Abstract
Phytochemicals from fruit and vegetables reduce systemic inflammation. This study examined the effects of an encapsulated fruit and vegetable (F&V) juice concentrate on systemic inflammation and other risk factors for chronic disease in overweight and obese adults. A double-blinded, parallel, randomized placebo-controlled trial was conducted in 56 adults aged ≥40 years with a body mass index (BMI) ≥28 kg/m2. Before and after eight weeks daily treatment with six capsules of F&V juice concentrate or placebo, peripheral blood gene expression (microarray, quantitative polymerase chain reaction (qPCR)), plasma tumour necrosis factor (TNF)α (enzyme-linked immunosorbent assay (ELISA)), body composition (Dual-energy X-ray absorptiometry (DEXA)) and lipid profiles were assessed. Following consumption of juice concentrate, total cholesterol, low-density lipoprotein (LDL) cholesterol and plasma TNFα decreased and total lean mass increased, while there was no change in the placebo group. In subjects with high systemic inflammation at baseline (serum C-reactive protein (CRP) ≥3.0 mg/mL) who were supplemented with the F&V juice concentrate (n = 16), these effects were greater, with decreased total cholesterol, LDL cholesterol and plasma TNFα and increased total lean mass; plasma CRP was unchanged by the F&V juice concentrate following both analyses. The expression of several genes involved in lipogenesis, the nuclear factor-κB (NF-κB) and 5′ adenosine monophosphate-activated protein kinase (AMPK) signalling pathways was altered, including phosphomevalonate kinase (PMVK), zinc finger AN1-type containing 5 (ZFAND5) and calcium binding protein 39 (CAB39), respectively. Therefore, F&V juice concentrate improves the metabolic profile, by reducing systemic inflammation and blood lipid profiles and, thus, may be useful in reducing the risk of obesity-induced chronic disease.
Keywords: obesity, systemic Inflammation, fruit and vegetable concentrate, blood lipids
1. Introduction
The global epidemic of obesity, “globesity”, is rapidly becoming a major public health problem in many parts of the world [1]. In the U.S., 78.6 million adults (35% of the population) are obese [2]. Australia is not far behind, with 28% of adults being obese [3]. Obesity develops due to high caloric intake and/or inadequate energy expenditure over time, with genetic susceptibility also likely to have contributed to obesity rates [4]. Overweight and obesity are associated with an epidemic of many chronic diseases, including type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD), stroke, hypertension and certain cancers [1].
Obesity is characterised by chronic, low-grade systemic inflammation, due to the release of pro-inflammatory mediators from adipose tissue [5,6]. Adipose tissue from lean individuals contains small, insulin-sensitive adipocytes, as well as tissue-resident macrophages of the alternatively-activated phenotype (M2) [6,7]. With the development of obesity, adipose tissue becomes characterised by large insulin-resistant adipocytes accompanied by the presence of classically-activated macrophages (M1), which are pro-inflammatory [6,7]. Adipocyte hypertrophy and death, adipose tissue hypoxia and changes in immune cell populations alter adipokine secretory patterns [8]. This process leads to the development of insulin resistance [6].
In obese individuals, adipose tissue produces and secretes adipokines, such as leptin and adiponectin, and pro-inflammatory mediators, including tumour necrosis factor (TNF)α [9]. The production and release of TNFα is the first step in a cascade of pro-inflammatory mediator release [10,11]. TNFα is a mediator in energy metabolism, immune function and apoptosis [12] and increases the activation of neutrophils [13]. C-reactive protein (CRP) is another inflammatory mediator that is produced by both adipocytes and the liver, in response to cytokines, such as TNFα and interleukin (IL)-6 [14]. CRP is also positively associated with the degree of obesity [10,11] and is an independent predictor of myocardial infarction risk, stroke and T2DM [10].
Toll-like receptors (TLRs) and nuclear factor-κB (NF-κB)-associated mechanisms have been proposed as primary molecular pathways mediating adipose tissue inflammation [15]. TLRs activate NFκB [15], a transcription factor and potent inducer of gene transcription of pro-inflammatory cytokines. A plethora of inflammatory pathways is activated in obesity with mechanisms, such as, phosphorylation of mitogen-activated protein kinases (MAPKs) [16] and AMP-activated protein kinase (AMPK) activity modulating adipose tissue inflammation [17]. Peroxisome proliferator-activated receptor-α (PPARα), a nuclear receptor primarily expressed in the liver and potent inducer of fat oxidizing genes, has also been reported to reduce adipose tissue inflammation [8,18].
Systemic inflammation puts obese individuals at increased risk of chronic diseases that have an inflammatory pathology, including CVD, diabetes and cancer [19,20]. Weight loss, achieved via energy restriction, is one approach, which has been shown to correct or improve the overall metabolic profile. Weight loss significantly reduces circulating CRP [21,22], TNFα [21,22,23,24,25] and leptin [22]. Indeed, for every 1 kg of weight lost, there is a corresponding reduction in CRP of 0.13 mg/L [26], accompanied by improvements in systemic insulin resistance [27,28]. However, it is well accepted that achieving and maintaining weight loss is unattainable for some individuals. Hence, alternative approaches for improving the metabolic profile of obese individuals are needed.
Many studies, in both humans and animals, demonstrate that the consumption of dietary bioactive compounds found in fruit and vegetables, such as polyphenols and carotenoids, is able to improve the metabolic profile and reduce the risk of chronic diseases via signalling pathways involving NF-κB, MAPKs and AMPK [8]. A recent study in overweight individuals found that a two-fold increase in fruit and vegetable intake for 16 weeks significantly decreased body mass index (BMI) and supine systolic blood pressure and increased plasma concentrations of the antioxidants α-carotene, β-carotene and lutein [29]. Another study found that supplementing healthy individuals with 500 g of strawberries, rich in vitamin C and anthocyanins, daily for one month improved the lipid profile by decreasing total cholesterol, low-density lipoprotein (LDL) cholesterol and triglycerides, as well as decreasing markers of oxidative stress, including plasma malondialdehyde, urinary 8-OHdG and isoprostane levels [30]. There is also emerging evidence that supplements containing dietary bioactive compounds can provide similar benefits. A recent review, including 18 human trials with a total of 1363 adults, reported that fruit and vegetable concentrate supplements significantly increased serum levels of antioxidants (β-carotene, vitamins C and E), as well as folate. In addition, the supplements reduced homocysteine and markers of oxidative stress [31].
In this study, we hypothesised that obesity-associated inflammation could be suppressed using a fruit and vegetable concentrate supplement. The aim of this study was to examine the effects of an encapsulated fruit and vegetable juice concentrate on systemic inflammation and other risk factors for chronic disease in overweight and obese adults.
2. Materials and Methods
2.1. Study Design
This was a double-blinded, parallel, randomised, placebo-controlled trial in 56 subjects aged ≥40 years and with a BMI ≥28 kg/m2, recruited from March 2014 to September 2014. Subjects were randomised to receive a fruit and vegetable (F&V) concentrate supplement (Juice Plus+® Orchard, Garden and Berry Blends) (n = 28) or placebo (n = 28) for 8 weeks. Subjects commenced a low fruit and vegetable diet (≤3 serves per day of fruit and vegetables combined) two weeks before randomisation as a washout period, and continued this diet for the duration of the study. Subjects were assessed at commencement of the study (Week 0) and again after supplementation (Week 8) at the Clinical Trials Facility at the Hunter Medical Research Institute. At both visits, anthropometric measures, blood pressure, pulse wave velocity and body composition were measured, and blood samples were collected. Subjects were non-smokers and had an absence of or irregular menses if they were female. Subjects were excluded if they were unwilling or unable to limit the intake of fruit and vegetables to no more than 3 servings/day, if they had used dietary or nutritional supplements within the previous 4 weeks, if they were current smokers, were currently participating in a weight management program or actively attempting to lose weight, were currently using any medication known to significantly influence inflammation, had an allergy to the supplement ingredients or chronic excessive alcohol consumption, which is associated with a high risk of chronic health problems, defined as ≥43 standard drinks per week for men and ≥29 drinks per week for women [32]. Unused capsules were collected at the end of the study to determine adherence with the intervention. Participants with adherence of >85% were included in the analysis, determined by the pill count back method.
2.2. Randomization
Subjects were randomly allocated to the treatment or placebo group. Subjects were screened to determine eligibility, and those eligible were assigned a unique study number according to the randomisation schedule. The randomisation schedule was computer generated with blocks of variable size and stratified by BMI and gender. Randomisation was managed by an independent statistician at the Hunter Medical Research Institute. During the treatment phase, both the subjects and the investigators were blinded to the allocation.
2.3. Study Supplement
The F&V concentrate capsules contained a blended fruit, vegetable and berry juice powder concentrate derived from the following: acerola, cherry, apple, bilberry, blackberry, black currant, blueberry, beetroot, broccoli, cabbage, carrot, concord grape, cranberry, elderberry, kale, orange, peach, papaya, parsley, pineapple, raspberry, red currant, spinach and tomato (Juice Plus+® Orchard, Garden and Berry Blends) as described previously [33]. Briefly, the F&V concentrate capsules provided; β-carotene 3.05 mg/day, α-tocopherol 4.8 mg/day, vitamin C 300 mg/day, folate 350 mg/day and polyphenols ~600 mg/day [34]. The placebo capsules were identical in appearance, opaque white capsules containing microcrystalline cellulose. All subjects were instructed to take three capsules twice daily with meals, in agreement with the label use instructions for the retail product, for a total of six capsules per day.
2.4. Ethics
The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Hunter New England Health Human Research Ethics Committee (14/02/19/3.01) and registered with the University of Newcastle Human Research Ethics Committee. Written informed consent was obtained from all subjects. The trial was prospectively registered with the Australian New Zealand Clinical Trials Registry (ANZCTRN12614000079640).
2.5. Anthropometric Measures and Quality of Life
Anthropometric assessments (height and weight) were performed following an overnight fast. Body mass index (BMI) was calculated as body weight (kg)/(height (m))2. Height (metres) was measured using the stretch stature method to 2 decimal places using a wall-mounted stadiometer (Seca 220, Seca, Hamburg, Germany). Body weight (kg) was measured to 1 decimal place with subjects wearing light clothing and without shoes, using calibrated electronic scales. Quality of life was assessed by the short form health survey (SF-36) questionnaire [35]. Waist circumference (WC) was measured to the nearest 0.1 cm at the midpoint between the lower costal edge and the iliac crest, using a non-extensible steel tape (Lufkin W606PM, Apex Tool Group, Sparks, MD, USA).
2.6. Blood Pressure
A blood pressure cuff was placed firmly around the upper arm of the participant, centred over the brachial artery. After resting quietly in a seated position for 10 min, four consecutive blood pressure and heart rate readings were taken at one-minute intervals by a single observer using an electronic vital signs monitor (6000 Series, Welch Allyn, Skaneateles Falls, NY, USA). The first reading was discarded and an average of the remaining measurements recorded for analysis.
2.7. Dual Energy X-ray Absorptiometry
Body composition was measured using a dual energy X-ray absorptiometry (DXA) machine and associated software (DXA Lunar Prodigy; Encore 2007 Version 11.40.004, GE Medical Systems, Madison, WI, USA). Total and regional absolute and percentage of fat and lean mass were calculated (kg).
2.8. Peripheral Blood Biomarkers
Fasting blood was collected into ethylenediaminetetraacetic acid (EDTA) tubes, then centrifuged at 3000× g, at 10 °C for 10 min. Plasma was separated and stored at −80 °C for batched analysis at the end of the study. TNFα, soluble (s)TNF receptor (R) 1 and sTNFR2 were measured by Human Quantikine ELISA Kits (R&D Systems, Minneapolis, Minnesota, USA). Oxidised (ox)-LDL was also analysed by ELISA (Mercodia, Uppsala, Sweden). Serum CRP, total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides and haemoglobin A1c (HbA1c) were measured in peripheral blood by an accredited pathology service (Hunter Area Pathology Service, Newcastle, NSW, Australia).
2.9. Plasma Antioxidant Levels
Plasma carotenoids (α-carotene, β-carotene, lutein, lycopene, β-cryptoxanthin) and plasma α-tocopherol were analysed by reverse phase high-performance liquid chromatography (HPLC) (Agilent LC 1200 System, Agilent Technologies, Santa Clara, CA, USA) using methods established in our laboratory [36,37,38]. Briefly, ethanol:ethyl acetate (1:1) containing internal standards (canthaxanthin and α-tocopherol acetate) and butylated hydroxytoluene were added to the sample. The solution was centrifuged (3000× g, 48 °C, 5 min), and the supernatant was collected; this was repeated 3 times adding ethyl acetate twice and then hexane to the pellet. Ultrapure water was then added to the pooled supernatant fluid, and the mixture was centrifuged. The supernatant was then decanted, and the solvents were evaporated with nitrogen. The sample was then reconstituted in dichloromethane:methanol (1:2). Chromatography was performed on a Hypersil ODS column with a flow rate of 0.3 mL/min, using the mobile phase of acetonitrile:dichloromethane:methanol 0.05% ammonium acetate (85:10:5). Carotenoids and tocopherols were detected at 450 nm and 290, respectively, using a photodiode array.
2.10. Dietary Analysis
Usual dietary intake over the previous 12 months was assessed at Week 0 by food frequency questionnaire using the Dietary Questionnaire for Epidemiological Studies v2 (DQES) (Victorian Cancer Council [39]). A 24-h food recall was also recorded at Week 0 and Week 8 by a dietitian and analysed using nutrient analysis software (FoodWorks Version 7, Xyris Software P/L, Kenmore Hills, QLD, Australia). Serving sizes were those as defined by the National Health and Medical Research Council Australian Dietary Guidelines [40]. Briefly, 75 grams of vegetables are equivalent to one serving and 150 grams of fruit counted as one serving.
2.11. Microarray Analysis
Microarray analysis was conducted in the F&V concentrate group only, to detect differences in gene expression before and after the intervention. Whole blood RNA was extracted from PAXgene tubes using the PAXgene Blood RNA Extraction Kit (Qiagen, Hilden, Germany), as per the manufacturer’s instructions. RNA was quantitated and the quality assessed using Bioanalyser and Quant-iT Ribogreen RNA Reagent (Molecular Probes, Invitrogen, Carlsbad, CA, USA); the average RNA integrity number was 8.07. Whole genome gene expression was determined in 500 ng of blood RNA, which was amplified using the Illumina TotalPrep RNA Amplification Kit (Ambion, Waltham, MA, USA). Seven hundred fifty nanograms of amplified RNA were then hybridised to HumanHT-12 Version 4 Expression BeadChip (Illumina, San Diego, CA, USA). Microarrays were then scanned using the Illumina Bead Station. The microarray primary data used in this study are available at the national centre for biotechnology information (NCBI) Gene Expression Omnibus [41] under Accession Number GSE87454.
2.12. PCR Analysis
Real-time PCR testing was performed on 2 key genes, which demonstrated the greatest fold difference in expression and had biological relevance to the study hypothesis. Two hundred nanograms of extracted RNA (see above) were converted to cDNA using the High Capacity cDNA Reverse Transcription Hit (Applied Biosystems, Foster city, CA, USA). Standard Taqman methods were used. Taqman qPCR primer and probes of target genes were purchased in kit form (Applied Biosystems, Foster city, CA, USA). Target gene expression was measured relative to the housekeeping gene 18S, and the stability value of 18S was calculated as 0.045 using NormFinder [42]. All reactions were carried out using the ABI 7500 Real-Time PCR Machine (Applied Biosystems, Foster city, CA, USA). Taqman Assay IDs are as follows; phosphomevalonate kinase (PMVK) (Hs00559915_m1) and zinc finger AN1-type containing 5 (ZFAND5) (Hs04400278_g1).
2.13. Sample Size and Statistical Analysis
Sample size was determined using the Power and Sample Size calculation programme (PS Version 3.0.43, Vanderbilt University, Nashville, TN, USA) [43]. Based on our previous studies of the expression of multiple genes involved in inflammatory pathways, we required n = 26 subjects per group to have 80% power to detect a mean difference in expression of genes of 20%, with standard deviation (SD) = 25%. Our initial recruitment target of n = 64 allowed for 20% dropouts; however, recruitment was ceased at n = 61, as a very low dropout rate of 8% was achieved.
Per protocol analysis was conducted in 2 stages; Part 1: analysis of the full cohort; Part 2: analysis of the subset of individuals who are at increased risk of chronic disease due to high systemic inflammation levels at baseline, defined as serum CRP ≥3.0 mg/mL. The normality of all data was assessed using the D’Agostino–Pearson omnibus normality test.
Baseline data: demographics, biomarkers and dietary intake were compared using the unpaired Student’s t-test (parametric data) or Mann–Whitney U-test (non-parametric data).
Intervention results: following the intervention, within-group differences were compared using paired t-tests for parametric data or the Wilcoxon rank sum test for non-parametric data. Between-group differences were analysed using analysis of covariance (ANCOVA) to test for differences between treatment groups after adjusting for baseline values. Associations between variables were assessed using Pearson’s correlation for normally distributed variables and Spearman’s rank correlation coefficient for non-parametric data.
Microarray whole genome gene expression analysis: data were exported to RStudio Version 3.2.2 (RStudio, Boston, MA, USA) using Illumina’s Genome Studio 3.0 (Illumina, San Diego, CA, USA) and processed using the R packages lumi [44] and limma [45]. Data were normalised via log transformation and the baseline converted to the median of all samples. Data were filtered based on whether the gene was detected as present in 28 or more samples, as this was half the samples available from the intervention group. Gene profiles were analysed for differential expression by paired t-test for significance and fold change in RStudio Version 3.2.2.
3. Results
3.1. Part 1: Analysis of Full Cohort
3.1.1. Participant Flow
Sixty-one subjects were randomised, with five withdrawing after visit 1 (Figure 1). Of those who withdrew, one was suffering from constipation and fluid retention, one was suffering from diarrhoea and stomach pain, one had difficulty swallowing the study medication, one required anti-inflammatory treatment for a prior knee injury and one no longer wished to participate in the study. Of the 56 subjects who completed the study, 28 were in the F&V concentrate group and 28 were in the placebo group. The mean adherence rate was 97.7%.
Figure 1.
Patient flow consolidated standards of reporting trials (CONSORT) diagram. A total of 395 patients were screened for eligibility to participate; 61 of those who were eligible and wished to participate were randomised to either the fruit and vegetable juice concentrate intervention group or placebo, each taking three capsules twice daily.
3.1.2. Subject Demographics, Dietary Intake and Plasma Nutrient Levels
At baseline, there were no differences between groups in demographics or nutrient intakes (Table 1). Daily intake of fruit and vegetables was also not different at baseline between the groups and was well below the recommended levels (Table 2). Fruit and vegetable intake did not change during the intervention (Table 2). After eight weeks, plasma β-carotene and total carotenoids increased significantly within the F&V concentrate group and compared to the placebo group (Table 2). Plasma lycopene levels decreased in the placebo group compared to the F&V concentrate group.
Table 1.
Baseline subject demographics and nutrient intake. F&V, fruit and vegetables.
| F&V Concentrate (n = 28) | Placebo (n = 28) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Gender (male/female) | 11/17 | 13/15 | 0.788 |
| Age (years) a | 61.4 ± 1.5 | 57.9 ± 1.4 | 0.091 |
| BMI (kg/m2) a | 34.6 ± 0.7 | 37.0 ± 1.3 | 0.271 |
| Smoking Status (never/ex) | 14/14 | 16/12 | 0.592 |
| Nutrient Intake | |||
| Total Energy (KJ/day) b | 7785 (6145, 10,371) | 6986 (5573, 8448) | 0.110 |
| Total fat (g/day) b | 78 (63, 107) | 70 (59, 87) | 0.229 |
| SFA (g/day) b | 32 (25, 45) | 28 (24, 37) | 0.205 |
| PUFA (g/day) a | 12 ± 1 | 12 ± 1 | 0.626 |
| MUFA (g/day) a | 31 ± 2 | 28 ± 2 | 0.286 |
| Protein (g/day) a | 101 ± 7 | 77 (73, 99) | 0.103 |
| Carbohydrates (g/day) a | 202 ± 15 | 177 ± 11 | 0.169 |
| Fibre (g/day) a | 23 ± 2 | 21 ± 1 | 0.285 |
| Calcium (mg/day) a | 965 ± 50 | 857 ± 50 | 0.134 |
| Folate (µg/day) a | 342 ± 17 | 238 ± 14 | 0.046 |
| Iron (mg/day) b | 14 (10, 21) | 11 (9, 14) | 0.103 |
| Magnesium (mg/day) a | 319 ± 21 | 270 ± 14 | 0.060 |
| Niacin (mg/day) b | 22 (17, 29) | 18 (14, 25) | 0.126 |
| Phosphorus (mg/day) a | 1732 ± 118 | 1459 ± 77 | 0.058 |
| Potassium (mg/day) b | 3078 (2312, 3534) | 2409 (2065, 3014) | 0.051 |
| Retinol (µg/day) b | 351 (303, 443) | 340 (277, 435) | 0.651 |
| Riboflavin (mg/day) a | 3 ± 0 | 2 ± 0 | 0.055 |
| Sodium (mg/day) a | 2647 ± 197 | 2476 ± 158 | 0.500 |
| Thiamine (mg/day) a | 2 ± 0 | 1 ± 0 | 0.064 |
| Vitamin C (mg/day) a | 105 ± 11 | 95 ± 8 | 0.417 |
| Vitamin E (mg/day) a | 7 ± 1 | 6 ± 0 | 0.273 |
| Zinc (mg/day) b | 13 (9, 18) | 10 (9, 12) | 0.114 |
| α-carotene (µg/day) b | 811 (416, 1244) | 643 (324, 830) | 0.122 |
| β-carotene (µg/day) a | 4068 ± 387 | 3255 ± 273 | 0.092 |
| β-cryptoxanthin (µg/day) b | 125 (48, 284) | 169 (85, 234) | 0.675 |
| Lutein/zeaxanthin (µg/day) a | 862 ± 78 | 723 ± 65 | 0.177 |
| Lycopene (µg/day) b | 3967 (2602, 6338) | 3807 (2324, 7564) | 0.950 |
BMI: body mass index; SFA: saturated fatty acids; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; a mean ± standard error of the mean (SEM); b median (Q1, Q3).
Table 2.
Fruit and vegetable intake, plasma carotenoids and plasma α-tocopherol before and after the intervention.
| F&V Concentrate (n = 28) | Placebo (n = 28) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 8 Weeks | p-Value * | 0 | 8 Weeks | p-Value * | ANCOVA ǂ p-Value | |
| Fruit and Vegetable Intake (Servings/Day) | |||||||
| Fruit b | 1.00 (0.00, 1.88) |
0.75 (0.00, 1.00) |
0.517 | 1.00 (0.00, 1.00) |
0.50 (0.00, 1.00) |
0.149 | 0.222 |
| Vegetables a | 1.45 ± 0.19 | 1.52 ± 0.18 | 0.784 | 1.34 ± 0.20 | 1.46 ±. 023 | 0.683 | 0.945 |
| Total a | 2.38 ± 0.21 | 2.30 ± 0.16 | 0.788 | 2.14 ± 0.23 | 2.00 ± 0.23 | 0.665 | 0.383 |
| Plasma Carotenoids (mg/L) | |||||||
| Lutein b | 0.43 (0.36, 0.52) |
0.45 (0.34, 0.59) |
0.545 | 0.37 (0.30, 0.52) |
0.37 (0.28, 0.49) |
0.922 | 0.127 |
| β-cryptoxanthin b | 0.09 (0.05, 0.15) |
0.07 (0.05, 0.12) |
0.375 | 0.07 (0.04, 0.16) |
0.06 (0.03, 0.13) |
0.200 | 0.269 |
| Lycopene b | 0.10 (0.08, 0.14) |
0.12 (0.08, 0.18) |
0.213 | 0.11 (0.07, 0.17) |
0.08 (0.04, 0.11) |
0.007 | 0.005 |
| α-carotene b | 0.01 (0.00, 0.15) |
0.01 (0.00, 0.01) |
0.188 | 0.01 (0.00, 0.02) |
0.01 (0.00, 0.01) |
0.018 | 0.095 |
| β-carotene b | 0.11 (0.07, 0.15) |
0.16 (0.11, 0.26) |
<0.001 | 0.07 (0.00, 0.16) |
0.06 (0.00, 0.11) |
0.127 | <0.001 |
| Total Carotenoids b | 0.73 (0.60, 1.05) |
0.85 (0.76, 0.99) |
0.017 | 0.72 (0.51, 1.01) |
0.59 (0.49, 0.80) |
0.019 | <0.001 |
| Plasma α-Tocopherol (mg/L) b | 13.6 (11.3, 15.9) |
14.2 (12.1, 15.4) |
0.849 | 14.3 (11.3, 25.6) |
14.4 (11.6, 17.7) |
0.360 | 0.568 |
* The p-value refers to the change between baseline and eight weeks within each group; ǂ analysis of covariance (ANCOVA) was used to compare the change in the F&V concentrate group to the change in the placebo group; a mean ± standard error of the mean (SEM); b median (Q1, Q3).
3.1.3. Lipid Profile, Glycated Haemoglobin and Systemic Inflammatory Markers
Total cholesterol and LDL cholesterol decreased within the F&V concentrate group only, while triglycerides increased in the placebo group. Comparison of the two groups showed that only the change (Δ) in triglycerides was different between the F&V concentrate and placebo groups. Glycated haemoglobin (HbA1c) was unchanged after the intervention in both the F&V concentrate and placebo groups (Table 3). Plasma TNFα decreased following the F&V concentrate supplementation, and the difference in ΔTNFα between the F&V concentrate and placebo groups approached significance (p = 0.071). There was a negative correlation between Δβ-carotene and ΔTNFα (r = −0.352, p = 0.018). Plasma sTNFR2 concentration increased in the placebo group, while plasma sTNFR1, ox-LDL and CRP were unchanged in the F&V concentrate and placebo groups at Week 8 (Table 3).
Table 3.
Lipid profiles, glycated haemoglobin and systemic inflammation before and after the intervention.
| F&V Concentrate (n = 28) | Placebo (n = 28) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 8 Weeks | p-Value * | 0 | 8 Weeks | p-Value * | ANCOVA ǂ
p-Value |
|
| Total Cholesterol (mmol/L) | 5.70 (5.00, 6.30) |
5.50 (4.83, 6.15) |
0.015 | 5.90 (4.50, 6.70) |
5.60 (4.60, 6.20) |
0.532 | 0.359 |
| LDL Cholesterol (mmol/L) | 3.63 (3.17, 4.36) |
3.50 (2.95, 4.27) |
0.032 | 4.15 (3.03, 4.63) |
3.51 (2.89, 4.10) |
0.089 | 0.904 |
| HDL Cholesterol (mmol/L) | 1.20 (1.10, 1.40) |
1.20 (1.10, 1.30) |
0.815 | 1.20 (1.00, 1.40) |
1.20 (1.00, 1.40) |
0.941 | 0.308 |
| Total/HDL Cholesterol | 4.50 (4.00, 5.10) |
4.55 (4.05, 5.00) |
0.221 | 4.60 (3.90, 5.60) |
4.60 (3.90, 5.50) |
0.587 | 0.634 |
| Triglycerides (mmol/L) | 1.24 (0.86, 1.81) |
1.23 (1.01, 1.67) |
0.344 | 1.36 (0.97, 1.83) |
1.53 (1.06, 1.84) |
0.012 | 0.022 |
| HbA1c (%) | 5.40 (5.20, 5.60) |
5.30 (5.10, 5.50) |
0.570 | 5.40 (5.20, 5.70) |
5.30 (5.10, 5.50) |
0.149 | 0.407 |
| TNFα (pg/mL) | 1.04 (0.87, 1.41) |
1.02 (0.55, 1.41) |
0.037 | 1.07 (0.79, 1.22) |
0.94 (0.82, 1.26) |
0.797 | 0.071 |
| sTNFR1 (pg/mL) | 1140 (1017, 1382) |
1108 (992, 1335) |
0.829 | 1120 (967, 1319) |
1147 (953, 1383) |
0.378 | 0.668 |
| sTNFR2 (pg/mL) | 2479 (2257, 3034) |
2345 (2209, 3130) |
0.467 | 2332 (1986, 2677) |
2547 (2047, 2754) |
0.006 | 0.299 |
| Ox-LDL (mU/L) | 48,620 (41,249, 62,976) |
48,352 (41,937, 57,960) |
0.990 | 50,053 (41,262, 64,661) |
48,911 (43,400, 59,733) |
0.551 | 0.781 |
| CRP (mg/mL) | 3.1 (1.7, 5.1) |
3.9 (1.2, 5.9) |
0.536 | 3.2 (1.7, 5.2) |
2.5 (1.5, 5.4) |
0.769 | 0.301 |
* The p-value refers to the change between baseline and eight weeks within each group; ǂ ANCOVA was used to compare the change in the F&V concentrate group to the change in the placebo group; all data are presented as the median (Q1, Q3). Low-density lipoprotein (LDL); high-density lipoprotein (HDL); haemoglobin A1c (HbA1c); tumour necrosis factor (TNF) α; soluble TNF receptor 1 (sTNFR1); soluble TNF receptor 2 (sTNFR2); oxidized-LDL (ox-LDL); C-reactive protein (CRP).
3.1.4. Body Composition, Blood Pressure and Quality of Life before and after the Intervention
Weight, BMI and waist circumference did not change in both the F&V concentrate and placebo groups (Table 4). Total lean mass increased within the F&V concentrate group, and the change compared to the placebo group approached significance (p = 0.057). Systolic blood pressure was significantly decreased after eight weeks in both the F&V concentrate and placebo groups; however, the difference between groups was not significant. Diastolic blood pressure, pulse and quality of life (SF36) were not changed after the F&V concentrate supplementation or the placebo (Table 4).
Table 4.
Body composition, blood pressure and quality of life before and after the intervention.
| F&V Concentrate (n = 28) | Placebo (n =28) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 8 Weeks | p-Value * | 0 | 8 Weeks | p-Value * | ANCOVA ǂ
p-Value |
|
| BMI (kg/m2) a | 34.6 ± 0.7 | 34.6 ± 0.8 | 0.076 | 37.0 ± 1.3 | 36.1 ± 1.2 | 0.781 | 0.336 |
| Waist circumference (cm) a | 113.7 ± 2.2 | 112.5 ± 2.3 | 0.649 | 116.6 ± 2.8 | 116.0 ± 2.9 | 0.863 | 0.699 |
| Body Composition | |||||||
| Total Body Fat (kg) a | 42.6 ± 1.7 | 42.7 ± 1.7 | 0.728 | 45.1 ± 2.4 | 44.8 ± 2.5 | 0.231 | 0.618 |
| % Body Fat a | 46.7 ± 1.5 | 46.4 ± 1.5 | 0.168 | 45.0 ± 1.5 | 44.4 ± 1.6 | 0.384 | 0.134 |
| Total Lean Mass (kg) a | 49.3 ± 2.4 | 50.0 ± 2.4 | 0.018 | 54.4 ± 2.0 | 55.0 ± 2.1 | 0.836 | 0.057 |
| % Lean Mass a | 51.4 ± 1.4 | 51.9 ± 1.4 | 0.078 | 53.5 ± 1.5 | 54.0 ± 1.5 | 0.560 | 0.096 |
| Android: Gynoid Fat Ratio a | 1.2 ± 0.0 | 1.2 ± 0.0 | 0.499 | 1.1 ± 0.0 | 1.1 ± 0.0 | 0.270 | 0.649 |
| Systolic BP (mmHg) a | 131.7 ± 2.3 | 125.9 ± 1.9 | 0.005 | 136.7 ± 2.8 | 132.4 ± 2.3 | 0.037 | 0.110 |
| Diastolic BP (mmHg) a | 80.6 ± 1.2 | 79.0 ± 1.0 | 0.097 | 82.6 ± 1.0 | 82.3 ± 1.0 | 0.665 | 0.055 |
| Pulse (BPM) a | 66.1 ± 1.7 | 68.6 ± 1.7 | 0.175 | 67.8 ± 1.7 | 66.4 ± 1.6 | 0.709 | 0.178 |
| Quality of Life (SF-36) | |||||||
| Physical a | 47.2 ± 1.6 | 47.8 ± 1.5 | 0.788 | 46.6 ± 1.9 | 49.2 ± 1.6 | 0.678 | 0.682 |
| Mental b | 51.6 (43.6, 58.7) |
54.1 (48.3, 59.1) |
0.211 | 56.4 (40.1, 59.8) |
54.0 (45.3, 58.1) |
0.897 | 0.290 |
BMI: body mass index; BP: blood pressure; * the p-value refers to the change between baseline and eight weeks within each group; ǂ ANCOVA was used to compare the change in the F&V concentrate group to the change in the placebo group; a mean ± SEM; b median (Q1, Q3). 36 item short form health survey (SF-36).
3.2. Part 2: Analysis of a Subgroup with High Baseline CRP (≥3.0 mg/mL)
3.2.1. Subject Demographics, Dietary Intake and Plasma Nutrient Levels
To determine whether the effects of the F&V concentrate supplement were more evident in subjects with elevated systemic inflammation, a subgroup analysis was performed in subjects with baseline CRP ≥3.0 mg/mL. By using this criteria, the number of participants in the F&V concentrate and placebo groups was reduced to n = 17 and n = 15, respectively. The baseline demographics and dietary intakes of these groups were analysed (Table 5) and found to be similar, except for BMI, which was higher in the placebo group. Baseline daily intake of fruit and vegetables was not different between the groups and was well below recommended levels. Fruit and vegetable intake did not change during the intervention (Table 6). Plasma β-carotene and total carotenoids were both increased within the F&V concentrate group compared to the placebo group at Week 8. Lycopene was significantly decreased within the placebo group compared to the F&V concentrate group (Table 6).
Table 5.
Baseline subject demographics and nutrient intake of subjects with high baseline CRP (≥3.0 mg/mL).
| F&V Concentrate (n = 16) | Placebo (n = 15) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Gender (male/female) | 3/13 | 6/9 | 0.252 |
| BMI (kg/m2) a | 35.6 ± 1.1 | 40.2 ± 1.5 | 0.016 |
| Age (years) a | 60.8 ± 1.5 | 56.6 ± 2.0 | 0.097 |
| Smoking Status (never/ex) | 9/8 | 8/7 | 1.000 |
| Nutrient Intake | |||
| Total Energy (KJ/day) a | 7884 ± 792 | 7569 ± 625 | 0.758 |
| Total fat (g/day) a | 83 ± 9 | 81 ± 7 | 0.857 |
| SFA (g/day) b | 30 (24, 45) | 28 (24, 43) | 0.688 |
| PUFA (g/day) a | 11 ± 1 | 12 ± 1 | 0.609 |
| MUFA (g/day) a | 30 ± 3 | 30 ± 3 | >0.999 |
| Protein (g/day) b | 94 (67, 120) | 77 (73, 110) | 0.858 |
| Carbohydrates (g/day) a | 190 ± 20 | 181 ± 16 | 0.738 |
| Fibre (g/day) a | 22 ± 2 | 20 ± 2 | 0.429 |
| Calcium (mg/day) a | 926 ± 64 | 871 ± 67 | 0.559 |
| Folate (µg/day) b | 267 (194, 329) | 213 (189, 294) | 0.418 |
| Iron (mg/day) b | 12 (9, 20) | 10 (9, 14) | 0.509 |
| Magnesium (mg/day) a | 312 ± 31 | 271 ± 20 | 0.271 |
| Niacin (mg/day) b | 20 (14, 25) | 18 (14, 26) | 0.800 |
| Phosphorus (mg/day) b | 1500 (1250, 2002) | 1357 (1140, 1859) | 0.377 |
| Potassium (mg/day) b | 2847 (2090, 3609) | 2501 (2081, 2896) | 0.533 |
| Retinol (µg/day) b | 342 (254, 507) | 376 (311, 473) | 0.688 |
| Riboflavin (mg/day) b | 2 (2, 3) | 2 (2, 3) | 0.397 |
| Sodium (mg/day) b | 2177 (1797, 2738) | 2286 (1845, 3623) | 0.463 |
| Thiamine (mg/day) b | 2 (1, 2) | 1 (1, 2) | 0.558 |
| Vitamin C (mg/day) b | 88 (63, 111) | 90 (57, 111) | 0.883 |
| Vitamin E (mg/day) a | 7 ± 1 | 6 ± 1 | 0.702 |
| Zinc (mg/day) b | 13 (8, 16) | 10 (9, 14) | 0.716 |
| α-Carotene (µg/day) a | 916 ± 128 | 684 ± 121 | 0.198 |
| β-Carotene (µg/day) a | 351 ± 36 | 3350 ± 412 | 0.352 |
| β-Cryptoxanthin (µg/day) b | 105 (48, 193) | 164 (89, 225) | 0.222 |
| Lutein/zeaxanthin (µg/day) b | 803 (584, 903) | 744 (332, 1134) | 0.509 |
| Lycopene (µg/day) b | 3523 (2409, 4931) | 2829 (2040, 9169) | 0.887 |
BMI: body mass index; SFA: saturated fatty acids; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; a mean ± SEM; b median (Q1, Q3).
Table 6.
Fruit and vegetable intake, plasma carotenoids and plasma α-tocopherol before and after the intervention in subjects with high baseline CRP (≥3.0 mg/mL).
| F&V Concentrate (n = 16) | Placebo (n = 15) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 8 Weeks | p-Value * | 0 | 8 Weeks | p-Value * | ANCOVA ǂ p-Value |
|
| Fruit and Vegetable Intake (Servings/Day) | |||||||
| Fruit b | 1.00 (0.00, 1.00) |
0.75 (0.00, 2.00) |
0.899 | 0.75 (0.00, 1.38) |
0.50 (0.00, 1.00) |
0.475 | 0.283 |
| Vegetables b | 1.25 (0.63, 2.00) |
1.75 (1.00, 2.00) |
0.606 | 1.00 (0.13, 1.88) |
1.00 (0.50, 2.00) |
0.435 | 0.893 |
| Total a | 2.13 ± 0.26 | 2.38 ± 0.23 | 0.478 | 1.78 ± 0.25 | 2.00 ± 0.32 | 0.596 | 0.475 |
| Plasma Carotenoids (mg/L) | |||||||
| Lutein b | 0.41 (0.37, 0.47) |
0.45 (0.33, 0.55) |
0.519 | 0.36 (0.29, 0.42) |
0.35 (0.28, 0.40) |
0.855 | 0.204 |
| β-cryptoxanthin b | 0.08 (0.05, 0.13) |
0.07 (0.05, 0.12) |
0.900 | 0.07 (0.02, 0.13) |
0.06 (0.02, 0.13) |
0.500 | 0.491 |
| Lycopene b | 0.10 (0.08, 0.15) |
0.12 (0.08, 0.14) |
0.850 | 0.11 (0.09, 0.19) |
0.10 (0.03, 0.12) |
0.003 | 0.026 |
| α-carotene b | 0.01 (0.00, 0.02) |
0.01 (0.00, 0.01) |
0.148 | 0.01 (0.00, 0.02) |
0.00 (0.00, 0.02) |
0.059 | 0.410 |
| β-carotene b | 0.11 (0.06, 0.14) |
0.15 (0.10, 0.26) |
0.003 | 0.09 (0.00, 0.17) |
0.00 (0.00, 0.15) |
0.393 | 0.002 |
| Total Carotenoids b | 0.72 (0.60, 0.88) |
0.89 (0.61, 0.98) |
0.035 | 0.72 (0.44, 0.94) |
0.54 (0.48, 0.80) |
0.066 | <0.0001 |
| Plasma α-Tocopherol (mg/L) b | 14.1 (12.5, 16.3) |
14.8 (13.6, 16.7) |
0.677 | 14.5 (12.8, 17.2) |
14.5 (11.8, 17.3) |
0.761 | 0.826 |
* The p-value refers to the change between baseline and eight weeks within each group; ǂ ANCOVA was used to compare the change in the F&V concentrate group to the change in the placebo group; a mean ± SEM; b median (Q1, Q3).
3.2.2. Blood Lipids, Glycated Haemoglobin and Systemic Inflammatory Markers
Cholesterol and LDL cholesterol were significantly decreased within the F&V concentrate group, but were not different compared to the placebo group (Table 7). Triglycerides, HDL cholesterol, total cholesterol/HDL ratio and HbA1c were unchanged within either the F&V concentrate or placebo groups. Plasma levels of TNFα were significantly decreased within the F&V concentrate group and also compared to the placebo group (Table 7). The soluble receptors, sTNFR1 and sTNFR2, were significantly different between groups following the intervention, due to an increase in concentration in the placebo group. Oxidised-LDL and CRP were unchanged in both groups (Table 7).
Table 7.
Blood lipids, glycated haemoglobin and systemic inflammation before and after the intervention in subjects with high baseline CRP (≥3.0 mg/mL).
| F&V Concentrate (n = 16) | Placebo (n = 15) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 8 Weeks | p-Value * | 0 | 8 Weeks | p-Value * | ANCOVA ǂ
p-Value |
|
| Total Cholesterol (mmol/L) | 6.10 (5.30, 6.45) |
5.65 (5.40, 6.20) |
0.016 | 6.30 (4.70, 6.70) |
5.95 (4.95, 6.43) |
0.538 | 0.549 |
| LDL Cholesterol (mmol/L) | 3.98 (3.37, 4.48) |
3.82 (3.34, 4.33) |
0.016 | 4.21 (2.87, 4.67) |
3.91 (3.22, 4.50) |
0.279 | 0.854 |
| HDL Cholesterol (mmol/L) | 1.30 (1.10, 1.40) |
1.20 (1.13, 1.38) |
0.656 | 1.20 (1.00, 1.40) |
1.20 (1.00, 1.40) |
0.941 | 0.804 |
| Total/HDL Cholesterol | 4.90 (3.95, 5.40) |
4.80 (4.40, 5.08) |
0.324 | 5.20 (4.30, 5.90) |
4.80 (3.95, 5.73) |
0.398 | 0.944 |
| Triglycerides (mmol/L) | 1.30 (0.98, 2.24) |
1.41 (1.17, 1.82) |
0.520 | 1.58 (1.16, 1.85) |
1.77 (1.09, 1.91) |
0.268 | 0.205 |
| HbA1c (mmol/mol) | 36.0 (32.5, 38.0) |
34.0 (33.0, 37.0) |
0.197 | 36.5 (33.8, 40.3) |
36.0 (33.0, 37.5) |
0.783 | 0.662 |
| HbA1c (%) | 5.40 (5.15, 5.60) |
5.30 (5.13, 5.48) |
0.128 | 5.45 (5.28, 5.83) |
5.40 (5.20, 5.55) |
0.629 | 0.284 |
| TNFα (pg/mL) | 1.13 (0.95, 1.52) |
0.95 (0.70, 1.40) |
0.007 | 1.07 (0.79, 1.30) |
0.96 (0.87, 1.26) |
0.632 | 0.035 |
| sTNFR1 (pg/mL) | 1140 (980, 1491) |
1143 (988, 1414) |
0.324 | 1294 (1047, 1371) |
1347 (1176, 1442) |
0.212 | 0.031 |
| sTNFR2 (pg/mL) | 2698 (2371, 3152) |
2389 (2205, 3005) |
0.198 | 2554 (2313, 2881) |
2711 (2532, 2998) |
0.002 | 0.009 |
| Ox-LDL (mU/L) | 48,833 (43,191, 63,536) |
50,719 (42,229, 61,437) |
0.357 | 52,683 (39,688, 64,661) |
45,559 (43,009, 62,543) |
0.536 | 0.852 |
| CRP (mg/mL) | 4.8 (3.6, 9.4) |
5.2 (3.8, 6.6) |
0.930 | 5.0 (4.1, 7.4) |
5.4 (3.8, 7.1) |
0.820 | 0.861 |
* The p-value refers to the change between baseline and eight weeks within each group; ǂ ANCOVA was used to compare the change in the F&V concentrate group to the change in the placebo group; all data are presented as the median (Q1, Q3).
3.2.3. Body Composition, Blood Pressure and Quality of Life Before and after the Intervention
At the completion of the trial, weight, BMI, waist circumference, blood pressure and quality of life were unchanged in both groups (Table 8). Total lean mass and pulse rate significantly increased within the F&V concentrate group, but were not different compared to the placebo group.
Table 8.
Body composition, blood pressure and quality of life before and after the intervention in subjects with high baseline CRP (≥3.0 mg/mL).
| F&V Concentrate (n = 16) | Placebo (n = 15) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 8 Weeks | p-Value * | 0 | 8 Weeks | p-Value * | ANCOVA ǂ
p-Value |
|
| BMI (kg/m2) a | 35.6 ± 1.1 | 35.5 ± 1.2 | 0.263 | 40.2 ± 1.5 | 40.3 ± 1.5 | 0.721 | 0.531 |
| Waist circumference (cm) a | 113.8 ± 3.4 | 113.5 ± 3.7 | 0.480 | 123.4 ± 3.9 | 124.3 ± 3.6 | 0.806 | 0.939 |
| Body Composition | |||||||
| Total Body Fat (kg) a | 45.5 ± 2.3 | 44.6 ± 2.4 | 0.362 | 52.9 ± 2.7 | 53.2 ± 2.8 | 0.345 | 0.421 |
| % Body Fat a | 49.9 ± 1.7 | 48.9 ± 1.8 | 0.062 | 47.7 ± 1.7 | 47.8 ± 1.7 | 0.615 | 0.069 |
| Total Lean Mass (kg) a | 46.1 ± 3.0 | 46.9 ± 3.2 | 0.049 | 58.1 ± 3.1 | 58.2 ± 3.2 | 0.919 | 0.221 |
| % Lean Mass a | 48.5 ± 1.6 | 49.5 ± 1.7 | 0.077 | 50.7 ± 1.6 | 50.7 ± 1.6 | 0.811 | 0.115 |
| Android: Gynoid Fat Ratio a | 1.1 ± 0.0 | 1.1 ± 0.0 | 0.892 | 1.1 ± 0.0 | 1.1 ± 0.0 | 0.951 | 0.996 |
| Systolic BP (mmHg) a | 131.8 ± 3.5 | 129.1 ± 2.8 | 0.243 | 140.1 ± 3.3 | 134.0 ± 3.4 | 0.052 | 0.953 |
| Diastolic BP (mmHg) a | 80.3 ± 1.8 | 80.0 ± 1.1 | 0.999 | 84.7 ± 1.6 | 83.5 ± 1.2 | 0.371 | 0.306 |
| Pulse (BPM) b | 67.0 (61.5, 71.8) |
71.0 (67.0, 78.0) |
0.021 | 68.0 (63.0, 71.0) |
69.0 (62.0, 72.0) |
0.988 | 0.138 |
| Quality of Life (SF-36) | |||||||
| Physical a | 45.9 ± 2.4 | 46.4 ± 2.0 | 0.862 | 44.4 ± 2.4 | 44.4 ± 2.1 | 0.843 | 0.665 |
| Mental a | 48.8 ± 3.3 | 53.7 ± 2.3 | 0.076 | 48.6 ± 3.4 | 49.6 ± 2.9 | 0.596 | 0.259 |
BMI: body mass index; BP: blood pressure; * the p-value refers to the change between baseline and eight weeks within each group; ǂ ANCOVA was used to compare the change in the F&V concentrate group to the change in the placebo group; a mean ± SEM; b median (Q1, Q3).
3.2.4. Peripheral Blood Gene Expression
In the subgroup of participants with high baseline CRP (n = 16), microarray analysis revealed that 1632 genes were differentially expressed after supplementation with the F&V concentrate. One thousand one hundred forty six genes were upregulated, while 486 genes were downregulated, with the highest absolute fold change being 1.44 and the lowest fold change being 1.03.
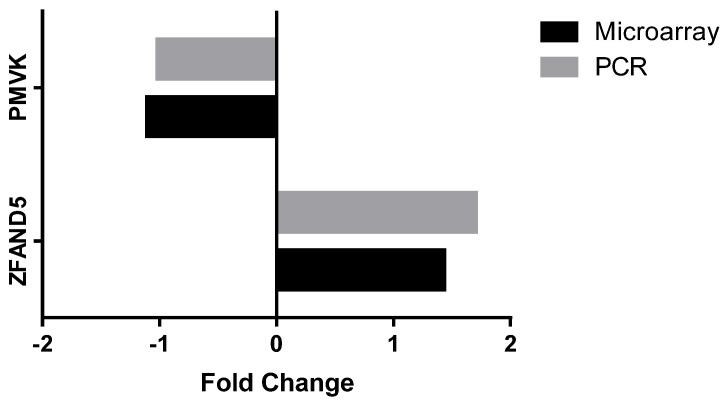
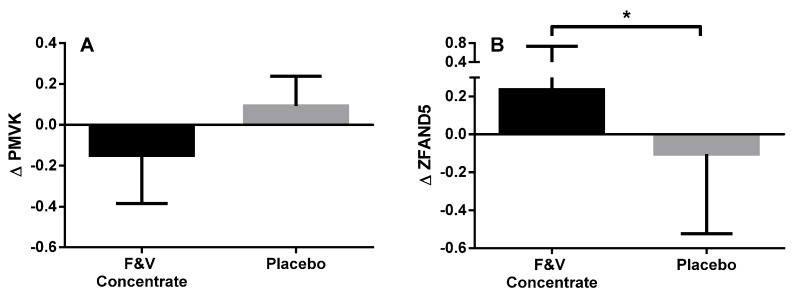
From the genes that were significantly differentially expressed, several genes were identified that are involved in biologically-relevant signalling pathways, including: lipogenesis, NF-kB and AMPK pathways (Table 9). Pathways analysis was also performed on genes that were differentially expressed with a p < 0.05, using gene ontology tool to help explain relationships (GATHER) [46]. The upregulated genes were found to be involved in five different pathways, those being: the insulin signalling pathway, neuroactive ligand-receptor interaction, adherens junction, regulation of the actin cytoskeleton and the wnt signalling pathway. There were 18 upregulated genes involved in the insulin signalling pathway: calmodulin 1 (CALM1), casitas B-lineage lymphoma (CBL), crk-like protein (CRKL), hexokinase 2 (HK2), insulin receptor substrate 2 (IRS2), mitogen-activated protein kinase kinase 1 (MAP2K1), mitogen-activated protein kinase 6 (MAPK6), phosphodiesterase 3B (PDE3B), phosphorylase kinase regulatory subunit beta (PHKB), phosphatidylinositol-4, 5-biphosphate 3-kinase catalytic subunit beta isoform (PIK3CB), protein phosphatase 1 catalytic subunit gamma (PPP1CC), 5′-AMP-activated protein kinase subunit gamma-2 (PRKAG2), cAMP-dependent protein kinase type I-alpha regulatory subunit (PRKAR1A), tyrosine-protein phosphatase non-receptor type 1 (PTPN1), glycogen phosphorylase, liver form (PYGL), raf-1 proto-oncogene, serine/threonine kinase (RAF1), ras homolog enriched in brain (RHEB), ras-related protein R-Ras2 (RRAS2). Two gene targets for qPCR analysis were chosen to validate the microarray data; ZFAND5 and PMVK. Figure 2 shows that ZFAND5 and PMVK mRNA expression had similar fold changes following the intervention when assessed using both microarray and qPCR. As seen in the microarray analysis, gene expression of ZFAND5 as determined by PCR analysis was also significantly upregulated following supplementation with the F&V concentrate. While PMVK expression was downregulated in the microarray analysis, the downregulated expression in the PCR analysis was not statistically significant (Figure 3).
Table 9.
Microarray analysis: biologically-relevant genes that were differentially regulated following F&V concentrate supplementation in subjects with high baseline CRP (≥3.0 mg/mL).
| Gene | Gene Name | Gene Function | Fold Change | p-Value |
|---|---|---|---|---|
| Lipogenesis | ||||
| PMVK | Phosphomevalonate kinase | Catalyses the conversion of mevalonate 5-phosphate into mevalonate 5-diphosphate, the fifth reaction of the cholesterol biosynthetic pathway | −1.102 | 0.005 |
| FDFT1 | Farnesyl-diphosphate farnesyltransferase 1 | First specific enzyme in cholesterol biosynthesis, catalyses dimerization farnesyl diphosphate to form squalene | 1.08 | 0.014 |
| FDPS | Farnesyl diphosphate synthase | Catalyses the production of intermediates in cholesterol biosynthesis | −1.06 | 0.034 |
| NF-κB | ||||
| ZFAND5 | Zinc finger, AN1-type domain 5 | Inhibits TNF, IL-1 and TLR-induced NF-κB activation | 1.438 | 0.005 |
| ATM | ATM serine/threonine kinase | Regulates tumour suppressor and DNA repair genes | 1.242 | 0.006 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 | Key enzyme in prostaglandin biosynthesis | 1.199 | 0.042 |
| PARP1 | Poly (adenosine diphosphate (ADP)-ribose) polymerase 1 | Differentiation, proliferation and tumour transformation | 1.194 | 0.008 |
| PLCG2 | Phospholipase C, gamma 2 (phosphatidylinositol-specific) | Catalyses the conversion of 1-phosphatidyl-1D-myo-inositol 4,5-bisphosphate to 1D-myo-inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), important second messenger molecules | 1.161 | 0.019 |
| BCL2 | B-cell chronic lymphoid leukemia (CLL)/lymphoma 2 | Important anti-apoptotic protein, classified as an oncogene | 1.141 | 0.003 |
| TLR4 | Toll-like receptor 4 | Pattern recognition receptor implicated in LPS signal transduction | 1.118 | 0.017 |
| MAP3K7 | Mitogen-activated protein kinase kinase kinase 7 | Forms complex with Transforming growth factor beta activated kinase (TAB)-1 or TAB2 which is required for NF-κB | 1.112 | 0.042 |
| PRKCQ | Protein kinase C, theta | Important for T-cell activation and NF-κB transcription factor activation | 1.082 | 0.020 |
| PRKCB | Protein kinase C, beta | Phosphorylates protein targets involved in B cell activation, apoptosis, endothelial cell proliferation, intestinal sugar absorption | 1.072 | 0.048 |
| MALT1 | Mucosa-associated lymphoid tissue lymphoma translocation protein 1 | Proteolytic activity, many targets involved in regulation of inflammation | 1.067 | 0.028 |
| TNFAIP3 | Tumour necrosis factor-induced protein 3 | Induced by TNF, inhibits NF-κB and TNF-mediated apoptosis | 1.059 | 0.042 |
| AMPK | ||||
| CAB39 | Calcium binding protein 39 | Stimulates STK11 activity, which is an upstream kinase of AMPK | 1.418 | 0.004 |
| RAB10 | RAB10; RAS oncogene family | Regulates intracellular vesicle trafficking | 1.279 | 0.020 |
| SIRT1 | Sirtuin 1 | Involved in regulating AMPK expression | 1.152 | 0.019 |
| IRS2 | Insulin receptor substrate 2 | Mediates effects of insulin, insulin-like growth factor 1, and cytokines | 1.137 | 0.009 |
| RHEB | Ras homolog enriched in brain | involved in the mechanistic targeting of rapamycin (mTOR) pathway and the regulation of the cell cycle | 1.119 | 0.048 |
| MAP3K7 | Mitogen-activated protein kinase kinase kinase 7 | Forms complex with TAB1 or TAB2, which is required for NF-κB | 1.112 | 0.042 |
Figure 2.
Validation of microarray analysis; fold changes in gene expression following the intervention as determined by microarray versus quantitative polymerase chain reaction (qPCR). Zinc finger, AN1-type domain 5 (ZFAND5); Phosphomevalonate kinase (PMVK).
Figure 3.
Change in peripheral blood mRNA expression of PMVK (A) and ZFAND5 (B) at baseline compared to Week 8 in the fruit and vegetable juice concentrate (F&V concentrate) and placebo groups; measured using qPCR; the data presented are the median (IQR); the statistical test used was the Mann–Whitney test; * p < 0.05 versus baseline.
4. Discussion
In this study, we found that in older subjects who are overweight or obese, F&V concentrate supplementation led to decreases in total cholesterol, LDL cholesterol, TNFα and systolic blood pressure. We conducted a secondary analysis of the subgroup of participants who had high systemic inflammation at baseline, as we hypothesised that these participants were more likely to obtain an improvement in metabolic health as a result of the supplement. Similar results were observed in the subgroup analysis, with F&V concentrate supplementation leading to a decrease in total cholesterol, LDL cholesterol and TNFα, and indeed, the size of the effects was greater. A number of genes associated with lipogenesis, AMPK and NF-κB signalling pathways were differentially expressed following the intervention. Pathways analysis also revealed that several genes involved in the insulin signalling pathway were upregulated in the subgroup of participants with high baseline CRP.
Total and LDL cholesterol were both found to be significantly decreased after the F&V concentrate intervention with greater changes seen in the subgroup with high baseline CRP. A recent Cochrane review used meta-analyses to demonstrate that increased fruit and vegetable consumption can reduce cholesterol levels [47]. Included studies achieved an average increase in fruit and vegetable intake of 1.88 (95% confidence interval (CI): 1.07 to 2.70) servings per day and total blood cholesterol levels reduced by 0.11 mmol/L (95% CI: −0.19 to −0.03). We observed a much greater decrease in total cholesterol in the high baseline CRP subgroup in this study. Importantly, the size of the decreases in total and LDL cholesterol that were observed in this study are substantial and clinically important. In the full cohort analysis, we observed a 0.2 mmol/L (3.5%) reduction in total cholesterol, which is estimated to be equivalent to a weight loss of 4 kg and an 8% to 9% reduction in CVD risk [48,49]. We also observed a 0.13 mmol/L (3.5%) decrease in LDL cholesterol, estimated to be equivalent to a 6.5 kg weight loss and 5% reduction in CVD risk [48,49]. In subjects who had elevated systemic inflammation at baseline, we observed a 0.45 mmol/L (7.4%) reduction in total cholesterol, which is estimated to be equivalent to a weight loss of 9 kg and an 18% to 19% reduction in CVD risk [48,49]. In this group, we also observed a 0.16 mmol/L (4.0%) decrease in LDL cholesterol, estimated to be equivalent to an 8 kg weight loss and a 4% reduction in CVD risk [48,49].
Serum triglycerides were found to be unchanged by the F&V concentrate in both the full cohort analysis and the high baseline CRP subgroup analysis. However, we did observe an inverse correlation between Δβ-carotene and Δtriglycerides. There is previous evidence that F&V concentrate supplementation and polyphenol-rich diets can lower triglyceride levels in overweight boys with elevated baseline triglycerides [50] and in overweight or obese adults [51]. It is likely that F&V concentrate supplementation would have the greatest effect in individuals with elevated triglycerides, which may explain the lack of change in this study, as the majority of subjects had normal triglyceride levels at baseline. Interestingly, in the placebo group, triglycerides significantly increased in the full cohort analysis after the intervention. This may be related to the decrease in total carotenoids that was observed in the placebo group after eight weeks, which indicates that bioactive compounds continued to washout during the intervention period. This may have also masked the effect of the F&V concentrate, if dietary sources of carotenoids and other phytochemicals were continuing to washout during the intervention phase.
Microarray analysis revealed differential expression of several key genes involved in lipogenesis following the F&V concentrate supplementation. PMVK (or phosphomevalonate kinase) was found to be significantly downregulated. PMVK catalyses conversion of mevalonate 5-phosphate into mevalonate 5-diphosphate, which is the fifth reaction of the cholesterol biosynthetic pathway [52]. Similarly, gene expression of farnesyl diphosphate synthase (FDPS) was downregulated. This may also lead to reduced cholesterol production as the gene encodes an enzyme that catalyses the production of farnesyl diphosphate (FPP), a key biosynthetic intermediate used in the formation of cholesterol [53]. Interestingly, a recent study has shown that supplementation with the same F&V concentrate used in this study led to increased levels of circulating fatty acid binding protein 4 (FABP4), possibly via modulation of PPAR activity, resulting in altered lipid metabolism and accumulation [54]. Hence, there are several mechanisms by which the F&V concentrate may have led to the reduced blood lipid levels that were observed in this study.
Following intervention with the F&V concentrate, there was a small, but statistically-significant reduction in TNFα in the full cohort analysis, with a much greater reduction seen in the high baseline CRP subgroup analysis. TNFα is a cell signalling protein involved in systemic inflammation, with a primary role of regulating immune cells. Previous studies have shown that increasing fruit and vegetable intake reduces systemic inflammation, specifically CRP concentration [55,56], while a previous study of F&V concentrate supplementation showed that monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein 1b (MIP-1b) and regulated on activation, normal T cell expressed and secreted (RANTES) were reduced [57]. The F&V concentrate used in this study is rich in β-carotene, which has been shown to reduce inflammatory mediator production, including TNFα [58,59]. For example, a study in mice found that β-carotene reduced the inflammatory response to LPS, including TNFα production [59]. Another study in a non-alcoholic fatty liver model in rats found that TNFα levels were markedly ameliorated with the administration of β-carotene [58]. Hence, the potential for carotenoids to reduce inflammation has previously been demonstrated.
TNFR1 and TNFR2 are the receptors to TNFα. Upon binding, inflammation is induced via various pathways, including activation of NF-kB and MAPK pathways. In this study, TNFR2 increased in the placebo group, in both the full cohort analysis and the subgroup analysis. Again, we speculate that this may have been a result of the decrease in total carotenoids that occurred in the placebo group during the intervention period, which suggests that the two-week washout period was not long enough to washout background levels of carotenoids prior to the intervention period.
A number of key genes involved in adenosine monophosphate-activated protein kinase (AMPK) and NF-κB signalling were found to be differentially expressed following intervention with F&V concentrate, and these may have contributed to the anti-inflammatory effect that we observed. AMPK is a key regulator of energy metabolism homeostasis, as AMPK inhibits energy consuming activities, such as protein, fatty acid and cholesterol synthesis, and stimulates energy production through glucose and lipid catabolism [60]. AMPK activation can also inhibit NF-κB signalling, thus reducing inflammation [60], and impaired AMPK activity has been shown to lead to insulin resistance [61,62]. AMPK signalling genes that were upregulated following the F&V concentrate intervention that have known anti-inflammatory effects include: IRS2, CAB39 and SIRT1. Previously, a study found that knockdown of IRS2 expression resulted in mice developing insulin resistance due to increased inflammation [63], while another study in mice found that AMPK signalling activated by CAB39 inhibits NF-κB activation [64]. SIRT1, a downstream target of AMPK, inhibits NF-κB signalling, thus reducing inflammation [60,65,66,67]. Other genes that were upregulated in our analysis include BCL2 and TNF-AIP3, which are known inhibitors of NF-κB signalling [68,69,70,71,72,73,74,75,76,77]. Another gene found to be upregulated following intervention with the F&V concentrate was ZFAND5, also known as ZNF216 [78]. A previous study on ZFAND5 found that it was an inhibitor of NF-κB activation, by reducing TNFα, IL-1 and TLR4 in a dose-dependent manner [79]. Hence, our data show several pathways by which the F&V concentrate appears to be having an anti-inflammatory effect, with several AMPK and NF-κB signalling pathway genes contributing to this effect.
F&V concentrate supplementation has previously been shown to reduce homeostatic model assessment-insulin resistance (HOMA-IR) in a group of overweight boys [50]. While there was no change in HbA1C in the intervention group in our study, pathways analysis using GATHER found that a number of genes involved in the insulin signalling pathway were upregulated following intervention with the F&V concentrate. In the high baseline CRP subgroup analysis, the increased expression of the genes HK2, IRS2, PDE3B, PHKB, PRKAG2, PTPN1 and RHEB suggests that the intervention with the F&V concentrate increases insulin sensitivity, as these genes are all involved in reducing blood glucose levels via the insulin signalling pathway [80,81,82,83,84,85,86]. This provides further evidence of the ability of the F&V concentrate to improve the metabolic profile of obese individuals.
Following the intervention with the F&V concentrate, systolic blood pressure (SBP) was found to be reduced in the full cohort analysis. A previous observational study found there was an inverse relationship between β-carotene levels and SBP [87]. In another intervention study, increasing fruit and vegetable consumption to at least five daily portions significantly reduced SBP [88]. This reduction in SBP indicates a decreased risk of cardiovascular diseases, such as myocardial infarction, stroke and congestive heart failure [15]. Interestingly, however, there was also a significant reduction in SBP in the placebo group. Hence, we cannot rule out the possibility that subjects in the trial experienced the ‘white coat effect’ prior to their baseline visit, with their blood pressure becoming elevated due to anxiety about attending the research centre for the first time [89].
F&V concentrate supplementation led to an increase in total lean mass in both the full cohort analysis and the high baseline CRP subgroup analysis, likely due, at least in part, to the decrease in TNFα that we observed. TNFα plays a central role in muscle wasting [90]. Circulating TNFα binds to peripheral muscle cell receptors, stimulating the production of reactive oxygen species (ROS) and cellular apoptosis. In addition, the receptor binding stimulates NF-κB activation, possibly enhanced by ROS. The result is protein loss, caused directly via increased ubiquitin activity and indirectly via decreased myogenic differentiation (MyoD) expression, which decreases myofibril synthesis [90]. This observation suggests that F&V concentrate supplementation may be beneficial in settings where muscle wasting is undesirable, e.g., during aging or cachexia.
Baseline intake of fruit and vegetables was well below recommended levels for both the F&V concentrate and placebo groups. Following the intervention with the F&V concentrate, β-carotene and total carotenoids were significantly increased in the full cohort analysis, as well as the high baseline CRP subgroup analysis. α-tocopherol was found to be unchanged by the F&V concentrate intervention. The F&V concentrate used in this study provides a daily dose of 3.5 mg β-carotene and 4.8 mg α-tocopherol. These daily doses correspond approximately to 100% of the usual daily intake of β-carotene, but only ~50% of the usual daily intake of α-tocopherol [3]. Hence, it is not surprising that we observed an increase in circulating levels of β-carotene, but not α-tocopherol. The supplement also contains polyphenols (at least 119 compounds), including ellagitannins, gallotannins, dihydrochalcones, flavan-3-ols including proanthocyanidins, flavanones, flavones, flavonols, anthocyanins, hydroxybenzoic acids, hydroxycinnamic acids, phenylethanoids and lignans [34]. Whilst we were unable to analyse the circulating levels of these compounds or their metabolites in the samples from this study, it is likely that they would have contributed to the effects that we observed, as the dose delivered (~600 mg/day) is approaching the usual daily dose for most populations [34]. As previously discussed, total carotenoids decreased in the placebo group following the eight-week intervention. This suggests that the two-week run-in period on the low fruit and vegetable washout diet was not long enough, and hence, carotenoids continued to decrease during the eight-week intervention period. Importantly, if the washout period was not adequate, this would also have affected the results in the intervention group, as the potential benefits of the supplement may have been masked, as background levels of carotenoids continued to wash out throughout the intervention period. In future trials, a longer wash out period is recommended.
5. Summary and Conclusions
To summarise, this randomised controlled trial in obese, older individuals shows that F&V concentrate supplementation has the potential to improve the metabolic profile of overweight and obese individuals by reducing blood lipid levels and systemic inflammation, as well as improving body composition. The size of the improvements is clinically significant, as the reduction in total cholesterol that we observed in the full cohort is estimated to be equivalent to a weight loss of 4 kg and an 8% to 9% reduction in CVD risk [48,49]. In the subset of participants who had elevated systemic inflammation at baseline, the reduction in total cholesterol was equivalent to a 9 kg weight loss and an 18% to 19% reduction in CVD risk [48,49]. Interestingly, while significant changes were seen in the whole group of participants, the changes were more pronounced in subjects who had elevated CRP levels at baseline. Hence, while all obese individuals are likely to gain some benefit from supplementation with F&V concentrate, those with high baseline systemic inflammation or blood lipids appear likely to obtain the greatest improvements. We conclude that in obese individuals, who typically have a low fruit and vegetable intake, F&V concentrate supplementation may be beneficial for improving the metabolic profile, thus reducing the risk of developing chronic inflammatory disease.
Acknowledgments
The authors acknowledge the contribution of the Hunter Medical Research Institute Respiratory Research group sample processing team. Financial support for this project, including the supply of study supplements, was received from NSA LLC (Collierville, TN, USA). NSA LLC had no role in the design, analysis nor writing of this article.
Author Contributions
Evan Williams contributed to the data acquisition, analysis and interpretation, drafting of the manuscript and final approval of the version of the manuscript to be published. Katherine Baines contributed to the study design, data analysis and interpretation, manuscript review and final approval of the version to be published. Bronwyn Berthon contributed to the data acquisition, analysis and interpretation, manuscript review and final approval of the version to be published. Lisa Wood contributed to the study design, data analysis and interpretation, drafting of the manuscript and final approval of the version to be published.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.WHO Global Database on Body Mass Index. [(accessed on 11 July 2013)]. Available online: http://apps.who.int/bmi/
- 2.Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. PRevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Australian Bureau of Statistics . Australian Health Survey: Updated Results. Australian Bureau of Statistics; Canberra, Australia: 2011–2012. [Google Scholar]
- 4.Damcott C.M., Sack P., Shuldiner A.R. The genetics of obesity. Endocrinol. Metab. Clin. N. Am. 2003;32:761–786. doi: 10.1016/S0889-8529(03)00076-8. [DOI] [PubMed] [Google Scholar]
- 5.Mraz M., Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014;222:R113–R127. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- 6.Kalupahana N.S., Moustaid-Moussa N., Claycombe K.J. Immunity as a link between obesity and insulin resistance. Mol. Aspects Med. 2012;33:26–34. doi: 10.1016/j.mam.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siriwardhanaa N., Kalupahanab N.S., Cekanovac M., LeMieuxa M., Greerd B., Moustaid-Moussaa N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013;24:613–623. doi: 10.1016/j.jnutbio.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Bulló M., Casas-Agustench P., Amigó-Correig P., Aranceta J., Salas-Salvadó J. Inflammation, obesity and comorbidities: The role of diet. Public Health Nutr. 2007;10:1164–1172. doi: 10.1017/S1368980007000663. [DOI] [PubMed] [Google Scholar]
- 11.Poulain M., Doucet M., Major G.C., Drapeau V., Sériès F., Boulet L.-P., Tremblay A., Maltais F. The effect of obesity on chronic respiratory diseases: Pathophysiology and therapeutic strategies. CMAJ. 2006;174:1293–1299. doi: 10.1503/cmaj.051299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cawthorn W.P., Sethi J.K. TNF-α and adipocyte biology. FEBS Lett. 2008;582:117–131. doi: 10.1016/j.febslet.2007.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarkesh-Esfahani H., Pockley A.G., Wu Z., Hellewell P.G., Weetman A.P., Ross R.J. Leptin indirectly activates human neutrophils via induction of TNF-α. J. Immunol. 2004;172:1809–1814. doi: 10.4049/jimmunol.172.3.1809. [DOI] [PubMed] [Google Scholar]
- 14.Das U.N. Is Obesity an Inflammatory Condition? Nutrition. 2001;17:953–966. doi: 10.1016/S0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 15.Vitseva O.I., Tanriverdi K., Tchkonia T.T., Kirkland J.L., McDonnell M.E., Apovian C.M., Freedman J., Gokce N. Inducible Toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity (Silver Spring) 2008;16:932–937. doi: 10.1038/oby.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bost F., Aouadi M., Caron L., Binetruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87:51–56. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier M.S., O’Brien E.L., Bigornia S., Mott M., Cacicedo J.M., Xu X.J., Gokce N., Apovian C., Ruderman N. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem. Biophys. Res. Commun. 2011;404:382–387. doi: 10.1016/j.bbrc.2010.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moller D.E., Berger J.P. Role of PPARs in the regulation of obesity-related insulin sensitivity and inflammation. Int. J. Obes. Relat. Metab. Disord. 2003;27:S17–S21. doi: 10.1038/sj.ijo.0802494. [DOI] [PubMed] [Google Scholar]
- 19.Dandona P., Aljada A., Bandyopadhyay A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends. Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Franks P.W. Obesity, inflammatory markers and cardiovascular disease: Distinguishing causality from confounding. J. Hum. Hypertens. 2006;20:837–840. doi: 10.1038/sj.jhh.1002059. [DOI] [PubMed] [Google Scholar]
- 21.Nicklas B.J., Ambrosius W., Messier S.P., Miller G.D., Penninx B.W.J.H., Loeser R.F., Palla S., Bleecker E., Pahor M. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: A randomized controlled clinical trial. Am. J. Clin. Nutr. 2004;79:544–551. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 22.Forsythe L.K., Wallace J.M., Livingstone M.B.E. Obesity and inflammation: The effects of weight loss. Nutr. Res. Rev. 2008;21:117–133. doi: 10.1017/S0954422408138732. [DOI] [PubMed] [Google Scholar]
- 23.Jung S.H., Park H.S., Kim K.-S., Choi W.H., Ahn C.W., Kim B.T., Kim S.M., Lee S.Y., Ahn S.M., Kim Y.K., et al. Effect of weight loss on some serum cytokines in human obesity: Increase in IL-10 after weight loss. J. Nutr. Biochem. 2008;19:371–375. doi: 10.1016/j.jnutbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Barinas-Mitchell E., Kuller L.H., Sutton-Tyrrell K., Hegazi R., Harper P., Mancino J., Kelley D.E. Effect of Weight Loss and Nutritional Intervention on Arterial Stiffness in Type 2 Diabetes. Diabetes Care. 2006;29:2218–2222. doi: 10.2337/dc06-0665. [DOI] [PubMed] [Google Scholar]
- 25.Marfella R., Esposito K., Siniscalchi M., Cacciapuoti F., Giugliano F., Labriloa D., Ciotola M., Di Palo C., Misso L., Giugliano D. Effect of Weight Loss on Cardiac Synchronization and Proinflammatory Cytokines in Premenopausal Obese Women. Diabetes Care. 2004;27:47–52. doi: 10.2337/diacare.27.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Selvin E., Paynter N.P., Erlinger T.P. The Effect of Weight Loss on C-Reactive Protein: A Systematic Review. Arch. Intern. Med. 2007;167:31–39. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 27.Higami Y., Barger J.L., Page G.P., Allison D.B., Smith S.R., Prolla T.A., Weindruch R. Energy restriction lowers the expression of genes linked to inflammation, the cytoskeleton, the extracellular matrix, and angiogenesis in mouse adipose tissue. J. Nutr. 2006;136:343–352. doi: 10.1093/jn/136.2.343. [DOI] [PubMed] [Google Scholar]
- 28.Larson-Meyer D.E., Heilbronn L.K., Redman L.M., Newcomer B.R., Frisard M.I., Anton S., Smith S.R., Alfonso A., Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarvi A., Karlstrom B., Vessby B., Becker W. Increased intake of fruits and vegetables in overweight subjects: Effects on body weight, body composition, metabolic risk factors and dietary intake. Br. J. Nutr. 2016;115:1760–1768. doi: 10.1017/S0007114516000970. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez-Suarez J.M., Giampieri F., Tulipani S., Casoli T., Di Stefano G., González-Paramás A.M., Santos-Buelga C., Busco F., Quiles J.L., Cordero M.D., et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014;25:289–294. doi: 10.1016/j.jnutbio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Esfahani A., Wong J.M., Truan J., Villa C.R., Mirrahimi A., Srichaikul K., Kendall C.W. Health effects of mixed fruit and vegetable concentrates: A systematic review of the clinical interventions. J. Am. Coll. Nutr. 2011;30:285–294. doi: 10.1080/07315724.2011.10719971. [DOI] [PubMed] [Google Scholar]
- 32.Heale P., Stockwell T., Dietze P., Chikritzhs T., Catalano P. Patterns of Alcohol Consumption in Australia, 1998. Curtin University of Technology; Perth, Australia: 2000. National Alcohol Indicators Project, Bulletin No. 3. National Drug Research Institute. [Google Scholar]
- 33.Lamprecht M., Obermayer G., Steinbauer K., Cvirn G., Hofmann L., Ledinski G., Greilberger J.F., Hallstroem S. Supplementation with a juice powder concentrate and exercise decrease oxidation and inflammation, and improve the microcirculation in obese women: Randomised controlled trial data. Br. J. Nutr. 2013;110:1685–1695. doi: 10.1017/S0007114513001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bresciani L., Calani L., Cossu M., Mena P., Sayegh M., Ray S., Del Rio D. (Poly)phenolic characterization of three food supplements containing 36 different fruits, vegetables and berries. PharmaNutrition. 2015;3:11–19. doi: 10.1016/j.phanu.2015.01.001. [DOI] [Google Scholar]
- 35.Ware J.E., Jr., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). Conceptual framework and item selection. Med. Care. 1992;30:473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Wood L.G., Gibson P.G. Reduced circulating antioxidant defences are associated with airway hyper-responsiveness, poor control and severe disease pattern in asthma. Br. J. Nutr. 2010;103:735–741. doi: 10.1017/S0007114509992376. [DOI] [PubMed] [Google Scholar]
- 37.Wood L.G., Garg M.L., Powell H., Gibson P.G. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: Proof of concept. Free Radic. Res. 2008;42:94–102. doi: 10.1080/10715760701767307. [DOI] [PubMed] [Google Scholar]
- 38.Wood L.G., Garg M.L., Blake R.J., Garcia-Caraballo S., Gibson P.G. Airway and circulating levels of carotenoids in asthma and healthy controls. J. Am. Coll. Nutr. 2005;24:448–455. doi: 10.1080/07315724.2005.10719490. [DOI] [PubMed] [Google Scholar]
- 39.Giles G., Ireland P. Dietary Questionnaire for Epidemiological Studies. The Cancer Council Victoria; Melbourne, Australia: 1996. version 2. [Google Scholar]
- 40.National Health and Medical Research Council . Australian Dietary Guidelines. National Health and Medical Research Council; Canberra, Australia: 2013. [Google Scholar]
- 41.National Center for Biotechnology Information Gene Expression Omnibus (GEO) 2017. [(accessed on 8 September 2016)]. Available online: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE87454.
- 42.Andersen C.L., Jensen J.L., Orntoft T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 43.Dupont W.D., Plummer W.D. Power and Sample Size Calculations for Studies Involving Linear Regression. Controll. Clin. Trials. 1998;19:589–601. doi: 10.1016/S0197-2456(98)00037-3. [DOI] [PubMed] [Google Scholar]
- 44.Du P., Kibbe W.A., Lin S.M. Lumi: A pipeline for processing Illumina microarray. Bioinformatics. 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 45.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucl. Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang J.T., Nevins J.R. GATHER: A systems approach to interpreting genomic signatures. Bioinformatics. 2006;22:2926–2933. doi: 10.1093/bioinformatics/btl483. [DOI] [PubMed] [Google Scholar]
- 47.Rees K., Dyakova M., Ward K., Thorogood M., Brunner E. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson J.W., Konz E.C. Obesity and disease management: Effects of weight loss on comorbid conditions. Obes. Res. 2001;9:326S–334S. doi: 10.1038/oby.2001.138. [DOI] [PubMed] [Google Scholar]
- 49.Dattilo A.M., Kris-Etherton P.M. Effects of weight reduction on blood lipids and lipoproteins: A meta-analysis. Am. J. Clin. Nutr. 1992;56:320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 50.Canas J.A., Damaso L., Altomare A., Killen K., Hossain J., Balagopal P. Insulin resistance and adiposity in relation to serum beta-carotene levels. J. Pediatr. 2012;161:58–64. doi: 10.1016/j.jpeds.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 51.Annuzzi G., Bozzetto L., Costabile G., Giacco R., Mangione A., Anniballi G., Vitale M., Vetrani C., Cipriano P., Della Corte G., et al. Diets naturally rich in polyphenols improve fasting and postprandial dyslipidemia and reduce oxidative stress: A randomized controlled trial. Am. J. Clin. Nutr. 2014;99:463–471. doi: 10.3945/ajcn.113.073445. [DOI] [PubMed] [Google Scholar]
- 52.Olivier L.M., Chambliss K.L., Michael Gibson K., Krisans S.K. Characterization of phosphomevalonate kinase: Chromosomal localization, regulation, and subcellular targeting. J. Lipid Res. 1999;40:672–679. [PubMed] [Google Scholar]
- 53.Aripirala S., Gonzalez-Pacanowska D., Oldfield E., Kaiser M., Amzel L.M., Gabelli S.B. Structural and thermodynamic basis of the inhibition of Leishmania major farnesyl diphosphate synthase by nitrogen-containing bisphosphonates. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014;70:802–810. doi: 10.1107/S1399004713033221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Canas J.A., Damaso L., Hossain J., Balagopal P.B. Fatty acid binding proteins 4 and 5 in overweight prepubertal boys: Effect of nutritional counselling and supplementation with an encapsulated fruit and vegetable juice concentrate. J. Nutr. Sci. 2015;4:e39. doi: 10.1017/jns.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Esposito K., Marfella R., Ciotola M., Di Palo C., Giugliano F., Giugliano G., D’Armiento M., D’Andrea F., Giugliano D. Effect of Mediterranean-Style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 56.Watzl B., Kulling S.E., Moseneder J., Barth S.W., Bub A. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am. J. Clin. Nutr. 2005;82:1052–1058. doi: 10.1093/ajcn/82.5.1052. [DOI] [PubMed] [Google Scholar]
- 57.Jin Y., Cui X., Singh U.P., Chumanevich A.A., Harmon B., Cavicchia P., Hofseth A.B., Kotakadi V., Stroud B., Volate S.R., et al. Systemic inflammatory load in humans is suppressed by consumption of two formulations of dried, encapsulated juice concentrate. Mol. Nutr. Food Res. 2010;54:1506–1514. doi: 10.1002/mnfr.200900579. [DOI] [PubMed] [Google Scholar]
- 58.Seif El-Din S.H., El-Lakkany N.M., El-Naggar A.A., Hammam O.A., Abd El-Latif H.A., Ain-Shoka A.A., Ebeid F.A. Effects of rosuvastatin and/or beta-carotene on non-alcoholic fatty liver in rats. Res. Pharm. Sci. 2015;10:275–287. [PMC free article] [PubMed] [Google Scholar]
- 59.Bai S.K., Lee S.J., Na H.J., Ha K.S., Han J.A., Lee H., Kwon Y.G., Chung C.K., Kim Y.M. beta-Carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-kappaB activation. Exp. Mol. Med. 2005;37:323–334. doi: 10.1038/emm.2005.42. [DOI] [PubMed] [Google Scholar]
- 60.Salminen A., Hyttinen J.M.T., Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lage R., Dieguez C., Vidal-Puig A., Lopez M. AMPK: A metabolic gauge regulating whole-body energy homeostasis. Trends Mol. Med. 2008;14:539–549. doi: 10.1016/j.molmed.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Ruderman N.B., Cacicedo J.M., Itani S., Yagihashi N., Saha A.K., Ye J.M., Chen K., Zou M., Carling D., Boden G., et al. Malonyl-CoA and AMP-activated protein kinase (AMPK): Possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochem. Soc. Trans. 2003;31:202–206. doi: 10.1042/bst0310202. [DOI] [PubMed] [Google Scholar]
- 63.Vinué Á., Andrés-Blasco I., Herrero-Cervera A., Piqueras L., Andrés V., Burks D.J., Sanz M.J., González-Navarro H. Ink4/Arf locus restores glucose tolerance and insulin sensitivity by reducing hepatic steatosis and inflammation in mice with impaired IRS2-dependent signalling. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015;1852:1729–1742. doi: 10.1016/j.bbadis.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Hur W., Lee J.H., Kim S.W., Kim J.H., Bae S.H., Kim M., Hwang D., Kim Y.S., Park T., Um S.J., et al. Downregulation of microRNA-451 in non-alcoholic steatohepatitis inhibits fatty acid-induced proinflammatory cytokine production through the AMPK/AKT pathway. Int. J. Biochem. Cell Biol. 2015;64:265–276. doi: 10.1016/j.biocel.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 65.Yoshizaki T., Schenk S., Imamura T., Babendure J.L., Sonoda N., Bae E.J., Oh D.Y., Lu M., Milne J.C., Westphal C., et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010;298:E419–E428. doi: 10.1152/ajpendo.00417.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salminen A., Kauppinen A., Suuronen T., Kaarniranta K. SIRT1 longevity factor suppresses NF-kappaB -driven immune responses: Regulation of aging via NF-kappaB acetylation? Bioessays. 2008;30:939–942. doi: 10.1002/bies.20799. [DOI] [PubMed] [Google Scholar]
- 67.Yang H., Zhang W., Pan H., Feldser H.G., Lainez E., Miller C., Leung S., Zhong Z., Zhao H., Sweitzer S., et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-kappaB activity. PLoS ONE. 2012;7:e46364. doi: 10.1371/journal.pone.0046364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elton L., Carpentier I., Staal J., Driege Y., Haegman M., Beyaert R. MALT1 cleaves the E3 ubiquitin ligase HOIL-1 in activated T cells, generating a dominant negative inhibitor of LUBAC-induced NF-kappaB signaling. FEBS J. 2016;283:403–412. doi: 10.1111/febs.13597. [DOI] [PubMed] [Google Scholar]
- 69.Ba X., Garg N.J. Signaling mechanism of poly(ADP-ribose) polymerase-1 (PARP-1) in inflammatory diseases. Am. J. Pathol. 2011;178:946–955. doi: 10.1016/j.ajpath.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Piret B., Schoonbroodt S., Piette J. The ATM protein is required for sustained activation of NF-kappaB following DNA damage. Oncogene. 1999;18:2261–2271. doi: 10.1038/sj.onc.1202541. [DOI] [PubMed] [Google Scholar]
- 71.Macrae K., Stretton C., Lipina C., Blachnio-Zabielska A., Baranowski M., Gorski J., Marley A., Hundal H.S. Defining the role of DAG, mitochondrial function, and lipid deposition in palmitate-induced proinflammatory signaling and its counter-modulation by palmitoleate. J. Lipid Res. 2013;54:2366–2378. doi: 10.1194/jlr.M036996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim H., Zamel R., Bai X.-H., Liu M. PKC Activation Induces Inflammatory Response and Cell Death in Human Bronchial Epithelial Cells. PLoS ONE. 2013;8:e64182. doi: 10.1371/journal.pone.0064182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hellmann J., Tang Y., Zhang M.J., Hai T., Bhatnagar A., Srivastava S., Spite M. Atf3 negatively regulates Ptgs2/Cox2 expression during acute inflammation. Prostaglandins Other Lipid Mediat. 2015;116–117:49–56. doi: 10.1016/j.prostaglandins.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Badrichani A.Z., Stroka D.M., Bilbao G., Curiel D.T., Bach F.H., Ferran C. Bcl-2 and Bcl-X(L) serve an anti-inflammatory function in endothelial cells through inhibition of NF-κB. J. Clin. Investig. 1999;103:543–553. doi: 10.1172/JCI2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zehavi L., Schayek H., Jacob-Hirsch J., Sidi Y., Leibowitz-Amit R., Avni D. MiR-377 targets E2F3 and alters the NF-kB signaling pathway through MAP3K7 in malignant melanoma. Mol. Cancer. 2015;14:68. doi: 10.1186/s12943-015-0338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guijarro-Munoz I., Compte M., Alvarez-Cienfuegos A., Alvarez-Vallina L., Sanz L. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-kappaB signaling pathway and proinflammatory response in human pericytes. J. Biol. Chem. 2014;289:2457–2468. doi: 10.1074/jbc.M113.521161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu J., Zhu L., Xie G.L., Bao J.F., Yu Q. Let-7 miRNAs Modulate the Activation of NF-kappaB by Targeting TNFAIP3 and Are Involved in the Pathogenesis of Lupus Nephritis. PLoS ONE. 2015;10:e0121256. doi: 10.1371/journal.pone.0121256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wullaert A., Heyninck K., Janssens S., Beyaert R. Ubiquitin: Tool and target for intracellular NF-kappaB inhibitors. Trends Immunol. 2006;27:533–540. doi: 10.1016/j.it.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 79.Huang J., Teng L., Li L., Liu T., Li L., Chen D., Xu L.G., Zhai Z., Shu H.B. ZNF216 Is an A20-like and IkappaB kinase gamma-interacting inhibitor of NFkappaB activation. J. Biol. Chem. 2004;279:16847–16853. doi: 10.1074/jbc.M309491200. [DOI] [PubMed] [Google Scholar]
- 80.Sears D.D., Hsiao G., Hsiao A., Yu J.G., Courtney C.H., Ofrecio J.M., Chapman J., Subramaniam S. Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization. Proc. Natl. Acad. Sci. USA. 2009;106:18745–18750. doi: 10.1073/pnas.0903032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Valverde A.M., Burks D.J., Fabregat I., Fisher T.L., Carretero J., White M.F., Benito M. Molecular mechanisms of insulin resistance in IRS-2-deficient hepatocytes. Diabetes. 2003;52:2239–2248. doi: 10.2337/diabetes.52.9.2239. [DOI] [PubMed] [Google Scholar]
- 82.Ahmad F., Lindh R., Tang Y., Ruishalme I., ÖSt A., Sahachartsiri B., StrÅLfors P., Degerman E., Manganiello V.C. Differential regulation of adipocyte PDE3B in distinct membrane compartments by insulin and the β(3)-adrenergic receptor agonist CL316243: Effects of caveolin-1 knockdown on formation/maintenance of macromolecular signalling complexes. Biochem. J. 2009;424:399–410. doi: 10.1042/BJ20090842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Terashima M., Fujita Y., Togashi Y., Sakai K., De Velasco M.A., Tomida S., Nishio K. KIAA1199 interacts with glycogen phosphorylase kinase beta-subunit (PHKB) to promote glycogen breakdown and cancer cell survival. Oncotarget. 2014;5:7040–7050. doi: 10.18632/oncotarget.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang B.L., Xu R.L., Zhang J., Zhao X.X., Wu H., Ma L.P., Hu J.Q., Zhang J.L., Ye Z., Zheng X., et al. Identification and functional analysis of a novel PRKAG2 mutation responsible for Chinese PRKAG2 cardiac syndrome reveal an important role of non-CBS domains in regulating the AMPK pathway. J. Cardiol. 2013;62:241–248. doi: 10.1016/j.jjcc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 85.Galic S., Hauser C., Kahn B.B., Haj F.G., Neel B.G., Tonks N.K., Tiganis T. Coordinated Regulation of Insulin Signaling by the Protein Tyrosine Phosphatases PTP1B and TCPTP. Mol. Cell. Biol. 2005;25:819–829. doi: 10.1128/MCB.25.2.819-829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saucedo L.J., Gao X., Chiarelli D.A., Li L., Pan D., Edgar B.A. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat. Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- 87.Chen J., He J., Hamm L., Batuman V., Whelton P.K. Serum antioxidant vitamins and blood pressure in the United States population. Hypertension. 2002;40:810–816. doi: 10.1161/01.HYP.0000039962.68332.59. [DOI] [PubMed] [Google Scholar]
- 88.John J.H., Ziebland S., Yudkin P., Roe L.S., Neil H.A.W. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: A randomised controlled trial. Lancet. 2002;359:1969–1974. doi: 10.1016/S0140-6736(02)98858-6. [DOI] [PubMed] [Google Scholar]
- 89.Pickering T. Blood pressure measurement and detection of hypertension. Lancet. 1994;344:31–35. doi: 10.1016/S0140-6736(94)91053-7. [DOI] [PubMed] [Google Scholar]
- 90.Sevenoaks M.J., Stockley R.A. Chronic obstructive pulmonary disease, inflammation and co-morbidity—A common inflammatory phenotype. Respir. Res. 2006;7:70. doi: 10.1186/1465-9921-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]