Abstract
Nanotechnology-enabled sensors (or nanosensors) will play an important role in enabling the progression toward ubiquitous information systems as the Internet of Things (IoT) emerges. Nanosensors offer new, miniaturized solutions in physiochemical and biological sensing that enable increased sensitivity, specificity, and multiplexing capability, all with the compelling economic drivers of low cost and high-energy efficiency. In the United States, Federal agencies participating in the National Nanotechnology Initiative (NNI) “Nanotechnology for Sensors and Sensors for Nanotechnology: Improving and Protecting Health, Safety, and the Environment” Nanotechnology Signature Initiative (the Sensors NSI), address both the opportunity of using nanotechnology to advance sensor development and the challenges of developing sensors to keep pace with the increasingly widespread use of engineered nanomaterials. This perspective article will introduce and provide background on the NNI signature initiative on sensors. Recent efforts by the Sensors NSI aimed at promoting the successful development and commercialization of nanosensors will be reviewed and examples of sensor nanotechnologies will be highlighted. Future directions and critical challenges for sensor development will also be discussed.
Keywords: nanosensors, engineered nanomaterials, commercialization, data quality, life cycle
SENSING AND THE NANOSCALE: A NATURAL FIT
A sensor produces a measurable signal as a result of physical, chemical, biological, or any combination of the aforementioned stimuli.1 This process inherently relies on engineering components within the sensor system that can sample, transduce, and analyze a given signal.1 Engineered nanomaterials (ENMs) possess unique physiochemical characteristics that can fundamentally enhance known measures of sensor performance related to sensitivity and specificity by orders of magnitude, while offering unique opportunities to miniaturize devices in order to achieve ubiquitous sensing.2–5 First, the high surface-to-volume ratio of materials at the nanoscale allows for enhanced chemical reactivity, a feature that can be modulated by particle type, shape, and surface topography.4 Second, the ability to precisely craft nanomaterials with functional ligands can confer single-molecule sensitivity and specificity.6 Third, an important attribute of ENMs is the possibility to engineer them as highly integrated systems that can offer more rapid and multiplexed detection of analytes using advanced transduction mechanisms.7–9
The ability to synthesize a vast range of nanomaterial types has broadened the sets of tools available for researchers to explore a range of sensing application areas, including: medicine, workplace safety, environmental monitoring, agriculture and food industries, energy, manufacturing, transportation, and defense.1 Several types of ENMs have been demonstrated for sensing applications, from carbon-based nanomaterials,10 metallic and semiconducting nanoparticles11 and nanowires12 to nanopore13,14 and nucleic acid-based15 platforms (see Figure 1). Metallic nanoparticles (including metal alloys and oxides) have been broadly adopted to detect chemical and biological agents; gold nanoparticles in particular have been demonstrated as suitable sensing platforms for the detection of pathogens and biomolecules due to their tunable plasmonic properties.16 Variations in ENM shape also present an opportunity to further modulate sensing behavior. For instance, detection sensitivity in nanowires (and nanotubes) has been shown to vary inversely with their radius.17 Nanowires also confer long optical absorption path lengths and strong light trapping in higher density arrays,18 which is optimal for photonic-based applications. On the other hand, spherical nanoparticles can provide distinctive ligand surface coverage from nanowires (up to a certain size in similar chemical conditions) due to differences in the radius of curvature.19 In the case of two-dimensional ENMs (e.g., graphene or transition metal dichalcogenides), charge carrier concentrations induced by adsorption or binding events can be used for the detection of individual molecules.20
Figure 1.
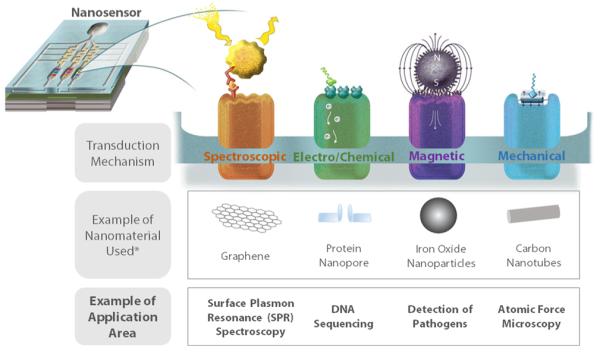
Examples of nanomaterials used by transduction mechanism and application area, including surface plasmon resonance (SPR) spectroscopy,89 DNA sequencing,90 pathogen detection,91 and atomic force (AFM) microscopy.92 (*) The nanoparticle types described in this figure can be used for other type(s) of transduction mechanisms (e.g., CNTs can be used for spectroscopic sensors). Reprinted (Adapted or Reprinted in part) with permission from Nanoscale Science, Engineering, and Technology Subcommittee of the Committee on Technology. Nanotechnology-Enabled Sensing: Report of the National Nanotechnology Initiative Workshop; National Science and Technology Council: Washington, District of Columbia, 2010. Copyright 2010/NNCO.
NATIONALLY COORDINATED EFFORT TO SUPPORT THE DEVELOPMENT OF NANOTECHNOLOGY FOR SENSORS AND SENSORS FOR NANOTECHNOLOGY
In 2012, recognizing the considerable potential for nanotechnology to facilitate the development of inexpensive portable devices for the rapid detection, identification, and quantification of biological and chemical substances, as well as the need to develop sensors to detect nanomaterials in complex media, the U.S. National Nanotechnology Initiative (NNI) launched its fifth Nanotechnology Signature Initiative (NSI), entitled Nanotechnology for Sensors and Sensors for Nanotechnology: Improving and Protecting Health, Safety, and the Environment (or the Sensors NSI).3 The NSIs place a spotlight on areas that can be more quickly advanced through enhanced coordination and collaboration of the participating Federal agencies, seed communities of interest, and develop public–private partnerships as appropriate.21 The Federal agencies participating in the Sensors NSI include the Consumer Product Safety Commission (CPSC), Department of Defense (DOD), Environmental Protection Agency (EPA), Food and Drug Administration (FDA), National Aeronautics and Space Administration (NASA), National Institutes of Health (NIH), National Institute for Occupational Safety and Health (NIOSH), National Institute of Standards and Technology (NIST), National Science Foundation (NSF), and the United States Department of Agriculture (USDA).22
The priorities and goals of the Sensors NSI reflect the interests and activities of the participating agencies, which span the sensor development life cycle (Figure 2) from mission evaluation and research and development to sensor testing, deployment, and use.3,23 An important function of the initiative is to bring together agency representatives to identify resources and technologies supported by one agency that can further the objectives of another. Enhanced communication between agencies has enabled interactions and collaborations in areas such as standards and devices of shared relevance, and facilitated development of cross-agency initiatives dedicated to sensors. Beyond interagency activities, the Sensors NSI has engaged the sensors community through a targeted Request for Information (RFI);24 a sensors web portal;25 workshops and town hall meetings embedded in international conferences;26 and webinars.27 The use of these mechanisms and the resulting impact on the direction of the initiative are discussed in more detail below.
Figure 2.
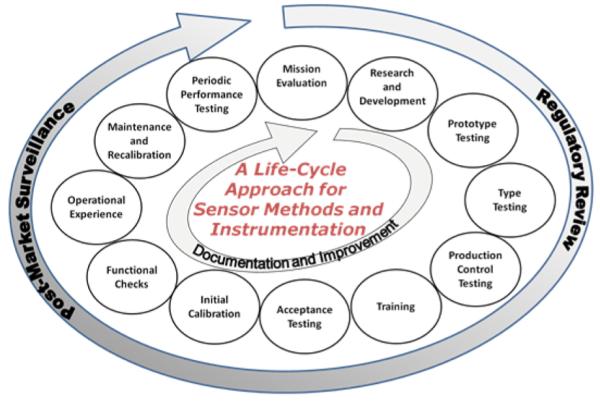
Life cycle approach to development and application of sensor sampling methods and instrumentation.3,23 Reprinted (Adapted or Reprinted in part) with permission from Nanoscale Science, Engineering, and Technology Subcommittee of the Committee on Technology. Nanotechnology for Sensors and Sensors for Nanotechnology: Improving and Protecting Health, Safety, and the Environment; National Science and Technology Council: Washington, District of Columbia, 2012. Copyright 2012/NNCO.
Over the past several years, significant progress has been made toward the goals of this initiative. Sensing devices and capabilities have been developed that are enabled by nanotechnology, and that are designed to sense nanomaterials in complex media. A few examples are described herein, followed by a discussion of future directions as well as key challenges.
ADVANCING THE SUCCESSFUL DEVELOPMENT AND COMMERCIALIZATION OF SENSOR NANOTECHNOLOGIES
The spotlight provided by the signature initiative has resulted in several collaborative activities as illustrated in the following examples. Food safety and quality is an area of sustained common interest among agency participants of the Sensors NSI. The U.S. FDA Center for Veterinary Medicine (CVM) has a long-standing interest in rapid identification of species in food samples, to detect both counterfeiting and contamination in the food supply. To this end, CVM has pursued the use of DNA barcoding in regulatory science, in which short chains of genetic material are used to uniquely identify species.28 CVM was interested in collaborating with external partners to develop a hand-held device with the ability to extract and amplify DNA to compare to reference libraries for species identification. Through the initiative, CVM researchers were connected with a group developing similar technologies with support from USDA, and were able to initiate a project to test the feasibility of combining FDA’s barcode database with the group’s device.
Another topic of interest to both regulatory and mission-oriented agencies is the appropriate use of standards and standardized methods when evaluating nanosensor performance. For example, methods for characterizing nanoparticle surface properties in the context of biological sensing and the confounding effects of nanoparticles on optical protein assays are of interest to both FDA and National Cancer Institute (NCI) representatives. Discussions stemming from the Sensors NSI interactions led to the sharing of techniques developed by NCI supported researchers and laboratories,29 as well as heightened participation by FDA staff members in NCI planning, education, and outreach efforts, such as the caNanoPlan 2015.30
The Sensors NSI activities intersect with and benefit other Federal efforts such as NSF’s recently launched Innovations at the Nexus of Food, Energy and Water Systems (INFEWS) program,31 particularly in the areas of precision agriculture and food and water monitoring. INFEWS is intended to support both fundamental studies to understand food, energy, and water systems and technological approaches to enable sustainable use of natural resources for a growing population. Major challenges are being identified and being addressed through workshops and Dear Colleague Letters. The development of new sensing modalities and field-deployable sensors for real-time monitoring of analytes of interest, such as phosphorus and nitrogen containing species, is a priority.32 Representatives of the Sensors NSI are actively engaged in these efforts and enable leveraging of these and other agency activities.
In addition to enhancing communication between representatives of member agencies, the Sensors NSI has provided a means for agencies to reach out to the research community and provide information about resources and opportunities in nanosensor development. The initiative has participated in the annual TechConnect World Innovation Conference each year since 2013,33 hosting symposia and Town Hall sessions, primarily as a means to share information about funding opportunities, facilities, and resources hosted or supported by agencies that are available for external researchers.34
As the initiative progressed, it became clear from interactions with sensor and device developers as well as participating agency representatives that numerous areas of uncertainty and debate exist in sensor development, particularly in the later stages of the development life cycle. To get feedback from the community regarding the state of the field, a Request for Information (RFI) centered on five crucial areas to sensor development and commercialization—standards, testing, manufacturing, commercialization, and regulation—was released.24 Questions were aimed at elucidating the purpose-built protocols and practices being used by the nanosensor community as well as the community’s sense of the usefulness of sanctioned standards, protocols, and facilities.
Common themes emerged from the wide variety of responses, representing constituents from industry and standards organizations to academic centers.35 These themes included the centrality of sensors to the emerging IoT, and the associated importance of data security for acceptance of ubiquitous sensing. This indicates an important role for regulators and a need to understand the implications of potentially wide access to data from personal or commercially hosted sensors. A paucity of realistic test beds was noted in order to create conditions beyond standard lab capabilities or provide access to expensive and highly specialized testing equipment for device specification. One of the most serious challenges noted was the lack of reproducibility in the synthesis and packaging of nanomaterials, a concern with relevance to both nanotechnology enabled chemical and biological sensors and sensors for nanomaterials. Respondents also noted a need for better communication and collaboration between stakeholders, and for assistance in identifying contacts and appropriate guidance in regulatory agencies.
Beyond the common themes noted, specific concerns of significant relevance to the initiative were also identified.35 These included a general lack of sanctioned standards for important measurements in sensor performance, such as the unit of measurement of sensitivity, and well-defined nanomaterial properties. Measurement protocols and disposal procedures are also missing or ad hoc for nanomaterials. Related to the oncoming era of widespread, distributed sensing, there is no clear distinction in standards for individual sensors versus networks of sensors and a lack of standards for interoperability and data security. This last issue exists both up and down the supply chain and laterally, as complementary applications and devices are linked to provide distributed sensing capability.
The results of the RFI were used to plan a Sensor Fabrication, Integration and Commercialization Workshop for September 11–12, 2014.36 The findings of this workshop, which brought together representatives from funding and regulatory agencies, large industry, small companies, venture capital, and academic researchers interested in sensor development, were published in a report in June 2015.37 Workshop participants identified a number of needs that must be met to accelerate the development and commercialization of nanosensors. These needs included facilities for device testing and manufacture, and better information regarding resources available for these tasks. It was clear from the workshop discussions that the community was not aware of the vast array of available resources supported by the Federal government and that there was considerable confusion over regulatory requirements, especially regarding use of nanomaterials in commercial devices.
In response to community feedback from the RFI and workshop, participants in the Sensors NSI worked with the NNCO to gather information and resources for sensor development into a sensors web portal.25 The web portal is a dynamic resource for the sensor development community, with frequent updates of news related to sensor research and commercialization and up-to-date information on federal funding opportunities and facilities. The portal hosts extensive information on existing regulatory guidance for sensor development (e.g., for medical diagnostics) and use of nanomaterials in devices, international standards for sensor performance, reference nanomaterials, and federal programs and private organizations supporting sensor development. Links are also provided to user facilities that can be used to design, characterize, and prototype sensor devices, such as the Center for Nanoscale Science and Technology at NIST,38 as well as privately owned facilities for fabrication of devices on small and medium scales. Databases of nanoparticle information and potential federal partners for studying nanomaterials are also accessible through this portal.
Furthermore, the Sensors NSI also conducted a series of webinars, in coordination with the Nanotechnology Knowledge Infrastructure (NKI) NSI, to provide technical and regulatory guidance on the development of nanosensors, along with information on data resources for nanomaterials. Nanosensors webinars focused on “Nanotechnology Sensors and Applications,” (October 16, 2015, Dr. Meyya Meyyappan, NASA) and “A Regulatory Case Study for the Development of Nanosensors,” (November 3, 2015, Dr. Kim Sapsford, FDA) along with a joint webinar with the NKI NSI, “All Hands on Deck for Data Quality” (December 11, 2015). Archived versions of these webinars are available on nano.gov.27
The Sensors NSI will continue to work together and engage with the community to advance the commercialization of nanosensors. The collaborative activities described above illustrate the ability of the signature initiative mechanism to bring together representatives to leverage agency programs, and respond to and support the needs of the sensors community.
EXAMPLES OF RESEARCH EFFORTS AIMED AT DEVELOPING NANOTECHNOLOGY-ENABLED SENSORS AND SENSORS FOR NANOTECHNOLOGY
Nanotechnology-Enabled Sensors
The attractive properties of ENMs have enabled the development of chemical and biosensors with superior sensitivity and other figures of merit relative to the state-of-the-art sensors. Commercial sensors for gas sensing are based on tin oxide thin films or conducting polymers; most ENMs can offer better sensitivity and lower power consumption in addition to smaller size and weight due to their large surface area and excellent electronic properties.4 Recent work from Mulchandani and colleagues have exploited the large surface area of single-walled carbon nanotubes (SWCNTs) to detect the presence of volatile organic compounds,39 in this case by functionalizing SWCNTs with iron tetraphenyl porphyrin40 or with a conducting polymer.41 Several other examples of nanosensor arrays for environmental monitoring and aided by pattern recognition tools have been shown to detect gases such as CH4, CO, H2S, Cl2, NH3, or landfill gas.42–44 More recently, some research efforts have focused on exploiting the unique physiochemical properties of graphene and other two-dimensional nanomaterials for sensing applications. For example, boron-doped graphene sensors have been reported with the ability to detect NO2 and NH3 at parts per billion levels or lower.45
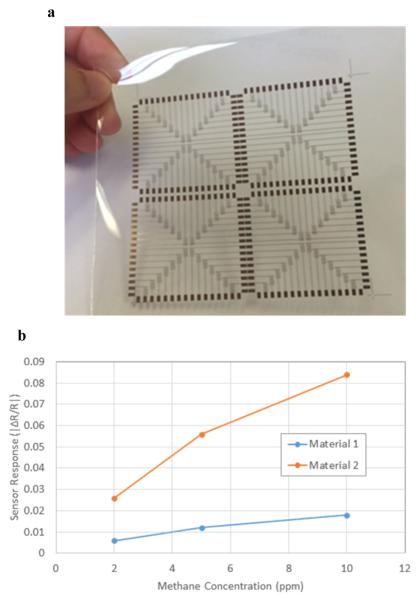
Often, sensor construction has been on silicon substrates using conventional microfabrication techniques with the nanomaterials serving as the sensing medium. Recently, flexible substrates such as paper, plastic, and textiles have been considered for sensor fabrication. Inkjet printing and other unconventional techniques have been developed to deposit the nanomaterial on soft substrates in order to allow large scale manufacturing (for example, roll-to-toll) of sensors and reduce the fabrication costs. Figure 3a depicts a chemical sensor platform printed on poly(ethylene) naphthalate (PEN) foil for the detection of methane leaks from natural gas wells. Other constituents in the leaks may include small quantities of ethane, propane, butane, and H2S. SWCNTs functionalized with COOH groups and coated with polyaniline respond well to methane with a change in conductivity as seen in Figure 3b. The sensor foil consists of interdigitated electrodes covered with the above two nanosensing materials and attempts to mimic the performance of an earlier generation methane sensor constructed on a silicon wafer.46
Figure 3.
(a) Image of a chemical sensor platform printed on poly(ethylene) naphthalate (PEN) foil for the detection of methane leaks from natural gas wells. (b) Sensor calibration data using the chemical sensor for methane detection. Material 1 is SWCNTs coated with polyaniline and material 2 is COOH-functionalized SWCNTs. Figure courtesy of Xerox PARC.
The need for biosensors is ubiquitous in clinical diagnostics and point-of-care health services.12,47,48 Significant progress has been achieved in the development of sensing platforms that can reliably detect protein analyte for applications in gene-sequencing,49 cancer therapy,50 and neuroscience.51 Over the past two decades, the Mirkin laboratory has developed a broad set of ultrasensitive methods based on deoxyribonucleic acid (DNA) gold nanoparticle conjugates.52–54 These studies have led to the launch of the Verigene system, which is now commercially used for molecular diagnostics applications.55 Research by Lieber and colleagues has enabled the development of nanowire-based sensor systems for electrophysiological applications.12 This includes nanowire-based field-effect transistors that can record potentials from single cells56 to multiplexed local field potentials in vivo.57 For DNA and protein analysis, significant attention has been placed on graphene-based sensors thanks to their atomic thickness and unique electrochemical properties.58 Next-generation nanopores using graphene, for instance, have been under development at Oxford Nanopore Technologies.59 These “solid-state” nanopore sensors aim to improve on the performance of commercially used protein nanopores by optimizing spatial resolution and reliability.59–61
More recently, applications of biosensors have been geared toward the detection of infectious disease. In response to the 2014–2015 Ebola outbreak, the U.S. FDA authorized the emergency use of the Corgenix ReEBOV Antigen Rapid Test for the presumptive detection of Ebola Zaire virus infection in whole blood, plasma, or serum from individuals with signs and symptoms of Ebola virus infection.62 The test kit uses antigens conjugated to gold nanoparticles to detect the virus.63 In 2014, FDA also cleared two T2 Biosystems, Inc., products—T2Candida Panel and the T2Dx Instrument—for the direct detection of Candida species in human whole blood specimens from patients with symptoms of, or medical conditions predisposing the patient to, invasive fungal infections.64 The technology utilizes magnetic biosensor nanotechnology designed to enable detection of nucleic acids.65
Due to the extensive range of societal applications, chemical and biological sensors have received much attention from the nanotechnology community. In contrast, the utility of nanomaterials for radiation sensing has not been extensively investigated. Interest in radiation sensors ranges from threat detection to monitoring radiation levels in crew cabins in planetary exploration. Properties of some inorganic nanowires are modified when exposed to energetic radiation,66,67 and this behavior can be exploited in developing radiation sensors. Bulk single crystal CdZnTe (CZT) is currently used in commercial gamma ray detectors. High detection efficiency requires a large volume of material with low defect density, which makes the manufacturing expensive. Preliminary results show that electrodeposited CZT nanowires can be used as radiation detector material at room temperature with a low bias (below 2 V).67 Metal oxide semiconductor field effect transistors (MOSFETs) are typically used as radiation detectors with semiconductor thin or thick films.
Sensors for the Detection and Monitoring of Engineered Nanomaterials
As applications for ENMs continue to rise and nanomaterial production scales up, the need to directly monitor nanoscale materials across their entire life cycle becomes greater. Direct monitoring of ENMs throughout the full cradle-to-grave life cycle, including the material manufacturing process, product integration, application, and disposal, will require precise detection strategies and advanced sensor platforms to fully meet environmental, health, and safety (EHS) standards. The multifaceted nature of this problem is exemplified by the wide variety of ENM types (e.g., varying compositions, morphologies, surface functional groups, etc.), complex sampling media (e.g., air, biological fluids, wastewater, soil, etc.), and unknown reactivity/aggregation dynamics during the materials life cycle that can alter the material’s structure/signature.68 As such, no single detection strategy will be viable for all situations, and multiple sensor platforms/methodologies will need to be employed to span the ENM life cycle.
From an exposure viewpoint, NIOSH has developed Recommended Exposure Limits (RELs) for select ENMs including CNTs (<1.0 μg/m3, 8 h TWA)69 and nanoscale TiO2 (<0.3 mg/m3, 10 h TWA).70 It is important to note that exposure limits for many ENM compositions and morphologies have yet to be fully developed. The National Institute of Environmental Health Science (NIEHS) of the NIH has developed a keen focus on gaining fundamental understanding of the interactions of ENMs within biological systems, to better understand the potential health risks associated with ENM exposure through the Nanotechnology Environmental Health and Safety program and NIEHS Centers for Nanotechnology Health Implications Research consortium. Knowledge gained through programs such as these is archived in the NIH-led nanomaterials registry,71 where research data on ENMs and their biological and environmental implications can be found to guide the safe use of nanomaterials and highlight unique properties that can be leveraged toward new ENM sensor designs.
To date, multiple ENM detection strategies have been employed depending on the targeted material and environmental conditions. Standard approaches commonly involve analytical techniques such as aerosol mass spectrometry, size-exclusion chromatography, electron microscopy, dynamic light scattering, X-ray crystallography, UV–vis-nIR absorption/fluorescence, Raman, and atomic force microscopy. These methodologies have demonstrated a great deal of efficacy but may not be amenable for wide deployment due to the requirement for advanced scientific instrumentation operated by highly skilled technicians, the time-consuming nature of the measurements, the large dependence on sample preparation/collection procedures, and the inability to perform target detection in real time. Currently the EPA has yet to approve any standardized methods for sampling, detecting, or quantifying nanomaterials in aqueous media, indicating the critical need for new sensor designs and approaches.72
In the case of CNTs, for example, a new detection scheme is emerging that holds the potential to detect and quantify CNT exposure/contamination across the material’s entire life cycle. Harnessing the sharp thermal signature of microwave-irradiated single and multiwalled CNTs, quantifiable detection at levels below 0.1 μg has been demonstrated.73 The simple setup, as schematically represented in Figure 4, has been shown to be amenable to directly detect the presence of CNTs and quantify material loading in complex, heterogeneous structures including plant roots73 and bioaccumulation levels in earthworm models.74 Current efforts to validate CNT detection/quantification in air collection samples could further demonstrate the viability of microwave-based CNT detection across the material life cycle.
Figure 4.
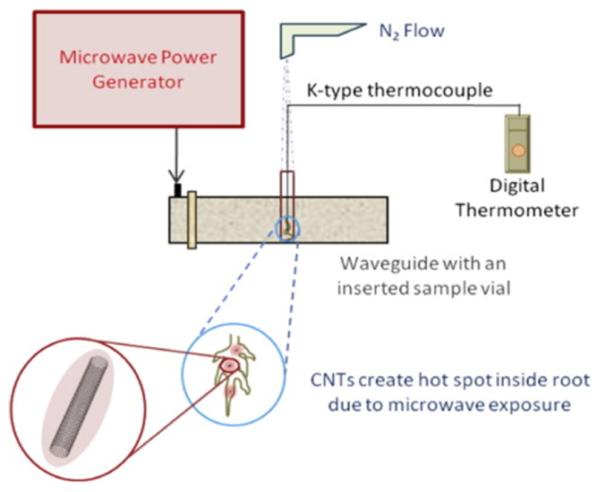
Schematic of microwave-based CNT detection scheme and corresponding thermal heating within biological samples.73 Reprinted (Adapted or Reprinted in part) with permission from Irin, F.; Shrestha, B.; Cañas, J. E.; Saed, M. A.; Green, M. J. Detection of Carbon Nanotubes in Biological Samples through Microwave-Induced Heating. Carbon 2012, 50, 4441–4449. Copyright 2012/Elsevier.
All Hands on Deck to Improve Data Quality
A challenge across the entire sensor life cycle is how to define and confirm data quality in a manner that is mission-relevant.Figure 5 illustrates an activity in response to that challenge, which the Sensors NSI is pursuing in collaboration with the NKI.75 The goal is to define the concept of “Data Readiness Levels (DRLs)” in a manner that conveys the maturity of the data and the suitability of the data for a specific purpose. This approach has similarities to the method used widely in the government to assign Technology Readiness Levels for equipment and devices.
Figure 5.

Conceptual approach to “Data Readiness Level” as a measure of data maturity.75 Reprinted (Adapted or Reprinted in part) with permission from The Nanotechnology Knowledge Infrastructure (NKI) Signature Initiative: Enabling National Leadership in Sustainable Design. Data Readiness Levels: Draft Discussion Document, 2013. Copyright 2013/NNCO.
The conceptual levels of data readiness range from invalid data at the DRL 0 level (which have little to no value, and should be flagged so they can be avoided) to standards-quality data at the DRL 6 level (which have a quality that merits their use for the refinement of theories and the validation of methods). The obvious challenge is that the assignment of data readiness levels is intrinsically application dependent. For example, sensor data from materials science studies of the durability of carbon nanotubes under various pH conditions might be of standards quality, i.e., DRL 6, for structural applications but of a lower, or even indeterminate, quality for biological applications. An overarching informatics framework for concepts such as DRLs is illustrated in Figure 6. The interactive network of nodes and interfaces describes roles and responsibilities for a data customer, a data creator, a data curator, or a data analyst. Credible actions are required within each of the four nodes and across each of the six interfaces (twelve interfaces, taking into account the fact that each interface must work in both directions). For example, clear articulation by an analyst of the data needs for running an applicable model does not ensure that the data creators or the data curators are able to appropriately translate and respond to those data needs.
Figure 6.
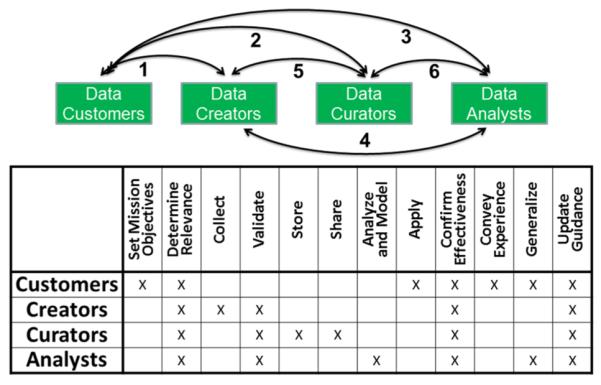
Roles and responsibilities for data customers, creators, curators, and analysts in a nanoinformatics life cycle.93
FUTURE DIRECTIONS FOR THE DEVELOPMENT OF NANOSENSORS
Looking ahead, it is important to recognize that ongoing progress in the development of nanosensors hinges on the miniaturization and decrease in cost of electronic components, advances in characterizing the effects of nanomaterials in biological and environmental media, and improved manufacturing processes for nanomaterials and their integration into various sensor components.37 In the private sector, research investments are shifting toward the development of data-driven processes that aim to improve decision-making and productivity. For reference, the McKinsey Global Institute recently predicted that technologies associated with the IoT will create up to $11 trillion in economic benefits globally in the year 2025.76 Yet, current processes have not been engineered to exploit the full potential of connected, interoperable sensor networks.76 Rather, nanosensing platforms have aimed to deliver on expectations of high sensitivity, selectivity, and minimal power consumption.77–79 On the research side, a notable example is the development of arrays of complementary metal oxide semiconductor (CMOS) nanocapacitors that can sense beyond the Debye screening length (the distance over which a charge can be sensed in liquid media).80 Commercially, a successful nanosensing tool is Veridex’s CellSearch platform, which utilizes a ferrofluid reagent that consists of targeted, polymer-coated magnetic nanoparticles that can detect as little as five circulating tumor cells per 7.5 mL of blood.81
Achieving robust, data-driven processes relies on nanosensors that are not only sensitive, selective, and energy-efficient, but also distributed via seamless integration in a wide range of platforms (Figure 7). In the U.S., an emerging resource for the development and manufacturing of next-generation sensors has been the National Network for Manufacturing Innovation (NNMI), which provides a manufacturing research infrastructure where U.S. industry, government laboratories, and academia collaborate to solve industry-relevant problems.82 In 2015, three Manufacturing Innovation Institutes (MIIs) with particular relevance to sensor deployment were announced: the Revolutionary Fibers and Textiles MII, which serves as a platform for the development and scaling of technical textile technologies; the Flexible Hybrid Electronics MII, which is focused on developing a new era in hybrid electronics manufacturing—including domestic foundry access, integrated design tools, automated packaging, assembly and test, and workforce development;83 and the MII on Smart Manufacturing, which aims to reduce the cost of deployments of advanced sensors, controls, platforms, and modeling for manufacturing by as much as 50%.84 Together, these three MIIs have accumulated so far over $390 million of allocated public–private funding. Ultimately, future research efforts should continue to broaden the “application footprint” of nanosensors and respond to current and projected data needs for the agricultural (food and water), energy, environmental, and clinical sectors in order to fulfill the needs of an IoT-based society.
Figure 7.
Illustration of next-generation, distributed sensing devices enabled by nanotechnology.
As nanomaterials become broadly available in commercial products and processes, an important need remains in the development of methods and devices that can detect and identify engineered nanomaterials across their life cycles.85 Detection strategies should hone in on multiple methodologies that span the ENM life cycle. Such activities would not only inform researchers about the potential exposure to nanomaterials from commercial products, but also link sensor measurements to toxicological data where harmful exposure levels are known. For example, NIOSH has developed RELs for certain nanomaterials including CNTs as mentioned above.69 These recommendations were supported by several rounds of toxicology studies on exposure and dose responses in mice.86 The development of reliable sensing methods for detecting nanomaterials could also leverage parallel activities in metrology, including tools for characterizing nanomaterials in physiological and environmental media. A notable example is the use of hyperspectral microscopes (e.g., CytoViva) for real-time optical imaging and spectral characterization of nanomaterials. Although this method was originally intended for imaging of nonfluorescent plasmonic nanoparticles, it has now been successfully adapted as a tool for detecting nanoparticles in complex media for biomedical and environmental applications.87
CHALLENGES AHEAD
Advancing Nanosensors from Potential to Practice
Although there has been significant progress, nanosensor development is still in its early stages. There has been an accumulation of strong experimental evidence supporting the advantages of nanomaterials in sensor construction. However, much needs to be done before realizing the promise of nanosensors and navigating these platforms through the developmental “valley of death” funding gap that hinders nanotechnology advancements beyond laboratory-scale prototypes.88
Achieving Relevant and Reliable Performance
The challenges for achieving relevant and reliable sensor performance can be best described for each type of sensors. In the case of gas/vapor sensors, the correlation between sensitivity or detection limit and nanomaterial properties such as type, geometry, size, purity, and preparation technique is well established by now. However, demonstration of selective identification of an analyte needs greater attention to enable implementation of sensors in practical scenarios. There are numerous other sensor metrics that also need to be addressed such as sensor drift, accuracy (through calibration against a standard), stability, repeatability, and reproducibility.
Sensor performance is tightly tied to nanomaterial quality including impurities and defect levels. Commercial supplies of nanomaterials, especially SWCNTs, continue to have batch-to-batch variability, which will have a significant impact on sensor reliability. In addition, the electronic nose construction relies on the ability to transfer the training data from a limited number of “test sensors” to a large batch of manufactured sensors, and this will be difficult with material quality issues.
Biosensors, while facing some of the same issues as above, have other unique challenges. Probe lifetime is a serious consideration in all types of biosensors. Large-scale fabrication, integration with microfluidics, demonstration with clinical samples, reliability, and multiplexing for multiple targets or biomarkers are other significant issues. The sensor signal should be reproducible and immune from parasitic effects to ensure reliability. As the size of the individual sensor decreases in an array, immobilization of different receptors in adjacent sensors becomes increasingly complex. Finally, capturing efficiency becomes a concern as the sensing area becomes smaller.
CONCLUSION
Advances in nanoscience could enhance the development of inexpensive, portable devices for the broad detection, identification, and quantification of biological and chemical substances. Translating nanosensors from a mere “opportunity” to common practice should consider manufacturability and seamless integration early on in the product development cycle. A focus on data quality is paramount in order to address issues related to sensor drift, accuracy, stability, repeatability, and reproducibility. Methods to detect nanomaterials across their life cycle could also leverage parallel activities in other sciences to broaden the set of detection and characterization tools. Federal agencies will continue to participate in all stages of the sensor development life cycle as they work together toward achieving the goals of the Sensors NSI for the responsible development of sensor nanotechnologies.
ABBREVIATIONS
- CPSC
Consumer Product Safety Commission
- CVM
U.S. FDA Center for Veterinary Medicine
- DOD
Department of Defense
- DRLs
Data Readiness Levels
- EHS
environmental, health, and safety
- ENM
engineered nanomaterials
- EPA
U.S. Environmental Protection Agency
- FDA
U.S. Food and Drug Administration
- INFEWS
Nexus of Food, Energy and Water Systems
- IoT
Internet of Things
- MII
Manufacturing Innovation Institute
- NASA
National Aeronautics and Space Administration
- NCI
National Cancer Institute of the National Institutes of Health
- NIEHS
National Institute of Environmental Health Science of the National Institutes of Health
- NIH
National Institutes of Health
- NIOSH
National Institute for Occupational Safety and Health
- NIST
National Institute of Standards and Technology
- NKI
Nanotechnology Knowledge Infrastructure
- NNCO
National Nanotechnology Coordination Office
- NNI
U.S. National Nanotechnology Initiative
- NNMI
National Network for Manufacturing Innovation
- NSF
National Science Foundation
- NSI
Nanotechnology Signature Initiative
- RFI
Request for Information
- NSI
Nanotechnology Signature Initiative
- Sensors NSI
The “Nanotechnology for Sensors and Sensors for Nanotechnology: Improving and Protecting Health, Safety, and the Environment” Nanotechnology Signature Initiative
- USDA
United States Department of Agriculture
Footnotes
Notes
The opinions shared in this article should not be considered as official position or policy of the U.S. Federal government. The mention of trade names or manufacturers does not constitute endorsement.
The authors declare no competing financial interest.
REFERENCES
- (1).Nanoscale Science, Engineering, and Technology Subcommittee of the Committee on Technology . Nanotechnology-Enabled Sensing: Report of the National Nanotechnology Initiative Workshop. National Science and Technology Council; Washington, DC: 2010. [Google Scholar]
- (2).Devreese JT. Importance of Nanosensors: Feynman’s Vision and the Birth of Nanotechnology. MRS Bull. 2007;32:718–725. [Google Scholar]
- (3).Nanoscale Science, Engineering, and Technology Subcommittee of the Committee on Technology . Nanotechnology for Sensors and Sensors for Nanotechnology: Improving and Protecting Health, Safety, and the Environment. National Science and Technology Council; Washington, DC: 2012. [Google Scholar]
- (4).Zhang A, Lieber CM. Nano-Bioelectronics. Chem. Rev. 2016;116:215–257. doi: 10.1021/acs.chemrev.5b00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Zhu C, Yang G, Li H, Du D, Lin Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015;87:230–249. doi: 10.1021/ac5039863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Zijlstra P, Paulo PMR, Orrit M. Optical Detection of Single Non-Absorbing Molecules Using the Surface Plasmon Resonance of a Gold Nanorod. Nat. Nanotechnol. 2012;7:379–382. doi: 10.1038/nnano.2012.51. [DOI] [PubMed] [Google Scholar]
- (7).Bargatin I, Myers EB, Aldridge JS, Marcoux C, Bri-anceau P, Duraffourg L, Colinet E, Hentz S, Andreucci P, Roukes ML. Large-Scale Integration of Nanoelectromechanical Systems for Gas Sensing Applications. Nano Lett. 2012;12:1269–1274. doi: 10.1021/nl2037479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Wei F, Lillehoj PB, Ho C-M. DNA Diagnostics: Nanotechnology-Enhanced Electrochemical Detection of Nucleic Acids. Pediatr. Res. 2010;67:458–468. doi: 10.1203/PDR.0b013e3181d361c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Walcarius A, Minteer SD, Wang J, Lin Y, Merkoçi A. Nanomaterials for Bio-Functionalized Electrodes: Recent Trends. J. Mater. Chem. B. 2013;1:4878–4908. doi: 10.1039/c3tb20881h. [DOI] [PubMed] [Google Scholar]
- (10).Qi P, Vermesh O, Grecu M, Javey A, Wang Q, Dai H, Peng S, Cho KJ. Toward Large Arrays of Multiplex Functionalized Carbon Nanotube Sensors for Highly Sensitive and Selective Molecular Detection. Nano Lett. 2003;3:347–351. doi: 10.1021/nl034010k. [DOI] [PubMed] [Google Scholar]
- (11).Segev-Bar M, Haick H. Flexible Sensors Based on Nanoparticles. ACS Nano. 2013;7:8366–8378. doi: 10.1021/nn402728g. [DOI] [PubMed] [Google Scholar]
- (12).Patolsky F, Zheng G, Lieber CM. Nanowire Sensors for Medicine and the Life Sciences. Nanomedicine. 2006;1:51–65. doi: 10.2217/17435889.1.1.51. [DOI] [PubMed] [Google Scholar]
- (13).Venkatesan BM, Bashir R. Nanopore Sensors for Nucleic Acid Analysis. Nat. Nanotechnol. 2011;6:615–624. doi: 10.1038/nnano.2011.129. [DOI] [PubMed] [Google Scholar]
- (14).Haywood DG, Saha-Shah A, Baker LA, Jacobson SC. Fundamental Studies of Nanofluidics: Nanopores, Nanochannels, and Nanopipets. Anal. Chem. 2015;87:172–187. doi: 10.1021/ac504180h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Liu J, Cao Z, Lu Y. Functional Nucleic Acid Sensors. Chem. Rev. 2009;109:1948–1998. doi: 10.1021/cr030183i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Ginger DS, Charles CY, Mirkin CA. Next-Generation Biosensing with Gold Nanoparticles. Biophot. Int. 2003;10:48–51. [Google Scholar]
- (17).Fan Z, Lu JG. Chemical Sensing with ZnO Nanowire Field-Effect Transistor. IEEE Trans. Nanotechnol. 2006;5:393–396. [Google Scholar]
- (18).Yang P, Yan R, Fardy M. Semiconductor Nanowire: What’s Next? Nano Lett. 2010;10:1529–1536. doi: 10.1021/nl100665r. [DOI] [PubMed] [Google Scholar]
- (19).Hill HD, Millstone JE, Banholzer MJ, Mirkin CA. The Role Radius of Curvature Plays in Thiolated Oligonucleotide Loading on Gold Nanoparticles. ACS Nano. 2009;3:418–424. doi: 10.1021/nn800726e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Zhang H. Ultrathin Two-Dimensional Nanomaterials. ACS Nano. 2015;9:9451–9469. doi: 10.1021/acsnano.5b05040. [DOI] [PubMed] [Google Scholar]
- (21).Nanotechnology Signature Initiatives [accessed Nov 12, 2015]; http://www.nano.gov/signatureinitiatives.
- (22).About the Sensors NSI: Participating Agencies [accessed Nov 12, 2015]; http://www.nano.gov/SensorsNSIPortal/ParticipatingAgencies.
- (23).Hoover MD, Debord GD. Turning Numbers into Knowledge: Sensors for Safety Health, Well-Being, and Productivity. Synergist. 2015;26:22–26. [PMC free article] [PubMed] [Google Scholar]
- (24).Offce of Science and Technology Policy [accessed Nov 12, 2015];Request for Information: NNI Nanotechnology for Sensors and Sensors for Nanotechnology Signature Initiative. https://federalregister.gov/a/2013-23916.
- (25).Sensors NSI Portal [accessed Feb 27, 2015]; http://www.nano.gov/SensorsNSIPortal.
- (26).Nanotechnology for Sensors and Sensors for Nanotechnology [accessed Nov 12, 2015]; http://www.techconnectworld.com/Nanotech2014/sym/Nanotechnology_Sensors_Sensors_Nanotechnology.html.
- (27).Public Webinars [accessed Nov 12, 2015]; http://www.nano.gov/PublicWebinars.
- (28).Hebert PDN, Cywinska A, Ball SL, deWaard JR. Biological Identifications through DNA Barcodes. Proc. R. Soc. London, Ser. B. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Ndong C, Toraya-Brown S, Kekalo K, Baker I, Gerngross TU, Fiering SN, Griswold KE. Antibody-Mediated Targeting of Iron Oxide Nanoparticles to the Folate Receptor Alpha Increases Tumor Cell Association In Vitro and In Vivo. Int. J. Nanomed. 2015;10:2595–2617. doi: 10.2147/IJN.S79367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Cancer Nanotechnology Plan [accessed Nov 12, 2015]; http://nano.cancer.gov/about/plan.
- (31).National Science Foundation . FY 2016 NSF Budget Request to Congress. The National Science Foundation; Arlington, VA: 2015. pp. 17–20. [Google Scholar]
- (32).National Science Foundation [accessed Nov 12, 2015];Dear Colleague Letter: FY 2016 Innovations at the Nexus of Food, Energy and Water Systems (INFEWS) Funding Opportunity on Nitrogen, Phosphorus, and Water. http://www.nsf.gov/pubs/2015/nsf15108/nsf15108.jsp.
- (33).Nanotechnology for Sensors and Sensors for Nanotechnology: Improving and Protecting Health, Safety, and the Environment: NNI Signature Initiative Symposium; [accessed Nov 12, 2015]. http://www.techconnectworld.com/Nanotech2013/sym/nanotechnology_sensors.html. [Google Scholar]
- (34).Farrell D. Overview of Resources and Support for Nanotechnology for Sensors and Sensors for Nanotechnology: Improving and Protecting Health, Safety, and the Environment, TechConnect World Innovation Conference; [accessed Nov 12, 2015]. 2013. http://www.nano.gov/sites/default/files/pub_resource/nsi_nanosensors_resources_for_web.pdf. [Google Scholar]
- (35).Farrell D. Summary of Request for Information, Sensor Fabrication, Integration, and Commercialization Workshop; [accessed Nov 12, 2015]. 2014. http://www.nano.gov/sites/default/files/farrell_request_for_information_summary.pdf. [Google Scholar]
- (36).Sensor Fabrication, Integration, and Commercialization Workshop; [accessed Nov 12, 2015]. http://www.nano.gov/2014SensorsWorkshop. [Google Scholar]
- (37).Nanoscale Science, Engineering, and Technology Subcommittee of the Committee on Technology . Sensor Fabrication, Integration, and Commercialization Workshop Report. National Science and Technology Council; Washington, DC: 2015. [Google Scholar]
- (38).National Institute of Standards and Technology [accessed Nov 5, 2014];Center for Nanoscale Science and Technology. http://www.nist.gov/cnst.
- (39).Sarkar T, Gao Y, Mulchandani A. Carbon Nanotubes-Based Label-Free Affinity Sensors for Environmental Monitoring. Appl. Biochem. Biotechnol. 2013;170:1011–1025. doi: 10.1007/s12010-013-0233-z. [DOI] [PubMed] [Google Scholar]
- (40).Rushi A, Datta K, Ghosh P, Mulchandani A, Shirsat MD. Iron Tetraphenyl Porphyrin Functionalized Single Wall Carbon Nanotubes for the Detection of Benzene. Mater. Lett. 2013;96:38–41. [Google Scholar]
- (41).Badhulika S, Myung NV, Mulchandani A. Conducting Polymer Coated Single-Walled Carbon Nanotube Gas Sensors for the Detection of Volatile Organic Compounds. Talanta. 2014;123:109–114. doi: 10.1016/j.talanta.2014.02.005. [DOI] [PubMed] [Google Scholar]
- (42).Star A, Joshi V, Skarupo S, Thomas D, Gabriel J-CP. Gas Sensor Array Based on Metal-Decorated Carbon Nanotubes. J. Phys. Chem. B. 2006;110:21014–21020. doi: 10.1021/jp064371z. [DOI] [PubMed] [Google Scholar]
- (43).Penza M, Rossi R, Alvisi M, Serra E. Metal-Modified and Vertically Aligned Carbon Nanotube Sensors Array for Landfill Gas Monitoring Applications. Nanotechnology. 2010;21:105501. doi: 10.1088/0957-4484/21/10/105501. [DOI] [PubMed] [Google Scholar]
- (44).Lu Y, Partridge C, Meyyappan M, Li J. A Carbon Nano-tube Sensor Array for Sensitive Gas Discrimination Using Principal Component Analysis. J. Electroanal. Chem. 2006;593:105–110. [Google Scholar]
- (45).Lv R, Chen G, Li Q, McCreary A, Botello-Mendez A, Morozov SV, Liang L, Declerck X, Perea-Lopez N, Cullen DA, et al. Ultrasensitive Gas Detection of Large-Area Boron-Doped Graphene. Proc. Natl. Acad. Sci. U. S. A. 2015;112:14527–14532. doi: 10.1073/pnas.1505993112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Lu Y, Li J, Han J, Ng H-T, Binder C, Partridge C, Meyyappan M. Room Temperature Methane Detection Using Palladium Loaded Single-Walled Carbon Nanotube Sensors. Chem. Phys. Lett. 2004;391:344–348. [Google Scholar]
- (47).Mirkin C. Opinion: Miniaturizing Medicine. Scientist. 2011 [Google Scholar]
- (48).Bishop GW, Rusling JF. Nanoelectrochemistry. CRC Press; 2015. Nanobioelectrochemistry: Proteins, Enzymes, and Biosensors; pp. 469–512. [Google Scholar]
- (49).Xie P, Xiong Q, Fang Y, Qing Q, Lieber CM. Local Electrical Potential Detection of DNA by Nanowire-Nanopore Sensors. Nat. Nanotechnol. 2012;7:119–125. doi: 10.1038/nnano.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Munge BS, Coffey AL, Doucette JM, Somba BK, Malhotra R, Patel V, Gutkind JS, Rusling JF. Nanostructured Immunosensor for Attomolar Detection of Cancer Biomarker Interleukin-8 Using Massively Labeled Superparamagnetic Particles. Angew. Chem., Int. Ed. 2011;50:7915–7918. doi: 10.1002/anie.201102941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Xie C, Liu J, Fu T-M, Dai X, Zhou W, Lieber CM. Three-Dimensional Macroporous Nanoelectronic Networks as Minimally Invasive Brain Probes. Nat. Mater. 2015;14:1286–1292. doi: 10.1038/nmat4427. [DOI] [PubMed] [Google Scholar]
- (52).Nam J-M, Thaxton CS, Mirkin CA. Nanoparticle-Based Bio-Barcodes for the Ultrasensitive Detection of Proteins. Science. 2003;301:1884–1886. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- (53).Young KL, Ross MB, Blaber MG, Rycenga M, Jones MR, Zhang C, Senesi AJ, Lee B, Schatz GC, Mirkin CA. Using DNA to Design Plasmonic Metamaterials with Tunable Optical Properties. Adv. Mater. 2014;26:653–659. doi: 10.1002/adma.201302938. [DOI] [PubMed] [Google Scholar]
- (54).Ross MB, Ku JC, Vaccarezza VM, Schatz GC, Mirkin CA. Nanoscale Form Dictates Mesoscale Function in Plasmonic DNA–nanoparticle Superlattices. Nat. Nanotechnol. 2015;10:453–458. doi: 10.1038/nnano.2015.68. [DOI] [PubMed] [Google Scholar]
- (55).Nanosphere [accessed Oct 6, 2015];Verigene System. http://www.nanosphere.us/products/verigene-system.
- (56).Gao R, Strehle S, Tian B, Cohen-Karni T, Xie P, Duan X, Qing Q, Lieber CM. Outside Looking in: Nanotube Transistor Intracellular Sensors. Nano Lett. 2012;12:3329–3333. doi: 10.1021/nl301623p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Liu J, Fu T-M, Cheng Z, Hong G, Zhou T, Jin L, Duvvuri M, Jiang Z, Kruskal P, Xie C, et al. Syringe-Injectable Electronics. Nat. Nanotechnol. 2015;10:629–636. doi: 10.1038/nnano.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Heerema SJ, Dekker C. Graphene Nanodevices for DNA Sequencing. Nat. Nanotechnol. 2016;11:127–136. doi: 10.1038/nnano.2015.307. [DOI] [PubMed] [Google Scholar]
- (59).Oxford Nanopore Technologies [accessed Feb 17, 2016];Introduction to Nanopore Sensing - Solid-State Nanopores. https://nanoporetech.com/science-technology/introduction-to-nanopore-sensing/solid-state-nanopores.
- (60).Gershow M, Golovchenko JA. Recapturing and Trapping Single Molecules with a Solid-State Nanopore. Nat. Nanotechnol. 2007;2:775–779. doi: 10.1038/nnano.2007.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Garaj S, Hubbard W, Reina A, Kong J, Branton D, Golovchenko JA. Graphene as a Subnanometre Trans-Electrode Membrane. Nature. 2010;467:190–193. doi: 10.1038/nature09379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Hamburg MA. Corgenix: FDA Letter of Authorization. 2015 [Google Scholar]
- (63). [accessed Nov 13, 2015];ReEBOV Antigen Rapid Test: Instructions for Use. http://www.fda.gov/downloads/MedicalDevices/Safety/EmergencySituations/UCM435528.pdf.
- (64).The U.S. Food and Drug Administration [accessed Nov 13, 2015];FDA allows marketing of the first test to identify five yeast pathogens directly from a blood sample. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm415728.htm.
- (65).Neely LA, Audeh M, Phung NA, Min M, Suchocki A, Plourde D, Blanco M, Demas V, Skewis LR, Anagnostou T, et al. T2Magnetic Resonance Enables Nanoparticle-Mediated Rapid Detection of Candidemia in Whole Blood. Sci. Transl. Med. 2013;5:182ra54–ra182ra54. doi: 10.1126/scitranslmed.3005377. [DOI] [PubMed] [Google Scholar]
- (66).Kar A, Ahern R, Gopalsami N, Raptis AC, Stroscio MA, Dutta M. Preliminary Investigation on the Modification of Electronic Properties in Surface Passivated SnO2 Nanowires with Schottky Contacts on Being Exposed to 137Cs Y-Radiation. J. Appl. Phys. 2012;111:084319–084319. [Google Scholar]
- (67).Gandhi T, Raja KS, Misra M. Cadmium Zinc Telluride (CZT) Nanowire Sensors for Detection of Low Energy Gamma-Ray Detection. In Proc. SPIE, Proceedings for the International Society for Optics and Photonics (SPIE); Orlando, Florida. April 16, 2008; pp. 695904–695904–13. [DOI] [Google Scholar]
- (68).Bekker C, Kuijpers E, Brouwer DH, Vermeulen R, Fransman W. Occupational Exposure to Nano-Objects and Their Agglomerates and Aggregates Across Various Life Cycle Stages; A Broad-Scale Exposure Study. Ann. Occup. Hyg. 2015;59:681–704. doi: 10.1093/annhyg/mev023. [DOI] [PubMed] [Google Scholar]
- (69).National Institute for Occupational Safety and Health . Current Intelligence Bulletin 65: Occupational Exposure to Carbon Nanotubes and Nanofibers; Current Intelligence Bulletin. Department of Health and Human Services, Centers for Disease Control and Prevention; Cincinnati, OH: 2013. p. 156. DHHS (NIOSH) Publication No. 2013–145. [Google Scholar]
- (70).National Institute for Occupational Safety and Health . Current Intelligence Bulletin 63: Occupational Exposure to Titanium Dioxide; Current Intelligence Bulletin. Department of Health and Human Services, Centers for Disease Control and Prevention; Cincinnati, OH: 2011. p. 119. DHHS (NIOSH) Publication No. 2011–160. [Google Scholar]
- (71).Nanomaterial Registry [accessed Nov 12, 2015]; https://www.nanomaterialregistry.org.
- (72).Office of Water, Environmental Protection Agency . Final 2014 Effluent Guidelines Program Plan. United States Environmental Protection Agency; Washington, DC: 2015. EPA-821-R-15–002. [Google Scholar]
- (73).Irin F, Shrestha B, Cañas JE, Saed MA, Green MJ. Detection of Carbon Nanotubes in Biological Samples through Microwave-Induced Heating. Carbon. 2012;50:4441–4449. [Google Scholar]
- (74).Li S, Irin F, Atore FO, Green MJ, Cañas-Carrell JE. Determination of Multi-Walled Carbon Nanotube Bioaccumulation in Earthworms Measured by a Microwave-Based Detection Technique. Sci. Total Environ. 2013:445–446. 9–13. doi: 10.1016/j.scitotenv.2012.12.037. [DOI] [PubMed] [Google Scholar]
- (75).The Nanotechnology Knowledge Infrastructure (NKI) Signature Initiative: Enabling National Leadership in Sustainable Design. Data Readiness Levels: Draft Discussion Document. 2013.
- (76).Manyika J, Chui M, Bisson P, Woetzel J, Dobbs R, Bughin J, Aharon D. Unlocking the Potential of the Internet of Things. McKinsey Global Institute; 2015. [Google Scholar]
- (77).Rana S, Le NDB, Mout R, Saha K, Tonga GY, Bain RES, Miranda OR, Rotello CM, Rotello VM. A Multichannel Nanosensor for Instantaneous Readout of Cancer Drug Mechanisms. Nat. Nanotechnol. 2014;10:65–69. doi: 10.1038/nnano.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Ma R-M, Ota S, Li Y, Yang S, Zhang X. Explosives Detection in a Lasing Plasmon Nanocavity. Nat. Nanotechnol. 2014;9:600–604. doi: 10.1038/nnano.2014.135. [DOI] [PubMed] [Google Scholar]
- (79).Wang ZL. Self-Powered Nanosensors and Nanosystems. Adv. Mater. 2012;24:280–285. doi: 10.1002/adma.201102958. [DOI] [PubMed] [Google Scholar]
- (80).Laborde C, Pittino F, Verhoeven HA, Lemay SG, Selmi L, Jongsma MA, Widdershoven FP. Real-Time Imaging of Microparticles and Living Cells with CMOS Nanocapacitor Arrays. Nat. Nanotechnol. 2015;10:791–795. doi: 10.1038/nnano.2015.163. [DOI] [PubMed] [Google Scholar]
- (81).Lowes LE, Hedley BD, Keeney M, Allan AL. User-Defined Protein Marker Assay Development for Characterization of Circulating Tumor Cells Using the CellSearch® System. Cytometry, Part A. 2012;81:983–995. doi: 10.1002/cyto.a.22158. [DOI] [PubMed] [Google Scholar]
- (82).National Network for Manufacturing Innovation (NNMI) [accessed Nov 13, 2015]; http://www.manufacturing.gov/nnmi.html.
- (83).Flexible Hybrid Electronics Manufacturing Innovation Institute (FHE-MII) [accessed Nov 13, 2015]; http://manufacturing.gov/fhe-mii.html.
- (84). [accessed Nov 13, 2015];Energy Department Announces $70 Million for Innovation Institute on Smart Manufacturing. http://www.energy.gov/articles/energy-department-announces-70-million-innovation-institute-smart-manufacturing.
- (85).National Nanotechnology Initiative Environmental, Health, and Safety Research Strategy. National Science and Technology Council; Washington, DC: 2011. Nanoscale Science, Engineering, and Technology Subcommittee of the Committee on Technology. [Google Scholar]
- (86).Erdely A, Dahm M, Chen BT, Zeidler-Erdely PC, Fernback JE, Birch ME, Evans DE, Kashon ML, Deddens JA, Hulderman T, et al. Carbon Nanotube Dosimetry: From Workplace Exposure Assessment to Inhalation Toxicology. Part. Fibre Toxicol. 2013;10:53. doi: 10.1186/1743-8977-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Malysheva A, Lombi E, Voelcker NH. Bridging the Divide Between Human and Environmental Nanotoxicology. Nat. Nanotechnol. 2015;10:835–844. doi: 10.1038/nnano.2015.224. [DOI] [PubMed] [Google Scholar]
- (88).United States Government Accountability Office . Nanomanufacturing and U.S. Competitiveness: Challenges and Opportunities. GAO-14-618T; Washington, DC: 2014. [Google Scholar]
- (89).Rodrigo D, Limaj O, Janner D, Etezadi D, Garcia de Abajo FJ, Pruneri V, Altug H. Mid-Infrared Plasmonic Biosensing with Graphene. Science. 2015;349:165–168. doi: 10.1126/science.aab2051. [DOI] [PubMed] [Google Scholar]
- (90).Clarke J, Wu H-C, Jayasinghe L, Patel A, Reid S, Bayley H. Continuous Base Identification for Single-Molecule Nanopore DNA Sequencing. Nat. Nanotechnol. 2009;4:265–270. doi: 10.1038/nnano.2009.12. [DOI] [PubMed] [Google Scholar]
- (91).Chung HJ, Castro CM, Im H, Lee H, Weissleder R. A Magneto-DNA Nanoparticle System for Rapid Detection and Phenotyping of Bacteria. Nat. Nanotechnol. 2013;8:369–375. doi: 10.1038/nnano.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Wilson NR, Macpherson JV. Carbon Nanotube Tips for Atomic Force Microscopy. Nat. Nanotechnol. 2009;4:483–491. doi: 10.1038/nnano.2009.154. [DOI] [PubMed] [Google Scholar]
- (93).Hoover MD, Myers DS, Cash LJ, Guilmette RA, Kreyling WG, Oberdörster G, Smith R, Cassata JR, Boecker BB, Grissom MP. Application of an Informatics-Based Decision-Making Framework and Process to the Assessment of Radiation Safety in Nanotechnology. Health Phys. 2015;108:179–194. doi: 10.1097/HP.0000000000000250. [DOI] [PubMed] [Google Scholar]