Abstract
Importance
While some countries have implemented widespread colonoscopy screening, most European countries have not introduced it because of uncertainty with regard to participation rates, procedure-related pain and discomfort, endoscopist performance, and effectiveness. No randomized trials on colonoscopy screening exist today.
Objective
To investigate participation rate, yield, performance and adverse events of population-based colonoscopy screening.
Design
Randomized controlled trial in Poland, Norway, the Netherlands, and Sweden
Setting
Average-risk population, population-based.
Participants
94,958 men and women aged 55 to 64 years.
Intervention
Colonoscopy screening or no-screening.
Outcome measures
This paper reports on screening participation, yield, and subject experience. Study outcomes were compared by country and endoscopist.
Results
Of 31,420 eligible subjects randomized to colonoscopy 12,574 (40%) attended screening. Participation rates were 60.7% in Norway, 39·8% in Sweden, 33% in Poland, and 22.9% in the Netherlands (p<0.001). The cecum intubation rate was 97.2%, with 9,726 (77.3%) of subjects not receiving sedation. We observed one perforation (0.01%), two post-polypectomy serosal burns and 18 bleedings due to polypectomy (0.15%). 62 subjects (0.5%) were diagnosed with colorectal cancer and 3,861 (30.7%) had adenomas, of which 1,304 (10.4%) high-risk adenomas. Detection rates were similar in the proximal and distal colon. Performance differed significantly between endoscopists; recommended benchmarks for caecal intubation (95%) and adenoma detection (25%) were not met by 6 (17.1%) and 10 endoscopists (28.6%), respectively. Moderate or severe abdominal pain after colonoscopy was reported by 16.6% examined with standard air insufflation versus 4.0% with carbon dioxide insufflation (p<0.001).
Conclusion and relevance
Colonoscopy screening entails high detection rates in the proximal and distal colon. Participation rates and endoscopist performance screening vary significantly. Post-procedure abdominal pain is common with standard air insufflation and can be significantly reduced by using carbon dioxide.
Trial registration
Introduction
Colorectal cancer is the second most common cancer in high- income countries with more than 730,000 new cases diagnosed globally each year.1 The disease is expected to become a large burden also for less developed countries in the near future.2
Randomized trials have shown that screening with guaiac fecal occult blood testing (gFOBT) reduces colorectal cancer mortality by 15%.3 One study has also shown an effect of gFOBT screening on colorectal cancer incidence, presumably due to a high colonoscopy rate after positive gFOBT.4 Guaiac FOBT is being replaced by more sensitive fecal immunochemical testing but data on cancer incidence and mortality are lacking.3
Because most colorectal cancers develop from benign adenomas, endoscopic screening, which allows detection and removal of adenomas, may have a larger impact on colorectal cancer incidence and mortality than FOBT screening. Four large-scale randomized trials have shown that flexible sigmoidoscopy screening reduces colorectal cancer incidence by 18 to 23% and mortality by 22 to 31%.5–8
Because colonoscopy is believed to be more effective than sigmoidoscopy, colonoscopy screening is widely endorsed in the United States and Canada.9 However, colonoscopy is invasive and expensive, and entails a risk of complications. Large population-based studies investigating patient participation and experience, detection rates for adenomas and cancer, and screening colonoscopy effectiveness are lacking. To carefully evaluate the balance of benefits and harms of colonoscopy screening, randomized trials are imperative. Therefore, European guidelines currently do not recommend colonoscopy screening.10
The Nordic-European Initiative on Colorectal Cancer (NordICC) study is a multinational, population-based randomized controlled trial to investigate the effectiveness of colonoscopy screening on CRC incidence and CRC mortality in different European countries. This paper reports on participation, patient experience, yield, and complications of colonoscopy screening in the different participating countries.
Methods
Study Design
Details of the NordICC trial rationale, its pragmatic (also called management) design, randomization, intervention and outcomes have been described elsewhere.11–13 The primary outcome is colorectal cancer mortality and incidence in an intention-to-treat analysis after 15 years of follow-up. Secondary aims include participation rates, patient experience, yield, and complications. Eligible individuals were all men and women aged 55 to 64 years living in defined geographical areas in Norway, Poland, Sweden, and the Netherlands. All colonoscopies were performed at dedicated endoscopy centres.
Figure 1 shows the trial flowchart. Poland was the only country with an ongoing colorectal cancer screening program.14, but not amongst the individuals recruited for this trial No other country had organized CRC screening of any kind in the trial areas.
Figure 1.
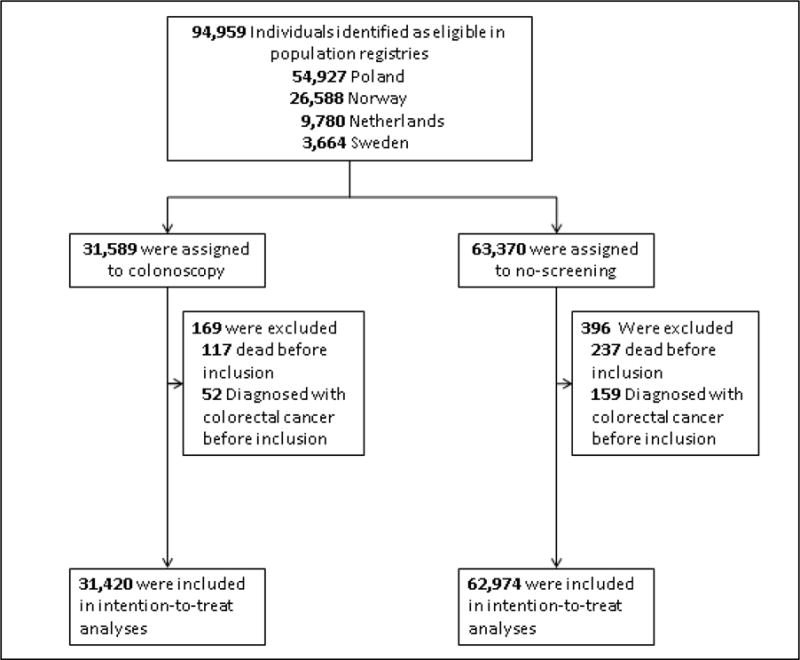
NordICC study flowchart
Randomization and Intervention
Subjects were randomly assigned to either colonoscopy screening or no-screening (control group) in a one-to-two ratio. Individuals randomized to colonoscopy screening were offered colonoscopy if they did not have any of the pre-specified comorbidities11. All individuals randomized to the screening group received a personal letter of invitation with an information leaflet about the study, and an informed consent form. An identical information leaflet was used in Norway, Sweden and Poland (translated into the local languages). In the Netherlands, we used a similar, but not entirely identical leaflet derived for a randomized trial comparing colonoscopy versus CT colonography11. All individuals attending colonoscopy screening provided written informed consent. Individuals randomized to the control group did not receive any intervention, and were not contacted at study enrolment.
Screening intervention
The colonoscopy and the bowel preparation were provided without cost sharing. No compensation was paid for participantion. Trial endoscopists had performed at least 300 colonoscopies before entering the trial, and had a minimum workload of 200 colonoscopies per year. Standard video colonoscopes were used for all procedures. All centres were encouraged to use carbon dioxide (CO2) insufflation whenever possible; otherwise standard air insufflation was used. All lesions detected during colonoscopy were removed whenever feasible, and all tumors were biopsied. Data from colonoscopy examinations were registered using an electronic case report form accessible online from the participating centres, and stored at the central trial database (Frontier Science (Scotland), Kincraig, UK).
Dedicated pathologists were responsible for histopathological classification according to the World Health Organization.15 Polyps were categorized as adenomas, serrated polyps (including hyperplastic polyps, sessile serrated polyps and traditional serrated adenomas), inflammatory, neuroendocrine, or other. Adenomas measuring 10 mm or more in diameter, or with villous architecture, or high-grade dysplasia were classified as advanced adenomas. Patients with advanced adenomas or three or more adenomas were classified as high-risk. We defined lesions with submucosal invasion as cancer. Patients were classified according to the most advanced lesion detected at screening.
We assessed patients’ abdominal pain during the colonoscopy, and in the 24 hours after the colonoscopy, using a validated patient questionnaire in Norway, Poland, and Sweden.11 Patients scored abdominal pain on a 4-point visual rating scale as either none, light, moderate, or severe. Similar questions were applied for pain during the colonoscopy, and for the 24- hour period after the procedure. All participants in Norway, Poland, and Sweden were asked to fill in the questionnaire 24 hours after the screening examination and return to the central secretariat. Thirty day morbidity and mortality after screening was assessed from the electronic case report forms and by linkage to patient registries in the participating countries.
Statistical analysis
For this report, we assessed study outcomes for the whole study cohort, and for the comparison of participating countries. Colonoscopy yield and patient satisfaction was compared between participating endoscopists who had performed at least 30 colonoscopies in the trial. Patients’ abdominal pain scores during and after the colonoscopy, respectively, were dichotomized for analyses (none or slight pain versus moderate or severe pain). Adenoma yield per endoscopist is defined as the percentage of patients with at least one adenoma (corresponding to what is commonly called adenoma detection rate). Differences between the groups in baseline variables which could influence study outcomes were adjusted for age and sex by multiple logistic-regression analyses and reported as odds ratios with 95% confidence intervals. We fitted a logistic regression model to estimate the association between country, sedation and insufflation gas (air or CO2) on abdominal pain, and tested country-wise heterogeneity by including a product (“interaction”) term between sedation and country. We estimated mean performance indicators and 95% confidence intervals using a random effects model to account for clustering at endoscopist level.
For patient questionnaire data, we present percentages among those who responded to the particular questions. All analyses were performed with the use of Stata statistical software version 14.0 (StataCorp, College Station, Texas, USA).
The study was approved by the ethical committees at all participating centres. Approvals were also obtained from the National Swedish Ethics Council, and from the Dutch National Health Council.
Results
Study population
At study start, 94,958 individuals were identified as eligible in the population registries. During the course of the screening period from June 8, 2009 to June 23, 2014, 169 individuals assigned to the screening group and 369 individuals assigned to the control group were excluded because they were dead or diagnosed with colorectal cancer before study entry (but not yet identified as such in the registries), figure 1. Thus, our analyses are based on 94,394 individuals; 31,420 in the screening group and 62,974 in the control group; 47,135 (49.9%) women, and 47,259 (50.1%) men, with a median age of 60.0 years.
Screening participation
Among the 31,420 individuals who were assigned to colonoscopy, 12,574 subjects (40.0%) accepted the invitation and underwent colonoscopy. 662 individuals did not undergo colonoscopy due to one or several comorbidities which precluded screening11 (these individuals are included on the estimates of participation, according to the intention-to-treat principle). Participation rates were slightly higher in men than women (41.2% versus 38.8%), and in the age group 60–64 years as compared to 55 to −59 years (41.0% versus 39.1%), table 1. Participation varied substantially between the participating countries from 60.7% in Norway, 39.8% in Sweden, 33.0% in Poland, to 22.9% in the Netherlands (p<0.001).
Table 1.
Baseline and procedural characteristics, and screening colonoscopy acceptance
| TOTAL patients | Norway | Poland | Sweden | Netherlands | |
|---|---|---|---|---|---|
| n (%) | |||||
| No. Randomized | |||||
| Total | 94,394 | 26,417 | 54,533 | 3,664 | 9,780 |
| Screening group | 31,420 (33.3) | 8,816 | 18,188 | 1,222 | 3,194 |
| Control group | 62,974 (66.7) | 17,601 | 36,345 | 2,442 | 6,586 |
| Sex | |||||
| Women | 47,135 (49.9) | 13,195 | 27,334 | 1,671 | 4,935 |
| Men | 47,259 (50.1) | 13,222 | 27,199 | 1,993 | 4,845 |
| Age at study entry | |||||
| 54–59 | 48,024 (50.9) | 12,526 | 28,794 | 1,791 | 4,913 |
| 60–64 | 46,370 (49.1) | 13,891 | 25,739 | 1,873 | 4,867 |
| Screening Participation | |||||
| Total | 12,574 (40.0) | 5,354 (60.7) | 6,004 (33.0) | 486 (39.8) | 730 (22.9) |
| Women | 6,081 (38.8) | 2,580 (58.8) | 2,919 (32.0) | 226 (40.4) | 356 (22.2) |
| Men | 6,493 (41.2) | 2,774 (62.7) | 3,085 (34.0) | 260 (39.3) | 374 (23.5) |
| 54–59 | 6,241 (39.1) | 2,497 (59.8) | 3,174 (33.1) | 207 (34.7) | 363 (22.8) |
| 60–64 | 6,333 (41.0) | 2,857 (61.6) | 2,830 (33.0) | 279 (44.6) | 367 (22.9) |
| Performance | |||||
| Cecum intubation | 12,217 (97.2) | 5,157 (96.3) | 5,869 (97.8) | 472 (97.1) | 719 (98.5) |
| Sedation given | 2,848 (22.7) | 579 (10.8) | 1,389 (23.1) | 223 (45.9) | 657 (90.0) |
| Withdrawal time*, median (IQR) | 10 (8–15) | 10 (8–15) | 8 (6–12) | 11 (9–17) | |
| Adverse events | |||||
| Perforations | 1 (0.01) | 0 | 0 | 0 | 1 (0.14) |
| Major bleedings | 18 (0.15) | 8 (0.15) | 7 (0.12) | 0 | 3 (0.41) |
| Vasovagal reactions | 51 (0.41) | 28 (0.52) | 14 (0.23) | 2 (0.41) | 7 (0.96) |
Given cecum intubation (calculated as total procedure time – time to reach cecum)
Overall performance and diagnostic yield
The overall cecum intubation rate was 97.2%, and the median withdrawal time was 10 minutes (IQR 8–15 minutes), table 1. Reflecting differences in colonoscopy traditions in the different countries, sedation was administered in 10.8% of patients in Norway, 23.1% in Poland, 45.9% in Sweden, and in 90.0% in the Netherlands. Most commonly used drugs were propofol (1,433 individuals, 11.4%), midazolam (2,126 individuals, 16.9%) or a combination of midazolam and fentanyl (2,398 individuals, 19.1%). The quality of bowel preparation was judged as very good or good in more than 90% of colonoscopies (table 2).
Table 2.
Bowel preparation quality and diagnostic yield at screening colonoscopy
| TOTAL patients, n (%) | Sex | Age group | |||
|---|---|---|---|---|---|
| Women | Men | 55–59 | 60–64 | ||
| Split dose bowel preparation* | 9,845 | 4,316 | 5,529 | 4,868 | 4.977 |
| Very good cleansing | 6,643 (67.5) | 3,078 (71.3) | 3,565 (64.5) | 3,245 (66.7) | 3,398 (68.3) |
| Good cleansing | 2,289 (23.3) | 899 (20.8) | 1,390 (25.1) | 1,162 (23.9) | 1,127 (22.6) |
| Partially poor cleansing | 301 (3.1) | 129 (3.0) | 172 (3.1) | 143 (2.9) | 158 (3.2) |
| Generally poor cleansing | 534 (5.4) | 173 (4.0) | 361 (6.5) | 287 (5.9) | 247 (5.0) |
| Day before bowel preparation* | 2,332 | 1,549 | 783 | 1,205 | 1,127 |
| Very good cleansing | 1,252 (53.7) | 898 (58.0) | 354 (45.2) | 654 (54.3) | 598 (53.1) |
| Good cleansing | 855 (36.7) | 532 (34.3) | 323 (41.3) | 441 (36.6) | 414 (36.7) |
| Partially poor cleansing | 93 (4.0) | 44 (2.8) | 49 (6.3) | 45 (3.7) | 48 (4.3) |
| Generally poor cleansing | 126 (5.4) | 71 (4.6) | 55 (7.0) | 61 (5.1) | 65 (5.8) |
| Participants | 12,574 | 6,081 | 6,493 | 6,241 | 6,333 |
| Polyps | 6,049 (48.1) | 2,535 (41.7) | 3,514 (54.1) | 2,958 (47.4) | 3,091 (48.8) |
| Proximal** | 3,341 (26.6) | 1,356 (22.3) | 1,985 (30.6) | 1,571 (25.2) | 1,770 (28.0) |
| Distal** | 4,402 (35.0) | 1,776 (29.2) | 2,626 (40.4) | 2,174 (34.8) | 2,228 (35.2) |
| Adenomas | 3,861 (30.7) | 1,490 (24.5) | 2,371 (36.5) | 1,836 (29.4) | 2,025 (32.0) |
| Proximal | 2,273 (18.1) | 818 (13.5) | 1,455 (22.4) | 1,035 (16.6) | 1,238 (19.6) |
| Distal | 2,407 (19.1) | 907 (14.9) | 1,500 (23.1) | 1,143 (18.3) | 1,264 (20.0) |
| High-risk adenomas | 1,304 (10.4) | 430 (7.1) | 874 (13.5) | 566 (9.1) | 738 (11.7) |
| Proximal | 562 (4.5) | 176 (2.9) | 386 (5.9) | 230 (3.7) | 332 (5.2) |
| Distal | 725 (5.8) | 255 (4.2) | 470 (7.2) | 312 (5.0) | 413 (6.5) |
| Serrated polyps | 3,095 (24.6) | 1,325 (21.8) | 1,770 (27.3) | 1,507 (24.2) | 1,588 (25.1) |
| Proximal | 1,078 (8.6) | 510 (8.4) | 568 (8.8) | 503 (8.1) | 575 (9.1) |
| Distal | 2,439 (19.4) | 998 (16.4) | 1,441 (22.2) | 1,211 (19.4) | 1,228 (19.4) |
| Colorectal cancer | 62 (0.5) | 23 (0.4) | 39 (0.6) | 29 (0.5) | 33 (0.5) |
| Proximal | 14 (0.1) | 4 (0.1) | 10 (0.2) | 7 (0.1) | 7 (0.1) |
| Distal | 50 (0.4) | 19 (0.3) | 31 (0.5) | 24 (0.4) | 26 (0.4) |
| Neuroendocrine tumor | 7 | 4 | 3 | 3 | 4 |
| Proximal | 1 | 1 | 0 | 0 | 1 |
| Distal | 6 | 3 | 3 | 3 | 3 |
The total number of subjects with proximal and distal lesions may exceed the total number of subjects because subjects could have lesions in both locations.
Cleansing regimen missing for 124 participants and cleansing quality missing for 190 participants
Proximal colon defined as cecum to splenic flexure; distal colon defined as descending colon to rectum
As shown in table 2, 62 individuals were diagnosed with colorectal cancer at screening (0.5%). Of these, 14 (0.1%) had tumors in the proximal colon (cecum, ascending or transverse colon, or splenic flexure), and 50 (0.4%) had distal tumors (descending or sigmoid colon, rectum). The overall prevalence of colorectal polyps was 48.1% (6,049 individuals); 3,861 individuals (30.7%) had adenomas, and of these, 1,304 (10.4%) were high-risk. The adenoma yield was similar in the distal as compared to the proximal colon (table 2). In total, 3095 patients were diagnosed with serrated polyps (24.6%); thereof 285 (2.3%) with a size of 10 mm or larger. 221 patients had large (10 mm or larger) serrated polyps in the proximal colon and 73 in the distal colon.
During screening colonoscopy, 58 patients were diagnosed with previously unknown inflammatory bowel disease, and seven patients had neuroendocrine tumors removed.
Adverse events
We observed one colonoscopy perforation (0.01%). The patient returned to the hospital the evening after the colonoscopy with abdominal pain and fever. CT-scan revealed free air; laparotomy with surgical suture of the perforation was performed. The patient fully recovered. Two post-polypectomy serosal burns were observed, both resolved without intervention. 18 patients developed bleeding due to polypectomy (0.15%). All were treated endoscopically. No deaths or other major complications related to the screening intervention occurred within 30 days after screening. Fifty-one patients experienced minor vasovagal reactions during colonoscopy (0.41%). All were short-term, without need of extra measures for the patient after the procedure.
Patient pain and satisfaction
Patient pain and satisfaction questionnaires were used in Norway, Poland and Sweden. Overall, 10,907 patient questionnaires were issued (92.1% of all participants), and 9,201 patients (84.4%) returned the questionnaire; 98.9% of patients were generally satisfied with the screening intervention. Figure 2 (panel A) shows patient pain during colonoscopy; 79.7% had none or slight abdominal pain during colonoscopy (no pain 45.9%; slight pain 33.8%), while 20.3% reported moderate or severe pain (moderate pain 12.5%; severe pain 7.8%). Figure 2 and table 2 in the supplementary appendix show the association of patient pain with sedation and insufflation gas used in the different participating countries. Overall, pain during colonoscopy was not significantly associated with the use of sedation (adjusted odds ratio 0.91; 95%CI 0.61–1.35), but with differences between the countries (due to variation in clinical practice). There was no difference in per-procedural patient pain between the two insufflation gases used, but significant differences between countries (p<0.001). A higher proportion of women experienced moderate or severe pain as compared to men (26.3% versus 14.6%, p<0.001).
Figure 2. Patients’ self-reported abdominal pain during and after screening colonoscopy.
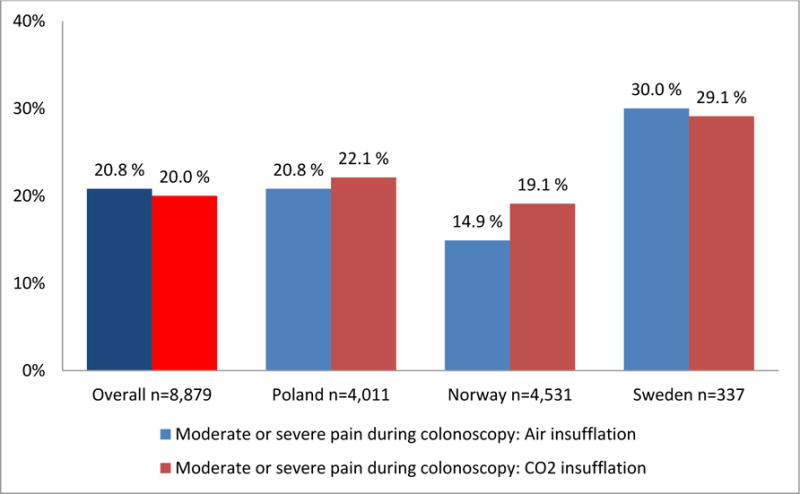
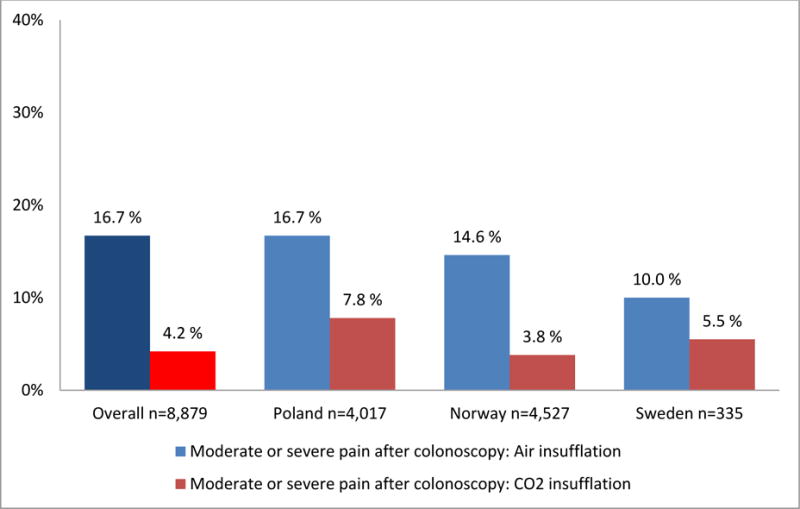
Percentages of patients with moderate or severe abdominal pain during (panel A) and within 24 hours after (panel B) colonoscopy, displayed by country and insufflation gas used (air or carbon dioxide (CO2)). Patient pain was self-reported using a validated questionnaire with a 4-point visual rating scale (VRS-4).
Panel A: Patients’ self-reported abdominal pain during screening colonoscopy (p<0·001 for difference between countries. P=0.40 for difference between insufflation gases after adjustment for country).
Panel B: Patients’ self-reported abdominal pain after screening colonoscopy. Moderate or severe pain after colonoscopy related to insufflation gas used during examination (carbon dioxide (CO2) or air); (p<0.001 for difference between insufflation gas after adjustment for country)
During the 24 hours after colonoscopy, 9.4% of all patients experienced moderate or severe abdominal pain (moderate pain 6.5%; severe pain 2.9%.) A 4-fold higher proportion of patients examined with air insufflation reported abdominal pain as compared to CO2 insufflation (16.7% versus 4.2%, p<0,001), (figure 2, panel B). Severe pain after colonoscopy was reported by 1% of patients examined with CO2 insufflation as compared to 5.6% with air insufflation. This finding did not change after adjustment for country, and there was no significant heterogeneity between countries.
Individual performance and diagnostic yield
We found substantial variations in individual endoscopist performance regarding cecum intubation rate, adenoma yield, and patient pain and discomfort during and after colonoscopy (figure 3, panel C and D). There was also a significant difference in adenoma yield between the participating countries (p<0.001).
Figure 3. Individual endoscopist performance in screening colonoscopy.
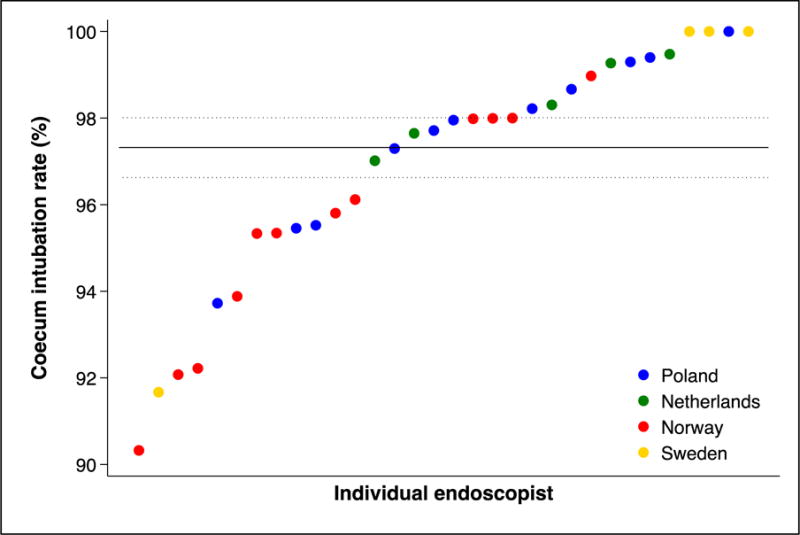
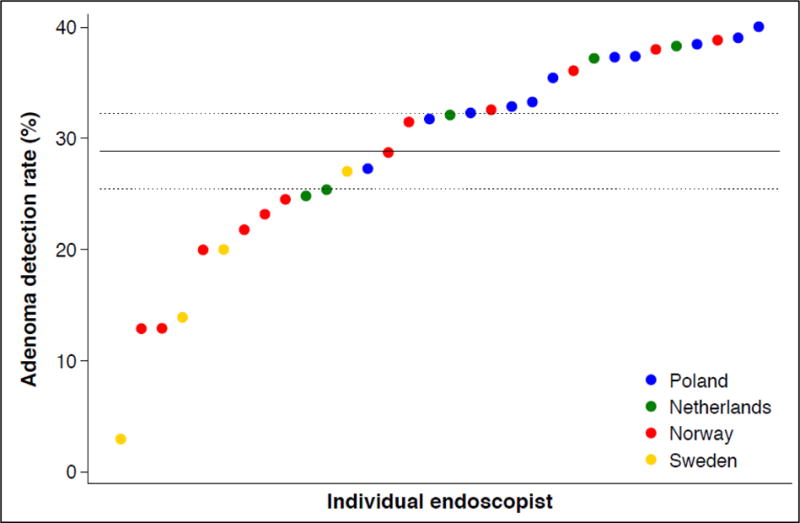
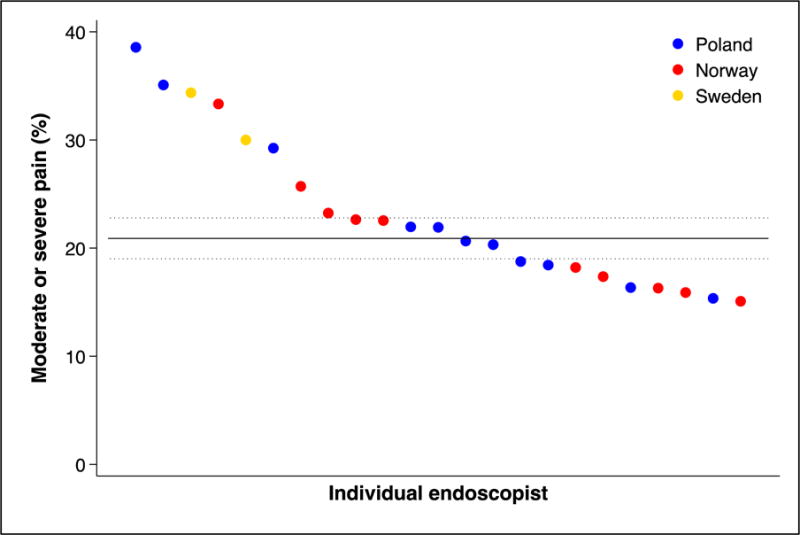
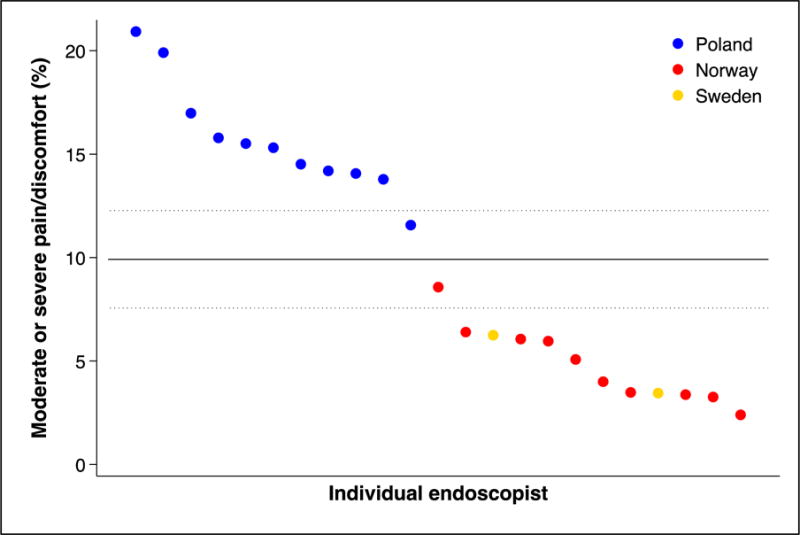
Performance indicators (panel A: cecum intubation rate; panel B: adenoma yield; panel C: percentage of patients with moderate or severe pain during colonoscopy; panel D: percentage of patients with moderate or severe pain during the 24 hours after colonoscopy) for endoscopists (by country) who performed at least 30 examinations in the NordICC trial. The horizontal lines represent the mean value (solid) with 95% confidence interval (dashed). These are estimated with a random effects model to account for clustering at the endoscopist level.
Panel A: cecum intubation rate
Panel B: adenoma yield (percentage of patients with adenomas)
Panel C: percentage of patients with moderate or severe pain during colonoscopy
Panel D: percentage of patients with moderate or severe pain 24 hours after colonoscopy
Discussion
In this randomized, population-based trial on colonoscopy screening, we found satisfactory participation, performance and yield for both distal and proximal polyps, but with large differences between endoscopists. We further reveal that post-procedural abdominal pain is common in screening colonoscopy using standard air insufflation, and that it can be significantly reduced by using CO2.
While many Americans regularly undergo colonoscopy screening, no randomized trials have been performed to quantify the effectiveness of colonoscopy screening on colorectal cancer incidence and mortality. The NordICC trial is the first to investigate the effectiveness of colonoscopy screening compared to no screening. The main results are expected in 15 years’ time.
The overall performance of colonoscopy was well above thresholds for adenoma detection and cecum intubation. This is reassuring with regards to future achievement of the trial endpoints. However, as figure 3 shows, there was considerable inter-endoscopist variation. Recommended benchmarks for cecum intubation rate (95%) and adenoma yield (25% adenoma detection rate) were not met by 6 (17.1%) and 10 endoscopists (28.6%), respectively. Future analysis will reveal if endoscopist performance is related to differences in ultimate patient outcomes. Further, although high adenoma yield is desirable, most non-advanced adenomas do not harbor a large risk of malignant transformation, but its detection leads to a larger number of surveillance colonoscopies. Future analysis may reveal if the effect on colorectal cancer incidence and death outweighs the increased burden in colonoscopy capacity due to high adenoma yield.
The rate of major adverse events was 0.15%, which we consider acceptable. Our rate is lower than that observed in a recently published population-based trial comparing colonoscopy with fecal immunochemical testing in Spain (0.51%)16. The higher adverse event rate may be related to the higher sedation rate in the Spanish trial (96% sedation rate) as compared to ours.
Some studies have suggested a smaller effect of colonoscopy in the proximal as compared to the distal colon. In our study, detection rates for low and high-risk adenomas were as high in the proximal as in the distal colon. This is in accordance withthe Spanish colonoscopy screening trial.16 The high adenoma yield in the proximal colon may translate into higher effectiveness of colonoscopy screening as compared to sigmoidoscopy for prevention of proximal cancer. Site-specific age- categorized colorectal cancer incidence and mortality will be investigated during follow-up, but to be able to achieve sufficient power, data pooling from the four currently ongoing randomized colonoscopy trials will be necessary.17
Our trial randomized individuals directly from the population registries. The intention-to-treat estimates obtained under this design are more helpful to assess population effectiveness than to inform individual decision making because the magnitude of the effect depends on the proportion of participants who accept to receive the screening. Interestingly, participation rates differed considerably between the different countries. This may be grounded in differences in cultural settings and beliefs, and expectations around endoscopic procedures. We used the same information and invitation routines in Poland, Sweden and Norway. The Netherlands used a slightly different invitation brochure, and due to national requirements, individuals were invited to an outpatient visit before the colonoscopy for verbal information on participation in the trial, the colonoscopy procedure and its preparation at the hospital before the date of the colonoscopy. While this may explain a lower participation rate in the Netherlands, observed rates were also significantly different between the other three countries. Thus, we cannot fully explain the difference in participation between the four countries by different approaches within the study organization. We believe that cultural differences such as public awareness or shame for colorectal disease, or perception about colonoscopy as painful or uncomfortable may play a role in the observed differences. The remarkable high 60% participation rate in Norway correlates with the previous participation rates for flexible sigmoidoscopy screening8, the low 23% participation in the Netherlands contrasts with the 70% uptake in FIT screening in that country. Notably, high cecum intubation rates were achieved with a low sedation rate.
The overall participation rate (40%) was somewhat lower than expected, but is higher than in other population-based trials: 24·6% for colonoscopy and 34·2% for fecal immunochemical screening in the Spanish randomized trial16, and 33·6% for CT colonography in the Dutch COCOS trial.18 As shown in Figure 1 of the supplementary appendix, the NordICC trial will likely have sufficient power to detect differences in colorectal cancer mortality with the achieved participation.
Patient pain and discomfort may be a major barrier for participation in screening colonoscopy. We found that 80% of patients reported no or only light pain during the procedure, while 20% reported moderate or severe pain. This is comparable to reported patient pain in flexible sigmoidoscopy screening trials.19,20 Interestingly, about 16% of patients suffered from moderate or severe pain after colonoscopy using standard air insufflation. The use of carbon dioxide insufflation reduced the absolute risk of post-colonoscopy abdominal pain significantly to 4%, see figure 2. It is important to disentangle the effects of CO2 from the effects of sedation; while the main effect of CO2 occurs after the examination (in the hours after the colonoscopy has ended, often after the patient is discharged from the endoscopy unit), sedation relates to pain and discomfort during the colonoscopy. Further, while pain and discomfort during colonoscopy often is short in duration, pain after colonoscopy lasts longer (for up to 24 hours) and may affect patient compliance more than intra-procedural pain. Thus, CO2 is equally relevant to use in sedated and non-sedated patients. Therefore; also in countries in which colonoscopy is performed with sedation (such as in the US), CO2 insufflation is beneficial.
The profound effect of CO2 to reduce post-colonoscopy pain and discomfort is intriguing, although not novel. Our observation is in accordance with previous evidence from smaller randomized trials21. Further, CO2 eliminates the risk of explosion during polypectomy. Although explosion during polypectomy is very rare event with air insufflation, cases have been reported in the literature until recently22. However, despite strong evidence for the superiority of CO2, air insufflation is still the standard gas used around the world. The lack of implementation of CO2 is a concern for patient safety and comfort. Abdominal pain after colonoscopy may also be an ignored cause for poor participation in endoscopic screening.
We found no significant correlation between patient pain and the use of sedation, but with different patterns in the different countries (see suppl. Fig 2). This is in accordance with previous evidence and suggests that pain and discomfort during colonoscopy is more related to local practice, endoscopist training and patient characteristics23,24. Colonoscopy without sedation is performed technically differently than with sedation, and local traditions for training are guiding local practice. Both have advantages and disadvantages23. For many patients, unsedated colonoscopy is feasible. However, some patient groups (e.g. women with previous abdominal surgery) have a higher risk of experiencing pain during colonoscopy and unsedated colonoscopy may be more challenging24.
In conclusion, we report satisfactory participation, high adenoma yield, and adequate performance for screening colonoscopy in the NordICC trial. The observed large differences between countries and individual endoscopists deserve further investigation. Air insufflation should be abandoned in screening colonoscopy.
Supplementary Material
Acknowledgments
Michael Bretthauer had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding/Support: The NordICC study has received research grants from: Norway: the Nordic Cancer Union, the Norwegian Cancer Society, the Norwegian Research Council (grant no. 197309), and the Health Fund of South-East Norway (grant no. 5135); Poland: the National Centre for Research and Development of Poland (grant no. N R13 0024 04), Polish-Norwegian Research Programme (Pol-Nor/204233/30/2013), and the Polish Foundation of Gastroenterology; Netherlands: the Dutch Ministry of Health and Health Care Prevention; Program–Implementation (ZonMw 2008 The Netherlands Organisation for Health Research and Development of the Dutch Ministry of Health (ZonMw 120720012), and by the Centre for Translational Molecular Medicine (CTMM DeCoDe-project); Sweden: Swedish Cancer Foundation (grant no. 2010/345 and CAN 2013/553); Regional forskningsfond i Uppsala-Örebro regionen, Karolinska Institutet Distinguished Professor Award to Prof. Hans-Olov Adami (grant no. 2368/10-221), AFA (grant no. 130072); the USA: NIH (R01 P01 CA134294); and received equipment and bowel preparation from Falk Pharma, Olympus Europe and UCB Pharma. The funders were not involved in design and conduct of the study, collection, management, analysis and interpretation of the data, preparation, review or approval of the manuscript, or decision to submit the manuscript for publication.
Study organization
The NordICC Study group comprises: the NordICC board: Hans-Olov Adami (chair), Michael Bretthauer (principal investigator), Geir Hoff (co-principal investigator), Michal F. Kaminski (co-principal investigator), Ann G. Zauber. Miguel A. Hernán, Jaroslaw Regula, Ernst J. Kuipers, Evelien Dekker, Lars Påhlman, and Tryggvi Stefansson; the NordICC researchers Marek Bugajski, Iris Landsdorp-Vogelaar, Magnus Løberg, Mette Kalager, Maciej Rupinski, Manon van Spaander; the Data Safety and Monitoring board: Stephen Duffy, Jean Faivre, and Jack Mandel; the screening centers: Kristiansand (Øyvind Holme, Kjetil Garborg), Arendal (Ole Høie, Geir Noraberg), Warsaw (Michal F. Kaminski), Rotterdam (Ernst J. Kuipers), Amsterdam (Evelien Dekker), Uppsala (Lars Påhlman and Helen Siilin), Ørebro (Magnus Andersson), Karlstad (Stefan Willmarsson), Eskilstuna (Louise Olsson), Falun (Lars Strandberg), Gävle (Oskar Vandell), and Västerås (Abbas Chabok)
Footnotes
Author contributions: Drs Bretthauer, Kaminski, Løberg, Zauber had full access to all data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bretthauer, Regula, Kuipers, Stefansson, Påhlman, Hoff, Kaminski, Adami
Acquisition of data: All authors
Analysis and interpretation of data: All authors
Drafting of the manuscript: Bretthauer, Adami
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Løberg, Zauber, Sunde
Obtained funding: Bretthauer, Regula, Kuipers, Påhlman, Hoff, Adami
Study supervision: NordICC Data and Safety Monitoring Board.
Conflict of interest disclosure: Michael Bretthauer is member of the European scientific advisory board of Exact Sciences and has received equipment for testing in scientific studies from Olympus, Fujinon, Falk Pharma and CCS Healthcare. All other authors report no conflicts of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 Mar 1;136(5):E359–86. doi: 10.1002/ijc.29210. Epub 2014 Oct 9. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Holme Ø, Bretthauer M, Fretheim A, Odgaard-Jensen J, Hoff G. Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane Database of Syst Rev. 2013 Oct 1;9:CD009259. doi: 10.1002/14651858.CD009259.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–7. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 5.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicenter randomized trial. Lancet. 2010;375:1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 6.Schoen RE, Pinsky PF, Weissfeld JL, et al. PLCO Project Team Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–2357. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segnan N, Armaroli P, Bonelli L, et al. SCORE Working Group Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial–SCORE. J Natl Cancer Inst. 2011;103:1310–1322. doi: 10.1093/jnci/djr284. [DOI] [PubMed] [Google Scholar]
- 8.Holme Ø, Løberg M, Kalager M, Bretthauer M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: A randomized clinical trial. JAMA. 2014;312:1–10. doi: 10.1001/jama.2014.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreuders EH, Ruco A, Rabeneck L, et al. Recent advances in clinical practice. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015 doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 10.Segnan N, Patrick J, von Karsa L, editors. European Union. 2010. European guidelines for quality assurance in colorectal cancer screening and diagnosis. [DOI] [PubMed] [Google Scholar]
- 11.Kaminski MF, Bretthauer M, Zauber A, et al. The NordICC Study: Rationale and design of a randomized trial on colonoscopy screening for colorectal cancer. Endoscopy. 2012;44:695–702. doi: 10.1055/s-0032-1306895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Chron Dis. 1967;20:637–48. doi: 10.1016/0021-9681(67)90041-0. [DOI] [PubMed] [Google Scholar]
- 13.McMahon AD. Study control, violators, inclusion criteria and defining explanatory and pragmatic trials. Stat Med. 2002;21:1365–76. doi: 10.1002/sim.1120. [DOI] [PubMed] [Google Scholar]
- 14.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–72. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton SR, Aaltonen LA. Pathology and Genetics of Tumors of the Digestive Tract. IARC Press; Lyon: 2000. World Health Organisation Classification of Tumors; pp. 104–19. [Google Scholar]
- 16.Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 17.Robertson DJ, Kaminski MF, Bretthauer M. Effectiveness, training and quality assurance of colonoscopy screening for colorectal cancer. Gut. 2015;64:982–90. doi: 10.1136/gutjnl-2014-308076. [DOI] [PubMed] [Google Scholar]
- 18.Stoop EM, de Haan MC, de Wijkerslooth TR, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol. 2012;13:55–64. doi: 10.1016/S1470-2045(11)70283-2. [DOI] [PubMed] [Google Scholar]
- 19.UK Flexible Sigmoidoscopy Screening Trial Investigators. Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet. 2002;359:1291–300. doi: 10.1016/S0140-6736(02)08268-5. [DOI] [PubMed] [Google Scholar]
- 20.Larsen IK, Grotmol T, Bretthauer M, et al. Continuous evaluation of patient satisfaction in endoscopy centres. Scand J Gastroenterol. 2002;37:850–5. [PubMed] [Google Scholar]
- 21.Wu J, Hu B. The role of carbon dioxide insufflation in colonoscopy: a systematic review and meta-analysis. Endoscopy. 2012;44:128–136. doi: 10.1055/s-0031-1291487. [DOI] [PubMed] [Google Scholar]
- 22.Hofstad B. Tidsskr Nor Lægeforen. 2007;127:1789–90. [PubMed] [Google Scholar]
- 23.Rex DK, Khalfan HK. Sedation and the technical performance of colonoscopy. Gastrointest Endosc Clin N Am. 2005;15:661–72. doi: 10.1016/j.giec.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Hoff G, Bretthauer M, Huppertz-Hauss G, et al. The Norwegian Gastronet project: Continuous quality improvement of colonoscopy in 14 Norwegian centres. Scand J Gastroenterol. 2006;41:481–7. doi: 10.1080/00365520500265208. [DOI] [PubMed] [Google Scholar]
- 25.Holme O, Bretthauer M, de Lange T, et al. Risk stratification to predict pain during unsedated colonoscopy: results of a multicenter cohort study. Endoscopy. 2013;45:691–6. doi: 10.1055/s-0033-1344239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


