Abstract
Although serotonin (5-HT) signaling is known to regulate food intake and energy homeostasis, the roles of the 5-HT3 receptor in feeding processes has been elusive. 5-HT3 receptors are found throughout mesolimbic circuitry that promote feeding not only in response to hunger, but also to the palatable and rewarding properties of food. These experiments examined if stimulation or blockade of the 5-HT3 receptor of the nucleus accumbens (NAcc) or ventral tegmentum affected food intake in the rat in response to hunger or the presence of a palatable diet. Rats (N = 6–9/group) received bilateral injections of the 5-HT3 agonist m-chlorophenylbiguanide hydrochloride (mCPBG; at 0.0, 10.0, or 20.0 µg/0.5µl/side) or the 5-HT3 antagonist ondansetron hydrochloride (at 0.0, 1.0, 2.0, or 5.0 µg/0.5µl/side) into either the NAcc or the ventral tegmentum. NAcc 5-HT3 receptor stimulation significantly increased 2-hr food intake in food-deprived animals offered rat chow and in a separate group of unrestricted rats offered a sweetened fat diet. In contrast to the feeding increase seen with NAcc treatments, stimulation of 5-HT3 receptors of the ventral tegmentum significantly reduced food and water intake in food-restricted animals; reductions of intake in non-restricted rats offered the palatable diet did not approach significance. Blockade of the 5-HT3 receptor had no effect on feeding in either brain region. These data support a functional role for serotonergic signaling in the mesolimbic pathway on motivated behavior, and demonstrate that 5-HT3 receptors differentially modulate food consumption in a region-dependent manner.
Keywords: Nucleus accumbens, ventral tegmental area, serotonin, 5-HT3, food intake, motivation
1.0 Introduction
Although there are currently few approved pharmacological treatments to assist with long-term weight loss in overweight or obese patients, those that have targeted serotonergic signaling have a history of modest success. Two current FDA-approved drugs for weight loss, the phentermine/topiramate combination (Qsymia®) and lorcaserin (Belviq®), increase serotonin signaling. Phentermine increases serotonin (5-HT) tone in the synapse, whereas lorcaserin is a selective agonist at the 5-HT2C receptor. Systemic stimulation of 5-HT1B and 5-HT2C receptors decreases food intake, causing reductions in meal size and duration that have been interpreted as advancing satiety processes [e.g., 1–5]. The recent success of lorcaserin in assisting weight loss provides hope that serotonin-based therapies that target individual serotonin subtypes may be of use in combatting obesity, without the cardiovascular side effects that resulted in the market removal of prior agents such as sibutramine and the phentermine/fenfluramine combination (phen-fen). The effects of serotonergic agents on satiety and meal size appear to be the result of their actions on the central nervous system, as injections of 5-HT1B or 5-HT2C agonists into the parabrachial and hypothalamic nuclei, respectively, also reduce feeding [e.g. 4,6].
Recent work has begun to examine what role, if any, the various 5-HT receptors of the meso-accumbens pathway may have on feeding and food-directed motivation. This pathway is of particular interest, as it has been argued to promote the overconsumption of palatable diets based upon their rewarding and reinforcing properties, and is differentially activated by food cues in individuals who are obese or are at risk of becoming obese [7–11]. Within the nucleus accumbens (NAcc) shell, stimulation of the 5-HT1A (but not 5-HT1B or 5-HT7) receptors inhibits feeding, albeit with concomitant locomotor alterations [12,13]. Jean et al. reported that 5-HT4 receptors of the NAcc inhibit food intake and are responsible for the anorexia observed following systemic 3,4-N-methylenedioxymethamphetamine (MDMA, ecstasy) treatment in mice [14]. In contrast to the reduced feeding caused by 5-HT1A and 5-HT4 receptor stimulation, 5-HT6 receptor agonism of the NAcc increases food intake [13]. Additionally, stimulation of 5-HT1A, 5-HT1B, and 5-HT2B receptors (but not 5-HT2A or 5-HT2C receptors) within the ventral tegmentum alters feeding in rats [15]. Clearly, serotonin afferents to the mesoaccumbens pathway are well-positioned to regulate motivated behavior, and serotonin’s impact on food-directed motivation depends upon the particular receptor subtype engaged within these regions.
The 5-HT3 receptor is unique amongst the serotonin receptor classes, as it is the only serotonin receptor that directly opens a cation channel, rather than acting as a g-protein coupled receptor. 5-HT3 receptors are found peripherally and in the central nervous system, and drugs that antagonize this receptor have been useful for their anti-emetic properties [16]. With regards to the central control of food intake, it has been shown that 5-HT3 receptors of the nucleus of the solitary tract regulate meal size and suppress feeding in response to cholecystokinin [17,18]. 5-HT3 receptors are also found within the ventral tegmental area (VTA) and the NAcc in rats and humans [19–21], and it is likely that they serve important regulatory functions. Specifically, 5-HT3 receptor stimulation in either the VTA or NAcc increases dopamine output [22,23], and 5-HT3 receptor antagonists reduce meso-accumbens dopamine signaling in response to several abuse-prone drugs, such as alcohol [for review, see, 24], cocaine [e.g., 25,26], and morphine [e.g., 25,27]. Given that drugs of abuse impact the same neural pathways implicated in the reinforcing and rewarding properties of food, these experiments were conducted to test whether stimulation or blockade of 5-HT3 receptors of the NAcc and ventral tegmentum might regulate feeding, motivated either by energy need or by the presence of a palatable sweetened fat diet.
2.0 Materials and Methods
2.1 Animals and Housing
Sixty-six adult male Sprague-Dawley rats (Harlan, Madison, WI; 275–300 g at arrival) were acclimated to dual housing in a colony room maintained at ~21 °C with a 12-hr light–dark cycle (lights on at 7 a.m.). All experiments were conducted in accordance to NIH animal care guidelines and were approved by the Wake Forest University Animal Care and Use Committee.
2.2 Surgical Procedures
Following acclimation to the housing environment and daily handling, rats were anesthetized with a Ketamine-Xylazine cocktail (100 mg/kg–10mg/kg). Standard aseptic surgical procedures were used to implant indwelling stainless steel guide cannulas (23 gauge) bilaterally above the NAcc shell (with the nose bar set at 5 mm above interaural zero: 3.1 mm anterior and 1.0 mm lateral to bregma, 5.0 mm ventral to the skull surface) or the VTA (with the skull flat; −5.6 mm posterior and 0.6 mm lateral to bregma, 7.3 mm ventral to skull surface). Stylets were placed within the cannulas to maintain patency. Rats were given at least one full week of recovery before acclimation to the feeding chambers and subsequent behavioral testing.
2.3 Apparatus
Food intake was monitored during 2-hr feeding sessions in experimental chambers. The feeding chambers were constructed from clear acrylic, with internal dimensions 42 cm wide, 30.5 cm deep and 33 cm tall. A water bottle was hung at one end of the chamber, and a food hopper was available at the opposite end of the chamber. The hopper was filled with diet and mounted on a food intake monitor (head entry at 6.4 cm above the wire floor). Each food intake monitor consisted of a calibrated potentiometer which allowed for continuous monitoring of the mass/weight of the food hopper during the experimental sessions (Med Associates, St. Albans, VT). Infra-red eyebeams were located along the floor at three locations (5 cm above the wire floor) to measure ambulation; four additional IR beams were placed at a height of 16 cm above the floor to index rearing behavior. IR beam interruption (including at a sensor at the entry to the food intake monitor) was continually recorded by Med-PC software (Med Associates, St. Albans, VT). The weights of the food hoppers were monitored by the computer at 10-sec intervals. A speaker maintained an ambient level of white noise at 65 dB in the experimental room.
2.4 Food-restriction feeding paradigm
The effects of drug infusion on hunger-driven food intake were tested in four groups of rats given free access to rat chow during a food-restricted state. Following one week of surgical recovery, rats were gradually reduced and maintained at approximately 90% of their ad libitum body weight. The animals then received six consecutive days of habituation to the feeding chambers prior to pharmacological treatments. Each session consisted of 2 hours of free access to rat chow (Prolab RMH 3000, Purina Lab Diets, 3.46 Kcal/gr) and water. When required, rats were given additional chow outside of the experimental chambers to maintain their 90% weight; most rats consumed the daily ration required to maintain their weight within the 2-hr testing sessions.
On the final 2 days of habituation, rats received mock infusions to allow acclimation to microinfusion procedures, as previously described [13]. Experimental treatments began 48 hrs after the last mock infusion. During vehicle and drug infusions, injection cannulas (30 gauge) were lowered into the NAcc (2.5 mm beyond the end of the guide cannulas) or the ventral tegmentum (1.0 mm beyond the end of the guide cannulas), and 0.5 µl of solution was delivered (at a rate of 0.32 µl/min) by a Harvard Apparatus microinfusion pump (Holliston, MA). Injectors remained in place for one minute after drug delivery to allow for diffusion. Injectors were removed, stylets replaced, and rats were immediately placed into the feeding chambers. Dependent measures included the amount of chow eaten across the 2-hr period, ambulation within the chamber (assessed as the number of complete crossings of the chamber from end to end), number of rears recorded, and total water intake across the feeding session.
2.5 Feeding paradigm on a palatable fat/sucrose diet
Separate groups of rats were tested for the effects of NAcc or ventral tegmentum treatment with 5-HT3 receptor agonists and antagonists on the intake of a highly palatable diet in non-deprived rats. Following one week of surgical recovery, ad libitum fed rats were given at least six days of habituation to the palatable diet for 2-hr sessions in the feeding chambers. The high-fat diet contained 278.3 g/kg vitamin free casein, 100.0 g/kg sucrose, 4.2 g/kg DL-methionine, 441.2 g/kg shortening, 77.7 g/kg safflower oil, 26.3 g/kg cellulose, 53.3 g/kg mineral mix, 15.2 g/kg vitamin mix and 3.8 g/kg choline chloride (Kilocaloric value of diet=6.2 kcal/g; Teklad Diets, Madison, WI, USA). Rats typically eat this diet when available, and we have previously shown that its intake is sensitive to intracranial injections of serotonergic, opiate or cholinergic drugs [e.g., 12,15,28–30]. On experimental days, mock injections and drug infusions were delivered as described for the food restriction paradigm above.
2.6 Drugs and Experimental Groups
These experiments assessed the behavioral effects of 5-HT3 receptor stimulation or blockade of the NAcc or ventral tegmentum on food intake following injection of the selective agonist m-chlorophenylbiguanide (m-CPBG, at 0.0, 10.0, or 20.0 mg/0.5 µg/side) or the selective antagonist ondansetron (at 0.0, 1.0, 2.0, or 5.0 µg/side). Separate groups of animals were tested for each brain region (NAcc and ventral tegmentum), for each drug (agonist and antagonist), and for each dietary condition (food-restricted or palatable eating), for a total of eight experimental groups. Within each experiment, rats received all doses of a single drug in a randomized order over 3–4 infusion days. Drug infusions were separated by a minimum of 48 hrs to allow for washout and metabolism of drug between injections. Drug concentrations for each agent were chosen based upon solubility and consistency with behaviorally-effective doses in other paradigms, when available [17,31,32].
2.7 Histology
Once the experiments were complete, rats were euthanized and the brains were collected for standard histological analysis. Brain sections were taken through the extent of the NAcc or ventral tegmentum and stained with cresyl violet. Placement of the injection sites was confirmed by light microscopy and charted with reference to Paxinos and Watson [33]. Only the fifty-seven animals who completed all days of the experiment and whose injectors were bilaterally placed within the anterior medial NAcc or ventral tegmentum were included in the behavioral analysis.
2.8 Data analysis
Feeding data were analyzed utilizing two-way repeated measures ANOVAs, comparing food intake assessed across time (at 5 minute intervals within each 2-hr session) and drug doses. For groups that had significant drug and/or drug × time interaction effects, ANOVAs were run to compare the main effects of drug dose at 5 minute time points across the session, in order to further assess consistency and time course of the drug effects. Total ambulation, rearing behavior, and water intake was analyzed with one-way repeated measures ANOVAs with drug dose as the independent variable; Tukey HSD post-hoc analyses were conducted to compare behaviors between vehicle and drug treatment days, as appropriate.
3.0 Results
3.1 Effects of 5-HT3 receptor manipulations of the NAcc on intake and locomotion
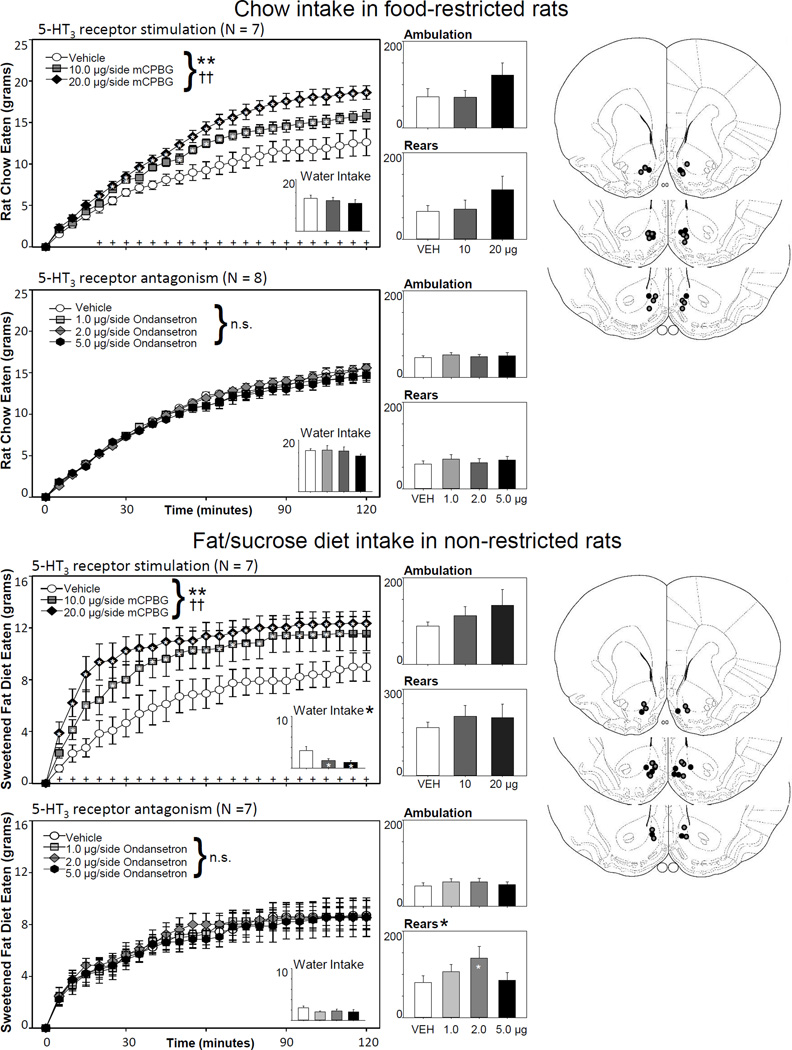
In food-restricted animals offered rat chow, 5-HT3 receptor stimulation of the NAcc yielded a significant effect of drug (N = 7; F2,12 = 17.27, p < .001, ηp2 = 0.74) and a significant drug X time interaction effect (F46,276= 7.09, p < .001, ηp2 = 0.54). As can be seen in the top panels of Figure 1, 5-HT3 receptor stimulation resulted in a dose-dependent increase in rat chow eaten over the 2-hr feeding test. Follow-up ANOVAs at individual time points determined that food intake began to diverge by 20 min into the session. After 1 hour of feeding, both doses of mCPBG had increased feeding as compared to the vehicle injection condition, and this effect continued throughout the remainder of the session for the 20.0 µg/side condition. Despite this increase of food intake, 5-HT3 receptor stimulation had no effect on ambulation (F2,12 = 2.20, p = .153, ηp2 = 0.27), rearing (F2,12 = 2.60, p = .115, ηp2 = 0.30), or water intake (F2,12 = 0.61, p = .559, ηp2 = 0.09) at these doses.
Figure 1.
Effects of NAcc stimulation or blockade of the 5-HT3 receptor on feeding, water intake, and locomotion. In food-restricted rats offered rat chow, 5-HT3 receptor stimulation dose-dependently increased food intake; water intake and locomotor measures were not affected. A similar dose-dependent increase in food intake was also observed following mCPBG treatment in non-deprived rats offered a palatable diet. In this case, water intake was significantly reduced, though locomotor measures were unaffected. Antagonism of NAcc 5-HT3 receptors with ondansetron did not impact feeding or water intake in either paradigm, though 2.0 µg/side increased rearing behavior in rats offered the palatable diet. Statistical symbols: *p < .05, **p < .01 for drug effects; single and double crosses demark p < .05 and p < .01 for drug X time interaction effect, respectively. Plus signs along the bottom of feeding graphs indicate times when there were significant simple effects of drug on food intake across drug doses. White stars within the graphs denote significant differences from the vehicle control injection, as assessed by Tukey’s HSD. The histology figures to the right represent the injection sites for each experiment, as charted within Paxinos and Watson [33]. Black-filled circles represent agonist injection sites; gray-filled circles denote antagonist injection sites.
A similar feeding increase was observed following NAcc 5-HT3 receptor stimulation in non-restricted rats offered a palatable sweetened fat diet (N = 7; drug effect: F2,12 = 20.20, p < .001, ηp2 = 0.77; drug X time interaction: F46,276 = 2.14, p < .001, ηp2 = 0.26). Compared to days on which the rats received a vehicle infusion, both 10.0 and 20.0 µg/side of mCPBG increased food intake by 10 minutes into the session, and this effect continued through the remainder of the session for the highest dose tested (bottom panels, Fig 1). Neither ambulation (F2,12 = 1.05, p = .380, ηp2 = 0.15) nor rearing (F2,12 = 0.42, p = .419, ηp2 = 0.07) were affected by drug treatment, although water intake was significantly reduced (F2,12 = 6.53, p = .012, ηp2 = 0.52; both doses significantly differed from vehicle according to Tukey’s HSD).
There were no effects of NAcc 5-HT3 receptor antagonism on the food intake of food-restricted rats offered rat chow (N = 8; drug effect: F3,21 = 1.03, p = .398, ηp2 = 0.13; drug × time interaction: F69,483 = 1.18, p = .171, ηp2 = 0.14) or in sated rats given access to a sweetened high fat diet (N = 7; drug effect: F3,18 = 0.10, p = .957, ηp2 = 0.02; drug × time interaction: F69,414 = 0.56, p = .998, ηp2 = 0.09). Ondansetron did not affect ambulation, rearing, or water intake for food-restricted rats (all p’s > .10); for rats offered the palatable diet, ondansetron increased rearing at 2.0 µg/side (F3,18 = 3.67, p = .041, ηp2 = 0.36), but did not impact ambulation or water intake (both p’s > .10).
3.1 Effects of 5-HT3 receptor manipulations of the ventral tegmentum on intake and locomotion
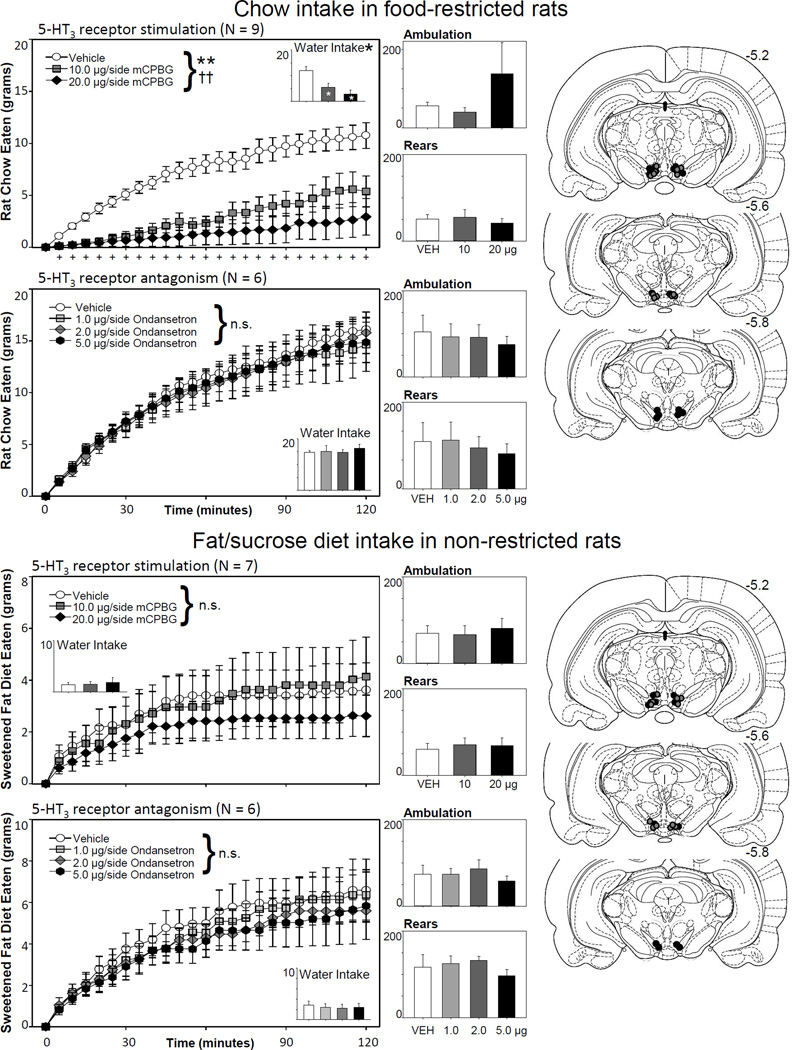
In contrast to the increases in food intake seen following NAcc treatment, stimulation of 5-HT3 receptors of the ventral tegmentum dose-dependently decreased food intake in food-restricted rats offered rat chow (N = 9; drug effect: F2,16 = 17.66, p < .001, ηp2 = 0.69; drug × time interaction: F46,368 = 5.60, p < .001, ηp2 = 0.41). As can be seen in Figure 2, inhibition of feeding occurred within the first 5 minutes of the session and continued through the remainder of the 2 hour test. Likewise, water intake was also reduced at both drug doses (F2,16 = 10.74, p = .001, ηp2 = 0.57). Neither ambulation (F2,16 = 1.32, p = .296, ηp2 = 0.141) nor rearing (F2,16 = 0.27, p = .765, ηp2 = 0.03) were impacted. Although a modest inhibition of feeding was observed following 20.0 µg/side mCPBG, this effect did not approach significance for non-restricted rats offered the sweetened fat diet (N = 7; drug effect: F2,12 = 0.51, p = .615, ηp2 = 0.08; drug × time interaction: F46,276 = 0.74, p =.896, ηp2 = .011). It is possible that this statistic was limited due to the low overall feeding on the palatable diet in this group (creating a possible floor effect). Nonetheless, there was a qualitative difference in the pattern of feeding inhibition between the group tested under restriction conditions as compared to the palatable feeding condition group. The restricted group’s feeding was most heavily inhibited early in the session, whereas the palatable diet group’s feeding was modestly inhibited in the second hour of the session. Other behavioral measures were unaffected; drug treatment did not alter water consumption (F2,12 = 0.34, p = .716, ηp2 = .05), ambulation (F2,12 = 0.16, p = .851, ηp2 = .03) or rearing (F2,12 = 0.14, p = .873, ηp2 = .02) in rats offered the palatable diet.
Figure 2.
Effects of ventral tegmentum stimulation or blockade of the 5-HT3 receptor on feeding, water intake, and locomotion. Stimulation of 5-HT3 receptors significantly inhibited food and water intake. This effect on feeding was seen within the first five minutes of food exposure for both doses of mCPBG, and continued through the remainder of the 2-hr session. No significant effects of the drug was observed on ambulation or rearing. Though a trend for a decrease in feeding was observed in sated animals offered a palatable diet for the 20.0 µg/side treatment of mCPBG, there was no significant effect of drug treatment on consumption measures or locomotion in the non-deprived rats. Antagonism of ventral tegmentum 5-HT3 receptors had no effect upon feeding or locomotion in either paradigm. Statistical symbols and histology as in Figure 1, with the exception that white stars were not placed in the figure to denote significant differences in feeding from the vehicle condition (as all time points were significantly different in the feeding condition).
Blockade of the ventral tegmental 5-HT3 receptor had no significant effects on behavior. Food intake was not altered by VTA treatment in food-restricted rats (N = 6; drug effect: F3,15 = 0.22, p = .882, ηp2 = 0.04; drug X time interaction: F69,345 = 0.54, p = .99, ηp2 = 0.10) or in non-restricted rats offered a palatable diet (N = 6; drug effect: F3,15 = 0.89, p = .467, ηp2 = 0.15; drug X time interaction: F69,345 = 0.403, p = 1.0, ηp2 = 0.08). Ondansetron treatments did not affect ambulation, rearing, or water intake measures (all p’s > .05).
4.0 Discussion
The goal of these experiments was to determine if 5-HT3 receptors of the meso-accumbens pathway influence feeding in response to energy need, or as motivated by the presence of a palatable diet. To our knowledge, this is the first report to examine the role of meso-accumbens 5-HT3 receptors on food-directed motivation. Our findings demonstrate that 5-HT3 receptor activation of the NAcc and ventral tegmentum affect feeding behavior in opposing manners. Specifically, stimulation of 5-HT3 receptors in the anterior medial NAcc dose-dependently increased both hunger-driven and palatable feeding. In contrast, agonist treatments of 5-HT3 receptors within the ventral tegmentum inhibited feeding in food-restricted rats offered rat chow, though intake was not significantly impacted in non-restricted animals presented with the palatable diet. Water intake was decreased by 5-HT3 receptor stimulation in two groups: the VTA-treated group offered rat chow, and the NAcc-treated group given access to the palatable diet. In the former case, it is likely that the water intake decrease was linked to the reduced consumption of the dry rat chow. The decreased water consumption observed here after NAcc treatment has not been observed with other treatments that enhance feeding on the palatable diet, such as 5-HT6 or mu-opioid receptor stimulation of the NAcc [13,29]. Despite the behavioral changes observed with 5-HT3 receptor stimulation, there were no effects of regional 5-HT3 receptor blockade on feeding following ondansetron injection into either the ventral tegmentum or the NAcc.
Although these experiments suggest that 5-HT3 receptors of the NAcc and VTA are involved in food intake, there is a possibility that the observed effects resulted from impacts of the drugs on nearby regions. It should be noted, however, that the volume and injection rate of the drug solutions used here have been successfully utilized by a number of laboratories to identify functional differences between the closely-positioned NAcc core and shell on learning and motivation [e.g., 34–36]. Additionally, Mena and colleagues [37] recently reported on the potential function of serotonergic agents of the medial prefrontal cortex in feeding. In their report, stimulation of serotonin receptors of the ventromedial prefrontal cortex (with serotonin hydrochloride) had no effect on feeding during a 60-minute session. Assuming that those injections stimulated 5-HT3 receptors (along with other 5-HT receptor subtypes), it seems unlikely that the effects we observed in the NAcc groups were due to diffusion of mCPBG to the nearby medial prefrontal cortex.
4.1 5-HT3 receptor modulation of the meso-accumbens circuit
The 5-HT3 receptor is an ionotropic cation channel, and its activation rapidly depolarizes the membrane [24,38]. Further experimental scrutiny will be required to determine which of the several cell populations within the tegmentum and ventral striatum possess the receptor, and how their activation may alter the functional output of the circuitry. Though 5-HT3 receptors are known to modulate multiple neurotransmitter systems in cortical and hindbrain systems, including both glutamate and GABA signaling [e.g., 39–42], the examination of their influence on signaling within the ventral tegmentum and NAcc has been primarily concentrated on dopamine output. 5-HT3 receptor stimulation of either region increases dopamine outflow [22,23], and mCPBG injections into the posterior VTA support self-administration behavior [32]. Despite the similar effects of 5-HT3 receptor stimulation on dopamine output in both regions, here we report that 5-HT3 receptor stimulation increased food intake when applied to the NAcc and inhibited feeding in restricted rats offered rat chow when injected into the ventral tegmentum. One possible reason for this difference across brain regions is that the temporal character of the dopamine release likely differs following tegmental or NAcc 5-HT3 agonism. For instance, NAcc dopamine release may be enhanced by 5-HT3 receptor stimulation in a manner that is consistent with afferent signaling of natural reinforcement by midbrain dopamine neurons, whereas VTA injections may directly impact dopamine output by altering the firing properties of dopaminergic neurons in a reinforcement-independent manner. Thus, the latter may be more disruptive of natural reward/reinforcement mechanisms than the former.
Additional insight into the functional role of 5-HT3-dopamine interactions has been provided by researchers interested in the possible effects of 5-HT3 receptor treatments on drug abuse. For instance, a robust literature has demonstrated that systemic alcohol increases dopamine output within the NAcc, and this increase can be reversed by blockade of 5-HT3 receptors [e.g., 24,25,43,44]. In a set of elegant studies, McBride and colleagues demonstrated that this may be mediated by 5-HT3 receptors within the ventral tegmentum, as VTA injections of a 5-HT3 antagonist reduces dopamine output within the NAcc in response to i.p. injections of ethanol [22]. Furthermore, 5-HT3 antagonist treatments also block lever-pressing for posterior VTA infusions of alcohol or cocaine [45]. Indeed, preclinical studies have shown that under certain access conditions, 5-HT3 receptor antagonism can reduce ethanol intake in rats [see, 24 for review]. Similar reduced dopamine outflow in the NAcc following 5-HT3 receptor antagonism has also been shown following injections of cocaine [25,26] or morphine [25,27], although the preclinical efficacy of 5-HT3 receptor antagonism is less compelling than it is for alcohol [24]. Regardless, such data suggest an important role for 5-HT3 receptors for modulating dopamine release in the meso-accumbens pathway.
Dopamine has a long history of being associated with motivated behavior. For instance, it regulates some aspects of incentive processes, the learning about natural reinforcers, and the allocation of effort expended to earn reinforcement [e.g., 10,46–48]. Furthermore, its signaling is a critical element for the addictive nature of drugs of abuse [e.g., 49]. Thus, it is interesting that the potential effects of 5-HT3 antagonists on the seeking and consumption of alcohol, which may be due in part to reductions of dopamine signaling, are not mirrored in this set of experiments or in other studies that have looked at the effects of 5-HT3 receptor antagonism on feeding. Several laboratories have failed to report changes in normal food intake following systemic 5-HT3 receptor antagonism in food-restricted rats [50–53]. Similarly, mice treated with the 5-HT3 antagonist tropistron showed no effects on food intake, even in a glucose-induced obesity model [54]. In a paradigm designed to test the effects of drug treatment on reinforcement, 5-HT3 receptor blockade of the ventral tegmentum was without effect on lever pressing for saccharin or water reinforcement, though the same treatments reduced alcohol seeking in an operant task [55]. Furthermore, systemic ondansetron treatment alone does not appear to impact lever pressing for a food-associated secondary reinforcer, though dopaminergic receptor blockade does [56]. That 5-HT3 receptor blockade does not affect normal feeding is striking given that 5-HT3 receptor antagonists are routinely used to ameliorate nausea, vomiting, and lack of appetite after chemotherapy treatment in human patients [16], and they also reverse anorexia in rats presented with diets with amino acid imbalances [57]. Together with the present data, this suggests that 5-HT3 receptor antagonism, which appears to reduce the rewarding properties of drugs of abuse under some conditions, may not impact reinforcement for natural rewards in non-addictive settings [but see, 50,53]. Although more work needs to be done to better understand when and how 5-HT3 receptor antagonism may interact with peripheral and central mechanisms of nausea and food intake, reports that demonstrate that 5-HT3 receptor antagonists reduce alcohol intake without affecting feeding is promising in terms of their potential for treating drug abuse without reducing motivation for natural reinforcers.
4.2 5-HT3 receptor stimulation, food intake, and motivation
The current data suggest that stimulation of 5-HT3 receptors may serve differential motivational functions across disparate brain regions. These findings may help clarify why prior studies that examined the impact of systemic 5-HT3 receptor stimulation on feeding or diet choice have yielded inconsistent outcomes. For instance, treatment with the peripheral agonist 2-methyl-5-HT does not affect food intake in deprived rats [58], suggesting a limited role for peripheral receptors in normal feeding. With drugs that penetrate brain, male and female Wistar rats maintained ad-libitum on a choice of diets (high-fat, high carbohydrate, and high-protein sources) reduced their carbohydrate ingestion following systemic 5-HT3 stimulation, though only females reduced their total diet consumption across a 2 or 12 hour test [59]. Mazzola-Pomietto and colleagues [52] reported a transient inhibition of feeding (within the first hour), followed by transient hyperphagia (between 1 and 4 hr), after systemic mCPBG treatment (10 mg/kg) in food-restricted male Wistar rats, though chin-rubbing behavior may have interfered with food intake early in the session. Recently, a similar transient inhibition of acute feeding was observed following SR 57227 (a 5-HT3 agonist) treatment in food restricted, but not fed, mice [60]. That 5-HT3 receptor stimulation does not appear to consistently reduce feeding when given systemically may suggest differential involvement of 5-HT3 receptors in food intake under different dietary and deprivation conditions across the hindbrain (see introduction), the ventral tegmentum, and the NAcc.
Both the ventral tegmentum and the striatum are important for regulating motivational and learning processes. In these experiments, we specifically targeted the anterior medial nucleus accumbens (as opposed to other striatal regions) for two prominent reasons: 1) this region of the NAcc connects directly and indirectly to hypothalamic regions responsible for regulating food intake, and 2) the anterior NAcc shell has been heavily implicated in both feeding and hedonic evaluation. Stimulation of mu-opioid receptors within this “hedonic hotspot” is known not only to increase food intake (“wanting”), but also enhance the hedonic responses that rats show to sapid sucrose solutions (“liking”), unlike other regions of striatum [61]. Little is known as to whether 5-HT receptors impact hedonic evaluation. This study suggests that mCPBG stimulation of 5-HT3 receptors in the anterior medial NAcc enhances “wanting”, but it cannot determine if “liking” is also increased by such treatment. Ventral tegmental drug treatments, although capable of altering food intake, have not yet been reported to increase hedonic evaluation [62]. Future studies, looking at affective facial reaction or licking microstructure to sapid solutions in response to local drug infusions, will be needed to determine if and how serotonin modulation within these circuits affects hedonic evaluation.
4.3 Concluding remarks
These experiments demonstrate for the first time that 5-HT3 receptors of the NAcc and ventral tegmentum are important modulators of feeding behavior. Stimulation, but not blockade, of these receptors impacted feeding in a differential manner dependent upon the region targeted and the motivational state of the animal. These data add to a growing understanding of the roles of serotonin receptors of the meso-accumbens pathway on food intake and motivation, and provide important basic insight to the functional impact of 5-HT3 receptor agents. Given current interest in the development of 5-HT3-targetting compounds (particularly antagonists) for potential use in the treatment of addiction and mental disorders [24,63–66], it remains of interest to understand how 5-HT3 (and other serotonin receptors) modulate the motivational functions of the meso-accumbens pathway in both normal and addictive-like conditions.
Highlights.
5-HT3 receptors are found in brain regions that regulate food-directed motivation
Nucleus accumbens 5-HT3 receptor stimulation increased feeding in rats
In contrast, 5-HT3 receptor stimulation of the ventral tegmentum reduced feeding
5-HT3 receptor blockade of either region did not alter food intake
The impact of 5-HT3 receptors on motivation depends upon the brain region affected
Acknowledgments
The work presented here was accomplished by undergraduate and Master’s-level students at Wake Forest University, some of whom contributed significantly to this work and are therefore on the author line of this manuscript. The opportunity to engage these students in this body of work was made possible by the Wake Forest Department of Psychology, and by financial support from the National Institutes of Health (R15 DA030618). We would like to thank Ian Rosner for his helpful comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clifton PG, Lee MD, Dourish CT. Similarities in the action of Ro 60-0175, a 5-HT2C receptor agonist, and d-fenfluramine on feeding patterns in the rat. Psychopharmacology (Berl.) 2000;152:256–267. doi: 10.1007/s002130000504. [DOI] [PubMed] [Google Scholar]
- 2.Halford JC, Blundell JE. The 5-HT1B receptor agonist CP-94,253 reduces food intake and preserves the behavioural satiety sequence. Physiol. Behav. 1996;60:933–939. doi: 10.1016/0031-9384(96)00073-x. [DOI] [PubMed] [Google Scholar]
- 3.Hewitt KN, Lee MD, Dourish CT, Clifton PG. Serotonin 2C receptor agonists and the behavioural satiety sequence in mice. Pharmacol. Biochem. Behav. 2002;71:691–700. doi: 10.1016/s0091-3057(01)00709-2. [DOI] [PubMed] [Google Scholar]
- 4.Lee MD, Aloyo VJ, Fluharty SJ, Simansky KJ. Infusion of the serotonin1B (5-HT1B) agonist CP-93,129 into the parabrachial nucleus potently and selectively reduces food intake in rats. Psychopharmacology (Berl.) 1998;136:304–307. doi: 10.1007/s002130050570. [DOI] [PubMed] [Google Scholar]
- 5.Lee MD, Simansky KJ. CP-94, 253: a selective serotonin1B (5-HT1B) agonist that promotes satiety. Psychopharmacology (Berl.) 1997;131:264–270. doi: 10.1007/s002130050292. [DOI] [PubMed] [Google Scholar]
- 6.López-Alonso VE, Mancilla-Díaz JM, Rito-Domingo M, González-Hernández B, Escartín-Pérez RE. The effects of 5-HT1A and 5-HT2C receptor agonists on behavioral satiety sequence in rats. Neurosci. Lett. 2007;416:285–288. doi: 10.1016/j.neulet.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Berridge KC, Ho C-Y, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berthoud H-R. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr. Opin. Neurobiol. 2011;21:888–896. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burger KS, Stice E. Variability in Reward Responsivity and Obesity: Evidence from Brain Imaging Studies. Curr. Drug Abuse Rev. 2011;4:182–189. doi: 10.2174/1874473711104030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol. Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 11.Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE. The contribution of brain reward circuits to the obesity epidemic. Neurosci. Biobehav. Rev. 2013;37:2047–2058. doi: 10.1016/j.neubiorev.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clissold KA, Choi E, Pratt WE. Serotonin 1A, 1B, and 7 receptors of the rat medial nucleus accumbens differentially regulate feeding, water intake, and locomotor activity. Pharmacol. Biochem. Behav. 2013;112:96–103. doi: 10.1016/j.pbb.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pratt WE, Blackstone K, Connolly ME, Skelly MJ. Selective serotonin receptor stimulation of the medial nucleus accumbens causes differential effects on food intake and locomotion. Behav. Neurosci. 2009;123:1046–1057. doi: 10.1037/a0016882. [DOI] [PubMed] [Google Scholar]
- 14.Jean A, Conductier G, Manrique C, Bouras C, Berta P, Hen R, Charnay Y, Bockaert J, Compan V. Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16335–16340. doi: 10.1073/pnas.0701471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pratt WE, Clissold KA, Lin P, Cain AE, Ciesinski AF, Hopkins TR, Ilesanmi AO, Kelly EA, Pierce-Messick Z, Powell DS, Rosner IA. A systematic investigation of the differential roles for ventral tegmentum serotonin 1- and 2-type receptors on food intake in the rat. Brain Res. 2016 doi: 10.1016/j.brainres.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hesketh PJ. Comparative review of 5-HT3 receptor antagonists in the treatment of acute chemotherapy-induced nausea and vomiting. Cancer Invest. 2000;18:163–173. doi: 10.3109/07357900009038248. [DOI] [PubMed] [Google Scholar]
- 17.Hayes MR, Covasa M. Dorsal hindbrain 5-HT3 receptors participate in control of meal size and mediate CCK-induced satiation. Brain Res. 2006;1103:99–107. doi: 10.1016/j.brainres.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 18.Hayes MR, Covasa M. Gastric distension enhances CCK-induced Fos-like immunoreactivity in the dorsal hindbrain by activating 5-HT3 receptors. Brain Res. 2006;1088:120–130. doi: 10.1016/j.brainres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Abi-Dargham A, Laruelle M, Wong DT, Robertson DW, Weinberger DR, Kleinman JE. Pharmacological and regional characterization of [3H]LY278584 binding sites in human brain. J. Neurochem. 1993;60:730–737. doi: 10.1111/j.1471-4159.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- 20.Bufton KE, Steward LJ, Barber PC, Barnes NM. Distribution and characterization of the [3H]granisetron-labelled 5-HT3 receptor in the human forebrain. Neuropharmacology. 1993;32:1325–1331. doi: 10.1016/0028-3908(93)90027-z. [DOI] [PubMed] [Google Scholar]
- 21.Parker RM, Barnes JM, Ge J, Barber PC, Barnes NM. Autoradiographic distribution of [3H]-(S)-zacopride-labelled 5-HT3 receptors in human brain. J. Neurol. Sci. 1996;144:119–127. doi: 10.1016/s0022-510x(96)00211-0. [DOI] [PubMed] [Google Scholar]
- 22.Campbell AD, Kohl RR, McBride WJ. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol Fayettev. N. 1996;13:569–574. doi: 10.1016/s0741-8329(96)00069-9. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Thielen RJ, McBride WJ. Effects of repeated daily treatments with a 5-HT3 receptor antagonist on dopamine neurotransmission and functional activity of 5-HT3 receptors within the nucleus accumbens of Wistar rats. Pharmacol. Biochem. Behav. 2006;84:370–377. doi: 10.1016/j.pbb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Engleman EA, Rodd ZA, Bell RL, Murphy JM. The role of 5-HT3 receptors in drug abuse and as a target for pharmacotherapy. CNS Neurol. Disord. Drug Targets. 2008;7:454–467. doi: 10.2174/187152708786927886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carboni E, Acquas E, Frau R, Di Chiara G. Differential inhibitory effects of a 5-HT3 antagonist on drug-induced stimulation of dopamine release. Eur. J. Pharmacol. 1989;164:515–519. doi: 10.1016/0014-2999(89)90259-8. [DOI] [PubMed] [Google Scholar]
- 26.Kankaanpää A, Lillsunde P, Ruotsalainen M, Ahtee L, Seppâlä T. 5-HT3 receptor antagonist MDL 72222 dose-dependently attenuates cocaine- and amphetamine-induced elevations of extracellular dopamine in the nucleus accumbens and the dorsal striatum. Pharmacol. Toxicol. 1996;78:317–321. doi: 10.1111/j.1600-0773.1996.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 27.Pei Q, Zetterström T, Leslie RA, Grahame-Smith DG. 5-HT3 receptor antagonists inhibit morphine-induced stimulation of mesolimbic dopamine release and function in the rat. Eur. J. Pharmacol. 1993;230:63–68. doi: 10.1016/0014-2999(93)90410-j. [DOI] [PubMed] [Google Scholar]
- 28.Pratt WE, Blackstone K. Nucleus accumbens acetylcholine and food intake: decreased muscarinic tone reduces feeding but not food-seeking. Behav. Brain Res. 2009;198:252–257. doi: 10.1016/j.bbr.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Skelly MJ, Guy EG, Howlett AC, Pratt WE. CB1 receptors modulate the intake of a sweetened-fat diet in response to μ-opioid receptor stimulation of the nucleus accumbens. Pharmacol. Biochem. Behav. 2010;97:144–151. doi: 10.1016/j.pbb.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Will MJ, Pratt WE, Kelley AE. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiol. Behav. 2006;89:226–234. doi: 10.1016/j.physbeh.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Monti JM, Ponzoni A, Jantos H, Lagos P, Silveira R, Banchero P. Effects of accumbens m-chlorophenylbiguanide microinjections on sleep and waking in intact and 6-hydroxydopamine-treated rats. Eur. J. Pharmacol. 1999;364:89–98. doi: 10.1016/s0014-2999(98)00826-7. [DOI] [PubMed] [Google Scholar]
- 32.Rodd ZA, Gryszowka VE, Toalston JE, Oster SM, Ji D, Bell RL, McBride WJ. The reinforcing actions of a serotonin-3 receptor agonist within the ventral tegmental area: evidence for subregional and genetic differences and involvement of dopamine neurons. J. Pharmacol. Exp. Ther. 2007;321:1003–1012. doi: 10.1124/jpet.106.112607. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego: Academic Press; 1998. Fourth Edition. [Google Scholar]
- 34.Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-D-aspartate receptor activation in the nucleus accumbens core. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12174–12179. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hauser SR, Deehan GA, Dhaher R, Knight CP, Wilden JA, McBride WJ, Rodd ZA. D1 Receptors in the Nucleus Accumbens-Shell, but not the Core, are Involved in Mediating Ethanol-Seeking Behavior of Alcohol-Preferring (P) Rats. Neuroscience. 2015;295:243–251. doi: 10.1016/j.neuroscience.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambroggi F, Ghazizadeh A, Nicola SM, Fields HL. Roles of nucleus accumbens core and shell in incentive-cue responding and behavioral inhibition. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:6820–6830. doi: 10.1523/JNEUROSCI.6491-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mena JD, Sadeghian K, Baldo BA. Induction of hyperphagia and carbohydrate intake by μ-opioid receptor stimulation in circumscribed regions of frontal cortex. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:3249–3260. doi: 10.1523/JNEUROSCI.2050-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson AJ, Lummis SCR. 5-HT3 Receptors. Curr. Pharm. Des. 2006;12:3615–3630. doi: 10.2174/138161206778522029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui RJ, Roberts BL, Zhao H, Zhu M, Appleyard SM. Serotonin Activates Catecholamine Neurons in the Solitary Tract Nucleus by Increasing Spontaneous Glutamate Inputs. J. Neurosci. 2012;32:16530–16538. doi: 10.1523/JNEUROSCI.1372-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner TJ, Mokler DJ, Luebke JI. Calcium influx through presynaptic 5-HT3 receptors facilitates GABA release in the hippocampus: in vitro slice and synaptosome studies. Neuroscience. 2004;129:703–718. doi: 10.1016/j.neuroscience.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 41.Fu Y, Yu S, Guo X, Li X, Li T, Li H, Dong Y. Fluvoxamine increased glutamate release by activating both 5-HT3 and sigma-1 receptors in prelimbic cortex of chronic restraint stress C57BL/6 mice. Biochim. Biophys. Acta BBA - Mol. Cell Res. 2012;1823:826–837. doi: 10.1016/j.bbamcr.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Ye JH, Hunt T, Wu WH, McArdle JJ. Ondansetron modulates GABA(A) current of rat central nervous system neurons. Eur. J. Pharmacol. 1997;337:87–94. doi: 10.1016/s0014-2999(97)01279-x. [DOI] [PubMed] [Google Scholar]
- 43.Imperato A, Angelucci L. 5-HT3 receptors control dopamine release in the nucleus accumbens of freely moving rats. Neurosci. Lett. 1989;101:214–217. doi: 10.1016/0304-3940(89)90533-8. [DOI] [PubMed] [Google Scholar]
- 44.Wozniak KM, Pert A, Linnoila M. Antagonism of 5-HT3 receptors attenuates the effects of ethanol on extracellular dopamine. Eur. J. Pharmacol. 1990;187:287–289. doi: 10.1016/0014-2999(90)90015-x. [DOI] [PubMed] [Google Scholar]
- 45.Rodd-Henricks ZA, McKinzie DL, Melendez RI, Berry N, Murphy JM, McBride WJ. Effects of serotonin-3 receptor antagonists on the intracranial self-administration of ethanol within the ventral tegmental area of Wistar rats. Psychopharmacology (Berl.) 2003;165:252–259. doi: 10.1007/s00213-002-1300-2. [DOI] [PubMed] [Google Scholar]
- 46.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl.) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 47.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schultz W. Dopamine reward prediction-error signalling: a two-component response. Nat. Rev. Neurosci. 2016;17:183–195. doi: 10.1038/nrn.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol. Biochem. Behav. 1982;17:901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- 50.Beczkowska IW, Bodnar RJ. Naloxone and serotonin receptor subtype antagonists: interactive effects upon deprivation-induced intake. Pharmacol. Biochem. Behav. 1991;38:605–610. doi: 10.1016/0091-3057(91)90021-s. [DOI] [PubMed] [Google Scholar]
- 51.Higgins GA, Tomkins DM, Fletcher PJ, Sellers EM. Effect of drugs influencing 5-HT function on ethanol drinking and feeding behaviour in rats: studies using a drinkometer system. Neurosci. Biobehav. Rev. 1992;16:535–552. doi: 10.1016/s0149-7634(05)80195-2. [DOI] [PubMed] [Google Scholar]
- 52.Mazzola-Pomietto P, Aulakh CS, Murphy DL. Temperature, food intake, and locomotor activity effects of a 5-HT3 receptor agonist and two 5-HT3 receptor antagonists in rats. Psychopharmacology (Berl.) 1995;121:488–493. doi: 10.1007/BF02246499. [DOI] [PubMed] [Google Scholar]
- 53.van der Hoek GA, Cooper SJ. Ondansetron, a selective 5-HT3 receptor antagonist, reduces palatable food consumption in the nondeprived rat. Neuropharmacology. 1994;33:805–811. doi: 10.1016/0028-3908(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 54.Weber S, Volynets V, Kanuri G, Bergheim I, Bischoff SC. Treatment with the 5-HT3 antagonist tropisetron modulates glucose-induced obesity in mice. Int. J. Obes. 2005. 2009;33:1339–1347. doi: 10.1038/ijo.2009.191. [DOI] [PubMed] [Google Scholar]
- 55.Rodd ZA, Bell RL, Oster SM, Toalston JE, Pommer TJ, McBride WJ, Murphy JM. Serotonin-3 receptors in the posterior ventral tegmental area regulate ethanol self-administration of alcohol-preferring (P) rats. Alcohol Fayettev. N. 2010;44:245–255. doi: 10.1016/j.alcohol.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fletcher PJ, Higgins GA. Differential effects of ondansetron and alpha-flupenthixol on responding for conditioned reward. Psychopharmacology (Berl.) 1997;134:64–72. doi: 10.1007/s002130050426. [DOI] [PubMed] [Google Scholar]
- 57.Hammer VA, Gietzen DW, Beverly JL, Rogers QR. Serotonin3 receptor antagonists block anorectic responses to amino acid imbalance. Am. J. Physiol. 1990;259:R627–R636. doi: 10.1152/ajpregu.1990.259.3.R627. [DOI] [PubMed] [Google Scholar]
- 58.Sugimoto Y, Yamada J, Yoshikawa T, Noma T, Horisaka K. Effects of peripheral 5-HT2 and 5-HT3 receptor agonists on food intake in food-deprived and 2-deoxy-D-glucose-treated rats. Eur. J. Pharmacol. 1996;316:15–21. doi: 10.1016/s0014-2999(96)00660-7. [DOI] [PubMed] [Google Scholar]
- 59.Mok E, Paquette M, Thibault L. Effect of quipazine, a selective 5-HT3 agonist, on dietary self-selection of different macronutrient diets in male and female rats. Appetite. 2000;34:313–325. doi: 10.1006/appe.2000.0321. [DOI] [PubMed] [Google Scholar]
- 60.Li B, Shao D, Luo Y, Wang P, Liu C, Zhang X, Cui R. Role of 5-HT3 receptor on food intake in fed and fasted mice. PloS One. 2015;10:e0121473. doi: 10.1371/journal.pone.0121473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peciña S, Berridge KC. Hedonic Hot Spot in Nucleus Accumbens Shell: Where Do μ-Opioids Cause Increased Hedonic Impact of Sweetness? J. Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castro DC, Berridge KC. Advances in the neurobiological bases for food “liking” versus “wanting,”. Physiol. Behav. 2014;136:22–30. doi: 10.1016/j.physbeh.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ellenbroek BA, Prinssen EPM. Can 5-HT3 antagonists contribute toward the treatment of schizophrenia? Behav. Pharmacol. 2015;26:33–44. doi: 10.1097/FBP.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 64.Kishi T, Mukai T, Matsuda Y, Iwata N. Selective serotonin 3 receptor antagonist treatment for schizophrenia: meta-analysis and systematic review. Neuromolecular Med. 2014;16:61–69. doi: 10.1007/s12017-013-8251-0. [DOI] [PubMed] [Google Scholar]
- 65.Serata D, Kotzalidis GD, Rapinesi C, Janiri D, Di Pietro S, Callovini G, Piacentino D, Gasperoni C, Brugnoli R, Ferri VR, Girardi N, Tatarelli R, Ferracuti S, Angeletti G, Girardi P, Del Casale A. Are 5-HT3 antagonists effective in obsessive-compulsive disorder? A systematic review of literature. Hum. Psychopharmacol. 2015;30:70–84. doi: 10.1002/hup.2461. [DOI] [PubMed] [Google Scholar]
- 66.Thompson AJ, Lummis SCR. The 5-HT3 receptor as a therapeutic target. Expert Opin. Ther. Targets. 2007;11:527–540. doi: 10.1517/14728222.11.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]