Summary
Brain enlargement has been observed in children with Autism Spectrum Disorder (ASD), but the timing of this phenomenon and its relationship to the appearance of behavioral symptoms is unknown. Retrospective head circumference and longitudinal brain volume studies of 2 year olds followed up at age 4 years, have provided evidence that increased brain volume may emerge early in development.1, 2 Studies of infants at high familial risk for autism can provide insight into the early development of autism and have found that characteristic social deficits in ASD emerge during the latter part of the first and in the second year of life3,4. These observations suggest that prospective brain imaging studies of infants at high familial risk for ASD might identify early post-natal changes in brain volume occurring before the emergence of an ASD diagnosis. In this prospective neuroimaging study of 106 infants at high familial risk of ASD and 42 low-risk infants, we show that cortical surface area hyper-expansion between 6-12 months of age precedes brain volume overgrowth observed between 12-24 months in the 15 high-risk infants diagnosed with autism at 24 months. Brain volume overgrowth was linked to the emergence and severity of autistic social deficits. A deep learning algorithm primarily using surface area information from brain MRI at 6 and 12 months of age predicted the diagnosis of autism in individual high-risk children at 24 months (with a positive predictive value of 81%, sensitivity of 88%). These findings demonstrate that early brain changes unfold during the period in which autistic behaviors are first emerging.
Keywords: autism, brain, neuroimaging, development
We first reported increased brain volume in adolescents and adults with ASD over twenty years ago5. Subsequent reports suggested that brain overgrowth in ASD may be most apparent in early childhood6-8. A study of infants at risk for ASD (33 high risk and 22 low risk), scanned from 6 to 24 months of age, found enlarged brain volume present at 12 and 24 months in the ten infants later diagnosed with autism at 24 months of age or later (mean age 32.5 months)9.
In the present study, we examined data from a subset of individuals from a longitudinal study comprising 318 infants at high familial risk for ASD (HR), of which 70 met clinical best-estimate criteria for ASD (HR-ASD) and 248 did not meet criteria for ASD (HR-neg) at 24 months of age, and 117 infants at low familial risk (LR) for ASD, who also did not meet criteria for ASD at 24 months (see Methods for diagnostic and exclusion criteria). The three groups were comparable in race/ethnicity (85% white), family income, maternal age at birth (33 years old), infant birth weight (8 lb), and gestational age at birth (39 weeks). The HR-ASD group had more males than the other two groups (83% vs. 59%) and mothers in the LR group had higher education level (Extended Data, Table 1).
Infants were evaluated at 6, 12 and 24 months of age with detailed behavioral assessments and high-resolution brain magnetic resonance imaging (MRI), to prospectively investigate brain and behavioral trajectories during infancy. The analyses described below were conducted on a subset of 106 high-risk (n = 15 HR-ASD; n=91 HR-neg) and 42 low-risk infants for whom all three MRI scans were successfully obtained. Based on our prior findings at 2 to 4 years of age2, we hypothesized that brain overgrowth in ASD begins before 24 months of age, that overgrowth is associated with hyper-expansion of cortical surface area, and that these early brain changes are temporally linked to the emergence of the defining behaviors of ASD. Finally, we sought to examine whether differences in the development of brain characteristics might suggest early biomarkers (i.e., occurring prior to the onset of the defining behaviors of ASD) for the detection of later emerging ASD.
Primary analyses examined group differences in the trajectories of brain growth rate (Figure 1). Total brain volume (TBV) growth rate did not differ between groups from 6-12 months of age. However, pairwise comparisons at 24 months showed large effect sizes for HR-ASD vs LR and HR-ASD vs HR-neg. The HR-ASD group demonstrated a significantly increased TBV growth rate in the second year compared to both the LR and HR-neg groups (Extended Data Table 2). In addition, the HR-ASD group showed a significantly increased surface area (SA) growth rate from 6 to 12 months of age compared to both the HR-neg and LR groups, with the most robust increases observed in left/right middle occipital gyrus, right cuneus and right lingual gyrus area (see Figure 2). No group differences were observed in cortical thickness (CT). We observed a significant correlation between SA growth rate from 6-12 months and enlargement in TBV at 24 months of age in all subjects (r (192) = 0.59, p <0.001), as well as the HR only subgroup (r (139) = 0.63, p < 0.001). Raw means, standard deviations, and effect size for the group comparisons of TBV and SA are provided in Extended Data (Table 3). Regional differences in SA change rate (from 6-12 months) were observed in the HR-ASD group (Figure 2).
Figure 1. Longitudinal trajectories of total brain volume (TBV), surface area (SA) and cortical thickness (CT) from 6 to 24 months.
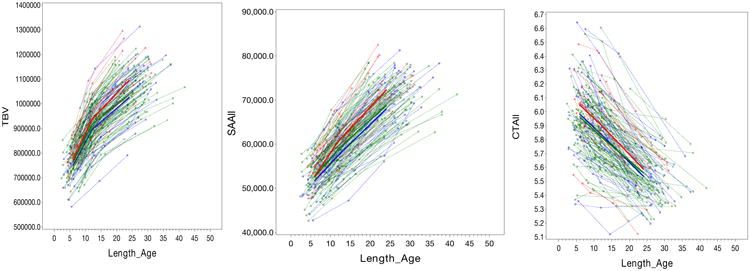
Figure 1 shows the longitudinal trajectories of total brain volume (TBV), cortical thickness (CT), and surface areas from 6 to 24 months for the three groups examined. Only individuals with complete longitudinal imaging (6, 12, and 24 months) were included in the analysis (HR-ASD, n=15; HR-neg, n=91; LR, n=42). Group trajectories were estimated from the random coefficient piece-wise linear model (see Methods). The HR-ASD group showed a significantly increased SA growth rate in the first year of life (from 6 to 12 months) compared to both the HR-neg (t (289) =2.01, p=0.04) and LR groups (t (289) = 2.50, p=0.01). There were no significant group differences in SA growth rates in the second year (Extended Data, Table 2). Pairwise comparisons of SA measured at 12 months of age showed medium to large effect sizes for HR-ASD vs LR (Cohen's d = 0.74) and HR-ASD vs HR-neg (Cohen's d = 0.41), becoming more robust by 24 months with HR-ASD vs LR (Cohen's d = 0.88) and HR-ASD vs HR-neg (Cohen's d = 0.70). There were no significant group differences in trajectories for cortical thickness (CT), with all groups showing a pattern of decreasing CT over time. No group differences were observed in trajectory of CT growth in either the first (F (2,289) = 0.00; p =0.99) or second year (F (2,289) = 1.44; p=0.24). Key: red = HR-ASD, green = HR-neg, blue = LR. TBV = total brain volume in mm3, Length_age refers to the age corrected by length (body size), SSAll = total surface area, CTAll = total cortical thickness. Surface area shown in mm2, Cortical thickness in mm.
Figure 2. Cortical regions showing significant expansion in surface area from 6-12 months in HR-ASD.
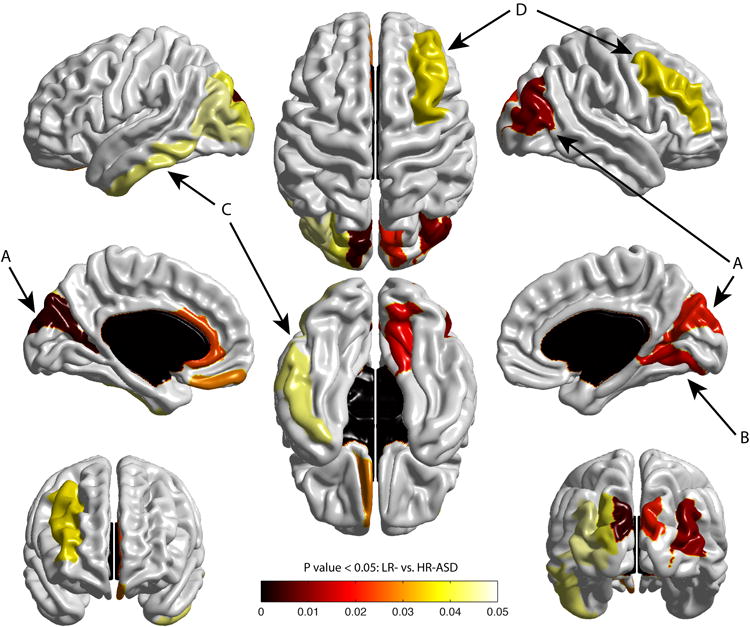
Figure 2 displays the map of significant group differences in surface area from 6 to 12 months. Exploratory analyses were conducted with a 78 region of interest surface map (see Supplementary Information), using an adaptive Hochberg method of p <0.05. The colored areas show the group effect for the HR-ASD versus LR subjects. Compared to the LR group, the HR-ASD group had significant expansion in cortical surface area in the left/right middle occipital gyrus and right cuneus (A), right lingual gyrus (B), and to a lesser extent the left inferior temporal gyrus (C), and middle frontal gyrus (D). HR-ASD, n = 34; LR, n = 84.
Given that the timing of TBV overgrowth in our study coincided with findings from other studies showing emergence of social deficits in the second year of life, we explored whether rate of volume overgrowth was linked to autism severity. Pearson correlations between TBV and behavioral measures of autism symptoms and social communication (on the Autism Diagnostic Observation Schedule10(ADOS) and Communication and Symbolic Behavior Scales11(CSBS)) were examined, adjusting for multiple comparisons.
We first examined the relationship between autistic behavior (ADOS severity score) at 24 months and TBV change rate from 6-12 and 12-24 months in the HR groups (HR-ASD and HR-neg). We found no significant correlation between 24 month ADOS severity score and 6-12 month TBV change rate (r (174) = 0.14; p =0.06); whereas a significant correlation was noted between 24 month ADOS severity score and 12-24 month TBV change rate (r (193) = 0.16; p =0.03). Subsequent analyses designed to examine the components of overall autism severity (ADOS) during the latter interval, revealed a significant correlation between 12-24 month TBV change rate and 24 month ADOS social affect score (r (194) = 0.17, p =0.01), but not ADOS restricted/repetitive behavior score (r (194) = 0.07; p =0.31).
To follow up on the above noted relationship between change in brain volume and social deficits in the second year, we examined the relationship between TBV change rates and social behavior at 24 months with an independent measure of social behavior, the CSBS. Consistent with the findings from the ADOS analysis, the CSBS social composite score was significantly correlated with a more rapid TBV change rate from 12-24 months (r (158) = 0.18, p =0.02) in HR subjects. No significant correlations were observed between CSBS social composite score at 24 months and TBV change rate from 6-12 months (r (143) = 0.11, p =0.17).
As opposed to the ADOS, which was only administered at 24 months (the ADOS was primarily designed as a tool for diagnosis), measurements of social behavior were available from the CSBS at both 12 and 24 months. We sought to examine change in social behavior during this time, considering our observation of changes in brain volume during that same period in the HR-ASD group, and a previous report that social deficits in ASD appear to unfold during the second year of life3. We observed a significant group (HR-ASD vs. HR-neg) × time (12-24 months) interaction for CSBS social composite score (F (2,130) = 10.0, p <0.0001). This finding was further supported by the observation that CSBS effect size almost tripled from 12 (d = 0.39) to 24 (d = 1.22) months.
Based on earlier findings from our group on surface area, cortical thickness and brain volume2, we examined whether selected MRI brain measurements at 6 and 12 months of age can be used to accurately identify those infants who later meet criteria for ASD at 24 months of age. Independent of knowledge about the results of the above analyses, a machine learning classification algorithm based on a deep learning network, was employed to investigate how well regional SA and CT at 6 and 12 months, intracranial volume (ICV), and sex predicted HR-ASD diagnosis at 24 months of age. We used only data from those infants for whom CT and SA data at both 6 and 12 months was available (HR-ASD = 34, HR-neg = 145). A ten-fold cross-validation was employed to compute classification performance, where the whole classification procedure, including network training was performed separately in each fold (see Supplementary Information for details on method, validation, and comparison to other approaches).
The classification scheme distinguished the HR-ASD group from the HR-neg group in the cross-validation with 94% accuracy (N=168/179), 88% sensitivity (N=30/34), 95% specificity (N=138/145), 81% positive predictive value (PPV) (N=30/37), and 97% negative predictive value (NPV) (N=138/142) (Extended Data, Table 4).
Additional inspection of the trained deep learning networks suggests that contributions to the discrimination are mostly due to SA and not CT (or TBV or sex), particularly at 6 months of age, as 11 of the top 12 measures contributing to the deep learning network are regional SA variables and the top six are variables from 6 months of age (Figure 3, Extended Data Figure 1).
Figure 3. Visualization of cortical regions with surface area measures among the top 40 features contributing to the deep learning (DL) dimensionality reduction.
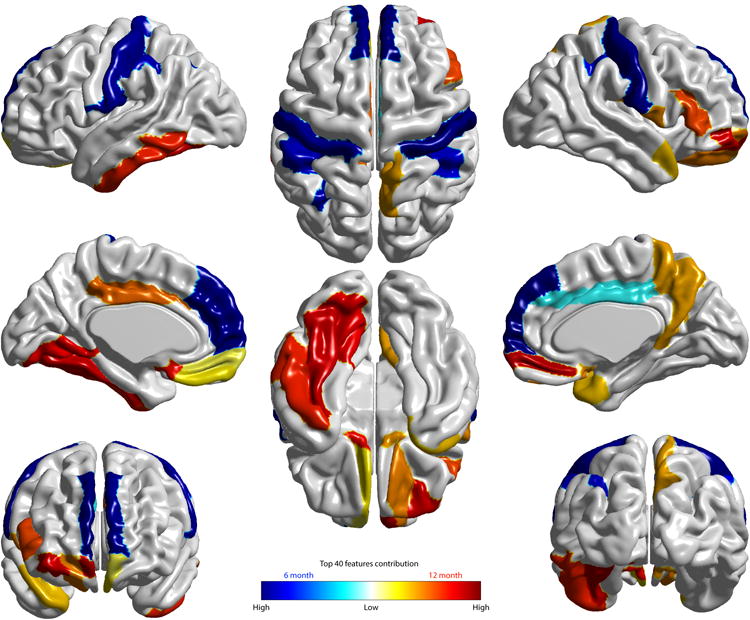
The cortical regions whose surface area measures are among these top 40 features obtained from the non-linear deep learning (DL) approach are visualized. The top 10 DL features observed include: surface area at 6 months in the right and left superior frontal gyrus, post-central gyrus, and inferior parietal gyri, and ICV at 6 months. These features produced by the DL approach are highly consistent with those observed using an alternative approach (linear sparse learning) (Extended Data, Figure 1). Two tables listing the top 40 features from the DL approach and sparse learning are provided in Supplementary Information (Tables 2 and 3).
Our data suggest that very early, post-natal hyper-expansion of cortical surface areas may play an important role in the development of autism. Rate of cortical surface area expansion from 6 to 12 months was significantly increased in individuals diagnosed with autism at 24 months, and was linked to subsequent brain overgrowth which, in turn, linked to the emergence of social deficits. This suggests a sequence whereby hyper-expansion of cortical surface area is an early event in a cascade leading to brain overgrowth and emerging autistic deficits. In infants diagnosed with autism at 24 months, surface area hyper-expansion in the first year was observed in cortical areas linked to processing sensory information (e.g., left middle occipital cortex), consistent with regions previously reported to show the earliest increase in SA growth rate in typically developing infants12, and with reports showing early sensory differences in infants who will later develop ASD13,14.
The finding of brain overgrowth in this sample of young children with ‘idiopathic’ ASD is consistent with an emerging literature demonstrating brain overgrowth in genetically-defined ASD subgroups (e.g., 16p11 deletions15, CHD816). Cellular mechanisms and heritability underlying SA expansion are thought to differ from mechanisms underlying cortical thickness17,18, and SA hyper-expansion has been reported in genetically-engineered mouse models of autism19. Our findings are not inconsistent with the mini-column hypothesis of autism20 which postulates that symmetrical proliferation of periventricular progenitor cells leads to an increased number of mini-columns, which may have a role in the pathogenesis of SA hyper-expansion and later emergence of the disorder18,21. Over-proliferation of cortical progenitor cells may impact other mechanisms of post-natal development (e.g., dendritic arborization and decreased pruning22). Fan et al. 23 showed that overproduction of upper-layer neurons in the neocortex was associated with autism-like features in mice, and the 16p11.2 deletion mouse has been shown to exhibit altered cortical progenitor proliferation24. Furthermore, an imaging study described increased brain volume in individuals with a 16p11 deletion, a genetically-defined subgroup of individuals often presenting with ‘syndromic autism’15. Expansion of basal progenitor cells in rodent models25 has been shown to regulate cerebral volume size and folding, while the dysregulation of neural progenitor cell proliferation has been observed in a genetically engineered mouse models involving ASD-associated genes (e.g., CHD8) 26. Work by Cotney et al.27 demonstrates the importance of CHD8 in mediating regulatory networks during neurodevelopment, and suggests the potential role of CHD8 in disrupting the proliferation and differentiation of neurons during early human brain development. Marchetto et al.28 observed increased rates of neural progenitor cell proliferation and neuron number compared to controls in induced pluripotent stem cells derived from individuals with ASD who also had increased brain volume on MRI28. Increased proliferation was due to dysregulation of a β-catenin/BRN2 transcriptional cascade and associated with reduced synaptogenesis leading to functional defects in neuronal networks, which could be rescued by insulin growth factor 128. The findings in the present study together with these recent reports suggest that understanding the mechanisms underlying surface area hyper-expansion in the first year in human infants is likely to provide important insights into the downstream pathogenesis of autism.
Prediction models developed from behaviorally-based algorithms during infancy have not provided sufficient predictive power to be clinically useful29. We found that a deep learning algorithm primarily using surface area information from brain MRI at 6 and 12 months of age predicted the 24 month diagnosis of autism in children at high familial risk for autism. This finding may have implications for early detection and intervention, given that this period is prior to consolidation of the defining features of ASD and the typical age for diagnosis30. The latter part of the first and early second years of life are characterized by greater neural plasticity relative to later ages and is a time when autistic social deficits are not yet well established. Intervention at this age may prove more efficacious than later in development. The fact that we demonstrate group differences in surface area growth rate from 6-12 months, that very early surface area changes are linked to later brain overgrowth in the second year, and that overgrowth is, in turn, linked to the emergence of core social deficits in autism during this period, provides additional context to support the validity of the prediction model we report. The positive predictive value findings from this high risk study are probably conservative in nature due to the likelihood that our HR-ASD group is milder than those who are clinically-referred and diagnosed with ASD at 24 months of age, and that HR-Neg groups are known to be more heterogeneous with respect to later development of cognitive, behavioral, social-communication and motor deficits than typical case-control studies29,31-33. The algorithm described in this paper will require replication before it could be considered a possible clinical tool for predicting ASD in high familial risk infants, as false diagnostic predictions have the potential to adversely impact individuals and families. In addition, we do not know whether the brain differences we observed are specific to so-called idiopathic autism or share characteristics with other neurodevelopmental disorders. While the findings of this study do not have direct application to the larger population of children with ASD who are not known to be at high familial risk for ASD, they provide a proof-of-principle that early prodromal detection using a brain biomarker may be possible. Future analyses incorporating complementary data from other relevant modalities (e.g., behavior, molecular genetics, electrophysiology and other imaging modalities such as whole brain functional MRI) may improve the accuracy of the prediction we observed.
Methods
Sample
This study includes data acquired from an NIH-funded Autism Centers of Excellence (ACE) network study, referred to as the ‘Infant Brain Imaging Study’ (IBIS). The network includes four clinical data collection sites (University of North Carolina at Chapel Hill, University of Washington, Children's Hospital of Philadelphia, Washington University in St. Louis), a Data Coordinating Center at the Montreal Neurological Institute (McGill University), and two image processing sites (University of Utah and UNC). Data collection sites had approved study protocols by their Institutional Review Boards (IRB), and all enrolled subjects had informed consent provided by parent/guardian. Infants at high (HR) and low familial risk (LR) entered the study at 6 months of age (a subset of HR infants entered at 12 months) and were followed-up at 12 and 24 months. Results from 6 month brain volume findings have previously been reported on a subset of this sample34.
Subjects were enrolled as HR if they had an older sibling with a clinical diagnosis of ASD confirmed on the Autism Diagnostic Interview35 (ADI-R). Subjects were enrolled in the LR group if they had an older sibling without evidence of ASD and no family history of a first or second-degree relative with ASD. Exclusion criteria for both groups included the following: (1) diagnosis or physical signs strongly suggestive of a genetic condition or syndrome (e.g., fragile × syndrome) reported to be associated with ASDs, (2) a significant medical or neurological condition affecting growth, development or cognition (e.g., CNS infection, seizure disorder, congenital heart disease), (3) sensory impairment such as vision or hearing loss, (4) low birth weight (<2000 grams) or prematurity (<36 weeks gestation), (5) possible perinatal brain injury from exposure to in-utero exogenous compounds reported to likely affect the brain adversely in at least some individuals (e.g., alcohol, selected prescription medications), (6) non-English speaking families, (7) contraindication for MRI (e.g., metal implants), (8) adopted subjects, and (9) a family history of intellectual disability, psychosis, schizophrenia or bipolar disorder in a first-degree relative. The sample for this analysis included all children with longitudinal imaging data processed thru 8/31/15. The final sample included 318 HR and 117 LR children, each with 2-3 MRI scans (Extended Data, Table 1).
Assessment Protocols
Behavioral assessment
Infants were assessed at ages 6, 12 and 24 months and received a brain MRI scan in addition to a battery of behavioral and developmental tests. The battery included measures of cognitive development, adaptive functioning, and behaviors associated with autism. Developmental level and adaptive functioning were assessed at each timepoint using the Mullen Scales of Early Learning36 and Vineland Scales of Adaptive Behavior37. Autism-oriented assessments included the Autism Diagnostic Interview-Revised35, Autism Diagnostic Observation Scale10 (ADOS-WPS) at 24 months and Communication and Symbolic Behavior Scales of Development Profile11 (CSBS-DP) at 12 and 24 months. From the CSBS, the total raw score and the social composite raw score were used in the brain-behavioral analyses. Raw scores were used to allow for better representation of the distribution of the data.
Diagnostic (outcome) classification
Diagnostic classification was made by an expert clinician at each site using all clinical, behavioral, and questionnaire data available at 24 months. A diagnosis of ASD was made using the DSM-IV-TR criteria for Autism and Pervasive Developmental Disorder, Not Otherwise Specified (PDD-NOS)38 by an expert clinician blind to the outcome of the imaging results. Across the IBIS Network, the expert clinicians met quarterly for diagnostic reliability meetings (via video/telephone) using the DSM-IV-TR criteria independently. The rationale for this conservative approach was to maximize validity of diagnosis at 24 months of age39. Reliability between diagnostic raters was maintained throughout the project period.
HR subjects were classified as HR-neg (i.e., negative for autism) if they did not meet either ASD or PDD-NOS criteria on the DSM-IV-TR. In order to have a LR comparison group representing typically developing infants without autism, we also assessed each LR subject at 24 months. The LR subjects included did not meet ASD or PDD-NOS criteria on the DSM-IV-TR clinical best estimate assessment at 24 months. Three LR subjects met DSM-IV criteria for ASD at their 24 month assessment (one for autism, two for PDD-NOS) and were excluded from the study (Extended Data, Table 5). There is strong evidence of differences in the underlying genetic architecture of multiple versus single incidence (or sporadic) cases, with the latter more often being attributed to de novo events, that support our exclusion of these LR-ASD subjects from a combined analysis with the HR-ASD subject group, who are HR infant siblings. The final HR groups included 70 HR-ASD and 248 HR-neg, and the LR group consisted of 117 children.
MRI acquisition
The brain MRI scans were completed on 3T Siemens Tim Trio scanners with 12-channel head coils and obtained while infants were naturally sleeping. The imaging protocol included (1) a localizer scan, (2) 3D T1 MPRAGE: TR=2400ms, TE=3.16ms, 160 sagittal slices, FOV=256, voxel size = 1mm3, (3) 3D T2 FSE TR=3200ms, TE=499ms, 160 sagittal slices, FOV=256, voxel size = 1mm3, and (4) a 25 direction DTI: TR=12800ms, TE=102ms, slice thickness = 2mm isotropic, variable b value = maximum of 1000s/mm2,, FOV=190.
A number of quality control procedures were employed to assess scanner stability and reliability across sites, time, and procedures. Geometry phantoms were scanned monthly and human phantoms (two adult subjects) were scanned annually to monitor scanner stability at each site across the study period. Details on the stability procedures for IBIS and scanner quality control checks are described elsewhere34.
We further examined the three subject groups (HR-ASD, HR-neg, LR) for any differences in scan success rates (i.e., proportion of completed scans). We found a significant difference between groups (X2(2, N=1,305) = 16.9, p=0.02). Overall, the HR-ASD subjects had proportionately fewer successful scans (69%) compared to the HR-neg (78%) and LR (76%) groups, particularly at the 12 month visit. We hypothesize that this may be due to more behavioral difficulties in the ASD group (e.g., problems with sleep disturbance).
Radiologic Review
All scans were reviewed locally by a pediatric neuroradiologist for radiologic findings that, if present, were communicated to the participant. In addition, a board certified pediatric neuroradiologist (R.C.M., Washington University) blindly reviewed all MRI scans across the IBIS network and rated the incidental findings. A third neuroradiologist (D.W. S., University of Washington) provided a second blind review for the Washington University site, and contributed to a final consensus rating if there were discrepancies between the local site reviews and the network review. The final consensus review was used to evaluate whether there were group differences in the number and/or type of incidental findings. Scans were rated as either normal, abnormal, or with incidental findings. No scans rated as abnormal were included in the analysis, and previous examinations of our data did not find group differences in incidental findings34. Scans rated as clinically abnormal by a site pediatric neuroradiologist, and independently confirmed by two study pediatric neuroradiologists, were excluded (N=3).
Image Processing
Image processing was performed to obtain global brain tissue volumes, regional brain tissue volumes, and cortical surface measures. All image processing was conducted blind to the subject group and diagnostic information. The brain volumes were obtained using a framework of atlas-moderated expectation-maximization including co-registration of multi-modal (T1w/T2w) MRI, bias correction, brain stripping, noise reduction, and multivariate classification with the AutoSeg toolkit40 (http://www.nitrc.org/projects/autoseg/). Population average templates and corresponding probabilistic brain tissue priors, for white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF) were constructed for the 6 to 24 month old brain. The following brain volumes were generated at all ages: intracranial volume (ICV), total brain volume = gray matter (GM) plus white matter (WM), total cerebrospinal fluid (CSF), cerebrum, cerebellum, and lateral ventricles. ICV was defined as the sum of WM, GM, and CSF. Total brain tissue volume (TBV) was defined as the sum of all WM and GM contained in the brain cavity (i.e., cerebrum, cerebellum, and portion of midbrain/brainstem). Subjects were included in the volumetric analyses if they had successfully segmented scans at 6, 12, and 24 months and corresponding body length measures.
Cortical thickness (CT) and surface area (SA) measures for 12 and 24 month data were obtained via a CIVET workflow41,42 adapted for this age using an age corrected automated anatomical labeling (AAL) atlas43,44. CIVET includes shrink-wrap deformable surface evolution of WM, local Laplacian distance and local SA, mapping to spherical domain, co-registration using cortical sulcal features and extraction of regional measurements via a deformably co-registered fine-scale lobar parcellation. SA was measured at the mid-cortical surface. CT and SA measures for 6 month data were extracted from surfaces propagated via deformable multi-modal, within-subject, co-registration44 of MRI data at 12 months.
Statistical Analysis
We used Chi-square tests to examine group differences in the categorical demographic variables, including race, gender, social economic income categories, and mother's education (see Extended Data, Table 1). For the continuous variables, including birth weight, mother's age at child birth, children's age at visits, and behavioral measures, we used ANOVA to test group differences. A random coefficient piecewise longitudinal mixed model was employed as a coherent framework to model brain growth trajectories in the first and second year and to test for group differences in growth trajectories. The three outcome variables under investigation were total brain volume (TBV), surface area (SA), and cortical thickness (CT). Each model included random coefficients for the first year growth rate (6-12 months) and change of growth rate in the second year (12-24 months), and random intercepts for each child to account for individual differences and correlated repeated measures collected at 6, 12 and 24 months. For subject i from group k at month j, the brain measure is:
The mean group growth rate in the first year will be β1k, and the growth rate for 12 months beyond will be β1k + β2k The inclusion of the change of slope after 12 months is to capture the change in growth rate from the first to the second year. The first two years of life is a period of rapid brain development, with growth rate being faster in the first than the second year45. The two-piece linear mixed model was chosen to capture the change in growth rate from the first to the second year. We required all subjects in this analysis to have 3 completed scans at 6, 12, and 24 months. This reduced the HR-ASD sample from 70 to 15 subjects. This requirement is to ensure that we captured the individual growth rate change from the first to the second year without the potential bias caused by partial visits and changes in study cohorts at different visits. We examined possible bias in the HR-ASD subjects with three completed visits versus those with only one or two visits and found no significant differences in demographic (e.g., sex, age) or outcome measure (e.g., TBV, SA). Results in Extended Data Tables 6 and 7.
To model the unique brain overgrowth separate from the general body growth, we modeled the brain growth relative to normative body growth in the first two years45,46. Normative age based on body length was used instead of chronological age in order to capture brain overgrowth in the context of body growth. The normative age for each infant's body size (tij length-age) was used in the model as the continuous growth variable. Length-age correlates highly with chronological age while taking into account the infant's sex and body size, which is necessary to determine the relative brain overgrowth. Sex-specific WHO height norms47 were used to determine length-age based on an infant's sex and height (length).
We addressed potential sex-related brain differences in two ways. First, in order to account for sex-related body size differences and their effects on brain volume48, we normalized differences in body size by using the sex specific WHO height norms. Second, we included sex as a covariate in the analysis model to account for remaining sex-related differences49. The approach to include sex as a model covariate will account for a linear, fixed effect of sex differences in brain volume. However, for developmental studies, the sex differences in body size and brain volume may be non-linear, with an unknown function form. Using a body size standardization based on normative sex-specific height data is more likely to account for non-linear sex-related differences.
The final model covariates include site and sex. Despite regular cross-site calibration in both behavioral and imaging protocols, a site covariate was included to account for the possibility of cohort differences or potential administrative differences in a multi-site study. Sex was included as a covariate in the analysis model to account for remaining sex-related differences not accounted for by sex-specific body growth. However, when we examined only males for group differences, our results remain unchanged (Extended Data Figure 2 and Extended Data Table 8).
As a sensitivity analysis, we also tested the model with other demographic, familial and child birth related covariates (race, social economic status, mother's education, mother's age at birth, birth weight, and gestational age), and only the site and sex remained in the model with p<0.01.
The association of 24-month clinical outcome (ADOS, CSBS) with brain growth rates (TBV) from 6-12 and 12-24 month intervals was assessed among HR subjects using Pearson correlation. Family income, mother's education, subject sex, and birth weight were examined as potential covariates, but none contributed significantly and were excluded from the final analysis.
Multiple comparison adjustments were performed for all pairwise comparisons and the correlation analyses, which followed the tests for overall group differences (F test, reported in Extended Data Table 2). All pairwise comparisons and correlation analyses used adaptive Hochberg multiple comparison adjustments50. Only those comparisons that survived the multiple comparison adjustment and remained significant are reported (Main Text Figure 1, Extended Data Table 2).
The machine learning analysis used a non-linear prediction model based on a standard three-stage deep learning network and included the following unbiased/unweighted information: sex, age-corrected ICV, and age-corrected SA and CT measurements from 39 left and 39 right cortical hemisphere regions at 6 months and 12 months (approximately 312 measurements). This analysis included 34 HR-ASD and 145 HR-neg subjects. The model was evaluated via a standard ten-fold cross-validation. The core of the prediction model is a weighted three-stage neural/deep learning network51, where the first stage reduces 315 measures to 100, the second stage reduces 100 to 10, and the third stage reduces 10 to only 2 such measures. At each stage, the measures (in the progressively smaller sets) are the weighted combination of input measures from the previous stage. In general, the training process determines a) those network weights that retain information that are capable of distinguishing the affected condition (e.g., HR-ASD) from the unaffected condition (HR-neg), as well as b) the linear support vector machine based classification decision that separates the group label (HR-ASD and HR-neg) in the two dimensional final network space. Thus to apply the prediction model, the data is first inserted into the two-dimension final network space using the trained deep learning network, and then classified in the final network space using the trained support vector machine. All training was performed purely on the training data in each fold. Once training was achieved, this prediction model was applied to the testing data in each fold. Classification measures of accuracy, sensitivity, specificity, PPV and NPV are combined and reported across the 10 folds. Details of our machine learning procedures and validity tests are provided in the Supplementary Information.
Data availability
The raw data that support the findings from this study are publically available from the NIH National Database for Autism Research (NDAR). Any additional data may be available from the corresponding author upon reasonable request. Source data for Figures 1-3 are provided with the paper.
Information on the following tools used in our analyses (AutoSeg, HeadCirc and ITK-SNAP) is freely available for download: http://www.med.unc.edu/psych/research/niral/download/download-software and http://www.nitrc.org.
Extended Data
Extended Data Figure 1. Visualization of cortical regions with surface area measures among the top 40 features contributing to the linear sparse learning classification.
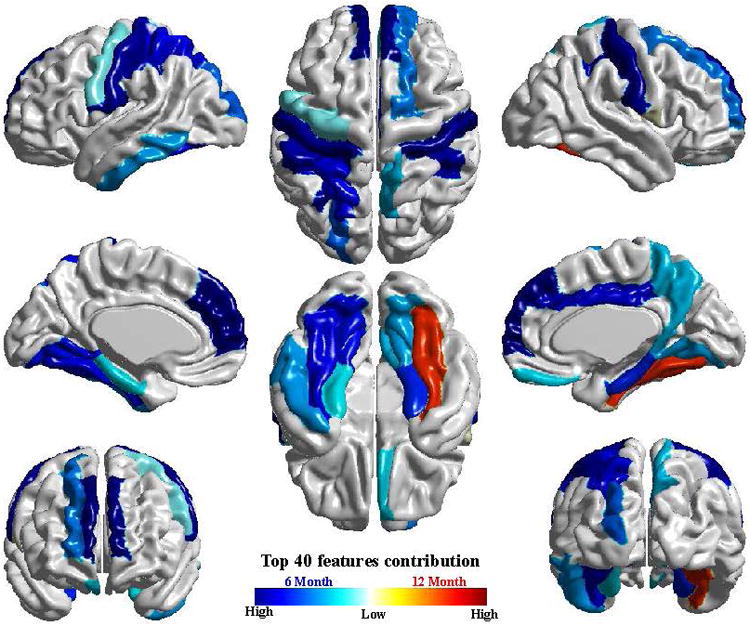
The cortical features produced by the deep learning approach (Main Text, Figure 3) are highly consistent with those observed using an alternative approach (linear sparse learning) shown here. Results from this alternative approach are included for comparison in the Supplementary Information (Supplementary Information, Tables 2 and 3).
Extended Data Figure 2. Trajectories of TBV for males (left) and females (right).
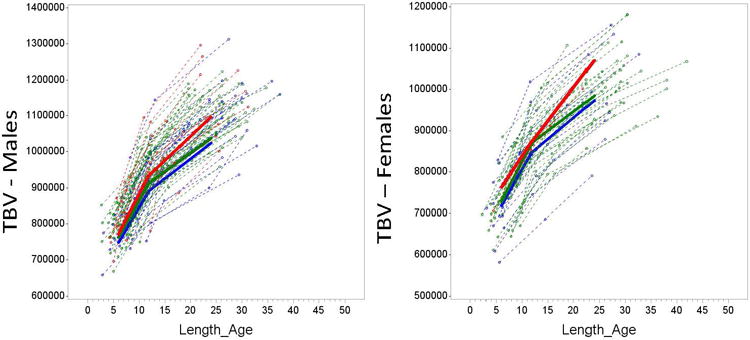
For illustrative purposes, we provide plots for Total Brain Volume (TBV) for males and females from the same sample. Figure 1 shows the longitudinal trajectories of total brain volume (TBV) from 6 to 24 months for the three groups examined, with males and females displayed separately. The trajectory of TBV for males only among the three groups is similar to the pattern we see in the full sample (Main Text, Figure 1). The female only HR-ASD group is quite small (n=2) which makes the pattern of trajectory difficult to interpret. These figures support the general similarity of the findings in the combined sample and the male-only sample. Key: red = HR-ASD, green = HR-neg, blue = LR. Total brain volume (TBV) shown in mm3. Length_age refers to the age corrected by length (body size).
Extended Data, Table 1. Subject demographics (including tests for group differences).
No significant group differences (between HR-ASD, HR-neg and LR) were observed in race/ethnicity, family income, maternal age at birth, infant birth weight, gestational age at birth, or age at visit. As expected based on the well-known disproportionately higher rates of ASD in males, the HR-ASD group contained significantly more males than the LR group (χ2 (2) = 15.7, p <.01). We also observed that the LR group had higher maternal education compared to the other two groups (χ2 (2) = 36.4, p <.01). As expected, based on the association between intellectual disability and ASD, the HR-ASD group had significantly lower Mullen and Vineland scores at 24 months than the other two groups. KEY: SD = standard deviation, % = percentage, Mullen ELC – Early Learning Composite standard score; Vineland ABC– Vineland Adaptive Behavior Composite standard score.
| LR (N=117) | HR-neg (N=248) | HR-ASD (N=70) | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | (%) | N | (%) | N | (%) | ||
| Sex | <0.01 | ||||||
| Female | 48 | (41) | 106 | (43) | 12 | (17) | |
| Male | 69 | (59) | 142 | (57) | 58 | (83) | |
| Race | 0.14 | ||||||
| Non-white | 15 | (13) | 29 | (12) | 9 | (13) | |
| White | 102 | (87) | 213 | (86) | 61 | (87) | |
| Not reported | 0 | (0) | 6 | (2) | 0 | (0) | |
| Family Income | 0.13 | ||||||
| Not Answered | 5 | (4) | 20 | (8) | 1 | (2) | |
| <50K | 17 | (15) | 52 | (21) | 19 | (27) | |
| 50-75K | 27 | (23) | 42 | (17) | 17 | (24) | |
| 75-100K | 20 | (17) | 40 | (16) | 12 | (17) | |
| 100-150K | 29 | (25) | 50 | (20) | 12 | (17) | |
| >150K | 19 | (16) | 44 | (18) | 9 | (13) | |
| Mother's Highest Education | <0.01 | ||||||
| Not Answered | 0 | (0) | 8 | (3) | 0 | (0) | |
| No College | 16 | (14) | 75 | (30) | 29 | (41) | |
| College Degree | 47 | (40) | 108 | (44) | 25 | (36) | |
| Graduate Degree | 54 | (46) | 57 | (23) | 16 | (23) | |
|
| |||||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | ||
|
| |||||||
| Mother's Age at Child Birth | 0.99 | ||||||
| Years | 33.2 | (4.2) | 33.2 | (4.7) | 33.3 | (4.1) | |
| Birth Weight | 0.52 | ||||||
| Pounds (LB) | 8.0 | (1.0) | 7.9 | (1.0) | 7.9 | (1.3) | |
| Gestational Age at Birth | 0.30 | ||||||
| Weeks | 39.3 | (1.3) | 39.1 | (1.2) | 38.9 | (1.1) | |
| Age at Study Visit | |||||||
| 6 month visit | 6.7 | (0.7) | 6.6 | (0.7) | 6.6 | (0.7) | 0.54 |
| 12 month visit | 12.7 | (0.7) | 12.7 | (0.6) | 12.7 | (0.7) | 0.48 |
| 24 month visit | 24.6 | (0.8) | 24.7 | (0.9) | 24.6 | (0.6) | 0.59 |
| 24 month Behavioral Scores | |||||||
| Mullen ELC | 109.7 | (13.8) | 101.8 | (15.9) | 79.3 | (17.6) | 0.01 |
| Vineland ABC | 105.0 | (7.4) | 101.0 | (9.0) | 88.1 | (10.0) | <0.01 |
Extended Data, Table 2. Group differences in developmental trajectories and cross-sectional volumes by age.
Key: LR = low risk, HR-neg = High Risk negative, HR-ASD = High Risk with Autism, Year 1 = 6-12 month period, Year 2 = 12-24 month period, Pairwise Group Comparison (p<.05), Adjusted Group Mean = Model estimated group means at the specified time point, SE = standard error, Unit of brain volume measures for trajectory and cross-sectional analyses is mm3. Unit for surface area is mm2, and for cortical thickness is mm. Slope is represented as change/months. The sample for the piecewise linear model included subjects with complete data at all three visits (6m, 12m, and 24m).
| LR(a) (N=42) | HR-neg(b) (N=91) | HR-ASD(c) (N=15) | Overall Group Comparison | Pairwise Group Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Trajectory | Slope | SE | Slope | SE | Slope | SE | df1, df2 | F | P | |
| Total brain volume | 1s1 Year | 24046 | 1416 | 25329 | 980 | 27298 | 2399 | 2,289 | 0.72 | 0.49 | |
| 2nd Year | 10928 | 523 | 10277 | 360 | 13318 | 1060 | 2,289 | 3.83 | 0.02 | c>(a, b) | |
| Surface Area | 1st Year | 972 | 71 | 1061 | 49 | 1331 | 125 | 2,289 | 3.13 | 0.04 | c>(a, b) |
| 2nd Year | 864 | 38 | 814 | 26 | 960 | 75 | 2,289 | 1.93 | 0.15 | ||
| Cortical Thickness | 1st Year | -0.024 | 0.003 | -0-024 | 0.002 | -0.024 | 0.006 | 2,289 | 0.00 | 0.99 | |
| 2nd Year | -0.025 | 0.002 | -0-021 | 0.001 | -0.026 | 0.004 | 2,289 | 1.44 | 0.24 | ||
| Cross-sectional Visit age | Adjusted Group Mean | SE | Adjusted Group Mean | SE | Adjusted Group Mean | SE | df1,df2 | F | P | ||
| Total brain volume | 6 months | 748890 | 9269 | 762420 | 6297 | 771403 | 15604 | 2,289 | 1.08 | 0.34 | |
| 12 months | 893167 | 11550 | 914391 | 7843 | 935189 | 19590 | 2,289 | 2.10 | 0.12 | ||
| 24 months | 1024297 | 12475 | 1037714 | 84442 | 1095002 | 21563 | 2,289 | 4.14 | 0.02 | c>(a, b) | |
| Surface area | 6 months | 51736 | 541 | 52611 | 367 | 52779 | 914 | 2,289 | 1.03 | 0.36 | |
| 12 months | 57570 | 671 | 58979 | 455 | 60764 | 1144 | 2,289 | 3.32 | 0.04 | c>a | |
| 24 months | 67933 | 765 | 68746 | 518 | 72281 | 1323 | 2,289 | 4.16 | 0.02 | c>(a, b) | |
| Cortical thickness | 6 months | 5.98 | 0.040 | 5.96 | 0.027 | 6.05 | 0.067 | 2,289 | 0.69 | 0.50 | |
| 12 months | 5.84 | 0.037 | 5.82 | 0.025 | 5.91 | 0.064 | 2,289 | 0.82 | 0.44 | ||
| 24 months | 5.54 | 0.029 | 5.56 | 0.020 | 5.59 | 0.051 | 2,289 | 0.48 | 0.62 | ||
Extended Data, Table 3. Raw means and standard deviations for TBV and SA group comparisons showing effect size and confidence intervals.
Key: Visit Age shown in months, HR-ASD = High Risk with Autism, HR-neg = High Risk without Autism, LR-neg = Low Risk, M = group mean, SD = standard deviation, Unit of brain volume measures is mm3. Unit for surface area is mm2.
| Total Brain Volume (TBV) Comparisons | |||||||
|---|---|---|---|---|---|---|---|
| Visit Age | Group 1 N | Raw Mean (SD) | Group 2 N | Raw Mean (SD) | t | Effect Size d′ | Effect size Confidence interval |
| 6 | HR-ASD N=15 | 800001 (69515) | HR-neg N=91 | 771089 (63012) | 0.54 | 0.15 | -0.40 - 0.70 |
| 6 | HR-ASD N=15 | 800001 (69515) | LR-neg N=42 | 770886 (77012) | 1.25 | 0.38 | -0.23 - 0.98 |
| 6 | HR-neg N=91 | 771089 (63012) | LR-neg N=42 | 770886 (77012) | 1.22 | 0.23 | -0.14 - 0.60 |
| 12 | HR-ASD N=15 | 959305 (83486) | HR-neg N=91 | 922692 (69138) | 0.99 | 0.28 | -0.28 - 0.83 |
| 12 | HR-ASD N=15 | 959305 (83486) | LR-neg N=42 | 917106 (85631) | 1.87 | 0.57 | -0.05 - 1.17 |
| 12 | HR-neg N=91 | 922692 (69138) | LR-neg N=42 | 917106 (85631) | 1.53 | 0.29 | -0.09 - 0.66 |
| 24 | HR-ASD N=15 | 1111639 (101094) | HR-neg N=91 | 1069671 (80844) | 2.48 | 0.70 | 0.13 - 1.25 |
| 24 | HR-ASD N=15 | 1111639 (101094) | LR-neg N=42 | 1066273 (99719) | 2.86 | 0.88 | 0.24 - 1.48 |
| 24 | HR-neg N=91 | 1069671 (80844) | LR-neg N=42 | 1066273 (99719) | 0.9 | 0.17 | -0.20 - 0.54 |
| Surface Area (SA) Comparisons | |||||||
| 6 | HR-ASD N=15 | 54886 (3671) | HR-neg N=91 | 53017 (3723) | 0.17 | 0.05 | -0.51 - 0.60 |
| 6 | HR-ASD N=15 | 54886 (3671) | LR-neg N=42 | 52785 (4102) | 1.0 | 0.31 | -0.30 - 0.91 |
| 6 | HR-neg N=91 | 53017 (3723) | LR-neg N=42 | 52785 (4102) | 1.35 | 0.25 | -0.12 - 0.62 |
| 12 | HR-ASD N=15 | 61745 (4206) | HR-neg N=91 | 59576 (4046) | 1.45 | 0.41 | -0.15 - 0.96 |
| 12 | HR-ASD N=15 | 61745 (4206) | LR-neg N=42 | 59011 (4652) | 2.44 | 0.75 | 0.12 - 1.35 |
| 12 | HR-neg N=91 | 59576 (4046) | LR-neg N=42 | 59011 (4652) | 1.75 | 0.33 | -0.04 - 0.70 |
| 24 | HR-ASD N=15 | 73254 (5293) | HR-neg N=91 | 70757 (4642) | 2.49 | 0.70 | 0.13 - 1.25 |
| 24 | HR-ASD N=15 | 73254 (5293) | LR-neg N=42 | 70686 (5450) | 2.87 | 0.88 | 0.24 - 1.49 |
| 24 | HR-neg N=91 | 70757 (70757) | LR-neg N=42 | 70686 (5450) | 0.88 | 0.17 | -0.21 - 0.53 |
Extended Data, Table 4. Prediction model using cortical data to classify groups at 24 months.
A non-linear prediction model included the following unbiased/unweighted information: sex, age-corrected ICV, and age-corrected SA and CT measurements from 39 left and 39 right cortical hemisphere regions at 6 months and 12 months. The prediction model was evaluated using a standard ten-fold cross-validation approach. Classification performance of the prediction model is at 94% overall accuracy, 88% sensitivity, 95% specificity, 81% positive predictive value and 97% negative prediction value. KEY: TP = true positive, FP = false positive, PPV = positive predictive value, NPV = negative predictive value, Diagnosis = outcome based on DSM-IV-TR.
| Prediction | Diagnosis HR+ N=34 | Diagnosis HR-N=145 | ||
|---|---|---|---|---|
| HR+ | 30 | 7 | 81% | 37 |
| HR- | 4 | 138 | 97% | 142 |
| 88% | 95% | |||
| 34 | 145 | 179 | ||
| A known | B known | |||
| A test | TP | FP | PPV | TP+FP |
| B test | FN | TN | NPV | FN+TN |
| Sensitivity | Specificity | |||
| TP+FN | FP+TN | (TP+FN+FN+TN) |
Extended Data Table 5. Clinical characteristics for LR subjects who met ASD criteria at 24 months.
Key: All data presented is for 24 month old visit. LR = low risk subjects who met criteria for ASD at 24 months, DSM = DSM-IV diagnostic criteria, PDD = pervasive developmental disorder, not otherwise specified, AUT = autism disorder, Mullen ELC = Mullen Early Learning Composite standard score, Vineland ABC = Vineland Adaptive Behavior Composite standard score, ADOS SA = ADOS social affective total score, ADOS RBx = ADOS Repetitive behavior total, ADOS Sev = ADOS severity score, TBV = total brain volume, SA = surface area, NA = no MRI data at 24 months.
| LR | DSM | Sex | Mullen ELC | Vineland ABC | ADOS SA | ADOS RBx | ADOS Sev | TBV | SA |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | PDD | F | 113 | 94 | 12 | 1 | 6 | 1034400 | 69839 |
| Case 2 | PDD | M | 82 | 78 | 8 | 4 | 4 | NA | NA |
| Case 3 | AUT | M | 59 | 89 | 12 | 4 | 7 | 1110231 | 72244 |
Extended Data, Table 6. Subject demographics (including tests for group differences) for subjects with all 3 longitudinal visits and those with 1-2 visits completed.
Key: LR = low risk, HR-neg = High Risk negative, HR-ASD = High Risk with Autism, M = group mean, SD = standard deviation, % = percentage.
| HR-ASD(c) | ||||||
|---|---|---|---|---|---|---|
| Measure | Visit | Subjects with All 3 Visits (N=15) | Subjects with Partial Visits (N=53) | |||
| N | % | N | % | P | ||
| Sex | .73 | |||||
| Male | 13 | 87 | 44 | 83 | ||
| Female | 2 | 13 | 9 | 17 | ||
| Race | .99 | |||||
| White | 13 | 87 | 46 | 87 | ||
| Non-white | 2 | 13 | 7 | 13 | ||
| Not reported | 0 | 0 | 0 | 0 | ||
| Family Income | .54 | |||||
| Not answered | 0 | 0 | 1 | 2 | ||
| <50K | 3 | 20 | 14 | 26 | ||
| 50K-75K | 3 | 20 | 14 | 26 | ||
| 75K-100K | 5 | 33 | 7 | 13 | ||
| 100K-150K | 3 | 20 | 9 | 17 | ||
| >150K | 1 | 7 | 8 | 15 | ||
| Mother's Highest Education | -58 | |||||
| No college | 5 | 33 | 23 | 43 | ||
| College Degree | 5 | 33 | 19 | 36 | ||
| Graduate Degree | 5 | 33 | 11 | 21 | ||
| Mean | SD | Mean | SD | P | ||
| Mother's Age at Childbirth (Years) | 34.5 | 3.6 | 32.9 | 4.2 | .20 | |
| Gestational Age at Birth (Weeks) | 38.6 | 1.0 | 39.1 | 1.1 | .24 | |
| Birth Weight in pounds (lbs) | 7.6 | 1.7 | 7.9 | 1.2 | .54 | |
| Age at study visit | 6 months | 6.7 | 0.8 | 6.6 | 0-7 | .61 |
| 12 months | 12.9 | 0.7 | 12.6 | 0.6 | .10 | |
| 24 months | 24.6 | 0.6 | 24.6 | 0.6 | .72 | |
Extended Data, Table 7. Group differences in developmental level, total brain volume, and surface area.
We further tested whether there were any group differences in developmental functioning (Mullen) and also the two main variables of interest in our longitudinal analyses, TBV and SA. Groups did not differ in developmental functioning at any visit age, indicating the subjects in the 3 visit and 1-2 visit subgroups are similar in their developmental capabilities. No group differences were observed for either TBV or SA at any visit age, suggesting both groups appear to have similar profiles in their brain measures. Key: HR-ASD = High Risk with Autism, Age – visit age (in months), M = group mean, SD = standard deviation, Unit of brain volume measures is mm3. Unit for surface area is mm2.
| HR-ASD(c) | ||||||
|---|---|---|---|---|---|---|
| Age | Subjects with All 3 Visits (N=15) | Subjects with Partial Visits (N=30, 26, 26) | ||||
| Mean | SD | Mean | SD | P | ||
| Mullen Scales of Early Learning Composite Standard Score | 6 | 98 | 12 | 97 | 15 | .73 |
| 12 | 97 | 14 | 91 | 14 | .22 | |
| 24 | 79 | 18 | 77 | 19 | .80 | |
| Total Brain Volume | 6 | 800001 | 69514 | 782607 | 64296 | .41 |
| 12 | 959305 | 83485 | 975594 | 75371 | .53 | |
| 24 | 1111639 | 101093 | 1093702 | 106536 | .60 | |
| Surface Area | 6 | 54886 | 3671 | 54312 | 3830 | .68 |
| 12 | 61745 | 4206 | 61545 | 4893 | .90 | |
| 24 | 73254 | 5293 | 71641 | 6388 | .41 | |
Extended Data, Table 8. Group differences in developmental trajectories and cross-sectional volumes by age: Males Only.
The primary analysis of brain volume trajectories included only those male and female study participants, with three completed visits (6-12-24 months), to best depict longitudinal trajectories over time. Separate analyses on males and females are likely to be inadequately powered due to small subsample size (males = 13, females = 2) and therefore provide inconclusive results. With that caveat, we provide the results of our male-only three group analysis for total brain volume. We do not see any group differences in the first year (6-12 months). The HR-ASD males show a pattern of TBV brain enlargement by the end of the second year, compared to the LR and HR-neg groups. Key: LR = low risk, HR-neg = High Risk negative, HR-ASD = High Risk with Autism, 1st Year = 6-12 month period, 2nd Year = 12-24 month period, Pairwise Group Comparison (p<.05), SE = standard error, LSM = least square means, Unit of brain volume measures for trajectory and cross-sectional analyses is mm3. Unit for surface area is mm2, and for cortical thickness is mm. Slope is represented as change/months. Sample for piecewise linear model included subjects with complete data at all three visits (6m, 12m, 24m).
| LR(a) (N=28) | HR-neg(b) (N=51) | HR-ASD(c) (N=13) | Overall Group Comparison | Pairwise Group Contrast | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Trajectory | Slope | SE | Slope | SE | Slope | SE | df1, df2 | F | P | |
| Total brain volume | 1st Year | 21559 | 1683 | 24038 | 1124 | 18633 | 4522 | 2, 106 | 1.25 | 0.29 | |
| 2nd Year | 10398 | 896 | 9396 | 450 | 16255 | 3057 | 2, 106 | 2.83 | 0.06 | c>b | |
| Surface Area | 1st Year | 1020 | 105 | 1070 | 75 | 1421 | 153 | 2, 177 | 2.56 | 0.08 | c>(a,b) |
| 2nd Year | 858 | 47 | 891 | 37 | 921 | 80 | 2, 177 | 0.29 | 0.75 | ||
| Cortical Thickness | 1st Year | -0.020 | 0.004 | -0.021 | 0.003 | -0.025 | 0.006 | 2, 177 | 0.19 | 0.83 | |
| 2nd Year | -0.026 | 0.002 | -0.023 | 0.002 | -0.026 | 0.004 | 2, 177 | 0.41 | 0.67 | ||
| Cross-Sectional | Visit age | LSM | SE | LSM | SE | LSM | SE | df1,df2 | F | P | |
| Total brain volume | 6 months | 718589 | 16333 | 727226 | 9585 | 762604 | 42125 | 2, 106 | 0.49 | 0.62 | |
| 12 months | 847944 | 20012 | 871452 | 11539 | 874399 | 52450 | 2, 106 | 0.53 | 0.59 | ||
| 24 months | 972725 | 21498 | 984204 | 12307 | 1069460 | 57570 | 2, 106 | 1.24 | 0.29 | ||
| Surface area | 6 months | 53167 | 660 | 54885 | 473 | 54463 | 939 | 2, 177 | 2.32 | 0.10 | |
| 12 months | 59287 | 781 | 61307 | 588 | 62990 | 1170 | 2, 177 | 4.01 | 0.02 | c>a | |
| 24 months | 69582 | 886 | 72001 | 667 | 74036 | 1354 | 2, 177 | 4.42 | 0.01 | c>a | |
| Cortical thickness | 6 months | 6.04 | 0.05 | 5.99 | 0.033 | 6.09 | 0.067 | 2, 177 | 1.10 | 0.33 | |
| 12 months | 5.92 | 0.045 | 5.86 | 0.034 | 5.95 | 0.07 | 2, 177 | 0.81 | 0.45 | ||
| 24 months | 5.61 | 0.035 | 5.58 | 0.026 | 5.63 | 0.05 | 2, 177 | 0.37 | 0.69 | ||
Supplementary Material
Acknowledgments
This work was supported by an NIH Autism Center of Excellence grant (NIMH and NICHD #HD055741 to J. Piven), Autism Speaks (#6020) and the Simons Foundation (#140209). Further support was provided by the National Alliance for Medical Image Computing (NA-MIC), funded by the NIH through grant U54 EB005149, the IDDRC Imaging and Participant Registry cores (NICHD #HD003110 to J. Piven), and R01 MH093510 (to J. Pruett). We thank Margaret Burchinal and Kinh Young Truong for their consultation on the statistical methods and approach. Given the large commitment of time and effort required by this study, we extend our sincere appreciation to the families who have participated in this study and the numerous research assistants and staff who have contributed to this work.
Footnotes
Contributions: All co-authors discussed the results, made critical contributions to the work and contributed to the writing of the manuscript. HCH, KNB, SRD, AME, RCM, SP, JP, RTS, and DWS contributed to the data collection. ACE, PK provided support for data management. BCM, SHK, MS, DLC, ACE, VSF, and GG conducted image processing. HG, BCM, SHK, MS, HZ, analyzed the data. HCH wrote the manuscript with JP, HG, BCM, MS and with JJW, JTE, MRS, JNC, JRP, AME, RTS and LZ providing additional feedback.
Disclosures: The authors declare no competing financial interests.
References
- 1.Hazlett HC, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62(12):1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 2.Hazlett HC, et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry. 2011;68(5):467–476. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozonoff S, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49(3):256–266. [PMC free article] [PubMed] [Google Scholar]
- 4.Zwaigenbaum L, et al. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23(2-3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Piven J, et al. An MRI study of brain size in autism. Am J Pyschiatry. 1995;152(8):1145–1149. doi: 10.1176/ajp.152.8.1145. [DOI] [PubMed] [Google Scholar]
- 6.Courchesne E, et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 7.Schumann CM, et al. Longitudinal magnetic resonance imaging study of cortical development throuhg early childhood in autism. J Neurosci. 2010;30(12):4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparks B, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 9.Shen MD, et al. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain. 2013;136(9):2825–2835. doi: 10.1093/brain/awt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lord C, Rutter M, DiLavore PC, Risi S. The Autism Diagnostic Observation Schedule. Los Angeles, CA: Western Psychological Services; 2000. [Google Scholar]
- 11.Wetherby A, Prizant B. Communication and symbolic behavior scales developmental profile- First normed edition. Baltimore, MD: Paul H. Brookes; 2002. [Google Scholar]
- 12.Li G, et al. Mapping longitudinal hemispheric structural asymmetries of the human cerebral cortex from birth to 2 years of age. Cereb Cortex. 2014;24(5):1289–1300. doi: 10.1093/cercor/bhs413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elison JT, et al. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am J Psychiatry. 2013;170(8):899–908. doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estes A, et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first two years of life. J Neurodev Disorders. 2015;7(1):24. doi: 10.1186/s11689-015-9117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qureshi AY, et al. Opposing brain differences in 16p11.2 deletion and duplication carriers. J Neurosci. 2014;34(34):11199–11211. doi: 10.1523/JNEUROSCI.1366-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernier R, et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell. 2014;158(2):263–276. doi: 10.1016/j.cell.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panizzon MS, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;11:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 19.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 20.Casanova MF, et al. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006;112(3):287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- 21.Hill J, et al. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci. 2010;107(29):13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Packer A. Neocortical neurogenesis and the etiology of autism spectrum disorder. Neurosci Biobehav Rev. 2016;64:185–196. doi: 10.1016/j.neubiorev.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Fan WQ, et al. Overproduction of upper-layer neurons in the neortex leads to autism-like features in mice. Cell Rep. 2014;9(5):1635–1643. doi: 10.1016/j.celrep.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Pucilowska J, Puzery PA, Karlo JC, Galan RF, Landreth GE. The 16p11.2 deletion mouse model of autism exhibits altered cortical progenitor proliferation and brain cytoarchitecture linked to the ERK MAPK patheway. J Neurosci. 2015;35(7):3190–2100. doi: 10.1523/JNEUROSCI.4864-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nonaka-Kinoshita M, et al. Regulation of cerebral cortex size and folding by expansion of basal progenitors. EMBO J. 2013;32(13):1817–1828. doi: 10.1038/emboj.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugathan A, et al. CHD8 regulates neurodevelopmental pathways associated with autism spectrum disorder in neural progenitors. Proc Natl Acad Sci. 2014;111(42):E4468–E4477. doi: 10.1073/pnas.1405266111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotney J, et al. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nature Commun. 2015;6:6406. doi: 10.1038/ncomms7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchetto MC, et al. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.95. E pub head of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charman T. Early identification and intervention in autism spectrum disorders: Some progress but not as much as we hoped. Int J Speech Lang Pathol. 2014;16(1):15–18. doi: 10.3109/17549507.2013.859732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landa RJ, Gross AL, Stuart EA, Faherty A. Developmental trajectories in children with and without autism spectrum disorders: the first 3 years. Child Dev. 2013;84(2):429–442. doi: 10.1111/j.1467-8624.2012.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messinger D, et al. Beyond autism: a baby siblings research consortium study of high-risk children at three years of age. J Am Acad Child Adolesc Psychiatry. 2013;52(3):300–308. doi: 10.1016/j.jaac.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Georgiades S, et al. A prospective study of autistic-like traits in unaffected siblings of probands with autism spectrum disorder. JAMA Psychiatry. 2013;70(1):42–48. doi: 10.1001/2013.jamapsychiatry.1. [DOI] [PubMed] [Google Scholar]
- 33.Ozonoff S, et al. The broader autism phenotype in infancy: When does it emerge? J Am Acad Child Adolesc Psychiatry. 2014;53(4):398–407. doi: 10.1016/j.jaac.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
References (Methods)
- 34.Hazlett HC, et al. Brain volume findings in 6-month-old infants at high familial risk for autism. Am J Psychiatry. 2012;169:601–608. doi: 10.1176/appi.ajp.2012.11091425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 36.Mullen EM. Mullen Scales of Early Learning (AGS ed) Circle Pines, MN: American Guidance Service, Inc; 1995. [Google Scholar]
- 37.Sparrow S, Balla D, Cicchetti D. Vineland scales of adaptive behavior: A survey form manual. Circle Pines, MN: American Guidance Service, Inc; 1984. [Google Scholar]
- 38.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, D.C: 2000. text rev. [Google Scholar]
- 39.Guthri W, Swineford LB, Nottke C, Wetherby A. Early diagnosis of autism spectrum disorder: stability and change in clinical diagnosis and symptom presentation. J Child Psychol Psychiatry. 2013;54(5):582–590. doi: 10.1111/jcpp.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, et al. Multi-atlas segmentation of subcortical brain structures via the AutoSeg software pipeline. Front Neuroinform. 2014;8:7. doi: 10.3389/fninf.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw P, et al. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72(3):191–197. doi: 10.1016/j.biopsych.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw P, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28(14):3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 44.Kim SH, et al. Adaptive prior probability and spatial temporal intensity change estimation for segmentation of the one-year-old human brain. J Neurosci Methods. 2013;212(1):43–455. doi: 10.1016/j.jneumeth.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilmore JH, et al. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex. 2011;22(11):2478–2485. doi: 10.1093/cercor/bhr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chawarska K, et al. Early generalized overgrowth in boys with autism. Arch of Gen Psych. 2011;68(10):1021–31. doi: 10.1001/archgenpsychiatry.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.WHO. Multicentre Growth Reference Study Group. WHO Child Growth Standards: growth velocity based on weight, length and head circumference: methods and development. Geneva (Switzerland): WHO; 2009. [Google Scholar]
- 48.Im K, et al. Brain size and cortical structure in the adult human brain. Cerebral Cortex. 2008;18:2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- 49.Gilmore JH, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007;27(6):1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometricka. 2006;93(3):491–507. [Google Scholar]
- 51.Hinton GE, Salakhutkinov RR. Reducing the dimensionality of data with neural networks. Science. 2006;313(5786):504–507. doi: 10.1126/science.1127647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data that support the findings from this study are publically available from the NIH National Database for Autism Research (NDAR). Any additional data may be available from the corresponding author upon reasonable request. Source data for Figures 1-3 are provided with the paper.
Information on the following tools used in our analyses (AutoSeg, HeadCirc and ITK-SNAP) is freely available for download: http://www.med.unc.edu/psych/research/niral/download/download-software and http://www.nitrc.org.


