Abstract
Despite promising therapeutic avenues, there is currently no effective treatment for Duchenne muscular dystrophy (DMD), a lethal monogenic disorder caused by the loss of the large cytoskeletal protein, dystrophin. A highly promising approach to therapy, applicable to all DMD patients irrespective to their genetic defect, is to modulate utrophin, a functional paralogue of dystrophin, able to compensate for the primary defects of DMD restoring sarcolemmal stability. One of the major difficulties in assessing the effectiveness of therapeutic strategies is to define appropriate outcome measures. In the present study, we utilised an aptamer based proteomics approach to profile 1,310 proteins in plasma of wild-type, mdx and Fiona (mdx overexpressing utrophin) mice. Comparison of the C57 and mdx sera revealed 83 proteins with statistically significant >2 fold changes in dystrophic serum abundance. A large majority of previously described biomarkers (ANP32B, THBS4, CAMK2A/B/D, CYCS, CAPNI) were normalised towards wild-type levels in Fiona animals. This work also identified potential mdx markers specific to increased utrophin (DUS3, TPI1) and highlights novel mdx biomarkers (GITR, MYBPC1, HSP60, SIRT2, SMAD3, CNTN1). We define a panel of putative protein mdx biomarkers to evaluate utrophin based strategies which may help to accelerate their translation to the clinic.
Duchenne muscular dystrophy (DMD) is a lethal X-linked recessive disorder caused by mutations in the dystrophin gene1. This disorder affects 1 in 5000 boys2 and is characterized by a progressive muscle wasting leading to loss of ambulation by 8–12 years of age3 and death by early adulthood due to cardiorespiratory failure4. Dystrophin, an essential link between the dystrophin associated protein complex (DAPC) at the sarcolemma and the cytoskeleton, maintains the strength, flexibility and stability in skeletal muscles5. In the absence of dystrophin, the myofibres are more susceptible to contraction-induced injury which results in muscle wasting and premature death6.
There is currently no effective treatment for the disease. Glucocorticoid treatment is the current standard of care which delays the loss of ambulation by 3–4 years7,8 but shows no long treatment benefit and is often associated with debilitating side effects9,10,11. The urgency to seek a therapy for DMD has resulted in parallel efforts to develop exon skipping12,13, termination codon read through14, dystrophin gene replacement or editing therapies15,16 and non-dystrophin strategies17,18,19 such as utrophin modulation20,21. However, despite the recent accelerated approval of Exondys 51 (eteplirsen) in US, disappointing clinical trials results22 and failure of approval from the FDA for Ataluren23 and Kyndrisa24 drugs rekindle discussions about clinical trials designs and endpoints.
We have focused on utrophin modulation because it is applicable to all DMD patients irrespective of their dystrophin mutation. Utrophin is found at the sarcolemma in utero and is progressively replaced by dystrophin during development25,26,27. In adult skeletal muscles, utrophin is expressed and enriched at the neuromuscular and myotendinous junctions28 and found at the sarcolemma in regenerating myofibres29. Despite subtle differences, utrophin shares 80% of homology30 with the dystrophin protein and has functional redundancy31,32,33,34. Utrophin is increased 1.8 fold in the mouse mdx model of the disease due mainly to regenerating fibres. Using transgenic mdx mice expressing high levels of utrophin (Fiona), we have demonstrated that increasing utrophin expression 3–4 fold prevents the development of pathology35,36. In partnership with Summit Therapeutics, we have developed small molecules which increase the levels of utrophin and prevent pathology in the mdx mouse model21,37. One of these, Ezutromid (formerly known as SMT C1100) has progressed into clinical development. Ezutromid has an excellent safety profile20,38, and recently entered into phase 2 trial39. We have reported a second generation compound, chemically related to Ezutromid, with improved physicochemical properties and a robust metabolism profile which ameliorates sarcolemmal stability and prevents the pathology through a significant reduction of regeneration, necrosis and fibrosis and provides functional enhancement21. These data emphasize the potential of utrophin modulation as a disease-modifying therapeutic strategy for all DMD patients.
Current clinical trials have used the analysis of the restoration of dystrophin as a biomarker. However this relies on invasive muscle biopsies which only provide semi-quantitative measures due to the small size of the tissue sample. The utility of the quantification of dystrophin as a biomarker is still under debate and limited by current western blot and immunofluorescence microscopy methodologies40. Furthermore, therapeutic strategies deliver different efficacy depending on the muscle type. In consequence, the correlation between the dystrophin level in a biopsy of one muscle type and the overall clinical improvement is under question. Currently, most clinical trials for DMD rely on standardized physical assessments such as the 6 minute walk distance test (6MWDT)41, the North Star Ambulatory Assessment (NSAA)42 as well as quantitative muscle strength tests43,44. These physical tests are useful readouts for determining the whether a treatment slows disease progression but these endpoints are limited to ambulatory patients only, often challenging to implement specially in young patients and suffer from high inter-patient variability due to the variable natural history of the disease.
Recently, less invasive approaches to monitor disease progression and response to treatment in DMD patients have emerged with Magnetic resonance imaging and T2 mapping45,46. While these approaches are useful in monitoring muscle loss, fat and progression of the disease as cardiac function, they do not provide a direct muscle function read-out, are laborious and subject to a number of limitations such as high cost and low through-put.
Thus there is an urgent need for minimally invasive biomarkers47,48, which can be used as outcome measures in pre-clinical studies and clinical trials for DMD therapeutics. Blood fluid samples are simple to obtain and provide insight into the circulating protein in the entire body in normal as pathological conditions49. Initially, serological level of creatine kinase (CK) was used to screen for DMD in newborns50,51. However whereas serum muscle CK reflects sarcolemma damage and is a useful tool for diagnosis, this marker is not suitable for monitoring the extent of the pathology, disease progression and response to therapy as it is influenced by age, exercise and stress52. In addition, serum CK may also be elevated in asymptomatic individuals. Over the last 5 years, work profiling different types of molecular biomarkers including miRNA, protein and metabolites in serum, plasma and/or urine of DMD patients have been reported53,54,55. Circulating protein markers such as carbonic anhydrase III (CA-III), myoglobin, TIMP-155,56, MMP957 or MYOM-358, which are simple to integrate into clinical workflows are emerging as valuable and useful biomarkers.
Current methodologies such as two-dimensional gel electrophoresis are limited to highly expressed proteins. Mass spectrometry is challenging to analyse with serum/plasma samples due to high dynamic range in abundance of different proteins as albumin, fibrinogen, immunoglobulins and macroglobulin which represent 90% of the circulating proteome. This can mask any less abundant potential biomarkers. To overcome these limitations, sensitive high throughput “omic” platforms such as affinity proteomics approach were recently introduced. This multiplexing methodology using 384 antibodies directed against 315 different proteins identified new protein biomarker as Troponin T, fast skeletal muscle (TNNT3), myosin light chain-3 (MYL3) and plastin-2 (LCP1) in a total of 190 blood samples from DMD patients59. Another serum proteome profiling study using a combination of high precision mass spectrometry and stable isotope labelling in mammals (SILAM) strategy quantify levels of 1,500 proteins in 15 DMD sera and two independent mdx model for DMD and successfully identified 20 protein biomarkers55. More recently, a new aptamer-based affinity purification approach (SOMAscan) was developed60 and successfully used to query 1,129 proteins in serum samples from 93 DMD patients56. Forty-four were identified at a False Discovery Rate of 1.0% to be significantly increased (24 proteins) or decreased (20 proteins) in DMD patients vs. controls. The SOMAscan technology was also used with mdx mice to define novel protein biomarkers61. These recent highly sensitive high throughput technologies provide a comprehensive panel of serum protein biomarkers which should be valuable tool to monitor treatment efficacy in future pre-clinical and clinical studies.
In this study, we carried out comprehensive serum proteome profiling using the 1,310-plex SomaScan assay in wild-type, mdx and Fiona mice to define panels of serum protein DMD markers applicable to utrophin modulation based therapies. The definition of therapeutic monitoring serum biomarkers in different utrophin level contexts should accelerate the development of small oral utrophin drugs for a quicker translation to DMD patients.
Results
Serum protein biomarkers profiling in mdx
In order to define robust circulating biomarkers for utrophin based strategies, we collected serum from 7 week old C57, mdx and utrophin transgenic mdx Fiona mice (n = 7). In dystrophic tibialis anterior (TA) muscle, mainly due to regeneration29, utrophin is increased by 2 fold in mdx compared to C57 animals and inconsistently localised at the muscle membrane of small regenerating fibres (Fig. S1A,C). In the transgenic mdx Fiona mouse, utrophin is expressed at a high level (4 fold compared to mdx) and uniformly distributed at the sarcolemma (Fig. S1A,B). Consequently, muscle membrane stability is improved, regeneration and necrosis are reduced to wild-type levels and muscle function is fully restored (Fig. S1C–E).
In this study, we used the SOMAscan platform to profile serum abundance of 1,310 SOMAmers (Slow Off-rate Modified Aptamers) in C57, mdx and Fiona samples (n = 7). These complexes, able to recognize with high specificity and sensitivity specific conformational epitopes of proteins, were precipitated and protein concentrations quantified on an Agilent hybridisation chip60. SOMAscan methodologies used three dilutions (0.5%, 2% and 5%) per sample to increase the dynamic range of detection and a unique set of SOMAmer reagents were assigned to one of three dilution sets. Quality controls showed that probe hybridization was in the acceptable range 0.4–2.5 for all the samples (Fig. S2A,B) and that median normalization scale factor was similar between groups of animals (Fig. S2A,C). All samples successfully passed quality control checks and were included in subsequent analyses.
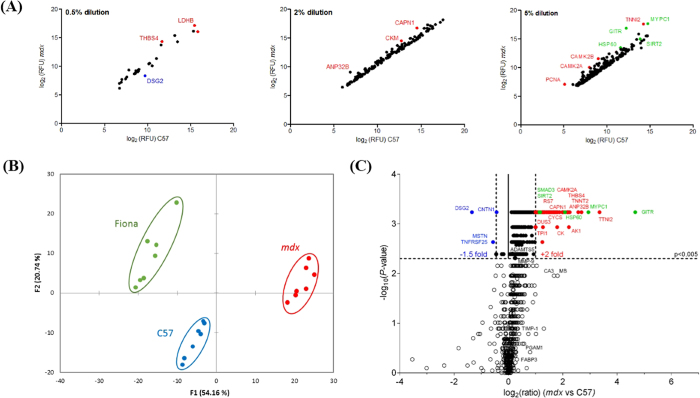
We first compared C57 and mdx animals and identified 83 serum protein significantly changed in abundance by at least 2 fold difference (Mann-Whitney U test, FDR correction, P < 0.005, q < 0.01) (Table S1). Levels of a large majority of these markers were upregulated (79; >2 fold) and abundance of 4 serum protein were significantly decreased (CNTN1, Contactin-1; TNFRSF25, Tumor necrosis factor receptor superfamily member 25; MSTN, Myostatin; DSG2, Desmoglein-2; <0.7 fold). As previously reported in the mdx model55,56,61 and in DMD patients55,56, levels of serological proteins with statistically significant changes in abundance are increased rather than decreased. Analysis of their abundance showed that a greater number of these highly differential mdx markers were identified from the highest 5% dilution group and are expressed at a low abundance, whereas only a few highly abundant markers were identified (LDHB, Lactate dehydrogenase B; THBS4, Thrombospondin 4 and DSG2, Desmoglein- 2) (Fig. 1A). A clear separation between dystrophic and healthy animals was noted using principal component analysis (Fig. 1B) suggesting that serum protein could be used to differentiate these experimental groups. Both highly differentially expressed and statically significant factors were identified by Volcano plot (Fig. 1C). Among the serological level of these 83 highly significantly expressed or repressed factors, most are muscle leakage proteins which have been previously reported to be elevated in DMD boys55,56 and mdx mice55,56,61 relative to healthy volunteers and wild-type animals respectively. Notably, we confirm that at 7 weeks of age, levels of circulating protein biomarkers linked to muscle function (MB, Myoglobin; TNNI2, Troponin I, fast skeletal muscle), metabolic dysregulation (LDHB, L-lactate dehydrogenase; TPI1, Triosephosphate isomerase; CYCS, Cytochrome C), calcium metabolism (CAMK2A, CAMK2B, Calcium/calmodulin-dependent protein kinase type II subunit alpha/beta; CAPN1, Calpain) and extracellular matrix remodelling/fibrogenesis (THBS4, Thrombospondin-4) are highly and significantly increased in mdx animals. We also identified several potential new serum biomarkers, notably GITR/TNFRSF18 (glucocorticoid-induced TNFR-related protein; Tumor necrosis factor receptor superfamily member 18) showing a 25.4 fold enrichment in dystrophic serum. Among these serological biomarkers identified, we also demonstrate high serological level for MYBPC1 (Myosin Binding Protein C, Slow Type; 7.7x), HSP60 (Heat shock protein 60; 3.7x), SIRT2 (NAD-dependent deacetylase sirtuin-2; 2.2x), SMAD3 (Mothers against decapentaplegic homolog 3; 2.1x) and a significant decrease in abundance for CNTN1 (0.7x), MSTN (0.7x) and DSG2 (0.4x).
Figure 1. Identification of protein biomarkers in dystrophic serum.
Serum samples from C57, mdx and Fiona mice were analysed using the SOMAscan methodology. (A) Scatter plots showing abundance of differentially expressed protein by dilution groups. (B) Principal components analysis (the first two components representing 74.9% of the data are shown). (C) Volcano plot visualizing significant protein changes in mdx serum determined by Mann-Whitney U test (p < 0.005). Red indicates up-regulated protein (>2.0 fold) previously described as potential mdx/DMD serological biomarkers. Blue indicates down-regulated proteins (<0.70 fold) and green are newly discovered markers with a >2.0 fold abundance in dystrophic serum.
Definition of top candidate protein biomarkers for utrophin based strategies
Statistical analysis including all experimental groups identified 89 proteins highly differentiated in sera of C57, mdx and Fiona animals (Kruskal-Wallis one-way ANOVA, P < 0.005, q < 0.01) of which 78 showed at least a 2 fold increase and 11 at least a 0.7 fold decrease in mdx sera abundance (Table S2). Importantly, a very uniform overlap between Mann-Whitney U test and Kruskal-Wallis one-way ANOVA was noted and revealed a total of 80 common protein markers (76 > 2.0; 4 < 0.70) (Table 1).
Table 1. Serum mdx protein biomarkers and rescue in mdx transgenic Fiona mice.
| Rank | Protein | Target | Uniprot | Dilution | Fold change (mdx vs C57) | pValue | Recovery score (Fiona) |
|---|---|---|---|---|---|---|---|
| 1 | Tumor necrosis factor receptor superfamily member 18 | TNFRSF18/GITR | Q9Y5U5 | 5 | 25.4 | 0.0001 | +++ |
| 2 | Troponin I, fast skeletal muscle | TNNI2 | P48788 | 5 | 10.2 | 0.0002 | ++ |
| 3 | Myosin-binding protein C, slow-type | MYBPC1 | Q00872 | 5 | 7.7 | 0.0003 | ++ |
| 4 | Fibrinogen | FGA | P02671 | 0.5 | 6.5 | 0.0001 | − |
| 5 | Calcium/calmodulin-dependent protein kinase type II subunit beta | CAMK2B | Q13554 | 5 | 5.9 | 0.0001 | ++ |
| 6 | Fibrinogen gamma chain | FGG | P02679 | 0.5 | 4.8 | 0.0001 | − |
| 7 | Adenylate kinase isoenzyme 1 | AK1 | P00568 | 2 | 4.7 | 0.0007 | ++ |
| 8 | Acidic leucine-rich nuclear phosphoprotein 32 family member B | ANP32B | Q92688 | 2 | 4.6 | 0.0003 | +++ |
| 9 | Troponin T, cardiac muscle | TNNT2 | P45379 | 5 | 4.6 | 0.0011 | +++ |
| 10 | Thrombospondin-4 | THBS4 | P35443 | 0.5 | 4.5 | 0.0002 | +++ |
| 11 | Calcium/calmodulin-dependent protein kinase type II subunit delta | CAMK2D | Q13557 | 5 | 4.5 | 0.0001 | ++ |
| 12 | Serine protease HTRA2, mitochondrial | HTRA2 | O43464 | 5 | 4.2 | 0.0001 | +++ |
| 13 | Calcium/calmodulin-dependent protein kinase type II subunit alpha | CAMK2A | Q9UQM7 | 5 | 4.2 | 0.0003 | +++ |
| 14 | Tyrosine-protein kinase Lyn | LYN | P07948 | 5 | 4 | 0.0003 | ++ |
| 15 | Tyrosine-protein kinase Lyn, isoform B | LYN | P07948 | 5 | 4 | 0.0003 | +++ |
| 16 | Proliferating cell nuclear antigen | PCNA | P12004 | 5 | 4 | 0.0012 | +++ |
| 17 | Eukaryotic translation initiation factor 4 gamma 2 | EIF4G2 | P78344 | 5 | 4 | 0.0012 | − |
| 18 | 60 kDa heat shock protein, mitochondrial | HSP60 | P10809 | 5 | 3.7 | 0.0006 | +++ |
| 19 | Tyrosine-protein kinase Fyn | FYN | P06241 | 5 | 3.5 | 0.0004 | +++ |
| 20 | Tyrosine-protein kinase Yes | YES1 | P07947 | 5 | 3.5 | 0.0003 | ++ |
| 21 | Creatine kinase M-type | CKM | P06732 | 2 | 3.5 | 0.0011 | + |
| 22 | Mitogen-activated protein kinase 8 | MAPK8 | P45983 | 5 | 3.4 | 0.0007 | + |
| 23 | L-lactate dehydrogenase B chain | LDHB | P07195 | 0.5 | 3.3 | 0.0005 | ++ |
| 24 | 40S ribosomal protein S3 | RPS3 | P23396 | 5 | 3.2 | 0.0005 | − |
| 25 | PIK3CA/PIK3R1 | PIK3CA PIK3R1 | P42336 | 5 | 3.1 | 0.0001 | ++ |
| 26 | 40S ribosomal protein S3a | RPS3A | P61247 | 5 | 3.1 | 0.0006 | +++ |
| 27 | Cytochrome c | CYCS | P99999 | 5 | 3 | 0.0013 | +++ |
| 28 | Proto-oncogene tyrosine-protein kinase Src | SRC | P12931 | 5 | 3 | 0.0007 | ++ |
| 29 | D-dimer | FGA FGB FGG | P02671 | 0.5 | 2.9 | 0.0001 | − |
| 30 | Calpain I | CAPN1 | P07384 | 2 | 2.9 | 0.0001 | +++ |
| 31 | RAC-beta serine/threonine-protein kinase | AKT2 | P31751 | 5 | 2.8 | 0.001 | − |
| 32 | Mitogen-activated protein kinase 14 | MAPK14 | Q16539 | 2 | 2.8 | 0.0005 | + |
| 33 | Peptidyl-prolyl cis-trans isomerase D | PPID | Q08752 | 5 | 2.8 | 0.0008 | − |
| 34 | Troponin I, cardiac muscle | TNNI3 | P19429 | 5 | 2.8 | 0.0036 | − |
| 35 | RAC-alpha/beta/gamma serine/threonine-protein kinase | AKT1 AKT2 AKT3 | P31749 | 2 | 2.7 | 0.0007 | − |
| 36 | Coiled-coil domain-containing protein 80 | CCDC80 | Q76M96 | 2 | 2.7 | 0.0002 | ++ |
| 37 | Tyrosine-protein phosphatase non-receptor type 11 | PTPN11 | Q06124 | 5 | 2.6 | 0.001 | ++ |
| 38 | 40S ribosomal protein S7 | RPS7 | P62081 | 5 | 2.5 | 0.0009 | +++ |
| 39 | Heat shock protein HSP 90-alpha/beta | HSP90AA1/AB1 | P07900 | 2 | 2.5 | 0.0004 | + |
| 40 | Ribosomal protein S6 kinase alpha-3 | RPS6KA3 | P51812 | 5 | 2.5 | 0.0005 | + |
| 41 | cAMP-dependent protein kinase catalytic subunit alpha | PRKACA | P17612 | 5 | 2.5 | 0.0012 | − |
| 42 | Dual specificity mitogen-activated protein kinase kinase 1 | MAP2K1 | Q02750 | 5 | 2.4 | 0.0001 | − |
| 43 | Dual specificity protein phosphatase 3 | DUSP3 | P51452 | 5 | 2.4 | 0.002 | +++ |
| 44 | Heat shock 70 kDa protein 1A | HSPA1A | P0DMV8 | 5 | 2.4 | 0.0004 | + |
| 45 | Casein kinase II 2-alpha’:2-beta heterotetramer | CSNK2A2 CSNK2B | P19784 | 5 | 2.4 | 0.0043 | +++ |
| 46 | Glycogen synthase kinase-3 alpha/beta | GSK3A GSK3B | P49840 | 5 | 2.4 | 0.0011 | − |
| 47 | Mitogen-activated protein kinase 12 | MAPK12 | P53778 | 5 | 2.4 | 0.0004 | ++ |
| 48 | 3-phosphoinositide-dependent protein kinase 1 | PDPK1 | O15530 | 5 | 2.3 | 0.002 | ++ |
| 49 | Heat shock cognate 71 kDa protein | HSPA8 | P11142 | 2 | 2.3 | 0.0003 | ++ |
| 50 | ATP-dependent RNA helicase DDX19B | DDX19B | Q9UMR2 | 5 | 2.3 | 0.0006 | + |
| 51 | 14–3–3 protein family | YWHAB | P31946 | 2 | 2.3 | 0.0003 | ++ |
| 52 | Protein FAM3D | FAM3D | Q96BQ1 | 5 | 2.2 | 0.0001 | +++ |
| 53 | NAD-dependent protein deacetylase sirtuin-2 | SIRT2 | Q8IXJ6 | 5 | 2.2 | 0.0014 | +++ |
| 54 | Xaa-Pro aminopeptidase 1 | XPNPEP1 | Q9NQW7 | 5 | 2.2 | 0.0008 | +++ |
| 55 | Receptor-type tyrosine-protein kinase FLT3 | FLT3 | P36888 | 5 | 2.2 | 0.0018 | + |
| 56 | Protein kinase C beta type (splice variant beta-II) | PRKCB | P05771 | 2 | 2.2 | 0.0016 | − |
| 57 | Tumor necrosis factor receptor superfamily member 27 | EDA2R | Q9HAV5 | 5 | 2.2 | 0.0001 | +++ |
| 58 | Osteocalcin | BGLAP | P02818 | 2 | 2.2 | 0.0012 | +++ |
| 59 | Histone H2A.z | H2AFZ | P0C0S5 | 5 | 2.2 | 0.0001 | +++ |
| 60 | Tyrosine-protein kinase CSK | CSK | P41240 | 2 | 2.1 | 0.0027 | − |
| 61 | Mothers against decapentaplegic homolog 3 | SMAD3 | P84022 | 5 | 2.1 | 0.0016 | +++ |
| 62 | Secreted and transmembrane protein 1 | SECTM1 | Q8WVN6 | 2 | 2.1 | 0.0003 | +++ |
| 63 | Follistatin-related protein 1 | FSTL1 | Q12841 | 2 | 2.1 | 0.0004 | +++ |
| 64 | beta-adrenergic receptor kinase 1 | ADRBK1 | P25098 | 5 | 2.1 | 0.0012 | +++ |
| 65 | Hemojuvelin | HFE2 | Q6ZVN8 | 2 | 2 | 0.001 | +++ |
| 66 | Intercellular adhesion molecule 2 | ICAM2 | P13598 | 2 | 2 | 0.0001 | +++ |
| 67 | Alpha-soluble NSF attachment protein | NAPA | P54920 | 2 | 2 | 0.0006 | + |
| 68 | Protein S100-A12 | S100A12 | P80511 | 2 | 2 | 0.0013 | +++ |
| 69 | MAP kinase-activated protein kinase 3 | MAPKAPK3 | Q16644 | 2 | 2 | 0.0031 | − |
| 70 | Repulsive guidance molecule A | RGMA | Q96B86 | 2 | 2 | 0.0005 | +++ |
| 71 | Mitochondrial import inner membrane translocase subunit TIM14 | DNAJC19 | Q96DA6 | 5 | 2 | 0.0001 | +++ |
| 72 | Triosephosphate isomerase | TPI1 | P60174 | 2 | 2 | 0.0034 | ++ |
| 73 | Growth factor receptor-bound protein 2 | GRB2 | P62993 | 5 | 2 | 0.0008 | − |
| 74 | Hsp90 co-chaperone Cdc37 | CDC37 | Q16543 | 5 | 2 | 0.001243 | − |
| 75 | Methionine aminopeptidase 2 | METAP2 | P50579 | 5 | 2 | 0.00098 | +++ |
| 76 | Nidogen-1 | NID1 | P14543 | 2 | 2 | 0.000276 | + |
| 77 | Contactin-1 | CNTN1 | Q12860 | 0.5 | 0.7 | 0.001295 | +++ |
| 78 | Growth/differentiation factor 8 | MSTN | O14793 | 0.5 | 0.7 | 0.003311 | +++ |
| 79 | Tumor necrosis factor receptor superfamily member 25 | TNFRSF25 | Q93038 | 5 | 0.7 | 0.00498 | − |
| 80 | Desmoglein-2 | DSG2 | Q14126 | 0.5 | 0.4 | 0.000456 | ++ |
High significant increased and decreased protein were defined using Mann-Witney U test and Kruskal-Wallis one-way ANOVA (p < 0.005; q < 0.01). (+++) restored to wild-type levels (recovery score ≥70%), (++) restored towards wild-type levels (50≤ recovery score <70, (+) low and inconsistent restoration towards wild-type (25≤ recovery score <50), (−) not restored towards wild-type levels (0≤ recovery score <25).
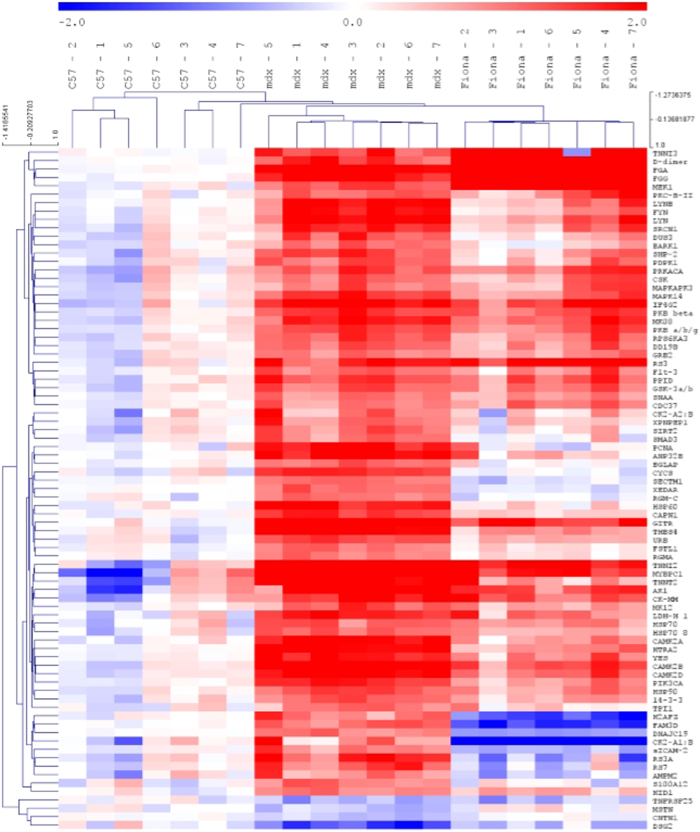
To appreciate potential rescue of these serological biomarkers in transgenic mdx Fiona animals expressing high levels of utrophin, we used the Recovery score, a common, quantitative and comparative scoring system36,62 (Table 1). This score is based on the measurements of the parameters studied on three different specimens and calculation follows the equation: RS = (“treated” − “untreated”)/(“normal” − “untreated)*100] = [(Fiona − mdx)/(C57 − mdx) * 100]. The recovery score ranges from 0%, when the increased utrophin in Fiona mice has no effect to 100% when the increased utrophin in Fiona animals display the same parameter value as the wild-type one. To rank serological biomarkers, thresholds were arbitrary defined following these criteria: (+++), RS ≥ 70, restored to wild-type levels; (++), 50 ≤ RS > 70, restored towards wild-type levels; (+), 25 ≤ RS > 50, low and inconsistent restoration towards wild-type; (−), 0 ≤ RS > 25, not restored towards wild-type levels. From the 80 serum markers previously defined, more than 75% were partially or fully rescued towards a wild-type level in Fiona mice due to high levels of utrophin. Hierarchical clustering illustrates profiles of wild-type, dystrophic and high utrophin context (Fig. 2). We next defined a set of 15 candidates (Table 2) as the most promising candidate DMD biomarkers for utrophin based strategies based on the following criteria: (i) differentially abundant with a 2 fold difference, (ii) statistically significant (p < 0.005) as determined by Kruskal-Wallis one-way ANOVA and Mann-Whitney U tests, (iii) recovery score >70% in transgenic mdx Fiona mice expressing high content of utrophin, (iv) previously described as potential biomarkers in DMD patients and/or mdx animals, (iv) known to be associated with pathways involved in modulation of utrophin expression and (v) function and protein group to obtain an homogeneous and representative panel of robust biomarkers. Table 2 and Fig. 3 present results in C57, mdx and Fiona mice for these 15 biomarkers candidates all rescue in high utrophin context. Importantly, Hathout et al., recently reported ANP32B (Acidic Nuclear Phosphoprotein 32 Family Member B), CAMK2A, RS7 (40S ribosomal protein S7), CYCS, THBS4 as serological proteins significantly increased in DMD patients55,56. TNNT2 (Troponin T, cardiac muscle) was also recently reported as increased in Becker63 and Duchenne patients and carriers64. In mdx mice, abundance of HTRA2 (Serine protease HTRA2, mitochondrial), PCNA (Proliferating cell nuclear antigen), DUS3 (Dual specificity protein phosphatase 3), CAPN1 as ANP32B, CAMK2A, THBS4 and CYCS is enriched in dystrophic sera61. Alongside these described markers, we have included five new undescribed potential circulating protein biomarkers in this panel: GITR/TNFRSF18, serum protein with the highest deregulation observed in this study (25.4x); SIRT2, a potential therapeutic avenue involving key utrophin pathways65 CNTN1, a neural adhesion and neuromuscular junction protein significantly reduced in dystrophic serum66 and two factors previously described as increased in muscle biopsies of DMD patients67 and mdx tissue68 but not yet in blood: HSP60 and a key intracellular signalling mediator for both transforming growth factor-β and myostatin, SMAD3. All these biomarker candidates are rescued in the high utrophin context of the Fiona mouse (Table 2, Fig. 3) and therefore represent interesting markers to evaluate benefits of utrophin based strategies.
Figure 2. Wild-type, mdx and Fiona protein marker profiles.
High significant protein changes determined by Mann-Witney U test and Kruskal-Wallis one-way ANOVA (p < 0.005, q < 0.01) were analysed by hierarchical clustering in all experimental groups. Red indicates up-regulated proteins and blue indicates down-regulated proteins.
Table 2. Selection of serum protein biomarkers for utrophin based DMD therapy.
| Rank | Protein | Target | Uniprot | Groups | Dilution | Fold change (mdx vs C57) | pValue | Recovery score (Fiona) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Tumor necrosis factor receptor superfamily member 18 | GITR | Q9Y5U5 | Immune response | 5 | 25.4 | 0.000135 | 89.9 | |
| 8 | Acidic leucine-rich nuclear phosphoprotein 32 family member B | ANP32B | Q92688 | Multifunctional/Other | 2 | 4.6 | 0.000306 | 84.9 | 55, 56, 61 |
| 9 | Troponin T, cardiac muscle | TNNT2 | P45379 | Muscle function | 5 | 4.6 | 0.001146 | 71.4 | 63, 64 |
| 10 | Thrombospondin-4 | THBS4 | P35443 | ECM remodeling | 0.5 | 4.5 | 0.000162 | 79.4 | 61 |
| 12 | Serine protease HTRA2, mitochondrial | HTRA2 | O43464 | Metabolism (Mitochondria) | 5 | 4.2 | 0.000135 | 75 | 61 |
| 13 | Calcium/calmodulin-dependent protein kinase type II subunit alpha | CAMK2A | Q9UQM7 | Metabolism (Calcium) | 5 | 4.2 | 0.000264 | 77.9 | 61 |
| 15 | Tyrosine-protein kinase Lyn, isoform B | LYN | P07948 | Multifunctional/Other | 5 | 4 | 0.000264 | 71.2 | 61 |
| 16 | Proliferating cell nuclear antigen | PCNA | P12004 | DNA repair/maintenance | 5 | 4 | 0.001243 | 94.7 | 61 |
| 18 | 60 kDa heat shock protein, mitochondrial | HSP60 | P10809 | Metabolism (Mitochondria) | 5 | 3.7 | 0.000575 | 92.9 | 67 |
| 19 | Tyrosine-protein kinase Fyn | FYN | P06241 | Immune response | 5 | 3.5 | 0.000389 | 75.5 | |
| 26 | 40S ribosomal protein S3a | RS3A | P61247 | Ribosomal biogenesis | 5 | 3.1 | 0.000637 | 113.4 | 56 |
| 27 | Cytochrome c | CYCS | P99999 | Metabolism (Mitochondria) | 5 | 3 | 0.001252 | 103.1 | 61 |
| 30 | Calpain I | CAPN1 | P07384 | Calcium metabolism | 2 | 2.9 | 0.000135 | 83.7 | 61 |
| 38 | 40S ribosomal protein S7 | RS7 | P62081 | Ribosomal biogenesis | 5 | 2.5 | 0.000897 | 110.2 | 56 |
| 43 | Dual specificity protein phosphatase 3 | DUS3 | P51452 | Multifunctional/Other | 5 | 2.4 | 0.001984 | 75.1 | 61 |
| 45 | Casein kinase II 2-alpha’:2-beta heterotetramer | CSNK2A2 CSNK2B | P19784 | Multifunctional/Other | 5 | 2.4 | 0.004261 | 80.8 | |
| 52 | Protein FAM3D | FAM3D | Q96BQ1 | Multifunctional/Other | 5 | 2.2 | 0.000135 | 153.1 | |
| 53 | NAD-dependent protein deacetylase sirtuin-2 | SIRT2 | Q8IXJ6 | Metabolism (Mitochondria) | 5 | 2.2 | 0.001369 | 85.7 | |
| 54 | Xaa-Pro aminopeptidase 1 | XPNPEP1 | Q9NQW7 | Multifunctional/Other | 5 | 2.2 | 0.000779 | 76.8 | |
| 57 | Tumor necrosis factor receptor superfamily member 27 | XEDAR | Q9HAV5 | Multifunctional/Other | 5 | 2.2 | 0.000135 | 112 | 61 |
| 58 | Osteocalcin | BGLAP | P02818 | Metabolism (Calcium) | 2 | 2.2 | 0.001243 | 96.3 | |
| 59 | Histone H2A.z | H2AFZ | P0C0S5 | Nucleosome structure | 5 | 2.2 | 0.000135 | 153.3 | |
| 61 | Mothers against decapentaplegic homolog 3 | SMAD3 | P84022 | Multifunctional/Other | 5 | 2.1 | 0.001636 | 95.2 | 68 |
| 62 | Secreted and transmembrane protein 1 | SECTM1 | Q8WVN6 | Immune response | 2 | 2.1 | 0.000306 | 114.5 | |
| 63 | Follistatin-related protein 1 | FSTL1 | Q12841 | Inflammation | 2 | 2.1 | 0.000402 | 82.2 | |
| 64 | Beta-adrenergic receptor kinase 1 | ADRBK1 | P25098 | Multifunctional/Other | 5 | 2.1 | 0.001198 | 79.4 | |
| 65 | Hemojuvelin | RGM-C | Q6ZVN8 | Multifunctional/Other | 2 | 2 | 0.000959 | 108.3 | |
| 66 | Intercellular adhesion molecule 2 | ICAM2 | P13598 | Inflammation/Immune response | 2 | 2 | 0.000135 | 131.8 | |
| 68 | Protein S100-A12 | S100A12 | P80511 | Inflammation/Immune response | 2 | 2 | 0.001252 | 98.5 | |
| 70 | Repulsive guidance molecule A | RGMA | Q96B86 | Multifunctional/Other | 2 | 2 | 0.000514 | 83.5 | |
| 71 | Mitochondrial import inner membrane translocase subunit TIM14 | DNAJC19 | Q96DA6 | Metabolism (Mitochondria) | 5 | 2 | 0.000135 | 141.6 | |
| 75 | Methionine aminopeptidase 2 | METAP2 | P50579 | Mutilfunctional/Other | 5 | 2 | 0.00098 | 128.5 | |
| 77 | Contactin-1 | CNTN1 | Q12860 | Mutilfunctional/Other | 0.5 | 0.7 | 0.001295 | 79.4 | |
| 78 | Growth/differentiation factor 8 | MSTN | O14793 | Muscle function | 0.5 | 0.7 | 0.003311 | 118.6 |
Potential serological marker to estimate efficacy of utrophin based strategies were defined using Mann-Witney U test and Kruskal-Wallis one-way ANOVA (p < 0.005; q < 0.01). 32 biomarkers significantly increased (>2.0) in mdx mice presenting a high recovery score >70% were selected as Contactin-1 and Myostatin (<0.7). Targets in red were selected in the final 15 set of selected markers and targets in red bold were studied by ELISA.
Figure 3. Top candidate biomarkers in dystrophic and Fiona serum.
Protein abundance per individual biological replicate was plotted for the top 15 ranked candidate biomarkers identified by SOMAscan. mdx vs C57 fold changes, Recovery score (RS) in Fiona compared to mdx and C57, Kruskal-Wallis one-way ANOVA p values are indicated for each protein. Error bars indicate mean +/− SEM.
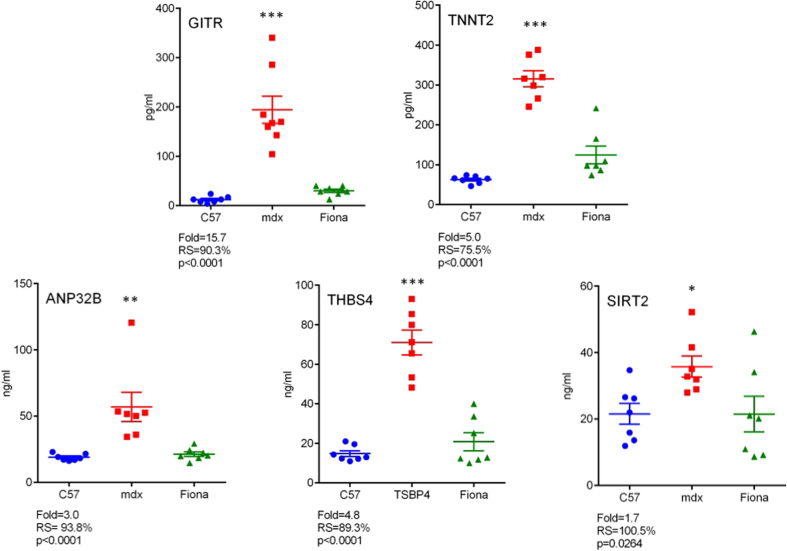
Finally, we confirmed a subset of five serological markers by ELISA to define an homogeneous set of markers based on function and pathophysiological features including muscle function (TNNT2), extracellular matrix remodelling (THBS4), immune response (GITR) and metabolic regulation (SIRT2). The multifunctional protein ANP32B was also included in this panel. Despite some small discrepancies, protein abundance was found to be significantly enriched in mdx serum relative to wild-type controls for all these targets (Fig. 4). In mdx transgenic Fiona mouse expressing a high level of utrophin, the levels of all serological markers were restored toward C57 levels as previously noted in the SOMAscan assay. CAMK2A protein was not detectable by ELISA. Details for the ELISA are specified in Table S3.
Figure 4. ELISA confirmation of candidate biomarkers.
Five of the top candidate serological marker proteins were validated by ELISA. Individual biological replicates are shown and mdx vs C57 fold changes, Recovery score in Fiona compared to mdx and C57 and Kruskal-Wallis one-way ANOVA p values are indicated for each protein. Bonferroni post hoc test significance values are indicated as (*)p < 0.05; (**)p < 0.01 and (***)p < 0.001. Error bars indicate mean +/− SEM.
Discussion
The effective execution of clinical trials in DMD has been severely hampered by the lack of robust biomarkers. In the present study, we have addressed the development of biomarkers for utrophin modulation. Ezutromid, a drug which shows efficacy in the mdx mouse has recently entered clinical trials and biomarkers are needed to facilitate these trials and provide robust end points. We utilised an aptamer-based proteomic screening approach to profile candidate biomarkers in serum of 7 weeks old C57, mdx and Fiona (mdx transgenic overexpressing high level of utrophin) mice. This technology, complementary to mass spectrometry and antibody-based arrays previously used with success to investigate serum protein abundance in DMD patients56 and dystrophic animals61, measures concentration of a higher number of predetermined 1,310 proteins with high affinity. Whereas some putative serological markers as F13A1, MYOM3 or TNNT3, are absent from the SOMAscan assay, SOMAmer reagents were defined to bind to recombinant human protein. Consequently, potential interspecies differences in protein sequences may alter results obtained with the SOMAscan platform and murine samples.
In our study, analysis of dystrophic sera revealed a clear separation between dystrophic and healthy animals, and 83 circulating protein in mdx sera, mostly of muscle origin, were significantly altered with a 2 fold change in abundance compared to wild-type animal. A majority of the identified factors - TNNI2, CAMK2A/B, ANP32B, THBS4, PCNA, CKM - were previously described in DMD boys and in mdx mouse55,56,61,69, confirming a rich set of common protein biomarkers to assess pre-clinical and clinical studies and supporting the usefulness of the SOMAscan assay as a discovery biomarker platform. Importantly, levels of some well-documented biomarkers, previously described at 12-weeks of age (PGAM1, TIMP-1, CA3 or ADAMTS5), were not significantly increased in our study with 7 weeks old dystrophic animals, suggesting an important age dependence for these markers, only deregulated at later stages of the pathology. Similar to the study performed by Hathout et al. with 2 months old animals55, we observed a significantly high induction of THBS4, CYSC or CKM in 7 weeks old mdx animals. Nevertheless, in our study, serological level of FABP3 was not deregulated. Additionally, we identified GITR, MYPC1, HSP60, SMAD3, SIRT2, CNTN1 and MSTN as new potential putative serum dystrophic markers. Notably, in our study, GITR, a co-stimulatory immune checkpoint molecule, and member of the tumour necrosis factor receptor (TNF-R) superfamily, induced with activation of T cells70, is the most deregulated factor with a 25 fold enrichment in dystrophic serum. Activated immune cell infiltrates (e.g., T lymphocytes and macrophages) are evident during early disease stages in dystrophic muscle and play a critical role in muscle wasting71. Thus, serum level of GITR, unchanged in previous studies with 12 weeks old mdx mice55,61, may be a potent marker for early dysregulation of immune system in Duchenne muscular dystrophy. Interestingly, we noted significant changes in the abundance of other members of the Tumor necrosis factor receptor superfamily as XEDAR/TNFRSF27 (2.0x), TNR4/TNFRSF4, TWEAKR/TNFRSF12A, BAFF Receptor/TNFRSF13C, RANK/TNFRSF11A, OPG/TNFRSF11B (1.4x; data not shown) and DR3/TNFRSF25 (0.7x). RELT/ TNFRSF19 was previously described as significantly decreased in DMD patient56 highlighting importance of this group of cytokine receptors primarily involved in pleatoric activities as inflammation, apoptosis, proliferation, survival and differentiation. Another intriguing protein that emerged from our study is the neural immunoglobulin family adhesion molecule Contactin-1, significantly reduced in abundance in mdx sera. CTNT1 was previously documented as expressed in the central and peripheral nervous system72 and at the neuromuscular junction in skeletal muscle66. Interestingly, CTNT1 deficiency was associated to the Compton-North congenital myopathy (CNCM) [MIM:612540], a familial lethal form of congenital myopathy, inherited in an autosomal-recessive fashion and characterized by ataxia, progressive muscle weakness and postnatal lethality66. It was proposed that loss of contactin-1 could impair communication or adhesion between nerve and muscle, resulting in severe myopathic phenotype. Interestingly, level of Contactin-5 was previously described as significantly decreased in serum from DMD patients56. Another protein of interest, significantly reduced in our study, is Growth Differentiation Factor 8/Myostatin (GDF8/MSTN), a member of the transforming growth factor beta (TGFβ) superfamily, acting as a major negative regulator of skeletal muscle mass73. MSTN and approaches to limit the activity of this secreted factor have been under extensive investigation for decades in dystrophic animal models74 and DMD patients19. Several reports failed to show induction of myostatin in DMD75,76 and in correlation with our findings, two studies reported a marker fourfold down-regulation of myostatin in mdx mice77,78. As myostatin abundance may not reflect myostatin activity, and that common adaptation of the myostatin level did not occur in dystrophic muscles, the usefulness of MSTN as serological biomarker can be questioned. Furthermore, GDF11 (unchanged in our study), highly homologous to myostatin, was recently reported as reduced in serum from DMD patients56. Whereas further studies are required to address the therapeutic potential of the balance GDF8 (inhibition)/11 (upregulation), a possible limitation of the SOMAscan assay is the cross-reactivity of SOMAmer reagents with closely homologous proteins.
To define a panel of robust serological biomarkers for utrophin modulation strategies, we next compared levels of these proteins in C57, mdx and Fiona sera. A first essential point to note is the specific context of the mdx transgenic Fiona mice expressing a high level of utrophin. These mice benefit from constant utrophin upregulation during developmental stages and after birth, and are histologically and functionally indistinguishable from wild-type animals35. Whereas systemic oral treatment with utrophin modulators aim to correct the pathology at later stages of the disease in animal models21,37 as DMD patients39, the disease is delayed from the initial stages in Fiona mice. Thus, this high level utrophin context serves as positive control for mdx mice treated with small utrophin inducers. Whereas further analysis will be required to define the sensitivity and robustness of potential serological biomarkers in therapies aiming to overexpress utrophin in pre-clinical and clinical settings, it is expected that the recovery of serological marker levels is dependent on the levels of induced utrophin expression after drug treatment. Among the initial 1,310 serum proteins analysed, a statistical analysis including all experimental groups, revealed 89 proteins highly differentiated in sera of C57, mdx and Fiona mice. Interestingly, a very positive correlation was noted between these markers and the previous panel of 83 serological protein biomarkers defined in mdx mice compared to wild-type. From the subsequent set of 80 common protein markers, profiles of these three distinct genotype/phenotypes showed a clear separation supporting the usefulness of monitoring the effectiveness of therapeutic interventions with utrophin modulators. Using a recovery score, we observed that more than 75% of the defined serological proteins were restored towards wild-type in Fiona mice, due to high levels of utrophin. However, the serum biomarker profile of the Fiona mice is not identical to the wild-type animal, suggesting that the biomarker rescue is either not complete or not necessary to obtain significant histological and functional benefits. Among these 80 markers, some were previously described in mdx mice as fully or partially rescued after dystrophin restoration61. These serum biomarkers are of interest as they may provide a common and comparative panel of serum biomarkers to therapeutic strategies aiming to rescue the sarcolemma stability by rescuing dystrophin or increasing utrophin expression.
More interesting are the unique markers specific to each strategy. A previous study showed that levels of TNNI3 (Troponin I, cardiac muscle) but not DUS3 and TPI1 were rescued in response to dystrophin restoration61. In Fiona mice, levels of DUS3 and TPI1 are normalised toward wild-type level, whereas TNNI3 is unchanged. Despite different contexts and the need for further studies, these results could emphasise specific serum biomarkers for dystrophin and utrophin strategies. As the first difference between utrophin and dystrophin is the specific spatio-temporal expression of each protein26, it is very likely utrophin fulfils specific roles and actions different from dystrophin, and vice versa, and that benefits of high level of utrophin may not only be restricted to improvement due to stabilisation of the muscle membrane. Thus, all these proteins may provide important insights in roles of utrophin protein and undiscovered benefits of high utrophin levels.
Importantly in our definition of robust biomarkers for utrophin modulation strategies, 34 serological protein markers were fully rescued to C57 level in Fiona mice. A set of 15 candidates was therefore defined. We noted the full restoration to wild-type level of previously biomarkers described as enriched in DMD (ANP32B, TNNT2, THBS4, CAMK2A, RS7, CYCS,) and mdx (HTRA2, PCNA, SMAD3, CAPN1, DUS3). Importantly, some of the common DMD associated biomarkers such as CK and TNNI3 are not included in the top 15 utrophin responsive biomarker candidates as they are poorly recovered in mdx transgenic Fiona mice (Table S2). Furthermore, despite a high increased level in mdx animals and a full rescue in Fiona mice, myoglobin was not selected as this marker does meet initial statistical significance criteria (Table S2). We also identified new mdx biomarkers as GITR, HSP60, SMAD3, SIRT2 and Contactin-1, all fully rescued in Fiona mice. Finally, despite some minor discrepancies, five top candidate protein biomarkers (ANP32B, THBS4, TNNT2, GITR and SIRT2) were validated by ELISA. Of great interest is the NAD-dependent deacetylase sirtuin-2 (SIRT2), a cytoplasmic enzyme involved in a large range of phenomenon as metabolic homeostasis, microtubule reorganisation, inflammatory responses and mitochondrial biogenesis65, notably by deacetylating PGC-1α, well known to induce utrophin expression79. SIRT2 is an emerging target in neurodegenerative diseases as inhibition of this sirtuin was beneficial. Interestingly, overexpression of SIRT1, another member of the sirtuin family, was recently reported to increase utrophin expression and ameliorate pathophysiology in mdx mouse80,81. Pharmacological modulation of the SIRT1/SIRT2 balance is therefore an interesting avenue for DMD.
In conclusion, we have identified several serological protein biomarkers to assist in the development of utrophin modulation strategies for DMD. Among 1,310 proteins, we progressively selected well-documented and undiscovered mdx markers, all rescued in the mdx transgenic Fiona mice. We therefore defined a final panel of 15 therapeutic monitoring biomarkers and confirmed five markers, easily measured in an automated fashion by ELISA. This work may help in the evaluation of Ezutromid currently in a clinical phase 2 trials and should accelerate development of future generations of utrophin modulators for a more rapid translation to DMD patients.
Methods
Animal samples
All animal procedures were performed in accordance with UK Home Office regulations which conform with the European Community Directive published in 1986 (86/609/ EEC). The work was performed under certificate of designation number 30/2306 and project license number 30/3104 following approval by the University of Oxford Departments of Physiology, Anatomy & Genetics and Experimental Psychology Joint Departmental Ethics Review Committee. 7 week old male C57/Bl10, C57/Bl10ScSn-Dmdmdx/J (mdx) and C57/Bl10ScSn-Dmdmdx/J-Tg(ACTA1-Utrn)2Ked (Fiona, Fio) mice (n = 7) were sacrificed. Each mouse used in this study was pentobarbital and blood immediately collected from the jugular vein and process according to the recommended Sample Handling and Processing SSM-001 Rev 5 Sop (SomaLogic, Inc). Briefly, whole blood was allowed to clot for 60 minutes at room temperature prior to centrifugation 2200 × g for 15 minutes. Typically 400 ul of blood could be collected per mouse aged from 7 weeks and 140 ul of serum obtained. Each sample was then aliquot in 70 ul volume and store at −80 °C for proteome profiling as described below. After blood collection, muscle samples were frozen in liquid nitrogen-chilled isopentane, and stored at −80 °C.
SOMAscan Assay
Serum proteome profiling was performed at SomaLogic, Inc (Boulder, CO, USA) using the 1,310-plex SomaScan platform. In this assay, each protein is targeted by a unique SOMAmer (Slow Off-rate Modified Aptamer) - a chemically modified nucleic acid ligand - attached to streptavidin beads via a tail consisting of a Cyanine3 fluorophore used for detection and quantification, a photocleavable linker and a photo-cleavable biotin molecule in order to capture specific proteins of interest. For each samples, three dilutions (0.5%, 2% and 5%) were prepared with a set of SOMAmers complexes and immobilised on streptavidin-coated beads. Once bound and after washes to reduce non-specific binding, proteins remain captured are biotinylated using NHS-PE04-Biotin and specifically photocleaved by ultraviolet light to releases the SOMAmer-biotinylated protein complexes. Biotin-labelled proteins-SOMAmers are then bound to a different set of streptavidin beads, washed to remove free SOMAmers and precipitated. Using a high-pH denaturing wash, SOMAmers are removed from their protein targets and eluted to be finally quantified using standard DND microarrays. Samples were randomly assigned to plates. Quality control procedures with intra-run normalisation and inter-run calibration were performed according to the SomaLogic good laboratory practice quality system (SSM-020 Rev 3). All samples passed quality control criteria for biases in SOMAmer hybridization.
ELISA
Enzyme-linked immunosorbent assay (ELISA) kits were purchased from antibodies-online (Aachen, Germany) and run to validate selected candidate serum protein biomarkers. ELISA kits used were: Acidic (Leucine-Rich) Nuclear phosphoprotein 32 Family, Member B, ANP32B (ABIN1745100); Calcium/calmodulin-Dependent Protein Kinase II alpha, CAMK2A, (ABIN426487); Cardiac Troponin T2 ELISA Kit, TNNT2, (ABIN426397); Tumor Necrosis Factor Receptor Superfamily, Member 18, TNFRSF18/GITR, (ABIN1672796); Sirtuin 2, SIRT2 (ABIN1144096), and Thrombospondin 4, THBS4 (ABIN426909). Sera of animals involved in the SOMAscan assay were used and diluted to fall within the linear range of each respective assay. Due to limited amount of sample, additional age matched animals were included in the study. ELISAs were performed according to manufacturer’s instructions. Samples concentrations were calculated using a four parameter logistic (4-PL) curve fit of the standard curves with MasterPlex 2.0.0.76 software.
Statistics
SOMAscan proteomic data reported in relative fluorescence units (RFU) were analysed using the SOMAsuite analysis software (V1.0.3). To identify differentially expressed protein between C57 and mdx animals, Mann-Witney U test (two-sided) tests was used. Comparison of C57, mdx and Fiona groups were performed using Kruskal-Wallis one-way ANOVA in correlation with Mann-Witney U test. Non-parametric tests were used as Shapiro-Wild test (GraphPad Prism 6.01) defined data as not normally distributed. Heatmap visualisation and hierarchical clustering were performed using log-transformed RFU and MeV (Multiple Experiment Viewer 4.9.0; The Institute for Genomic Research, Rockville, MD, USA)82. Principal Component Analysis (PCA) was studied using XLSTAT 2014.5.03. Using SOMAscan values, the percentage recovery score was calculated as described on the TREAT-NMD M. 1.1_001 SOP. Additional statistical analyses were performed using GraphPad Prism 6.01 software (GraphPad Software Inc, La Jolla, CA) as one-way ANOVA, Bonferroni post hoc test. Data are presented as mean ± SEM (standard error of mean), with n indicating the number of independent biological replicates used in each group for comparison. Differences were considered significant at (*) p < 0.05; (**)p < 0.01 and (***)p < 0.001.
Additional Information
How to cite this article: Guiraud, S. et al. Identification of serum protein biomarkers for utrophin based DMD therapy. Sci. Rep. 7, 43697; doi: 10.1038/srep43697 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We acknowledge G.E. Morris (Oswestry, UK) for the MANCHO3 antibody. This work was supported by grants from the Medical Research Council, Muscular Dystrophy UK, the Muscular Dystrophy Association USA and Summit Therapeutics plc.
Footnotes
K.E.D. is a shareholder of Summit Therapeutics plc.
Author Contributions The study was conceived and designed by S.G. and K.E.D. Experimental work was performed by S.G., B.E., S.E.S., A.B., N.S., A.B. and H.C. Data were analysed by S.G. and K.E.D. The manuscript was written by S.G. and K.E.D.
References
- Guiraud S., Aartsma-Rus A., Vieira N. M. et al. The Pathogenesis and Therapy of Muscular Dystrophies. Annual review of genomics and human genetics 16, 281–308, doi: 10.1146/annurev-genom-090314-025003 (2015). [DOI] [PubMed] [Google Scholar]
- Mendell J. R. et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Annals of neurology 71, 304–313, doi: 10.1002/ana.23528 (2012). [DOI] [PubMed] [Google Scholar]
- Emery A. E. Duchenne muscular dystrophy–Meryon’s disease. Neuromuscular disorders: NMD 3, 263–266 (1993). [DOI] [PubMed] [Google Scholar]
- Bach J. R., O’Brien J., Krotenberg R. & Alba A. S. Management of end stage respiratory failure in Duchenne muscular dystrophy. Muscle & nerve 10, 177–182, doi: 10.1002/mus.880100212 (1987). [DOI] [PubMed] [Google Scholar]
- Davies K. E. & Nowak K. J. Molecular mechanisms of muscular dystrophies: old and new players. Nat Rev Mol Cell Biol 7, 762–773 (2006). [DOI] [PubMed] [Google Scholar]
- Emery A. E. Dystrophin function. Lancet 335, 1289 (1990). [DOI] [PubMed] [Google Scholar]
- McAdam L. C., Mayo A. L., Alman B. A. & Biggar W. D. The Canadian experience with long-term deflazacort treatment in Duchenne muscular dystrophy. Acta myologica: myopathies and cardiomyopathies: official journal of the Mediterranean Society of Myology/edited by the Gaetano Conte Academy for the study of striated muscle diseases 31, 16–20 (2012). [PMC free article] [PubMed] [Google Scholar]
- Henricson E. K. et al. The cooperative international neuromuscular research group Duchenne natural history study: glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures. Muscle & nerve 48, 55–67, doi: 10.1002/mus.23808 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B. L. & Christopher C. Corticosteroids in Duchenne muscular dystrophy: a reappraisal. Journal of child neurology 17, 183–190 (2002). [DOI] [PubMed] [Google Scholar]
- Manzur A. Y., Kuntzer T., Pike M. & Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. The Cochrane database of systematic reviews, CD003725, doi: 10.1002/14651858.CD003725.pub3 (2008). [DOI] [PubMed] [Google Scholar]
- Ricotti V. et al. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. Journal of neurology, neurosurgery, and psychiatry 84, 698–705, doi: 10.1136/jnnp-2012-303902 (2013). [DOI] [PubMed] [Google Scholar]
- Voit T. et al. Safety and efficacy of drisapersen for the treatment of Duchenne muscular dystrophy (DEMAND II): an exploratory, randomised, placebo-controlled phase 2 study. The Lancet. Neurology 13, 987–996, doi: 10.1016/S1474-4422(14)70195-4 (2014). [DOI] [PubMed] [Google Scholar]
- Mendell J. R. et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Annals of neurology 79, 257–271, doi: 10.1002/ana.24555 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushby K. et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle & nerve 50, 477–487, doi: 10.1002/mus.24332 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. et al. Successful regional delivery and long-term expression of a dystrophin gene in canine muscular dystrophy: a preclinical model for human therapies. Molecular therapy: the journal of the American Society of Gene Therapy 20, 1501–1507, doi: 10.1038/mt.2012.111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nik-Ahd F. & Bertoni C. Ex vivo gene editing of the dystrophin gene in muscle stem cells mediated by peptide nucleic acid single stranded oligodeoxynucleotides induces stable expression of dystrophin in a mouse model for Duchenne muscular dystrophy. Stem Cells 32, 1817–1830, doi: 10.1002/stem.1668 (2014). [DOI] [PubMed] [Google Scholar]
- Nelson M. D. et al. PDE5 inhibition alleviates functional muscle ischemia in boys with Duchenne muscular dystrophy. Neurology 82, 2085–2091, doi: 10.1212/WNL.0000000000000498 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J. R. et al. A phase 1/2a follistatin gene therapy trial for becker muscular dystrophy. Molecular therapy: the journal of the American Society of Gene Therapy 23, 192–201, doi: 10.1038/mt.2014.200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C. et al. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: Results of a randomized, placebo-controlled clinical trial. Muscle & nerve, doi: 10.1002/mus.25268 (2016). [DOI] [PubMed] [Google Scholar]
- Ricotti V. et al. Safety, Tolerability, and Pharmacokinetics of SMT C1100, a 2-Arylbenzoxazole Utrophin Modulator, following Single- and Multiple-Dose Administration to Pediatric Patients with Duchenne Muscular Dystrophy. PloS one 11, e0152840, doi: 10.1371/journal.pone.0152840 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiraud S. et al. Second-generation compound for the modulation of utrophin in the therapy of DMD. Human molecular genetics 24, 4212–4224, doi: 10.1093/hmg/ddv154 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchenne A. Eli Lilly announce end of Tadalifil study due to lack of evidence of efficacy. http://www.actionduchenne.org/eli-lilly-announce-end-of-tadalifil-study-due-to-lack-of-evidence-of-efficacy/ (2016).
- Therapeutics P. PTC Receives Refuse to File Letter from FDA for Translarna™ (ataluren). http://ir.ptcbio.com/releasedetail.cfm?releaseid=956451 (2016).
- Pharmaceutical B. FDA Issues Complete Response Letter for KyndrisaTM for Duchenne Muscular Dystrophy Amenable to Exon 51 Skipping. http://investors.bmrn.com/releasedetail.cfm?ReleaseID=950309 (2016).
- Clerk A. et al. Dystrophin-related protein, utrophin, in normal and dystrophic human fetal skeletal muscle. The Histochemical journal 25, 554–561 (1993). [PubMed] [Google Scholar]
- Tome F. M. et al. Expression of dystrophin-associated glycoproteins during human fetal muscle development: a preliminary immunocytochemical study. Neuromuscular disorders: NMD 4, 343–348 (1994). [DOI] [PubMed] [Google Scholar]
- Schofield J. et al. Expression of the dystrophin-related protein (utrophin) gene during mouse embryogenesis. Developmental dynamics: an official publication of the American Association of Anatomists 198, 254–264, doi: 10.1002/aja.1001980403 (1993). [DOI] [PubMed] [Google Scholar]
- Man N. t., Ellis J. M. et al. Localization of the DMDL gene-encoded dystrophin-related protein using a panel of nineteen monoclonal antibodies: presence at neuromuscular junctions, in the sarcolemma of dystrophic skeletal muscle, in vascular and other smooth muscles, and in proliferating brain cell lines. J Cell Biol. 115, 1695–1700. (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell T. R., Man N. T., Morris G. E. & Davies K. E. The dystrophin-related protein, utrophin, is expressed on the sarcolemma of regenerating human skeletal muscle fibres in dystrophies and inflammatory myopathies. Neuromuscular disorders: NMD 2, 177–184 (1992). [DOI] [PubMed] [Google Scholar]
- Tinsley J. M. et al. Primary structure of dystrophin-related protein. Nature 360, 591–593, doi: 10.1038/360591a0 (1992). [DOI] [PubMed] [Google Scholar]
- Love D. R. et al. An autosomal transcript in skeletal muscle with homology to dystrophin. Nature 339, 55–58, doi: 10.1038/339055a0 (1989). [DOI] [PubMed] [Google Scholar]
- Squire S. et al. Prevention of pathology in mdx mice by expression of utrophin: analysis using an inducible transgenic expression system. Human molecular genetics 11, 3333–3344 (2002). [DOI] [PubMed] [Google Scholar]
- Rafael J. A. et al. Skeletal muscle-specific expression of a utrophin transgene rescues utrophin-dystrophin deficient mice. Nature genetics 19, 79–82, doi: 10.1038/ng0598-79 (1998). [DOI] [PubMed] [Google Scholar]
- Krag T. O. et al. Heregulin ameliorates the dystrophic phenotype in mdx mice. Proceedings of the National Academy of Sciences of the United States of America 101, 13856–13860, doi: 10.1073/pnas.0405972101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley J. et al. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nature medicine 4, 1441–1444 (1998). [DOI] [PubMed] [Google Scholar]
- Gillis J. M. Multivariate evaluation of the functional recovery obtained by the overexpression of utrophin in skeletal muscles of the mdx mouse. Neuromuscular disorders: NMD 12 Suppl 1, S90–94 (2002). [DOI] [PubMed] [Google Scholar]
- Tinsley J. M. et al. Daily treatment with SMTC1100, a novel small molecule utrophin upregulator, dramatically reduces the dystrophic symptoms in the mdx mouse. PloS one 6, e19189, doi: 10.1371/journal.pone.0019189 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley J., Robinson N. & Davies K. E. Safety, tolerability, and pharmacokinetics of SMT C1100, a 2-arylbenzoxazole utrophin modulator, following single- and multiple-dose administration to healthy male adult volunteers. Journal of clinical pharmacology, doi: 10.1002/jcph.468 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therapeutics S. First Patient Enrolled in Summit’s PhaseOut DMD, a Phase 2 Clinical Trial of Ezutromid in Boys with DMD. http://www.summitplc.com/media/press-releases/ (2016).
- Wilton S. D., Fletcher S. & Flanigan K. M. Dystrophin as a therapeutic biomarker: are we ignoring data from the past? Neuromuscular disorders: NMD 24, 463–466, doi: 10.1016/j.nmd.2014.03.007 (2014). [DOI] [PubMed] [Google Scholar]
- McDonald C. M. et al. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle & nerve 48, 343–356, doi: 10.1002/mus.23902 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone E. S. et al. Reliability of the North Star Ambulatory Assessment in a multicentric setting. Neuromuscular disorders: NMD 19, 458–461, doi: 10.1016/j.nmd.2009.06.368 (2009). [DOI] [PubMed] [Google Scholar]
- Pane M. et al. The 6 minute walk test and performance of upper limb in ambulant duchenne muscular dystrophy boys. PLoS currents 6, doi: 10.1371/currents.md.a93d9904d57dcb08936f2ea89bca6fe6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone E. S. et al. Timed Rise from Floor as a Predictor of Disease Progression in Duchenne Muscular Dystrophy: An Observational Study. PloS one 11, e0151445, doi: 10.1371/journal.pone.0151445 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth K. G. et al. Magnetic resonance imaging in Duchenne muscular dystrophy: longitudinal assessment of natural history over 18 months. Muscle & nerve 48, 586–588, doi: 10.1002/mus.23879 (2013). [DOI] [PubMed] [Google Scholar]
- Willcocks R. J. et al. Longitudinal measurements of MRI-T2 in boys with Duchenne muscular dystrophy: effects of age and disease progression. Neuromuscular disorders: NMD 24, 393–401, doi: 10.1016/j.nmd.2013.12.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlini A. et al. 204th ENMC International Workshop on Biomarkers in Duchenne Muscular Dystrophy 24–26 January 2014, Naarden, The Netherlands. Neuromuscular disorders: NMD 25, 184–198, doi: 10.1016/j.nmd.2014.09.004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aartsma-Rus A., Ferlini A. & Vroom E. Biomarkers and surrogate endpoints in Duchenne: meeting report. Neuromuscular disorders: NMD 24, 743–745, doi: 10.1016/j.nmd.2014.03.006 (2014). [DOI] [PubMed] [Google Scholar]
- Hathout Y. et al. Clinical utility of serum biomarkers in Duchenne muscular dystrophy. Clinical proteomics 13, 9, doi: 10.1186/s12014-016-9109-x (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okinaka S. et al. Serum creatine phosphokinase. Activity in progressive muscular dystrophy and neuromuscular diseases. Archives of neurology 4, 520–525 (1961). [DOI] [PubMed] [Google Scholar]
- Mendell J. R. & Lloyd-Puryear M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle & nerve 48, 21–26, doi: 10.1002/mus.23810 (2013). [DOI] [PubMed] [Google Scholar]
- Zatz M. et al. Serum creatine-kinase (CK) and pyruvate-kinase (PK) activities in Duchenne (DMD) as compared with Becker (BMD) muscular dystrophy. Journal of the neurological sciences 102, 190–196 (1991). [DOI] [PubMed] [Google Scholar]
- Cacchiarelli D. et al. miRNAs as serum biomarkers for Duchenne muscular dystrophy. EMBO molecular medicine 3, 258–265, doi: 10.1002/emmm.201100133 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boca S. M. et al. Discovery of Metabolic Biomarkers for Duchenne Muscular Dystrophy within a Natural History Study. PloS one 11, e0153461, doi: 10.1371/journal.pone.0153461 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathout Y. et al. Discovery of serum protein biomarkers in the mdx mouse model and cross-species comparison to Duchenne muscular dystrophy patients. Human molecular genetics 23, 6458–6469, doi: 10.1093/hmg/ddu366 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathout Y. et al. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proceedings of the National Academy of Sciences of the United States of America 112, 7153–7158, doi: 10.1073/pnas.1507719112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah V. D. et al. Serum matrix metalloproteinase-9 (MMP-9) as a biomarker for monitoring disease progression in Duchenne muscular dystrophy (DMD). Neuromuscular disorders: NMD 21, 569–578, doi: 10.1016/j.nmd.2011.05.011 (2011). [DOI] [PubMed] [Google Scholar]
- Rouillon J. et al. Serum proteomic profiling reveals fragments of MYOM3 as potential biomarkers for monitoring the outcome of therapeutic interventions in muscular dystrophies. Human molecular genetics 24, 4916–4932, doi: 10.1093/hmg/ddv214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoglu B. et al. Affinity proteomics within rare diseases: a BIO-NMD study for blood biomarkers of muscular dystrophies. EMBO molecular medicine 6, 918–936, doi: 10.15252/emmm.201303724 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PloS one 5, e15004, doi: 10.1371/journal.pone.0015004 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen-Stass A. M. et al. Identification of novel, therapy-responsive protein biomarkers in a mouse model of Duchenne muscular dystrophy by aptamer-based serum proteomics. Scientific reports 5, 17014, doi: 10.1038/srep17014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deconinck N. et al. Expression of truncated utrophin leads to major functional improvements in dystrophin-deficient muscles of mice. Nature medicine 3, 1216–1221 (1997). [DOI] [PubMed] [Google Scholar]
- Rittoo D., Jones A., Lecky B. & Neithercut D. Elevation of cardiac troponin T, but not cardiac troponin I, in patients with neuromuscular diseases: implications for the diagnosis of myocardial infarction. Journal of the American College of Cardiology 63, 2411–2420, doi: 10.1016/j.jacc.2014.03.027 (2014). [DOI] [PubMed] [Google Scholar]
- Yilmaz A. et al. Images in cardiovascular medicine. Cardiomyopathy in a Duchenne muscular dystrophy carrier and her diseased son: similar pattern revealed by cardiovascular MRI. Circulation 121, e237–239, doi: 10.1161/CIR.0b013e3181d74468 (2010). [DOI] [PubMed] [Google Scholar]
- Donmez G. & Outeiro T. F. SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO molecular medicine 5, 344–352, doi: 10.1002/emmm.201302451 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton A. G. et al. Mutations in contactin-1, a neural adhesion and neuromuscular junction protein, cause a familial form of lethal congenital myopathy. American journal of human genetics 83, 714–724, doi: 10.1016/j.ajhg.2008.10.022 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacatterina F. et al. Quantitative analysis of proteins of metabolism by reverse phase protein microarrays identifies potential biomarkers of rare neuromuscular diseases. Journal of translational medicine 13, 65, doi: 10.1186/s12967-015-0424-1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeman T. et al. Prevention of muscle fibrosis and improvement in muscle performance in the mdx mouse by halofuginone. Neuromuscular disorders: NMD 18, 857–868, doi: 10.1016/j.nmd.2008.06.386 (2008). [DOI] [PubMed] [Google Scholar]
- Burch P. M. et al. Muscle-Derived Proteins as Serum Biomarkers for Monitoring Disease Progression in Three Forms of Muscular Dystrophy. Journal of neuromuscular diseases 2, 241–255, doi: 10.3233/JND-140066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchetti S. et al. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. European journal of immunology 34, 613–622, doi: 10.1002/eji.200324804 (2004). [DOI] [PubMed] [Google Scholar]
- Spencer M. J., Montecino-Rodriguez E., Dorshkind K. & Tidball J. G. Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol 98, 235–243, doi: 10.1006/clim.2000.4966 (2001). [DOI] [PubMed] [Google Scholar]
- Colakoglu G., Bergstrom-Tyrberg U., Berglund E. O. & Ranscht B. Contactin-1 regulates myelination and nodal/paranodal domain organization in the central nervous system. Proceedings of the National Academy of Sciences of the United States of America 111, E394–403, doi: 10.1073/pnas.1313769110 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron A. C., Lawler A. M. & Lee S. J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387, 83–90, doi: 10.1038/387083a0 (1997). [DOI] [PubMed] [Google Scholar]
- Bogdanovich S. et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature 420, 418–421, doi: 10.1038/nature01154 (2002). [DOI] [PubMed] [Google Scholar]
- Chen Y. W. et al. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology 65, 826–834, doi: 10.1212/01.wnl.0000173836.09176.c4 (2005). [DOI] [PubMed] [Google Scholar]
- Castro-Gago M. et al. Myostatin expression in muscular dystrophies and mitochondrial encephalomyopathies. Pediatric neurology 34, 281–284, doi: 10.1016/j.pediatrneurol.2005.08.009 (2006). [DOI] [PubMed] [Google Scholar]
- Tseng B. S. et al. Regenerated mdx mouse skeletal muscle shows differential mRNA expression. Journal of applied physiology 93, 537–545, doi: 10.1152/japplphysiol.00202.2002 (2002). [DOI] [PubMed] [Google Scholar]
- Tkatchenko A. V., Le Cam G., Leger J. J. & Dechesne C. A. Large-scale analysis of differential gene expression in the hindlimb muscles and diaphragm of mdx mouse. Biochimica et biophysica acta 1500, 17–30 (2000). [DOI] [PubMed] [Google Scholar]
- Handschin C. et al. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes & development 21, 770–783, doi: 10.1101/gad.1525107 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkiadaki A. et al. Muscle-specific SIRT1 gain-of-function increases slow-twitch fibers and ameliorates pathophysiology in a mouse model of duchenne muscular dystrophy. PLoS genetics 10, e1004490, doi: 10.1371/journal.pgen.1004490 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubicic V., Burt M., Lunde J. A. & Jasmin B. J. Resveratrol induces expression of the slow, oxidative phenotype in mdx mouse muscle together with enhanced activity of the SIRT1-PGC-1alpha axis. American journal of physiology. Cell physiology 307, C66–82, doi: 10.1152/ajpcell.00357.2013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed A. I. et al. TM4: a free, open-source system for microarray data management and analysis. BioTechniques 34, 374–378 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.