Abstract
The ability to stop ongoing movement is fundamental to animal survival. Behavioural arrest involves the hierarchical integration of information throughout the forebrain, which ultimately leads to the coordinated inhibition and activation of specific brainstem motor centres. Recent advances have shed light on multiple regions and pathways involved in this critical behavioural process. Here, we synthesize these new findings together with previous work to build a more complete understanding of the circuit mechanisms underlying suppression of ongoing action. We focus on three specific conditions leading to behavioural arrest: goal completion, fear and startle. We outline the circuitry responsible for the production of these behaviours and discuss their dysfunction in neurological disease.
This article is part of the themed issue ‘Movement suppression: brain mechanisms for stopping and stillness’.
Keywords: stopping, behavioural arrest, action suppression, freezing, startle
1. Introduction
An animal will stop ongoing movement for a variety of reasons, including reaching a goal, freezing during perceived threat and stopping to evaluate a surprising stimulus. In each of these situations, the animal will terminate the current action, such as locomotion or grooming, and transition its energy into postural control. This requires coordination between the neural signals that cease the current action and those that enable and maintain postural control during motionlessness. There is no single circuit in the brain responsible for the termination of movement. Instead, multiple neural systems appear to carry out the process depending on the specific set of circumstances that require it [1–8].
In this review, we highlight recent work on the neural circuits implicated in different types of behavioural arrest. We start with action termination upon goal completion, which putatively involves inhibitory control of downstream motor circuitry by the basal ganglia (BG). This type of motor suppression should be clearly distinguished from action cancellation (or countermanding), which occurs at or near the initiation of action and is reviewed elsewhere in this issue. We next consider fear-induced freezing, which is controlled by the amygdala and involves both activation of muscle tone and inhibition of other motor programmes. Following this, we examine circuits involved in startle-induced behavioural arrest that arises from surprising visual or auditory stimuli. Finally, we discuss diseases in which this circuitry malfunctions leading to aberrant arrest and movement symptoms.
2. Termination of action upon goal completion
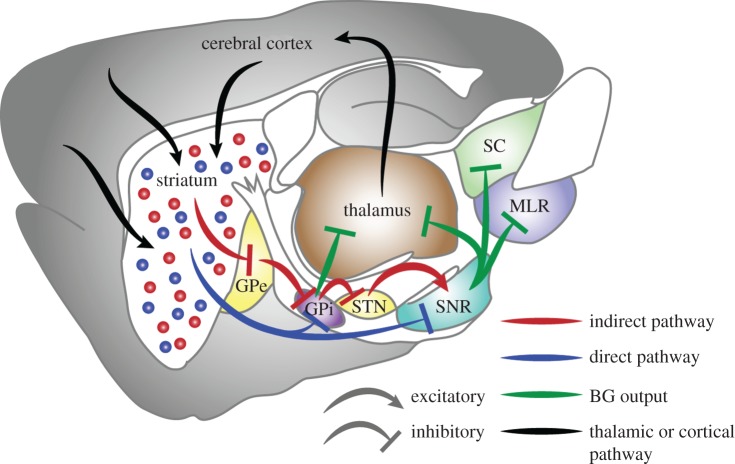
One of the most common instances in which ongoing movement must be terminated is when an animal reaches a goal, such as a food or water source. However, few studies have looked specifically at this aspect of natural behaviour. A strong candidate system for action termination upon goal completion is the BG, which are a set of subcortical nuclei that are involved in adaptive motor planning and action selection [4,5,9–12]. Two major circuits through the BG originate from two populations of GABAergic striatal projection neurons: direct pathway medium spiny neurons (dMSNs) and indirect pathway medium spiny neurons (iMSNs) [10,13–15]. dMSNs directly inhibit the output nuclei of the BG, the substantia nigra pars reticulata (SNr) and globus pallidus internal portion (GPi), whereas iMSNs disinhibit the SNr/GPi through a series of intermediate nuclei (figure 1). The two pathways have long been hypothesized to serve opposing functions [16,17], and recent optogenetic experiments have established opposite behavioural effects of activating each of these circuits [18–21]. The SNr and GPi contain tonically active GABAergic projection neurons that regulate motor behaviour through inhibition of regions downstream of the BG, including the thalamus, superior colliculus, dorsal raphe and brainstem motor centres [4,22–25]. A simple model for BG regulation of target regions is that activation of dMSNs releases tonic inhibition by BG output, allowing action initiation, whereas iMSN activity increases inhibition and suppresses motor programmes, enabling an animal to stop. Indeed, a pause in the firing rate of some SNr neurons precedes increased firing in the superior colliculus and saccades in monkeys [26]. However, both increases and decreases in the firing of BG output neurons are observed during limb movements [27,28] or optogenetic activation of BG circuitry [29], reflecting more complex control of downstream circuitry.
Figure 1.
Simplified basal ganglia circuit diagram shown in sagittal view with many connections omitted for simplicity. Blue arrows and dots, direct pathway and dMSNs; red arrows and dots, indirect pathway and iMSNs; green arrows, BG output; black arrows, thalamic or cortical connections. GPe, globus pallidus, pars externa; GPi, globus pallidus, pars interna; MLR, mesencephalic locomotor region; SC, superior colliculus; SNr, substantia nigra, pars reticulata; STN, subthalamic nucleus.
More recently, the effects of dMSN and iMSN stimulation on brainstem regions involved in locomotion were tested. Consistent with the model proposed above, Roseberry et al. [25] showed that optogenetic activation of dMSNs resulted in a selective increase in firing of glutamatergic neurons in the mesencephalic locomotor region (MLR), whereas iMSN activation resulted in decreased firing of MLR glutamatergic neurons. This modulation of the glutamatergic population was quite homogeneous, in contrast to the heterogeneous effects of dMSN and iMSN stimulation on SNr neurons [29], indicating functional specificity in projections from BG output. However, much remains unknown, particularly with regard to modulation of thalamocortical circuits, where BG circuit activation exerts complex actions that depend on time, cell location, functional specialization and training [30].
Although the basal ganglia have long been implicated in movement initiation, their role in termination of motivated behaviours is less appreciated. Indeed, there are cells in the striatum that become active at the termination of a motor sequence or at the cessation of locomotion during goal-directed behaviour [31–37]. As the striatum receives input from regions involved in motor planning and outcome evaluation [8,38–40], and the SNr/GPi send outputs that regulate downstream motor structures [9,24,41], it would follow that information about task progress could be integrated here to cease ongoing action. In support of this, patients with Parkinson's disease (PD), which strongly disrupts striatal circuitry and function [10,11,13], have deficits in terminating ongoing movement [42,43]. In addition, optogenetic activation of dMSNs during lever-pressing causes mice to continue lever-pressing beyond the number of presses required to receive reward [44]. Taken together, the stop signals seen in the striatum and other BG nuclei appear to be critical for terminating ongoing movement.
The simplest explanation for how the BG could stop movement would be an increase in the activity of the indirect pathway. This would lead to enhanced firing in SNr/GPi and the inhibition of downstream structures. However, many aspects of this simple model are not borne out by the available evidence. First, both pathways are active simultaneously during movement [35,45] and at the cessation of movement [35]. Second, Jin et al. [35] found that more optogenetically identified dMSNs encoded the termination of a lever-pressing sequence than identified iMSNs. Indeed, termination of movement could be considered an action in and of itself that requires increased drive to muscles to counteract ongoing behaviour and subsequently stabilize posture. The basal ganglia are therefore likely to coordinate both the initiation and termination of actions through similar processes.
3. Fear-induced freezing
Perhaps the most widely studied form of behavioural arrest occurs during conditioned fear paradigms [2,3]. When presented with a cue (CS) predicting an aversive stimulus, animals will stop moving and their muscles will become rigid in preparation for escape [6,46,47]. In the context of behavioural arrest, it is important to note that these are two separate processes: one to suppress movement and one to induce the characteristic muscle tone associated with freezing [48]. This freezing state has been used for decades to measure an animal's state of fear [49–52]. A principal structure in the expression of freezing is the central nucleus of the amygdala (CeA; figure 2) [53–56]. This structure is considered the output of the amygdala and projects to the hypothalamus, periaqueductal grey (PAG), and pontine reticular formation among other structures [57]. The CeA can be divided into two separate subregions, a lateral portion (CeL) and a medial portion (CeM) [3,58]. It appears that both structures regulate fear behaviours through their projections to downstream areas [56,59].
Figure 2.
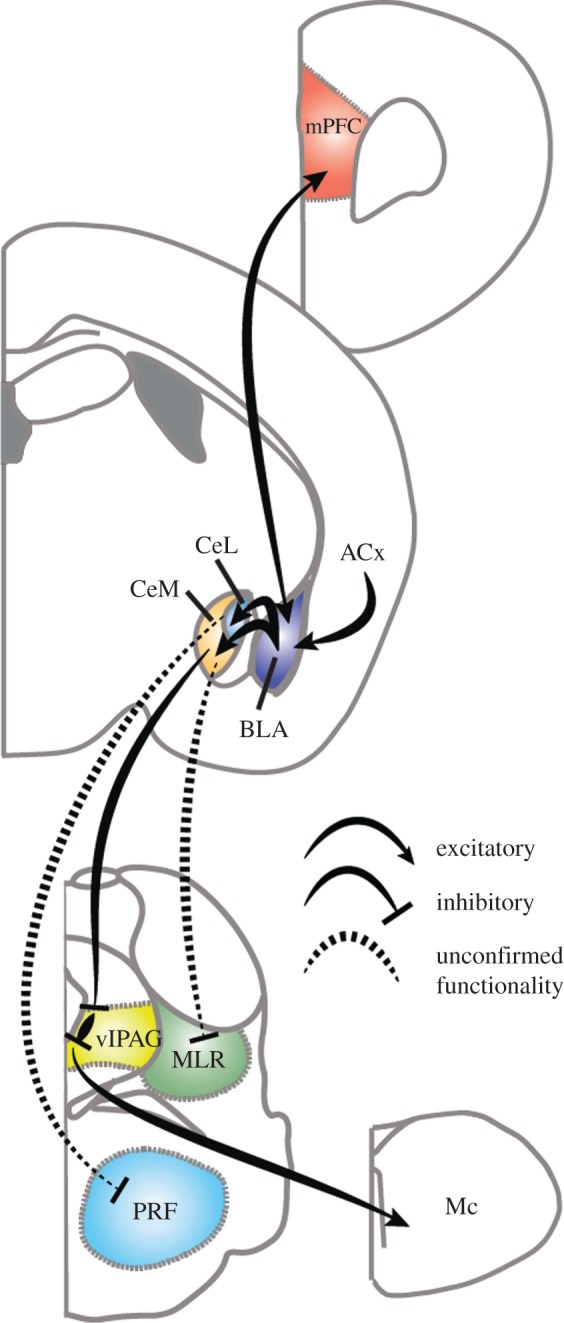
Schematic of fear-induced behavioural arrest pathways. Much of the amygdala circuitry is omitted for simplicity. ACx, auditory cortex; BLA, basolateral amygdala; CeL, centrolateral nucleus of the amygdala; CeM, centromedial nucleus of the amygdala; Mc, magnocellular nucleus; MLR, mesencephalic locomotor region; mPFC, medial prefrontal cortex; PRF, pontine reticular formation.
A recent study elegantly mapped how fear-induced freezing could result in increased muscle tone. CeM projection neurons were previously shown to be excited by a CS predicting a foot shock [55]. The authors demonstrated that GABAergic cells within the ventrolateral PAG (vlPAG) receive inhibitory input from CeM projection neurons and that activation or inhibition of these GABAergic neurons suppresses or drives freezing, respectively (figure 2). These GABAergic cells, in turn, synapse onto local glutamatergic cells within the PAG that target pre-motor neurons in the magnocellular nucleus and drive freezing-like behaviours [60]. This pathway increases muscle tone during presentation of a CS and likely participates in generating the rigid muscles characteristic of the freezing state [46,61]. The purpose of rigid muscles during freezing is still unknown, but it may enable faster escape than if only the postural and axial muscles used for regular standing were active [6,62]. It is interesting to note that in the same mouse line, activation of vlPAG GABAergic neurons increased the probability that a mouse would enter non-rapid eye movement (NREM) sleep [63]. These divergent results indicate that further work is needed to dissect out which neurons are responsible for these effects.
Although in theory, freezing could override ongoing actions, current evidence suggests that suppression of ongoing actions in response to a CS is a separate process. For example, suppression of lever-pressing has been shown to be independent of the PAG [48], but could still involve the CeL. Somatostatin-expressing (SOM+) cells in the CeL have been shown to project to the paraventricular nucleus of the thalamus (PVT) and the vlPAG [64]. The PVT lies adjacent to the intralaminar nuclei of the thalamus, which was recently shown to be a site for behavioural arrest induced by stimulation of glycinergic fibres from the pontine reticular formation (PRF; figure 2) [65]. This could be part of an arrest coordination centre as PRF terminal stimulation also resulted in cortical slow wave activity, which is a hallmark of the stationary state [66]. In a follow-up study using optogenetics, CeL SOM+ cells were shown to be sufficient to suppress licking behaviour to an aversive cue [64]. Interestingly, Penzo et al. [64] also found that inhibiting SOM+ cells in the CeL increased a locomotor response to aversive stimuli. Our laboratory recently demonstrated a direct connection from the CeA to glutamatergic neurons in MLR using cell-type-specific rabies viral tracing methods [25,67]. These glutamatergic cells are both necessary and sufficient to drive locomotion, and it is possible that SOM+ CeL neurons inhibit the MLR directly to stop locomotion during freezing. Finally, the CeM projects preferentially to direct pathway neurons in the striatum [38], which have been shown to initiate movement and locomotion [18,25]. As CeM neurons are GABAergic [57], this provides yet another pathway through which a CS could induce fear-mediated behavioural suppression in multiple brain regions through the CeA (figure 2).
Another mechanism for coordinated suppression of behaviour could rely on coherent oscillations across brain regions. While cortical slow-wave activity has usually been thought to result from the freezing state [68], recent evidence suggests that slow waves in the mPFC can actually drive freezing. Karalis et al. [61] took these results further and demonstrated that 4 Hz oscillations originating in the dorsomedial prefrontal cortex (dmPFC) could predict the freezing state and temporally lead similar oscillations in the basolateral amygdala (BLA; figure 2). Driving this specific frequency using channelrhodopsin in parvalbumin-expressing interneurons in the dmPFC also resulted in increased freezing behaviour. It is interesting to note that specific basolateral amygdala (BLA) neuron populations also project to specific portions of the dmPFC and that these projection patterns specify whether a BLA neuron encodes fear expression or extinction [59]. These reciprocal connections could be part of an integrated signal that evaluates the necessity of freezing against the opportunity for other actions such as escape that could be mediated by the dmPFC projection to the striatum for coordinating orientation and locomotor initiation [6,38].
Together, these data suggest that brain-wide coordinated signalling involving both activation and inhibition of specific structures and cell types is necessary to execute fear-induced freezing. In spite of recent work in this area [3,58], many questions remain as to how these signals are integrated to result in behavioural arrest.
4. Startle-induced behavioural arrest
When an animal is surprised by a bright light or loud noise, it activates the startle reflex, which causes a strong increase in muscle tone and termination of ongoing behaviour. This is an unconscious action that occurs prior to cognitive assessment of a situation, but which can be modulated by conditions such as fear or experience [7,69–71]. Most studies have focused on the jump-like reflex that occurs during presentation of the surprising stimulus, but recent work has looked at the arresting effects of startle in ongoing behaviour.
Light-induced startle is relatively unstudied [72]. It is important to differentiate it from freezing behaviour observed under other visually aversive stimuli such as looming objects, which produce long-lasting immobilization. The temporary arrest behaviour seen during light-induced startle is transient and the animal rapidly transitions back to the behaviour it was performing previously [72]. Using a head-fixed preparation, Liang et al. [72] were able to demonstrate a novel pathway from visual cortex (V1) to the superficial superior colliculus (SC) involved in locomotor inhibition during light-induced startle. Silencing the SC eliminated the locomotor arrest, while silencing V1 input decreased the amount of locomotor inhibition indicating that while the SC is required for the behaviour, V1 may be playing a modulatory role. The SC receives direct input from the retina [73] and directly interfaces with spinally projecting motor systems [4,9,74,75], making it an excellent substrate to carry out visually induced startle arrest. In addition, the SC is thought to be part of the defensive response system along with the PAG [76–78]. In agreement with this role, the superficial SC was shown to contain a population of parvalbumin-positive excitatory neurons that could drive defensive behaviours including freezing and escape [79]. This population projects to the parabigeminal nucleus of the brainstem, which neighbours the MLR and PRF and has been implicated in the startle response [7]. In both these neighbouring regions, GABAergic neuron activity has been shown to halt ongoing behaviour and locomotion [25,65], suggesting a generalized role for GABAergic activity in this area.
Acoustic-mediated startle is generated through a separate pathway beginning at the cochlear nucleus (VCN) [7,70,80]. The VCN then synapses with the ventrolateral lemniscus, which in turn connects to reticulospinal neurons in the caudal portion of the PRF [80,81]. Little work has been done on the arresting effects of acoustic startle; however, both the SC [80] and the MLR [82] become active during the acoustic startle response independently of the reflexive action. GABAergic neurons in the MLR could pause ongoing behaviour while glutamatergic neuron activity remains just below running threshold so a quick transition to locomotion could occur if deemed necessary after the brief pause in behaviour [25].
5. Behavioural arrest in disease
A number of neurological diseases involve aberrant activity in the regions and pathways described in §§2–4. Perhaps most prominently, PD arises from a progressive loss of dopamine neurons and is characterized by basal ganglia circuit dysfunction [10,11,13,16,17]. Patients with the disease can have difficulty both initiating and stopping movement [42,43]. Among the most effective treatments for PD is deep brain stimulation (DBS) of the subthalamic nucleus (STN; figure 1), which sends excitatory inputs to the SNr and GPi. DBS in the STN was shown to increase performance in the stop-signal reaction time test [83], likely by increasing the inhibitory output of SNr or GPi neurons. It is important to note that this is action cancellation, a different form of behavioural arrest. Nevertheless, the same circuitry could be active in both forms of arrest. Interestingly, dopamine signalling in the dorsal striatum was recently shown to decrease rapidly during locomotion termination [84]. Given the poor performance in stopping tasks in PD patients, it is tempting to speculate that loss of phasic dopamine signals is involved in both initiation and termination of actions.
Cataplexy is a condition related to narcolepsy in which a person suddenly loses muscle tone while maintaining consciousness. Strong emotional or surprising stimuli can trigger these attacks, potentially through the amygdalar–PAG–ventromedial medulla circuit outlined above, which can aberrantly drive REM sleep circuitry and trigger sleep paralysis [56,60,85]. When these sleep paralysis circuits fail, the result is REM sleep disorder, which is characterized by acting out dreams and hallucinations [85,86]. Paradoxically, increased activity in the CeA, which occurs during highly emotional stimuli [87], should result in disinhibition of glutamatergic vlPAG neurons projecting to the PRF and spinal cord [60]. This would result in increased, rather than decreased, muscle tone, the opposite of what is seen in cataplexy. In addition, vlPAG GABAergic neurons that were shown to drive NREM sleep [63] would be inhibited. It is therefore likely that the circuitry underlying cataplexy involves other brain regions. One candidate region is the MLR, which has been hypothesized to be involved in postural control and muscle tone, in addition to its well-established role in locomotion [88–90]. Indeed, cataplexy can be produced by lesions of the MLR [88,89], and DBS of the MLR in PD patients can increase awareness during moderate stimulation or drive sleep during over-stimulation [91]. This observation, along with newly found connections from the amygdala [25,67] (figure 2), suggests that the MLR could be involved in cataplectic attacks. The CeA projects most strongly to glutamatergic neurons in the MLR [25], and it is these glutamatergic neurons that drive locomotion, project to the spinal cord [88] and modulate brain state [92,93]. An inhibitory signal from the CeA could inhibit MLR glutamatergic neurons during strong emotional stimuli to shut down all of these functions.
6. Conclusion
The findings outlined here summarize how multiple circuits and pathways can result in behavioural arrest or cessation of ongoing movement. When the circuits involved in this process become damaged or malfunction, they can have highly detrimental effects. It should be noted that while we separated the circuits based on hypothesized function, these circuits are most likely acting in parallel and simultaneously during all phases of behavioural arrest, adding specific or even redundant contributions. Future work will continue to refine and tease out these contributions while integrating them into the overall function of the nervous system.
Acknowledgements
We thank the Kreitzer Laboratory and Marielena Sosa for valuable discussions.
Authors' contributions
T.K.R. and A.C.K. conceptualized and wrote the manuscript.
Competing interests
We have no competing interests.
Funding
The Kreitzer laboratory is supported by the NIH.
References
- 1.Klemm WR. 2001. Behavioral arrest: in search of the neural control system. Prog. Neurobiol. 65, 453–471. ( 10.1016/S0301-0082(01)00016-8) [DOI] [PubMed] [Google Scholar]
- 2.Pare D, Quirk GJ, Ledoux JE. 2004. New vistas on amygdala networks in conditioned fear. J. Neurophysiol. 92, 1–9. ( 10.1152/jn.00153.2004) [DOI] [PubMed] [Google Scholar]
- 3.Herry C, Johansen JP. 2014. Encoding of fear learning and memory in distributed neuronal circuits. Nat. Neurosci. 17, 1644–1654. ( 10.1038/nn.3869) [DOI] [PubMed] [Google Scholar]
- 4.Hikosaka O, Takikawa Y, Kawagoe R. 2000. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol. Rev. 80, 953–978. [DOI] [PubMed] [Google Scholar]
- 5.Graybiel AM. 1997. The basal ganglia and cognitive pattern generators. Schizophr. Bull. 23, 459–469. ( 10.1093/schbul/23.3.459) [DOI] [PubMed] [Google Scholar]
- 6.Mogenson GJ, Jones DL, Yim CY. 1980. From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol. 14, 69–97. ( 10.1016/0301-0082(80)90018-0) [DOI] [PubMed] [Google Scholar]
- 7.Davis M. 1989. Neural systems involved in fear-potentiated startle. Ann. N.Y. Acad. Sci. 563, 165–183. ( 10.1111/j.1749-6632.1989.tb42197.x) [DOI] [PubMed] [Google Scholar]
- 8.Gourley SL, Taylor JR. 2016. Going and stopping: dichotomies in behavioral control by the prefrontal cortex. Nat. Neurosci. 19, 656–664. ( 10.1038/nn.4275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA. 2005. Mechanisms for selection of basic motor programs: roles for the striatum and pallidum. Trends Neurosci. 28, 364–370. ( 10.1016/j.tins.2005.05.004) [DOI] [PubMed] [Google Scholar]
- 10.Kreitzer AC, Malenka RC. 2008. Striatal plasticity and basal ganglia circuit function. Neuron 60, 543–554. ( 10.1016/j.neuron.2008.11.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson AB, Kreitzer AC. 2014. Reassessing models of basal ganglia function and dysfunction. Annu. Rev. Neurosci. 37, 117–135. ( 10.1146/annurev-neuro-071013-013916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin HH, Knowlton BJ. 2006. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 7, 464–476. ( 10.1038/nrn1919) [DOI] [PubMed] [Google Scholar]
- 13.Gerfen CR, Surmeier DJ. 2011. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441–466. ( 10.1146/annurev-neuro-061010-113641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerfen CR, Engber T, Mahan L, Susel Z, Chase T, Monsma F, Sibley D. 1990. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432. ( 10.1126/science.2147780) [DOI] [PubMed] [Google Scholar]
- 15.Smith Y, Bevan MD, Shink E, Bolam JP. 1998. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience 86, 353–387. ( 10.1016/S0306-4522(97)00608-8) [DOI] [PubMed] [Google Scholar]
- 16.Albin RL, Young AB, Penney JB. 1989. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375. ( 10.1016/0166-2236(89)90074-X) [DOI] [PubMed] [Google Scholar]
- 17.DeLong MR. 1990. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285. ( 10.1016/0166-2236(90)90110-V) [DOI] [PubMed] [Google Scholar]
- 18.Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. 2010. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626. ( 10.1038/nature09159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobo MK, et al. 2010. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330, 385–390. ( 10.1126/science.1188472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yttri EA, Dudman JT. 2016. Opponent and bidirectional control of movement velocity in the basal ganglia. Nature 533, 402–406. ( 10.1038/nature17639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. 2012. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat. Neurosci. 15, 1281–1289. ( 10.1038/nn.3188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang Y, Kitai ST. 1990. Electrophysiological properties of pedunculopontine neurons and their postsynaptic responses following stimulation of substantia nigra reticulata. Brain Res. 535, 79–95. ( 10.1016/0006-8993(90)91826-3) [DOI] [PubMed] [Google Scholar]
- 23.Saitoh K, Hattori S, Song WJ, Isa T, Takakusaki K. 2003. Nigral GABAergic inhibition upon cholinergic neurons in the rat pedunculopontine tegmental nucleus. Eur. J. Neurosci. 18, 879–886. ( 10.1046/j.1460-9568.2003.02825.x) [DOI] [PubMed] [Google Scholar]
- 24.Takakusaki K, Shiroyama T, Yamamoto T, Kitai ST. 1996. Cholinergic and noncholinergic tegmental pedunculopontine projection neurons in rats revealed by intracellular labeling. J. Comp. Neurol. 371, 345–361. ( 10.1002/(SICI)1096-9861(19960729)371:3%3C345::AID-CNE1%3E3.0.CO;2-2) [DOI] [PubMed] [Google Scholar]
- 25.Roseberry TK, Lee AM, Lalive AL, Wilbrecht L, Bonci A, Kreitzer AC. 2016. Cell-type-specific control of brainstem locomotor circuits by basal ganglia. Cell 164, 526–537. ( 10.1016/j.cell.2015.12.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hikosaka O, Wurtz RH. 1983. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J. Neurophysiol. 49, 1285–1301. [DOI] [PubMed] [Google Scholar]
- 27.Mink JW. 1996. The basal ganglia: focused selection and inhibition of competing motor programs. Prog. Neurobiol. 50, 381–425. ( 10.1016/S0301-0082(96)00042-1) [DOI] [PubMed] [Google Scholar]
- 28.Turner RS, Anderson ME. 1997. Pallidal discharge related to the kinematics of reaching movements in two dimensions. J. Neurophysiol. 77, 1051–1074. [DOI] [PubMed] [Google Scholar]
- 29.Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. 2013. Control of basal ganglia output by direct and indirect pathway projection neurons. J. Neurosci. 33, 18 531–18 539. ( 10.1523/JNEUROSCI.1278-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oldenburg IA, Sabatini BL. 2015. Antagonistic but not symmetric regulation of primary motor cortex by basal ganglia direct and indirect pathways. Neuron 86, 1174–1181. ( 10.1016/j.neuron.2015.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atallah HE, McCool AD, Howe MW, Graybiel AM. 2014. Neurons in the ventral striatum exhibit cell-type-specific representations of outcome during learning. Neuron 82, 1145–1156. ( 10.1016/j.neuron.2014.04.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith KS, Graybiel AM. 2013. A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron 79, 361–374. ( 10.1016/j.neuron.2013.05.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thorn CA, Atallah H, Howe M, Graybiel AM. 2010. Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron 66, 781–795. ( 10.1016/j.neuron.2010.04.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujii N, Graybiel AM. 2003. Representation of action sequence boundaries by macaque prefrontal cortical neurons. Science 301, 1246–1249. ( 10.1126/science.1086872) [DOI] [PubMed] [Google Scholar]
- 35.Jin X, Tecuapetla F, Costa RM. 2014. Basal ganglia subcircuits distinctively encode the parsing and concatenation of action sequences. Nat. Neurosci. 17, 423–430. ( 10.1038/nn.3632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin X, Costa RM. 2010. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature 466, 457–462. ( 10.1038/nature09263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apicella P, Scarnati E, Ljungberg T, Schultz W. 1992. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J. Neurophysiol. 68, 945–960. [DOI] [PubMed] [Google Scholar]
- 38.Wall NR, De La Parra M, Callaway EM, Kreitzer AC. 2013. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron 79, 347–360. ( 10.1016/j.neuron.2013.05.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sippy T, Lapray D, Crochet S, Petersen CC. 2015. Cell-type-specific sensorimotor processing in striatal projection neurons during goal-directed behavior. Neuron 88, 298–305. ( 10.1016/j.neuron.2015.08.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hintiryan H, et al. 2016. The mouse cortico-striatal projectome. Nat. Neurosci. 19, 1100–1114. ( 10.1038/nn.4332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hikosaka O. 2007. Basal ganglia mechanisms of reward-oriented eye movement. Ann. N.Y. Acad. Sci. 1104, 229–249. ( 10.1196/annals.1390.012) [DOI] [PubMed] [Google Scholar]
- 42.Gauggel S, Rieger M, Feghoff TA. 2004. Inhibition of ongoing responses in patients with Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 75, 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rieger M, Gauggel S, Burmeister K. 2003. Inhibition of ongoing responses following frontal, nonfrontal, and basal ganglia lesions. Neuropsychology 17, 272–282. ( 10.1037/0894-4105.17.2.272) [DOI] [PubMed] [Google Scholar]
- 44.Tecuapetla F, Jin X, Lima SQ, Costa RM. 2016. Complementary contributions of striatal projection pathways to action initiation and execution. Cell 166, 703–715. ( 10.1016/j.cell.2016.06.032) [DOI] [PubMed] [Google Scholar]
- 45.Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. 2013. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238–242. ( 10.1038/nature11846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steenland HW, Zhuo M. 2009. Neck electromyography is an effective measure of fear behavior. J. Neurosci. Methods 177, 355–360. ( 10.1016/j.jneumeth.2008.10.020) [DOI] [PubMed] [Google Scholar]
- 47.Fanselow MS. 1994. Neural organization of the defensive behavior system responsible for fear. Psychon. Bull. Rev. 1, 429–438. ( 10.3758/BF03210947) [DOI] [PubMed] [Google Scholar]
- 48.Amorapanth P, Nader K, LeDoux JE. 1999. Lesions of periaqueductal gray dissociate conditioned freezing from conditioned suppression behavior in rats. Learn. Mem. 6, 491–499. ( 10.1101/lm.6.5.491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lorenz K, Tinbergen N. 1938. Taxis und Instinkthandlung in der Eirollbewegung der Graugans. Z. Tierpsychol. 2, 1–29. ( 10.1111/j.1439-0310.1939.tb01558.x) [DOI] [Google Scholar]
- 50.Blanchard RJ, Blanchard DC. 1969. Passive and active reactions to fear-eliciting stimuli. J. Comp. Physiol. Psychol. 68, 129–135. ( 10.1037/h0027676) [DOI] [PubMed] [Google Scholar]
- 51.Fanselow MS. 1980. Conditioned and unconditional components of post-shock freezing. Pavlov J. Biol. Sci. 15, 177–182. [DOI] [PubMed] [Google Scholar]
- 52.Bolles RC, Riley A. 1976. The effect of predictive cues on freezing in rats. Anim. Learn. Behav. 4, 6–8. ( 10.3758/BF03211975) [DOI] [Google Scholar]
- 53.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. 1988. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 8, 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johansen JP, Tarpley JW, LeDoux JE, Blair HT. 2010. Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nat. Neurosci. 13, 979–986. ( 10.1038/nn.2594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciocchi S, et al. 2010. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282. ( 10.1038/nature09559) [DOI] [PubMed] [Google Scholar]
- 56.Yu K, Garcia da Silva P, Albeanu DF, Li B. 2016. Central amygdala somatostatin neurons gate passive and active defensive behaviors. J. Neurosci. 36, 6488–6496. ( 10.1523/JNEUROSCI.4419-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oka T, Tsumori T, Yokota S, Yasui Y. 2008. Neuroanatomical and neurochemical organization of projections from the central amygdaloid nucleus to the nucleus retroambiguus via the periaqueductal gray in the rat. Neurosci. Res. 62, 286–298. ( 10.1016/j.neures.2008.10.004) [DOI] [PubMed] [Google Scholar]
- 58.Duvarci S, Pare D. 2014. Amygdala microcircuits controlling learned fear. Neuron 82, 966–980. ( 10.1016/j.neuron.2014.04.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Senn V, et al. 2014. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81, 428–437. ( 10.1016/j.neuron.2013.11.006) [DOI] [PubMed] [Google Scholar]
- 60.Tovote P, et al. 2016. Midbrain circuits for defensive behaviour. Nature 534, 206–212. ( 10.1038/nature17996) [DOI] [PubMed] [Google Scholar]
- 61.Karalis N, et al. 2016. 4-Hz oscillations synchronize prefrontal-amygdala circuits during fear behavior. Nat. Neurosci. 19, 605–612. ( 10.1038/nn.4251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gladwin TE, Hashemi MM, van Ast V, Roelofs K. 2016. Ready and waiting: Freezing as active action preparation under threat. Neurosci. Lett. 619, 182–188. ( 10.1016/j.neulet.2016.03.027) [DOI] [PubMed] [Google Scholar]
- 63.Weber F, Chung S, Beier KT, Xu M, Luo L, Dan Y. 2015. Control of REM sleep by ventral medulla GABAergic neurons. Nature 526, 435–438. ( 10.1038/nature14979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penzo MA, Robert V, Li B. 2014. Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J. Neurosci. 34, 2432–2437. ( 10.1523/JNEUROSCI.4166-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giber K, et al. 2015. A subcortical inhibitory signal for behavioral arrest in the thalamus. Nat. Neurosci. 18, 562–568. ( 10.1038/nn.3951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poulet JF, Petersen CC. 2008. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454, 881–885. ( 10.1038/nature07150) [DOI] [PubMed] [Google Scholar]
- 67.Liang H, Watson C, Paxinos G. 2015. Projections from the central amygdaloid nucleus to the precuneiform nucleus in the mouse. Brain Struct. Funct. 220, 263–271. ( 10.1007/s00429-013-0653-0) [DOI] [PubMed] [Google Scholar]
- 68.Buzsaki G. 2006. Rhythms of the brain. New York, NY: Oxford University Press. [Google Scholar]
- 69.Ison JR, Hammond GR. 1971. Modification of the startle reflex in the rat by changes in the auditory and visual environments. J. Comp. Physiol. Psychol. 75, 435–452. ( 10.1037/h0030934) [DOI] [PubMed] [Google Scholar]
- 70.Ison JR, McAdam DW, Hammond GR. 1973. Latency and amplitude changes in the acoustic startle reflex of the rat produced by variation in auditory prestimulation. Physiol. Behav. 10, 1035–1039. ( 10.1016/0031-9384(73)90185-6) [DOI] [PubMed] [Google Scholar]
- 71.Davis M, Gendelman PM. 1977. Plasticity of the acoustic startle response in the acutely decerebrate rat. J. Comp. Physiol. Psychol. 91, 549–563. ( 10.1037/h0077345) [DOI] [PubMed] [Google Scholar]
- 72.Liang F, Xiong XR, Zingg B, Ji XY, Zhang LI, Tao HW. 2015. Sensory cortical control of a visually induced arrest behavior via corticotectal projections. Neuron 86, 755–767. ( 10.1016/j.neuron.2015.03.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wassle H, Illing RB. 1980. The retinal projection to the superior colliculus in the cat: a quantitative study with HRP. J. Comp. Neurol. 190, 333–356. ( 10.1002/cne.901900208) [DOI] [PubMed] [Google Scholar]
- 74.Gandhi NJ, Katnani HA. 2011. Motor functions of the superior colliculus. Annu. Rev. Neurosci. 34, 205–231. ( 10.1146/annurev-neuro-061010-113728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Redgrave P, Mitchell IJ, Dean P. 1987. Descending projections from the superior colliculus in rat: a study using orthograde transport of wheatgerm-agglutinin conjugated horseradish peroxidase. Exp. Brain Res. 68, 147–167. ( 10.1007/BF00255241) [DOI] [PubMed] [Google Scholar]
- 76.Schenberg LC, Povoa RM, Costa AL, Caldellas AV, Tufik S, Bittencourt AS. 2005. Functional specializations within the tectum defense systems of the rat. Neurosci. Biobehav. Rev. 29, 1279–1298. ( 10.1016/j.neubiorev.2005.05.006) [DOI] [PubMed] [Google Scholar]
- 77.Sahibzada N, Dean P, Redgrave P. 1986. Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J. Neurosci. 6, 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dean P, Mitchell IJ, Redgrave P. 1988. Responses resembling defensive behaviour produced by microinjection of glutamate into superior colliculus of rats. Neuroscience 24, 501–510. ( 10.1016/0306-4522(88)90345-4) [DOI] [PubMed] [Google Scholar]
- 79.Shang C, et al. 2015. Brain circuits. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science 348, 1472–1477. ( 10.1126/science.aaa8694) [DOI] [PubMed] [Google Scholar]
- 80.Davis M, Gendelman DS, Tischler MD, Gendelman PM. 1982. A primary acoustic startle circuit: lesion and stimulation studies. J. Neurosci. 2, 791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee Y, Lopez DE, Meloni EG, Davis M. 1996. A primary acoustic startle pathway: obligatory role of cochlear root neurons and the nucleus reticularis pontis caudalis. J. Neurosci. 16, 3775–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ebert U, Ostwald J. 1991. The mesencephalic locomotor region is activated during the auditory startle response of the unrestrained rat. Brain Res. 565, 209–217. ( 10.1016/0006-8993(91)91651-G) [DOI] [PubMed] [Google Scholar]
- 83.van den Wildenberg WP, van Boxtel GJ, van der Molen MW, Bosch DA, Speelman JD, Brunia CH. 2006. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson's disease. J. Cogn. Neurosci. 18, 626–636. ( 10.1162/jocn.2006.18.4.626) [DOI] [PubMed] [Google Scholar]
- 84.Howe MW, Dombeck DA. 2016. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535, 505–510. ( 10.1038/nature18942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fraigne JJ, Torontali ZA, Snow MB, Peever JH. 2015. REM sleep at its core - circuits, neurotransmitters, and pathophysiology. Front. Neurol. 6, 123 ( 10.3389/fneur.2015.00123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mathis J, Hess CW, Bassetti C. 2007. Isolated mediotegmental lesion causing narcolepsy and rapid eye movement sleep behaviour disorder: a case evidencing a common pathway in narcolepsy and rapid eye movement sleep behaviour disorder. J. Neurol. Neurosurg. Psychiatry 78, 427–429. ( 10.1136/jnnp.2006.099515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. 1996. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383, 812–815. ( 10.1038/383812a0) [DOI] [PubMed] [Google Scholar]
- 88.Sherman D, Fuller PM, Marcus J, Yu J, Zhang P, Chamberlin NL, Saper CB, Lu J. 2015. Anatomical location of the mesencephalic locomotor region and its possible role in locomotion, posture, cataplexy, and Parkinsonism. Front. Neurol. 6, 140 ( 10.3389/fneur.2015.00140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu J, Sherman D, Devor M, Saper CB. 2006. A putative flip-flop switch for control of REM sleep. Nature 441, 589–594. ( 10.1038/nature04767) [DOI] [PubMed] [Google Scholar]
- 90.Martinez-Gonzalez C, Bolam JP, Mena-Segovia J. 2011. Topographical organization of the pedunculopontine nucleus. Front. Neuroanat. 5, 22 ( 10.3389/fnana.2011.00022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arnulf I, Ferraye M, Fraix V, Benabid AL, Chabardes S, Goetz L, Pollak P, Debû B. 2010. Sleep induced by stimulation in the human pedunculopontine nucleus area. Ann. Neurol. 67, 546–549. ( 10.1002/ana.21912) [DOI] [PubMed] [Google Scholar]
- 92.Lee AM, Hoy JL, Bonci A, Wilbrecht L, Stryker MP, Niell CM. 2014. Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron 83, 455–466. ( 10.1016/j.neuron.2014.06.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alessandro S, et al. 2010. Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive domains. J. Neurol. Sci. 289, 44–48. ( 10.1016/j.jns.2009.08.017) [DOI] [PubMed] [Google Scholar]