Abstract
Unresolved experimental Lyme arthritis in C3H 5-lipoxygenase (5-LOX)−/− mice is associated with impaired macrophage phagocytosis of Borrelia burgdorferi. In the present study, we further investigated the effects of the 5-LOX metabolite, leukotriene (LT)B4 on phagocytosis of B. burgdorferi. Bone marrow-derived macrophages (BMDMs) from 5-LOX−/− mice were defective in the uptake and killing of B. burgdorferi from the earliest stages of spirochete internalization. BMDMs from mice deficient for the LTB4 high-affinity receptor (BLT1−/−) were also unable to efficiently phagocytose B. burgdorferi. Addition of exogenous LTB4 augmented the phagocytic capability of BMDMs from both 5-LOX−/− and BLT1−/− mice, suggesting that the low-affinity LTB4 receptor, BLT2, might be involved. Blocking BLT2 activity with the specific antagonist, LY255283, inhibited phagocytosis in LTB4-stimulated BLT1−/− BMDMs, demonstrating a role for BLT2. However, the lack of a phagocytic defect in BLT2−/− BMDMs suggested that this was a compensatory effect. In contrast, 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid, a natural BLT2-specific high-affinity ligand, and resolvin E1, a BLT1 agonist, were both unable to boost phagocytosis in BMDMs from either 5-LOX−/− or BLT1−/− mice, suggesting a specific role for LTB4 in mediating phagocytosis in murine macrophages. This study demonstrates that LTB4 promotes macrophage phagocytosis of bacteria via BLT1, and that BLT2 can fulfill this role in the absence of BLT1.
Keywords: eicosanoids, immunology, leukotrienes, receptors, phagocytosis, bacteria, leukotriene B4, bone marrow-derived macrophage, leukotriene B4 receptor 1, leukotriene B4 receptor 2
The enzyme, 5-lipoxygenase (5-LOX), is predominantly expressed in inflammatory cells such as polymorphonuclear cells (PMNs), macrophages, and mast cells and catalyzes the oxidation of arachidonic acid into leukotrienes (LTs) (1–3). LTB4 was first described as a powerful chemoattractant for neutrophils (4), but is now recognized to play critical roles in both host defense and inflammatory disease (5–8). In addition to its potent effect on neutrophil chemotaxis, LTB4 can recruit and activate eosinophils, monocytes, macrophages, mast cells, dendritic cells, and T cells (9). Two cell-surface G protein-coupled seven transmembrane-domain receptors for LTB4 have been molecularly identified. BLT1 is a high-affinity receptor for LTB4 that is almost exclusively expressed in leukocytes (10–12); whereas the low-affinity receptor, BLT2, is expressed more ubiquitously (13). LTB4 is the primary ligand for BLT1, but other hydroxyeicosanoids are known to bind to and activate BLT2 (14). The 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid (12-HHT), a product of the cyclooxygenase-1 enzyme, is a high-affinity ligand for BLT2 and is involved in skin wound healing and protection against colitis (15–17). While the role of BLT1 in immune responses is fairly well understood, the impact of BLT2 signaling is not clear.
We have previously demonstrated that infection of WT C3H/HeJ mice with Borrelia burgdorferi induced the production of numerous pro- and anti-inflammatory lipid mediators in their heart and joint tissues (18). B. burgdorferi infection increased the expression of 5-LOX in joint tissues and induced the production of several 5-LOX metabolites, including LTB4 (18). In other models of inflammatory arthritis, inhibiting the production of LTB4 or interfering with its signaling through BLT1 prevents arthritis development (19–21). In contrast, B. burgdorferi infection of C3H 5-LOX−/− mice results in exacerbated and prolonged arthritis (22). This did not appear to be due to a failure of spirochete clearance, as the production of Borrelia-specific antibodies and tissue loads were similar to those found in WT mice, although C3H 5-LOX−/− tissues did tend to contain higher numbers of B. burgdorferi (22). Bone marrow-derived macrophages (BMDMs) from 5-LOX−/− mice were defective in the uptake and clearance of both B. burgdorferi and apoptotic neutrophils (22), a process important for the induction of anti-inflammatory mechanisms (23). Others have shown that alveolar macrophages and PMNs from mice deficient in 5-LOX activity had reduced bacterial phagocytosis and microbicidal activity (24–26), and these defects were restored by exogenous LTB4 treatment (27, 28). Specific deletion of BLT1 in mice revealed important roles for LTB4 and its receptors in regulating pathologic inflammation (29). Moreover, addition of LTB4 to mouse peritoneal macrophages infected with Salmonella typhimurium or Pseudomonas aeruginosa resulted in enhanced bacterial uptake and killing, suggesting a possible therapeutic use for LTB4 in bacterial diseases where phagocytes play a key role in host defense (8, 30). Thus, LTB4 plays an important role in inducing the uptake and destruction of bacterial pathogens during the course of inflammation. This most likely occurs through interaction with its high-affinity receptor, BLT1; however, because macrophages express both BLT1 and BLT2, a role for BLT2 cannot be discounted.
In the current study, we further investigated the role of LTB4 on the phagocytosis of B. burgdorferi. We demonstrate that exogenous addition of LTB4 is capable of rescuing the phagocytic defect in 5-LOX−/− macrophages, and this effect can be mediated through either BLT1 or BLT2. Other agonists for these receptors were unable to rescue the phagocytic defect, suggesting that LTB4 may have a unique role in the clearance of microbial pathogens.
MATERIALS AND METHODS
Mice
Female C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Breeder pairs of BLT1−/− and 5-LOX−/− mice on a C57BL/6J background were also purchased from the Jackson Laboratory and were maintained in our mouse colony. C57BL/6J BLT2−/− and BLT1/BLT2−/− (BLT1/2−/−) mice were a kind gift from Haribabu Bodduluri (University of Louisville). All experiments were conducted under an approved protocol from the University of Missouri Animal Care and Use Committee.
Bacteria
A virulent B. burgdorferi N40 clone harboring a transcriptional fusion of the green fluorescent protein (GFP) gene (gfp) and the constitutive flaB promoter (gfp-PflaB) (a kind gift from James Carroll, National Institutes of Health) was used for all experiments (31). Frozen stocks were cultured in 7 ml Barbour-Stoenner-Kelly (BSK)-H medium containing 6% rabbit serum (Sigma-Aldrich, St. Louis, MO) and grown to log phase at 32°C. Spirochetes were enumerated using a Petroff-Hausser counting chamber (Hausser Scientific, Horsham, PA) via dark field microscopy.
Antibodies
Lysosome-associated membrane proteins (LAMPs) were stained with Alexa Fluor 647-conjugated rat monoclonal anti-mouse LAMP-1 (1D4B; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Rabbit polyclonal antibodies (1:100) against early endosome antigen 1 (EEA1), small GTPases, Rab5, and Rab7, respectively, were used to detect different stages of phagosomal maturation, followed by incubation with Alexa Fluor 488-conjuagated rat monoclonal anti-rabbit IgG (1:500) (Invitrogen, Carlsbad, CA). Host cell nuclei were stained by Hoechst 33258 (1:1,000) (Invitrogen). Anti-mouse CD16/32 was used to block Fc receptors (eBioscience, San Diego, CA). BMDMs were stained with Alexa Fluor 488-conjugated monoclonal rat anti-mouse F4/80A (Invitrogen).
Cell isolation and culture
BMDMs were isolated from mouse femurs using sterile PBS and differentiated on 100 × 15 mm plastic Petri dishes in medium containing RPMI 1640 supplemented with 30% L929 cell-conditioned medium, 10% FBS, and 2% penicillin-streptomycin at 37°C in 5% CO2. The medium was refreshed every 2 days, and the cells were incubated for 5–7 days. Bone marrow PMNs were purified from mouse femurs using a three-layer Percoll gradient centrifugation, as described previously (32, 33) with some modifications. Briefly, bone marrow cells were collected and red blood cells were removed by hypotonic lysis. Cells were then washed and laid on top of a discontinuous three-layer Percoll gradient (1.095, 1.085, and 1.070 g/ml). After centrifugation at 500 g for 30 min at 25°C, the lowest interface (1.085/1.095 g/ml interface) was collected as the PMN fraction. Purity of PMNs was 95%, as determined by Quik-Dip differential staining (Mercedes Medical, Sarasota, FL). BLT1 and BLT2 expression in BMDMs was determined using quantitative (q)RT-PCR and primers as described (34). Expression levels were normalized to GAPDH.
Phagocytosis assay of B. burgdorferi
BMDMs were harvested from Petri dishes by gentle scraping and plated on glass coverslips at 0.5 × 106 cells per well in 24-well tissue culture plates containing antibiotic-free RPMI 1640 and 10% FBS. Live GFP-B. burgdorferi were added to the macrophage cultures at a multiplicity of infection of 10 in a small volume of complete BSK-H medium (Sigma-Aldrich). A larger volume of BSK-H medium was not required, as B. burgdorferi remain viable in RPMI for at least 4 h (35). Plates were incubated for 1 or 4 h at 37°C with 5% CO2. Freshly isolated bone marrow PMNs were cultured on coverslips in 24-well tissue culture plates in antibiotic-free RPMI medium and incubated with immune serum-opsonized or nonopsonized live GFP-B. burgdorferi at multiplicity of infection of 10. At the indicated time points, both BMDMs and PMNs were washed three times with PBS to remove extracellular spirochetes and fixed in 4% paraformaldehyde for 15 min at 25°C. Cells were then washed and permeabilized with cold methanol for 10 min at 4°C and blocked with 5% BSA in PBS overnight with Fc blocking antibody. LAMPs were stained with Alexa Fluor 647-conjugated rat monoclonal anti-mouse LAMP-1 (1D4B; Santa Cruz Biotechnology, Inc.). Host cell nuclei were stained by Hoechst (1:1,000) and examined using fluorescence microscopy. A minimum of 300 cells were counted in random fields from at least two independent coverslips. In some experiments, phagocytosis was measured by flow cytometry, as described (36), using F4/80-labeled BMDMs with similar results. The phagocytic index was calculated by determining the percentage of BMDMs containing at least one spirochete per 300 cells and dividing this number by the value for WT cells × 100. Comparable results were obtained in two or more independent experiments. Data are expressed as mean + SEM.
LTB4 immunoassay
BMDMs were cultured with B. burgdorferi at a ratio of 10:1 for 1 or 4 h. Cell supernatants were collected and supernatant from uninfected cells was used as control. LTB4 concentration in the supernatant was measured by using a Parameter LTB4 immunoassay (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions. Comparable results were obtained in two or more independent experiments. Data are expressed as mean + SEM.
LTB4 stimulation
BMDMs were isolated and plated in 24-well tissue culture plates with glass coverslips, as described earlier. LTB4 (Cayman Chemical, Ann Arbor, MI) was added to the cells at concentrations of 0.1 nM and 1 nM along with B. burgdorferi at a ratio of 10:1 for 1 or 4 h. WT BMDMs were cultured with B. burgdorferi for 1 or 4 h as a phagocytic control. Phagocytosis assays were performed and slides were viewed under fluorescence microscopy as described earlier. Phagocytic indexes were calculated as above. Comparable data were obtained in two or more independent experiments. Data are expressed as mean + SEM.
BLT2 blocking
BMDMs were isolated and plated in 24-well tissue culture plates with glass coverslips as described earlier. The BLT2 antagonist, LY255283 (Cayman Chemical) , at concentrations of 0.1–10 μM (37) was added to B. burgdorferi-infected BLT1−/− BMDMs, with or without exogenous LTB4 (1 nM), for 1 or 4 h. WT BMDMs were infected with B. burgdorferi for 1 or 4 h as a phagocytic control. A phagocytosis assay was performed and slides were viewed under fluorescence microscopy as described earlier. Phagocytic indexes were calculated as above. Comparable data were obtained in two or more independent experiments. Data are expressed as mean + SEM.
BLT2 stimulation
BMDMs were isolated and plated in 24-well tissue culture plates with glass coverslips as described earlier. The 12-HHT (Cayman Chemical) at a concentration of 0.1 μM or 1 μM was added to B. burgdorferi-infected 5-LOX−/− or BLT1−/− BMDMs with or without exogenous LTB4 (1 nM) for 1 or 4 h. WT BMDMs were infected with B. burgdorferi for 1 or 4 h as a phagocytic control. A phagocytosis assay was performed and slides were viewed under fluorescence microscopy as described earlier. Phagocytic indexes were calculated as above. Comparable results were obtained in two or more independent experiments. Data are expressed as mean + SEM.
Resolvin E1 stimulation
BMDMs were isolated and plated in 24-well tissue culture plates with glass coverslips as described earlier. Prior to stimulation of cells with resolvin E1 (RvE1), a chemokine-like receptor 1 blocking peptide (100 μg, 1 mg) (Cayman Chemical) was used to block the activity of the RvE1 receptor, ChemR23. RvE1 (Cayman Chemical) at a concentration of 0.1 μM or 1 μM was then added to 5-LOX−/− macrophages with B. burgdorferi for 1 or 4 h. WT macrophages were infected with B. burgdorferi for 1 or 4 h as phagocytic control. A phagocytosis assay was performed and slides were viewed under fluorescence microscopy as described earlier. Phagocytic indexes were calculated as above. Comparable results were obtained in two or more independent experiments. Data are expressed as mean ± SEM.
cAMP assay
WT BMDMs were isolated and plated in 24-well tissue culture plates at 1 × 106 cells/well. The cells were treated with vehicle (ethanol), 1 nM LTB4, 1 μM Butaprost, 1 μM 12-HHT, or 1 μM RvE1 and incubated at 37°C for 2 h. Supernatants were removed and the cells lysed in 0.1 M HCl. Levels of cAMP were then determined using a cAMP ELISA kit (Enzo). The protein concentration in the samples was determined using a BCA assay (Pierce). Results are depicted as picomoles of cAMP per milligram protein.
Statistical analysis
Each experiment was completed at least twice. A two-tailed Student’s t-test or one-way ANOVA followed by Dunnett’s test was used to compare the significance of each group. Statistical significance was analyzed using GraphPad Prism software with P < 0.05.
RESULTS
The 5-LOX metabolites are required for efficient macrophage phagocytosis of B. burgdorferi
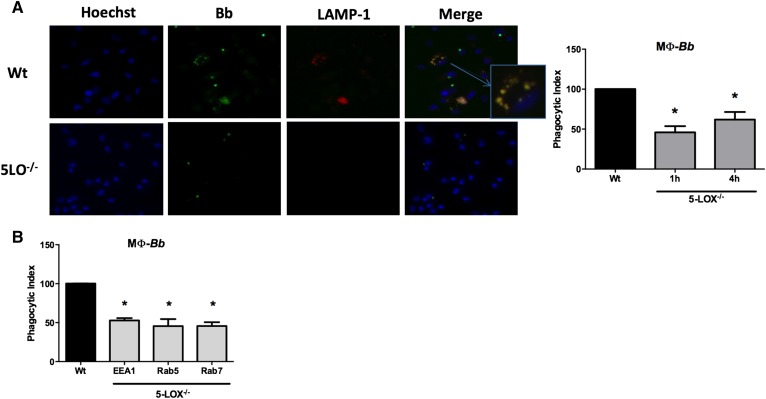
Metabolites of the 5-LOX pathway, especially LTB4, have been shown to be important mediators of phagocytosis of several bacterial pathogens (8, 24, 25). Because we previously demonstrated an exacerbated and prolonged Lyme arthritis in B. burgdorferi-infected 5-LOX−/− mice that was associated with impaired phagocytosis in mouse leukocytes (22), we further assessed the role of 5-LOX−/− metabolites in mediating macrophage phagocytosis. We tested the ability of primary BMDMs from WT or 5-LOX−/− mice to phagocytose B. burgdorferi. WT BMDMs were capable of the efficient uptake and destruction of B. burgdorferi spirochetes, with degraded organisms colocalizing with LAMP-1-containing phagolysosomes (Fig. 1A). In contrast, 5-LOX−/− BMDM cultures had only a few cells containing internalized B. burgdorferi and no identified LAMP-1-positive vacuoles. The phagocytic index shows that 5-LOX−/− BMDMs were significantly deficient (P < 0.01) in their uptake of B. burgdorferi at the 1 and 4 h time points, taking up only about half as many spirochetes as WT cells. These results suggest that products from the 5-LOX metabolic pathway are crucial to the efficient phagocytosis of microbes and also to the development and function of the phagolysosome.
Fig. 1.
Defective phagocytosis in macrophages from 5-LOX−/− mice occurs at early stages of phagosome development. BMDMs from WT or 5-LOX−/− mice were cocultured with GFP-B. burgdorferi (Bb) for 1 or 4 h. A: Images from fluorescence microscopy of WT or 5-LOX−/− macrophages cocultured with GFP-Bb for 4 h and stained with Hoechst (nuclear stain) or LAMP-1 (lysosomal stain). The inset shows colocalization of GFP-Bb and LAMP-1 in macrophage endosomes. The phagocytic index shows the level of uptake of Bb by 5-LOX−/− macrophages (MΦ) as compared with WT macrophages. Because the phagocytosis levels of WT macrophages at 1 and 4 h was arbitrarily set to 100%, only a single WT bar is shown. B: Phagocytic index of 5-LOX−/− macrophages using different markers of phagosome progression (EEA, early endosome; Rab5, early endosome; Rab7, late endosome). Bars represent mean + SEM and are the results from two independent experiments (n = 3 per group). *P < 0.01 versus corresponding WT control cells.
To determine how early in the phagocytic process 5-LOX metabolites had an impact, we monitored progression of phagolysosome development by staining for the EEA1 and the small GTPases, Rab5 and Rab7, which are markers for early phagolysosomal development. We found that 5-LOX−/− macrophage phagocytosis of B. burgdorferi was significantly defective (P < 0.01) at the earliest stage of endosome formation (EEA1) (Fig. 1B). Therefore, efficient macrophage phagocytosis of live B. burgdorferi is dependent upon metabolites from the 5-LOX pathway from the earliest stage of internalization through phagolysosomal development.
BLT1 signaling is required for efficient phagocytosis of B. burgdorferi
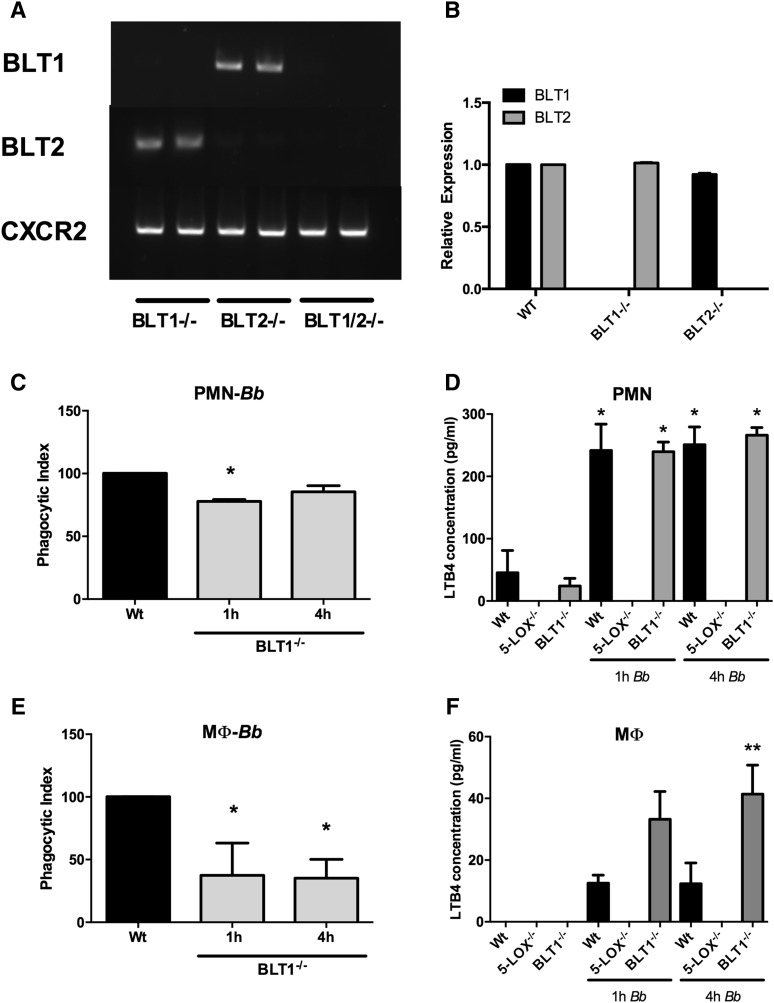
Several products of the 5-LOX metabolic pathway have been shown to enhance phagocytosis in alveolar macrophages, including LTB4, LTC4, and 5-HETE (24, 27). Here we focused on the contribution of endogenous LTB4 signaling through BLT1 in our model. We isolated PMNs and BMDMs from mice deficient in the high-affinity LTB4 receptor, BLT1−/−, and determined their phagocytic capacity. In Fig. 2A, we demonstrate genotyping of the BLT KO mouse strains used in this study. The G protein-coupled receptor, CXCR2, was used as a positive control. We also examined BLT expression levels in the KO mice to determine whether there were compensatory effects. We show that levels of BLT2 expression in BLT1−/− BMDMs were similar to those in WT mice, and BLT1 expression in BLT2−/− BMDMs was also similar to WT levels, indicating no compensatory effects on receptor expression (Fig. 2B). No reverse transcriptase controls were negative for all samples (supplemental Fig. S1). The phagocytic uptake of B. burgdorferi by BLT1−/− PMNs was slightly, but significantly (P < 0.01), less than that of WT PMNs at 1 h of coculture (Fig. 2C). However, by 4 h, the phagocytic capacity of BLT1−/− PMNs was similar to WT PMNs. In contrast, phagocytosis of B. burgdorferi by BLT1−/− BMDMs was significantly defective (P < 0.01) compared with WT BMDMs at both the 1 and 4 h time points (Fig. 2E), suggesting differential requirements for BLT1 signaling for phagocytosis between macrophages and PMNs. We then measured the production of LTB4 from both cell types. Coculture of both PMNs and BMDMs with B. burgdorferi stimulated the production of LTB4, but PMNs from both WT and BLT1−/− mice produced approximately 10-fold more LTB4 (Fig. 2D) than BMDMs (Fig. 2F) when stimulated with B. burgdorferi. In addition, while LTB4 levels were roughly equivalent between WT and BLT1−/− PMNs, culture supernatants from BLT1−/− BMDMs contained two to three times more LTB4 and were significantly higher (P < 0.05) at 4 h than WT supernatants in response to B. burgdorferi stimulation. LTB4 levels in BMDMs at 1 h were higher, but did not reach statistical significance (P = 0.07). These results suggest that the higher levels of LTB4 produced by PMNs allow for stimulation of phagocytosis through the low-affinity BLT2 receptor, while this process is much less efficient in the BMDMs due to their lower production of LTB4.
Fig. 2.
Receptor expression, phagocytosis, and LTB4 production by PMNs and BMDMs from BLT1−/− mice. Genotyping of KO mice used in this study (A). BLT1 and BLT2 expression levels in macrophages from WT and KO mice (B). BMDMs and PMNs were isolated from WT or BLT1−/− animals and phagocytosis assays were performed. Freshly isolated PMNs were cultured with B. burgdorferi (Bb) (PMN-Bb) (C) and levels of LTB4 in cell-free supernatants were determined (D). BMDMs from WT or BLT1−/− animals were cultured with Bb [macrophage (MФ)-Bb)] (E) and levels of LTB4 in cell-free supernatants were determined (F). Phagocytic indexes were determined after 1 or 4 h incubation. In (D) and (F), the control cultures contained the same volume of BSK-H medium as the cultures containing Bb. Bars represent mean + SEM and are the results from two independent experiments (n = 3 per group). *P < 0.01 versus corresponding WT control cells. **P < 0.05 versus WT control at the same time point.
Exogenous LTB4 restores phagocytic ability of both 5-LOX−/− and BLT1−/− BMDMs
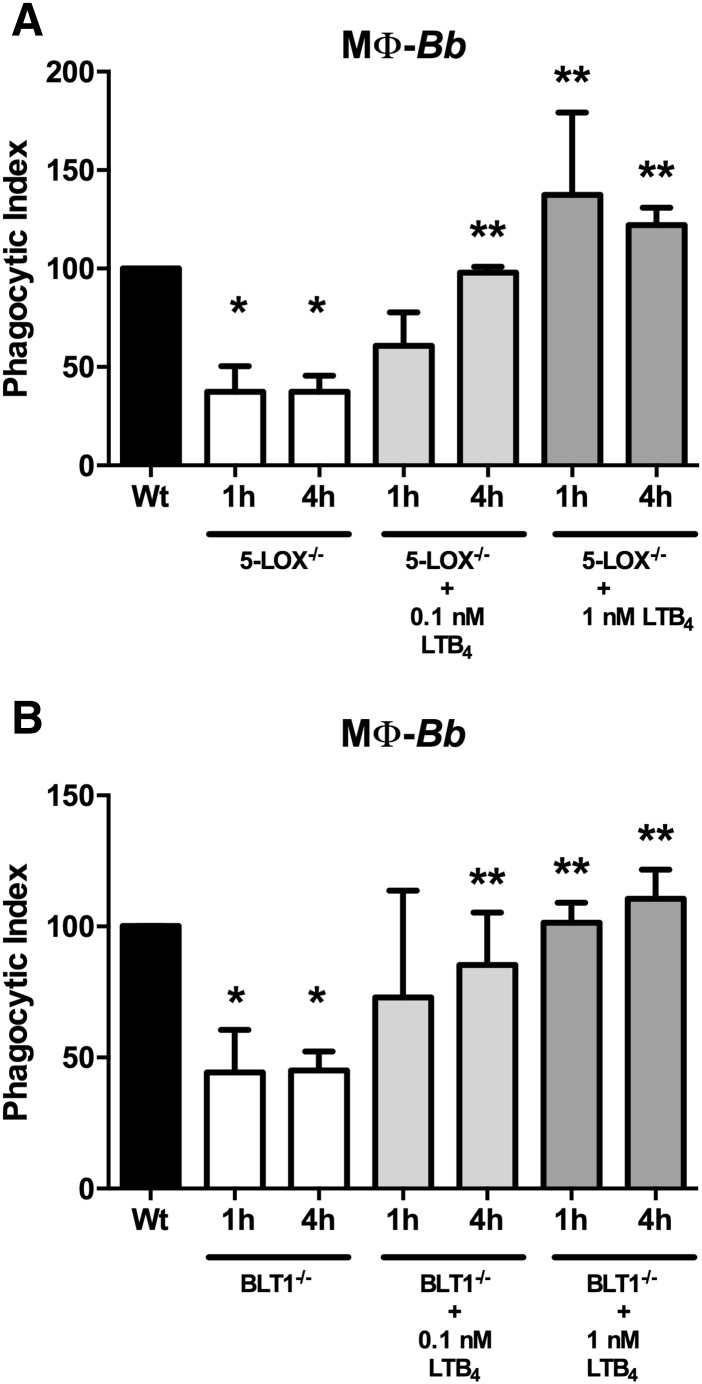
The 5-LOX catalyzes the conversion of arachidonic acid into LTA4, which is then converted to LTB4 by LTA4 hydrolase. Exogenous addition of LTB4 to 5-LOX−/− alveolar macrophages can rescue their defective phagocytosis of Klebsiella pneumoniae (27). However, because LTB4 can signal through both BLT1 and BLT2 receptors, it is not clear which signaling pathway was used for this response. The differential response we observed between the 5-LOX−/− and BLT1−/− BMDMs suggests an additional role for BLT2 in mediating phagocytic responses. To examine this, we added exogenous LTB4 to 5-LOX−/− and BLT1−/− BMDMs at levels roughly equivalent to those produced by BMDMs and PMNs in Fig. 2. Addition of 0.1 nM exogenous LTB4 (roughly 33 pg/ml) was able to completely restore phagocytosis of B. burgdorferi in 5-LOX−/− macrophages, and addition of 1 nM LTB4 (roughly 330 pg/ml) appeared to augment B. burgdorferi engulfment (Fig. 3A). Surprisingly, exogenous addition of LTB4 was also able to restore phagocytosis of B. burgdorferi in BLT1−/− macrophages (Fig. 3B), indicating that BLT2 can also mediate phagocytic processes in macrophages.
Fig. 3.
Exogenous LTB4 augments the phagocytic abilities of 5-LOX−/− and BLT1−/− macrophages (MΦ). BMDMs were isolated from WT and 5-LOX−/− (A) or BLT1−/− (B) mice and cultured with B. burgdorferi (Bb) in the presence or absence of exogenous LTB4. Phagocytic indexes were determined after 1 or 4 h incubation. Bars represent mean + SEM and are the results from two independent experiments (n = 3 per group). *P < 0.01 versus corresponding WT control cells. **P < 0.05 versus untreated KO cells from the same time point.
BLT2 antagonism prevents LTB4-mediated phagocytosis in BLT1−/− macrophages
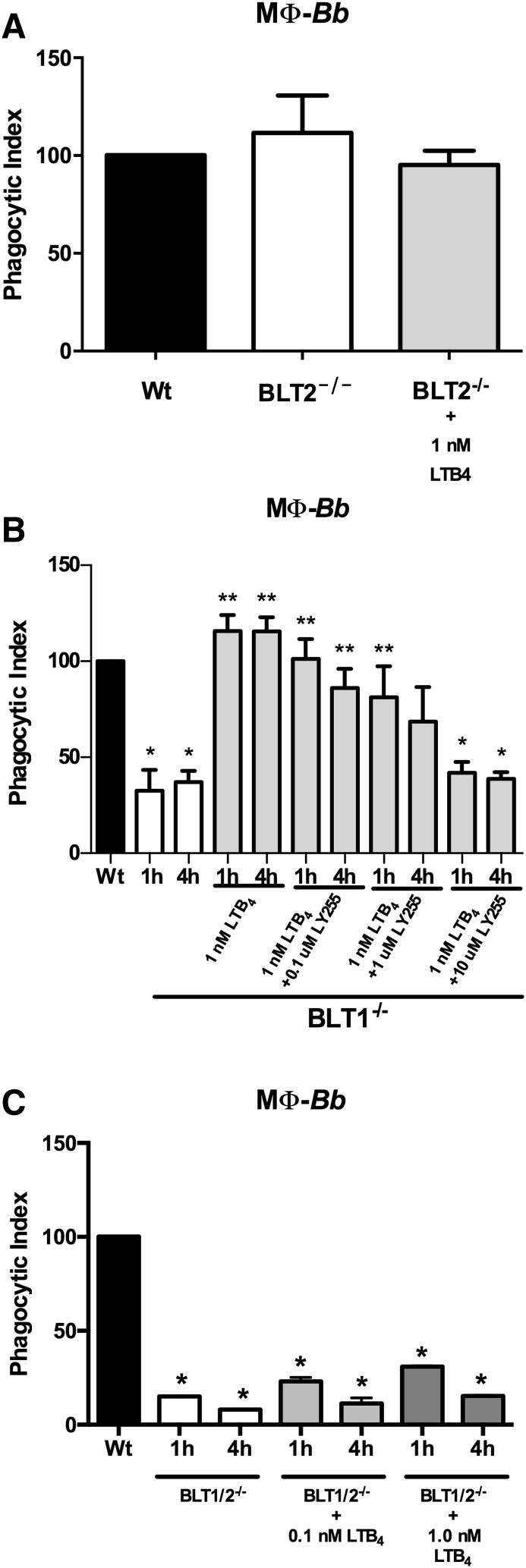
BLT2 is a ubiquitously expressed low-affinity nonspecific LTB4 receptor (14) that has been suggested to mediate biological and pathophysiological responses distinct from BLT1 (17). Its exact role in inflammatory responses, however, remains unclear. BMDMs from BLT2−/− mice were not impaired in their phagocytosis of B. burgdorferi and addition of exogenous LTB4 did not enhance the uptake of spirochetes (Fig. 4A). To formally implicate a role for BLT2 in LTB4-mediated phagocytosis, we blocked BLT2 activity with a specific antagonist, LY255283, in BLT1−/− BMDMs. At high concentrations, LY255283 is also known to inhibit signaling through BLT1 (14); however, because we were using this compound below its IC50 for BLT1 and also in BLT1−/− cells, an inhibitory effect should thus be mediated via blocking of BLT2. Addition of increasing concentrations of LY255283 to cultures of B. burgdorferi-stimulated BLT1−/− BMDMs decreased LTB4-mediated phagocytosis in a dose-dependent manner (Fig. 4B). In addition, we measured the phagocytic capability of BMDMs from BLT1/2−/− mice (21). Addition of exogenous LTB4 was unable to rescue phagocytosis of B. burgdorferi and demonstrated that this effect was mediated through BLT1 and BLT2, and not through another unknown LTB4 receptor. Thus, in the absence of BLT1 signaling, BLT2 is capable of driving LTB4-mediated phagocytosis of B. burgdorferi by BMDMs. However, when BLT1 is present, LTB4 signaling through BLT2 appears to be dispensable.
Fig. 4.
BLT2 mediates LTB4-augmented phagocytosis in BLT1−/− macrophages (MΦ). A: BMDMs were isolated from WT or BLT2−/− mice and cultured with B. burgdorferi (Bb) for 4 h in the presence or absence of exogenous LTB4. B: The BLT2-specific antagonist, LY255283 (LY255), was added to B. burgdorferi-stimulated BLT1−/− macrophages treated with exogenous LTB4 (1 nM) and their phagocytic index determined after 1 or 4 h coculture. C: BMDMs were isolated from WT and BLT1/2−/− mice and cultured with B. burgdorferi in the presence or absence of exogenous LTB4. Phagocytic indexes were determined after 1 or 4 h incubation. Bars represent mean + SEM and are the results from two independent experiments (n = 3 per group). *P < 0.01 versus corresponding WT control cells. **P < 0.05 versus untreated KO cells from the same time point.
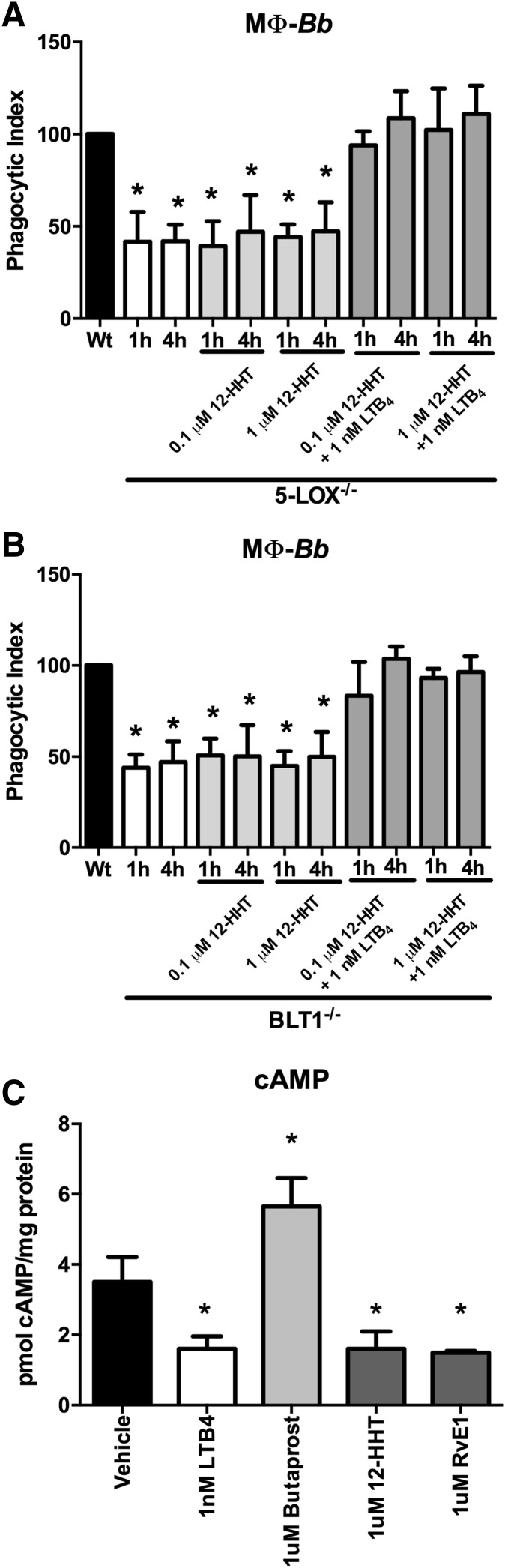
Exogenous 12-HHT stimulation of BLT2 does not affect BMDM phagocytic activity
BLT2 is known to bind several eicosanoids in addition to LTB4 (9, 10). Recently, 12-HHT, which is produced via the COX-1 metabolic pathway as a byproduct of thromboxane A2 synthesis, was identified as a high-affinity ligand of BLT2 (14, 15). To test the specificity of LTB4 in BLT2-mediated phagocytic signaling, exogenous 12-HHT was used to stimulate B. burgdorferi cocultures of 5-LOX−/− or BLT1−/− BMDMs, with or without the addition of exogenous LTB4. We found that unlike LTB4, 12-HHT treatment was unable to influence phagocytosis of spirochetes in either 5-LOX−/− or BLT1−/− BMDMs (Fig. 5A, B, respectively). When both BLT2 ligands were added together, LTB4 was still able to completely restore phagocytosis of B. burgdorferi in both 5-LOX−/− and BLT1−/− BMDMs, even though 12-HHT is the high-affinity BLT2 ligand. These results demonstrate that even though LTB4 and 12-HHT both signal through BLT2, they may have differential binding sites and differential effects on the induction of phagocytosis by BMDMs. To demonstrate that the 12-HHT treatment was effective, we measured levels of cAMP (Fig. 5C). Butaprost is an agonist of the EP2 prostanoid receptor and increases cAMP levels, while LTB4 decreases cAMP levels. A previous report has demonstrated that both LTB4 and 12-HHT activate the Gi family of G proteins and inhibit cAMP levels (15). Our results in Fig. 5C demonstrate that the 12-HHT treatment was working as expected. In addition, we analyzed the activity of RvE1 used in the next section. Stimulation of BMDMs with RvE1 also decreased cAMP levels similarly to LTB4 and 12-HHT, indicating that the treatment was working.
Fig. 5.
The high-affinity BLT2 ligand 12-HHT agonist does not influence macrophage (MΦ) bacterial phagocytosis. BMDMs were isolated from WT, 5-LOX−/−, or BLT1−/− mice. The BLT2-specific agonist, 12-HHT (0.1 μM, 1 μM), was added to B. burgdorferi (Bb)-stimulated 5-LOX−/− (A) or BLT1−/− (B) macrophages with or without LTB4 (1 nM) and their phagocytic index determined after 1 or 4 h coculture. C: Activation of cAMP by treatments. Bars represent mean + SEM and are the results from two independent experiments (n = 3 per group). *P < 0.01 versus corresponding WT control cells.
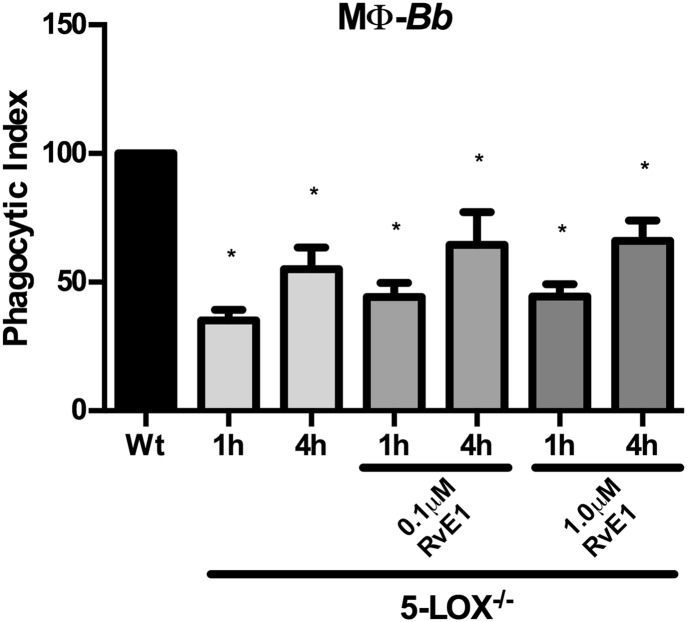
Exogenous RvE1 stimulation of BLT1 does not affect BMDM phagocytic activity
RvE1 is a potent anti-inflammatory lipid mediator that has been shown to bind to BLT1 and ChemR23 receptors (38). Exogenous stimulation of murine peritoneal macrophages or human monocyte-derived macrophages with RvE1 was shown to increase phagocytosis of zymosan-coated beads and apoptotic neutrophils (39, 40). Because the receptors used to mediate these effects have not been defined, we determined whether RvE1 signaling through BLT1 could stimulate BMDM phagocytosis of B. burgdorferi in an LTB4-independent manner. The 5-LOX−/− BMDMs were treated with exogenous RvE1 and cocultured with B. burgdorferi for 1 or 4 h. ChemR23 signaling was blocked with a chemokine-like receptor 1 blocking peptide. Exogenous addition of RvE1 slightly increased phagocytic uptake of B. burgdorferi by 5-LOX−/− macrophages, but these changes were not significantly different from untreated cells (Fig. 6). Thus, RvE1-mediated increase in BMDM phagocytosis does not appear to be mediated via signaling through the BLT1 receptor.
Fig. 6.
Exogenous RvE1 signaling through BLT1 does not activate macrophage (MΦ) phagocytosis of B. burgdorferi (Bb). BMDMs from WT or 5-LOX−/− mice were treated with exogenous RvE1 (0.1 μM, 1 μM) and their phagocytic index determined after 1 or 4 h coculture with B. burgdorferi. Signaling through ChemR23 was blocked in all cells by the addition of chemokine-like receptor 1 blocking peptide. Bars represent mean ± SEM and are the results from two independent experiments (n = 3 per group). *P < 0.01 versus corresponding WT control cells.
DISCUSSION
Phagocytic cells, such as macrophages and PMNs, play important roles in host defense during infection. They initiate and promote the development of inflammation, take up and destroy microbial invaders, promote the resolution of inflammation by secreting pro-resolution lipid mediators and cytokines, and mediate the clearance of dead cells and debris from the infection site. Disruption of these processes can lead to increased pathogen burden and disease severity, and may contribute to the development of chronic inflammatory diseases (41). We previously reported a prolonged inflammatory response in B. burgdorferi-infected 5-LOX−/− mice that was associated with impaired phagocytosis (22). In the present study, we further investigated the cellular mechanisms of 5-LOX-mediated phagocytic clearance of bacteria.
Products of the 5-LOX metabolic pathway, such as LTB4, the cysLT, and 5-HETE, have been shown to mediate the phagocytic uptake of several pathogens including: S. typhimurium, Streptococcus pneumoniae, K. pneumoniae, and Candida albicans (25–27). We confirmed our previous report that BMDMs from 5-LOX−/− mice were unable to efficiently phagocytose B. burgdorferi (22). The phagocytic process is considered to consist of multiple steps, and each stage acquires different markers as it progresses toward the lysosome (42). Using early endosomal markers, we found that phagocytosis of B. burgdorferi by 5-LOX−/− BMDMs was defective very early during endosome formation. This is likely due to LT-mediated deactivation of the actin depolymerizing factor, cofilin-1, that allows F-actin polymerization and mediates phagocytosis (43). In addition, a recent study reported that LTB4 was required for MyD88-dependent macrophage responses both in vitro and in vivo (44). Because B. burgdorferi lipoproteins are recognized by Toll-like receptors (TLRs), especially TLR-2 (45, 46), the absence of LTB4 signaling may influence macrophage activation and recognition of microbial pathogens resulting in inefficient phagocytosis.
Exogenous addition of LTB4 has been shown to restore phagocytosis in 5-LOX−/− macrophages and to augment phagocytic uptake and destruction of bacteria in WT macrophages (8, 24), thus we sought to determine the role of LTB4 in macrophage phagocytosis of B. burgdorferi. LTB4 mediates its effects by signaling through two G protein-coupled receptors: the high-affinity BLT1 and the low-affinity BLT2 (11, 13). Phagocytosis of both immune serum-opsonized and unopsonized B. burgdorferi by bone marrow PMNs from BLT1−/− mice was inhibited at 1 h of coculture. Similar results were reported for the uptake of K. pneumoniae by human peripheral blood PMNs treated with a LT receptor inhibitor (8). However, by 4 h of coculture, the phagocytic ability of the BLT1−/− PMNs was recovered, suggesting involvement of the low-affinity BLT2 in mediating phagocytosis. BLT1−/− macrophage phagocytosis of B. burgdorferi was significantly impaired and did not recover with increased culture time, similar to the results with the 5-LOX−/− macrophages. These results support previous work suggesting that endogenous LTB4-mediated phagocytosis of microbes and efferocytosis may be mediated via different mechanisms (47).
Exogenous addition of LTB4 has been demonstrated to increase phagocytosis in K. pneumoniae-infected 5-LOX−/− PMNs and macrophages (24, 26). In our model, exogenous LTB4 stimulation of 5-LOX−/− BMDMs demonstrated a similar dose-dependent enhancement of the phagocytosis of B. burgdorferi. Surprisingly, however, exogenous addition of LTB4 also enhanced the phagocytosis of B. burgdorferi in BLT1−/− macrophages, suggesting a role for BLT2 in mediating LTB4-induced phagocytosis. To test this hypothesis, we treated BLT1−/− macrophages with LTB4 in the presence of increasing concentrations of a BLT2-specific inhibitor and measured the ability of these cells to phagocytose B. burgdorferi. Blocking BLT2 inhibited the LTB4-mediated phagocytosis of B. burgdorferi in a dose-dependent manner, demonstrating a role for BLT2 in this process. The biological role of BLT2 is not clear, although it has been suggested to represent a novel target for the therapeutic treatment of inflammation associated with arthritis. In an autoantibody-induced inflammatory arthritis model, mice deficient in BLT2 displayed a reduced incidence and severity of disease (48). The 12-HHT is a natural lipid agonist for BLT2 with a higher affinity than LTB4 and was suggested to be a preferred ligand for inducing mast cell migration through BLT2 (15). Endogenous addition of 12-HHT to 5-LOX−/− or BLT1−/− macrophages did not stimulate phagocytosis of B. burgdorferi, nor did it compete with LTB4 for receptor binding. Similarly, RvE1 reportedly binds to the receptors BLT1 and ChemR23, and exogenous addition of RvE1 to macrophages was reported to enhance phagocytosis of apoptotic cells and zymosan-coated particles (39, 40). However, addition of RvE1 to 5-LOX−/− BMDMs did not increase their uptake of B. burgdorferi. Therefore, LTB4 seems to be a specific ligand capable of stimulating leukocyte phagocytosis via both LTB4-specific BLT1 and nonspecific BLT2 receptors.
In summary, the results presented in this study suggest that the 5-LOX product, LTB4, which is released during the inflammatory response, not only functions as a pro-inflammatory lipid mediator, but also may play an important role in the resolution phase of inflammation by stimulating the clearance of apoptotic cells. A better understanding of the context of LTB4/BLT signaling might help to increase our knowledge of the inflammatory process, which may bring profound advances in therapies and in prevention of chronic inflammation.
Supplementary Material
Footnotes
Abbreviations:
- BLT1/2−/−
- BLT1/BLT2−/−
- BMDM
- bone marrow-derived macrophage
- BSK
- Barbour-Stoenner-Kelly
- EEA1
- early endosome antigen 1
- GFP
- green fluorescent protein
- 12-HHT
- 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid
- LAMP
- lysosome-associated membrane protein
- 5-LOX
- 5-lipoxygenase
- LT
- leukotriene
- PMN
- polymorphonuclear cell
- RvE1
- resolvin E1
This work was supported by a University of Missouri, College of Veterinary Medicine Faculty Research Award.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Gerstmeier J., Weinigel C., Rummler S., Rådmark O., Werz O., and Garscha U.. 2016. Time-resolved in situ assembly of the leukotriene-synthetic 5-lipoxygenase/5-lipoxygenase-activating protein complex in blood leukocytes. FASEB J. 30: 276–285. [DOI] [PubMed] [Google Scholar]
- 2.Rådmark O., Werz O., Steinhilber D., and Samuelsson B.. 2007. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem. Sci. 32: 332–341. [DOI] [PubMed] [Google Scholar]
- 3.Ford-Hutchinson A. W., Gresser M., and Young R. N.. 1994. 5-Lipoxygenase. Annu. Rev. Biochem. 63: 383–417. [DOI] [PubMed] [Google Scholar]
- 4.Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., and Smith M. J.. 1980. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 286: 264–265. [DOI] [PubMed] [Google Scholar]
- 5.Rao N. L., Riley J. P., Banie H., Xue X., Sun B., Crawford S., Lundeen K. A., Yu F., Karlsson L., Fourie A. M., et al. . 2010. Leukotriene A4 hydrolase inhibition attenuates allergic airway inflammation and hyperresponsiveness. Am. J. Respir. Crit. Care Med. 181: 899–907. [DOI] [PubMed] [Google Scholar]
- 6.Sadik C. D., and Luster A. D.. 2012. Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J. Leukoc. Biol. 91: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavares N. M., Araujo-Santos T., Afonso L., Nogueira P. M., Lopes U. G., Soares R. P., Bozza P. T., Bandeira-Melo C., Borges V. M., and Brodskyn C.. 2014. Understanding the mechanisms controlling Leishmania amazonensis infection in vitro: the role of LTB4 derived from human neutrophils. J. Infect. Dis. 210: 656–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancuso P., Lewis C., Serezani C. H., Goel D., and Peters-Golden M.. 2010. Intrapulmonary administration of leukotriene B4 enhances pulmonary host defense against pneumococcal pneumonia. Infect. Immun. 78: 2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tager A. M., and Luster A. D.. 2003. BLT1 and BLT2: the leukotriene B4 receptors. Prostaglandins Leukot. Essent. Fatty Acids. 69: 123–134. [DOI] [PubMed] [Google Scholar]
- 10.Goldman D. W., and Goetzl E. J.. 1982. Specific binding of leukotriene B4 to receptors on human polymorphonuclear leukocytes. J. Immunol. 129: 1600–1604. [PubMed] [Google Scholar]
- 11.Yokomizo T., Izumi T., Chang K., Takuwa Y., and Shimizu T.. 1997. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 387: 620–624. [DOI] [PubMed] [Google Scholar]
- 12.Huang W. W., Garcia-Zepeda E. A., Sauty A., Oettgen H. C., Rothenberg M. E., and Luster A. D.. 1998. Molecular and biological characterization of the murine leukotriene B4 receptor expressed on eosinophils. J. Exp. Med. 188: 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokomizo T., Kato K., Terawaki K., Izumi T., and Shimizu T.. 2000. A second leukotriene B4 receptor, BLT2: a new therapeutic target in inflammation and immunological disorders. J. Exp. Med. 192: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokomizo T., Kato K., Hagiya H., Izumi T., and Shimizu T.. 2001. Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. J. Biol. Chem. 276: 12454–12459. [DOI] [PubMed] [Google Scholar]
- 15.Okuno T., Iizuka Y., Okazaki H., Yokomizo T., Taguchi R., and Shimizu T.. 2008. 12(S)-hydroxyheptadeca-5Z, 8E, 10E-trienoic acid is a natural ligand for leukotriene B4 receptor 2. J. Exp. Med. 205: 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M., Saeki K., Matsunobu T., Okuno T., Koga T., Sugimoto Y., Yokoyama C., Nakamizo S., Kabashima K., Narumiya S., et al. . 2014. 12-Hydroxyheptadecatrienoic acid promotes epidermal wound healing by accelerating keratinocyte migration via the BLT2 receptor. J. Exp. Med. 211: 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iizuka Y., Okuno T., Saeki K., Uozaki H., Okada S., Misaka T., Sato T., Toh H., Fukayama M., Takeda N., et al. . 2010. Protective role of the leukotriene B4 receptor BLT2 in murine inflammatory colitis. FASEB J. 24: 4678–4690. [DOI] [PubMed] [Google Scholar]
- 18.Blaho V. A., Buczynski M. W., Brown C. R., and Dennis E. A.. 2009. Lipidomic analysis of dynamic eicosanoid responses during the induction and resolution of Lyme arthritis. J. Biol. Chem. 284: 21599–21612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen M., Lam B. K., Kanaoka Y., Nigrovic P. A., Audoly L. P., Austen K. F., and Lee D. M.. 2006. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J. Exp. Med. 203: 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim N. D., Chou R. C., Seung E., Tager A. M., and Luster A. D.. 2006. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J. Exp. Med. 203: 829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao W. H., Del Prete A., Bock C. B., and Haribabu B.. 2006. Targeted disruption of leukotriene B4 receptors BLT1 and BLT2: A critical role for BLT1 in collagen-induced arthritis in mice. J. Immunol. 176: 6254–6261. [DOI] [PubMed] [Google Scholar]
- 22.Blaho V. A., Zhang Y., Hughes-Hanks J. M., and Brown C. R.. 2011. 5-Lipoxygenase-deficient mice infected with Borrelia burgdorferi develop persistent arthritis. J. Immunol. 186: 3076–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fadok V. A., Bratton D. L., Konowal A., Freed P. W., Westcott J. Y., and Henson P. M.. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101: 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancuso P., Standiford T. J., Marshall T., and Peters-Golden M.. 1998. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect. Immun. 66: 5140–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demitsu T., Katayama H., Saito-Taki T., Yaoita H., and Nakano M.. 1989. Phagocytosis and bactericidal action of mouse peritoneal macrophages treated with leukotriene B4. Int. J. Immunopharmacol. 11: 801–808. [DOI] [PubMed] [Google Scholar]
- 26.Coffey M. J., Phare S. M., and Peters-Golden M.. 2004. Role of leukotrienes in killing of Mycobacterium bovis by neutrophils. Prostaglandins Leukot. Essent. Fatty Acids. 71: 185–190. [DOI] [PubMed] [Google Scholar]
- 27.Bailie M. B., Standiford T. J., Laichalk L. L., Coffey M. J., Strieter R., and Peters-Golden M.. 1996. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J. Immunol. 157: 5221–5224. [PubMed] [Google Scholar]
- 28.Mancuso P., Nana-Sinkam P., and Peters-Golden M.. 2001. Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae. Infect. Immun. 69: 2011–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haribabu B., Verghese M. W., Steeber D. A., Sellars D. D., Bock C. B., and Snyderman R.. 2000. Targeted disruption of the leukotriene B4 receptor in mice reveals its role in inflammation and platelet-activating factor-induced anaphylaxis. J. Exp. Med. 192: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto F., Saeki K., Sumimoto H., Yamasaki S., and Yokomizo T.. 2010. Leukotriene B4 augments and restores FcgRs-dependent phagocytosis in macrophages. J. Biol. Chem. 285: 41113–41121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carroll J. A., Stewart P. E., Rosa P., Elias A. F., and Garon C. F.. 2003. An enhanced GFP reporter system to monitor gene expression in Borrelia burgdorferi. Microbiology. 149: 1819–1828. [DOI] [PubMed] [Google Scholar]
- 32.Boxio R., Bossenmeyer-Pourie C., Steinckwich N., Dournon C., and Nusse O.. 2004. Mouse bone marrow contains large numbers of functionally competent neutrophils. J. Leukoc. Biol. 75: 604–611. [DOI] [PubMed] [Google Scholar]
- 33.Maderna P., Yona S., Perretti M., and Godson C.. 2005. Modulation of phagocytosis of apoptotic neutrophils by supernatant from dexamethasone-treated macrophages and annexin-derived peptide Ac(2-26). J. Immunol. 174: 3727–3733. [DOI] [PubMed] [Google Scholar]
- 34.Iizuka Y., Yokomizo T., Terawaki K., Komine M., Tamaki K., and Shimizu T.. 2005. Characterization of a mouse second leukotriene B4 receptor, mBLT2: BLT2-dependent ERK activation and cell migration of primary mouse keratinocytes. J. Biol. Chem. 280: 24816–24823. [DOI] [PubMed] [Google Scholar]
- 35.Lazarus J. J., Kay M. A., McCarter A. L., and Wooten R. M.. 2008. Viable Borrelia burgdorferi enhances interleukin-10 production and suppresses activation of murine macrophages. Infect. Immun. 76: 1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lasky C. E., Jamison K. E., Sidelinger D. R., Pratt C. L., Zhang G., and Brown C. R.. 2015. Infection of interleukin 17 receptor A-deficient C3H mice with Borrelia burgdorferi does not affect their development of Lyme arthritis and carditis. Infect. Immun. 83: 2882–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi J. A., Kim E. Y., Song H., Kim C., and Kim J. H.. 2008. Reactive oxygen species are generated through a BLT2-linked cascade in Ras-transformed cells. Free Radic. Biol. Med. 44: 624–634. [DOI] [PubMed] [Google Scholar]
- 38.Arita M., Ohira T., Sun Y. P., Elangovan S., Chiang N., and Serhan C. N.. 2007. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and chemR23 to regulate inflammation. J. Immunol. 178: 3912–3917. [DOI] [PubMed] [Google Scholar]
- 39.Fredman G., Oh S. F., Ayilavarapu S., Hasturk H., Serhan C. N., and Van Dyke T. E.. 2011. Impaired phagocytosis in localized aggressive periodontitis: rescue by resolvin E1. PLoS One. 6: e24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwab J. M., Chiang N., Arita M., and Serhan C. N.. 2007. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 447: 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herold S., Mayer K., and Lohmeyer J.. 2011. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front. Immunol. 2: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flannagan R. S., Cosio G., and Grinstein S.. 2009. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 7: 355–366. [DOI] [PubMed] [Google Scholar]
- 43.Morato-Marques M., Campos M. R., Kane S., Rangel A. P., Lewis C., Ballinger M. N., Kim S. H., Peters-Golden M., Jancar S., and Serezani C. H.. 2011. Leukotrienes target F-actin/cofilin-1 to enhance alveolar macrophage anti-fungal activity. J. Biol. Chem. 286: 28902–28913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serezani C. H., Lewis C., Jancar S., and Peters-Golden M.. 2011. Leukotriene B4 amplifies NF-κB activation in mouse macrophages by reducing SOCS1 inhibition of MyD88 expression. J. Clin. Invest. 121: 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirschfeld M., Kirschning C. J., Schwandner R., Wesche H., Weis J. H., Wooten R. M., and Weis J. J.. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163: 2382–2386. [PubMed] [Google Scholar]
- 46.Alexopoulou L., Thomas V., Schnare M., Lobet Y., Anguita J., Schoen R. T., Medzhitov R., Fikrig E., and Flavell R. A.. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8: 878–884. [DOI] [PubMed] [Google Scholar]
- 47.Canetti C., Hu B., Curtis J. L., and Peters-Golden M.. 2003. Syk activation is a leukotriene B4-regulated event involved in macrophage phagocytosis of IgG-coated targets but not apoptotic cells. Blood. 102: 1877–1883. [DOI] [PubMed] [Google Scholar]
- 48.Mathis S. P., Jala V. R., Lee D. M., and Haribabu B.. 2010. Nonredundant roles for leukotriene B4 receptors BLT1 and BLT2 in inflammatory arthritis. J. Immunol. 185: 3049–3056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.