Summary
Cellular senescence is a state of stable proliferation arrest of cells. The senescence pathway has many beneficial effects and is seen to be activated in damaged/stressed cells, as well as during embryonic development and wound healing. However, the persistence and accumulation of senescent cells in various tissues can also impair function and have been implicated in the pathogenesis of many age‐related diseases. Osteoarthritis (OA), a severely debilitating chronic condition characterized by progressive tissue remodeling and loss of joint function, is the most prevalent disease of the synovial joints, and increasing age is the primary OA risk factor. The profile of inflammatory and catabolic mediators present during the pathogenesis of OA is strikingly similar to the secretory profile observed in ‘classical’ senescent cells. During OA, chondrocytes (the sole cell type present within articular cartilage) exhibit increased levels of various senescence markers, such as senescence‐associated beta‐galactosidase (SAβGal) activity, telomere attrition, and accumulation of p16ink4a. This suggests the hypothesis that senescence of cells within joint tissues may play a pathological role in the causation of OA. In this review, we discuss the mechanisms by which senescent cells may predispose synovial joints to the development and/or progression of OA, as well as touching upon various epigenetic alterations associated with both OA and senescence.
Keywords: cellular senescence, epigenetics, osteoarthritis
Introduction
Osteoarthritis (OA)
Osteoarthritis (OA) is the most prevalent disease of synovial joints (around 4.7% of global population for knee and hip OA alone), afflicting many millions worldwide with pain and disability (Cross et al., 2014), and thus represents an enormous healthcare and socioeconomic burden. Advancing age is a major risk factor, thus the burden of OA is set to increase dramatically as populations continue to age. Gender is also recognized as a contributing factor, with the female population generally being at a higher risk of developing OA. Females are seen to develop more severe knee and hand OA compared to their male counterparts, especially when ≥55 years old (Srikanth et al., 2005). It has been implied that hormones may play a role in the increased incidence of OA in females, particularly a postmenopausal decrease in estrogen levels. Other risk factors contributing to the burden of OA are summarized in Fig. 1.
Figure 1.
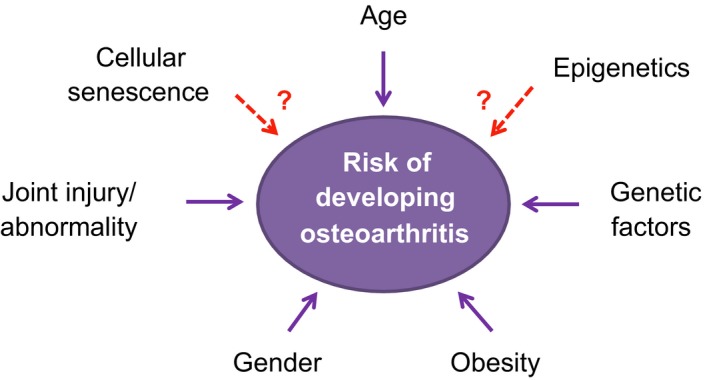
Various factors believed to contribute to the onset of OA.
A generalized structure for a synovial joint is illustrated in Fig. 2, characterized by the presence of connective tissues such as articular cartilage, subchondral bone, ligaments, and in some joints menisci (fibrocartilage structures that provide stability and load dispersal), encapsulated by the synovial membrane (Fig. 2A). A joint affected by OA exhibits progressive degeneration of the articular cartilage, formation of bony peripheral outgrowths (osteophytes), changes in subchondral bone and thickening of both the synovium and ligaments (Fig. 2B), and in many cases synovial inflammation (synovitis), which is thought to be an important driver of early pathology (Benito et al., 2005). Pathologic roles for multiple tissues in deteriorating joint function therefore define OA as a whole joint disease, driven by various biomechanical and inflammatory factors. There are currently no treatments available to effectively prevent or reverse progressive joint damage; therefore, new and innovative treatments are urgently required to improve treatment options. This will require continued improvements in our understanding of the molecular mechanisms underlying OA pathology.
Figure 2.
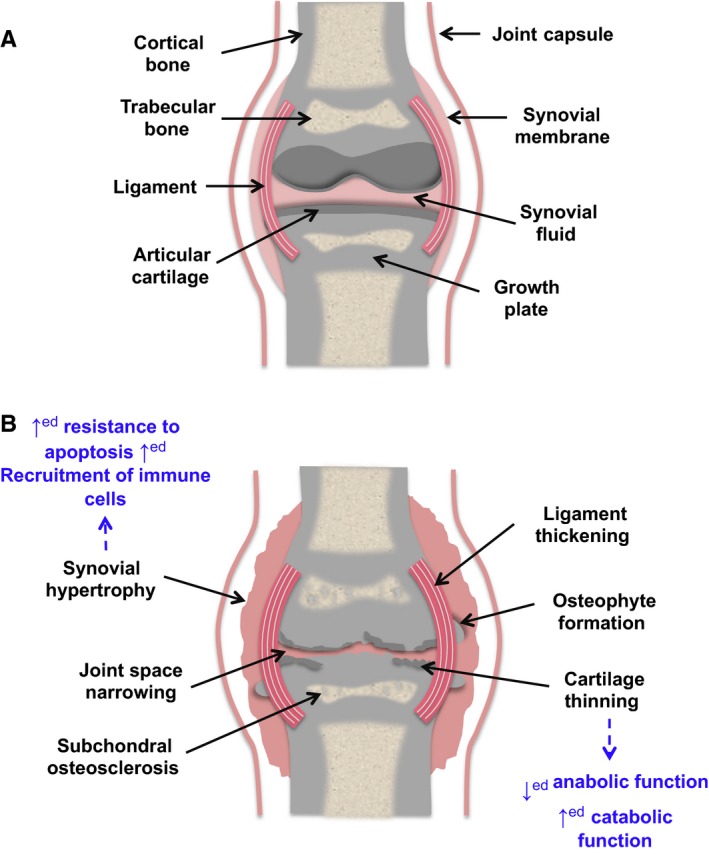
A comparison of a normal ‘healthy’ articular joint with that of a joint with OA. We also highlight the characteristics of the senescent phenotype that could potentially play a role in specific alterations seen in OA (seen in blue). (A) A diagram of a normal healthy joint. (B) A diagram of an OA‐affected joint, highlighting common changes, for example, cartilage degradation; synovial hypertrophy often accompanied by inflammation (synovitis); formation of peri‐articular osteophytes; osteosclerosis of subchondral bone.
Cellular senescence
In 1961, Leonard Hayflick and Paul Moorhead first described the phenomenon known as ‘cellular senescence’, a form of ‘senescence at the cellular level’ (Hayflick & Moorhead, 1961), stating that primary human fibroblasts have a restricted lifespan of around 50 cell divisions in culture. This was once believed to be purely an in vitro phenomenon caused by cell culture shock; however, many research groups observed senescent cells in premalignant tissues, and this was soon discovered to be an important process in vivo (Dimri et al., 1995; Serrano et al., 1997; Sherr & DePinho, 2000; Michaloglou et al., 2005; Narita & Lowe, 2005). Cellular senescence is now considered a signal transduction process that results in cells entering a stable state of growth arrest while remaining metabolically active. Senescent cells most commonly enter this stable state in G1 phase, or early S phase, of the cell cycle (Di Leonardo et al., 1994; Ogryzko et al., 1996; Serrano et al., 1997; Herbig et al., 2004). However, senescent cells have also been observed to undergo arrest in G2 phase (Mao et al., 2012). Senescence ultimately results in the loss of cellular replicative capacity due to the inability of these cells to express genes required for proliferation (Dimri et al., 1994, 1996). Senescence is not characterized by a specific set of markers, but rather by association with a collection of cellular phenotypes that often coexist in a stressed cellular environment, such as altered morphology, chromatin structure and gene expression patterns, and an activated DNA damage response (d'Adda di Fagagna et al., 2003; Di Micco et al., 2006, 2011; d'Adda di Fagagna, 2008; Rodier et al., 2009). Senescent cells secrete a variety of inflammatory cytokines, growth factors and many more soluble and insoluble factors known as the senescence‐associated secretory phenotype (SASP) (Campisi, 2005), or the senescence‐messaging secretome (SMS) (Kuilman & Peeper, 2009). These factors are secreted into the cell microenvironment, with cytokines such as IL‐6 and IL‐8 enforcing the stable growth arrest of senescent cells (Acosta et al., 2008; Kuilman et al., 2008). Various features of senescent cells, such as the SASP, can cause damage to surrounding tissue (Burton et al., 2007). SASP secreted by senescent cells can alter the tissue microenvironment, while the senescence of stem or progenitor cells can impair tissue regeneration (Koobatian et al., 2015). Cells undergo senescence in response to various detrimental stimuli, including but not limited to oncogene activation; radiation; oxidative stress; shortened telomeres; and unscheduled DNA replication. Senescence is known to evoke tumor suppression, and it is widely accepted that senescence functions as a protective mechanism against cancer due to its ability to induce the proliferation arrest of damaged cells (Michaloglou et al., 2005; Dhomen et al., 2009; Goel et al., 2009). Aside from cancer, senescence‐associated growth arrest is also important in normal physiological processes such as wound healing (Krizhanovsky et al., 2008).
Over the past decade, many studies have linked cellular senescence to aging (Krishnamurthy et al., 2004; Baker et al., 2008, 2013, 2016) and age‐related pathologies (Baker et al., 2011), thus leading to an overlap in research between the fields of disease processes and gerontology.
Cellular senescence and disease
In healthy individuals, the body utilizes various systems that help to prevent and/or repair cellular, molecular, and physiological damage to cells. However, these repair systems become progressively weaker during aging and the organism becomes more vulnerable to the development of a variety of diseases. Mammals such as mice, baboons, and humans have been reported to accumulate senescent cells as they age (Dimri et al., 1995; Krishnamurthy et al., 2004; Jeyapalan et al., 2007). Moreover, the accumulation of senescent cells in tissues contributes to both aging and the promotion of age‐related diseases (Krishnamurthy et al., 2004; Baker et al., 2008, 2016). For example, researchers have observed the presence of senescent cells (endothelial‐like cells, vascular smooth muscle cells, and macrophage‐like cells) in mice induced to develop atherosclerosis (Childs et al., 2016). Senescent vascular endothelial cells are present in human atherosclerotic lesions and contribute to atherogenesis (Minamino et al., 2002). In the context of OA, senescent cells were observed near the osteoarthritic lesions, but not in intact cartilage from the same patients and normal donors (Price et al., 2002; Erusalimsky & Kurz, 2005). Consistent with this, transplanted senescent cells induce an OA‐like state in mice (Xu et al., 2016).
We discuss below the potential mechanisms by which accumulation of senescent cells may predispose articular joints to the development and/or progression of OA. We will also discuss the role of epigenetic changes in senescent cells in the context of OA pathology and highlight potential epigenetic treatment options.
Senescence in osteoarthritis pathology
Although there are multiple joint tissues and cell types involved in OA pathology, chondrocytes have been the focus of the vast majority of studies to date that address a role for senescence. Chondrocytes are the only cell type present in articular cartilage, a highly specialized avascular and aneural tissue whose structural and mechanical properties are largely defined by the two predominant extracellular matrix (ECM) components, type II collagen, and aggrecan. Chondrocytes are responsible for producing and maintaining this ECM and receive nutrients and external chemical signals from the synovial fluid via secretions of fibroblast‐like synoviocytes of the intimal synovial layer.
Senescent cells exhibit a SASP that enables them to communicate with other cells, as well as the microenvironment, stimulating neighboring cells to senesce (Acosta et al., 2013). One characteristic feature of SASP is enhanced production of vascular endothelial growth factor (VEGF), a signal protein that promotes blood vessel formation via the processes of vasculogenesis and angiogenesis. VEGF and its cognate receptors are expressed in OA cartilage and may contribute to dysregulated osteogenesis and the formation of osteophytes (Pfander et al., 2001; Hashimoto et al., 2002; Enomoto et al., 2003). Chondrocyte SASP is known to include production of matrix‐degrading proteases including the matrix metalloproteinases MMP‐1, and ‐13 (Philipot et al., 2014). MMP‐13 is thought to be central to the irreversible degradation of the cartilage type II collagen lattice in OA, partly on the basis of exogenous expression or deficiency in murine studies (Neuhold et al., 2001; Little et al., 2009).
Obesity is a major risk factor in OA, and oxidative stress resulting from excess adiposity (Keaney et al., 2003; Furukawa et al., 2004) could therefore contribute to disease partly through reactive oxygen species (ROS)‐induced pathways. Excess adiposity is also associated with an increased accumulation of senescent cells and associated SASP factors (Schafer et al., 2016), as proposed (Tchkonia et al., 2010). Further, exercise prevents diet‐induced cellular senescence as well as the SASP within visceral adipose tissue (Schafer et al., 2016). This suggests a possible mechanism whereby exercise‐mediated health benefits may be mediated by the prevention of senescence.
Senescence in OA chondrocytes & cartilage
It is thought that cellular senescence may play a significant role in the pathology of OA, with OA chondrocytes exhibiting a variety of senescent‐associated phenotypes (discussed below and Fig. 3). Despite recent traction for views of OA as a whole joint disease rather than merely dysfunctional cartilage, chondrocytes remain regarded as key players in OA pathology and are understood to exhibit during disease a perturbation of the normal balance between synthesis and degradation of extracellular matrix (ECM) components. This involves upregulating the production of matrix‐degrading metalloproteinases such as MMP‐13, exogenous activity of which was sufficient to recapitulate key OA features in mice (Neuhold et al., 2001). Senescence of chondrocytes would be expected to lead similarly to shifting of the balance between ECM synthesis and degradation, through metalloproteinase components of the SASP response. Moreover, enhanced degradation of cartilage ECM by chondrocytes during OA may be partly due to demethylation of CpG sites in the promoter regions of genes encoding key cartilage‐degrading proteases, and therefore contributing to disease progression by increasing their production (Roach et al., 2005).
Figure 3.
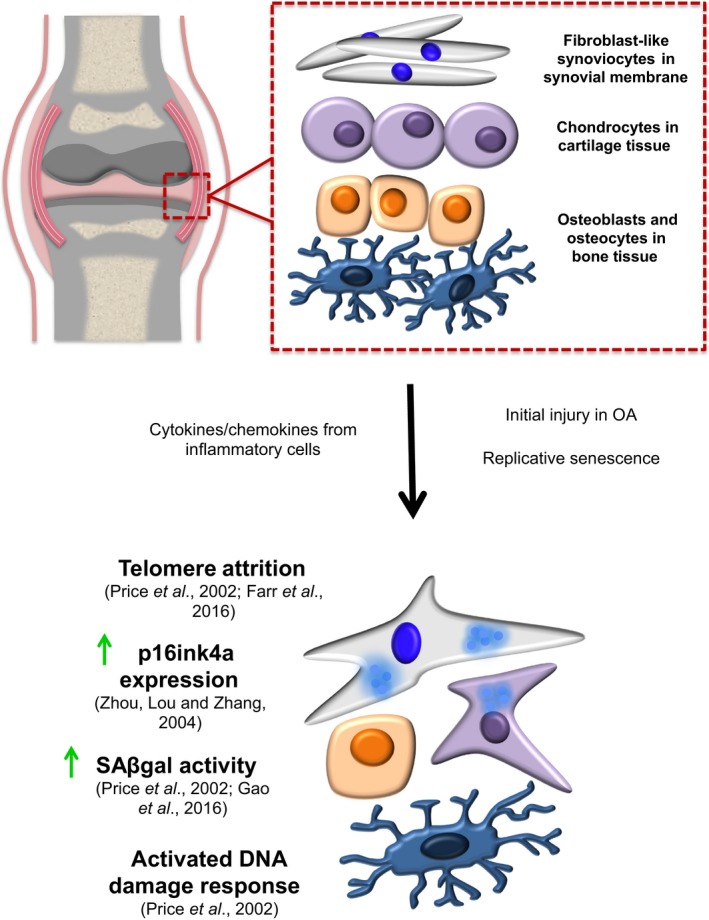
A comparison of the different characteristics observed in cell types found within joints of healthy subjects and patients with OA.
Aging and OA are not always interdependent, and many cases of OA in younger adults stem from joint injury (Gelber et al., 2000). However, as cellular senescence can result from a chronically stressed environment, it remains an interesting possibility that posttraumatic OA may be characterized or even partly triggered by an accumulation of senescent cells within damaged tissue. This view was supported, albeit in vitro, by the observation that mechanical stress accelerated chondrocyte senescence through increased oxidative stress (Martin et al., 2004a). Chondrocyte senescence is observed to be triggered by oxidative stress (Martin et al., 2004b) and is expected to contribute to the abnormal inflammatory environment present in OA. Chondrocytes exhibit very low metabolic activity and are well adapted to the hypoxic conditions of the joint, although exacerbated hypoxia may drive synovial inflammation in rheumatoid arthritis (RA) (an age‐related autoimmune inflammatory joint disease characterized by joint destruction, chronic inflammation, and dysfunction of innate and adaptive immune responses) contributing to pathology (Ng et al., 2010). As synovitis is an acknowledged feature of OA, it is plausible that enhanced hypoxia could play a similar pathologic role in this scenario (Giatromanolaki et al., 2003). Similarly, chronic oxidative stress experienced by other joint tissues during disease may also lead to cellular senescence.
When compared to isolated chondrocytes from normal cartilage, OA chondrocytes positively express a variety of senescence‐associated markers, for example, telomere attrition (Martin & Buckwalter, 2001); activated DNA damage response (DDR); ROS secretion; SAβGal activity (Price et al., 2002); increased p16ink4a expression (Zhou et al., 2004); accumulation of MMPs induced by pro‐inflammatory cytokines (Billinghurst et al., 1997; Shlopov et al., 1997; Fig. 3). This has led to speculation that the integrity and function of the cartilage becomes impaired due in part to the age‐related accumulation of senescent chondrocytes. In 2001, Martin and Buckwalter described telomere erosion in OA chondrocytes (Martin & Buckwalter, 2001). However, in normal articular cartilage, the rate of chondrocyte mitosis is very low (Aigner et al., 2001). This limited proliferative capacity in normal cartilage would suggest that other stress factors, such as oxidative stress (Yudoh et al., 2005) and abnormal mechanical loading (Harbo et al., 2012, 2013), may contribute to the shortening of telomeres of OA chondrocytes, contributing to pathology via premature senescence. Premature senescence of chondrocytes could then be a consequence of both intrinsic (limited replicative capacity in situ) and extrinsic (stress‐induced) factors provoking the age‐dependent deterioration of chondrocytes. On the other hand, as proliferation of chondrocytes is actually increased in OA cartilage (Aigner et al., 2001), this might help explain observed telomere erosion in disease. In knee OA, Gao et al. (2016) demonstrated a correlation between SAβGal expression and disease severity of patients. They investigated the levels of SAβGal expression in normal cartilage compared with OA cartilage of differing severity (mild, moderate, and severe). In normal articular cartilage, no staining was observed. However, in lesions taken from mild, moderate, and severely damaged knee OA cartilage, they observed SAβGal staining in a subset of chondrocytes close to the lesion. It is important to highlight that the elevation of SAβGal in cultured cells isolated from diseased joints is not a reliable indicator of pathological involvement of senescence in vivo. Hence, more functional experiments are required to investigate role of senescent cells in the in vivo development of OA.
Chondrocyte turnover is thought be a rare event in cartilage; however, these cells proliferate when removed from the tissue and placed in culture. In human OA lesions, senescent cells are often found near clusters of cells, indicating increased mitotic activity prior to senescence (Price et al., 2002). Further, senescent cells are known to accumulate in tissues as we age and the mere presence of them in a disease context could be a consequence of the normal aging process. For example, Martin and Buckwalter (2001) showed that an association between OA and aging is due in part to replicative senescence of chondrocytes in vivo. However, evidence for direct involvement of senescent cells in cartilage damage comes from the senescent cell transplantation mouse model (Xu et al., 2016). In a recent study, Xu et al. injected either senescent or nonsenescent cells into the knee joint area of mice. Authors showed that transplanting senescent cells into the knee region caused pathological features suggestive of OA (Xu et al., 2016). More specifically, knee joints injected with senescent cells exhibited severe articular cartilage damage at the lateral and medial tibial plateaus, as well as the femoral condyles. This would suggest targeting senescent cells might be an attractive therapeutic modality for treatment of OA. However, it is not yet fully understood how the mechanisms of chondrocyte senescence contribute to cartilage degradation, and further mechanistic studies are urgently needed.
Senescence in the bone microenvironment
Recently senescent cells have been identified within the bone microenvironment (Farr et al., 2016). Comparing the presence of senescence and SASP markers in young (6 month) and old (24 month) mice, osteoblasts and osteocytes retrieved from trabecular and cortical skeletal tissue in older animals showed an increase in expression of p16Ink4a, a cell cycle inhibitor seen to increase with age (Krishnamurthy et al., 2004; Waaijer et al., 2012; Burd et al., 2013; Farr et al., 2016), concomitant with an increase in senescent osteocytes present within the bone cortex. Telomere dysfunction‐induced foci were also more prevalent in osteocytes from old mice. These findings suggest age‐related bone loss could be, in part, caused by cellular osteocyte senescence, given the vital role of these cells in bone remodeling.
Autophagy
Autophagy is a cellular process thought to be a mechanism for cell survival when cells become stressed, for example under hypoxia or nutrient deprivation, in which cells degrade dysfunctional proteins and macromolecules and recycle them to produce the necessary raw materials for protein synthesis (Narita et al., 2011). There is an increasing interest in the role of autophagy in cartilage biology; this process may provide a key link between aging, cell survival, and OA. For instance, autophagy appears to be constitutively active in articular cartilage but decreases with age; an increase in apoptotic chondrocyte death was associated with a decline in autophagy and increased cartilage damage (Carames et al., 2010, 2015). Furthermore, chondrocyte‐specific deficiency of the autophagy factor ATG5 was recently shown to promote age‐related OA features in mice, concomitant with increased chondrocyte apoptosis (Bouderlique et al., 2016).
Advancing age is associated with dysregulated autophagy in cells such as cardiac myocytes (Terman et al., 2003); this dysregulation appears to result in oxidative stress and subsequent cellular senescence (Wu et al., 2009; Toshima et al., 2014). It is possible that these processes observed in other cell types also play a role in the cellular senescence observed in OA. Age‐related loss of skeletal muscle is a major cause of movement impairment during later stages of human life (García‐Prat et al., 2016). It is thought that stem cells in the muscle lose their regenerative function as we age and contribute to structural and functional decline of the muscle tissue, reviewed in detail (Grounds, 2014). In 2016, García‐Prat et al. (2016) described in detail the relationship between cell survival and autophagy in muscle stem/progenitor cells, with physiologically aged satellite cells undergoing senescence due to a decrease in autophagy. Young satellite cells entered senescence due to increased mitochondrial dysfunction, oxidative stress, and failure of proteostasis. By restoring the autophagy pathway in aged cells, these workers were able to show the reversal of senescence and restoration of regenerative functions (García‐Prat et al., 2016). Extrapolating to the context of OA, this suggests that targeting improved autophagy in joint tissues could provide a potential therapy that may lead to a decrease in inflammation, along with enhanced regeneration of joint tissues. This is a tempting idea given not only the poor regenerative capacity of articular cartilage in OA, but also with regard to senescence suppression strategies aimed at enhancing the capacity for potential use of autologous chondrocyte populations for tissue regeneration/engineering applications (Ashraf et al., 2016).
It is clear from the studies described above that an increased understanding of the senescent program in relation to OA would provide beneficial insight into the molecular mechanisms occurring within OA joints, which could serve to broaden the spectrum of therapeutic opportunities for the treatment of this debilitating disease. Furthermore, a renewed effort to understand cellular senescence in joint tissues other than cartilage might contribute to a more holistic view of the role of this process in OA pathology.
Epigenetic changes in OA and cellular senescence
Both OA and cellular senescence are characterized by various epigenetic changes thought to contribute to altered cellular phenotypes and disease progression. Typically, epigenetic mechanisms can be clustered into three categories: DNA methylation involving the methylation of CpG islands; histone modifications such as acetylation, methylation, ubiquitination, and phosphorylation; and regulatory micro RNAs (small noncoding sequences involved in gene expression). We have already discussed various striking similarities of senescent cells with cells found in joint tissues during OA (particularly chondrocytes). In the following section, we will discuss the role of epigenetic changes in the context of OA, highlighting the similarities with those seen in senescence.
In human OA chondrocytes, DNA demethylation of MMP13 promoter region CpG sites is observed to enhance the expression of MMP‐13 (Bui et al., 2012), a key enzyme contributing to irreversible cartilage matrix destruction. Furthermore, demethylation of the promoter sites of various other matrix‐degrading enzymes such as MMP‐3, MMP‐9 and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)‐4 has been associated with their enhanced expression in OA (Roach et al., 2005). Expression of MMP‐3, an important proteolytic activator of pro‐collagenases, is extensively used as confirmation of the senescent phenotype in vitro and is recognized as a ‘marker’ of senescence. In fibroblasts, loss of DNA methylation activity may cause p21‐dependent cell cycle withdrawal and senescence (Young & Smith, 2001). Interestingly, chemically mediated DNA demethylation in chondrocytes induces terminal hypertrophic differentiation (Cheung et al., 2001), a process essential to long bone growth in development that is recapitulated during OA (von der Mark et al., 1992). Beyond a commonality of enhanced MMP‐13 expression (D'Angelo et al., 2000), it is unclear whether there is any significant relationship between this state and cellular senescence, although cellular hypertrophy has been linked to senescence in fibroblasts (Demidenko & Blagosklonny, 2008).
It is yet to be determined whether changes in DNA methylation are causative in OA or a consequence of the disease. In human mesenchymal stem cells, workers have reported (Ezura et al., 2009) the presence of low levels of DNA methylation at the CpG promoters of SOX9, whose product maintains the chondrocyte phenotype (Lefebvre et al., 1997) and RUNX2, whose product promotes chondrocyte hypertrophy (an important factor in driving cartilage destruction) and subsequent mineralization or apoptosis (von der Mark et al., 1992; Enomoto et al., 2000; Yang et al., 2001; Cheung et al., 2003; Zheng et al., 2003; Kamekura et al., 2006). While SOX9 and RUNX2 gene products are critical master transcriptional regulators of chondrocyte differentiation, profoundly regulating chondrogenesis and osteogenesis, the consequences of differential methylation of their gene promoters in terms of OA are as yet unclear.
Interleukin(IL)‐1β, IL‐6, and TNF‐α, all present in the SASP of senescent cells, are key pro‐inflammatory cytokines associated with OA. In addition to their confirmed role in driving destructive MMP expression, IL‐1β and TNF‐α (in synergistic combination with the IL‐6 type cytokine, oncostatin M) have been shown to stimulate changes in the methylation pattern of the IL1B gene (Hashimoto et al., 2009), leading to potent and sustained mRNA induction. This then poses an important question of how inflammatory stimuli alter the DNA methylation pattern seen in OA. Could an influx or accumulation of senescent cells and their SASP within the joints promote these epigenetic changes?
Interestingly, hypomethylation changes are also observed in senescent cells, as well as in cancer. For example, Cruickshanks et al. (2013) performed whole‐genome single‐nucleotide bisulfide sequencing on human replicative senescent cells and showed widespread DNA hypomethylation, as well as focal hypermethylation of senescent cells. It is well known that one of the hallmarks of cancer is an alteration in DNA methylation resulting in the destabilization of both genome integrity and function (Matsuo et al., 1993; de Wind et al., 1995). In human cancer cells, one of the first epigenetic alterations to be observed was the low level of methylation in tumors when compared to non‐neoplastic cells (Feinberg & Vogelstein, 1983). As OA is an age‐associated disease, it is tempting to speculate that these strikingly similar features of DNA methylation could reflect the involvement of senescence gene expression programs in the pathogenesis of OA.
Gaining an understanding of the pathways and mechanisms involved in epigenetic alterations, as well as investigating the effect of various epigenetic drugs in the context of OA, would provide a promising future research area with the potential of uncovering innovative therapeutic advances. Such a method of treatment could potentially revolutionize how age‐related diseases such as OA are treated and possibly even prevented in the future.
Future perspectives
This review explores the potential of the senescent phenotype to predispose joint tissues to the development and/or progression of OA. Senescent cells have the ability to synergize with inflammation and inflammaging, already present within the OA joint microenvironment, to drive further cellular senescent conversion and compound existing age‐related tissue damage, thus exacerbating and accelerating joint destruction. During OA, chondrocytes are seen to become ‘activated’ and express a variety of senescence‐associated markers. The exact mechanism by which senescence of chondrocytes and especially other joint cells such as synovial fibroblasts, osteoblasts, and osteocytes contribute to OA is not fully understood, although the accumulation of senescent chondrocytes in the joints is widely thought to impair cartilage integrity. Activated chondrocytes secrete a potent cocktail of cartilage‐degrading MMPs into the cartilage matrix, driving the development and progression of OA (Price et al., 2002). A study demonstrating the presence of senescent osteoblasts and osteocytes within the bone microenvironment of aged mice suggests an interesting potential to target these cell types to delay, or even prevent, age‐related bone loss (Farr et al., 2016).
Given this potential to play a key role in OA pathology, innovative treatments could be developed by gaining an understanding of the underlying mechanisms by which cellular senescence contributes to OA. Recent studies have exposed numerous common characteristics between OA cartilage and preneoplastic tissues, one of the most prevalent being cellular senescence. These recent findings have inspired researchers to explore the potential of using anticancer treatments to slow or prevent the development and/or progression of OA.
Potential innovative therapeutic approaches
Senotherapeutic agents are used to target specific properties of cellular senescence; more specifically, senolytics are used to target anti‐apoptotic mechanisms and induce cell death within senescent cells (Zhu et al., 2015a,b; Chang et al., 2016). Senolytic drugs may therefore also be potentially used to provide an innovative therapeutic approach to treatment of various conditions. Dasatinib is currently used in the treatment of cancer. It is widely accepted that cancer cells and senescent cells share common anti‐apoptotic characteristics, and the combination treatment of dasatinib and quercetin has already been observed to reduce the burden of senescent cells, as well as enhance cardiovascular function, in aged mice (Zhu et al., 2015b). These workers reported, in Ercc1 −/∆ mice exhibiting an accelerated aging condition, that periodic administration of dasatinib and quercetin was seen to delay bone loss and neurological dysfunction and to enhance health span (Zhu et al., 2015b). We would emphasize that not all senolytic compounds are anticancer and not all anticancer compounds are senolytic. Even among senolytics there seems to be cell type specific responses, for example, dasatinib is more effective in killing senescent human preadipocytes than human umbilical vein endothelial cells (HUVECs), whereas quercetin is effective in killing senescent HUVECs rather than senescent adipocytes. More mechanistic work is needed to clarify the exact relationship of senolytics to age‐related disorders such as cancer and OA.
We have reviewed a body of work that, taken together, strongly suggests that senescence could play a significant role in the pathogenesis of OA. Therefore, if dasatinib/quercetin combination therapy is effective in eliminating senescent cells, it could provide an extremely appealing therapeutic target for OA. Although OA, unlike osteoporosis, involves not loss but mostly local increases in bone synthesis, the bisphosphonate anti‐osteoporosis drug, alendronate, has been reported to ameliorate experimental OA progression (Hayami et al., 2004). This suggests the possibility that senolytic drugs could also be of therapeutic value in both diseases, perhaps by preventing (likely via reducing inflammatory signaling) aberrant bone metabolism.
Epigenetic alterations have cellular impacts potentially contributing to OA development and progression, such as histone modifications that regulate catabolic mediators in cartilage (Culley et al., 2013), and DNA methylation present in genes known to regulate cartilage development (Ezura et al., 2009). In RA, the effects of epigenetic drugs are already being investigated. For instance, JQ1 is a BET bromodomain inhibitor known to downregulate Myc transcription (Delmore Jake et al., 2011). Myc is a transcription factor known to be upregulated in various cancers as well as aiding in the growth and invasiveness of RA synovial fibroblasts (RASFs) (Pap et al., 2004), a key cell type mediating joint inflammation and destruction seen in RA (Bucala et al., 1991; Lefevre et al., 2009). Recently, Zhang et al. (2015) showed that inhibition of bromodomain‐containing protein 4 (BRD4) by JQ1 caused a reduction in the inflammatory response, joint damage, and also autoantibody production in collagen‐induced arthritis (CIA) murine models. Xiao et al. (2016) further highlighted the therapeutic potential of JQ1 in inflammatory arthritis, stating JQ1 decreased the proliferation of RASFs, as well as decreasing the production of various cytokines, MMPs, and synovial inflammation in CIA. These findings, although in the context of RA, provide promising experimental evidence that targeting various epigenetic alterations could be used in the future for OA treatment, especially as synovial inflammation is now recognized to be a prominent feature of OA. Further research into the effect of epigenetic alterations in OA offers the potential to uncover novel pathways whose targeting could aid in improved treatment, delayed progression, or even possible reversal of OA.
Conclusion
The research of recent years has established that OA is not simply a passive ‘wear and tear’ disorder, but rather a complex age‐related disease involving various different effectors, ranging from inflammatory mediators to epigenetic alterations. This complexity has undoubtedly contributed to the current lack of effective treatment options for OA, with pain management and eventual joint replacement surgery a common endpoint for a large proportion of patients. In this review, we have highlighted the potential mechanisms by which senescent cells can predispose joints of the body to the development and/or progression of OA, and explored the potential of using senolytic drugs to target senescent cells present in OA. In addition, targeting the epigenetic alterations observed in OA provides a promising approach to treatment as, unlike genetic changes, epigenetic changes can be reversed. In conclusion, gaining a better understanding of molecular mechanisms by which the senescence pathway and epigenetic changes underpin OA pathogenesis could open up novel and innovative therapeutic approaches.
Author contributions
KM and TSR came up with the ideas and the theories. KM and TSR wrote the review with the help of GJL.
Funding
No funding information provided.
Conflict of interest
Authors declare there is no conflict of interest.
Acknowledgments
We thank members of the CMS group at IBEHR, University of the West of Scotland, and Institute of Infection and Immunity at the University of Glasgow for scientific discussions.
References
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, d'Adda di Fagagna F, Bernard D, Hernando E, Gil J (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018. [DOI] [PubMed] [Google Scholar]
- Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J (2013) A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Adda di Fagagna F (2008) Living on a break: cellular senescence as a DNA‐damage response. Nat. Rev. Cancer 8, 512–522. [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay‐Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP (2003) A DNA damage checkpoint response in telomere‐initiated senescence. Nature 426, 194–198. [DOI] [PubMed] [Google Scholar]
- Aigner T, Hemmel M, Neureiter D, Gebhard PM, Zeiler G, Kirchner T, McKenna L (2001) Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 44, 1304–1312. [DOI] [PubMed] [Google Scholar]
- Ashraf S, Ahn J, Cha BH, Kim JS, Han I, Park H, Lee SH (2016) RHEB: a potential regulator of chondrocyte phenotype for cartilage tissue regeneration. J. Tissue Eng. Regen. Med. DOI: 10.1002/term.2148. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Perez‐Terzic C, Jin F, Pitel K, Niederlander NJ, Jeganathan K, Yamada S, Reyes S, Rowe L, Hiddinga HJ, Eberhardt NL, Terzic A, van Deursen JM (2008) Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat. Cell Biol. 10, 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM (2011) Clearance of p16Ink4a‐positive senescent cells delays ageing‐associated disorders. Nature 479, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Weaver RL, van Deursen JM (2013) p21 both attenuates and drives senescence and aging in BubR1 progeroid mice. Cell Rep. 3, 1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM (2016) Naturally occurring p16(Ink4a)‐positive cells shorten healthy lifespan. Nature 530, 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B (2005) Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 64, 1263–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole AR (1997) Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J. Clin. Invest. 99, 1534–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouderlique T, Vuppalapati KK, Newton PT, Li L, Barenius B, Chagin AS (2016) Targeted deletion of Atg5 in chondrocytes promotes age‐related osteoarthritis. Ann. Rheum. Dis. 75, 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R, Ritchlin C, Winchester R, Cerami A (1991) Constitutive production of inflammatory and mitogenic cytokines by rheumatoid synovial fibroblasts. J. Exp. Med. 173, 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui C, Barter MJ, Scott JL, Xu Y, Galler M, Reynard LN, Rowan AD, Young DA (2012) cAMP response element‐binding (CREB) recruitment following a specific CpG demethylation leads to the elevated expression of the matrix metalloproteinase 13 in human articular chondrocytes and osteoarthritis. FASEB J. 26, 3000–3011. [DOI] [PubMed] [Google Scholar]
- Burd CE, Sorrentino JA, Clark KS, Darr DB, Krishnamurthy J, Deal AM, Bardeesy N, Castrillon DH, Beach DH, Sharpless NE (2013) Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)‐luciferase model. Cell 152, 340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DG, Sheerin AN, Ostler EL, Smith K, Giles PJ, Lowe J, Rhys‐Williams W, Kipling DG, Faragher RG (2007) Cyclin D1 overexpression permits the reproducible detection of senescent human vascular smooth muscle cells. Ann. N. Y. Acad. Sci. 1119, 20–31. [DOI] [PubMed] [Google Scholar]
- Campisi J (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522. [DOI] [PubMed] [Google Scholar]
- Carames B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M (2010) Autophagy is a protective mechanism in normal cartilage, and its aging‐related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 62, 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carames B, Olmer M, Kiosses WB, Lotz MK (2015) The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol. 67, 1568–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Wang Y, Shao L, Laberge R‐M, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin‐Burns N, Krager K, Ponnappan U, Hauer‐Jensen M, Meng A, Zhou D (2016) Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung JOP, Hillarby MC, Ayad S, Hoyland JA, Jones CJP, Denton J, Thomas JT, Wallis GA, Grant ME (2001) A novel cell culture model of chondrocyte differentiation during mammalian endochondral ossification. J. Bone Miner. Res. 16, 309–318. [DOI] [PubMed] [Google Scholar]
- Cheung JO, Grant ME, Jones CJ, Hoyland JA, Freemont AJ, Hillarby MC (2003) Apoptosis of terminal hypertrophic chondrocytes in an in vitro model of endochondral ossification. J. Pathol. 201, 496–503. [DOI] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM (2016) Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L (2014) The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 73, 1323–1330. [DOI] [PubMed] [Google Scholar]
- Cruickshanks HA, McBryan T, Nelson DM, Vanderkraats ND, Shah PP, van Tuyn J, Singh Rai T, Brock C, Donahue G, Dunican DS, Drotar ME, Meehan RR, Edwards JR, Berger SL, Adams PD (2013) Senescent cells harbour features of the cancer epigenome. Nat. Cell Biol. 15, 1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley KL, Hui W, Barter MJ, Davidson RK, Swingler TE, Destrument APM, Scott JL, Donell ST, Fenwick S, Rowan AD, Young DA, Clark IM (2013) Class I histone deacetylase inhibition modulates metalloproteinase expression and blocks cytokine‐induced cartilage degradation. Arthritis Rheum. 65, 1822–1830. [DOI] [PubMed] [Google Scholar]
- D'Angelo M, Yan Z, Nooreyazdan M, Pacifici M, Sarment DS, Billings PC, Leboy PS (2000) MMP‐13 is induced during chondrocyte hypertrophy. J. Cell. Biochem. 77, 678–693. [PubMed] [Google Scholar]
- Delmore Jake E, Issa Ghayas C, Lemieux Madeleine E, Rahl Peter B, Shi J, Jacobs Hannah M, Kastritis E, Gilpatrick T, Paranal Ronald M, Qi J, Chesi M, Schinzel Anna C, McKeown Michael R, Heffernan Timothy P, Vakoc Christopher R, Bergsagel PL, Ghobrial Irene M, Richardson Paul G, Young Richard A, Hahn William C, Anderson Kenneth C, Kung Andrew L, Bradner JE, Mitsiades CS (2011) BET bromodomain inhibition as a therapeutic strategy to target c‐Myc. Cell 146, 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidenko ZN, Blagosklonny MV (2008) Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle 7, 3355–3361. [DOI] [PubMed] [Google Scholar]
- Dhomen N, Reis‐Filho JS, da Rocha Dias S, Hayward R, Savage K, Delmas V, Larue L, Pritchard C, Marais R (2009) Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell 15, 294–303. [DOI] [PubMed] [Google Scholar]
- Di Leonardo A, Linke SP, Clarkin K, Wahl GM (1994) DNA damage triggers a prolonged p53‐dependent G1 arrest and long‐term induction of Cip1 in normal human fibroblasts. Genes Dev. 8, 2540–2551. [DOI] [PubMed] [Google Scholar]
- Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre M, Giovanni Nuciforo P, Bensimon A, Maestro R, Giuseppe Pelicci P, d'Adda di Fagagna F (2006) Oncogene‐induced senescence is a DNA damage response triggered by DNA hyper‐replication. Nature 444, 638–642. [DOI] [PubMed] [Google Scholar]
- Di Micco R, Sulli G, Dobreva M, Liontos M, Botrugno OA, Gargiulo G, dal Zuffo R, Matti V, d'Ario G, Montani E, Mercurio C, Hahn WC, Gorgoulis V, Minucci S, d'Adda di Fagagna F (2011) Interplay between oncogene‐induced DNA damage response and heterochromatin in senescence and cancer. Nat. Cell Biol. 13, 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Hara E, Campisi J (1994) Regulation of two E2F‐related genes in presenescent and senescent human fibroblasts. J. Biol. Chem. 269, 16180–16186. [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira‐Smith O, Peacocke M, Campisi J (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo . Proc. Natl Acad. Sci. USA 92, 9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Testori A, Acosta M, Campisi J (1996) Replicative senescence, aging and growth‐regulatory transcription factors. Biol. Signals 5, 154–162. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Enomoto‐Iwamoto M, Iwamoto M, Nomura S, Himeno M, Kitamura Y, Kishimoto T, Komori T (2000) Cbfa1 is a positive regulatory factor in chondrocyte maturation. J. Biol. Chem. 275, 8695–8702. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Inoki I, Komiya K, Shiomi T, Ikeda E, Obata K‐I, Matsumoto H, Toyama Y, Okada Y (2003) Vascular endothelial growth factor isoforms and their receptors are expressed in human osteoarthritic cartilage. Am. J. Pathol. 162, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erusalimsky JD, Kurz DJ (2005) Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp. Gerontol. 40, 634–642. [DOI] [PubMed] [Google Scholar]
- Ezura Y, Sekiya I, Koga H, Muneta T, Noda M (2009) Methylation status of CpG islands in the promoter regions of signature genes during chondrogenesis of human synovium–derived mesenchymal stem cells. Arthritis Rheum. 60, 1416–1426. [DOI] [PubMed] [Google Scholar]
- Farr JN, Fraser DG, Wang H, Jaehn K, Ogrodnik MB, Weivoda MM, Drake MT, Tchkonia T, LeBrasseur NK, Kirkland JL, Bonewald LF, Pignolo RJ, Monroe DG, Khosla S (2016) Identification of senescent cells in the bone microenvironment. J. Bone Miner. Res. 31, 1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B (1983) Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature 301, 89–92. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114, 1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S‐G, Zeng C, Li L‐J, Luo W, Zhang F‐J, Tian J, Cheng C, Tu M, Xiong Y‐L, Jiang W, Xu M, Lei G‐H (2016) Correlation between senescence‐associated beta‐galactosidase expression in articular cartilage and disease severity of patients with knee osteoarthritis. Int. J. Rheum. Dis. 19, 226–232. [DOI] [PubMed] [Google Scholar]
- García‐Prat L, Martínez‐Vicente M, Perdiguero E, Ortet L, Rodríguez‐Ubreva J, Rebollo E, Ruiz‐Bonilla V, Gutarra S, Ballestar E, Serrano AL, Sandri M, Muñoz‐Cánoves P (2016) Autophagy maintains stemness by preventing senescence. Nature 529, 37–42. [DOI] [PubMed] [Google Scholar]
- Gelber AC, Hochberg MC, Mead LA, Wang N, Wigley FM, Klag MJ (2000) Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann. Intern. Med. 133, 321–328. [DOI] [PubMed] [Google Scholar]
- Giatromanolaki A, Sivridis E, Maltezos E, Athanassou N, Papazoglou D, Gatter KC, Harris AL, Koukourakis MI (2003) Upregulated hypoxia inducible factor‐1alpha and ‐2alpha pathway in rheumatoid arthritis and osteoarthritis. Arthritis. Res. Ther. 5, R193–R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel VK, Ibrahim N, Jiang G, Singhal M, Fee S, Flotte T, Westmoreland S, Haluska FS, Hinds PW, Haluska FG (2009) Melanocytic nevus‐like hyperplasia and melanoma in transgenic BRAFV600E mice. Oncogene 28, 2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grounds MD (2014) Therapies for sarcopenia and regeneration of old skeletal muscles: more a case of old tissue architecture than old stem cells. Bioarchitecture 4, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbo M, Bendix L, Bay‐Jensen A‐C, Graakjaer J, Søe K, Andersen TL, Kjaersgaard‐Andersen P, Koelvraa S, Delaisse J‐M (2012) The distribution pattern of critically short telomeres in human osteoarthritic knees. Arthritis. Res. Ther. 14, R12–R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbo M, Delaisse JM, Kjaersgaard‐Andersen P, Soerensen FB, Koelvraa S, Bendix L (2013) The relationship between ultra‐short telomeres, aging of articular cartilage and the development of human hip osteoarthritis. Mech. Ageing Dev. 134, 367–372. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Creighton‐Achermann L, Takahashi K, Amiel D, Coutts RD, Lotz M (2002) Development and regulation of osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage 10, 180–187. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Oreffo ROC, Gibson MB, Goldring MB, Roach HI (2009) DNA De‐methylation at specific CpG sites in the IL1B promoter in response to inflammatory cytokines in human articular chondrocytes. Arthritis Rheum. 60, 3303–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayami T, Pickarski M, Wesolowski GA, McLane J, Bone A, Destefano J, Rodan GA, Duong LT (2004) The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 50, 1193–1206. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621. [DOI] [PubMed] [Google Scholar]
- Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM (2004) Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol. Cell 14, 501–513. [DOI] [PubMed] [Google Scholar]
- Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U (2007) Accumulation of senescent cells in mitotic tissue of aging primates. Mech. Ageing Dev. 128, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamekura S, Kawasaki Y, Hoshi K, Shimoaka T, Chikuda H, Maruyama Z, Komori T, Sato S, Takeda S, Karsenty G, Nakamura K, Chung UI, Kawaguchi H (2006) Contribution of runt‐related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 54, 2462–2470. [DOI] [PubMed] [Google Scholar]
- Keaney JF, Larson MG, Vasan RS, Wilson PWF, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ (2003) Obesity and systemic oxidative stress. Arterioscler. Thromb. Vasc. Biol. 23, 434. [DOI] [PubMed] [Google Scholar]
- Koobatian MT, Liang MS, Swartz DD, Andreadis ST (2015) Differential effects of culture senescence and mechanical stimulation on the proliferation and leiomyogenic differentiation of MSC from different sources: implications for engineering vascular grafts. Tissue Eng. Part A 21, 1364–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al‐Regaiey K, Su L, Sharpless NE (2004) Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114, 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW (2008) Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Peeper DS (2009) Senescence‐messaging secretome: SMS‐ing cellular stress. Nat. Rev. Cancer 9, 81–94. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS (2008) Oncogene‐induced senescence relayed by an interleukin‐dependent inflammatory network. Cell 133, 1019–1031. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B (1997) SOX9 is a potent activator of the chondrocyte‐specific enhancer of the pro alpha1(II) collagen gene. Mol. Cell. Biol. 17, 2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre S, Knedla A, Tennie C, Kampmann A, Wunrau C, Dinser R, Korb A, Schnaker EM, Tarner IH, Robbins PD, Evans CH, Sturz H, Steinmeyer J, Gay S, Scholmerich J, Pap T, Muller‐Ladner U, Neumann E (2009) Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat. Med. 15, 1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, Shah M, Thompson EW (2009) Matrix metalloproteinase 13‐deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 60, 3723–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Ke Z, Gorbunova V, Seluanov A (2012) Replicatively senescent cells are arrested in G1 and G2 phases. Aging 4, 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Mark K, Kirsch T, Nerlich A, Kuss A, Weseloh G, Gluckert K, Stoss H (1992) Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 35, 806–811. [DOI] [PubMed] [Google Scholar]
- Martin JA, Buckwalter JA (2001) Telomere erosion and senescence in human articular cartilage chondrocytes. J. Gerontol. A Biol. Sci. Med. Sci. 56, B172–B179. [DOI] [PubMed] [Google Scholar]
- Martin JA, Brown T, Heiner A, Buckwalter JA (2004a) Post‐traumatic osteoarthritis: the role of accelerated chondrocyte senescence. Biorheology 41, 479–491. [PubMed] [Google Scholar]
- Martin JA, Klingelhutz AJ, Moussavi‐Harami F, Buckwalter JA (2004b) Effects of oxidative damage and telomerase activity on human articular cartilage chondrocyte senescence. J. Gerontol. A Biol. Sci. Med. Sci. 59, 324–337. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Tang SH, Zeki K, Gutman RA, Fagin JA (1993) Aberrant deoxyribonucleic acid methylation in human thyroid tumors. J. Clin. Endocrinol. Metab. 77, 991–995. [DOI] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS (2005) BRAFE600‐associated senescence‐like cell cycle arrest of human naevi. Nature 436, 720–724. [DOI] [PubMed] [Google Scholar]
- Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I (2002) Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105, 1541–1544. [DOI] [PubMed] [Google Scholar]
- Narita M, Lowe SW (2005) Senescence comes of age. Nat. Med. 11, 920–922. [DOI] [PubMed] [Google Scholar]
- Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, Yoshida S, Hong S, Berry LS, Reichelt S, Ferreira M, Tavare S, Inoki K, Shimizu S (2011) Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 332, 966–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, Poole AR, Babij P, DeGennaro LJ (2001) Postnatal expression in hyaline cartilage of constitutively active human collagenase‐3 (MMP‐13) induces osteoarthritis in mice. J. Clin. Invest. 107, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CT, Biniecka M, Kennedy A, McCormick J, Fitzgerald O, Bresnihan B, Buggy D, Taylor CT, O'Sullivan J, Fearon U, Veale DJ (2010) Synovial tissue hypoxia and inflammation in vivo . Ann. Rheum. Dis. 69, 1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogryzko VV, Hirai TH, Russanova VR, Barbie DA, Howard BH (1996) Human fibroblast commitment to a senescence‐like state in response to histone deacetylase inhibitors is cell cycle dependent. Mol. Cell. Biol. 16, 5210–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap T, Nawrath M, Heinrich J, Bosse M, Baier A, Hummel KM, Petrow P, Kuchen S, Michel BA, Gay RE, Muller‐Ladner U, Moelling K, Gay S (2004) Cooperation of Ras‐ and c‐Myc‐dependent pathways in regulating the growth and invasiveness of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheum. 50, 2794–2802. [DOI] [PubMed] [Google Scholar]
- Pfander D, Kortje D, Zimmermann R, Weseloh G, Kirsch T, Gesslein M, Cramer T, Swoboda B (2001) Vascular endothelial growth factor in articular cartilage of healthy and osteoarthritic human knee joints. Ann. Rheum. Dis. 60, 1070–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipot D, Guerit D, Platano D, Chuchana P, Olivotto E, Espinoza F, Dorandeu A, Pers YM, Piette J, Borzi RM, Jorgensen C, Noel D, Brondello JM (2014) p16INK4a and its regulator miR‐24 link senescence and chondrocyte terminal differentiation‐associated matrix remodeling in osteoarthritis. Arthritis. Res. Ther. 16, R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, Clark IM (2002) The role of chondrocyte senescence in osteoarthritis. Aging Cell 1, 57–65. [DOI] [PubMed] [Google Scholar]
- Roach HI, Yamada N, Cheung KSC, Tilley S, Clarke NMP, Oreffo ROC, Kokubun S, Bronner F (2005) Association between the abnormal expression of matrix‐degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 52, 3110–3124. [DOI] [PubMed] [Google Scholar]
- Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J (2009) Persistent DNA damage signalling triggers senescence‐associated inflammatory cytokine secretion. Nat. Cell Biol. 11, 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MJ, White TA, Evans G, Tonne JM, Verzosa GC, Stout MB, Mazula DL, Palmer AK, Baker DJ, Jensen MD, Torbenson MS, Miller JD, Ikeda Y, Tchkonia T , van Deursen JM, Kirkland JL, LeBrasseur NK (2016) Exercise prevents diet‐induced cellular senescence in adipose tissue. Diabetes 65, 1606–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, DePinho RA (2000) Cellular senescence. Cell 102, 407–410. [DOI] [PubMed] [Google Scholar]
- Shlopov BV, Lie W‐R, Mainardi CL, Cole AA, Chubinskaya S, Hasty KA (1997) Osteoarthritic lesions. Involvement of three different collagenases. Arthritis Rheum. 40, 2065–2074. [DOI] [PubMed] [Google Scholar]
- Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G (2005) A meta‐analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage 13, 769–781. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, von Zglinicki T, van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL (2010) Fat tissue, aging, and cellular senescence. Aging Cell 9, 667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman A, Dalen H, Eaton JW, Neuzil J, Brunk UT (2003) Mitochondrial recycling and aging of cardiac myocytes: the role of autophagocytosis. Exp. Gerontol. 38, 863–876. [DOI] [PubMed] [Google Scholar]
- Toshima T, Shirabe K, Fukuhara T, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Okano S, Maehara Y (2014) Suppression of autophagy during liver regeneration impairs energy charge and hepatocyte senescence in mice. Hepatology 60, 290–300. [DOI] [PubMed] [Google Scholar]
- Waaijer ME, Parish WE, Strongitharm BH, van Heemst D, Slagboom PE, de Craen AJ, Sedivy JM, Westendorp RG, Gunn DA, Maier AB (2012) The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell 11, 722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wind N, Dekker M, Berns A, Radman M, te Riele H (1995) Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell 82, 321–330. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, Rovira II, Gutkind S, Daniels MP, Komatsu M, Finkel T (2009) Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging 1, 425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Liang L, Huang M, Qiu Q, Zeng S, Shi M, Zou Y, Ye Y, Yang X, Xu H (2016) Bromodomain and extra‐terminal domain bromodomain inhibition prevents synovial inflammation via blocking IkappaB kinase‐dependent NF‐kappaB activation in rheumatoid fibroblast‐like synoviocytes. Rheumatology 55, 173–184. [DOI] [PubMed] [Google Scholar]
- Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, Curran GL, Ogrodnik M, Jurk D, Johnson KO, Lowe V, Tchkonia T, Westendorf JJ, Kirkland JL (2016) Transplanted senescent cells induce an osteoarthritis‐like condition in mice. J. Gerontol. A Biol. Sci. Med. Sci. DOI: 10.1093/gerona/glw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Chen L, Xu X, Li C, Huang C, Deng CX (2001) TGF‐beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J. Cell Biol. 153, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JI, Smith JR (2001) DNA methyltransferase inhibition in normal human fibroblasts induces a p21‐dependent cell cycle withdrawal. J. Biol. Chem. 276, 19610–19616. [DOI] [PubMed] [Google Scholar]
- Yudoh K, Nguyen VT, Nakamura H, Hongo‐Masuko K, Kato T, Nishioka K (2005) Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis. Res. Ther. 7, R380–R391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Qian J, Zhu YC (2015) Targeting bromodomain‐containing protein 4 (BRD4) benefits rheumatoid arthritis. Immunol. Lett. 166, 103–108. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Zhou G, Morello R, Chen Y, Garcia‐Rojas X, Lee B (2003) Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte‐specific expression in vivo . J. Cell Biol. 162, 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HW, Lou SQ, Zhang K (2004) Recovery of function in osteoarthritic chondrocytes induced by p16INK4a‐specific siRNA in vitro . Rheumatology 43, 555–568. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Fuhrmann‐Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL (2015a) Identification of a novel senolytic agent, navitoclax, targeting the Bcl‐2 family of anti‐apoptotic factors. Aging Cell 15, 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O'Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann‐Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL (2015b) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]


