SUMMARY
Malignant cells utilize diverse strategies that enable them to thrive under adverse conditions while simultaneously inhibiting the development of anti-tumor immune responses. Hostile microenvironmental conditions within tumor masses, such as nutrient deprivation, oxygen limitation, high metabolic demand and oxidative stress disturb the protein folding capacity of the Endoplasmic Reticulum (ER), thereby provoking a cellular state of “ER stress”. Sustained activation of ER stress sensors endows malignant cells with greater tumorigenic, metastatic and drug resistant capacity. Additionally, recent studies have uncovered that ER stress responses further impede the development of protective anti-cancer immunity by manipulating the function of myeloid cells in the tumor microenvironment. Here, we discuss the tumorigenic and immunoregulatory effects of ER stress in cancer, and we explore the concept of targeting ER stress responses to enhance the efficacy of standard chemotherapies and evolving cancer immunotherapies in the clinic.
Keywords: ER stress, UPR, IRE1, XBP1, PERK, CHOP, ATF6, cancer, immunotherapy
INTRODUCTION
Tumor growth persists despite many cell-intrinsic and cell-extrinsic stresses, including dysregulated proliferation, oxidative stress, nutrient and lipid deprivation, hypoxia, and acidic extracellular pH. Tumor progression despite these challenges requires frequent adaptation. The ER regulates this adaptive capacity by coordinating a wide array of fundamental cellular processes, including transmembrane and secretory protein folding, lipid biosynthesis, drug detoxification, and calcium storage and signaling. At steady state, the ER protein folding machinery readily handles secretory pathway requirements. However, if misfolded proteins accumulate beyond a tolerable threshold, ER-resident sensors trigger an unfolded protein response (UPR) to transcriptionally and translationally improve ER protein folding capacity. If these corrective efforts are insufficient, the cell will undergo apoptosis (Wang and Kaufman, 2014).
Despite these potentially fatal outcomes, robust ER stress responses have been documented in most major types of human cancer, including breast, pancreatic, lung, skin, prostate, brain, and even liquid malignancies (Wang and Kaufman, 2014). Furthermore, ER stress in situ frequently correlates with advanced stage disease and chemoresistance. The ability to tolerate persistent ER stress enhances cancer cell survival, angiogenesis, metastatic capacity, drug resistance, and immunosuppression. Yet this risky balancing act also endows cancer cells with selective vulnerabilities that could be harnessed to therapeutic advantage. In this review, we explore the causes and consequences of ER stress in malignancy within individual tumor cells and across their larger microenvironments.
ORCHESTRATING AN ER STRESS RESPONSE
Detecting and resolving ER stress requires three major ER-spanning transmembrane proteins, inositol-requiring enzyme 1α (IRE1α, encoded by ERN1), PKR-like ER kinase (PERK, encoded by EIF2AK3), and activating transcription factor 6α (ATF6α, encoded by ATF6). These sensors exhibit a broadly similar activation mechanism and regulate many unique and overlapping facets of the ER stress response. Each is bound intra-luminally by the chaperone protein BiP, which locks them in monomeric, inactive states. If the level of intraluminal misfolded proteins exceeds the folding capacity of ER-resident chaperones, glycosylases, and oxido-reductases, BiP dissociates from IRE1α, PERK and ATF6α (Bertolotti et al., 2000; Shen et al., 2002). These sensors subsequently drive mutually reinforcing signaling pathways to correct the protein misfolding stress. If the burden can be reduced quickly, the cells successfully adapt to the insult, while insufficient clearance results in apoptotic cell death.
IRE1α-XBP1
IRE1α is a highly conserved dual enzyme possessing both kinase and endoribonuclease activity. After BiP dissociation, IRE1α dimerizes and autophosphorylates, triggering a conformational shift that allosterically activates its endoribonuclease domain. This nuclease then catalyzes a unique cytoplasmic mRNA splicing reaction, specifically cleaving out 26 nucleotides from the XBP1 mRNA, which is subsequently re-ligated by the tRNA ligase RctB (Lu et al., 2014; Yoshida et al., 2001). Re-ligation causes a reading frame shift and translation of the highly active transcription factor XBP1, which upregulates multiple foldases, oxido-reductases, intracellular trafficking components, ER-associated degradation machinery and glycosylases to correct ER homeostasis (Shoulders et al., 2013). XBP1 also upregulates UPR-independent pathways, including pro-inflammatory cytokine production, lipid and hexosamine biosynthesis, and the hypoxia response (Bettigole and Glimcher, 2015). XBP1 induction favors cell survival, as enforced overexpression rescues cell viability in vitro and in a transgenic rat model of retinitis pigmentosa (Lin et al., 2007). However, under severe ER stress, IRE1α can also oligomerize and sequence-specifically degrade multiple ER-localized mRNAs and microRNAs in a pro-apoptotic process known as regulated IRE1α-dependent decay (RIDD) (Hollien et al., 2009; Lerner et al., 2012). Independently of its endoribonuclease function, phosphorylated IRE1α recruits TRAF2 to facilitate JNK and NFκB activation upon pharmacological ER stress (Tam et al., 2012; Urano et al., 2000). Similarly, IRE1α constitutively associates with the transcription factor STAT3 in mouse primary hepatocytes, and this interaction is crucial for enhancing STAT3 phosphorylation both in vitro and in vivo (Liu et al., 2015). IRE1α is thus well positioned to influence several key regulators of tumorigenesis independently of XBP1.
Interestingly, decreased ER membrane fluidity resulting from increased ER phospholipid saturation induces IRE1α activation by forcing transmembrane domains of neighboring IRE1α monomers into contact. Exogenous saturated lipids such as palmitate or loss of lipid desaturases like SCD1 perturbs ER membrane composition and can induce this activation mode (Volmer et al., 2013). Elegant studies using truncated IRE1α variants unable to bind BiP revealed that this lipid-mediated activation mode proceeds in the absence of misfolded proteins. Interestingly, IRE1α cannot oligomerize when activated by membrane lipid saturation, potentially favoring cell survival outcomes (Kitai et al., 2013). However, given the immense difficulty in quantifying intracellular protein misfolding, the relative contribution of membrane rigidity versus unfolded protein accumulation to IRE1α activation in vivo remains unknown.
PERK
Like IRE1α, PERK homodimerizes and autophosphorylates upon BiP dissociation or reduced ER membrane fluidity (Volmer et al., 2013). Activated PERK phosphorylates the translation initiation factor eIF2α, which reduces the influx of nascent proteins into the ER by restricting 5′ cap-dependent mRNA translation (Harding et al., 1999). Reduced translation rates facilitate focused refolding efforts by ER-localized chaperones. Paradoxically, global translational inhibition increases selective translation of the transcription factor ATF4, which directly upregulates the transcription factor CHOP. Subsequently, ATF4 and CHOP cooperatively induce multiple genes involved in amino acid biosynthesis, amino acid transport, and the intracellular recycling system autophagy (B’Chir et al., 2013; Han et al., 2013). Accelerated amino acid biosynthesis generates significant amounts of reactive oxygen species (ROS), which induces apoptosis if left unabated. However, PERK limits ROS accumulation by phosphorylating and stabilizing NRF2 (Cullinan et al., 2003), enhancing glutathione synthesis (Rouschop et al., 2013) and upregulating heme oxygenase-1 (HO-1) (Dey et al., 2015). PERK also activates NFκB by repressing translation of the NFκB inhibitor IκBα (Tam et al., 2012).
ATF6α
After BiP dissociation, ATF6α translocates to the Golgi apparatus, where it is cleaved intramembranously by site 1 and site 2 proteases to liberate an active, soluble ATF6α transcription factor (Shen et al., 2002). Disulfide bonding additionally regulates this ER-to-Golgi trafficking, and only monomeric, reduced ATF6α can properly access COPII endosomes (Schindler and Schekman, 2009). Unlike PERK and IRE1α, reduced ER membrane fluidity does not activate ATF6α, perhaps because dimerization is unfavorable for ATF6α activation. ATF6α fine-tunes the UPR by upregulating BiP and a subset of XBP1-dependent chaperones, oxidoreductases, and quality control and degradation machinery(Shoulders et al., 2013). While IRE1α and PERK conditional or germline knockout mice often exhibit pronounced phenotypes, ATF6α germline knockout mice only yield clear phenotypes under pharmacological or pathological stresses (Yamamoto et al., 2010), suggesting that ATF6α fine-tunes the UPR which is largely controlled by the more dominant PERK/IRE1α responses.
SOURCES OF ER STRESS IN TUMORS
Multiple cell-intrinsic and cell-extrinsic mechanisms initiate and amplify ER stress within the cancer cell and the larger tumor microenvironment (Figure 1). Spatiotemporal differences in ER stress burden, driven by genetic, epigenetic and microenvironmental heterogeneity, likely result in a range of pro-survival and anti-apoptotic responses. Anticancer interventions such as chemotherapy can also modulate UPR signaling, though the clinical implications are only beginning to be understood.
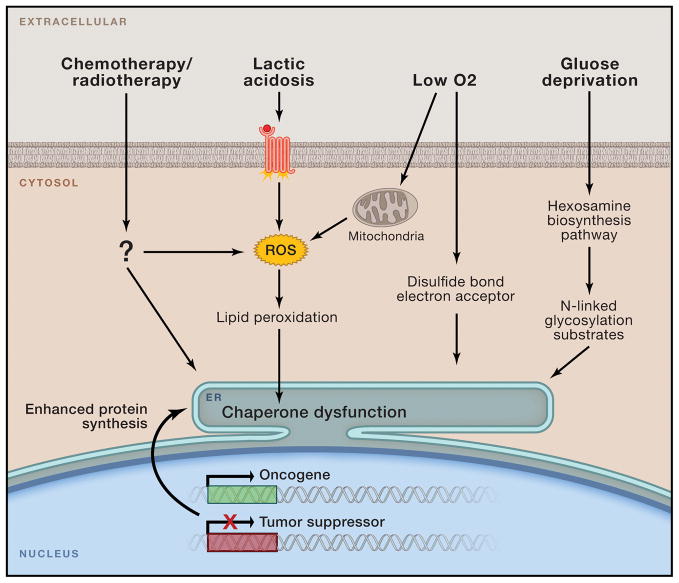
Figure 1. Tumor microenvironmental factors and conditions perturbing ER homeostasis.
Malignant transformation mediated by oncogene activation and loss of tumor suppressor function places intense biosynthetic and bioenergetic demands on available cellular resources, triggering initial ER stress. Cancer cells may eventually adapt to these early challenges, yet as tumors expand they encounter a host of new environmental stresses including oxygen and nutrient deprivation, lactic acidosis, and multiple forms of clinical intervention. These stimuli can disrupt ER protein folding by limiting crucial reaction intermediates (i.e. O2, N-acetylglucosamine) or by directly disrupting chaperone function via ROS-mediated lipid peroxidation and covalent adduct formation.
Cell intrinsic sources
Cancer initiation and development require both inactivation of tumor suppressors and/or the acquisition of oncogenic mutations that uncouple proliferative from extracellular, growth factor-mediated regulation. Transformation-associated increases in protein synthesis often overwhelm ER protein folding capacity. Consequently, highly secretory cancers such as the B cell malignancy multiple myeloma, which produces extremely high levels of immunoglobulins, often undergo persistent ER stress (Obeng et al., 2006). Specific cellular behaviors can also influence protein secretory rates, as evidenced by PERK activation during epithelial-mesenchymal transition (EMT) (Feng et al., 2014). Similarly, oncogenic transformation driven by loss of the tumor suppressors p53, PTEN, TSC1 or TSC2 dramatically enhances protein synthesis rates, leading to ER stress (Hart et al., 2012; Namba et al., 2015; Signer et al., 2014). Enhanced protein synthesis and concomitant ER stress are also observed upon overexpression of oncogenic HRAS (G12E), BRAF (V600E), c-Myc or Src (Chen et al., 2014; Corazzari et al., 2015; Denoyelle et al., 2006). Importantly, both the UPR signaling and leukemia development induced by conditional PTEN deletion (Signer et al., 2014) or c-Myc overexpression (Hart et al., 2012) were dramatically reduced or entirely abrogated upon heterozygous deletion of the key translation rate regulator ribosomal protein L24. This strongly implicates protein synthesis rate as a key driver of ER stress and tumorigenicity in vivo.
However, oncogene expression does not always induce ER stress. In contrast to c-Myc transgene-driven B cell lymphoma (Hart et al., 2012), high Myc expression insulated a large panel of human cancer cell lines from ER stress upon exogenous proline depletion (Sahu et al., 2016). Similarly, Ras-transformed, Mychigh cells exhibited low basal ER stress, but activated the UPR upon Ras inhibition, suggesting that additional layers of regulation coordinate Myc expression with ER homeostasis (Yaari-Stark et al., 2010). Furthermore, exogenous desaturated lipids protected TSC2−/− MEFs from ER stress (Young et al., 2013). Recently transformed cells may initially undergo ER stress in response to the higher replicative and metabolic demands, but may adapt by enhancing steady state ER protein folding capacity (Huber et al., 2013). However, de novo genetic mutations and other cell-intrinsic and cell-extrinsic stresses likely contribute to the active UPR observed in most major cancer types (Wang and Kaufman, 2014). Fundamental differences in the experimental approaches used such as overexpression versus endogenous expression, primary cells versus cell lines, and in vivo versus in vitro models may have also contributed to these discrepant findings. Future work should address how protein translation rates and related processes such as copy number alterations, epigenetic modifications and microRNA-mediated regulatory mechanisms influence the prevalence and intensity of ER stress responses in human tumors. Furthermore, identifying genetic defects and cell biological changes that influence the saturated:unsaturated ER phospholipid ratio will help distinguish protein misfolding from lipotoxic sources of ER stress.
Nonsynonymous mutations can also directly destabilize intrinsic protein folding, triggering the UPR by overwhelming ER-resident chaperone capacity. Consistent with this, overexpressing certain destabilized smoothened (SMO) mutants induces robust ER stress in Drosophila in vivo (Marada et al., 2013). Solid tumors possess dozens of nonsynonymous mutations, with certain cancers such as melanoma and lung cancers harboring upwards of 200 mutations (Vogelstein et al., 2013). Identifying the spectrum of protein destabilizing mutations that can trigger ER stress will help clarify the physiological relevance of this mechanism.
Microenvironmental sources
The tumor microenvironment (TME) predominantly fuels ER stress via oxygen and nutrient deprivation and acidic waste accumulation, though hypernutrition can also contribute during obesity (Nakagawa et al., 2014). While normal cells primarily rely on oxidative phosphorylation or anaerobic glycolysis to generate ATP, cancer cells often favor aerobic glycolysis in a phenomenon known as the Warburg effect. Consequently, rapidly dividing cancer cells aggressively consume glucose and release large quantities of lactic acid waste regardless of local oxygen concentration, which lowers local extracellular pH. Tumors initially rely on resident tissue microvasculature to supply key nutrients and oxygen, but eventually must generate their own local neovasculature to sustain growth. Though normal tissues possess highly ordered and efficient vasculature, tumor-generated neovasculature is generally leaky and torturous with slow, inconsistent blood flow. Such intermittent circulation limits nutrient accessibility, oxygen delivery and waste drainage, thereby driving sporadic, acute hypoxia and lactic acidosis (Vaupel et al., 1989).
Each of these extracellular conditions can induce ER stress, though responsiveness varies depending on cell type. Low oxygen tension activates complex III of the mitochondrial electron transport chain to increase cytosolic ROS production, required for stabilizing the key hypoxia response transcription factor HIF1α (Guzy et al., 2005). ROS can also generate highly reactive peroxidized lipid byproducts, which form destructive covalent adducts with various ER chaperones (Cubillos-Ruiz et al., 2015; Vladykovskaya et al., 2012). Furthermore, both ER disulphide bond formation and lipid desaturation require molecular oxygen. Nutrient deprivation, particularly of glucose and glutamine, limits metabolic intermediates required for the hexosamine biosynthetic pathway (HBP). The HBP generates substrates for N-linked protein glycosylation, which is required for successful ER protein folding (Huber et al., 2013). Proline starvation can also induce ER stress, potentially by inducing excessive ROS accumulation (Sahu et al., 2016). Lastly, extracellular acidosis can induce ER stress in a ROS-dependent manner, potentially by driving lipid peroxidation-mediated chaperone dysfunction (Xie et al., 2015). Whether acid-sensing GPCRs can trigger ER stress-inducing calcium fluxes and ROS generation in the TME remains to be determined.
Clinical sources
Multiple anticancer drugs induce potent ER stress responses in vitro, and ER stress can facilitate anticancer drug efficacy or the development of chemoresistance depending on context and tumor type. Paclitaxel, doxorubicin, the BRAF (V600E) inhibitor vemurafenib, and the EGFR inhibitor cetuximab potently induce eIF2α phosphorylation and downstream signaling, though the involvement of PERK in some cases remains unclear (Jeon et al., 2015; Ma et al., 2014; Pozzi et al., 2016). Several anthracyclines can also trigger lethal ER stress by enhancing ROS levels and depleting ER calcium stores, leading to PERK-dependent immunogenic cell death ICD (Kepp et al., 2013). Additionally, the proteasome inhibitor Bortezomib induces ER stress and CHOP expression, but paradoxically reduces XBP1 protein accumulation by diminishing IRE1α-mediated XBP1 mRNA splicing and stabilizing the dominant negative unspliced XBP1 protein (Lee et al., 2003). Critically, Bortezomib-resistant multiple myelomas partially de-differentiate to XBP1low, providing clinical evidence that Bortezomib efficacy likely relies on UPR inhibition (Leung-Hagesteijn et al., 2013). Thus, interactions between anticancer drugs and ER stress signaling significantly alter disease progression. Whether additional anticancer drugs induce intratumoral ER stress in vivo remains to be determined. If they do, intratumoral ER stress may be a valuable biomarker for determining whether to use UPR-activating or inhibiting compounds. However, as ER stress is also highly immunosuppressive in many leukocyte populations (described below), ER stress-inducing drug dosages must be carefully optimized to enable selective cancer killing without compromising anti-tumor immune responses.
MECHANISMS OF ER STRESS-MEDIATED TUMOR PROGRESSION
Irremediable ER protein folding defects are often lethal, yet tolerable levels of ER stress paradoxically facilitate multiple mechanisms of tumor development. These include bolstering viability under hypoxia and nutrient deprivation, enhancing metastatic spread by supporting EMT, tumor cell dormancy and tumor initiating cell (TIC) function, and stimulating angiogenesis (Figure 2). Many of these beneficial adaptations arise from intimate links between the ER stress response and fundamental cell biological processes such as autophagy and ER-mitochondrial crosstalk (Wang and Kaufman, 2014). As ER stress is a common feature of aggressive cancers, understanding how the UPR modulates disease is critical for identifying promising new clinical strategies.
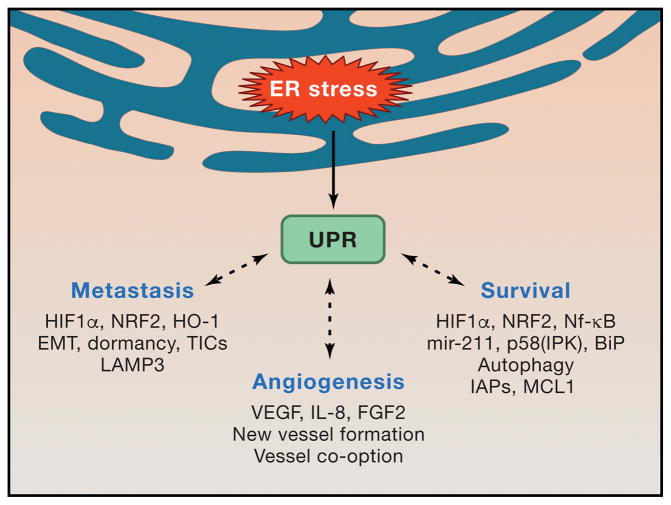
Figure 2. Consequences of ER stress in cancer cells.
Sublethal UPR activation and signaling via IRE1α, PERK and ATF6α sustain multiple cell-intrinsic and cell-extrinsic mechanisms of tumor progression. ER stress mediated activation of central signaling hubs such as HIF1α, STAT3, NRF2 and NFκB facilitate cell survival under harsh microenvironmental conditions and preserve tumor initiating cell function. Cytokine-driven angiogenesis delivers much needed oxygen and nutrients into the tumor bed, though IRE1α-deficient cells can also utilize vessel co-option. Intrinsic cancer cell apoptotic resistance is likely crucial for harnessing ER stress to enhance tumor growth.
Cell survival
ER stress dictates cell fate depending on context and signal strength. Prolonged and severe pharmacological ER stress can trigger caspase-mediated cell death through several IRE1α and PERK-dependent mechanisms. IRE1α-mediated JNK activation represses anti-apoptotic BCL2 activity and enhances pro-apoptotic BIM function, thereby favoring cell death (Wang and Kaufman, 2014). In parallel, RIDD de-represses pro-apoptotic caspase-2 and TXNIP expression in MEFs and pancreatic β-cells, respectively, by specifically cleaving microRNAs miR-17, miR-34a, miR-96, and miR-125b (Lerner et al., 2012; Upton et al., 2012). ATF4 and CHOP accelerate protein synthesis by tRNA synthetase expression, which elevates ROS production from ER oxidative protein folding. Importantly, the anti-oxidant butylated hydroxyanisole and silencing Rpl24 significantly reduced cell death by reducing ROS and protein translation rates (Han et al., 2013). Excessive intratumoral ER stress can also indirectly induce cancer cell death by enhancing immunosurveillance (Kepp et al., 2013). Chromosomal tetraploidy enhances protein translation rates and induces ER stress-dependent calreticulin translocation to the plasma membrane, which serves as a phagocyte “eat me” signal to facilitate ICD. Therefore, immunosurveillance selects against cancer cells undergoing intense ER stress (Senovilla et al., 2012), and suggests that selectively exacerbating cancer cell ER stress could enhance cell-intrinsic and immune-mediated tumor regression.
Mammalian cells have evolved multiple adaptive mechanisms to limit pro-apoptotic UPR outputs. MEFs exposed to persistent low-grade pharmacological ER stress resist subsequent ER insults, likely due to higher pro-survival BiP mRNA stability and reduced pro-apoptotic CHOP mRNA stability (Rutkowski et al., 2006). Furthermore, STAT3 and NFκB, which can be activated by IRE1α and PERK, transcriptionally upregulate multiple anti-apoptotic proteins, including BCL2 family members, the caspase-8 inhibitor c-FLIP, MCL1, and inhibitor of apoptosis proteins (IAPs)(Grivennikov and Karin, 2010). Independently, ATF4-induced miR-211 decreases CHOP expression by enhancing histone methylation at the CHOP promoter (Chitnis et al., 2012). Additionally, ATF6α-dependent p58 (IPK) limits apoptosis during oncogenic transformation by repressing PERK activity (Huber et al., 2013). Consistent with these findings, germline CHOP deletion enhanced lung lesion development in an immunocompetent K-rasG12V-driven murine model of lung cancer (Huber et al., 2013). BAX, BAK, PP2A, and GADD34 additionally modulate UPR signal strength, though how these factors influence cell fate under chronic ER stress in the TME is unknown (Hetz, 2012).
If cells successfully limit pro-apoptotic UPR outputs, ER stress confers survival advantages during tumor progression in vivo. Hypoxia and nutrient deprivation induce XBP1 splicing, which sustains cell growth and viability in human breast cancer cell lines in vitro and in vivo (Chen et al., 2014; Romero-Ramirez et al., 2004). PERK-mediated NRF2 stabilization, glutathione synthesis, and HO-1 upregulation collectively reduce cytotoxic ROS levels to facilitate cancer cell growth in vitro and in vivo (Bi et al., 2005; Dey et al., 2015; Rouschop et al., 2013). Sub-lethal ER stress also enhances survival by sustaining autophagy, an intracellular recycling system charged with eliminating toxic cytosolic protein aggregates and damaged organelles. The UPR and autophagy are intimately linked, and pharmacological ER stress triggers autophagosome formation as an adaptive mechanism to remove damaged ER and restrict ER expansion (Bernales et al., 2006). Furthermore, PERK-mediated eIF2α phosphorylation is required for LC3 lipidation, autophagy initiation and survival in cells overexpressing aggregation-prone expanded polyglutamine 72 repeat (polyQ72) (Kouroku et al., 2007), while ATF4 and CHOP upregulate numerous genes involved in autophagophore formation and maturation such as ATG5, ATG12, ATG16L1, and BECN1 (B’Chir et al., 2013). Invasive cancer cells require PERK-mediated autophagy to resist anoikis, a form of cell death triggered by extracellular matrix (ECM) detachment. Substrate detachment activates PERK-ATF4 signaling in human breast cancer, fibrosarcoma, and colorectal adenocarcinoma cell lines, and PERK-mediated autophagy limits damaging ROS accumulation (Avivar-Valderas et al., 2011; Dey et al., 2015). Accordingly, silencing ATF4 strongly reduced fibrosarcoma lung metastasis in a xenograft mouse model (Dey et al., 2015). Furthermore, human breast ductal carcinomas exhibit higher PERK phosphorylation than normal breast tissue, attesting to the physiological relevance of this mechanism (Avivar-Valderas et al., 2011).
ER stress-mediated autophagy likely also supports therapeutic resistance. A wide variety of anticancer drugs can induce autophagy, which can exert pro-survival or pro-apoptotic effects, depending on the drug used and tumor type (Sui et al., 2013). Like PERK, IRE1α–JNK signaling can sustain autophagy (Ogata et al., 2006), and this pathway facilitates sorafenib resistance in hepatocellular carcinoma cell lines (Shi et al., 2011). In melanoma, immuno-histochemical analyses of paired pre-treatment and post-resistance tumor biopsies revealed a marked increase in autophagy markers upon the development of vemurafenib resistance (Ma et al., 2014). BRAF (V600E) inhibition induced ER stress-dependent cytoprotective autophagy, which was completely abrogated with the small molecule PERK inhibitor GSK2606414 or by silencing PERK (Ma et al., 2014). Critically, PERK inhibition alone had no effect on cell viability, while simultaneously blocking BRAF (V600E) and PERK induced apoptosis in chemoresistant cell lines. Vemurafenib-mediated ER stress is also a therapeutic vulnerability that can be exploited to sensitize chemoresistant melanoma to pharmacological ER stress-induced cell death (Beck et al., 2013). Thus, blocking UPR-mediated cytoprotective autophagy or pharmacologically exacerbating chemotherapy-induced ER stress can overcome chemoresistance. ER stress-induced autophagy can also be highly cytotoxic, as was recently shown with the BiP inhibitor HA15. This compound induced apoptosis in a variety of chemoresistant cancer cell lines in vitro and in vivo, though the mechanism remains incompletely understood (Cerezo et al., 2016). In sum, optimal cancer growth and survival relies on carefully balanced UPR signaling pathways that interact with other cell processes such as autophagy, to result in cancer cell death or survival. Hence, depending on circumstances, either inhibition or overactivation of UPR pathways can lead to cell death.
Metastasis
Metastasis is a multi-step process in which cancer cells break away from the primary tumor site, infiltrate the surrounding ECM and stromal cell layers, enter the cardiovascular or lymphatic circulatory systems, colonize foreign tissues, and eventually grow into new tumor masses (Nieto et al., 2016). The UPR contributes to multiple steps along this invasion-metastasis cascade. EMT facilitates initial stromal invasion, and upregulates extracellular matrix protein production to facilitate migration and invasion. PERK buffers protein-folding stress during this increased secretory load and prevents anoikis during EMT-induced loss of cell-cell contact (Dey et al., 2015; Feng et al., 2014). Inducing EMT by silencing E-cadherin or overexpressing Twist strongly enhanced migration and tumorsphere formation, which was inhibited by a small molecule PERK inhibitor (Feng et al., 2014). Critically, ATF4 target gene expression strongly correlated with an EMT gene signature in breast, colon, gastric, lung, and mixed origin metastatic cancers. Consequently, pretreating cells with a PERK inhibitor or silencing ATF4 dramatically reduced in vivo lung metastasis (Dey et al., 2015; Feng et al., 2014). PERK also upregulates metastasis-associated LAMP3 to enhance migration and invasion in vitro and in vivo (Mujcic et al., 2013). PERK is therefore a potential therapeutic target to reduce EMT and invasiveness.
If invasive cells escape the stroma and successfully enter the circulatory system, they are often deposited in inhospitable tissue microenvironments. Pioneer tumor cells adapt by entering a p38-dependent program of anti-proliferative dormancy, which persists until microenvironmental conditions improve. Dormant cells are often quiescent and exhibit reduced metabolic rates, which can insulate them from many anticancer drugs that rely on active proliferation. Disseminated tumor cells in the bone marrow of breast cancer patients exhibit high expression of multiple ER chaperones, including BiP, which insulates these cells from hypoxia and glucose deprivation (Bartkowiak et al., 2010; Bartkowiak et al., 2015). Furthermore, comparative proteomic analyses of highly proliferative T-HEp3 human squamous carcinoma cell line and the D-HEp3 subclone, which becomes dormant in vivo despite growing in vitro, revealed a p38-dependent program sustaining high BiP expression and constitutive PERK phosphorylation. Dormancy-associated chemoresistance required both BiP and PERK, as silencing BiP or overexpressing a dominant-negative PERK variant sensitized D-HEp3 cells to doxorubicin and etoposide-mediated apoptosis (Ranganathan et al., 2006). Subsequent studies identified constitutive ATF6α nuclear translocation in the same dormant cell line, which was partially dependent on p38 signaling. Though ATF6α was not required for tumor cell growth in vitro, silencing ATF6 sensitized cells to rapamycin treatment and reduced tumor nodule size in vivo (Schewe and Aguirre-Ghiso, 2008). ATF6 knockdown reduced pro-survival Rheb and mTOR expression in the dormant cell line, though whether these genes are direct ATF6α transcriptional targets remains to be determined. Thus, multiple branches of the UPR contribute to tumor cell dormancy during metastasis.
Even if environmental conditions become favorable for metastatic outgrowth, only tumor initiating cells (TICs) possess the necessary proliferative capacity to generate clinically detectable tumor masses. We recently identified the IRE1α-XBP1 pathway as a key regulator of TIC function in human triple negative breast cancer (TNBC). Human basal-like breast cancer cell lines constitutively spliced XBP1, with highest splicing in patient-derived CD44+CD24low TICs. Silencing XBP1 potently inhibited mammosphere growth in multiple TNBC cell lines and primary patient samples (Chen et al., 2014). Inducible silencing of XBP1 in established TNBC xenografts significantly reduced primary tumor growth, angiogenesis, secondary metastases, and tumor recurrence after chemotherapy without enhancing tumor cell death. ChIP-sequencing and transcriptome analysis revealed that XBP1 and HIF1α–cooperatively upregulated hypoxia response genes to enhance TIC function. Critically, high expression of XBP1-target genes correlated with reduced overall survival in two independent TNBC patient cohorts, positioning XBP1 as an attractive clinical target in this malignancy. Taken together, all three UPR branches collectively promote metastasis by sustaining invasion, dormancy, and tumor-initiating cell function.
Angiogenesis
Solid cancers require vascularization, often mediated by new vessel growth, in order to supply sufficient oxygen and nutrients for growth while removing potentially toxic waste buildup. UPR induction stabilizes the VEGF mRNA via AMPK, though UPR-AMPK crosstalk is poorly understood (Pereira et al., 2010). PERK translationally upregulates the vessel growth and stabilization factors VCIP and PDGFRB. Consequently, K-Ras transformed PERK−/− MEFs exhibited extensive vascular hemorrhaging, failed microvasculature formation, and reduced tumor growth in vivo (Blais et al., 2006). XBP1, ATF4 and ATF6α can each transcriptionally upregulate VEGFA under hypoxia or glucose deprivation by directly binding the VEGF promoter or intronic enhancers (Ghosh et al., 2010). IRE1α also sustains expression of a broad array of pro-angiogenic cytokines including FGF2, IL-6, IL-8 and angiogenin, which likely contributes to the reduced in vivo growth and neovascularization observed upon IRE1α or XBP1 disruption in glioma and TNBC, respectively (Auf et al., 2010; Chen et al., 2014). Interestingly, glioma cells adapt to loss of IRE1α-mediated angiogenesis by enhancing mesenchymal differentiation and invasiveness to facilitate growth along established blood vessels, suggesting IRE1α may exert tumor type-specific angiogenic functions. The IRE1α kinase domain, but not the endoribonuclease domain, inhibited this vessel co-option, suggesting that vascularization can proceed independently of XBP1 or RIDD in certain cancer types (Jabouille et al., 2015). Thus, IRE1α can therefore promote new vessel formation at the expense of other vascularization modes, though whether this occurs in other cancer types remains to be determined. Like IRE1α, silencing PERK strongly suppressed tumor growth and vascularization in an orthotopic squamous cell carcinoma model, potentially due to PERK-mediated upregulation of FGF2, VEGF and IL-6 and suppression of the anti-angiogenic cytokines/chemokines THBS1, CXCL14 and CXCL10 (Wang et al., 2012). Interestingly, VEGF signaling directly activated PERK, IRE1α and ATF6α in HUVEC endothelial cells via a phospholipase Cγ-mTORC1 signaling pathway. Silencing eIF2α and ATF6α reduced VEGF-mediated AKT phosphorylation, cell survival, and neovascularization in vitro (Karali et al., 2014). Interestingly, silencing IRE1α did not inhibit these angiogenic processes in vitro. Therefore, VEGF signaling and the UPR may occasionally engage in a positive feedback loop to sustain angiogenic processes. In sum, tumors rely on cell-intrinsic ER stress signaling and outward ER stress transmission to access oxygen and nutrients in the blood.
ER STRESS RESPONSES IN TUMOR-ASSOCIATED IMMUNE CELLS
The microenvironment of most established tumors is formed by stromal cells including leukocytes, vascular cells and fibroblasts, whose normal functions are actively co-opted by cancer cells in order to promote malignant progression (Quail and Joyce, 2013). For instance, leukocyte recruitment to tumor sites can lead to unfavorable effects such as the secretion of growth factors enhancing cancer cell proliferation (Mantovani et al., 2008), the induction of tumor vascularization through paracrine mechanisms (De Palma et al., 2007), and the establishment of complex immunosuppressive networks that restrain the protective function of cancer-reactive T cells (Crespo et al., 2013). While the role of sustained ER stress in influencing the phenotype of cancer cells has been extensively studied during the last decade, the causes and consequences of ER stress in non-malignant cells that constitute the tumor microenvironment have just begun to be characterized.
Cancer cells undergoing ER stress actively modulate immune cell function
In vitro studies initially described some paracrine effects of ER-stressed malignant cells on innate immune cell populations. Pharmacological induction of ER stress prompted cancer cell lines to release unknown soluble factors that induced upregulation of UPR markers and pro-inflammatory cytokines in responder macrophages (Mahadevan et al., 2011). This process, termed “transmissible ER stress” was then shown to affect the antigen-presenting capacity of bone marrow-derived dendritic cells (DCs) while provoking overexpression of immunosuppressive molecules like Arginase (Mahadevan et al., 2012). These studies suggested that ER-stressed cancer cells secrete factors that actively modulate innate immune cell functions, but whether tumor-infiltrating leukocytes indeed experience physiological ER stress responses in vivo, and whether this process contributed to tolerance and/or immunosuppression in cancer hosts was unknown. Importantly, subsequent studies demonstrated that administration of the ER stressor thapsigargin to tumor-bearing mice accelerated cancer progression and stimulated the accumulation and immunosuppressive capability of myeloid-derived suppressor cells (MDSC), a process that could be alleviated upon treatment of cancer hosts with chemical chaperones that reduce ER stress (Lee et al., 2014).
While sustained but controlled ER stress responses apparently endow cancer cells with greater immunomodulatory capacity, intense/lethal ER stress responses instigated by interventions such as radiation or some chemotherapeutic agents can trigger ICD and protective anti-tumor immunity (Pol et al., 2015). Malignant cells exposed to drugs of the anthracycline family, for instance, experience irremediable ER stress characterized by ROS overproduction, increased cytoplasmic Ca2+ levels, and activation of ER stress sensors such as PERK and IRE1α, which can further promote activation of the inflammasome (Kepp et al., 2013; Lerner et al., 2012). Prior to evoking cell death via induction of caspase-8, BAX and BAK, these agents trigger exposure of the ER-associated chaperone calreticulin on the cancer cell surface, which acts as a classical “eat me” signal for neighboring immune cells (Kepp et al., 2013). Interestingly, eIF2α phosphorylation correlates with calreticulin expression in non-small cell lung cancer (NSCLC), and this phenomenon is further associated with enhanced anti-cancer immune responses and favorable prognosis (Fucikova et al., 2016). However, XBP1 was recently demonstrated to impede ICD in metastatic colorectal cancer cells exposed to epidermal growth factor receptor blockers and chemotherapy (Pozzi et al., 2016). Plant-derived polyphenol fractions have also been shown to induce potent ICD and T cell-dependent anti-tumor activity in preclinical models of melanoma and breast cancer (Gomez-Cadena et al., 2016; Uruena et al., 2015), but whether ER stress response factors mediate these effects remains to be determined.
p97 is an AAA family ATPase that promotes the egress of misfolded proteins from the ER to the cytosol for subsequent proteasomal-mediated degradation (Wolf and Stolz, 2012). Selective p97 inhibitors have recently been found to trigger intense ER stress responses, which interfere with autophagy and induce cancer cell death (Magnaghi et al., 2013). Yet, it is unknown whether this cytotoxic strategy can stimulate ICD or whether systemic p97 inhibition impacts the optimal function of anti-tumor immune cells in cancer hosts. The magnitude of ER stress in malignant cells therefore seems to define the development of either immunosuppressive or immunogenic responses.
Intrinsic ER stress responses in cancer-associated immune cells
Overexpression of ER stress markers in multiple cancer types is associated with unfavorable prognosis and poor clinical outcome (Chen et al., 2014; Dalton et al., 2013; Davies et al., 2008; Matsuo et al., 2013; Shimizu et al., 2016). Though most of these detrimental effects have been attributed to direct pro-tumoral roles of ER stress in the cancer cell, evaluating whether ER stress responses also operate in tumor stromal cells such as leukocytes, endothelial cells or fibroblasts to influence malignant progression has recently emerged as an area of active research.
A function for the IRE1α-XBP1 branch of the UPR in cells of the immune system has been well established (Bettigole and Glimcher, 2015). IRE1α–XBP1 signaling is required for the optimal differentiation of plasma cells, some dendritic cell populations and eosinophils in cancer-free hosts (Bettigole et al., 2015; Iwakoshi et al., 2007; Reimold et al., 2001). XBP1 expression in macrophages was necessary for optimal production of IL-6 in response to Toll-like receptor (TLR) agonists, and mice devoid of XBP1 showed increased bacterial burden in models of systemic Francisella tularensis infection (Martinon et al., 2010). Neutrophils infiltrating acute lung injury lesions exhibit XBP1 overactivation compared with lung-resident neutrophils in naïve mice. In this setting, XBP1 was necessary for optimal neutrophil granule secretion and ablation of XBP1-driven ER stress responses in these myeloid cells relieved acute lung injury (Hu et al., 2015). Nevertheless, the function of IRE1α–XBP1 signaling in cancer-associated myeloid cells had not been explored.
Ovarian cancer is a highly aggressive and lethal malignancy that subverts the normal function of host DCs as a key mechanism to suppress the development of protective immune responses (Conejo-Garcia et al., 2016; Scarlett et al., 2012). We postulated that hostile conditions within the tumor itself could trigger ER stress not only in cancer cells, but also in immune cells that reside in the same adverse milieu. We found that dysfunctional DCs commonly present in the ovarian cancer microenvironment in both humans and rodents demonstrated robust expression of ER stress markers and sustained activation of the IRE1α–XBP1 arm of the UPR, compared with DCs isolated from non-tumor locations (Cubillos-Ruiz et al., 2015). Ovarian tumor-infiltrating DCs (tDCs) showed high levels of ROS that promoted intracellular lipid peroxidation and the consequential generation of byproducts such as 4-hydroxynonenal (4-HNE). This highly diffusible and reactive aldehyde modified several ER-resident chaperones and proteins in DCs, thereby disrupting ER homeostasis and triggering the UPR (Cubillos-Ruiz et al., 2015). Of note, 4-HNE has been shown to promote vascular inflammation and atherogenesis by provoking ER stress in endothelial cells (Vladykovskaya et al., 2012). Numerous cytotoxic drugs induce oxidative stress and subsequent 4-HNE generation (Velez et al., 2011), suggesting that chemotherapy might promote immunosuppressive ER stress in myeloid cells of the tumor microenvironment. Supporting this notion, the status of lipid peroxidation at the time of tumor resection has been proposed as a biomarker of disease recurrence in breast cancer patients (Herrera et al., 2014). Treatment with antioxidants that control ROS overproduction, or hydrazine derivatives capable of sequestering 4-HNE, prevented the induction of ER stress in DCs exposed to tumor-derived factors present in ovarian cancer ascites supernatants (Cubillos-Ruiz et al., 2015). Conditional deletion of Xbp1 in DCs resulted in delayed ovarian cancer progression in various models of primary and metastatic ovarian cancer, and these effects were mediated by the generation of protective T cell responses (Cubillos-Ruiz et al., 2015). XBP1 overactivation in tDCs disrupted lipid biosynthetic processes and stimulated aberrant accumulation of triglycerides, a process that was associated with reduced tDC antigen-presenting capacity. Accordingly, extensive functional assays revealed that tDCs lacking XBP1 abandoned their usual tolerogenic phenotype and became immunostimulatory cells in vivo and in situ. Interestingly, uncontrolled lipid accumulation and the generation of oxidized fatty acids have been demonstrated to be common regulatory features of tumor-infiltrating myeloid cells (Herber et al., 2010; Hossain et al., 2015; Ramakrishnan et al., 2014). Consistent with the anti-tumoral effects elicited by tDCs lacking IRE1α or XBP1, controlling receptor-mediated lipid uptake or inhibiting fatty acid oxidation can boost anti-cancer immunity by enhancing myeloid cell function in the tumor microenvironment (Herber et al., 2010; Hossain et al., 2015; Ramakrishnan et al., 2014).
The key immunoregulatory role of aberrant IRE1α-XBP1 signaling in cancer-associated myeloid cells was supported by Gabrilovich and colleagues (Condamine et al., 2016), who found that overexpression of ER stress-related gene markers and surface expression of the lectin-type oxidized LDL receptor-1 (LOX-1) could effectively distinguish high-density neutrophils from low-density immunosuppressive polymorphonuclear MDSCs (PMN-MDSCs). More importantly, pharmacological induction of ER stress using thapsigargin triggered LOX-1 upregulation in human neutrophils and simultaneously transformed them into immunosuppressive cells, a process that could be prevented by targeting IRE1α–XBP1 activation using selective IRE1α inhibitors such as B-I09 (Condamine et al., 2016; Tang et al., 2014)
Tumor-associated macrophages promote malignant progression and support chemoresistance via multiple mechanisms (Quail and Joyce, 2013). For instance, macrophage-derived cathepsins can enhance cancer cell invasion and angiogenesis in the tumor microenvironment (Olson and Joyce, 2015). IL-4 had been demonstrated to control Xbp1 expression in B cells, and this process was required for optimal IL-6 synthesis by these lymphoid cells (Iwakoshi et al., 2003). Interestingly, IL-4 was recently found to synergize with IL-6 or IL-10 to trigger IRE1α-XBP1 activation in macrophages via STAT6 and STAT3, a process that promoted cathepsin secretion (Yan et al., 2016). Intriguingly, IRE1α was shown to be required for STAT3 phosphorylation under IL-6 stimulation (Liu et al., 2015), implying a potential positive feedback loop between these two factors. Transient silencing or pharmacological inhibition of IRE1α in bone marrow-derived macrophages stimulated with IL-6 and IL-4 impaired cathepsin secretion and blunted macrophage-mediated cancer cell invasion in vitro (Yan et al., 2016). However, genetic evidence is needed to ascertain whether IRE1α-XBP1 signaling controls cathepsin secretion by tumor-associated macrophages in vivo. The IRE1α-XBP1 arm of the UPR therefore emerges as a key modulator of tumor-associated myeloid cells such as DCs, neutrophils, MDSCs and macrophages (Figure 3).
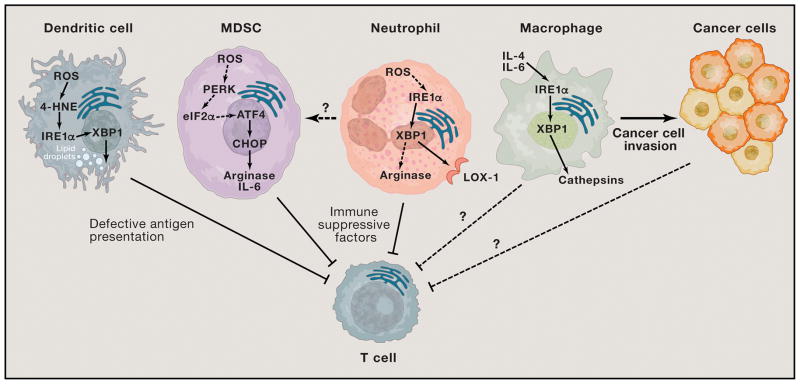
Figure 3. Effects of ER stress in cancer-associated myeloid cells.
IRE1α-XBP1 overactivation in tDCs is driven by lipid peroxidation byproducts like 4-HNE. This process disrupts their lipid metabolic homeostasis and cripples antigen presentation to T cells, thereby impeding the development of protective immune responses. Neutrophils and MDSCs use IRE1α-XBP1 and CHOP, respectively, to express factors such as Arginase that actively suppress T cell function. It is unknown whether IRE1α-XBP1 activation is required for the development of MDSCs in cancer hosts. IL-4 and IL-6 signaling triggers cathepsin expression in macrophages via IRE1α-XBP1 activation to promote cancer cell invasion. It is plausible to speculate that other tumor-associated myeloid cells, besides macrophages, could use the ER stress response to secrete factors that promote cancer cell survival and aggressiveness. Whether macrophages and/or cancer cells also use ER stress response factors to directly inhibit T cell function within tumor masses is unknown.
In addition to the protumoral role of the IRE1α-XBP1 arm in myeloid cells, the UPR downstream effector CHOP also operates as a regulator of MDSC activity and turnover in tumors (Thevenot et al., 2014). CHOP was initially found to control the polarization of macrophages into “alternatively activated” cells, and to directly regulate pro-inflammatory cytokines such as IL-23, IL-1β, and IL-6 (Chen et al., 2009; Goodall et al., 2010; Oh et al., 2012). Augmented CHOP expression was recently detected in MDSCs infiltrating mouse and human tumors, a process that directly correlated with the ability of MDSC to restrain T cell responses (Condamine et al., 2014; Thevenot et al., 2014). Chop-deficient hosts challenged with a variety of cancer types demonstrated delayed tumor growth compared with their wild-type counterparts, and these effects were mediated by the induction of protective CD8+ T cells (Thevenot et al., 2014). Notably, MDSCs isolated from tumor-bearing mice devoid of CHOP exhibited reduced immunosuppressive activity towards T cells due to defective expression of regulatory factors such as Arginase (Thevenot et al., 2014). Similar to their activating effects on IRE1α-XBP1 in tDCs (Cubillos-Ruiz et al., 2015), endogenous ROS also provoked CHOP overexpression in tumor-associated MDSCs (Thevenot et al., 2014), suggesting a conserved role for ROS in the induction of ER stress responses in cancer-associated myeloid cells.
Besides PERK, other kinases such as HRI, GCN2 and PKR can regulate the eIF2α/ATF4/CHOP axis of the UPR (Hetz et al., 2013). Thus, the precise upstream drivers of enhanced CHOP activity in cancer-associated MDSCs remain to be defined and characterized. In addition, it is unknown whether ER stress-induced PERK or ATF4 could play CHOP-independent roles that impact MDSCs or additional tumor-induced immunosuppressive mechanisms. Nonetheless, recent studies indicate that ER stress may also control MDSC survival in tumors (Condamine et al., 2014). UPR engagement was detected in tumor-infiltrating MDSCs and promoted their apoptosis through TNF-related apoptosis induced ligand receptor 2 (DR5) and caspase 8 activation (Condamine et al., 2014), suggesting that treatment with DR5 agonists could represent a potential strategy for targeting MDSCs in cancer. Notably, tumor-infiltrating MDSCs devoid of CHOP demonstrated delayed apoptosis and prolonged survival rates, indicating that CHOP acts as a key regulator of MDSC turnover in vivo (Thevenot et al., 2014).
Taken together, these recent findings suggest that ER stress responses driven by IRE1α-XBP1 signaling and CHOP are crucial modulators of myeloid cell activity and survival in tumors (Figure 3). It remains unknown whether the ATF6α branch of the UPR also contributes to myeloid cell dysfunction in cancer. Furthermore, whether ER stress responses also operate in other major cell types of the tumor microenvironment such as T cells, fibroblasts and endothelial cells remains to be tested.
THERAPEUTIC STRATEGIES TO CONTROL ER STRESS RESPONSES IN CANCER
Pharmacological inhibitors
Sustained IRE1α-XBP1 signaling promotes cancer cell-intrinsic growth, metastasis and chemoresistance, but its surprising role as a key modulator of myeloid cell function in tumors emerges as an attractive target for cancer immunotherapy. While direct pharmacological inhibition of nuclear XBP1 is difficult due to major technical limitations, targeting its upstream activator, IRE1α, represents a viable strategy. Indeed, the dual enzyme IRE1α is amenable to small molecule targeting, and two classes of direct inhibitors have been identified. The first group of compounds directly targets the IRE1α endoribonuclease domain, and some examples of this class include toyocamycin (Ri et al., 2012), STF-083010 (Papandreou et al., 2011), 4μ8C (Cross et al., 2012), MKC-3946 (Mimura et al., 2012), and B-I09 (Tang et al., 2014) (Figure 4). Notably, these direct IRE1α endonuclease inhibitors were capable of blocking Xbp1 splicing without affecting IRE1α phosphorylation or the PERK and ATF6α arms of the UPR. STF-083010, MKC-3946 and toyocamycin have demonstrated therapeutic efficacy in multiple myeloma xenograft models, and B-I09 has been shown to control the aggressiveness of chronic lymphocytic leukemia cells in vivo (Tang et al., 2014). Importantly, daily intraperitoneal administration of 4μ8C substantially decreased pathological joint swelling in the KBxN serum transfer murine model of rheumatoid arthritis (Qiu et al., 2013). This suggests that this class of inhibitors could be also used in cancer hosts to modulate the function of intratumoral myeloid cells.
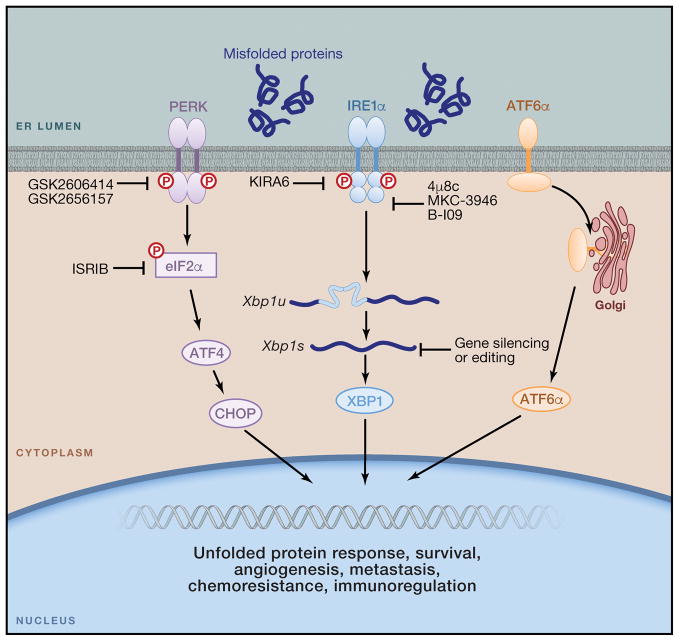
Figure 4. Therapeutic strategies to control ER stress responses in cancer.
The IRE1α kinase domain can be inactivated using compounds like KIRA6, leading to allosteric inhibition of the IRE1α RNAse domain. Other compounds such as 4μ8c, MKC-3946 and B-I09 can directly inhibit the IRE1α RNAse domain to prevent splicing of the Xbp1 mRNA. Treatment with these IRE1α inhibitory compounds could be effective to reduce tolerance to hypoxia, angiogenesis, drug resistance and metastatic capacity by cancer cells. These compounds could also be used to reprogram the function of cancer-associated myeloid cells, including macrophages, DCs and neutrophils. Furthermore, siRNA-loaded nanoparticles could be effective for selectively silencing ERN1 or XBP1 in ovarian cancer-associated DCs. Novel genome-editing technologies could be used to ablate IRE1α-XBP1 signaling in DC-based therapeutic vaccines for cancer. Recently developed inhibitors of PERK and eIF2α could be exploited to control CHOP overexpression by MDSCs and relieve immunosuppression in the tumor microenvironment. However, CHOP-independent roles of PERK/eIF2α in MDSCs have not been explored. Whether the ATF6α arm of the UPR also operates as a modulator of immune cell function in tumors has not been determined.
The second group of inhibitors targets the IRE1α kinase domain in order to allosterically disrupt its endoribonuclease function (Figure 4). A recent compound in this category is KIRA6 (Ghosh et al., 2014), which reduced plasma glucose levels and improved glucose tolerance in Ins2+/Akita mice that exhibit chronic ER stress in pancreatic β-cells (Ghosh et al., 2014). Moreover, intravitreal KIRA6 injection in the P23H transgenic rat model of retinitis pigmentosa preserved photoreceptor viability and function (Ghosh et al., 2014). Nevertheless, it has not been determined whether treatment with IRE1α inhibitors fully recapitulates the biological effects of IRE1α genetic ablation. Developing novel IRE1α inhibitors with potent in vivo efficacy in the tumor microenvironment could therefore be useful to directly restrain cancer cell survival, metastasis and chemoresistance while eliciting protective anti-tumor immune responses via myeloid cell reprogramming.
Since targeting CHOP in the nucleus using small molecule inhibitors would also involve major technical challenges, restraining the activity of its upstream activators, PERK or eIF2α, may represent a more practical approach (Figure 4). GSK2606414 was the first reported PERK inhibitor (Axten et al., 2012) and was found to be neuroprotective in mouse models of prion disease (Moreno et al., 2013). Another ATP-competitive inhibitor of PERK enzymatic activity, GSK2656157, was shown to impede ER stress-induced PERK autophosphorylation, eIF2α phosphorylation and subsequent overexpression of ATF4 and CHOP in multiple cell lines (Atkins et al., 2013). Oral administration of GSK2656157 to mice impaired PERK autophosphorylation in the pancreas and compromised xenograft tumor growth in immunodeficient hosts (Atkins et al., 2013). However, further studies indicate that inhibition of PERK activity by GSK2656157 does not always correlate with reduced eIF2α phosphorylation, and that this inhibitor fails to recreate the biological effects of PERK genetic inactivation (Krishnamoorthy et al., 2014). The Integrated Stress Response inhibitor (ISRIB) is a symmetric bisglycolamide that renders cells resistant to eIF2α phosphorylation, thereby blocking the activation of ATF4 and the accumulation of CHOP during conditions of ER stress (Sidrauski et al., 2015). Importantly, this compound showed significant in vivo effects by enhancing spatial and fear-associated learning in rodents (Sidrauski et al., 2015). Whether GSK2656157 or ISRIB could modulate the function or survival of MDSCs in the tumor microenvironment by impeding PERK/eIF2α-mediated CHOP activation is yet to be tested. Given the importance of PERK and IRE1α-XBP1 signaling in organ homeostasis of highly secretory tissues, careful optimization of these inhibitory compounds for in vivo use is essential to minimize potential side effects and toxicity in treated hosts.
Ceapins, a new class of pyrazole amides, were recently demonstrated to specifically inhibit the ATF6α branch of the UPR by blocking ATF6α processing and nuclear translocation in cells undergoing ER stress (Gallagher and Walter, 2016). Further optimization of Ceapins for in vivo use will hence be critical for determining the tumoricidal activity of these compounds alone or in combination with other agents that ablate PERK and/or IRE1α signaling in the tumor microenvironment. Additionally, developing new pharmacological interventions capable of triggering lethal ER stress and subsequent ICD selectively in malignant cells could also be useful for eliciting robust anti-tumor immune responses.
Controlling immune cell-intrinsic ER stress responses
Gene targeting strategies have proven effective for therapeutically disabling detrimental IRE1α-XBP1 signaling in DCs of cancer hosts (Cubillos-Ruiz et al., 2015). In the ovarian cancer microenvironment, DCs exhibit a remarkable phagocytic capacity that renders them exceptional targets for nanoparticle-mediated RNAi therapeutics (Cubillos-Ruiz et al., 2012; Cubillos-Ruiz et al., 2009). Since ovarian cancer metastasis and malignant ascites accumulation is confined within the peritoneal cavity, administration of DC-targeting siRNA-loaded nanocarriers in this anatomical location represents a novel and feasible immunotherapeutic strategy. In preclinical models of metastatic ovarian cancer, silencing Xbp1 or Ern1 using this approach transformed tolerogenic tDCs into highly immunostimulatory cells that extended host survival by evoking T cell-mediated anti-tumor immunity (Cubillos-Ruiz et al., 2015).
As a second strategy, we propose that IRE1α-XBP1 signaling could be genetically interrupted to enhance the efficacy of DC-based therapeutic vaccines in ovarian cancer, which unfortunately have shown limited success in recent clinical trials (Kandalaft et al., 2013). In proof-of-concept experiments, we found that transferring Xbp1-deficient BMDCs intraperitoneally into mice bearing established ovarian cancer significantly delayed tumor progression compared with infusion of wild type BMDCs (Cubillos-Ruiz et al., 2015). Notably, transplanted Xbp1-deficient DCs were dominantly immunostimulatory over the endogenous (wild type) regulatory DCs residing in the tumor microenvironment. Cutting-edge genome editing technologies such as CRISPR/Cas9, zinc finger nucleases, or TALENs (Gaj et al., 2013) should therefore enable precise and efficient inactivation of XBP1 or ERN1 in DCs prior to adoptive transfer (Figure 4), thereby protecting these transplanted DCs from the suppressive effects of aberrant ER stress responses in the tumor microenvironment. Interestingly, transplanted CHOP-deficient MDSCs also show enhanced antigen-presenting capacity and potent T cell stimulatory capacity (Thevenot et al., 2014), suggesting that the gene-targeting strategies described above may also be useful to re-program MDSC function in tumors.
CONCLUDING REMARKS
Tumors thrive under adverse conditions such as hypoxia, nutrient starvation and oxidative stress by adjusting their protein folding capacity via the ER stress response pathway. Activation of multiple ER stress sensors has been demonstrated to endow malignant cells with greater tumorigenic, metastatic and drug resistant capacity. However, recent studies have uncovered a second mechanism by which abnormal ER stress responses promote malignant progression: by subverting the protective function of innate immune cells in the tumor microenvironment to cripple the development of anti-tumor immunity. Harnessing the intrinsic ability of our immune system to recognize and eliminate malignant cells represents an extraordinarily promising anti-cancer strategy especially when combined with recently developed therapeutics such as Gleevec that precisely target genetic driver mutations in the tumor itself. Together, these two approaches offer the most exciting opportunities for cancer treatment since the development of chemotherapy. However, hostile microenvironmental conditions within aggressive solid tumors inhibit the optimal activity of protective immune cells. Targeting immunosuppression and re-programming immune cell function in the tumor microenvironment are fundamental requirements for developing successful cancer immunotherapies. Abnormal ER stress responses emerge as critical regulators of immune cell function in the tumor microenvironment and appear to integrate protumoral and immunosuppressive mechanisms in cancer hosts. Therefore, identifying, understanding and disabling the precise molecular mechanisms by which ER stress inhibits the natural function of innate immune cells in tumors could be a novel approach to complement and enhance the efficacy of both standard chemotherapies and evolving cancer immunotherapies such as checkpoint blockade and adoptive T cell transfer in the clinic.
Acknowledgments
Our research was supported by the Irvington Institute Fellowship Program of the Cancer Research Institute (J.R.C-R), the Ann Schreiber Mentored Investigator Award of the Ovarian Cancer Research Fund Alliance (J.R.C-R), the Ovarian Cancer Academy–Early-Career Investigator Award of the Department of Defense (J.R.C-R), the Stand Up to Cancer Innovative Research Grant (J.R.C-R), the Clinic and Laboratory Integration Program of the Cancer Research Institute (J.R.C-R), Weill Cornell Medical College Funds (L.H.G.) and NIH grant R01CA112663 (L.H.G.). We apologize to colleagues whose work was not cited in this review due to space limitations.
Footnotes
Disclosure of Potential Conflicts of Interest: J.R.C-R and L.H.G. are co-founders of and scientific advisors for Quentis Therapeutics, Inc. S.E.B. is co-founder and employee of Quentis Therapeutics, Inc. L.H.G. also serves on the board of directors of and holds equity in Bristol-Myers Squibb.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- Atkins C, Liu Q, Minthorn E, Zhang SY, Figueroa DJ, Moss K, Stanley TB, Sanders B, Goetz A, Gaul N, et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73:1993–2002. doi: 10.1158/0008-5472.CAN-12-3109. [DOI] [PubMed] [Google Scholar]
- Auf G, Jabouille A, Guerit S, Pineau R, Delugin M, Bouchecareilh M, Magnin N, Favereaux A, Maitre M, Gaiser T, et al. Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Proc Natl Acad Sci U S A. 2010;107:15553–15558. doi: 10.1073/pnas.0914072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, Aguirre-Ghiso JA. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Molecular and cellular biology. 2011;31:3616–3629. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, Li WH, Heerding DA, Minthorn E, Mencken T, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) Journal of medicinal chemistry. 2012;55:7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- B’Chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P, Bruhat A. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic acids research. 2013;41:7683–7699. doi: 10.1093/nar/gkt563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkowiak K, Effenberger KE, Harder S, Andreas A, Buck F, Peter-Katalinic J, Pantel K, Brandt BH. Discovery of a novel unfolded protein response phenotype of cancer stem/progenitor cells from the bone marrow of breast cancer patients. Journal of proteome research. 2010;9:3158–3168. doi: 10.1021/pr100039d. [DOI] [PubMed] [Google Scholar]
- Bartkowiak K, Kwiatkowski M, Buck F, Gorges TM, Nilse L, Assmann V, Andreas A, Muller V, Wikman H, Riethdorf S, et al. Disseminated Tumor Cells Persist in the Bone Marrow of Breast Cancer Patients through Sustained Activation of the Unfolded Protein Response. Cancer research. 2015;75:5367–5377. doi: 10.1158/0008-5472.CAN-14-3728. [DOI] [PubMed] [Google Scholar]
- Beck D, Niessner H, Smalley KS, Flaherty K, Paraiso KH, Busch C, Sinnberg T, Vasseur S, Iovanna JL, Driessen S, et al. Vemurafenib potently induces endoplasmic reticulum stress-mediated apoptosis in BRAFV600E melanoma cells. Science signaling. 2013;6:ra7. doi: 10.1126/scisignal.2003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS biology. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nature cell biology. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Bettigole SE, Glimcher LH. Endoplasmic reticulum stress in immunity. Annu Rev Immunol. 2015;33:107–138. doi: 10.1146/annurev-immunol-032414-112116. [DOI] [PubMed] [Google Scholar]
- Bettigole SE, Lis R, Adoro S, Lee AH, Spencer LA, Weller PF, Glimcher LH. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat Immunol. 2015;16:829–837. doi: 10.1038/ni.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I, Varia M, Raleigh J, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. The EMBO journal. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais JD, Addison CL, Edge R, Falls T, Zhao H, Wary K, Koumenis C, Harding HP, Ron D, Holcik M, et al. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Molecular and cellular biology. 2006;26:9517–9532. doi: 10.1128/MCB.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerezo M, Lehraiki A, Millet A, Rouaud F, Plaisant M, Jaune E, Botton T, Ronco C, Abbe P, Amdouni H, et al. Compounds Triggering ER Stress Exert Anti-Melanoma Effects and Overcome BRAF Inhibitor Resistance. Cancer cell. 2016;29:805–819. doi: 10.1016/j.ccell.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Chen L, Jarujaron S, Wu X, Sun L, Zha W, Liang G, Wang X, Gurley EC, Studer EJ, Hylemon PB, et al. HIV protease inhibitor lopinavir-induced TNF-alpha and IL-6 expression is coupled to the unfolded protein response and ERK signaling pathways in macrophages. Biochem Pharmacol. 2009;78:70–77. doi: 10.1016/j.bcp.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Iliopoulos D, Zhang Q, Tang Q, Greenblatt MB, Hatziapostolou M, Lim E, Tam WL, Ni M, Chen Y, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature. 2014;508:103–107. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis NS, Pytel D, Bobrovnikova-Marjon E, Pant D, Zheng H, Maas NL, Frederick B, Kushner JA, Chodosh LA, Koumenis C, et al. miR-211 is a prosurvival microRNA that regulates chop expression in a PERK-dependent manner. Molecular cell. 2012;48:353–364. doi: 10.1016/j.molcel.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine T, Dominguez GA, Youn JI, VKA, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Science Immunology. 2016;1:aaf8943. doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine T, Kumar V, Ramachandran IR, Youn JI, Celis E, Finnberg N, El-Deiry WS, Winograd R, Vonderheide RH, English NR, et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. JClinInvest. 2014;124:2626–2639. doi: 10.1172/JCI74056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejo-Garcia JR, Rutkowski MR, Cubillos-Ruiz JR. State-of-the-art of regulatory dendritic cells in cancer. Pharmacology & therapeutics. 2016;164:97–104. doi: 10.1016/j.pharmthera.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corazzari M, Rapino F, Ciccosanti F, Giglio P, Antonioli M, Conti B, Fimia GM, Lovat PE, Piacentini M. Oncogenic BRAF induces chronic ER stress condition resulting in increased basal autophagy and apoptotic resistance of cutaneous melanoma. Cell death and differentiation. 2015;22:946–958. doi: 10.1038/cdd.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Current opinion in immunology. 2013;25:214–221. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, Silverman RH, Neubert TA, Baxendale IR, Ron D, et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E869–878. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Baird JR, Tesone AJ, Rutkowski MR, Scarlett UK, Camposeco-Jacobs AL, Anadon-Arnillas J, Harwood NM, Korc M, Fiering SN, et al. Reprogramming tumor-associated dendritic cells in vivo using miRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. 2012;72:1683–1693. doi: 10.1158/0008-5472.CAN-11-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Fiering S, Conejo-Garcia JR. Nanomolecular targeting of dendritic cells for ovarian cancer therapy. Future Oncol. 2009;5:1189–1192. doi: 10.2217/fon.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell. 2015;161:1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Molecular and cellular biology. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton LE, Clarke HJ, Knight J, Lawson MH, Wason J, Lomas DA, Howat WJ, Rintoul RC, Rassl DM, Marciniak SJ. The endoplasmic reticulum stress marker CHOP predicts survival in malignant mesothelioma. Br J Cancer. 2013;108:1340–1347. doi: 10.1038/bjc.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MP, Barraclough DL, Stewart C, Joyce KA, Eccles RM, Barraclough R, Rudland PS, Sibson DR. Expression and splicing of the unfolded protein response gene XBP-1 are significantly associated with clinical outcome of endocrine-treated breast cancer. International journal of cancer. 2008;123:85–88. doi: 10.1002/ijc.23479. [DOI] [PubMed] [Google Scholar]
- De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28:519–524. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Denoyelle C, Abou-Rjaily G, Bezrookove V, Verhaegen M, Johnson TM, Fullen DR, Pointer JN, Gruber SB, Su LD, Nikiforov MA, et al. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nature cell biology. 2006;8:1053–1063. doi: 10.1038/ncb1471. [DOI] [PubMed] [Google Scholar]
- Dey S, Sayers CM, Verginadis II, Lehman SL, Cheng Y, Cerniglia GJ, Tuttle SW, Feldman MD, Zhang PJ, Fuchs SY, et al. ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. The Journal of clinical investigation. 2015;125:2592–2608. doi: 10.1172/JCI78031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YX, Sokol ES, Del Vecchio CA, Sanduja S, Claessen JH, Proia TA, Jin DX, Reinhardt F, Ploegh HL, Wang Q, et al. Epithelial-to-mesenchymal transition activates PERK-eIF2alpha and sensitizes cells to endoplasmic reticulum stress. Cancer discovery. 2014;4:702–715. doi: 10.1158/2159-8290.CD-13-0945. [DOI] [PubMed] [Google Scholar]
- Fucikova J, Becht E, Iribarren K, Goc J, Remark R, Damotte D, Alifano M, Devi P, Biton J, Germain C, et al. Calreticulin Expression in Human Non-Small Cell Lung Cancers Correlates with Increased Accumulation of Antitumor Immune Cells and Favorable Prognosis. Cancer Res. 2016;76:1746–1756. doi: 10.1158/0008-5472.CAN-15-1142. [DOI] [PubMed] [Google Scholar]
- Gaj T, Gersbach CA, Barbas CF., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends in biotechnology. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher CM, Walter P. Ceapins inhibit ATF6alpha signaling by selectively preventing transport of ATF6alpha to the Golgi apparatus during ER stress. eLife. 2016:5. doi: 10.7554/eLife.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, Urano F. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PloS one. 2010;5:e9575. doi: 10.1371/journal.pone.0009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Wang L, Wang ES, Perera BG, Igbaria A, Morita S, Prado K, Thamsen M, Caswell D, Macias H, et al. Allosteric inhibition of the IRE1alpha RNase preserves cell viability and function during endoplasmic reticulum stress. Cell. 2014;158:534–548. doi: 10.1016/j.cell.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cadena A, Uruena C, Prieto K, Martinez-Usatorre A, Donda A, Barreto A, Romero P, Fiorentino S. Immune-system-dependent anti-tumor activity of a plant-derived polyphenol rich fraction in a melanoma mouse model. Cell death & disease. 2016;7:e2243. doi: 10.1038/cddis.2016.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall JC, Wu C, Zhang Y, McNeill L, Ellis L, Saudek V, Gaston JS. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17698–17703. doi: 10.1073/pnas.1011736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine & growth factor reviews. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nature cell biology. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, Qiu B, Zhang H, Cerniglia G, Bi M, et al. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. The Journal of clinical investigation. 2012;122:4621–4634. doi: 10.1172/JCI62973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera AC, Victorino VJ, Campos FC, Verenitach BD, Lemos LT, Aranome AM, Oliveira SR, Cecchini AL, Simao AN, Abdelhay E, et al. Impact of tumor removal on the systemic oxidative profile of patients with breast cancer discloses lipid peroxidation at diagnosis as a putative marker of disease recurrence. Clinical breast cancer. 2014;14:451–459. doi: 10.1016/j.clbc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nature reviews Molecular cell biology. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nature reviews Drug discovery. 2013;12:703–719. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T, Zou W, et al. Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer immunology research. 2015;3:1236–1247. doi: 10.1158/2326-6066.CIR-15-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Chen ZF, Yan J, Li QF, Huang Y, Xu H, Zhang XP, Jiang H. Endoplasmic Reticulum Stress of Neutrophils Is Required for Ischemia/Reperfusion-Induced Acute Lung Injury. J Immunol. 2015;195:4802–4809. doi: 10.4049/jimmunol.1500073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AL, Lebeau J, Guillaumot P, Petrilli V, Malek M, Chilloux J, Fauvet F, Payen L, Kfoury A, Renno T, et al. p58(IPK)-mediated attenuation of the proapoptotic PERK-CHOP pathway allows malignant progression upon low glucose. Molecular cell. 2013;49:1049–1059. doi: 10.1016/j.molcel.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4:321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- Iwakoshi NN, Pypaert M, Glimcher LH. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J Exp Med. 2007;204:2267–2275. doi: 10.1084/jem.20070525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabouille A, Delugin M, Pineau R, Dubrac A, Soulet F, Lhomond S, Pallares-Lupon N, Prats H, Bikfalvi A, Chevet E, et al. Glioblastoma invasion and cooption depend on IRE1alpha endoribonuclease activity. Oncotarget. 2015;6:24922–24934. doi: 10.18632/oncotarget.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YJ, Khelifa S, Ratnikov B, Scott DA, Feng Y, Parisi F, Ruller C, Lau E, Kim H, Brill LM, et al. Regulation of glutamine carrier proteins by RNF5 determines breast cancer response to ER stress-inducing chemotherapies. Cancer cell. 2015;27:354–369. doi: 10.1016/j.ccell.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandalaft LE, Powell DJ, Jr, Chiang CL, Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Torigian DA, et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology. 2013;2:e22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karali E, Bellou S, Stellas D, Klinakis A, Murphy C, Fotsis T. VEGF Signals through ATF6 and PERK to promote endothelial cell survival and angiogenesis in the absence of ER stress. Molecular cell. 2014;54:559–572. doi: 10.1016/j.molcel.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Kepp O, Menger L, Vacchelli E, Locher C, Adjemian S, Yamazaki T, Martins I, Sukkurwala AQ, Michaud M, Senovilla L, et al. Crosstalk between ER stress and immunogenic cell death. Cytokine & growth factor reviews. 2013;24:311–318. doi: 10.1016/j.cytogfr.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Kitai Y, Ariyama H, Kono N, Oikawa D, Iwawaki T, Arai H. Membrane lipid saturation activates IRE1alpha without inducing clustering. Genes to cells : devoted to molecular & cellular mechanisms. 2013;18:798–809. doi: 10.1111/gtc.12074. [DOI] [PubMed] [Google Scholar]
- Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell death and differentiation. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy J, Rajesh K, Mirzajani F, Kesoglidou P, Papadakis AI, Koromilas AE. Evidence for eIF2alpha phosphorylation-independent effects of GSK2656157, a novel catalytic inhibitor of PERK with clinical implications. Cell Cycle. 2014;13:801–806. doi: 10.4161/cc.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci U S A. 2003;100:9946–9951. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Chang SY, Hong EH, Kwon BE, Kim HM, Kim YJ, Lee J, Cho HJ, Cheon JH, Ko HJ. Elevated endoplasmic reticulum stress reinforced immunosuppression in the tumor microenvironment via myeloid-derived suppressor cells. Oncotarget. 2014;5:12331–12345. doi: 10.18632/oncotarget.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M, et al. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16:250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung-Hagesteijn C, Erdmann N, Cheung G, Keats JJ, Stewart AK, Reece DE, Chung KC, Tiedemann RE. Xbp1s-negative tumor B cells and pre-plasmablasts mediate therapeutic proteasome inhibitor resistance in multiple myeloma. Cancer cell. 2013;24:289–304. doi: 10.1016/j.ccr.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shao M, Wu Y, Yan C, Jiang S, Liu J, Dai J, Yang L, Li J, Jia W, et al. Role for the endoplasmic reticulum stress sensor IRE1alpha in liver regenerative responses. Journal of hepatology. 2015;62:590–598. doi: 10.1016/j.jhep.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Lu Y, Liang FX, Wang X. A synthetic biology approach identifies the mammalian UPR RNA ligase RtcB. Molecular cell. 2014;55:758–770. doi: 10.1016/j.molcel.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XH, Piao SF, Dey S, McAfee Q, Karakousis G, Villanueva J, Hart LS, Levi S, Hu J, Zhang G, et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. The Journal of clinical investigation. 2014;124:1406–1417. doi: 10.1172/JCI70454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnaghi P, D’Alessio R, Valsasina B, Avanzi N, Rizzi S, Asa D, Gasparri F, Cozzi L, Cucchi U, Orrenius C, et al. Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nature chemical biology. 2013;9:548–556. doi: 10.1038/nchembio.1313. [DOI] [PubMed] [Google Scholar]
- Mahadevan NR, Anufreichik V, Rodvold JJ, Chiu KT, Sepulveda H, Zanetti M. Cell-extrinsic effects of tumor ER stress imprint myeloid dendritic cells and impair CD8(+) T cell priming. PLoS One. 2012;7:e51845. doi: 10.1371/journal.pone.0051845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, Zanetti M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. ProcNatlAcadSciUSA. 2011;108:6561–6566. doi: 10.1073/pnas.1008942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Marada S, Stewart DP, Bodeen WJ, Han YG, Ogden SK. The unfolded protein response selectively targets active smoothened mutants. Molecular and cellular biology. 2013;33:2375–2387. doi: 10.1128/MCB.01445-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Gray MJ, Yang DY, Srivastava SA, Tripathi PB, Sonoda LA, Yoo EJ, Dubeau L, Lee AS, Lin YG. The endoplasmic reticulum stress marker, glucose-regulated protein-78 (GRP78) in visceral adipocytes predicts endometrial cancer progression and patient survival. Gynecologic oncology. 2013;128:552–559. doi: 10.1016/j.ygyno.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura N, Fulciniti M, Gorgun G, Tai YT, Cirstea D, Santo L, Hu Y, Fabre C, Minami J, Ohguchi H, et al. Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood. 2012;119:5772–5781. doi: 10.1182/blood-2011-07-366633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Halliday M, Molloy C, Radford H, Verity N, Axten JM, Ortori CA, Willis AE, Fischer PM, Barrett DA, et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med. 2013;5:206ra138. doi: 10.1126/scitranslmed.3006767. [DOI] [PubMed] [Google Scholar]
- Mujcic H, Nagelkerke A, Rouschop KM, Chung S, Chaudary N, Span PN, Clarke B, Milosevic M, Sykes J, Hill RP, et al. Hypoxic activation of the PERK/eIF2alpha arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6126–6137. doi: 10.1158/1078-0432.CCR-13-0526. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Umemura A, Taniguchi K, Font-Burgada J, Dhar D, Ogata H, Zhong Z, Valasek MA, Seki E, Hidalgo J, et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer cell. 2014;26:331–343. doi: 10.1016/j.ccr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba T, Chu K, Kodama R, Byun S, Yoon KW, Hiraki M, Mandinova A, Lee SW. Loss of p53 enhances the function of the endoplasmic reticulum through activation of the IRE1alpha/XBP1 pathway. Oncotarget. 2015;6:19990–20001. doi: 10.18632/oncotarget.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Molecular and cellular biology. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Riek AE, Weng S, Petty M, Kim D, Colonna M, Cella M, Bernal-Mizrachi C. Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J Biol Chem. 2012;287:11629–11641. doi: 10.1074/jbc.M111.338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson OC, Joyce JA. Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat Rev Cancer. 2015;15:712–729. doi: 10.1038/nrc4027. [DOI] [PubMed] [Google Scholar]
- Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, Solow-Cordero DE, Bouley DM, Offner F, Niwa M, et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira ER, Liao N, Neale GA, Hendershot LM. Transcriptional and post-transcriptional regulation of proangiogenic factors by the unfolded protein response. PloS one. 2010:5. doi: 10.1371/journal.pone.0012521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol J, Vacchelli E, Aranda F, Castoldi F, Eggermont A, Cremer I, Sautes-Fridman C, Fucikova J, Galon J, Spisek R, et al. Trial Watch: Immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4:e1008866. doi: 10.1080/2162402X.2015.1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi C, Cuomo A, Spadoni I, Magni E, Silvola A, Conte A, Sigismund S, Ravenda PS, Bonaldi T, Zampino MG, et al. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat Med. 2016;22:624–631. doi: 10.1038/nm.4078. [DOI] [PubMed] [Google Scholar]
- Qiu Q, Zheng Z, Chang L, Zhao YS, Tan C, Dandekar A, Zhang Z, Lin Z, Gui M, Li X, et al. Toll-like receptor-mediated IRE1alpha activation as a therapeutic target for inflammatory arthritis. The EMBO journal. 2013;32:2477–2490. doi: 10.1038/emboj.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan R, Tyurin VA, Veglia F, Condamine T, Amoscato A, Mohammadyani D, Johnson JJ, Zhang LM, Klein-Seetharaman J, Celis E, et al. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immunol. 2014;192:2920–2931. doi: 10.4049/jimmunol.1302801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan AC, Zhang L, Adam AP, Aguirre-Ghiso JA. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer research. 2006;66:1702–1711. doi: 10.1158/0008-5472.CAN-05-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- Ri M, Tashiro E, Oikawa D, Shinjo S, Tokuda M, Yokouchi Y, Narita T, Masaki A, Ito A, Ding J, et al. Identification of Toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing. Blood cancer journal. 2012;2:e79. doi: 10.1038/bcj.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, Mori K, Glimcher LH, Denko NC, Giaccia AJ, et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64:5943–5947. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- Rouschop KM, Dubois LJ, Keulers TG, van den Beucken T, Lambin P, Bussink J, van der Kogel AJ, Koritzinsky M, Wouters BG. PERK/eIF2alpha signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4622–4627. doi: 10.1073/pnas.1210633110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS biology. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu N, Dela Cruz D, Gao M, Sandoval W, Haverty PM, Liu J, Stephan JP, Haley B, Classon M, Hatzivassiliou G, et al. Proline Starvation Induces Unresolved ER Stress and Hinders mTORC1-Dependent Tumorigenesis. Cell Metab. 2016 doi: 10.1016/j.cmet.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, Cubillos-Ruiz JR, Jacobs AC, Gonzalez JL, Weaver J, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012 doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schewe DM, Aguirre-Ghiso JA. ATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10519–10524. doi: 10.1073/pnas.0800939105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AJ, Schekman R. In vitro reconstitution of ER-stress induced ATF6 transport in COPII vesicles. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17775–17780. doi: 10.1073/pnas.0910342106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, Galluzzi L, Adjemian S, Kepp O, Niso-Santano M, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337:1678–1684. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]
- Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Developmental cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- Shi YH, Ding ZB, Zhou J, Hui B, Shi GM, Ke AW, Wang XY, Dai Z, Peng YF, Gu CY, et al. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy. 2011;7:1159–1172. doi: 10.4161/auto.7.10.16818. [DOI] [PubMed] [Google Scholar]
- Shimizu A, Kaira K, Yasuda M, Asao T, Ishikawa O. Clinical and Pathological Significance of ER Stress Marker (BiP/GRP78 and PERK) Expression in Malignant Melanoma. Pathology oncology research : POR. 2016 doi: 10.1007/s12253-016-0099-9. [DOI] [PubMed] [Google Scholar]
- Shoulders MD, Ryno LM, Genereux JC, Moresco JJ, Tu PG, Wu C, Yates JR, 3rd, Su AI, Kelly JW, Wiseman RL. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell reports. 2013;3:1279–1292. doi: 10.1016/j.celrep.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C, McGeachy AM, Ingolia NT, Walter P. The small molecule ISRIB reverses the effects of eIF2alpha phosphorylation on translation and stress granule assembly. eLife. 2015:4. doi: 10.7554/eLife.05033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signer RA, Magee JA, Salic A, Morrison SJ. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature. 2014;509:49–54. doi: 10.1038/nature13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, Han W, Lou F, Yang J, Zhang Q, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell death & disease. 2013;4:e838. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam AB, Mercado EL, Hoffmann A, Niwa M. ER stress activates NF-kappaB by integrating functions of basal IKK activity, IRE1 and PERK. PloS one. 2012;7:e45078. doi: 10.1371/journal.pone.0045078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CH, Ranatunga S, Kriss CL, Cubitt CL, Tao J, Pinilla-Ibarz JA, Del Valle JR, Hu CC. Inhibition of ER stress-associated IRE-1/XBP-1 pathway reduces leukemic cell survival. The Journal of clinical investigation. 2014;124:2585–2598. doi: 10.1172/JCI73448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenot PT, Sierra RA, Raber PL, Al-Khami AA, Trillo-Tinoco J, Zarreii P, Ochoa AC, Cui Y, Del VL, Rodriguez PC. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity. 2014;41:389–401. doi: 10.1016/j.immuni.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JP, Wang L, Han D, Wang ES, Huskey NE, Lim L, Truitt M, McManus MT, Ruggero D, Goga A, et al. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science. 2012;338:818–822. doi: 10.1126/science.1226191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Uruena C, Gomez A, Sandoval T, Hernandez J, Li S, Barreto A, Fiorentino S. Multifunctional T Lymphocytes Generated After Therapy With an Antitumor Gallotanin-Rich Normalized Fraction Are Related to Primary Tumor Size Reduction in a Breast Cancer Model. Integrative cancer therapies. 2015;14:468–483. doi: 10.1177/1534735415596425. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer research. 1989;49:6449–6465. [PubMed] [Google Scholar]
- Velez JM, Miriyala S, Nithipongvanitch R, Noel T, Plabplueng CD, Oberley T, Jungsuwadee P, Van Remmen H, Vore M, St Clair DK. p53 Regulates oxidative stress-mediated retrograde signaling: a novel mechanism for chemotherapy-induced cardiac injury. PLoS One. 2011;6:e18005. doi: 10.1371/journal.pone.0018005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladykovskaya E, Sithu SD, Haberzettl P, Wickramasinghe NS, Merchant ML, Hill BG, McCracken J, Agarwal A, Dougherty S, Gordon SA, et al. Lipid peroxidation product 4-hydroxy-trans-2-nonenal causes endothelial activation by inducing endoplasmic reticulum stress. J Biol Chem. 2012;287:11398–11409. doi: 10.1074/jbc.M111.320416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volmer R, van der Ploeg K, Ron D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4628–4633. doi: 10.1073/pnas.1217611110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nature reviews Cancer. 2014;14:581–597. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- Wang Y, Alam GN, Ning Y, Visioli F, Dong Z, Nor JE, Polverini PJ. The unfolded protein response induces the angiogenic switch in human tumor cells through the PERK/ATF4 pathway. Cancer research. 2012;72:5396–5406. doi: 10.1158/0008-5472.CAN-12-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH, Stolz A. The Cdc48 machine in endoplasmic reticulum associated protein degradation. Biochimica et biophysica acta. 2012;1823:117–124. doi: 10.1016/j.bbamcr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Xie WY, Zhou XD, Li Q, Chen LX, Ran DH. Acid-induced autophagy protects human lung cancer cells from apoptosis by activating ER stress. Experimental cell research. 2015;339:270–279. doi: 10.1016/j.yexcr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Yaari-Stark S, Shaked M, Nevo-Caspi Y, Jacob-Hircsh J, Shamir R, Rechavi G, Kloog Y. Ras inhibits endoplasmic reticulum stress in human cancer cells with amplified Myc. International journal of cancer. 2010;126:2268–2281. doi: 10.1002/ijc.25102. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Takahara K, Oyadomari S, Okada T, Sato T, Harada A, Mori K. Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Molecular biology of the cell. 2010;21:2975–2986. doi: 10.1091/mbc.E09-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Wang HW, Bowman RL, Joyce JA. STAT3 and STAT6 Signaling Pathways Synergize to Promote Cathepsin Secretion from Macrophages via IRE1alpha Activation. Cell reports. 2016;16:2914–2927. doi: 10.1016/j.celrep.2016.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Young RM, Ackerman D, Quinn ZL, Mancuso A, Gruber M, Liu L, Giannoukos DN, Bobrovnikova-Marjon E, Diehl JA, Keith B, et al. Dysregulated mTORC1 renders cells critically dependent on desaturated lipids for survival under tumor-like stress. Genes & development. 2013;27:1115–1131. doi: 10.1101/gad.198630.112. [DOI] [PMC free article] [PubMed] [Google Scholar]