells or correcting mutations in patient‐derived cell lines.
Genomic editing with programmable nucleases, especially CRISPR/Cas9 25, has been one of the major technological breakthroughs of recent years in biomedical research. Although CRISPR‐mediated genome editing has generated much excitement, genome editing without a nuclease was accomplished long before the development of the CRISPR technology (Fig 1). In the 1980s, a series of studies demonstrated that genomic sequences can be modified by homologous recombination (HR) between genomic DNA and an exogenous DNA template harboring homologous regions 26, 27, 28, 29, 30. Through HR, modifications made on the template DNA via standard molecular cloning techniques can be precisely introduced to the genomes of mammalian cells including mouse embryonic stem cells (ESCs). In fact, before the programmable nuclease era, HR‐mediated genome editing was a standard procedure in generating genomically modified mouse ESC lines as well as generating transgenic mouse strains via blastocyst injections of the genomically modified ESCs 31. A key disadvantage of HR‐mediated genome editing, however, is its discouragingly low efficiency, which is typically at 10−6 frequency. Because of this, co‐integration of antibiotic selection marker genes is usually required to enrich modified cells 32.
Figure 1. Genome editing mediated by conventional HR or programmable nucleases.
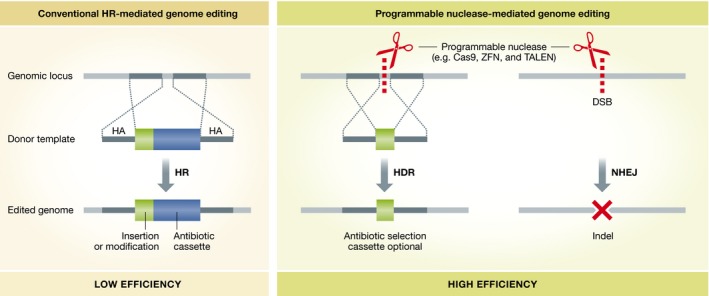
Genome editing has been traditionally achieved to low efficiency without the use of a programmable nuclease. Programmable nuclease‐mediated DNA double‐stranded breaks dramatically increased the efficiency of genomic editing.
It was later discovered that the HR efficiency can be greatly improved by introducing a DNA double‐stranded break (DSB) at the targeted locus 33, 34. While the elevated HR rate was limited to only a few special cut sites due to the non‐programmable nature of the nucleases used in these studies, the discovery of programmable nucleases led to the expansion of DSB‐stimulated HR to the genome level. Early programmable nucleases, including zinc finger nucleases (ZFNs) and transcription activator‐like effector nucleases (TALENs), require labor‐ and cost‐intensive construction of new nucleases for each targeting site. The emergence of the CRISPR‐associated RNA‐guided nuclease Cas9 has truly revolutionized genome engineering. Rather than assembling various domain arrays into new ZFN or TALEN nucleases to target a particular site, CRISPR/Cas9 only needs the expression of a specific 20‐bp guide RNA (gRNA), which can easily be made with standard cloning techniques. In addition to the advantages of simplicity and low cost, CRISPR is highly efficient 35, 36, 37, 38, 39 and enables multiplex targeting 36. Recently engineered high‐fidelity Cas9 variants have even reduced off‐target effects, once a big concern for CRISPR, down to below detection levels of the most sensitive genome‐wide methods available 40, 41. After Cas9, the “new kid on the block”, even newer programmable nucleases are being discovered, such as two other CRISPR‐associated nucleases Cpf1 42 and C2c1 43.
Programmable nucleases can be targeted to virtually anywhere on the genome to create a site‐specific DSB. Cells utilize one of two types of pathways to repair the DSB, either homology‐directed repair (HDR) or non‐homologous end‐joining (NHEJ). In the presence of a donor template, the HDR pathway repairs the DNA break via the above‐mentioned HR mechanism. Precise modifications, including specific base pair substitutions and insertions ranging from a single base pair to large gene cassettes, can be introduced into the genome through this pathway. The NHEJ pathway, on the other hand, does not use a donor template and is error prone, leading to random‐length insertion or deletion mutations (indels) that can often disrupt a gene, especially with frameshift indels 44 (Fig 1). When two DSBs are created on a chromosome, repairs through the NHEJ pathway can result in large genomic deletions (as large as 1,600 kb), inversions, and duplications 45.
Since the earliest HR studies 27, 30, genomic editing has been routinely performed on both embryonic and somatic stem cells. As organoids are derived from aggregates of these stem cells and they require genomic alteration for many applications, the marriage between organoid and genome editing technologies is unsurprising. However, the fact that the establishment of organoid models has been built upon the basis of genomically engineered stem cells is often overlooked. In this review, we will highlight how genomic engineering techniques was used for the development and optimization of the earlier organoid models. We will also discuss how genome editing has been catered to model embryonic organ development and disease progression in 3D culture. In addition, we will discuss several technical considerations and provide insights into future applications.
Development of 3D organoid models using genomically engineered reporter cell lines
Traditionally, stem cell differentiation experiments are performed in two‐dimensional (2D) monolayer culture conditions. While relatively homogenous populations of differentiated cells can be generated in 2D cultures, the 2D systems face the challenge of a lower degree of physiological relevance. Through several landmark papers published during the late 2000s and early 2010s, Yoshiki Sasai and his colleagues were among the first to not only add one dimension to the differentiation culture system, but also to take one step closer to accurately modeling in vivo physiology of an organ 1. By aggregating dissociated ESCs into spheres and allowing them to differentiate and self‐organize in 3D floating culture, Sasai and colleagues invented a new model system that recapitulated development of various organs, representing the cortical tissues, the retina, and the pituitary gland 3, 4, 46, 47. Though these organoid models were created before genome editing technologies became vastly popular, they relied extensively on genomically edited ESC lines while establishing these organoid models.
For example, a genomically engineered reporter ESC line was essential for deriving retinal organoids from aggregates of mouse ESCs 3. Previously, Sasai's laboratory established a protocol to promote retinal differentiation from mouse ESCs, but the differentiated cells lacked spatial patterning and did not morphologically resemble retinal epithelial structures 48. While they wisely used the transcription factor Rx as a marker for neural retinal precursors in their culture, the lack of a readily identifiable fluorescence reporter compromised their efforts in identifying optimal culture conditions to promote the formation of self‐organized retinal tissues. In their subsequent study, a mouse Rx‐GFP reporter ESC line was generated to overcome this hurdle. Using conventional HR, the investigators inserted GFP after the start codon of one of the two alleles of the Rx gene, so that cells would be GFP+ upon their differentiation into retinal lineages 49. This fluorescence reporter cell line not only allowed for prompt identification of retinal precursor cells arising in culture, but also enabled easy tracking of the morphological changes of their residing tissues. Indeed, with the help of the Rx‐GFP cell line, a stepwise differentiation method for 3D retinal organoids was developed. The derived organoids contained GFP+ cells that bore retinal marker gene signatures. Moreover, these cells self‐patterned into neural retina and retinal pigment epithelium domains. Remarkably, the self‐patterned retinal cells morphologically self‐organized into an optic cup structure that is strikingly similar to an optic cup developed in vivo 3. A number of assays in this study, including fluorescence‐activated cell sorting (FACS), multiphoton long‐term 3D live imaging, 3D reconstruction of tissue and cell morphology, and cell migration tracking, were made possible due to the Rx‐GFP fluorescent cell line.
Spurred by the success of optic cup organoid generation from mouse ESCs, human optic cup organoids were generated using genomically engineered fluorescent reporter cell lines as well (RX‐Venus and CRX‐Venus human ESC lines) 4. It should be noted that for these two optic cup organoid studies, conventional HR without a programmable nuclease was used to establish the mouse ESC line 49, while the programmable nuclease ZFNs were utilized for the genomic modifications to the human ESC lines 4. This is likely because conventional HR approaches, while considered standard in mouse ESCs before the era of the programmable nucleases, have been proven to be very difficult in human ESCs 24.
Besides the optic cup organoids, Sasai and his colleagues also took advantage of early genome editing technologies to establish a number of other reporter ESC lines to aid in the identification of other stem cell‐derived organs and tissues. Examples include Bf1‐Venus mouse ESC lines generated via conventional HR for the cerebral cortex organoids 47, Lim3‐Venus mouse ESC lines generated via ZFN for the pituitary organoids 46, and FOXG1‐Venus and PAX6‐Venus human ESC lines generated via ZFN for the human neocortex organoids 7.
In addition to these ES cell‐derived organoids, pioneering work on generating organoids from tissue‐resident somatic stem cells also used fluorescently labeled reporter cells. Hans Clevers and his colleagues built intestinal organoids with FACS‐sorted single Lgr5‐GFP somatic stem cells 8. The Lgr5‐GFP cells were isolated from transgenic mice created via HR‐mediated genomic editing in mouse ESCs followed by a blastocyst injection and transplantation into foster mice 50. Besides the mouse intestinal organoids, genomically engineered human ESC and iPSC lines, with LGR5‐GFP reporters built with ZFNs, have been used to generate human intestinal organoids 51.
Genome editing technologies used in organoid studies
As discussed above, organoid models were established with reporter PSC lines created by genomic engineering. Genome engineering was also applied to these newly established organoid systems for inducing or correcting mutations in order to elucidate pathological conditions (Table 1). Many of the above‐mentioned reporter cell lines were created by inserting GFP or Venus after the start codon of one of the two alleles of a gene of interest 4, 7, 46, 47, 49. This labeling strategy resulted in disruption of the inserted allele, making the cells heterozygous mutants for the targeted gene. The same labeling strategy can be used to create homozygous mutant cells, simply by screening for cell lines in which both alleles have been inserted with a fluorescence protein 52. In the pituitary organoid study 46, a mouse ESC line knocked out for the transcription factor gene Tbx19 was created. The knockout was achieved by selecting for ZFN‐mediated biallelic insertion of Venus at the start codon of Tbx19. During in vivo pituitary development, Tbx19 drives the differentiation of the cell lineage that produces the adrenocorticotropic hormone. To test whether the in vitro derived pituitary organoids recapitulate in vivo development, Tbx19 Venus/Venus knockout ESCs were guided toward pituitary development in 3D culture. As expected, the expression level of the adrenocorticotropic hormone was significantly reduced, confirming the same cell lineage specification requirements between 3D culture and in vivo pituitary development.
Table 1.
Published studies of genome engineering in organoids
| Main purpose of genome editing | Type of modification | Organoid type | Cell of origin | Genome editing method | Reference number |
|---|---|---|---|---|---|
| Generating new type of organoids | Fluorescence gene knockin | Retinal organoids | mESC | Conventional HR | 3 49 |
| hESC | ZFN | 4 | |||
| Cerebral cortex organoids | mESC | Conventional HR | 47 | ||
| Kidney organoids | hESC | Conventional HR | 15 95 | ||
| Pituitary organoids | mESC | ZFN | 46 | ||
| Neocortex organoids | hESC | ZFN | 7 | ||
| Intestinal organoids | hESC and hiPSC | ZFN | 51 | ||
| Modeling of development | Gene disruption via biallelic knockin of a fluorescence gene | Pituitary organoids | mESC | ZFN | 46 |
| Epiblast organoids | mESC | CRISPR (WT Cas9) | 52 | ||
| Modeling of disease | NHEJ‐mediated gene disruption and/or HDR‐mediated gene modification | Intestinal organoids | m/hSSC | CRISPR (WT Cas9) | 53 58 59 60 |
| hSSC | ZFN | 56 | |||
| hiPSC | CRISPR (Cas9n) | 57 | |||
| Kidney organoids | hESC | CRISPR (WT Cas9) | 13 | ||
| Small scale screen | NHEJ‐mediated gene disruption | Lung organoids | hSSC | CRISPR (WT Cas9) | 61 |
The discovery of CRISPR/Cas9 was a major milestone in genome engineering. Just a few months after the first studies of CRISPR‐mediated editing of mammalian genomes were published 36, 37, Clevers and colleagues successfully applied this technology to intestinal organoids for mutation correction 53. A mutation on the anion channel gene CFTR (F508del) is known to cause cystic fibrosis, a disease affecting multiple organs including lung and intestine. The function of CFTR can be assessed in a forskolin‐induced swelling assay 54, in which healthy intestinal organoids respond by rapid swelling due to fluid secretion through the forskolin‐activated CFTR channels, while organoids derived from patients with the CFTR mutation do not expand their surface area. The investigators used CRISPR to correct the CFTR mutation via co‐transfection of a repair template vector. The genetically corrected intestinal organoids demonstrated a functional repair, as they rapidly expanded the organoid surface area upon forskolin treatment 53. Together with previous successes of intestinal organoid transplantation in mice 55, this study provided a potential CRISPR/organoid‐based therapeutic strategy for intestine symptoms of cystic fibrosis. In addition to this study, functional correction of disease genes has also been performed in intestinal organoids on the CFTR gene using ZFN 56 and on a telomere maintenance dysfunction‐related gene DKC1 using CRISPR 57.
Before performing the CFTR gene correction, Clevers and colleagues first optimized the CRISPR system in intestinal organoid cultures by targeting the APC gene 53. As APC is a negative regulator of the Wnt pathway, APC null mutant organoids can grow in the absence of the normally essential ingredients Wnt and Wnt agonist R‐spondin. This system provided an excellent opportunity to perform functional selection on the CRISPR edited organoids, as only mutant intestinal organoids devoid of APC function can survive and expand in the culture medium lacking Wnt and R‐spondin. This elegantly designed study serves as a prime example of how targeted clones can be selected promptly and unequivocally based on the target gene function in the organoid culture.
Using the same functional selection strategies to isolate successfully edited organoids, researchers from two laboratories independently modeled colorectal cancer in intestinal organoids via CRISPR 58, 59. These groups demonstrated that by sequentially mutating tumor suppressor genes and oncogenes including APC, SMAD4, TP53, and KRAS with CRISPR, the mutant intestinal organoids grew independently of niche factors (e.g., Wnt) and formed tumors in hostile niche environments. When coupled with chromosome instability, the mutated intestinal organoids showed invasive and metastatic growth upon transplantation into mice. In another colorectal cancer‐related study, researchers induced mutations in a distal hot spot region of another negative Wnt regulator gene Rnf43 with CRISPR, and used the mutant intestinal organoids to study the importance of the Rnf43 distal hot spot region in colorectal cancer progression 60.
In addition to intestinal organoids, CRISPR genome editing technology has been used in kidney organoids for disease modeling 13, and gene function testing in lung organoids 61. In the latter example, the simplicity and effectiveness of CRISPR allowed investigators to rapidly mutate and screen the function of multiple genes. These genes were a subset of a previously identified pool of putative target genes of the master regulator of lung development, GRHL2. Using CRISPR‐enabled small scale screening in lung organoid cultures, the investigators discovered two new downstream effector genes of GRHL2 that play important roles in ciliogenesis and barrier function in the airway epithelium 61.
Technical considerations
CRISPR‐based genome editing technology, based on its versatility and broad application potential, is rapidly evolving, and many technical improvements are being made to make genome editing more specific and efficient.
WT Cas9 used in the first‐generation CRISPR platform, while highly efficient, is also prone to off‐target cleavages 62, 63. Most of the CRISPR‐organoid studies discussed above used WT Cas9 (Table 1), and off‐target indels were indeed found 53. Several Cas9 variants have been created with greatly reduced off‐target effects while retaining on‐target activities comparable to WT Cas9. These next‐generation Cas9s include the Cas9 nickase variants 64, and the two recently developed high‐fidelity Cas9 variants, eSpCas9(1.1) 41 and SpCas9‐HF1 40. More recently, another CRISPR‐associated endonuclease Cpf1 42, in its WT form, is reported to be highly specific 65, at levels approaching that of the high‐fidelity variants of Cas9 66.
The CRISPR components can be transfected into cells in the form of DNA vectors encoding Cas9 and gRNA, or as Cas9 mRNA or protein with in vitro transcribed gRNA. To deliver these CRISPR components and donor templates into cells of interest, nucleofection is a highly efficient method, especially for hard‐to‐transfect cells such as human PSCs 67, 68, 69. Other delivery methods, such as lipofectamine, conventional electroporation, and lentiviral infection, have also been successfully used to transfect embryonic or somatic stem cells 53, 69, 70, 71. In addition to transfecting dissociated stem cells followed by aggregation and differentiation into organoids, it is also possible to directly deliver constructs into organoids. For instance, adenoviruses have been shown to mediate efficient gene transfer into intestinal organoids 72, and retroviruses have been used to infect fragments of intestinal organoids 73.
When making precise genome modifications, small molecule inhibitors of the NHEJ pathway can be used to promote the efficiency of HDR 74, 75, 76, including the NHEJ inhibitor Scr7, which is reported to increase the HDR efficiency by up to 19‐fold 77. Recently, an impressive 60% HDR efficiency has been achieved using an asymmetric single‐stranded DNA (ssDNA) donor with optimal homology arm lengths 78. Chemically modified ssDNA donors with phosphorothioate linkages also enhance HDR efficiency 79. In addition, restricting Cas9 or Cas9 variants to specific cell cycle stages favors the HDR pathway over the NHEJ pathway, thus increasing the HDR rate nearly twofold 80, 81, 82. Combined use of these approaches may further improve the efficiency of HDR.
When making mutant organoids, it is important to note that not the entire targeted cell population contains indels, even after puromycin selection or FACS sorting when a Cas9‐puromycin/GFP co‐expressing vector is used. In addition, in‐frame indels may not disrupt gene function, as the alteration of a small number of amino acids may not affect the protein function. Even a frame‐shift indel, which disrupts the reading frame of a gene, may not be a complete gene knockout if it does not occur at the beginning of the gene. Therefore, to generate monoallelic/biallelic mutant organoids, it is essential to design gRNAs to target the beginning of the gene for all its splicing variants, establish cell lines from single cells, perform thorough genotyping and sequencing analysis, and use cell lines with frameshift indels. With regard to establishing clonal cell lines, single‐cell survival rate has been a hurdle for clonal cell line generation in human PSCs. This challenge has been largely overcome by the discovery that ROCK kinase inhibitors can dramatically increase the survival rate of single human PSCs 83. However, care should be taken as small clusters of cells due to incomplete single‐cell dissociation may survive better than single cells 84, giving rise to “clonal cell lines” with mixed genotypes.
To create a reporter cell line, a fluorescence gene (e.g., GFP) is integrated at a marker gene locus (e.g., Rx) whose expression coincides with the emergence of the cell type of interest. The marker genes often play essential roles for the differentiation or the function of the cell type of interest, and therefore, reduced expression levels of the marker genes may decrease the target cell type generation efficiency or may affect the function of these cells. Up to now, most of the reporter cell lines in the organoid field were created at the expense of disrupting one of the two alleles of a marker gene, by inserting a fluorescence‐antibiotic gene cassette right after the start codon of the marker gene. We recommend a non‐destructive labeling design for optimal differentiation efficiency and normal functioning of the organoids. For example, GFP can be fused in‐frame with the marker gene, or alternatively, a “self‐cleavage” 2A sequence 85 can be placed between the marker gene and GFP (Figs 2 and 3) so that GFP will be co‐expressed during transcription but later separated from the marker gene during translation. The 2A‐GFP labeling approach has been successfully used in human ESCs, demonstrating higher fluorescence intensity (indicating better protein stability) compared to the direct GFP fusion approach 86. Since the fluorescence protein is separated from the marker gene protein during translation, it will have a pan‐cellular localization pattern. If another cellular localization pattern is desired, short localization sequences can be added to the fluorescence gene. For instance, the nuclear localization signal (NLS) or nuclear export signal (NES) can be linked with GFP for localization inside or outside the nucleus, respectively, and a short sequence from the MARCKS gene can be added to GFP for plasma membrane localization, which can facilitate visualization of fine cellular morphologies 87.
Figure 2. Workflow of genomic engineering in organoids.

Before in vitro differentiation into organoids, pluripotent stem cells (ESCs and iPSCs) or multipotent somatic stem cells can be genomically modified in various ways. The resulting genomically edited organoids play critical roles in applications such as disease modeling and drug testing.
Figure 3. Generating new organoid models with reporter cell lines.
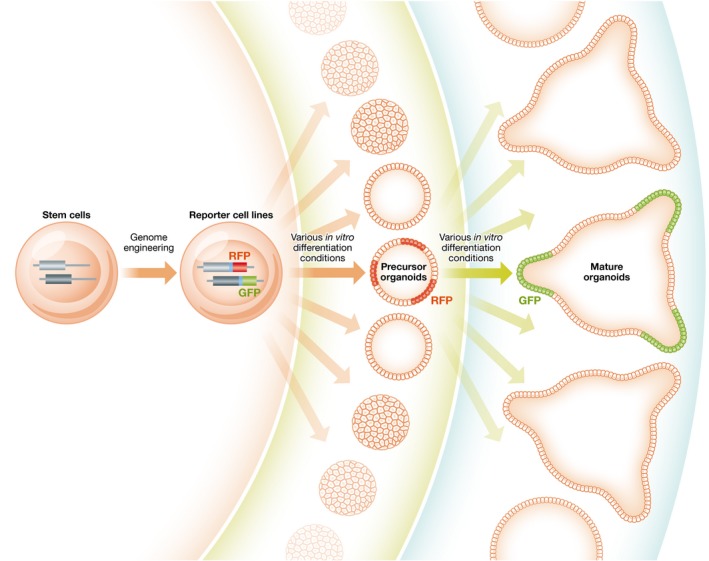
Development and optimization of new organoid generation methods can be greatly accelerated by genomically engineered fluorescence reporter stem cells. In this example, a red fluorescent protein (RFP) gene is integrated at a gene that is expressed in precursor cells. The successfully derived precursor cell‐containing organoids will exhibit red fluorescence, allowing quick identification of optimal deriving conditions. Similarly, via multiplex genome editing in the same stem cell line, a green fluorescence protein (GFP) gene integrated at a gene that is expressed in the mature target cell type will assist protocol development for generating mature organoids. A short 2A “self‐cleaving” peptide sequence (shown in blue) can be placed between the target gene and the fluorescence protein for non‐destructive labeling.
Box 1: In need of answers.
Organoids are typically derived from PSCs through modulation of key signaling pathways by stepwise treatments with small molecules and recombinant proteins. Can such directed differentiation be achieved with CRISPR‐based inducible gene activation/repression instead? Will the CRISPR‐generated organoid models solve the consistency and efficiency issues that many current organoid models are facing?
Various novel methods, such as Scr7, asymmetric ssDNA donors, and cell cycle synchronization, have been recently developed to promote HDR. Can any of these methods be combined to further enhance the HDR efficiency?
Cas9 and its variants are the most popular genome editing tool in academia, but they are not yet available for biopharma and biotech industries due to the ongoing CRISPR patent dispute. Will Cpf1, which has a distinct intellectual property, be the answer for industry users who wish to replace ZFNs and TALENs?
Future perspectives
The past several years have seen a surge in new organoid models, aided by fluorescent reporter stem cell lines which allow prompt identification and confirmation of lineage‐specific cell types arising in 3D culture, thereby greatly facilitating optimization of differentiation protocols. Now with the highly robust means to make genomic modifications at hand, we expect to see a significant increase in different organoid models being created with CRISPR‐based reporter cell lines (Fig 3). Aside from developing methods to generate new types of organoids, reporter cell lines can be used to isolate specific cell types for biochemical or genomic assays (e.g., RNA‐seq). Moreover, reporter cell lines are a must‐have tool to identify rare cell populations in organoids for functional assays. For example, Atoh1‐GFP (created by random genomic insertion of an Atoh1‐GFP construct in mouse ESCs 88) was used to identify sensory hair cells in mouse ESC‐derived inner ear organoids, and mechanoelectrical transduction currents as well as voltage‐gated currents were successful recorded from these GFP‐positive cells 89.
For research purposes, ex vivo CRISPR‐mediated mutant gene correction can be easily achieved by genotyping and selecting for corrected cell lines. For example, a 3‐bp deletion (CFTR F508 del) and a 1‐bp point mutation (DKC1 A386T) have been corrected with HDR in patient‐derived stem cells, and the correct clones were selected for further studies in intestinal organoids 53, 57. However, due to the low efficiency of the HDR pathway, precise gene corrections in vivo for therapeutic purposes are technically challenging. Recently, several groups discovered that deleting a mutant exon entirely through NHEJ, in lieu of correcting disease‐associated mutations via HDR, greatly improved muscle function in mouse models with Duchenne muscular dystrophy (DMD) 90, 91, 92. This exon deletion approach holds great therapeutic promise as NHEJ is more efficient than HDR. Also, patient iPSC‐derived organoids could serve as a beta‐test platform prior to clinical trials to validate the therapeutic potential of this approach for various diseases.
In addition to making changes to the DNA sequences, the CRISPR technology can also be repurposed to regulate gene expression. The nuclease‐deactivated mutant form of Cas9 (dCas9) can be fused with various effector domains. Bringing these effectors to specific genomic loci results in activation or repression of the genes, depending on the type of effectors 93. Coupled with commercially available gRNA libraries, genome‐wide CRISPR‐based gene activation/repression screening should be readily applicable to organoid tissues (Fig 2). For example, gRNA‐coding sequences integrated into the host genomes can be detected by deep‐sequencing 94. By comparing the abundance of gRNA‐coding sequences that lead to activation or repression of their corresponding genes, it is possible to identify a set of genes essential for the specification of certain cell types.
In conclusion, 3D organoid technology is a new and fast‐evolving field in stem cell biology. When combined with powerful programmable nuclease‐based genome engineering, this technology provides exciting opportunities for a wide range of biomedical research, from uncovering mechanisms of human organ development to exploiting future clinical applications.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
We would like to thank Rick Nelson and Emma Longworth‐Mills for their critical reading of the manuscript. This work was supported by an Action on Hearing Loss grant and a National Institutes of Health grant R01DC013294 (to E.H.).
EMBO Reports (2017) 18: 367–376
See the Glossary for abbreviations used in this article.
References
- 1. Clevers H (2016) Modeling development and disease with organoids. Cell 165: 1586–1597 [DOI] [PubMed] [Google Scholar]
- 2. Sasai Y (2013) Cytosystems dynamics in self‐organization of tissue architecture. Nature 493: 318–326 [DOI] [PubMed] [Google Scholar]
- 3. Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y (2011) Self‐organizing optic‐cup morphogenesis in three‐dimensional culture. Nature 472: 51–56 [DOI] [PubMed] [Google Scholar]
- 4. Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y (2012) Self‐formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10: 771–785 [DOI] [PubMed] [Google Scholar]
- 5. Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA (2013) Cerebral organoids model human brain development and microcephaly. Nature 501: 373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pasca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park JY, O'Rourke NA, Nguyen KD et al (2015) Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods 12: 671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y (2013) Self‐organization of axial polarity, inside‐out layer pattern, and species‐specific progenitor dynamics in human ES cell‐derived neocortex. Proc Natl Acad Sci USA 110: 20284–20289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ et al (2009) Single Lgr5 stem cells build crypt‐villus structures in vitro without a mesenchymal niche. Nature 459: 262–265 [DOI] [PubMed] [Google Scholar]
- 9. Ootani A, Li XN, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR et al (2009) Sustained in vitro intestinal epithelial culture within a Wnt‐dependent stem cell niche. Nat Med 15: 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM et al (2011) Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro . Nature 470: 105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fordham RP, Yui S, Hannan NRF, Soendergaard C, Madgwick A, Schweiger PJ, Nielsen OH, Vallier L, Pedersen RA, Nakamura T et al (2013) Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13: 734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Lopes SMCD et al (2015) Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526: 564–568 [DOI] [PubMed] [Google Scholar]
- 13. Freedman BS, Brooks CR, Lam AQ, Fu HX, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Vander Werff R et al (2015) Modelling kidney disease with CRISPR‐mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6: 8715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV (2015) Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33: 1193–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH (2014) Directing human embryonic stem cell differentiation towards a renal lineage generates a self‐organizing kidney. Nat Cell Biol 16: 118–126 [DOI] [PubMed] [Google Scholar]
- 16. Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N et al (2013) Vascularized and functional human liver from an iPSC‐derived organ bud transplant. Nature 499: 481–484 [DOI] [PubMed] [Google Scholar]
- 17. Huch M, Dorrell C, Boj SF, van Es JH, Li VSW, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ et al (2013) In vitro expansion of single Lgr5(+) liver stem cells induced by Wnt‐driven regeneration. Nature 494: 247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MMA, Ellis E, van Wenum M, Fuchs SA, de Ligt J et al (2015) Long‐term culture of genome‐stable bipotent stem cells from adult human liver. Cell 160: 299–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF (2014) Lung stem cell differentiation in mice directed by endothelial cells via a BMP4‐NFATc1‐thrombospondin‐1 axis. Cell 156: 440–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dye BR, Hill DR, Ferguson MAH, Tsai YH, Nagy MS, Dyal R, Wells JM, Mayhew CN, Nattiv R, Klein OD et al (2015) In vitro generation of human pluripotent stem cell derived lung organoids. Elife 4: e05098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Konishi S, Gotoh S, Tateishi K, Yamamoto Y, Korogi Y, Nagasaki T, Matsumoto H, Muro S, Hirai T, Ito I et al (2016) Directed induction of functional multi‐ciliated cells in proximal airway epithelial spheroids from human pluripotent stem cells. Stem Cell Reports 6: 18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koehler KR, Mikosz AM, Molosh AI, Patel D, Hashino E (2013) Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 500: 217–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koehler KR, Hashino E (2014) 3D mouse embryonic stem cell culture for generating inner ear organoids. Nat Protoc 9: 1229–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hockemeyer D, Jaenisch R (2016) Induced pluripotent stem cells meet genome editing. Cell Stem Cell 18: 573–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual‐RNA‐guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smithies O, Gregg RG, Boggs SS, Koralewski MA, Kucherlapati RS (1985) Insertion of DNA sequences into the human chromosomal beta‐globin locus by homologous recombination. Nature 317: 230–234 [DOI] [PubMed] [Google Scholar]
- 27. Thomas KR, Capecchi MR (1987) Site‐directed mutagenesis by gene targeting in mouse embryo‐derived stem‐cells. Cell 51: 503–512 [DOI] [PubMed] [Google Scholar]
- 28. Thomas KR, Folger KR, Capecchi MR (1986) High frequency targeting of genes to specific sites in the mammalian genome. Cell 44: 419–428 [DOI] [PubMed] [Google Scholar]
- 29. Thomas KR, Capecchi MR (1986) Introduction of homologous DNA sequences into mammalian cells induces mutations in the cognate gene. Nature 324: 34–38 [DOI] [PubMed] [Google Scholar]
- 30. Doetschman T, Gregg RG, Maeda N, Hooper ML, Melton DW, Thompson S, Smithies O (1987) Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature 330: 576–578 [DOI] [PubMed] [Google Scholar]
- 31. Capecchi MR (2005) Gene targeting in mice: functional analysis of the mammalian genome for the twenty‐first century. Nat Rev Genet 6: 507–512 [DOI] [PubMed] [Google Scholar]
- 32. Capecchi MR (1989) Altering the genome by homologous recombination. Science 244: 1288–1292 [DOI] [PubMed] [Google Scholar]
- 33. Rudin N, Sugarman E, Haber JE (1989) Genetic and physical analysis of double‐strand break repair and recombination in Saccharomyces cerevisiae . Genetics 122: 519–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rouet P, Smih F, Jasin M (1994) Expression of a site‐specific endonuclease stimulates homologous recombination in mammalian‐cells. Proc Natl Acad Sci USA 91: 6064–6068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ding QR, Regan SN, Xia YL, Oostrom LA, Cowan CA, Musunuru K (2013) Enhanced efficiency of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. Cell Stem Cell 12: 393–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cong L, Ran FA, Cox D, Lin SL, Barretto R, Habib N, Hsu PD, Wu XB, Jiang WY, Marraffini LA et al (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mali P, Yang LH, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM (2013) RNA‐guided human genome engineering via Cas9. Science 339: 823–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang LH, Guell M, Byrne S, Yang JL, De Los Angeles A, Mali P, Aach J, Kim‐Kiselak C, Briggs AW, Rios X et al (2013) Optimization of scarless human stem cell genome editing. Nucleic Acids Res 41: 9049–9061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. He ZY, Proudfoot C, Whitelaw CBA, Lillico SG (2016) Comparison of CRISPR/Cas9 and TALENs on editing an integrated EGFP gene in the genome of HEK293FT cells. Springerplus 5: 814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, Joung JK (2016) High‐fidelity CRISPR‐Cas9 nucleases with no detectable genome‐wide off‐target effects. Nature 529: 490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F (2016) Rationally engineered Cas9 nucleases with improved specificity. Science 351: 84–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A et al (2015) Cpf1 is a single RNA‐guided endonuclease of a class 2 CRISPR‐Cas system. Cell 163: 759–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shmakov S, Abudayyeh OO, Makarova KS, Wolf YI, Gootenberg JS, Semenova E, Minakhin L, Joung J, Konermann S, Severinov K et al (2015) Discovery and functional characterization of diverse class 2 CRISPR‐Cas systems. Mol Cell 60: 385–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sander JD, Joung JK (2014) CRISPR‐Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 32: 347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kraft K, Geuer S, Will AJ, Chan WL, Paliou C, Borschiwer M, Harabula I, Wittler L, Franke M, Ibrahim DM et al (2015) Deletions, inversions, duplications: engineering of structural variants using CRISPR/Cas in mice. Cell Rep 10: 833–839 [DOI] [PubMed] [Google Scholar]
- 46. Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N, Wataya T, Muguruma K, Miyoshi H et al (2011) Self‐formation of functional adenohypophysis in three‐dimensional culture. Nature 480: 57–62 [DOI] [PubMed] [Google Scholar]
- 47. Eiraku M, Watanabe K, Matsuo‐Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasail Y (2008) Self‐organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3: 519–532 [DOI] [PubMed] [Google Scholar]
- 48. Ikeda H, Osakada F, Watanabe K, Mizuseki K, Haraguchi T, Miyoshi H, Kamiya D, Honda Y, Sasai N, Yoshimura N et al (2005) Generation of Rx(+)/Pax(6 + ) neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci USA 102: 11331–11336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wataya T, Ando S, Muguruma K, Ikeda H, Watanabe K, Eiraku M, Kawada M, Takahashi J, Hashimoto N, Sasai Y (2008) Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc Natl Acad Sci USA 105: 11796–11801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ et al (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- 51. Forster R, Chiba K, Schaeffer L, Regalado SG, Lai CS, Gao Q, Kiani S, Farin HF, Clevers H, Cost GJ et al (2014) Human intestinal tissue with adult stem cell properties derived from pluripotent stem cells. Stem Cell Reports 2: 838–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takata N, Sakakura E, Kasukawa T, Sakuma T, Yamamoto T, Sasai Y (2016) Establishment of functional genomics pipeline in epiblast‐like tissue by combining transcriptomic analysis and gene knockdown/knockin/knockout, using RNA interference and CRISPR/Cas9. Hum Gene Ther 27: 436–450 [DOI] [PubMed] [Google Scholar]
- 53. Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK et al (2013) Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13: 653–658 [DOI] [PubMed] [Google Scholar]
- 54. Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter‐de Groot KM, Brandsma AM, de Jong NWM, Bijvelds MJC, Scholte BJ et al (2013) A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19: 939–945 [DOI] [PubMed] [Google Scholar]
- 55. Yui SR, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K et al (2012) Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med 18: 618–623 [DOI] [PubMed] [Google Scholar]
- 56. Bednarski C, Tomczak K, Vom Hovel B, Weber WM, Cathomen T (2016) Targeted integration of a super‐exon into the CFTR locus leads to functional correction of a cystic fibrosis cell line model. PLoS One 11: e0161072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Woo DH, Chen Q, Yang TB, Glineburg MR, Hoge C, Leu NA, Johnson FB, Lengner CJ (2016) Enhancing a Wnt‐telomere feedback loop restores intestinal stem cell function in a human organotypic model of dyskeratosis congenita. Cell Stem Cell 19: 397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, Sachs N, Overmeer RM, Offerhaus GJ, Begthel H et al (2015) Sequential cancer mutations in cultured human intestinal stem cells. Nature 521: 43–47 [DOI] [PubMed] [Google Scholar]
- 59. Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, Watanabe T, Kanai T, Sato T (2015) Modeling colorectal cancer using CRISPR‐Cas9‐mediated engineering of human intestinal organoids. Nat Med 21: 256–262 [DOI] [PubMed] [Google Scholar]
- 60. Yan HH, Lai JC, Ho SL, Leung WK, Law WL, Lee JF, Chan AK, Tsui WY, Chan AS, Lee BC et al (2016) RNF43 germline and somatic mutation in serrated neoplasia pathway and its association with BRAF mutation. Gut doi: 10.1136/gutjnl‐2016‐311849 [DOI] [PubMed] [Google Scholar]
- 61. Gao X, Bali AS, Randell SH, Hogan BLM (2015) GRHL2 coordinates regeneration of a polarized mucociliary epithelium from basal stem cells. J Cell Biol 211: 669–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O et al (2013) DNA targeting specificity of RNA‐guided Cas9 nucleases. Nat Biotechnol 31: 827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD (2013) High‐frequency off‐target mutagenesis induced by CRISPR‐Cas nucleases in human cells. Nat Biotechnol 31: 822–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y et al (2013) Double nicking by RNA‐Guided CRISPR Cas9 for enhanced genome editing specificity (vol 154, pg 1380, 2013). Cell 155: 479–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim D, Kim J, Hur JK, Been KW, Yoon SH, Kim JS (2016) Genome‐wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat Biotechnol 34: 863–868 [DOI] [PubMed] [Google Scholar]
- 66. Kleinstiver BP, Tsai SQ, Prew MS, Nguyen NT, Welch MM, Lopez JM, McCaw ZR, Aryee MJ, Joung JK (2016) Genome‐wide specificities of CRISPR‐Cas Cpf1 nucleases in human cells. Nat Biotechnol 34: 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (2013) Genome engineering using the CRISPR‐Cas9 system. Nat Protoc 8: 2281–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim S, Kim D, Cho SW, Kim J, Kim JS (2014) Highly efficient RNA‐guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res 24: 1012–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li K, Wang G, Andersen T, Zhou PZ, Pu WT (2014) Optimization of genome engineering approaches with the CRISPR/Cas9 system. PLoS One 9: e105779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, Maeder ML, Joung JK, Chen ZY, Liu DR (2015) Cationic lipid‐mediated delivery of proteins enables efficient protein‐based genome editing in vitro and in vivo . Nat Biotechnol 33: 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Onuma K, Ochiai M, Orihashi K, Takahashi M, Imai T, Nakagama H, Hippo Y (2013) Genetic reconstitution of tumorigenesis in primary intestinal cells. Proc Natl Acad Sci USA 110: 11127–11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang N, Zhang HY, Zhang BQ, Liu W, Zhang ZL, Qiao M, Zhang HM, Deng F, Wu NN, Chen X et al (2014) Adenovirus‐mediated efficient gene transfer into cultured three‐dimensional organoids. PLoS One 9: e93608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Koo BK, Stange DE, Sato T, Karthaus W, Farin HF, Huch M, van Es JH, Clevers H (2012) Controlled gene expression in primary Lgr5 organoid cultures. Nat Methods 9: 81–83 [DOI] [PubMed] [Google Scholar]
- 74. Chu VT, Weber T, Wefers B, Wurst W, Sander S, Rajewsky K, Kuhn R (2015) Increasing the efficiency of homology‐directed repair for CRISPR‐Cas9‐induced precise gene editing in mammalian cells. Nat Biotechnol 33: 543–548 [DOI] [PubMed] [Google Scholar]
- 75. Yu C, Liu Y, Ma T, Liu K, Xu S, Zhang Y, Liu H, La Russa M, Xie M, Ding S et al (2015) Small molecules enhance CRISPR genome editing in pluripotent stem cells. Cell Stem Cell 16: 142–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Song J, Yang D, Xu J, Zhu T, Chen YE, Zhang J (2016) RS‐1 enhances CRISPR/Cas9‐ and TALEN‐mediated knock‐in efficiency. Nat Commun 7: 10548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Maruyama T, Dougan SK, Truttmann MC, Bilate AM, Ingram JR, Ploegh HL (2015) Increasing the efficiency of precise genome editing with CRISPR‐Cas9 by inhibition of nonhomologous end joining. Nat Biotechnol 33: 538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Richardson CD, Ray GJ, DeWitt MA, Curie GL, Corn JE (2016) Enhancing homology‐directed genome editing by catalytically active and inactive CRISPR‐Cas9 using asymmetric donor DNA. Nat Biotechnol 34: 339–344 [DOI] [PubMed] [Google Scholar]
- 79. Renaud JB, Boix C, Charpentier M, De Cian A, Cochennec J, Duvernois‐Berthet E, Perrouault L, Tesson L, Edouard J, Thinard R et al (2016) Improved genome editing efficiency and flexibility using modified oligonucleotides with TALEN and CRISPR‐Cas9 nucleases. Cell Rep 14: 2263–2272 [DOI] [PubMed] [Google Scholar]
- 80. Lin S, Staahl B, Alla RK, Doudna JA (2014) Enhanced homology‐directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. Elife 3: e04766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gutschner T, Haemmerle M, Genovese G, Draetta GF, Chin L (2016) Post‐translational regulation of Cas9 during G1 enhances homology‐directed repair. Cell Rep 14: 1555–1566 [DOI] [PubMed] [Google Scholar]
- 82. Howden SE, McColl B, Glaser A, Vadolas J, Petrou S, Little MH, Elefanty AG, Stanley EG (2016) A Cas9 variant for efficient generation of indel‐free knockin or gene‐corrected human pluripotent stem cells. Stem Cell Reports 7: 508–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K et al (2007) A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 25: 681–686 [DOI] [PubMed] [Google Scholar]
- 84. Beers J, Gulbranson DR, George N, Siniscalchi LI, Jones J, Thomson JA, Chen GK (2012) Passaging and colony expansion of human pluripotent stem cells by enzyme‐free dissociation in chemically defined culture conditions. Nat Protoc 7: 2029–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Donnelly MLL, Luke G, Mehrotra A, Li XJ, Hughes LE, Gani D, Ryan MD (2001) Analysis of the aphthovirus 2A/2B polyprotein ‘cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: a putative ribosomal ‘skip’. J Gen Virol 82: 1013–1025 [DOI] [PubMed] [Google Scholar]
- 86. Hockemeyer D, Wang HY, Kiani S, Lai CS, Gao Q, Cassady JP, Cost GJ, Zhang L, Santiago Y, Miller JC et al (2011) Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol 29: 731–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo LQ (2007) A global double‐fluorescent cre reporter mouse. Genesis 45: 593–605 [DOI] [PubMed] [Google Scholar]
- 88. Helms AW, Abney AL, Ben‐Arie N, Zoghbi HY, Johnson JE (2000) Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development 127: 1185–1196 [DOI] [PubMed] [Google Scholar]
- 89. Liu XP, Koehler KR, Mikosz AM, Hashino E, Holt JR (2016) Functional development of mechanosensitive hair cells in stem cell‐derived organoids parallels native vestibular hair cells. Nat Commun 7: 11508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tabebordbar M, Zhu K, Cheng JK, Chew WL, Widrick JJ, Yan WX, Maesner C, Wu EY, Xiao R, Ran FA et al (2016) In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351: 407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, Madhavan S, Pan X, Ran FA, Yan WX et al (2016) In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351: 403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez‐Ortiz E, Bhattacharyya S, Shelton JM, Bassel‐Duby R, Olson EN (2016) Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351: 400–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dominguez AA, Lim WA, Qi LS (2016) Beyond editing: repurposing CRISPR‐Cas9 for precision genome regulation and interrogation. Nat Rev Mol Cell Biol 17: 5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang T, Birsoy K, Hughes NW, Krupczak KM, Post Y, Wei JJ, Lander ES, Sabatini DM (2015) Identification and characterization of essential genes in the human genome. Science 350: 1096–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Davis RP, Ng ES, Costa M, Mossman AK, Sourris K, Elefanty AG, Stanley EG (2008) Targeting a GFP reporter gene to the MIXL1 locus of human embryonic stem cells identifies human primitive streak‐like cells and enables isolation of primitive hematopoietic precursors. Blood 111: 1876–1884 [DOI] [PubMed] [Google Scholar]


