Abstract
Carbon coated TiO2 (TiO2@C) is fabricated by a convenient and green one-pot solvothermal method, in which ethylene glycol serve as both the reaction medium and carbon source without the addition of any other carbon additives. During the solvothermal process, ethylene glycol polymerize and coordinate with Ti4+ to form the polymeric ligand precursor, then the polymer brushes carbonize and convert to homogeneous carbon layer firmly anchored on the TiO2 nanoparticles (~1 nm thickness). The polymerization and carbonization process of the ethylene glycol is confirmed by FT-IR, Raman, TG and TEM characterizations. Benefiting from the well-dispersed nanoparticles and uniform carbon coating, the as-prepared TiO2@C demonstrate a high reversible capacity of 317 mAh g−1 (94.6% of theoretical value), remarkable rate capability of 125 mAh g−1 at 3.2 A g−1 and superior cycling stability over 500 cycles, possibly being one of the highest capacities reported for TiO2.
The development of advanced energy storage technology is of great importance to address the increasingly global concerns of energy shortage and environmental issues1,2. To date, Li-ion batteries (LIBs) represent the state-of-the-art technology due to their high energy density and have dominated the energy storage market of portable electronic devices. However, the limited lithium resources and high price of lithium-based compounds remains an obstacle for their expanded application in large-scale energy storage3,4. In contrast to lithium, sodium is widely distributed around the world and has a suitable redox potential (E0Na/Na+ = −2.71 V vs SHE), only 0.3 V above that of lithium. Sodium ion batteries (SIBs) with low cost and high efficiency seem to be the ideal alternative to LIBs, especially for grid-scale energy storage applications5,6,7.
Unfortunately, the large radius of sodium ions make it difficult to find appropriate Na-storage electrode materials with high capacity and rapid kinetics. With respect to the anode side, various types of hard carbon have been revealed to deliver considerable reversible capacity, but their low potential and large polarization raise safety issues for the practical battery applications8,9,10. Metallic anodes, such as Sn and Sb-based materials, have attracted significant attention due to their high Na-storage capacity11,12,13. However, the unavoidable volume change of these materials during the repeated sodiation/desodiation hinders their further applications. As a result, exploiting better anode materials with low cost and cycling stability is still necessary for the development of practically viable SIBs.
Titanium-based materials, a cost effective, structurally stable and sustainable material, is considered to be promising Na-storage anode14,15. Among various polymorphs of Ti-based anode materials, anatase TiO2 exhibit much better electrochemical performances owning to the three dimensional open structure, which is favorable for the Na+ transport and storage16. Nevertheless, the intrinsic low conductivity of pure TiO2 leads to low realizable capacity and poor rate performance. Considering that the electrochemical performances of TiO2 electrode is strongly depend on the morphology and pore size of the particles, varieties of TiO2 nanostructures have been designed and investigated as Na-storage materials with enhanced reversible Na-storage capacity, such as nanoparticles, nanotubes, nanorods, nanospheres, nanofibers17,18,19,20,21,22,23. However, the side reactions and structure instability of nanoparticles lead to low initial coulombic efficiency and poor cycling performances. Another effective strategy to increase the capacity utilization of TiO2 is heteratomic doping24,25,26,27. Doping elements with low charge states can create structure defects in the bulk TiO2 and thus enhance the electrical and ionic conductivities. Of significance, Pan et al.26 prepared Ni2+ doped TiO2 nanotubes with a maximum capacity of 286 mAh g−1 after 100 cycles at a current density of 50 mA g−1. However, the initial coulombic efficiency (CE), which are critical for practical SIBs, still need to be upgraded considerably. In attempt to further improve the electrochemical performances of TiO2, efforts have been devoted to combine the nanosized TiO2 with conductive carbon28,29,30,31,32. Carbon coating can provide conducting network and stabilize the SEI formation by restraining sodium ions, thus resulting in improved capacity utilization as well as initial coulombic efficiency and rate performances. Recently, Yang et al. reported a graphene supported TiO2 nanospheres with a superior Na storage capacity of 300 mAh g−1 at 20 mA g−1 and a high rate capability of 123.1 mAh g−1 at a high rate of 4.0 A g−1. Nevertheless, the long-term cycling stability of this material still need to be improved. Moreover, the high cost, low initial coulombic efficiency and complex synthesis route of graphene create a barrier for the large-scale applications of TiO2 anode.
Glycol is the common used reaction medium for the preparation of TiO2 with the advantages of effectively controlling the morphology and particle size33,34,35,36. In this work, we present a simple and green one-pot solvothermal method to fabricate carbon-coated TiO2 nanoparticles (TiO2@C), in which ethylene glycol serve as both the reaction medium and carbon source without the addition of any other carbon additives. During the solvothermal process, ethylene glycol polymerize and coordinate with Ti4+ to produce polymeric ligand precursor. Then in the subsequent annealing process, the polymer brushes pyrolyze and convert to a uniform and homogenous carbon layer firmly anchored on the surface of TiO2 nanoparticles. As expected, the as-prepared TiO2@C demonstrate a high reversible capacity of 317 mAh g−1 at 0.05 A g−1, strong rate capability of 125 mAh g−1 at 3.2 A g−1 and superior cycling stability over 500 cycles, offering a low cost and high performance anode material for SIBs.
Results and Discussion
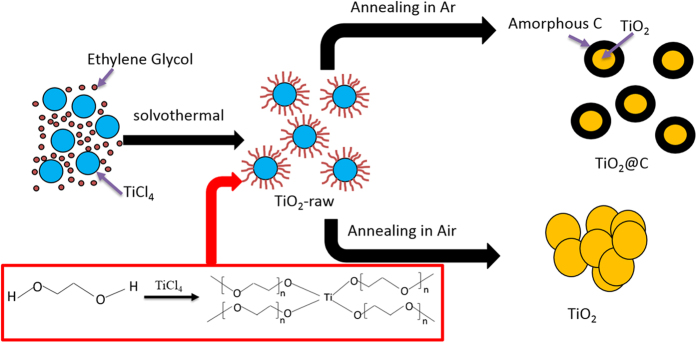
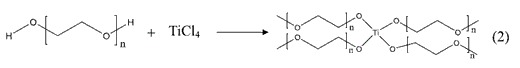
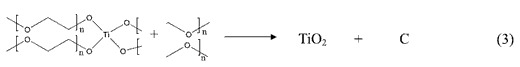
Figure 1 presents the typical synthesis route of the TiO2@C (TiO2) nanoparticles. The solvent (ethylene glycol) polymerized during the first solvothermal process (equation 1). As polyethyleneglycol is rich in –OH and –C–O–C– groups, it can easily coordinate with Ti4+ to firmly anchor the precursor molecules on their surfaces (equation 2). The FT-IR spectrum of the TiO2-raw material reflects all the characteristic absorptions of the typical precursor, confirming the polymerization and coordination reaction mechanism stated above (Fig. S1). In the final annealing process, the polymer brushes carbonize and convert to uniform and homogeneous carbon coating on the TiO2 nanoparticles (equation 3). It is worth noted that the ethylene glycol cannot polymerize in the same condition without the presence of TiCl4, indicating that the Ti4+ play a catalysis role in the polymerization of ethylene glycol.
Figure 1. Schematic illustration of the synthesis of TiO2@C nanoparticles.
 |
 |
 |
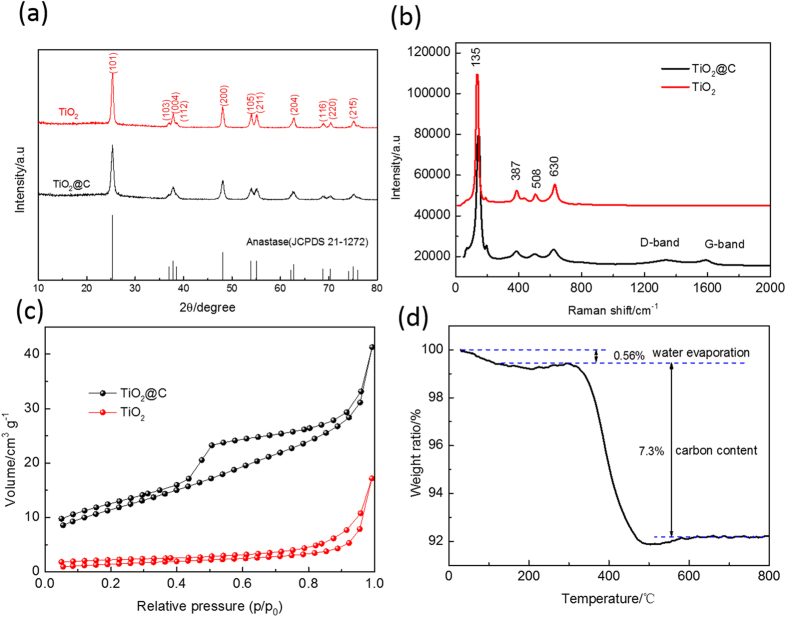
The crystalline structure of the TiO2@C and TiO2 are examined by X-ray diffraction spectrometry (XRD). As shown in Fig. 2a, all the diffraction peaks of TiO2@C and TiO2 can be well indexed to the anatase phase TiO2 (JCPDS: 21-1272) with the tetragonal crystal structure belonging to I41/amd space group, a = 3.784 ± 0.002 Å, and c = 9.514 ± 0.004 Å. The peak intensity of TiO2@C is weaker than that of TiO2, indicating the TiO2 nanoparticles embedded in amorphous carbon matrix. Based on the Debye Scherrer equation, the crystal sizes of TiO2@C and TiO2 are calculated to be ~24 nm and 27 nm, respectively. The X-ray photoelectron spectroscopy (XPS) spectra are recorded to analyze the chemical state of the TiO2@C. As shown in Fig. S2, there are two peaks of binding energies at 459 and 465 eV ascribed to Ti4+ 2p3/2 and Ti4+ 2p1/2 in the spectrum of the TiO2@C, suggesting the formation of TiO2. Raman spectra are recorded to investigate their surface composition and structures (Fig. 2b). The vibrational peaks at 135, 387, 508 and 630 cm−1 are observed in the Raman spectra of both TiO2@C and TiO2, well consistent with that of previously reported37,38. Besides, compared with TiO2, two extra characteristic peaks located at 1337 and 1587 cm−1 are detected in the Raman spectra of TiO2@C, corresponding to disorder carbon (D-band) and graphite carbon (G-band) attributed by the amorphous carbon coating layer39. The Brunauer–Emmett–Teller (BET) measurement is carried out to investigate the surface and porous structures of TiO2. As shown in Fig. 2c, N2 adsorption-desorption isotherms of TiO2@C and TiO2 can be identified as type IV isotherm (IUPAC), suggesting the mesoporous structure. According to the BET analysis, the specific surface areas of the TiO2 and TiO2@C are measured to be 5.5 and 41.3 m2 g−1, respectively. The large surface area and porous structure can not only increase the electrolyte/electrode contact areas, but also facilitate the kinetics of Na+ insertion/extraction and diffusion. Figure 2d presents the thermogravimetry analyses (TGA) curves of TiO2@C, the carbon contents in the TiO2@C is evaluated to be ~7.3 wt%.
Figure 2.
Physical characterizations of the TiO2@C and TiO2: (a) XRD pattern, (b) Raman spectra and (c) N2 adsorption–desorption isotherm of TiO2@C and TiO2; (d) TGA curve of TiO2@C.
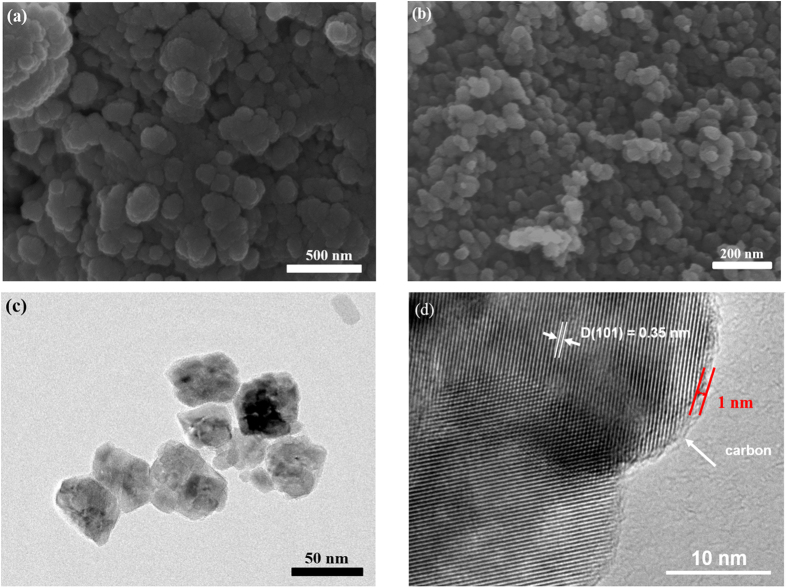
The as-prepared TiO2@C is in the form of black powders, while the color of the pure TiO2 is white (Fig. S3). The morphologies of the TiO2 and TiO2@C are presented in Fig. 3. As shown in Fig. 3a, the as-prepared TiO2 appeared as uneven particles with the average particle size of ~200 nm, which consist of aggregated primary crystallites with the crystal size of ~25 nm (Fig. S4). In contrast, the TiO2@C emerges as well-dispersed nanoparticles with much smaller size ranging from 30 to 50 nm (Fig. 3b,c), indicating the carbon coating can prevent the TiO2 nanoparticles from aggregating. The high-resolution TEM image of TiO2@C (Fig. 3d) reveals clear lattices with spacing of 0.35 nm, corresponding to the [101] planes of anatase TiO2, in accordance with the XRD result. Besides, it is clearly visualized that the amorphous carbon shell with the thickness of ~1 nm are well decorated on the surface of the TiO2 nanoparticles, ensuring the high conductivity and stable structure of the TiO2@C composites.
Figure 3.
Morphological features of the TiO2@C and TiO2 particles: (a) SEM image of TiO2; (b) SEM image of TiO2@C; (c) TEM images and (d) high resolution TEM image of TiO2@C.
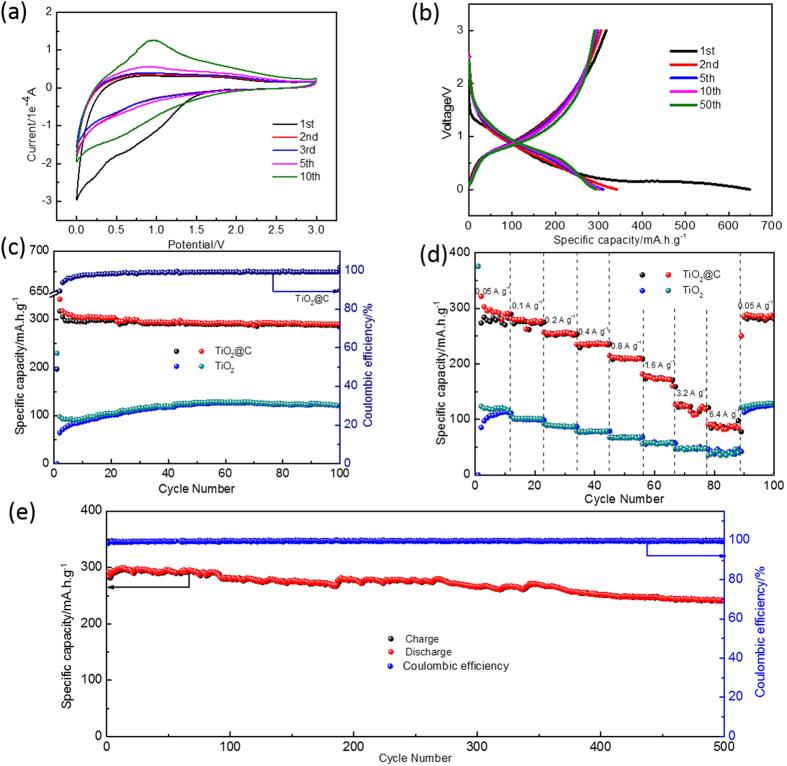
The electrochemical reactivity of the TiO2@C sample is investigated by cyclic voltammetry (CV) and galvanostatic charge-discharge cycling in 1 M NaPF6 in a mixed solvent of ethylene carbonate (EC) and diethyl carbonate (DEC). Figure 4a shows the CV curves of the TiO2@C electrode at the scan rate of 0.5 mV s−1. During the first cathodic scan, a large and broad reduction band appears at the potential region from 1.5 to 0 V, which considerably decreases its intensity during subsequent cycles, suggesting the formation of solid electrolyte interface (SEI) by decomposition of electrolyte. In the subsequent scans, a pair of redox peaks located at 0.7 and 0.85 V, referring the Na+ insertion/extraction reactions in the host lattice of anatase TiO2. It is noteworthy that the cathodic and anodic currents exhibit a gradual increase in the first ten cycles, possibly ascribed to an activation process of the TiO2@C electrode.
Figure 4.
Electrochemical characterizations of the TiO2@C electrode: (a) CV curves obtained at a scan rate of 0.5 mV s;−1 (b) charge-discharge profiles at a current rate of 0.05 A g−1 in the first 50 cycles; (c) cycling performance at a constant current of 0.05 A g;−1 (d) rate capability at various current rates from 0.05 A g−1 to 6.4 A g;−1 (e) long-term cycling performances at a constant current density of 400 mA g−1.
Figure 4b shows the typical charge/discharge profiles of the TiO2@C electrode at the current density of 0.05 A g−1. In accordance with the CV curves, the TiO2@C electrode demonstrates sloping charge/discharge profiles in the potential range of 0.3–1.3 V (vs Na/Na+). The initial charge and discharge capacities of TiO2@C are 649 and 317 mAh g −1 (based on the weight of TiO2@C composite), corresponding to an initial columbic efficiency of 48.9%. The irreversible capacity during the first several cycles is due to the formation of SEI film by electrolyte decomposition and some form of irreversible trapping of Na+ in the TiO2 lattice. As shown in Fig. 4c, the reversible capacities of the TiO2@C remain stably at 298 mA h g−1 over 100 cycles, suggesting an outstanding cycling stability. For comparison, the TiO2 deliver a much lower reversible capacity of less than 100 mAh g−1 with an inferior initial columbic efficiency of 28% (Fig. S5). Considering the possible capacity contribution of the carbon additives, we also measured the Na-storage capacities of the acetylene black (Fig. S6). The capacity contribution of the carbon additive is calculated to be to be less than 10 mAh g−1, which is negligible. It is noteworthy that the reversible capacity of TiO2@C is possibly one of the highest capacities reported for the TiO2 anodes23,26,28.
In addition to the remarkable high capacity, the TiO2@C electrode also exhibits superior high rate capability and long-term cycling stability. Figure 4d compares the rate capability of the TiO2 and TiO2@C electrodes. The TiO2@C electrode delivers a reversible capacity of 311.5, 277.8, 257, 235, 214.6, 181.8, 125.5, 91.3 mAh g−1 at different current densities of 0.05, 0.1, 0.2, 0.4, 0.8, 1.6, 3.2 and 6.4 A g−1, respectively. More encouragingly, after cycled at different current densities for 90 cycles, the TiO2@C electrode recovers a reversible capacity of 289 mAh g−1 when the current density returns back to 0.05 A g−1, about 93.2% of its initial capacity. In contrast, the TiO2 electrode shows much poor rate performances and can only deliver a reversible capacity of less than 70 mAh g−1 at the current density of 1.6 A g−1, indicating a significant enhancement in rate capability of the TiO2 after carbon coating. In order to further evaluate the long-term cycling stability of the TiO2@C, cells are assembled and galvanostatically charged and discharged at 0.4 A g−1 for 500 cycles. As shown in Fig. 4e, a reversible capacity of 241 mAh g−1 is obtained after 500 cycles with a capacity retention of 85.2%. The coulombic efficiency rapidly rise up to 99.2% in the first few cycles, indicating stable reversibility.
The excellent electrochemical performance of TiO2@C can be ascribed to the synergistic effect of the well-dispersed TiO2 nanoparticles and the homogeneous carbon coating. The nanostructured TiO2 are beneficial for Na storage on account of the large surface areas, short diffusion length and fast kinetic properties. The surrounding carbon matrix, derived from the polyethyleneglycol, can not only provide abundant active sites for Na+−insertion/desertion, but also offers high electric conduction paths for fast electron transport, leading to a remarkable reversible capacity and strong rate capability. Moreover, the uniform carbon layer can stabilize the SEI formation by preventing the TiO2 nanoparticles from aggregating and attacking by electrolyte, thus resulting in high initial coulombic efficiency and long cycle life.
To further provide a better understanding of the improved electrochemical performance by carbon coating, electrochemical impedance spectra (EIS) of the TiO2@C and TiO2 electrodes are obtained in the frequency range from 100 KHz to 0.1 Hz. As shown in Fig. S7, the semicircles in the high-frequency region is attributed to the interface reaction of SEI film, while the medium-frequency semicircle is assigned to the real axis corresponding to the sodium-diffusion process in the bulk phase. The TiO2@C electrode exhibits much lower SEI film resistance (RSEI, 13.3 Ω) and charge transfer resistance (Rct, 305.4 Ω) than those of the TiO2 electrode (87.2 Ω and 403.9 Ω) based on the equivalent circuit simulation, respectively, indicating better electronic and ionic conduction in the TiO2@C composite.
Conclusion
In summary, we present a convenient and green one-pot solvothermal method to fabricate carbon-coated TiO2 nanoparticles (TiO2@C). The ethylene glycol serve as both the reaction mediate and carbon source without adding any other carbon additives. Benefiting from the well-dispersed nanoparticles and homogeneous carbon coating, the as-prepared TiO2@C demonstrate a high reversible capacity of 317 mAh g−1, strong rate capability of 125 mAh g−1 at 3.2 A g−1 and superior cycling stability over 500 cycles, offering a low cost and high performance anode material for Na-storage. Particularly, the synthesis route described in this work is simple and intrinsically green, which provide new insights for the development of better host materials for practical SIBs.
Methods
Material Synthesis
The carbon-coated TiO2 nanoparticles were synthesized by solvothermal process as schematically illustrated in Fig. 1. Typically, 1 ml TiCl4 were added into 80 ml ethylene glycol dropwise with continuous stirring until the solution became clear. Then 2 ml ammonium hydroxide (25%) were added into the above solution and stirred for another 15 mins. The mixed solution was transferred into a 100 ml Teflon-lined stainless steel autoclave and treated at 180 °C for 24 h. After cooling down to room temperature, the products were collected by centrifugation and washed several times with ethanol and distilled water. Then the samples were dried at 80 °C under vacuum for 10 h to obtain the raw powders of TiO2 (denoted as TiO2-raw). This raw material was then calcined in Argon atmosphere at 700 °C for 2 h to obtain the carbon-coated TiO2 nanoparticles (denoted as TiO2@C). For comparison, TiO2 nanoparticles were also prepared in the same way as above except for annealing in air (denoted as TiO2).
Material characterization
The crystalline structure of the as-prepared materials were recorded on powder X-ray diffraction (XRD, PANalytical B.V., Holland) using Cu-Kα radiation. Particle morphologies were characterized by scanning electron microscopy (SEM, SIRION200) and transmission electron microscopy (TEM, JEOL2100). Fourier transformed infrared (FTIR) spectra were recorded on a Bruker VERTEX 70 FTIR spectrometer. Raman spectroscopic analysises were performed with a Horiba Jobin-Yvon LabRAM HR800 Raman system using laser excitation at 532 nm from an Nd-YAG laser. The specific surface area were determined by Brunauer–Emmett–Teller (BET) nitrogen adsorption–desorption measurement on TriStar II 3020. X-ray photoelectron spectroscopic (XPS) measurement was performed on an AXIS-ULTRA DLD X-ray photoelectron spectrometer. Carbon content of the carbon-coated anatase-phase TiO2 was confirmed by TG-DSC (Netzsch STA 449 F5) in an air atmosphere with a heating rate of 10 °C/min from room temperature to 800 °C.
Electrochemical measurements
The working electrodes were prepared by mixing the TiO2@C (TiO2), acetylene black and polyvinylidene difluoride (PVDF) in N-methyl-2-pyrrolidine (NMP) in a mass ratio of 80: 10: 10. The slurry was coated uniformly (doctor-blade) on Cu foil and vacuum-dried at 110 °C for more than 12 h. Electrochemical tests were carried out using CR2016 coin cells, which were assembled in glove box filled with highly pure argon gas (O2 and H2O levels <0.1 ppm). Sodium metal acted as the counter and reference electrode, Celgard 2400 membrane as the separator. The electrolyte was 1 M NaPF6 salt in a mixture of ethylene carbonate (EC) and dimethyl carbonate (DEC) solution (EC: DEC, 1:1 in volume) with the addition of 10 wt% fluoroethylene carbonate (FEC). Cyclic voltammetry (CV) was measured on an electrochemistry workstation (CHI 660E) using a scan rate of 0.5 mV s−1. Galvanostatic discharge/charge cycling was made on a LANHE battery test system (Wuhan, China) in the voltage range of 0 ~3 V (vs. Na/Na+). Electrochemical impedance spectroscopy (EIS) analysis was conducted using an electrochemical workstation (Autolab, PGSTAT302N) with the frequency range of 100 kHz to 0.1 Hz after operating the electrodes for 100 cycles.
Additional Information
How to cite this article: Tao, H. et al. Glycol Derived Carbon- TiO2 as Low Cost and High Performance Anode Material for Sodium-Ion Batteries. Sci. Rep. 7, 43895; doi: 10.1038/srep43895 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the Natural Science Foundation of China (Grant 51622703), 973 Program (2015CB258400) and the National Thousand Talents Program of China. The authors thank Analytical and Testing Center of HUST for XRD, SEM and FETEM measurements.
Footnotes
The authors declare no competing financial interests.
Author Contributions K.J. and K.L.W. proposed and designed the work. H.W.T. and M.Z. performed the experiments. H.W.T., M.Z., K.L.W., S.J.C. and K.J. drafted the manuscript.
References
- Dunn B., Kamath H. & Tarascon J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011). [DOI] [PubMed] [Google Scholar]
- Yang Z. et al. Electrochemical energy storage for green grid. Chemical reviews 111, 3577–3613 (2011). [DOI] [PubMed] [Google Scholar]
- Scrosati B., Hassoun J. & Sun Y.-K. Lithium-ion batteries. A look into the future. Energy & Environmental Science 4, 3287–3295 (2011). [Google Scholar]
- Scrosati B. Technology: Charging towards the superbattery. Nature 473, 448–449 (2011). [Google Scholar]
- Palomares V., Casas-Cabanas M., Castillo-Martínez E., Han M. H. & Rojo T. Update on Na-based battery materials. A growing research path. Energy & Environmental Science 6, 2312–2337 (2013). [Google Scholar]
- Kim S. W., Seo D. H., Ma X., Ceder G. & Kang K. Electrode materials for rechargeable sodium‐ion batteries: potential alternatives to current lithium‐ion batteries. Advanced Energy Materials 2, 710–721 (2012). [Google Scholar]
- Kundu D., Talaie E., Duffort V. & Nazar L. F. The emerging chemistry of sodium ion batteries for electrochemical energy storage. Angewandte Chemie International Edition 54, 3431–3448 (2015). [DOI] [PubMed] [Google Scholar]
- Stevens D. & Dahn J. The mechanisms of lithium and sodium insertion in carbon materials. Journal of The Electrochemical Society 148, A803–A811 (2001). [Google Scholar]
- Li W. et al. A high performance sulfur-doped disordered carbon anode for sodium ion batteries. Energy & Environmental Science 8, 2916–2921 (2015). [Google Scholar]
- Xiao L. et al. Hard carbon nanoparticles as high-capacity, high-stability anodic materials for Na-ion batteries. Nano Energy 19, 279–288 (2016). [Google Scholar]
- Li H. et al. Layered SnS2 cross-linked by carbon nanotubes as a high performance anode for sodium ion batteries. RSC Advances 6, 35197–35202 (2016). [Google Scholar]
- Li W. et al. Carbon-coated Sb2Se3 composite as anode material for sodium ion batteries. Electrochemistry Communications 60, 74–77 (2015). [Google Scholar]
- Li W. et al. Carbon-coated Mo3Sb7 composite as anode material for sodium ion batteries with long cycle life. Journal of Power Sources 307, 173−180 (2016). [Google Scholar]
- Guo S., Yi J., Sun Y. & Zhou H. Recent advances in titanium-based electrode materials for stationary sodium-ion batteries. Energy & Environmental Science (2016). [Google Scholar]
- Ni J. et al. Superior Sodium Storage in Na2Ti3O7 Nanotube Arrays through Surface Engineering. Advanced Energy Materials 6 (2016). [Google Scholar]
- Su D., Dou S. & Wang G. Anatase TiO2: Better Anode Material Than Amorphous and Rutile Phases of TiO2 for Na-Ion Batteries. Chemistry of Materials 27, 6022–6029 (2015). [Google Scholar]
- Kim K.-T. et al. Anatase titania nanorods as an intercalation anode material for rechargeable sodium batteries. Nano letters 14, 416–422 (2014). [DOI] [PubMed] [Google Scholar]
- Xiong H., Slater M. D., Balasubramanian M., Johnson C. S. & Rajh T. Amorphous TiO2 nanotube anode for rechargeable sodium ion batteries. The journal of physical chemistry letters 2, 2560–2565 (2011). [Google Scholar]
- Xu Y. et al. Nanocrystalline anatase TiO2: a new anode material for rechargeable sodium ion batteries. Chemical Communications 49, 8973–8975 (2013). [DOI] [PubMed] [Google Scholar]
- Oh S.-M. et al. High electrochemical performances of microsphere C-TiO2 anode for sodium-ion battery. ACS applied materials & interfaces 6, 11295–11301 (2014). [DOI] [PubMed] [Google Scholar]
- Ge Y. et al. High cyclability of carbon-coated TiO2 nanoparticles as anode for sodium-ion batteries. Electrochimica Acta 157, 142–148 (2015). [Google Scholar]
- Yang X. et al. Anatase TiO2 nanocubes for fast and durable sodium ion battery anodes. Journal of Materials Chemistry A 3, 8800–8807 (2015). [Google Scholar]
- Xiong Y., Qian J., Cao Y., Ai X. & Yang H. Electrospun TiO2/C Nanofibers As a High-Capacity and Cycle-Stable Anode for Sodium-Ion Batteries. ACS Applied Materials & Interfaces 8, 16684–16689 (2016). [DOI] [PubMed] [Google Scholar]
- Yang Y. et al. Carbon dots supported upon N-doped TiO2nanorods applied into sodium and lithium ion batteries. J. Mater. Chem. A 3, 5648–5655, doi: 10.1039/c4ta05611f (2015). [DOI] [Google Scholar]
- Yan D. et al. Sn-doped TiO2 nanotubes as superior anode materials for sodium ion batteries. Chemical Communications 51, 8261–8264 (2015). [DOI] [PubMed] [Google Scholar]
- Yan D. et al. Improved sodium-ion storage performance of TiO2 nanotubes by Ni2+ doping. Journal of Materials Chemistry A 4, 11077–11085 (2016). [Google Scholar]
- Chen J. et al. Black Anatase Titania with Ultrafast Sodium-Storage Performances Stimulated by Oxygen Vacancies. ACS applied materials & interfaces 8, 9142–9151 (2016). [DOI] [PubMed] [Google Scholar]
- Xiong Y., Qian J., Cao Y., Ai X. & Yang H. Graphene-supported TiO2 nanospheres as a high-capacity and long-cycle life anode for sodium ion batteries. Journal of Materials Chemistry A 4, 11351–11356 (2016). [Google Scholar]
- Lee J., Chen Y.-M., Zhu Y. & Vogt B. D. Fabrication of porous carbon/TiO2 composites through polymerization-induced phase separation and use as an anode for Na-ion batteries. ACS applied materials & interfaces 6, 21011–21018 (2014). [DOI] [PubMed] [Google Scholar]
- Ding C., Nohira T. & Hagiwara R. A high-capacity TiO2/C negative electrode for sodium secondary batteries with an ionic liquid electrolyte. Journal of Materials Chemistry A 3, 20767–20771 (2015). [Google Scholar]
- Zhang Y. et al. Enhanced sodium storage behavior of carbon coated anatase TiO2 hollow spheres. Journal of Materials Chemistry A 3, 18944–18952 (2015). [Google Scholar]
- Chen C. et al. Na+ intercalation pseudocapacitance in graphene-coupled titanium oxide enabling ultra-fast sodium storage and long-term cycling. Nature Communications 6 (2015). [DOI] [PubMed] [Google Scholar]
- Wang D. et al. High-performance, nano-structured LiMnPO4 synthesized via a polyol method. Journal of Power Sources 189, 624–628, doi: 10.1016/j.jpowsour.2008.09.077 (2009). [DOI] [Google Scholar]
- Chen C. et al. Ionic-Liquid-Assisted Synthesis of Self-Assembled TiO2-B Nanosheets under Microwave Irradiation and Their Enhanced Lithium Storage Properties. European Journal of Inorganic Chemistry 2013, 5320–5328, doi: 10.1002/ejic.201300832 (2013). [DOI] [Google Scholar]
- Chen C. et al. Controllable growth of TiO2-B nanosheet arrays on carbon nanotubes as a high-rate anode material for lithium-ion batteries. Carbon 69, 302–310, doi: 10.1016/j.carbon.2013.12.029 (2014). [DOI] [Google Scholar]
- Chen C. et al. TiO2-B nanosheets/anatase nanocrystals co-anchored on nanoporous graphene: in situ reduction-hydrolysis synthesis and their superior rate performance as an anode material. Chemistry 20, 1383–1388, doi: 10.1002/chem.201303734 (2014). [DOI] [PubMed] [Google Scholar]
- Cao F.-F. et al. Symbiotic coaxial nanocables: Facile synthesis and an efficient and elegant morphological solution to the lithium storage problem. Chemistry of Materials 22, 1908–1914 (2010). [Google Scholar]
- Wang B. et al. Mesoporous CNT@ TiO2-C nanocable with extremely durable high rate capability for lithium-ion battery anodes. Scientific reports 4, 3729 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. W., Fu H. B. & Zhu Y. F. Efficient TiO2 photocatalysts from surface hybridization of TiO2 particles with graphite‐like carbon. Advanced Functional Materials 18, 2180–2189 (2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.