Abstract
Evidence suggests that the infralimbic cortex (IL), a subregion of the ventromedial prefrontal cortex (vmPFC), suppresses cocaine-seeking behavior in a self-administration paradigm, whereas the more anterior vmPFC subregion, the medial orbitofrontal cortex (mOFC), has received very little attention in this regard. Despite the established dopaminergic innervation of the vmPFC, whether dopamine receptor blockade in each subregion alters the reinstatement of cocaine seeking is unclear. To address this issue, male Sprague-Dawley rats underwent two weeks of cocaine self-administration followed by extinction training and reinstatement testing. Immediately prior to each reinstatement test, rats received microinjections of the D1 receptor antagonist SCH 23390, the D2 receptor antagonist sulpiride, or their respective vehicles. D1 receptor blockade in the IL reduced cued reinstatement but had no effect on cocaine-prime and cue + cocaine-prime reinstatement, whereas D2 receptor blockade in the IL had no effect on reinstatement. For the mOFC, however, D1 receptor blockade reduced cocaine seeking in all reinstatement types, whereas blocking D2 receptors in the mOFC had no effect on any form of cocaine seeking. These findings suggest different roles for D1 receptors in the IL vs. the mOFC in regulating cocaine-seeking behavior. Moreover, even as previous work indicates that IL inactivation does not affect reinstatement but, rather, induces cocaine seeking during extinction, the present findings suggest that dopamine receptor activation in the IL is necessary for cocaine seeking under some circumstances.
Keywords: cocaine-prime, rat, SCH 23390, sulpiride, self-administration, ventromedial prefrontal cortex
Increasing evidence has pointed to a critical role for the ventromedial prefrontal cortex (vmPFC), and especially the infralimbic (IL) region of the vmPFC, in regulating drug seeking (LaLumiere et al., 2012; Peters et al., 2008; for a larger review of PFC involvement in drug seeking, see Van den Oever et al., 2010). Prior work indicates that IL inactivation induces cocaine seeking during an extinction session, whereas IL activation reduces cocaine seeking during cocaine-prime or cued reinstatement (LaLumiere et al., 2012; Peters et al., 2008). Together with other findings (LaLumiere et al., 2010), these results suggest that IL activity suppresses cocaine seeking following self-administration and extinction training and is involved in the consolidation of extinction learning for cocaine-seeking behavior.
However, prior studies have not investigated the role of IL dopamine receptors in cocaine seeking, despite known dopaminergic projections to the vmPFC (Hitchcott et al., 2007; Smiley et al., 1992; Van Eden et al., 1987). Previous work examining this region during non-drug related motivated behaviors indicates that intra-IL infusions of dopamine or a D1 receptor antagonist produce a shift from habitual to goal-oriented instrumental behaviors (Hitchcott et al., 2007). Additionally, D1 receptor antagonism and D2 receptor agonism within the IL reduce compulsive sucrose seeking (Barker et al., 2013). Given that addiction is conceptualized as a shift from goal-oriented behaviors to a habitual response (Koob and Volkow, 2010), such findings suggest that dopamine activity within the IL may be critical for the expression of relapse behaviors. Indeed, addressing this issue may be of importance to related controversial questions raised about the role of the IL in inhibiting drug seeking in general, as previous studies have shown blocking 5-HT2A receptors in the vmPFC decreases both cue and cocaine-primed reinstatement (Pockros et al., 2011). Moreover, IL/vmPFC activity drives, rather than inhibits, the reinstatement of heroin seeking (Bossert et al., 2011; Rogers et al., 2008), leading Peters and colleagues (2013) to suggest that dopamine activity within the IL may account for these discrepant results regarding the ability of the IL to regulate drug seeking but note that no data exist to address this question.
In addition to the IL, the vmPFC also includes a more anterior subregion known as the medial orbitofrontal cortex (mOFC). Previous research has found that identical manipulations in different vmPFC regions along the rostrocaudal axis produce distinct behavioral effects (Smith and Berridge, 2005). Indeed, IL activation appears to suppress feeding behavior that is induced via glutamate receptor blockade in the nucleus accumbens shell, whereas mOFC activation enhances this feeding behavior (Richard and Berridge, 2013). However, akin to prior work on the IL, mOFC inactivation using a GABAA/B agonist cocktail has no effect on the reinstatement of cocaine-seeking behavior (Fuchs et al., 2004). Nevertheless, previous research on the nucleus accumbens shell has found conflicting results using GABA receptor activation vs. dopamine receptor manipulations in terms of the reinstatement of cocaine seeking (Anderson et al., 2006; McFarland and Kalivas, 2001). Given that the mOFC receives dopaminergic innervation from the VTA (Swanson, 1982) and maintains efferent projections to the amygdala and nucleus accumbens (Brog et al., 1993; Ishikawa and Nakamura, 2003; Malkusz et al., 2015), we considered the possibility that dopaminergic manipulations in the mOFC would alter reinstatement. As D1 receptor blockade in the lateral OFC reduces context-induced cocaine seeking (Lasseter et al., 2014), we hypothesized that blocking these receptors in the medial region of the OFC would similarly result in a disruption of cocaine seeking. To examine these issues, the current study investigated the effects of D1 and D2 receptor blockade in the IL and mOFC during the reinstatement of cocaine seeking.
Materials and Methods
Subjects
Male Sprague-Dawley rats (250–275 g at time of arrival; Charles River Laboratories; n = 98) were single-housed on a 12-hour reverse light cycle (and kept at constant temperature) with food and water ad libitum in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-approved vivarium. All animals were allowed to acclimate to the vivarium for a minimum of 5 days before undergoing surgery. All procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by University of Iowa Institutional Animal Care and Use Committee.
Surgery
Rats were anaesthetized using ketamine (100 mg/kg, i.m.) and xylazine (6 mg/kg, i.m.). Additionally, ketorolac was given (3 mg/kg, i.p.) as an analgesic on both the day of surgery as well as the day immediately following surgery. Catheter implantation was performed, as described previously (Cosme et al., 2015). Rats were then placed in a small animal stereotaxic instrument (Kopf Instruments, Tujunga, CA, USA). Jewelers’ screws were affixed to the skull surface to serve as anchors. Double-barreled cannulae with a 1.2 mm center-to-center distance (Plastics One, Roanoke, VA, USA) were implanted, aimed at either the IL or mOFC and then secured using dental cement. Coordinates were as follows: IL: 2.8 mm anterior to bregma and 2.1 mm ventral from the skull surface (cannula aimed 3 mm above IL); mOFC: 3.5 mm anterior to bregma and 2.8 mm ventral from skull surface (cannula aimed 3 mm above mOFC). These coordinates were chosen based on previous work (LaLumiere et al., 2010) and refined in our laboratory.
After surgery, animals received 3 ml subcutaneous saline and a topical application of the anesthetic bupivacaine to the cranial and chest incision sites. Dummy injectors were placed in each cannula along with a tight fitting cap. Rats were then returned to their home cages where they were allowed to recover for 5–7 days. During recovery, catheters were flushed daily with 0.1 ml of heparinized saline (100 USP) to ensure catheter patency.
Cocaine self-administration and extinction
Self-administration was conducted in standard operant chambers (Med Associates, Fairfield, VT) with two retractable levers, a house light, a cue light, and a tone-generator (4500 Hz). Rats had all food removed 24 h prior to a single 15-h overnight food training session. During this session, a press on the active lever resulted in a single food pellet (45 mg) on a fixed-ratio 1 (FR1) schedule. Following the single food training session, rats were fed 10 g of rat chow and began self-administration training the following day. All rats received ~20 g of rat chow daily after each cocaine self-administration, extinction, or reinstatement session. After food training, rats’ catheters were assessed for patency using 0.1 ml of sodium brevital (10 mg/ml).
One day after food training, cocaine self-administration began. Rats were placed in operant chambers for 2 h/d for a minimum of 12 d, during which active lever presses produced an infusion of cocaine (50 μl infusion of 150 μg cocaine dissolved in sterile saline, given over 2.18 s; cocaine kindly provided by the National Institute on Drug Abuse) along with a 5 s light and tone cue on an FR1 schedule. A 20 s timeout period followed each infusion. Inactive lever presses had no consequence. Animals began extinction training if they received a minimum of 15 infusions of cocaine per day for at least 10 d, including the last 3 d of self-administration, and demonstrated discrimination between the active and inactive lever. During extinction training, active lever presses did not produce cocaine infusions or the light and tone cues. Each rat underwent extinction for a minimum of 7 d and entered reinstatement testing when it had 25 or fewer active lever presses for at least 2 consecutive days immediately prior to the reinstatement session. The final 2 d of extinction training prior to each reinstatement test served as the extinction baseline.
Microinjections
Immediately prior to each reinstatement test, rats received intra-IL or intra-mOFC microinjections. Microinjectors (with 3 mm projections beyond the end of the respective cannula) were connected to PE20 tubing, which was attached to 10-μl Hamilton syringes controlled by an infusion pump. Microinjections (0.3 μl/side) were given over 1 min, and injectors were left in place for an additional 2 min to permit drug diffusion. Following the microinjection, rats were immediately placed into the operant chamber for the appropriate reinstatement test. Microinjected drugs consisted of the D1 receptor antagonist SCH 23390 (0.1 μg/side) and the D2 receptor antagonist sulpiride (30 ng/side), each dissolved in artificial cerebral spinal fluid (aCSF) as the vehicle. Doses were chosen based on prior research (Lalumiere et al., 2004). Although some previous studies have infused higher concentrations of sulpiride (1–3 μg/side) into the mPFC (Granon et al., 2000; Winter et al., 2009), others have shown that smaller concentrations of sulpiride are equally as effective (Druzin et al., 2000), and, in some cases, lower doses of sulpiride in the mPFC and nucleus accumbens have proven to be more effective at revealing behavioral differences (Pakdel and Rashidy-Pour, 2007; Setlow and McGaugh, 1998).
Reinstatement testing
During each reinstatement test (2 h), active lever presses never produced a cocaine infusion. Following each reinstatement test, rats had their lever pressing re-extinguished to baseline levels for a minimum of 3 days using the same criteria described previously. Each rat underwent three reinstatement tests (cued, cocaine-prime, and cue + cocaine-prime) and completed each test twice, once following a vehicle microinjection and once following a drug microinjection in a counterbalanced design, resulting in a total of 6 reinstatement sessions per rat. For cued reinstatement, active lever presses produced the light and tone cues that were previously paired with the drug infusion during self-administration. Cocaine-prime reinstatement consisted of an injection of cocaine (10 mg/kg, i.p.) immediately before the reinstatement session. Cued + cocaine-prime reinstatement involved an injection of cocaine (10 mg/kg, i.p.) immediately prior to a cued reinstatement session. As cue + cocaine-prime reinstatement typically produces higher levels of cocaine-seeking behavior than cue or cocaine-prime reinstatement alone, we included this test in our experimental design to provide us with the optimal opportunity to observe behavioral differences following dopamine receptor blockade.
Experiment 1
Prior to reinstatement testing rats received intra-IL microinjections of the D1 receptor antagonist SCH 23390 (n = 11). All rats underwent reinstatement testing in the following order: cued, cocaine-prime, and cued + cocaine-prime as described above. Due to clogged cannula and illness, three rats were removed from the experiment after the cued reinstatement and one more was removed following the cocaine-prime test.
Experiment 2
Prior to reinstatement testing rats received intra-IL microinjections of the D2 receptor antagonist sulpiride (n = 14). All rats underwent reinstatement testing in the following order: cued, cocaine-prime, and cued + cocaine-prime, as described above.
Experiment 3
Prior to reinstatement testing rats received intra-mOFC microinjections of the D1 receptor antagonist SCH 23390 (n = 12). Rats underwent reinstatement testing in the following order: cued, cocaine-prime, and cued + cocaine-prime, except for a subset of rats that, unintentionally, received exposure to the cues during extinction training. Therefore, the data for this subset (n = 5) for the cued reinstatement were not included in the analysis due to concern about the robustness of the reinstatement levels. The data for this subset for the cued + cocaine-prime reinstatement were included, as the levels of this reinstatement were robust and the results were not different from those observed with the rest of the rats in this experiment. Due to clogged cannula and illness, two rats were removed from the experiment after the cued reinstatement and four more was removed following the cocaine-prime test.
Experiment 4
Prior to reinstatement testing rats received intra-mOFC microinjections of the D2 receptor antagonist sulpiride (n = 6). All rats underwent reinstatement testing in the following order: cued, cocaine-prime, and cued + cocaine-prime, as previously described. Due to a lack of significant cocaine-prime reinstatement, an additional group of rats underwent only cocaine-prime reinstatement with no prior reinstatement tests (n = 6).
Histological analysis
To verify cannula placement, rats were overdosed with sodium pentobarbital (100 mg/kg, i.p.) and intracardially perfused using phosphate-buffered saline. Brains were then placed in 3.7% formaldehyde for a minimum of 24 h before being sectioned. Coronal slices were 75 μm thick and were mounted directly onto a gelatin-coated slide. Sections were stained using Cresyl violet and analyzed for the correct termination site of the microinjectors. Any rat with an injector termination point outside the borders of the IL or mOFC was excluded from further analysis.
Data analysis
Two-way analyses of variance (ANOVA) were used to analyze reinstatement lever pressing data with both comparisons as within-subjects (extinction vs. reinstatement; vehicle vs. drug). Post-hoc analysis was completed using Holm-Sidak’s multiple comparison tests. p-values of less than 0.05 were considered significant. All measures were expressed as mean ± SEM. Each group’s n is indicated in the figure.
Results
Out of a total of 96 rats used in the present experiments, 51 rats were included in the final data. Rats were excluded due to misplaced (18 rats) or unverifiable (15 rats) microinjection termination locations, failure to acquire self-administration (1 rat), loss of cannula patency (7 rats), and loss of catheter patency (4 rats). Figure 1A shows the number of active and inactive lever presses and cocaine infusions over the final 12 days of cocaine self-administration. Active lever pressing was significantly higher than inactive lever pressing over the last 3 days of self-administration (t(50) = 9.55, p < 0.05). Figures 1B and C show the termination site of the microinjector tips in the IL and mOFC, respectively.
Figure 1.

Self-administration data and histological representations. A, Number of active and inactive lever presses and cocaine infusions for the last 12 days of cocaine self-administration for all rats included in the final analysis. B and C, Diagrams showing the termination of needle tracks for microinjections aimed at the IL and mOFC, respectively. Figures are adapted from Paxinos and Watson (2007), and A/P coordinates (in mm) are given relative to Bregma.
Experiment 1. D1 receptor blockade in the IL attenuates cued reinstatement
D1 receptor blockade within the IL significantly reduced active lever presses for cued reinstatement (Figure 2A). A two-way repeated-measures ANOVA of active lever presses indicated a significant effect of reinstatement (F(1, 10) = 7.78, p < 0.05), a significant effect of microinjection (F(1,10) = 7.23, p < 0.05) and a significant interaction (F(1,10) = 7.017, p < 0.05). Post hoc tests revealed that, although active lever pressing during reinstatement with vehicle treatment was significantly higher than the extinction baseline (p < 0.05), active lever pressing during reinstatement following SCH treatment was no different from extinction baseline and was significantly lower than what was observed with the vehicle treatment (p < 0.05). Intra-IL D1 receptor blockade had no effect on active lever pressing during cocaine-prime reinstatement (Figure 2B). A two-way repeated measures ANOVA of active lever presses indicated a significant effect of reinstatement (F(1,7) = 17.37, p < 0.01), no effect of microinjection (F(1,7) = 2.28, p > 0.05), and no interaction (F(1,7) = 0.96, p > 0.05). Post hoc tests revealed that active lever pressing during reinstatement was significantly higher than extinction for both treatments (p < 0.05). Intra-IL D1 receptor blockade had no effect on cue + cocaine-prime reinstatement (Figure 2C). A two-way repeated measures ANOVA of active lever presses indicated a significant effect of reinstatement (F(1,6) = 27.53, p < 0.01), no effect of microinjection (F(1,6) = 1.07, p > 0.05), and no interaction (F(1,6) = 1.48, p > 0.05). Post hoc tests revealed that active lever pressing during reinstatement was significantly higher than extinction for both treatments (p < 0.05). There were no significant differences in inactive lever presses across any type of reinstatement (Figures 2D–F).
Figure 2.
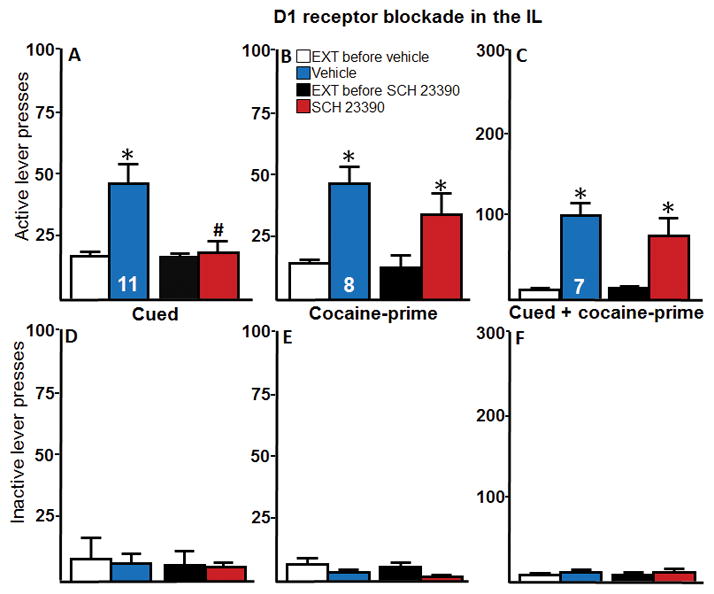
Intra-IL D1 receptor blockade via SCH 23390 (SCH) mediates cued reinstatement. (Note that the y-axis maximum is either 100 or 300 across the different experiments to accommodate the large differences in values.) A–C, The active lever presses and, D–F, the inactive lever presses for each reinstatement test for Experiment 1 are shown. A, Intra-IL microinjections of the D1 receptor antagonist SCH significantly reduced active lever pressing during cued reinstatement compared to vehicle controls (n = 11). B, Intra-IL microinjections of SCH had no effect on active lever presses during cocaine-prime reinstatement (n = 8). C, Intra-IL microinjections of SCH had no effect on active lever pressing during cued + cocaine-prime reinstatement (n = 7). D–F, There were no effects of reinstatement or microinjections on inactive lever presses for any type of reinstatement test. *p < 0.05 compared with extinction baseline. #p < 0.05 compared to vehicle-control group. EXT, extinction baseline.
Experiment 2. D2 receptor blockade in the IL has no effect on cocaine-seeking behavior
Intra-IL D2 receptor blockade had no effect on active lever pressing during cued reinstatement (Figure 3A). A two-way repeated measures ANOVA indicated a significant effect of reinstatement (F(1,13) = 13.60, p < 0.05), no effect of microinjections (F(1,13) = 1.37, p > 0.05), and no interaction (F(1,13) = 1.13, p > 0.05). Post hoc tests revealed that active lever pressing during reinstatement was significantly higher than extinction for both treatments (vehicle vs. sulpiride; p < 0.05). Intra-IL D2 receptor blockade had no effect on active lever pressing during cocaine-prime reinstatement (Figure 3B). A two-way repeated measures ANOVA indicated a significant effect of reinstatement (F(1,12) = 12.54, p < 0.05), no effect of microinjections (F(1,12) = 0.06, p > 0.05), and no significant interaction (F(1,12) = 0.23, p > 0.05). Post hoc tests revealed that active lever pressing during reinstatement was significantly higher than extinction for both treatments (p < 0.05). Intra-IL D2 receptor blockade had no effect on active lever pressing during cued + cocaine-prime reinstatement (Figure 3C). A two-way repeated measures ANOVA revealed a significant effect of reinstatement (F(1,11) = 17.18, p < 0.05) but no effect of microinjections (F(1,11) = 3.12, p > 0.05) or significant interaction (F(1,11) = 3.90, p > 0.05). Post hoc tests revealed that active lever pressing during reinstatement was significantly higher than extinction for both treatments (p < 0.05). There were no significant differences in inactive lever presses across any type of reinstatement (3D–F).
Figure 3.
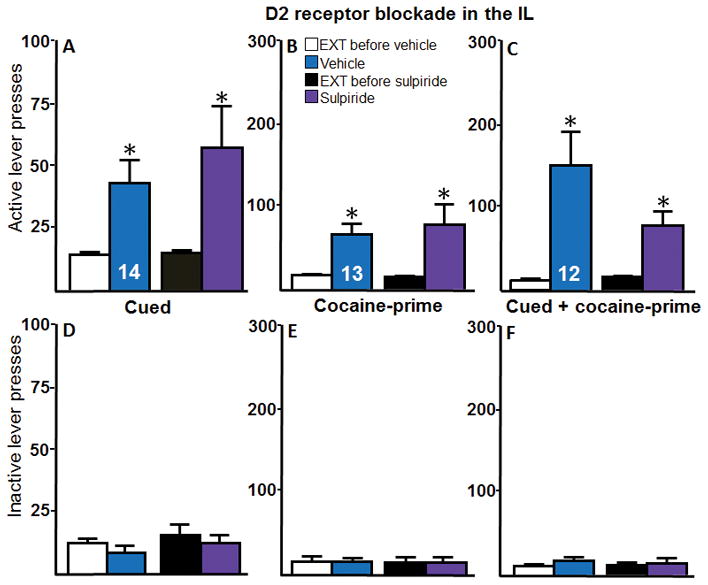
Intra-IL D2 receptor blockade via sulpiride does not alter cued or cocaine-prime reinstatement. A–C, The active lever presses and, D–F, inactive lever presses for each reinstatement test for Experiment 2 are shown. A, Intra-IL microinjections of the D2 receptor antagonist sulpiride had no effect on cued reinstatement (n = 14). B, Intra-IL microinjections of sulpiride had no effect on cocaine-prime reinstatement (n = 13). C, Intra-IL microinjections of sulpiride had no effect on cued + cocaine-prime reinstatement (n = 12). D–F, There were no effects of reinstatement or microinjections on inactive lever presses for any type of reinstatement. *p < 0.05 compared with extinction baseline. EXT, extinction baseline.
Experiment 3. D1 receptor blockade in the mOFC reduces cocaine-seeking behavior
Intra-mOFC D1 receptor blockade significantly reduced active lever pressing during cued-reinstatement (Figure 4A). A two-way repeated measures ANOVA indicated a significant effect of reinstatement (F(1,8) = 24.00, p < 0.05), a significant effect of microinjections (F(1,8) = 5.12, p < 0.05), and a significant interaction (F(1,8) = 7.51, p < 0.05). A post-hoc analysis revealed that, although vehicle-treated animals had significantly increased active lever pressing during reinstatement compared to the extinction baseline, active lever pressing during reinstatement following SCH treatment did not significantly differ from extinction baseline and was significantly lower than that observed with vehicle treatment (p < 0.05). Intra-mOFC D1 receptor blockade also resulted in a significant reduction of active lever pressing during cocaine-prime reinstatement (Figure 4B). A two-way repeated measures ANOVA indicated a significant effect of reinstatement (F(1,11) = 20.72, p < 0.05), a significant effect of microinjections (F(1,11) = 8.91, p < 0.05), and a significant interaction (F(1,11) = 10.07, p < 0.05). Post hoc analysis revealed the vehicle-treated rats demonstrated significantly increased active lever pressing during reinstatement compared to extinction baseline (p < 0.05). In contrast, SCH-treated rats showed no difference in active lever presses during reinstatement compared to extinction baseline and had significantly fewer active lever presses compared to the vehicle-treated group (p < 0.01). Intra-mOFC D1 receptor blockade significantly attenuated active lever pressing during cued + cocaine-prime reinstatement (Figure 4C). A two-way repeated measures ANOVA revealed a significant effect of reinstatement (F(1,7) = 11.87, p < 0.05), a significant effect of microinjections (F(1,7) = 9.90, p < 0.05), and a significant interaction (F(1,7) = 14.78, p < 0.05). Post hoc analysis showed that, although both the vehicle- and SCH- treated groups demonstrated significantly higher lever pressing during reinstatement compared to extinction baseline, the SCH-treated animals had significantly fewer active lever presses compared to the vehicle-treated group (p < 0.05).
Figure 4.
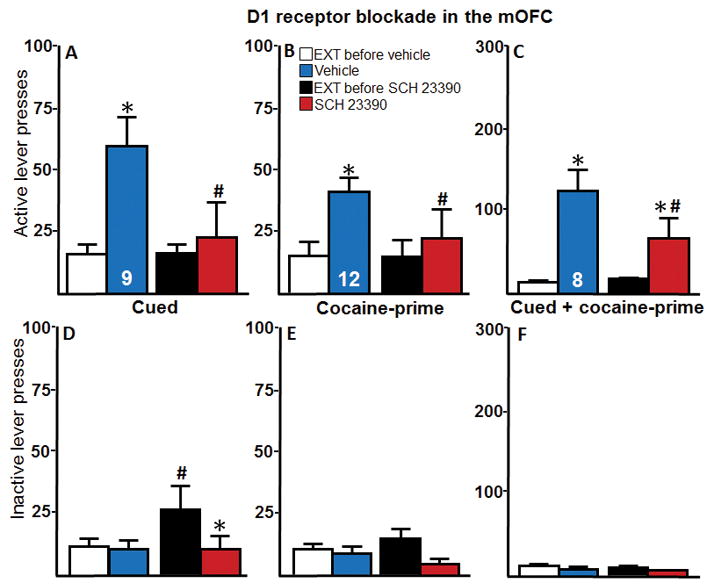
Intra-mOFC D1 receptor blockade via SCH 23390 (SCH) mediates cocaine-seeking behaviors. A–C, The active lever presses and, D–F, the inactive lever presses for each reinstatement test for Experiment 2 are shown. A, Intra-mOFC microinjections of SCH significantly reduced active lever pressing during cued reinstatement compared to vehicle controls (n = 9). B, Intra-mOFC microinjections of SCH significantly reduced active lever pressing during cocaine-prime reinstatement (n = 12). C, Intra-mOFC microinjections of SCH significantly reduced active lever pressing during cued + cocaine-prime reinstatement (n = 8). D, Intra-mOFC microinjections significantly reduced inactive lever presses compared to extinction baseline, though the extinction baseline itself was elevated. E and F, There were no effects of reinstatement or microinjections on inactive lever presses for cocaine-prime or cued + cocaine-prime reinstatement. *p < 0.05 compared with extinction baseline. #p < 0.05 compared to vehicle-control group. EXT, extinction baseline.
A two-way repeated measures ANOVA of inactive lever presses during cued reinstatement (Figure 4D) revealed a significant effect of reinstatement (F(1,8) = 6.24, p < 0.05), no effect of microinjections (F(1,8) = 2.04, p > 0.05), and a significant interaction (F(1,8) = 8.86, p < 0.05). Post hoc analysis indicated that the SCH-treated animals had significantly higher inactive lever presses during extinction baseline when compared to both the extinction baseline for the vehicle group and the SCH-treated animals during reinstatement testing. However, analyses of inactive lever pressing for the cocaine-prime and cued + cocaine-prime reinstatement showed no significant differences (Figures 4E–F). Because the statistically significant increase in inactive lever pressing shown in Figure 4D occurred during the extinction baseline when no manipulations were given, because there was no significant effect in the active lever presses during the same extinction baseline, and because there were no other statistically significant effects of inactive lever pressing either in the baseline or reinstatement tests in this experiment or across all the experiments, it is difficult to conclude that the observed effect is anything but a random statistical artifact.
Experiment 4. D2 receptor blockade in the mOFC has no effect on cocaine-seeking behavior
Intra-mOFC D2 receptor blockade had no effect on active lever presses during cued reinstatement (Figure 5A). A two-way repeated measures ANOVA of active lever presses during cued reinstatement revealed a significant effect of reinstatement (F (1,5) = 10.55, p < 0.05), no effect of microinjection (F(1,5) = 0.65, p > 0.05), and no interaction (F(1,5) = 0.89, p > 0.05). Post hoc tests revealed that active lever pressing during reinstatement was significantly higher than extinction for both treatments. Figure 5B shows the active lever presses during cocaine-prime reinstatement following intra-mOFC D2 receptor blockade. A two-way repeated measures ANOVA of active lever pressing during cocaine-prime reinstatement revealed no effect of reinstatement (F(1,5) = 5.87, p > 0.05), no effect of microinjection (F(1,5) = 0.17, p > 0.05), and no interaction (F(1,5) = 0.10, p > 0.05). Intra-mOFC D2 receptor blockade had no effect on active lever pressing during cued + cocaine-prime reinstatement (Figure 5C). A two-way repeated measures ANOVA of active lever presses revealed a significant effect of reinstatement (F(1,5) = 15.01, p < 0.05), no effect of microinjections (F(1,5) = 0.20, p > 0.05), and no significant interaction (F(1,5) = 0.31, p > 0.05). Post hoc tests revealed that active lever pressing during reinstatement was significantly higher than extinction for both treatments (p < 0.05).
Figure 5.
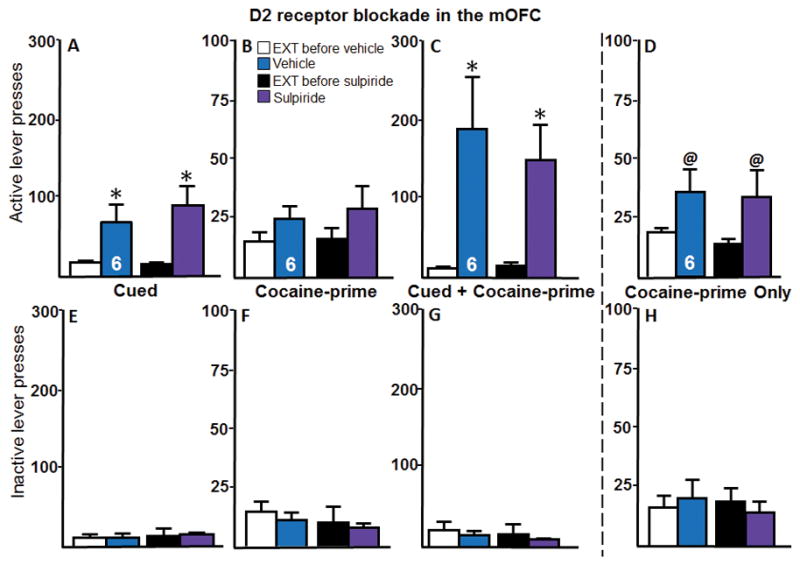
Intra-mOFC D2 receptor blockade via sulpiride has no effect on cocaine-seeking behaviors. A–D, The active lever presses and, E–H, inactive lever presses for each reinstatement test for Experiment 4 are shown. A, Intra-mOFC microinjections of sulpiride had no effect on active lever pressing during cued reinstatement compared to vehicle controls (n = 6). B, Although intra-mOFC microinjections of sulpiride had no effect on active lever pressing during cocaine-prime reinstatement, cocaine-prime reinstatement levels were not significantly above the extinction baseline (n = 6). C, Intra-mOFC microinjections of sulpiride had no effect on active lever pressing during cued + cocaine – prime reinstatement (n = 6). D, Due to the low levels of cocaine-prime reinstatement, a separate group of rats underwent only a cocaine-prime reinstatement (n = 6). Intra-mOFC microinjections of sulpiride did not affect active lever pressing when only a cocaine-prime reinstatement test was conducted. E–H, There were no effects of reinstatement or microinjections on inactive lever presses for any type of reinstatement. *p < 0.05 compared with extinction baseline. @p < 0.09 compared with extinction baseline. EXT, extinction baseline.
Because the cocaine-prime reinstatement did not produce significantly higher lever pressing compared to the extinction baseline, we conducted a separate experiment in which rats underwent only cocaine-prime reinstatement with the goal of achieving more robust levels of this form of reinstatement. Figure 5D shows the active lever presses for those rats that only underwent the cocaine-prime reinstatement. A two-way repeated measures ANOVA of active lever pressing during this cocaine-prime reinstatement test revealed no effect of microinjections (F(1,5) = 0.72, p > 0.05), no interaction (F(1,5) = 0.06, p > 0.05), and only a marginal effect of reinstatement (F(1,5) = 2.96, p < 0.15). Post hoc tests revealed that active lever pressing during reinstatement was marginally higher than extinction for both treatments (p < 0.09) and was not different between treatment groups. There were no significant differences in inactive lever presses across any type of reinstatement.
Discussion
In contrast to prior work using GABA receptor activation (Peters et al., 2008), the current findings indicate that D1 receptor activation in the IL and mOFC is involved in the reinstatement of cocaine seeking, though to different extents. Blocking D1 receptors in the IL reduced cued reinstatement but did not affect cocaine-prime or cued + cocaine-prime reinstatement. Similar blockade in the mOFC reduced cocaine seeking for all forms of reinstatement tested. In contrast, the present results suggest that D2 receptor activation in either structure played little role in the reinstatement of cocaine seeking as blocking D2 receptors in the IL and mOFC had no effect on reinstatement. Although it is possible that the sulpiride dose used in the present experiments was too low to produce any effects on drug seeking, sulpiride doses given in the mPFC within this range have revealed behavioral differences in the past (Druzin et al., 2000; Pakdel and Rashidy-Pour, 2007). Nonetheless, we cannot rule out the possibility that higher doses may be effective at altering drug seeking, though non-specific “off-target” effects may become a concern at higher doses. These findings suggest that activation of dopamine receptors within the vmPFC, particularly the D1 receptors, is involved in the reinstatement of cocaine seeking, though the precise role appears to depend on an interaction of the type of reinstatement and the vmPFC subregion.
Infralimbic results
Intra-IL administration of the D1 receptor antagonist reduced cue-induced cocaine seeking but did not alter reinstatement when a cocaine prime was given. D2 receptor blockade in the IL had no effect on any form of reinstatement. The results with IL dopamine receptor blockade show partial consistency with prior work, as IL inactivation via GABA receptor activation has no effect on cocaine-prime reinstatement (McFarland and Kalivas, 2001) and blocking 5HT2A receptors in the IL decreases cued and cocaine-prime reinstatement (Pockros et al., 2011). Moreover, several studies, including work from our laboratory, suggest that the IL inhibits cocaine seeking following self-administration and extinction and is also involved in the consolidation of the extinction of cocaine-seeking behavior (LaLumiere et al., 2010; LaLumiere et al., 2012; Peters et al., 2008). However, those studies also suggest that IL inactivation and activation have no effect and reduce, respectively, cued reinstatement of cocaine seeking.
The reasons underlying this discrepancy regarding the IL remain unclear, though to some degree, they parallel the discrepancies with the mOFC results. However, such conflicting findings are not unprecedented. Previous work investigating the nucleus accumbens shell has found that inactivation via GABA receptor activation does not alter the reinstatement of cocaine seeking and, akin to the IL, induces cocaine seeking during an extinction session (McFarland and Kalivas, 2001; Peters et al., 2008). In contrast, dopamine receptor blockade within the accumbens shell impairs the reinstatement of cocaine seeking (Anderson et al., 2006). Together with the present findings, this work suggests that conclusions drawn based on one type of pharmacological manipulation may not always predict the outcomes for a similar set of studies using a different pharmacological manipulation. Previous work indicates that dopamine in the accumbens shell appears to override excitatory inputs from the IL that would normally suppress cocaine seeking (LaLumiere et al., 2012). A similar process may occur within the IL itself. More speculatively, this process may also involve different neuronal ensembles. Indeed, recent findings suggest that a small minority of IL neurons is responsible for the suppression of alcohol seeking (Pfarr et al., 2015). However, there may also be IL neurons that promote cocaine seeking (e.g., Koya et al., 2009) and it is possible that D1 receptor blockade on these specific neurons may inhibit the neuronal ensembles that promote cocaine seeking, resulting in a decrease in the reinstatement of cocaine seeking observed in the present study. These neuronal ensembles that drive cocaine seeking, however, may typically be masked by the prepotent inhibitory drive within the structure with regard to cocaine seeking. Unfortunately, it is not clear whether this would explain the discrepancy for the mOFC, as prior work has not identified a role for this region in inhibiting cocaine seeking.
Alternatively, the actions of dopamine within the IL (and possibly mOFC) may have subtler effects on the type of behavior in which an animal is engaged and may explain the differences between the effects of D1 receptor blockade in the IL during cued and cocaine-prime reinstatement. Past studies have suggested that IL dopamine is critically involved in how the IL influences goal-seeking vs. habit-based behavior (Hitchcott et al., 2007). Cued reinstatement takes advantage of the ability of the cues to serve as conditioned reinforcers that increase cocaine-seeking behavior even when the reinforcement of cocaine itself is removed. Blockade of dopamine receptors in the IL may, therefore, reduce any reinforcing properties of the cues, thus preventing the goal-seeking behavior. In contrast, cocaine-prime reinstatement involves a single cocaine injection given prior to the reinstatement session with no reinforcer given during the session itself. Thus, cocaine-prime reinstatement likely does not involve the same type of goal-seeking behavior as cued reinstatement and, therefore, may not depend on IL dopamine receptors. Nonetheless, it may be a combination of type of reinstatement, and therefore goal-seeking behavior vs. habitual behavior, and the selective activation of specific ensembles within the IL that are responsible for the current results. Additionally, and perhaps in concert with the speculation above, the lack of a role for IL dopamine receptors during cocaine-prime reinstatement may be due to the direct actions of cocaine on PL dopamine, thereby directly activating the PL-nucleus accumbens core pathway that is necessary for reinstatement (Stefanik et al., 2013). Indeed, prior studies indicate that PL inactivation, dopamine receptor blockade in the PL, and optical inhibition of the PL inputs to the accumbens core attenuate cocaine-prime reinstatement (McFarland and Kalivas, 2001; Stefanik et al., 2013).
Medial orbitofrontal results
Blocking D1 receptors within the mOFC impaired all forms of reinstatement tested (cued, cocaine-prime, and cued + cocaine-prime reinstatement), whereas D2 receptor blockade in the mOFC did not alter any form of reinstatement. Previous work indicates mOFC activation potentiates the feeding behavior that is induced by AMPA receptor blockade in the nucleus accumbens shell which lies downstream from the mOFC (Hoover and Vertes, 2011; Richard and Berridge, 2013). Given that prior work indicates that inactivation of the accumbens shell induces cocaine-seeking behavior (Peters et al., 2008), it may be possible that activation of the mOFC would potentiate cocaine seeking in the same way that it potentiates feeding. Thus, the present results indicating a role for the mOFC in promoting cocaine seeking may be consistent with those examining feeding. Nonetheless, prior studies directly examining the role of the mOFC in cocaine seeking found no effect of mOFC inactivation on cued reinstatement or of mOFC lesions on cocaine-prime reinstatement (Fuchs et al., 2004), making the present study the first, to our knowledge, to show a role for this region in the reinstatement of cocaine seeking. Indeed, although much work has focused on the lateral OFC and medial PFC in general (PL and IL specifically) in drug seeking and reward-related behavior, there has been less attention directed toward examining the role of the mOFC in such behaviors. It is worth noting, however, that our results are consistent with previous studies indicating that D1 receptor blockade within the lateral OFC disrupts cocaine-seeking behavior (Lasseter et al., 2009), suggesting that these two distinct regions may be acting similarly to influence reinstatement. Moreover, relatively few studies consider differences along the rostrocaudal axis within the mPFC, which has likely obscured functional differences among these subregions. Thus, with a relative paucity of studies in the literature, it is difficult to reconcile the current results using dopamine receptor blockade with those examining lesions and inactivation of the mOFC. Nonetheless, the present study, along with previous work, provides a critical basis for future work to examine this vmPFC subregion as well as consider functional differences along the rostrocaudal axis during motivated behaviors including drug seeking and feeding.
D1 V. D2 Receptors in the mPFC
Although previous work indicates that systemic and intra-mPFC D2 receptor blockade alters drug-seeking behaviors (Liu et al., 2010; Sun and Rebec, 2005; Woolverton and Virus, 1989), the present results suggest that D2 receptor blockade in the IL and mOFC did not affect cocaine seeking. Given that D1 and D2 receptors typically have excitatory and inhibitory influences, respectively, via opposing actions on adenylyl cyclase activity and cAMP production (Gonon, 1997; Paul et al., 1992), differential localization of these receptors on IL and mOFC neurons may account for our results. Work using in situ hybridization indicates that both D1 and D2 receptor mRNAs are found in efferent cortical populations (Gaspar et al., 1995). However, the cortical populations expressing D1 and D2 receptors appear to differ significantly depending on the receptor type expressed. Indeed, Gaspar et al. estimated that D2 receptor mRNA-containing neurons were 3–4 times less numerous than those containing D1 receptor mRNA in the PFC and noted that, although 25% of corticothalamic neurons express D1 receptors, these projection neurons do not appear to express D2 receptors. Other work has suggested that D1 and D2 receptors within the mPFC are located in minimally overlapping neuronal populations (Vincent et al., 1995). Thus, the present results may reflect mPFC differences in D1 vs. D2 receptor expression level and/or localization on different neuronal populations or even on different parts of the neurons (dendrites vs. axon terminals). Consistent with the importance of the D1 receptor in particular in the mPFC, prior research indicates that mPFC functions such as working memory and mental flexibility depends on dopamine activity at D1 receptors (Goldman-Rakic et al., 2000; Okubo et al., 1997; Sawaguchi and Goldman-Rakic, 1991; Williams and Goldman-Rakic, 1995).
Conclusion
Examining the role of D1 and D2 receptors in the IL and mOFC during the reinstatement of cocaine seeking, the present study found that D1 receptor blockade in the mOFC reduced all forms of reinstatement tested but, in the IL, only reduced cued reinstatement. D2 receptor blockade had no effect in either structure. Together with previous work showing a role for the IL in inhibiting cocaine seeking, the present study suggests that the actions of dopamine within the IL may be quite different from those of the structure as a whole and introduces an additional layer of complexity in our attempt to understand how the IL regulates drug-seeking behavior. Moreover, the current results are among the first to identify the mOFC as a region driving the reinstatement of cocaine seeking and also confirm the existence of critical functional differences along the rostrocaudal axis within the vmPFC. Future studies examining the vmPFC should carefully consider such differences in drawing functional conclusions about this region.
Acknowledgments
The authors would like to thank Victoria Ewald for her assistance in conducting experiments. This research was supported by NIH grant DA034684 (RTL). The authors declare no conflict of interest.
Footnotes
Author Contributions
CVC and RTL designed the experiments. CVC, ALG, and WRW conducted the experiments. CVC analyzed the data. CVC and RTL wrote the manuscript, and ALG provided manuscript revisions. All authors critically reviewed content and approved final version for publication.
References
- Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2006;31:1452–1461. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Taylor JR. Bidirectional modulation of infralimbic dopamine D1 and D2 receptor activity regulates flexible reward seeking. Frontiers in neuroscience. 2013;7:126. doi: 10.3389/fnins.2013.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nature neuroscience. 2011;14:420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. The Journal of comparative neurology. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Cosme CV, Gutman AL, LaLumiere RT. The Dorsal Agranular Insular Cortex Regulates the Cued Reinstatement of Cocaine-Seeking, but not Food-Seeking, Behavior in Rats. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2015;40:2425–2433. doi: 10.1038/npp.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzin MY, Kurzina NP, Malinina EP, Kozlov AP. The effects of local application of D2 selective dopaminergic drugs into the medial prefrontal cortex of rats in a delayed spatial choice task. Behavioural brain research. 2000;109:99–111. doi: 10.1016/s0166-4328(99)00166-7. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. The European journal of neuroscience. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain research Brain research reviews. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Gonon F. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcott PK, Quinn JJ, Taylor JR. Bidirectional modulation of goal-directed actions by prefrontal cortical dopamine. Cerebral cortex. 2007;17:2820–2827. doi: 10.1093/cercor/bhm010. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Projections of the medial orbital and ventral orbital cortex in the rat. The Journal of comparative neurology. 2011;519:3766–3801. doi: 10.1002/cne.22733. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:9987–9995. doi: 10.1523/JNEUROSCI.23-31-09987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalumiere RT, Nguyen LT, McGaugh JL. Post-training intrabasolateral amygdala infusions of dopamine modulate consolidation of inhibitory avoidance memory: involvement of noradrenergic and cholinergic systems. The European journal of neuroscience. 2004;20:2804–2810. doi: 10.1111/j.1460-9568.2004.03744.x. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. The European journal of neuroscience. 2012;35:614–622. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. The European journal of neuroscience. 2009;30:1370–1381. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Arguello AA, Wells AM, Hodges MA, Fuchs RA. Contribution of a mesocorticolimbic subcircuit to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2014;39:660–669. doi: 10.1038/npp.2013.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jernigen C, Gharib M, Booth S, Caggiula AR, Sved AF. Effects of dopamine antagonists on drug cue-induced reinstatement of nicotine-seeking behavior in rats. Behavioural pharmacology. 2010;21:153–160. doi: 10.1097/FBP.0b013e328337be95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkusz DC, Yenko I, Rotella FM, Banakos T, Olsson K, Dindyal T, Vig V, Bodnar RJ. Dopamine receptor signaling in the medial orbital frontal cortex and the acquisition and expression of fructose-conditioned flavor preferences in rats. Brain research. 2015;1596:116–125. doi: 10.1016/j.brainres.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- Pakdel R, Rashidy-Pour A. Microinjections of the dopamine D2 receptor antagonist sulpiride into the medial prefrontal cortex attenuate glucocorticoid-induced impairment of long-term memory retrieval in rats. Neurobiology of learning and memory. 2007;87:385–390. doi: 10.1016/j.nlm.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Paul ML, Graybiel AM, David JC, Robertson HA. D1-like and D2-like dopamine receptors synergistically activate rotation and c-fos expression in the dopamine-depleted striatum in a rat model of Parkinson’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1992;12:3729–3742. doi: 10.1523/JNEUROSCI.12-10-03729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Pattij T, De Vries TJ. Targeting cocaine versus heroin memories: divergent roles within ventromedial prefrontal cortex. Trends in pharmacological sciences. 2013;34:689–695. doi: 10.1016/j.tips.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schonig K, Bartsch D, Hope BT, Spanagel R, Sommer WH. Losing Control: Excessive Alcohol Seeking after Selective Inactivation of Cue-Responsive Neurons in the Infralimbic Cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:10750–10761. doi: 10.1523/JNEUROSCI.0684-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockros LA, Pentkowski NS, Swinford SE, Neisewander JL. Blockade of 5-HT2A receptors in the medial prefrontal cortex attenuates reinstatement of cue-elicited cocaine-seeking behavior in rats. Psychopharmacology. 2011;213:307–320. doi: 10.1007/s00213-010-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Prefrontal cortex modulates desire and dread generated by nucleus accumbens glutamate disruption. Biological psychiatry. 2013;73:360–370. doi: 10.1016/j.biopsych.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Setlow B, McGaugh JL. Sulpiride infused into the nucleus accumbens posttraining impairs memory of spatial water maze training. Behavioral neuroscience. 1998;112:603–610. doi: 10.1037//0735-7044.112.3.603. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Williams SM, Szigeti K, Goldman-Rakic PS. Light and electron microscopic characterization of dopamine-immunoreactive axons in human cerebral cortex. The Journal of comparative neurology. 1992;321:325–335. doi: 10.1002/cne.903210302. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, LaLumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addiction biology. 2013;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology. 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain research bulletin. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neuroscience and biobehavioral reviews. 2010;35:276–284. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Hoorneman EM, Buijs RM, Matthijssen MA, Geffard M, Uylings HB. Immunocytochemical localization of dopamine in the prefrontal cortex of the rat at the light and electron microscopical level. Neuroscience. 1987;22:849–862. doi: 10.1016/0306-4522(87)92964-2. [DOI] [PubMed] [Google Scholar]
- Vincent SL, Khan Y, Benes FM. Cellular colocalization of dopamine D1 and D2 receptors in rat medial prefrontal cortex. Synapse. 1995;19:112–120. doi: 10.1002/syn.890190207. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Winter S, Dieckmann M, Schwabe K. Dopamine in the prefrontal cortex regulates rats behavioral flexibility to changing reward value. Behavioural brain research. 2009;198:206–213. doi: 10.1016/j.bbr.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Virus RM. The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacology, biochemistry, and behavior. 1989;32:691–697. doi: 10.1016/0091-3057(89)90019-1. [DOI] [PubMed] [Google Scholar]


