Abstract
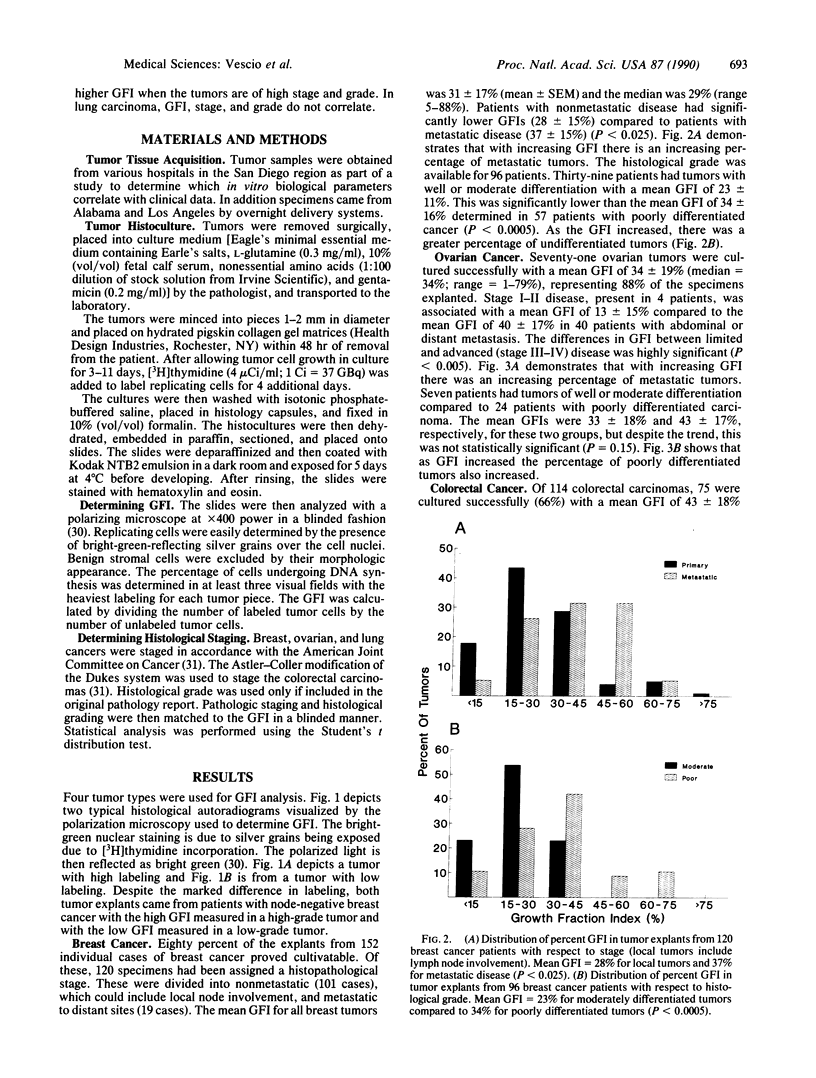
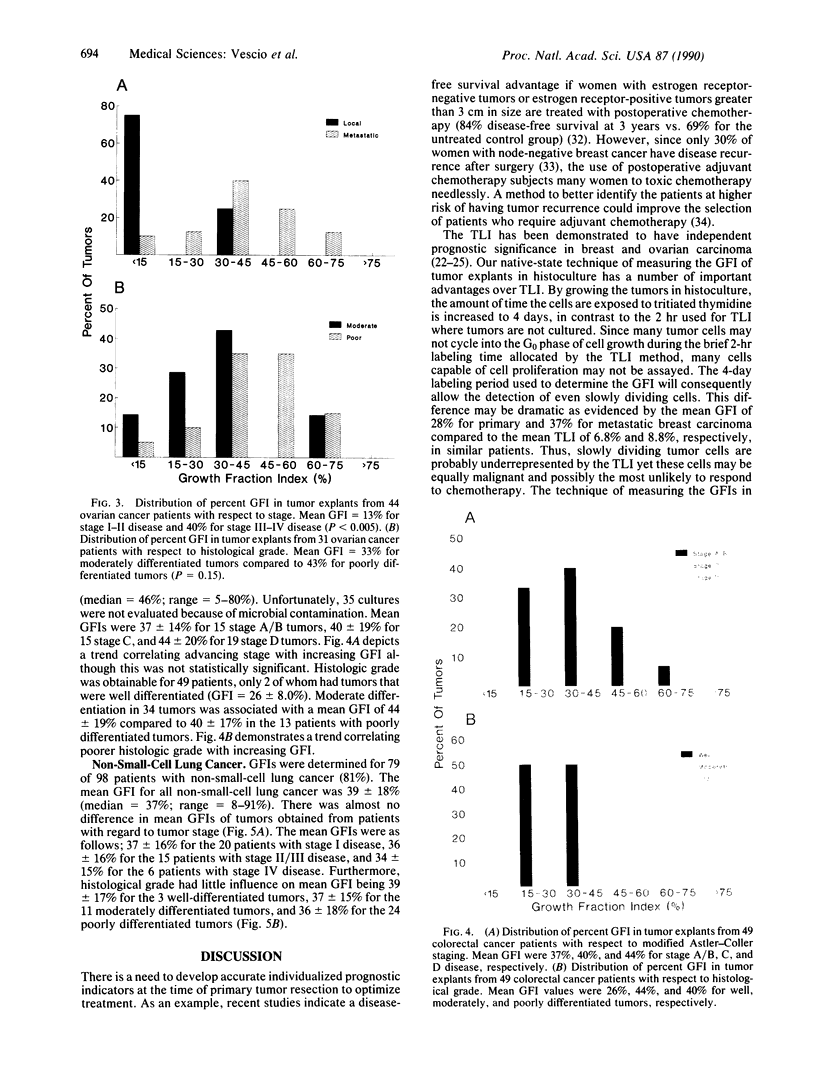
There is a need for individualization of all aspects of cancer therapy. Because of significant heterogeneity within a tumor class, there is a need to develop an in vitro test to accurately gauge tumor aggressiveness. Such a measurement would greatly aid treatment decision making. Current methodologies such as flow cytometry, which lacks unambiguous interpretation of cell-proliferative data, and determination of the thymidine-labeling index, which measures nucleotide uptake in a nonphysiological state, have not reproducibly attained this goal. We have developed an in vitro native-state three-dimensional gel-supported histoculture system that allows the growth of all human solid tumor types for relatively long time periods. The native-state system was used to identify the percent of cells capable of incorporating [3H]thymidine over a 4-day period, which we term the growth fraction index (GFI). We have compared the ability of cancer tissue to proliferate in native-state culture to the stage and histological grade of four major types of human carcinomas: breast, ovarian, colon, and lung. Eighty percent of tumor explants could be evaluated, even when sent from across the country. We have determined that the GFI correlates with tumor stage and grade for breast and ovarian carcinoma. In colon carcinoma, there is a trend toward higher GFIs in tumors of more advanced stage and grade. In non-small cell lung carcinomas, GFI, stage, and grade do not correlate. These results suggest the applicability of gel-supported three-dimensional native-state histoculture for prognostic purposes in patients with breast and ovarian cancers and demonstrate the clinical relevance of the native-state histoculture system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baak J. P., Langley F. A., Talerman A., Delemarre J. F. Interpathologist and intrapathologist disagreement in ovarian tumor grading and typing. Anal Quant Cytol Histol. 1986 Dec;8(4):354–357. [PubMed] [Google Scholar]
- Charpin C., Andrac L., Vacheret H., Habib M. C., Devictor B., Lavaut M. N., Toga M. Multiparametric evaluation (SAMBA) of growth fraction (monoclonal Ki67) in breast carcinoma tissue sections. Cancer Res. 1988 Aug 1;48(15):4368–4374. [PubMed] [Google Scholar]
- Christov K., Milev A., Todorov V. DNA aneuploidy and cell proliferation in breast tumors. Cancer. 1989 Aug 1;64(3):673–679. doi: 10.1002/1097-0142(19890801)64:3<673::aid-cncr2820640318>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Clark G. M., Dressler L. G., Owens M. A., Pounds G., Oldaker T., McGuire W. L. Prediction of relapse or survival in patients with node-negative breast cancer by DNA flow cytometry. N Engl J Med. 1989 Mar 9;320(10):627–633. doi: 10.1056/NEJM198903093201003. [DOI] [PubMed] [Google Scholar]
- De Vita V. T., Jr Breast cancer therapy: exercising all our options. N Engl J Med. 1989 Feb 23;320(8):527–529. doi: 10.1056/NEJM198902233200812. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Hoffman R. M. In vivo-like growth of human tumors in vitro. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2694–2698. doi: 10.1073/pnas.83.8.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander M. L., Hedley D. W., Taylor I. W., Russell P., Coates A. S., Tattersall M. H. Influence of cellular DNA content on survival in advanced ovarian cancer. Cancer Res. 1984 Jan;44(1):397–400. [PubMed] [Google Scholar]
- Hedley D. W., Rugg C. A., Gelber R. D. Association of DNA index and S-phase fraction with prognosis of nodes positive early breast cancer. Cancer Res. 1987 Sep 1;47(17):4729–4735. [PubMed] [Google Scholar]
- Hoffman R. M., Connors K. M., Meerson-Monosov A. Z., Herrera H., Price J. H. A general native-state method for determination of proliferation capacity of human normal and tumor tissues in vitro. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2013–2017. doi: 10.1073/pnas.86.6.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallioniemi O. P., Blanco G., Alavaikko M., Hietanen T., Mattila J., Lauslahti K., Lehtinen M., Koivula T. Improving the prognostic value of DNA flow cytometry in breast cancer by combining DNA index and S-phase fraction. A proposed classification of DNA histograms in breast cancer. Cancer. 1988 Nov 15;62(10):2183–2190. doi: 10.1002/1097-0142(19881115)62:10<2183::aid-cncr2820621019>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P., Punnonen R., Mattila J., Lehtinen M., Koivula T. Prognostic significance of DNA index, multiploidy, and S-phase fraction in ovarian cancer. Cancer. 1988 Jan 15;61(2):334–339. doi: 10.1002/1097-0142(19880115)61:2<334::aid-cncr2820610224>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Kikuyama S., Kubota T., Watanabe M., Ishibiki K., Abe O. Cell kinetic study of human carcinomas using bromodeoxyuridine. Cell Tissue Kinet. 1988 Jan;21(1):15–20. doi: 10.1111/j.1365-2184.1988.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Kikuyama S., Kubota T., Watanabe M., Isobe Y., Fukutomi T., Ishibiki K., Abe O. Cell kinetic analysis of human tumor xenografts using anti-bromodeoxyuridine monoclonal antibody. Jpn J Surg. 1987 Jan;17(1):28–32. doi: 10.1007/BF02470581. [DOI] [PubMed] [Google Scholar]
- Kokal W., Sheibani K., Terz J., Harada J. R. Tumor DNA content in the prognosis of colorectal carcinoma. JAMA. 1986 Jun 13;255(22):3123–3127. [PubMed] [Google Scholar]
- Lellé R. J., Heidenreich W., Stauch G., Gerdes J. The correlation of growth fractions with histologic grading and lymph node status in human mammary carcinoma. Cancer. 1987 Jan 1;59(1):83–88. doi: 10.1002/1097-0142(19870101)59:1<83::aid-cncr2820590119>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Mansour E. G., Gray R., Shatila A. H., Osborne C. K., Tormey D. C., Gilchrist K. W., Cooper M. R., Falkson G. Efficacy of adjuvant chemotherapy in high-risk node-negative breast cancer. An intergroup study. N Engl J Med. 1989 Feb 23;320(8):485–490. doi: 10.1056/NEJM198902233200803. [DOI] [PubMed] [Google Scholar]
- McGuire W. L. Adjuvant therapy of node-negative breast cancer. N Engl J Med. 1989 Feb 23;320(8):525–527. doi: 10.1056/NEJM198902233200811. [DOI] [PubMed] [Google Scholar]
- Merkel D. E., Dressler L. G., McGuire W. L. Flow cytometry, cellular DNA content, and prognosis in human malignancy. J Clin Oncol. 1987 Oct;5(10):1690–1703. doi: 10.1200/JCO.1987.5.10.1690. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Friedman E., McCrate M. M., Bauer W. C. Prediction of early course of breast carcinoma by thymidine labeling. Cancer. 1983 May 15;51(10):1879–1886. doi: 10.1002/1097-0142(19830515)51:10<1879::aid-cncr2820511021>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., McDivitt R. W. Reliability and stability of the thymidine labeling index of breast carcinoma. Lab Invest. 1986 Feb;54(2):160–164. [PubMed] [Google Scholar]
- Meyer J. S., Province M. Proliferative index of breast carcinoma by thymidine labeling: prognostic power independent of stage, estrogen and progesterone receptors. Breast Cancer Res Treat. 1988 Oct;12(2):191–204. doi: 10.1007/BF01805940. [DOI] [PubMed] [Google Scholar]
- Riccardi A., Danova M., Wilson G., Ucci G., Dörmer P., Mazzini G., Brugnatelli S., Girino M., McNally N. J., Ascari E. Cell kinetics in human malignancies studied with in vivo administration of bromodeoxyuridine and flow cytometry. Cancer Res. 1988 Nov 1;48(21):6238–6245. [PubMed] [Google Scholar]
- Rodenburg C. J., Ploem-Zaaijer J. J., Cornelisse C. J., Mesker W. E., Hermans J., Heintz P. A., Ploem J. S., Fleuren G. J. Use of DNA image cytometry in addition to flow cytometry for the study of patients with advanced ovarian cancer. Cancer Res. 1987 Aug 1;47(15):3938–3941. [PubMed] [Google Scholar]
- Shepherd N. A., Richman P. I., England J. Ki-67 derived proliferative activity in colorectal adenocarcinoma with prognostic correlations. J Pathol. 1988 Jul;155(3):213–219. doi: 10.1002/path.1711550306. [DOI] [PubMed] [Google Scholar]
- Silvestrini R., Daidone M. G., Gasparini G. Cell kinetics as a prognostic marker in node-negative breast cancer. Cancer. 1985 Oct 15;56(8):1982–1987. doi: 10.1002/1097-0142(19851015)56:8<1982::aid-cncr2820560816>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Tubiana M., Pejovic M. H., Chavaudra N., Contesso G., Malaise E. P. The long-term prognostic significance of the thymidine labelling index in breast cancer. Int J Cancer. 1984 Apr 15;33(4):441–445. doi: 10.1002/ijc.2910330404. [DOI] [PubMed] [Google Scholar]
- Vescio R. A., Redfern C. H., Nelson T. J., Ugoretz S., Stern P. H., Hoffman R. M. In vivo-like drug responses of human tumors growing in three-dimensional gel-supported primary culture. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5029–5033. doi: 10.1073/pnas.84.14.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. A., Camplejohn R. S. Comparison of monoclonal antibody Ki-67 reactivity with grade and DNA flow cytometry of breast carcinomas. Br J Cancer. 1988 Mar;57(3):281–283. doi: 10.1038/bjc.1988.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dierendonck J. H., Keijzer R., van de Velde C. J., Cornelisse C. J. Nuclear distribution of the Ki-67 antigen during the cell cycle: comparison with growth fraction in human breast cancer cells. Cancer Res. 1989 Jun 1;49(11):2999–3006. [PubMed] [Google Scholar]
- van de Vijver M. J., Peterse J. L., Mooi W. J., Wisman P., Lomans J., Dalesio O., Nusse R. Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med. 1988 Nov 10;319(19):1239–1245. doi: 10.1056/NEJM198811103191902. [DOI] [PubMed] [Google Scholar]