Abstract
Catheter-related bloodstream infections, exit-site infections, and tunnel infections are common complications related to hemodialysis central venous catheter use. The various definitions of catheter-related infections are reviewed, and various preventive strategies are discussed. Treatment options, for both empiric and definitive infections, including antibiotic locks and systemic antibiotics, are reviewed.
Keywords: tunneled catheter, central venous catheter, catheter-related bacteremia, antimicrobial locks, catheter-related infection complications, hemodialysis
Abrégé
Les bactériémies liées à l’utilisation d’un cathéter, les infections au point d’émergence de celui-ci ainsi que les infections du tunnel constituent les complications les plus courantes associées à l’utilisation d’un cathéter veineux central pour l’hémodialyse. Le présent chapitre expose les multiples définitions d’une infection liée à l’utilisation d’un cathéter pour la dialyse, et discute des stratégies préventives à adopter. On y présente également les options de traitement pour ces infections, empiriques ou définitives, notamment l’ajout d’antibiotiques dans le dispositif de verrouillage du cathéter et l’usage d’antibiotiques systémiques.
Introduction
Infections are common complications among patients on chronic hemodialysis. Hemodialysis patients with a catheter have a 2- to 3-fold increased risk of hospitalization for infection and death compared with patients with an arteriovenous fistula or graft.1 Catheter-related bloodstream infections (CRBSIs), exit-site infections, and tunnel infections are common complications related to hemodialysis central venous catheter use. Catheter-related bloodstream infections (BSIs) alone have a reported incidence of 1.1 to 5.5 episodes per 1000 catheter days and are associated with increased morbidity, hospitalization, and death.2-5 The most common causative pathogens are gram-positive bacteria, with Staphylococcus aureus and coagulase-negative staphylococci accounting for 40% to 80% of CRBSIs.6 Gram-negative organisms cause 20% to 40% CRBSIs, whereas polymicrobial infections (10%-20%) and fungal infections (<5%) are less common. Metastatic infectious complications of CRBSIs include endocarditis, osteomyelitis, spinal epidural abscess, septic arthritis, brain abscess, and septic pulmonary emboli.
Diagnosis
Clinical Features
Fever or chills are the most sensitive clinical features, associated with positive blood cultures in 60% to 80% of patients.7,8 Only 5% of patients with CRBSIs will have a concurrent exit-site or tunnel infection.9 Other clinical manifestations of CRBSIs include hemodynamic instability, altered mental status, catheter dysfunction, hypothermia, nausea/vomiting, and generalized malaise (Figure 1). In some cases, complications related to a CRBSI may be the first clues to the presence of a CRBSI (see section “Infection Complications”).
Figure 1.
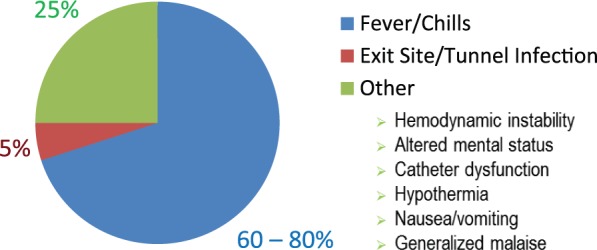
Clinical features associated with catheter-related bloodstream infections.
Clinical Definitions
Several definitions for catheter-related infections are cited in the literature; however, consensus is not attained.10,11 In addition to the many technical diagnostic challenges (Table 1), it is very important to recognize the difference between surveillance definitions for CRBSIs and clinical definitions. Surveillance definitions for CRBSI include all bacteremias that occur in patients with catheters, likely overestimating the true incidence of CRBSIs because not all bacteremias originate from the catheter. Clinical definitions of CRBSIs are those where other sources of infection are excluded by patient examination and review of patient record, and finding of positive catheter tip cultures (if available) with the same organism as that seen on blood cultures. To accurately compare health care facilities’ infection rates with each other, and with published data, comparable definitions should be used.
Table 1.
Diagnostic Challenges in Defining CRBSIs.
| Peripheral blood cultures are not obtained, either because their veins cannot be accessed or because an existing vein needs to be preserved for fistula. |
| Suboptimal handling of blood cultures obtained in the outpatient dialysis unit: ● Variable period before culture bottles are eventually placed in an incubator ● Differences in temperature during transport to a microbiology laboratory |
| The physician may not be available at the time of clinical presentation to exclude other sources of infection. |
| The increasing role of catheter salvage means catheter tips are not being sent for cultures. |
| The use of antibiotic locks for prevention may interfere with diagnosis. |
Note. CRBSI = catheter-related bloodstream infection.
Despite the challenges, there are several commonly used and accepted clinical definitions of catheter-related infections in the literature. The reader can find details of these definitions in the following references: Kidney Disease Outcomes Quality Initiative (KDOQI),12 Centers for Disease Control and Prevention (CDC),13 Infectious Diseases Society of America (IDSA),14 and Public Health Agency of Canada.15 A summary of these clinical definitions can be found in Table 2. To illustrate the differences in clinical and surveillance definitions, Table 3 provides the CDC surveillance definitions for primary BSIs associated with intravascular devices.
Table 2.
CRBSI Clinical Definitions.
| KDOQI12 | CDC13 | IDSA14 | Public Health Agency of Canada15 |
|---|---|---|---|
|
Definite: Same organism from a semiquantitative culture of the catheter tip (>15 CFU/catheter segment) and from a BC in a symptomatic patient with no other apparent source of infection. Probable: Defervescence of symptoms after antibiotic therapy with or without removal of the catheter, in the setting in which BC confirms infection, but catheter tip does not (or catheter tip does, but blood does not) in a symptomatic patient with no other apparent source of infection. Possible: Defervescence of symptoms after antibiotic treatment or after removal of catheter in the absence of laboratory confirmation of BSI in a symptomatic patient with no other apparent source of infection. |
Clinical manifestations and at least 1 positive BC from a peripheral vein and no other apparent source, with either positive semiquantitative (>15 CFU/catheter segment) or quantitative (>103 CFU/catheter segment) culture, whereby the same organism (species and antibiogram) is isolated from the catheter segment and a peripheral blood sample; Simultaneous quantitative cultures of blood samples with a ratio of ≥3:1 (catheter vs peripheral); Differential period of catheter culture versus peripheral BC positivity of 2 h; OR Isolation of the same organism from semiquantitative or quantitative culture segment and from blood (preferably from a peripheral vein) of a patient with accompanying symptoms of BSI and no other apparent source of infection. |
Bacteremia/fungemia in a patient with an intravascular catheter with at least 1 positive BC and with clinical manifestations of infections (ie, fever, chills, and/or hypotension) and no apparent source for the BSI except the catheter AND One of the following should be present: A positive semiquantitative (>15 CFU/catheter segment) or quantitative (>103 CFU/catheter segment) culture whereby the same organism (species and antibiogram) is isolated from the catheter segment and peripheral blood. Simultaneous quantitative BC with a >5:1 ratio catheter versus peripheral. Differential time period of catheter culture versus peripheral BC positivity of >2 h. |
Definite: Single positive BC and positive culture result of catheter segment with identical organism or ≥10-fold colony count difference in BC drawn from device and peripheral blood OR Single positive BC and positive culture from discharge from exit site or tunnel with identical organism. Probable: ≥2 positive BC results with no evidence for source other than the device or single positive BC for S aureus or Candida species with no evidence for source other than the device OR Single positive BC for coagulase-negative staphylococci, Bacillus, Corynebacterium jeikeium, Enterococcus, Trichophyton, or Malassezia species in immunocompromised or neutropenic host or patient receiving total parenteral nutrition with no evidence for source other than a centrally placed device. Possible: Single positive BC result with no evidence for source except a centrally placed device, and patient or organism does not fit criteria for probable infection. |
Note. CRBSI = catheter-related bloodstream infection; KDOQI = Kidney Disease Outcomes Quality Initiative; CDC = Centers for Disease Control and Prevention; IDSA = Infectious Diseases Society of America; CFU = colony-forming unit; BC = blood culture; S aureus = Staphylococcus aureus; BSI = bloodstream infection.
Table 3.
CDC Surveillance Definitions for CRBSI.13
| Laboratory-confirmed bloodstream infection | Clinical sepsis | Catheter-associated bloodstream infection |
|---|---|---|
| Should meet at least one of the following criteria: Criterion 1. Patient has a recognized pathogen cultured from 1 or more BC, and the pathogen cultured from the blood is not related to an infection at another site. Criterion 2. Patient has at least one of the following signs or symptoms: fever (>100.4°F [>38°C]), chills, or hypotension, and at least one of the following: 1. Common skin contaminant (eg, diphtheroids, Bacillus spp., Propionibacterium spp., coagulase-negative staphylococci, or micrococci) cultured from 2 or more BC drawn on separate occasions. 2. Common skin contaminant (as in 1 above) cultured from at least 1 BC from a patient with an intravenous line, and the physician institutes appropriate antimicrobial therapy. 3. Positive antigen test on blood (eg, Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, or group B streptococcus). AND 4. Signs and symptoms with positive laboratory results are not related to an infection at another site. |
Should meet at least one of the following criteria: Criterion 1. Patient has at least one of the following clinical signs with no other recognized cause: fever (>100.4°F [>38°C]), hypotension (systolic pressure <90 mm Hg), or oliguria (<20 mL/h), and BC not done or no organisms or antigen detected in blood and no apparent infection at another site, and physician institutes treatment for sepsis. Criterion 2. Patient aged <1 year has at least one of the following clinical signs or symptoms with no other recognized cause: fever (>100.4°F [>38°C]), hypothermia (<98.6°F [<37°C]), apnea, or bradycardia, and BC not done or no organisms or antigen detected in blood and no apparent infection at another site, and physician institutes treatment for sepsis. |
Vascular access device that terminates at or close to the heart or one of the great vessels. An umbilical artery or vein catheter is considered a central line. BSI is considered to be associated with a central venous catheter if the catheter was in use during the 48-h period before development of the BSI. If the time interval between onset of infection and device use is >48 h, there should be compelling evidence that the infection is related to the central venous catheter. |
Note. CDC = Centers for Disease Control and Prevention; CRBSI = catheter-related bloodstream infection; BC = blood culture.
The IDSA14 and CDC13 definitions for exit-site infection and tunnel infection are provided below:
Exit-site infection (Figure 2).
Figure 2.
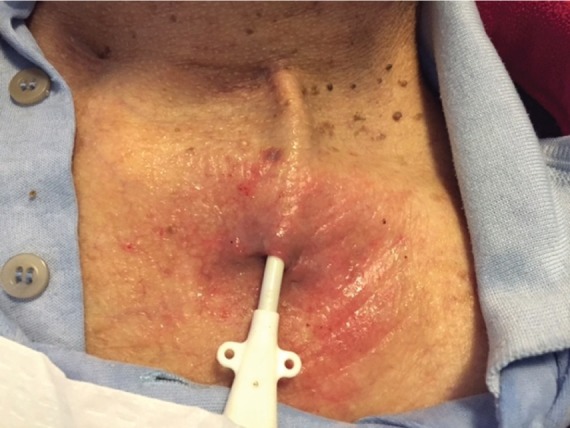
Exit-site infection.
IDSA—Hyperemia, induration, and/or tenderness ≤2 cm from catheter exit site. May be associated with fever and purulent drainage from the exit site. It may or may not be associated with bacteremia. If there is purulent drainage, it should be collected and sent for Gram staining and culture.
CDC—Erythema or induration within 2 cm of the catheter exit site, in the absence of concomitant BSI and without concomitant purulence.
Tunnel infection (Figure 3).
Figure 3.
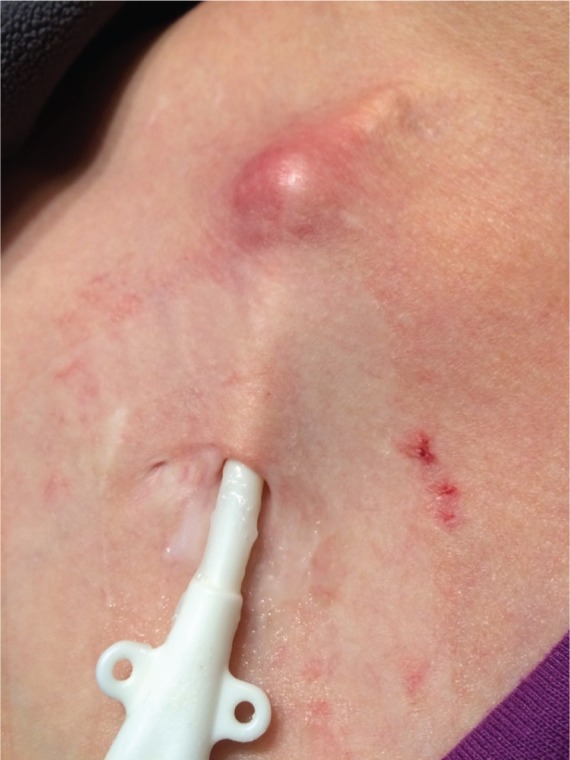
Tunnel infection.
IDSA—Tenderness, hyperemia, and/or induration that extends >2 cm from the exit site and along the subcutaneous tunnel. It may or may not be associated with bacteremia. If there is purulent drainage, it should be collected and sent for Gram staining and culture.
CDC—Tenderness, erythema, or site induration >2 cm from the catheter site along the subcutaneous tract of a tunneled catheter, in the absence of concomitant BSI.
Prevention of Catheter-Related Infections16
There are several risk factors for the development of infection, including conditions of catheter insertion, site of catheter insertion, and duration of use (Table 4).6,17,18 The most effective strategy for prevention of CRBSIs is reducing the use of catheters. Other basic measures include improved catheter care, good hand hygiene practices, and education for both patients and staff on vascular access care. As catheter use cannot be eliminated, CDC has recommended several core interventions to decrease infections (Table 5). Several of these recommendations are incorporated in catheter care bundles, which have been shown to reduce catheter-related infections in patients with central venous catheters.19-21
Table 4.
Risk Factors for the Occurrence of CRBSIs.
| Submaximal barrier precautions at the time of catheter insertion |
| Nontunneled catheter |
| Site of insertion—femoral > internal jugular > subclavian |
| Prolonged duration of catheter use |
| Previous episode of CRBSI |
| Staphylococcus aureus nasal carriage |
| Diabetes |
| Hypoalbuminemia |
| Recent surgery |
Note. CRBSI = catheter-related bloodstream infection.
Table 5.
Core Interventions for Dialysis BSI Prevention.
| Surveillance and feedback | ● Conduct monthly surveillance for BSIs and other dialysis events using CDC’s NHSN. ● Calculate facility rates and compare with rates in other NHSN facilities. ● Actively share results with front-line clinical staff. |
| Hand hygiene observations | ● Perform observations of hand hygiene opportunities monthly ● Share results with clinical staff. |
| Catheter/vascular access care observations | ● Perform observations of vascular access care and catheter accessing quarterly. ● Assess staff adherence to aseptic technique when connecting and disconnecting catheters and during dressing changes. ● Share results with clinical staff. |
| Staff education and competency | ● Train staff on infection control topics, including access care and aseptic technique. ● Perform competency evaluation for skills such as catheter care and accessing every 6 to 12 mo and upon hire. |
| Patient education/engagement | ● Provide standardized education to all patients on infection prevention topics including vascular access care, hand hygiene, risks related to catheter use, recognizing signs of infection, and instructions for access management when away from the dialysis unit. |
| Catheter reduction | ● Incorporate efforts (eg, through patient education, vascular access coordinator) to reduce catheters by identifying and addressing barriers to permanent vascular access placement and catheter removal. |
| Chlorhexidine for skin antisepsis | ● Use an alcohol-based chlorhexidine (>0.5%) solution as the first-line skin antiseptic agent for central line insertion and during dressing changes. |
| Catheter hub disinfection | ● Scrub catheter hubs with an appropriate antiseptic after cap is removed and before accessing. Perform every time catheter is accessed or disconnected. |
| Antimicrobial ointment | ● Apply antibiotic ointment or povidone-iodine ointment to catheter exit sites during dressing change. |
Source. Adapted from CDC Approach to BSI Prevention in Dialysis Facilities.22
Note. BSI = bloodstream infection; CDC = Centers for Disease Control and Prevention; NHSN = National Healthcare Safety Network.
Catheter Site Selection
The right internal jugular vein is the preferred initial insertion site for tunneled catheters (see “Catheter Site Selection” section in Clark et al23). Subclavian catheters are avoided if possible due to increased risk of central venous stenosis (see “Risk Factors for Central Vein Stenosis” section in Miller et al24). Femoral catheters have generally been considered less preferable to internal jugular catheters due to concerns of catheter dysfunction and increased risk of infection. However, the Cathedia study randomized 750 patients from 12 different intensive care units to either femoral or internal jugular nontunneled hemodialysis catheter insertion for acute renal replacement therapy (RRT), and showed similar infection rates between femoral and internal jugular catheterization.25 However, in a prespecified subgroup analysis according to body mass index (BMI), those in the highest BMI tertile (>28.4) had a higher risk of infection with femoral versus internal jugular nontunneled hemodialysis catheters, whereas patients in the lowest BMI tertile (<24.2) had lower risk of infection with femoral versus internal jugular catheters. This suggests that femoral catheters may have a role in this subset of patients requiring dialysis for acute kidney injury. It is unknown whether similar findings would be observed in noncritically ill hemodialysis patients with tunneled catheters. The left internal jugular, external jugular, subclavian, and femoral veins, and transhepatic and translumbar are other sites available for insertion of these catheters.
Maximal Barrier Precautions at the Time of Catheter Insertion
A cap, mask, sterile gown, and sterile gloves should be worn at the time of placing a catheter. The catheter insertion site should be prepped using sterile technique. A sterile full body drape should be used leaving only a small opening at the insertion site.26
Dressing Type and Replacement Intervals
There are no definitive recommendations for the optimal dressing or frequency of change for hemodialysis catheters (see “Tunneled Cuffed Catheter Care” section in Clark et al23). The Canadian Society of Nephrology guidelines recommend dressing changes with each hemodialysis treatment.27 Either sterile gauze or sterile, transparent, semipermeable dressing can be used to cover the exit site.16 Both patient and environmental factors should be considered when selecting dressing type. Dressings should not be submerged in water. Dressings should be changed when they become damp, loose, soiled, nonocclusive, or nonadherent, and only trained dialysis staff should change catheter dressings.
Antimicrobials/Antiseptic Application to Catheter Exit Site
Chlorhexidine skin preparation has been shown to be superior to povidone-iodine and alcohol in the prevention of catheter-related infection.28,29 A solution containing 1% to 2% chlorhexidine gluconate in ≥70% ethyl or isopropyl alcohol (alcoholic chlorhexidine) should be used to cleanse the area. If chlorhexidine is contraindicated (eg, sensitivity, allergy), povidone-iodine 10% in 70% ethanol should be used. The antiseptic solution should be applied using friction, for at least 30 seconds and allow to air-dry without wiping or blotting.
Topical Antimicrobial Application
Antimicrobial application to exit site with dressing changes can reduce catheter-related infections. A meta-analysis by James et al30 examined whether topical or intraluminal antibiotic instillations compared with no antibiotic therapy reduced CRBSIs in adults undergoing hemodialysis. They showed topical antibiotics, compared with no antibiotic therapy, lowered bacteremia rates (rate ratio [RR]: 0.22, 95% confidence interval [CI]: 0.12-0.40) and exit-site infection rates (RR: 0.17, 95% CI: 0.08-0.38). However, several types of antimicrobial agents or co-intervention were used in the trials, thereby making it difficult to determine the impact of the topical antibiotic alone in reducing infection. Furthermore, many studies were of short duration and important clinical outcomes such as hospitalization and death were not reported.
A Cochrane review of interventions to prevent infectious complications associated with hemodialysis catheters was recently published.31 It included randomized controlled trials (RCTs) and quasi-RCTs investigating interventions to prevent infection but excluded impregnated catheters and antimicrobial solutions. Ten trials were included in their analysis. Their findings included the following:
-
Mupirocin ointment
Reduced the risk of exit-site infection (RR: 0.18, 95% CI: 0.06-0.60) caused by S aureus.
Reduced the risk of CRBSIs (RR: 0.17, 95% CI: 0.07-0.43).
-
Polysporin triple
Reduced the risk of CRBSIs (RR: 0.40, 95% CI: 0.19-0.86).
Associated with a reduction in all-cause mortality (RR: 0.22, 95% CI: 0.07-0.74), but no effect on infection-related mortality. This was based on a single study (Hemodialysis Infection Prevention with Polysporin, HIPPO study),3 which enrolled 169 hemodialysis patients with a central venous catheter. Patients were randomized to polysporin triple antibiotic ointment or placebo over a 6-month trial.
-
Povidone-iodine ointment
Reduced the risk of CRBSIs (RR: 0.10, 95% CI: 0.01-0.72).
The CDC’s Dialysis BSI Prevention Collaborative recommends application of an antibiotic ointment or povidone-iodine ointment to catheter exit sites during dressing changes.22 However, the practice of antimicrobial ointment application has not been widely adopted, in part due to concerns of development of antimicrobial resistance. Use of antimicrobial agents should be based on local infection rates and practice.
Antimicrobial Locks
Multiple RCTs and meta-analyses have been done assessing the role of antibiotic locks in the prevention of CRBSIs.30,32-36 All meta-analyses have shown a reduction in bacteremia rates with the use of antimicrobial locks. However, catheter lock solutions were sometimes used in conjunction with other preventive measures such as topical antimicrobial ointment, likely impacting findings. Furthermore, many studies had a short duration of follow-up, so long-term benefit or loss of efficacy, development of antimicrobial resistance, or other adverse effects could not be evaluated. Heterogeneity between studies and publication bias was also evident, thereby limiting interpretation and conclusions.
The only study of antibiotic locks in the prevention of CRBSIs that has demonstrated a mortality benefit is an observational study comparing gentamicin-citrate with heparin published in 2014.37 There were 555 patients (1350 catheters) included in this study, for a total of 84 326 days in the heparin group and 71 192 days in the gentamicin-citrate group. Compared with heparin, gentamicin-citrate was associated with decreased CRBSI rates (0.45 per 1000 catheter days vs 1.68 per 1000 catheter days; RR: 0.23, 95% CI: 0.13-0.38) and mortality (Hazard Ratio, HR: 0.32, 95% CI: 0.14-0.95).
The main concerns with prolonged use of antimicrobial lock solutions are the potential development of antimicrobial resistance and other adverse effects. This has resulted in cautious use of antibiotic locks, highlighting the need for further study in this area with longer follow-up duration, examining clinically important outcomes such as antibiotic resistance, hospitalization, and mortality.
Treatment of Catheter-Related Infection
Exit-Site Infection
Obtain cultures of any drainage from the exit site before administration of antibiotics. Treat empirically with antibiotics to cover gram-positive organisms. Modify the antibiotic regimen once culture and sensitivity results are available. Exit-site infections are typically treated for 7 to 14 days, depending on the microorganism isolated and local practice.
Tunnel Infection
Obtain cultures of any drainage from the exit site and send blood cultures from the catheter. The catheter should always be removed, without exchange over a wire. A new catheter should be inserted at a separate site. Start empiric broad-spectrum antibiotics to cover both gram-positive and gram-negative organisms. Modify antibiotic regimen when culture and sensitivity results are available. Tunnel infections, in the absence of a concurrent CRBSI, are typically treated for 10 to 14 days, depending on the microorganism isolated and local practice. If a CRBSI is also present, then duration of therapy will be determined by the management of the CRBSI (see section “Definitive management”).
Catheter-Related Bloodstream Infection
Empiric management
Blood cultures should be sent from the catheter, dialysis circuit, and peripheral sites if possible. A recent prospective study of 178 suspected CRBSIs in hemodialysis patients showed that blood culture results are the most sensitive, specific, and accurate for diagnosis when taken from the hemodialysis circuit and the venous catheter hub, compared with any combination with peripheral vein cultures.38 Broad-spectrum antibiotics should be initiated to cover both gram-positive and gram-negative organisms.14 Antibiotics should generally cover methicillin-resistant S aureus (MRSA) and Pseudomonas but are also dictated by local infection rates, dialysis center policies, and center-specific antimicrobial resistance patterns. Following initiation of empiric antibiotic therapy, it is crucial that culture sensitivity data are followed up in a timely manner, so that the most appropriate antibiotics based on sensitivity results can be used.
Definitive management
Definitive management of CRBSIs must be tailored to the clinical presentation of the patient, the microorganism isolated, and vascular access options of the patient. For example, management of the patient with septic shock secondary to MRSA CRBSI will differ from that of a hemodynamically stable patient presenting with a fever and found to have coagulase-negative staphylococcus. Treatment can be categorized into 3 groups: systemic antibiotics, antimicrobial locking (instillation) solutions, and catheter management. Comparison of treatment strategies is very challenging because many studies are observational design with different methodologies, and have differences in CRBSI definitions, as well as different outcome measures.
Systemic antibiotics
All patients with a CRBSI should receive systemic antibiotics, which will typically be administered for 2 to 6 weeks depending on the microorganism, clinical presentation, and complications.
Final decision on specific antibiotic agent(s) is dependent on final blood culture result and sensitivities, and whether or not patient has any allergies. If methicillin-sensitive S aureus (MSSA) infection is isolated, cefazolin is the preferred choice over vancomycin because it is associated with decreased hospitalization and death secondary to infection.39
Ease of administration is also a factor, ideally choosing agents that can be given to patients 3 times weekly for patients receiving conventional thrice weekly dialysis.
Drug dose and timing vary for those who are not on conventional thrice weekly dialysis (eg, short daily or nocturnal dialysis).
Antibiotic locks
May be used as adjunctive therapy to systemic antibiotics.
There are no randomized trials on the role of antibiotic locks in the treatment of CRBSIs, but several observational studies have shown similar eradication of bacteremia in patients treated with systemic antibiotics plus antibiotic lock compared with systemic antibiotics and catheter exchange or removal.40
A recent systematic review and meta-analysis of hemodialysis patients with tunneled dialysis catheters, with a CRBSI, compared 3 treatment protocols for CRBSIs: (1) systemic antibiotics alone, (2) systemic antibiotics plus antibiotic lock (catheter not removed), and (3) systemic antibiotics plus guidewire exchange.41 It included 28 retrospective and prospective studies, with a total of 1596 patients. Patients treated with systemic antibiotics and antibiotic lock had similar cure rates to those treated with systemic antibiotics and guidewire exchange, and both were superior to the rates obtained when antibiotics were used alone. Recurrence of infection with the same organism was not different between the systemic antibiotics plus antibiotic lock group and the systemic antibiotics plus guidewire exchange but was much higher in patients treated with systemic antibiotics alone, which further supports the practice to use an antibiotic lock or guidewire exchange in conjunction with systemic antibiotics.
Antibiotic locks should be used when immediate catheter removal is not possible and when catheter salvage attempted.
Catheter removal with replacement in new site
-
One option is immediate catheter removal, followed by placement of a temporary catheter, then conversion back to tunneled catheter. Indications for immediate removal are the following:
Severe sepsis
Hemodynamic instability
If fever or bacteremia persists 48 to 72 hours after initiation of antibiotics to which the organism is susceptible
Metastatic infection
Signs of tunnel infection
Fungal organisms.
Consider catheter removal for patients with CRBSIs due to S aureus, Pseudomonas species, and fungus.
A temporary nontunneled catheter should be inserted into another anatomical site.
In some cases, patients may not have any alternative site available for catheter insertion, and in these patients, catheter exchange over a wire or catheter salvage might be considered instead of catheter removal, regardless of microorganism isolated.
Catheter exchange over a guidewire
For CRBSIs due to other pathogens (eg, gram-negative bacilli other than Pseudomonas species or coagulase-negative staphylococci), empirical intravenous antibiotic therapy may be started without immediate catheter removal. If the symptoms that prompted initiation of antibiotic therapy (fever, chills, hemodynamic instability, or altered mental status) resolve within 2 to 3 days and there is no metastatic infection, then the infected catheter can be exchanged over a guidewire for a new tunneled catheter.14
Catheter salvage
Catheter salvage, defined as continued use of the same catheter throughout an episode of CRBSI, may be considered in cases of uncomplicated bacteremia, and in hemodialysis patients with limited vascular access. If attempted, systemic antibiotics and an adjunctive antibiotic lock should both be used. A recent meta-analysis showed similar cure rates in patients treated with systemic antibiotics and antibiotic lock as those treated with systemic antibiotics and guidewire exchange.41
Success rates for curing CRBSIs with catheter salvage (antibiotic locks plus systemic antibiotics) are dependent on organism.
-
Catheter salvage should not be used in the following situations:
S aureus, pseudomonas, and fungal infections
Unresolved infection symptoms 48 to 72 hours after initiation of antibiotics
Metastatic complications
Concomitant tunnel infection.
Infection Complications
Infection complications are thought to occur in ~15 - 40% of CRBSIs.6 These are most common for S aureus infections, with endocarditis being the most common. Other complications include vertebral osteomyelitis or discitis (2%-15%), and less commonly, spinal epidural abscess, septic arthritis, and septic pulmonary emboli. Mortality rates are high: Reports in the literature vary between 6% and 34% in all cases of CRBSI. Mortality is highest with S aureus infection complicated by metastatic complications, associated with 30% to 50% of mortality in these patients.6,42-44
Endocarditis
The most frequent and severe complication of CRBSIs.
Most common with S aureus, reported in 25% to 35% of S aureus bacteremias in hemodialysis patients, significantly higher than in S aureus infection in the general population.45 Next most common organisms are coagulase-negative staphylococci, enterococci, and viridans group streptococci.
Consider transthoracic echocardiography in all patients with S aureus CRBSIs.
Requires minimum 6 weeks’ intravenous antibiotic therapy.
Vertebral Osteomyelitis or Discitis
Most commonly caused by S aureus.
Fever and back pain are the most common presenting symptoms.
Plain film x-ray may be helpful to start, but diagnosis made primarily by computed tomography (CT) or magnetic resonance imaging (MRI).
If blood cultures are nondiagnostic for the organism, then CT-guided percutaneous aspiration of the disk space should be undertaken.
Requires minimum 6 weeks’ antibiotic therapy, and may require up to 3 months of intravenous antibiotic treatment.
Spinal Epidural Abscess
Uncommon.
Most common symptoms are back pain, fever, and weakness.
MRI is the best diagnostic modality.
Requires minimum 6 weeks’ antibiotic therapy, and all patients should have neurosurgical evaluation.
Septic Arthritis
Usually presents as an acute inflammatory monoar-thritis.
Knee, hip, shoulder, and ankle are most commonly affected joints.
Diagnosis should be made by joint aspiration, with fluid sent for cell count, Gram stain, cultures, and crystals.
Requires joint irrigation and debridement and minimum 2 weeks’ antibiotic therapy.
Infection Surveillance Program
An infection surveillance program that can facilitate identification of catheter-related infections and lead to timely intervention is key to monitoring catheter-related infections and their complications. Several studies have demonstrated the clinical benefits of an active surveillance program.46,47 Surveillance programs require dedicated teams and resources, and should monitor outcome measures including CRBSI rates, hospitalizations, and death. A multidisciplinary team of infection control personnel plays a critical role in preventing infections and improving outcomes.48 Effective surveillance programs should continuously monitor for opportunities for improvement, and engage staff and patients in the implementation and maintenance of strategies to prevent CRBSIs. A CRBSI surveillance program should include the following components:
Track catheter placement
Date and place of catheter insertion or removal
Selected vein for insertion
Type of catheter inserted—temporary versus tunneled, heparin coated, and so forth
Reason for insertion or removal
Monitor CRBSI rates
Identify all exit-site infections, tunnel infections, and CRBSIs
Identify type of bacteria isolated in each infection
Calculate infection rates (eg, as CRBSI per 1000 catheter days)
Set a benchmark rate of infection (eg, less than 1 per 1000 catheter days)49
Analyze infection rates on a routine basis (eg, quarterly, semiannually)
Monitor for development of antibiotic resistance
Intervene
Determine if infection rates are too high (ie, above the set benchmark)
If rates are high, design and implement intervention (eg, catheter care protocol, antimicrobial application)
Reevaluate the intervention: Has the benchmark been achieved?
Continue education and staff engagement.
Summary
CRBSIs are a major cause of hospitalization and mortality in hemodialysis patients.
Prevention is key! The CDC outlines 9 core preventive measures to reduce CRBSIs, including maximal barrier precautions with catheter insertion and catheter care, topical antibiotics, education, and surveillance.
Gram-positive organisms are responsible for most CRBSIs, with S aureus and coagulase-negative staphylococci comprising 40% to 80%.
S aureus bacteremia is associated with 30% to 50% mortality in hemodialysis patients, and most likely to cause metastatic complications.
Treatment strategies for CRBSIs can be categorized into systemic antibiotics, antibiotics locks, and catheter management.
CRBSI management decisions depend on clinical presentation of the patient, microorganism isolated, and vascular access options of the patient.
All CRBSIs require a minimum 2 to 3 weeks’ systemic antibiotic therapy.
S aureus CRBSIs and complicated infections should be treated with systemic antibiotic therapy for minimum 4 to 6 weeks.
Antibiotics locks have been shown to be effective adjunctive therapy to systemic antibiotics in the treatment of CRBSIs.
CRBSI catheter management options include immediate catheter removal with insertion of a temporary catheter at another site, guidewire exchange, or catheter salvage with an antibiotic lock.
Catheters should be removed in patients who are hemodynamically unstable, have metastatic complications, or have the following organisms on blood culture—S aureus, Pseudomonas, fungus.
If catheter salvage is attempted, an adjunctive lock should be used in conjunction with the systemic antibiotics.
Every dialysis program should have an infection surveillance program with dedicated personnel and resources, to facilitate identification of catheter-related infections and timely interventions to reduce infection rates and improve patient clinical outcomes.
Acknowledgments
The authors thank Kirsten Campbell for her editing and management expertise.
Footnotes
Ethics Approval and Consent to Participate: Ethics approval and consent to participate was not required for this trial.
Consent for Publication Availability: Consent for publication was obtained from all authors.
Availability of Data and Materials: There is no data to share.
Author Contributions: L.M.M. designed and coordinated the review, drafted the manuscript, and critically revised the manuscript at all stages. E.C., S.H., J.K., C.L., and L.M. helped draft the manuscript and provided critical review. C.D., M.K., M.O., and R.L. provided critical review. J.M. conceived, designed, and coordinated the review, and critically revised the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Vascular Access Work Group was supported by the Canadian Society of Nephrology with an unrestricted educational grant from Roche Pharmaceuticals, Canada.
References
- 1. Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in US hemodialysis patients. Kidney Int. 2001;60(4):1443-1451. [DOI] [PubMed] [Google Scholar]
- 2. Lee T, Barker J, Allon M. Tunneled catheters in hemodialysis patients: reasons and subsequent outcomes. Am J Kidney Dis. 2005;46(3):501-508. [DOI] [PubMed] [Google Scholar]
- 3. Lok CE, Stanley KE, Hux JE, Richardson R, Tobe SW, Conly J. Hemodialysis infection prevention with polysporin ointment. J Am Soc Nephrol. 2003;14(1):169-179. [DOI] [PubMed] [Google Scholar]
- 4. Weijmer MC, van den Dorpel MA, Van de Ven PJ, et al. Randomized, clinical trial comparison of trisodium citrate 30% and heparin as catheter-locking solution in hemodialysis patients. J Am Soc Nephrol. 2005;16(9):2769-2777. [DOI] [PubMed] [Google Scholar]
- 5. Weijmer MC, Vervloet MG, ter Wee PM. Compared to tunnelled cuffed haemodialysis catheters, temporary untunnelled catheters are associated with more complications already within 2 weeks of use. Nephrol Dial Transplant. 2004;19(3):670-677. [DOI] [PubMed] [Google Scholar]
- 6. Lok CE, Mokrzycki MH. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int. 2011;79(6):587-598. [DOI] [PubMed] [Google Scholar]
- 7. Krishnasami Z, Carlton D, Bimbo L, et al. Management of hemodialysis catheter-related bacteremia with an adjunctive antibiotic lock solution. Kidney Int. 2002;61(3):1136-1142. [DOI] [PubMed] [Google Scholar]
- 8. Poole CV, Carlton D, Bimbo L, Allon M. Treatment of catheter-related bacteraemia with an antibiotic lock protocol: effect of bacterial pathogen. Nephrol Dial Transplant. 2004;19(5):1237-1244. [DOI] [PubMed] [Google Scholar]
- 9. Sychev D, Maya ID, Allon M. Clinical management of dialysis catheter-related bacteremia with concurrent exit-site infection. Semin Dial. 2011;24(2):239-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tomlinson D, Mermel LA, Ethier MC, Matlow A, Gillmeister B, Sung L. Defining bloodstream infections related to central venous catheters in patients with cancer: a systematic review. Clin Infect Dis. 2011;53(7):697-710. [DOI] [PubMed] [Google Scholar]
- 11. Mermel LA. Defining intravascular catheter-related infections: a plea for uniformity. Nutrition. 1997;13(4)(suppl):2S-4S. [DOI] [PubMed] [Google Scholar]
- 12. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(suppl 1):S248-S273. [DOI] [PubMed] [Google Scholar]
- 13. O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2002;35(11):1281-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nicolle L, Conly J, Johnston L, et al. Preventing infections associated with indwelling intravascular access devices. Can Commun Dis Rep. 1997;23(suppl 8):i-iii, 1,-32, i,-iv, 31-16. [PubMed] [Google Scholar]
- 16. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections; 2011. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3106267/pdf/cir138.pdf. Accessed August 26, 2016. [DOI] [PMC free article] [PubMed]
- 17. Lata C, Girard L, Parkins M, James MT. Catheter-related bloodstream infection in end-stage kidney disease: a Canadian narrative review. Can J Kidney Health Dis. 2016;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tokars JI, Light P, Anderson J, et al. A prospective study of vascular access infections at seven outpatient hemodialysis centers. Am J Kidney Dis. 2001;37(6):1232-1240. [DOI] [PubMed] [Google Scholar]
- 19. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732. [DOI] [PubMed] [Google Scholar]
- 20. Costello JM, Morrow DF, Graham DA, Potter-Bynoe G, Sandora TJ, Laussen PC. Systematic intervention to reduce central line-associated bloodstream infection rates in a pediatric cardiac intensive care unit. Pediatrics. 2008;121(5):915-923. [DOI] [PubMed] [Google Scholar]
- 21. Guerin K, Wagner J, Rains K, Bessesen M. Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control. 2010;38(6):430-433. [DOI] [PubMed] [Google Scholar]
- 22.CDC Approach to BSI Prevention in Dialysis Facilities (i.e., the Core Interventions for Dialysis Bloodstream Infection (BSI) Prevention). http://www.cdc.gov/dialysis/prevention-tools/core-interventions.html. Accessed August 26, 2016.
- 23. Clark E, Kappel J, MacRae JM, et al. ; on behalf of the Canadian Society of Nephrology Vascular Access Work Group. Practical aspects of nontunneled and tunneled hemodialysis catheters. Can J Kidney Health Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller L, MacRae JM, Kiaii M, et al. ; on behalf of the Canadian Society of Nephrology Vascular Access Work Group. Hemodialysis tunneled catheter noninfectious complications. Can J Kidney Health Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parienti JJ, Thirion M, Megarbane B, et al. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: a randomized controlled trial. JAMA. 2008;299(20):2413-2422. [DOI] [PubMed] [Google Scholar]
- 26. Raad II, Hohn DC, Gilbreath BJ, et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15(4, pt 1):231-238. [PubMed] [Google Scholar]
- 27. Jindal K, Chan CT, Deziel C, et al. Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. J Am Soc Nephrol. 2006;17(3)(suppl 1):S1-27. [DOI] [PubMed] [Google Scholar]
- 28. Onder AM, Chandar J, Billings A, et al. Chlorhexidine-based antiseptic solutions effectively reduce catheter-related bacteremia. Pediatr Nephrol. 2009;24(9):1741-1747. [DOI] [PubMed] [Google Scholar]
- 29. Chaiyakunapruk N, Veenstra DL, Lipsky BA, Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med. 2002;136(11):792-801. [DOI] [PubMed] [Google Scholar]
- 30. James MT, Conley J, Tonelli M, Manns BJ, MacRae J, Hemmelgarn BR. Meta-analysis: antibiotics for prophylaxis against hemodialysis catheter-related infections. Ann Intern Med. 2008;148(8):596-605. [DOI] [PubMed] [Google Scholar]
- 31. McCann M, Moore ZE. Interventions for preventing infectious complications in haemodialysis patients with central venous catheters. Cochrane Database Syst Rev. 2010(1):CD006894. [DOI] [PubMed] [Google Scholar]
- 32. Jaffer Y, Selby NM, Taal MW, Fluck RJ, McIntyre CW. A meta-analysis of hemodialysis catheter locking solutions in the prevention of catheter-related infection. Am J Kidney Dis. 2008;51(2):233-241. [DOI] [PubMed] [Google Scholar]
- 33. Labriola L, Crott R, Jadoul M. Preventing haemodialysis catheter-related bacteraemia with an antimicrobial lock solution: a meta-analysis of prospective randomized trials. Nephrol Dial Transplant. 2008;23(5):1666-1672. [DOI] [PubMed] [Google Scholar]
- 34. Yahav D, Rozen-Zvi B, Gafter-Gvili A, Leibovici L, Gafter U, Paul M. Antimicrobial lock solutions for the prevention of infections associated with intravascular catheters in patients undergoing hemodialysis: systematic review and meta-analysis of randomized, controlled trials. Clin Infect Dis. 2008;47(1):83-93. [DOI] [PubMed] [Google Scholar]
- 35. Rabindranath KS, Bansal T, Adams J, et al. Systematic review of antimicrobials for the prevention of haemodialysis catheter-related infections. Nephrol Dial Transplant. 2009;24(12):3763-3774. [DOI] [PubMed] [Google Scholar]
- 36. Snaterse M, Ruger W, Scholte Op, Reimer WJ, Lucas C. Antibiotic-based catheter lock solutions for prevention of catheter-related bloodstream infection: a systematic review of randomised controlled trials. J Hosp Infect. 2010;75(1):1-11. [DOI] [PubMed] [Google Scholar]
- 37. Moore CL, Besarab A, Ajluni M, et al. Comparative effectiveness of two catheter locking solutions to reduce catheter-related bloodstream infection in hemodialysis patients. Clin J Am Soc Nephrol. 2014;9(7):1232-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quittnat Pelletier F, Joarder M, Poutanen SM, Lok CE. Evaluating approaches for the diagnosis of hemodialysis catheter-related bloodstream infections. Clin J Am Soc Nephrol. 2016;11(5):847-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chan KE, Warren HS, Thadhani RI, et al. Prevalence and outcomes of antimicrobial treatment for Staphylococcus aureus bacteremia in outpatients with ESRD. J Am Soc Nephrol. 2012;23(9):1551-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Capdevila JA, Segarra A, Planes AM, et al. Successful treatment of haemodialysis catheter-related sepsis without catheter removal. Nephrol Dial Transplant. 1993;8(3):231-234. [PubMed] [Google Scholar]
- 41. Aslam S, Vaida F, Ritter M, Mehta RL. Systematic review and meta-analysis on management of hemodialysis catheter-related bacteremia. J Am Soc Nephrol. 2014;25(12):2927-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doulton T, Sabharwal N, Cairns HS, et al. Infective endocarditis in dialysis patients: new challenges and old. Kidney Int. 2003;64(2):720-727. [DOI] [PubMed] [Google Scholar]
- 43. Spies C, Madison JR, Schatz IJ. Infective endocarditis in patients with end-stage renal disease: clinical presentation and outcome. Arch Intern Med. 2004;164(1):71-75. [DOI] [PubMed] [Google Scholar]
- 44. Shroff GR, Herzog CA, Ma JZ, Collins AJ. Long-term survival of dialysis patients with bacterial endocarditis in the United States. Am J Kidney Dis. 2004;44(6):1077-1082. [DOI] [PubMed] [Google Scholar]
- 45. Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345(18):1318-1330. [DOI] [PubMed] [Google Scholar]
- 46. Axon RN, Engemann JJ, Butcher J, Lockamy K, Kaye KS. Control of nosocomial acquisition of vancomycin-resistant Enterococcus through active surveillance of hemodialysis patients. Infect Control Hosp Epidemiol. 2004;25(5):436-438. [DOI] [PubMed] [Google Scholar]
- 47. Hannah EL, Stevenson KB, Lowder CA, et al. Outbreak of hemodialysis vascular access site infections related to malfunctioning permanent tunneled catheters: making the case for active infection surveillance. Infect Control Hosp Epidemiol. 2002;23(9):538-541. [DOI] [PubMed] [Google Scholar]
- 48. Hess S, Bren V. Essential components of an infection prevention program for outpatient hemodialysis centers. Semin Dial. 2013;26(4):384-398. [DOI] [PubMed] [Google Scholar]
- 49. Beathard GA, Urbanes A. Infection associated with tunneled hemodialysis catheters. Semin Dial. 2008;21(6):528-538. [DOI] [PubMed] [Google Scholar]


