Abstract
Pre-storage leucoreduction has been universally adopted in most developed countries in Asia, Europe and the Americas. It decreases febrile transfusion reactions, alloimmunisation to HLA antigens, cytomegalovirus exposure, the accumulation of a number of pro-inflammatory mediators in the supernatant, including the accumulation of platelet-and leucocyte-derived proteins and metabolites during routine storage. This review will highlight the lipids and proteins, biological response modifiers (BRMs) that accumulate, their clinical effects in transfused hosts, and methods of mitigation.
Keywords: red blood cells, transfusion, transfusion-related acute lung injury (TRALI), leucoreduction, neutrophils
Introduction
Transfusion of red blood cells (RBCs) has saved numerous lives, far outnumbering any adverse events induced by their infusion. RBC transfusions allow for lengthy and complicated surgeries, survival from life-threatening injuries in both military and civilian settings, organ, bone marrow, and stem cell transplantation, treatment of malignancies with myelotoxic chemotherapy, survival from haemorrhagic diatheses, and haematologic disorders in which RBC production is significantly decreased or destroyed. While the benefits of transfusions far outweigh the risks of a reaction, these reactions still occur, and therefore efforts have been made to improve haemotherapy in order to further decrease clinical morbidity and mortality.
Pre-storage leucoreduction of RBCs (LR-RBCs) by buffy coat depletion, simple filtration, or a combination of the two removes leucocytes and platelets from the RBC units. Buffy coat depletion causes a one log depletion of both leucocytes and platelets while filtration decreases leucocytes by more than 3 logs and platelets by 2 logs1. Universal pre-storage leucoreduction significantly decreases febrile non-haemolytic transfusion reactions and decreases exposure to HLA antigens, HLA alloimmunisation, and decreases the accumulation of platelet and leucocyte derived pro-inflammatory mediators, biological response modifiers (BRMs), including: interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-18 (IL-18), soluble CD40 ligand (sCD40L), and lysophosphatidylcholines (lyso-PCs)2–8. Despite these decreases, pro-inflammatory mediators still accumulate during routine storage of LR-RBCs, a number of which have been implicated in the pathogenesis of transfusion-related acute lung injury (TRALI) and post-injury multiple organ failure (MOF), which includes acute lung injury (ALI)5,9. This review will detail the mediators/BRMs in question, their clinical effects and possible mitigation, as well as proposing novel strategies to inhibit their production during routine storage.
Transfusion-related acute lung injury
TRALI, which is a rare, adverse event, has been linked to the infusion of bioactive lipids which accumulate during the routine storage of RBCs, and are released into and accumulate in the supernatant of the RBC units5,10–12. In unmodified RBCs, there are two classes of lipids, based upon their retention time via normal phase with further characterisation by reverse phase high pressure liquid chromatography (HPLC) and identification by mass spectrometry: a mixture of lyso-PCs and non-polar lipids consisting of arachidonic acid, 5-hydroxyeicosotetraenoic acid (HETE), 12-HETE and 15-HETE. These data have been reported by a number of other groups5,8,13–20. These lipids were increased in patients at the time TRALI was recognised, and both the supernatants and the lipids from stored RBCs, both day (d)28 and d42 of storage, induced TRALI as the second event in a 2-event animal model5,12,21,22. Pre-storage leucoreduction by filtration, specifically the Haemonetics BPF4 filter, removes two logs of platelets and the lyso-PCs from LR-RBC units1,5,12. The neutral lipids are not affected and may still serve as the second event in a 2-event animal model of TRALI5,12,22. In addition, this removal of platelets also decreases the accumulation of sCD40L, a reported co-factor in TRALI, which has the capacity to alter PMN physiology, e.g. prime the PMNs through the CD40 receptor on the cellular membrane3. Importantly, animal models are employed to mimic human disease and to give relevance to suspected mediators; however, just because each and every rodent experiences TRALI, for example, does not mean that each and every human will also manifest this adverse event23.
The accumulation of bioactive lipids has been questioned; however, these studies measured lyso-PCs in LR-RBCs, by both buffy coat removal and filtration24. Pre-storage leucoreduction nullifies the accumulation of these lipids because of effective platelet removal (approximately 2 logs). Moreover, flow-based measurement of oxidase activity is a qualitative test, and because of time constraints, it is not amenable to quantification since the actual assays are not done simultaneously like the 96-well plate assays that measure superoxide dismutase-inhibitable reduction of cytochrome c1,24. Lastly, in a prospective clinical study of TRALI, bioactive lipids (lyso-PCs) were risk factors for TRALI in the univariate but not the multivariate analyses25. In addition, the bioactivity measurement on PMNs, increased surface expression of CD11b/CD66, did not demonstrate significant pro-inflammatory activity. However, the details of these assays are important because: 1) the bioactive lipids present: AA and 5-, 12-, and 15-HETEs affect the surface expression of CD11b in five minutes and with longer incubations the surface expression disappears; and 2) if fixed with paraformaldhyde prior to incubation with the antibodies to CD11b/CD66, the antigens are changed for CD11b such that the increased surface expression may be diminished by more than 30%25. As stated above, lyso-PCs and sCD40L do not accumulate in LR-RBCs due to platelet removal by the filter and the analyses looked at lipids that should not be present in the RBCs but would be in the platelet concentrates3,5,25.
TRALI mitigation and experimental filtration
Transfusion-related acute lung injury mitigation has centred on the male-only plasma donors to obviate female plasma which may contain antibodies to human lymphocyte antigens (HLA) or human neutrophil antigens (HNA) due to pregnancy. These efforts have significantly decreased TRALI secondary to plasma transfusion but have not eliminated it26–28. Nevertheless, there are few formal mitigation strategies for RBC transfusions and reported clinical series have shown that 20% of TRALI follows RBC transfusions, with this percentage likely to increase because of the decrease in TRALI to plasma26,27,29. RBCs contain 5–10 mL of plasma so the relative amount of antibodies to HLA or HNA antigens is relatively sparse compared to plasma or even apheresis platelets, although only 10–20 mL of antibody-containing plasma may elicit TRALI30,31. To this end, an experimental filter was developed that removes virtually two logs of IgG. Filtered plasma samples from multiparous females known to have antibodies to HLA or to HNA-3a were deemed negative via measurements with Luminex™ beads and flow cytometry at two HLA reference laboratories or for HNA-3a at the Granulocyte Laboratory, Blood Center of Southeastern Wisconsin, USA, employing standard techniques in a blinded fashion1. Lastly, these experimental filters also removed neutral lipid priming activity which accumulates during routine storage. (This will be discussed under the proteomics section)1.
TRALI modelling
The 2-event model of TRALI has been recently criticised because humans given endotoxin (LPS) from E. coli followed by stored LR-RBCs or the lipids from LR-RBCs did not manifest TRALI32,33. Unfortunately, these studies are marred by a number of factors, most of which appeared in the literature many years ago. In rats, LPS from E. coli may not be an effective first event; activation of the pulmonary endothelium did not result in PMN sequestration, which is to be expected because rats are known to live successfully in sewers, which have high levels of E. coli and E. coli LPS from human waste. Thus, for all rodent experiments, the first event was LPS from S. enteritides given via an intraperitoneal injection11,34,35. This first event caused the animals to become: 1) febrile with rigors and shaking; 2) tachypneic; and 3) despondent, although they respond to pain, with all rats having copious diarrhoea1,11,22,34–37. On the cellular level, IP S. enteritides LPS in rats causes activation of the pulmonary endothelium and sequestration of PMNs to the capillaries as evidenced by increased pulmonary myeloperoxidase and the lung histology without ALI1,11,22,37. The S. enteritides LPS concentration administered is 2 mg/kg with 99% animal survival1,11,22,37. Although critics of this model have deemed this dose to be supra-physiological, 2 individuals were injected with 2 μg-1 mg of either E. coli or S. enteritides and both became acutely ill with fevers, hypotension, gastroenteritis, increased respiratory rate, somnolence, and malaise, with one admitted to the intensive care unit with mild ALI and multi-organ dysfunction; both survived 38–40. Additionally, the treatment of human neurosyphilus was LPS infusion that reached 1 mg intravenous (IV) with the overwhelming majority of the patients surviving38. Recent human TRALI models gave E. coli LPS IV at a concentration of 2 ng/kg which corresponds to 40 pg/mL of plasma for males and 48 pg/mL of plasma for females and resulted in fever over 38 °C, pulse rates of over 90 beats/min, and mild tachypnea with respiratory rates over 20 breathes/min32,33. There was no evidence that any of the human subjects had pulmonary endothelial activation or PMN sequestration in the lung, prerequisites for the 2-event model of TRALI32,33. In vitro LPS, whether from E. coli or S. enteritides did not cause significant activation of human pulmonary microvascular endothelial cells (HMVECs), as measured by increased surface expression of intercellular adhesion molecule-1 (ICAM-1) or chemokine release, until a concentration of 20 ng/mL was reached9,41,42. In addition, LPS primes PMNs; however, E. coli LPS did not prime fMLF activation of the respiratory burst of human PMNs at concentrations of 2 ng/mL and did induce priming of the oxidase at 20 ng/mL but to a lesser extent compared to S. enteritides LPS, which was reported to have an almost identical concentration curve for PMN priming of the fMLF-activated respiratory burst and lyso-PC activation of the oxidase42. Unlike intact animals, there is no way to process or excrete the LPS, and the human modelling used concentrations much less than the concentrations needed to cause physiological changes in human cells; thus, clinical TRALI from the human modelling is unlikely because of an insufficient first event43–45. Lastly, the administration of LPS (intravenous vs intraperitoneal) may also have ramifications for its suitability as the first event of a 2-event model of TRALI in humans.
The proteome of the RBC supernatant
To determine the role of pre-storage leucoreduction on the release of proteins during routine storage, 5 units of red blood cells were drawn; 50% (by weight) were left unmodified and the other 50% was pre-storage leucoreduced by filtration. Both were stored in AS-546. The protein concentration increased 2–3-fold in both the unmodified- and LR-RBCs from day (d)1 to d42 of storage46. Leucoreduction decreased the total number of proteins in the supernatant from 401 to 231, and of these, 84 proteins increased (>2-fold increase) with 42 being unique to d42, 30 decreased (<2-fold decrease) with 7 being unique to d1, and 117 remained unchanged46. Preliminary data with 3 RBC/LR-RBC units from female donors compared to 3 RBC/LR-RBC units from male donors only demonstrated an increase in pregnancy zone protein, which is increased in the female sex46. As expected, the leucocyte and platelet-derived proteins, present in the unmodified RBCs, were not present in the LR-RBC supernatant. However, the glycolytic enzymes were more pronounced in LR-RBC supernatant, including: transaldolase, fructose-bisphosphate aldolase, phosphoglycerate kinase, and α-enolase46. Other proteins of interest that increased in the LR-RBC supernatant included: latexin (also known as endogenous carboxypeptidase inhibitor and implicated as a mediator of the haematopoietic stem cell compartment), Prdx1, Prdx2, and Prdx6. These all increased during storage in the LR-RBC supernatant likely due to protease activity. Importantly, Prdx6 contains a phospholipase domain which requires either acidic pH or T-phosphorylation for activity; immunoblotting of the Prdx6 in LR-RBCs showed T-phosphorylation indicating an active enzyme38,47–50. There was also significant accumulation of MMP-8 and MMP-9, which display extracellular protease activity, most proteosome subunits, and a drastic decrease in cystatin C46,51,52. The presence of an active phospholipase in LR-RBCs may explain the accumulation of AA and 5-, 12-, and 15-HETEs, which have been implicated in TRALI5. In addition, these lipids can be used as not only the second event, but also the first event in a 2-event animal model of ALI.
RBC supernatant lipids and proteins and the injured patient
Massive RBC transfusion, more than 6 units in the first 12 hours, was an independent risk factor for the development of post-injury MOF53–56. With a more conservative transfusion target, haemoglobin of 7.0 g/dL, the transfusion of fewer RBCs has resulted in less MOF, despite increasing patient age and increased injury severity scores, both risk factors for MOF57. In these early studies that controlled for the number of RBC units transfused, older, stored RBCs were implicated in MOF56. As stated, MOF has decreased; however, post-injury ALI still plagues more than 12.5% of severely injured patients ISS more than 1758. In older LR-RBCs, neutral lipids accumulate, notably AA and 5-, 12-, and 15-HETEs, and pilot data have demonstrated that they induce activation of HMVECs and human liver sinusoidal endothelial cells (LSECs) at concentrations that would be reached by 2, 4 and 6 units of LR-RBCs transfused5.
The proteome of LR-RBCs and that of the injured patients may provide some insight into the development of trauma-induced coagulopathy (TIC). Recent work on TIC has subdivided trauma patients based on their thrombolytic phenotype: systemic hyperfibrinolysis, physiological fibrinolysis and fibrinolysis shutdown59,60. A number of proteins in the LR-RBC supernatants have an affinity for plasminogen, especially α-enolase which is the plasminogen cellular receptor, and may be involved in the prolongation of TIC with respect to fibrinolysis: shutdown, physiological or hyperfibrinolysis9.
Preliminary data have implicated a role for α-enolase in injured patients at risk for ALI (based on the number of transfusions) who also have evidence of fibrinolysis shutdown. These patients are also prone to organ injury, as well as venous thromboembolism (VTE). In vitro, α-enolase significantly increased ICAM-1 surface expression on HMVECs and induced the adherence of PMNs to these activated endothelial cells9. This HMVEC activation was inhibited by anti-proteases, required human plasma, and served as the first event in a 2-event model of PMN cytotoxicity9. α-enolase was shown to also co-precipitate with PAR-2 and plasminogen/plasmin in HMVECs and enzymatic activity was not required9. Thus, proteins that accumulate during RBC storage as a risk factor for ALI, such as α-enolase, may also elicit previously unrecognised adverse clinical events, both TIC and ALI.
Possible mitigation
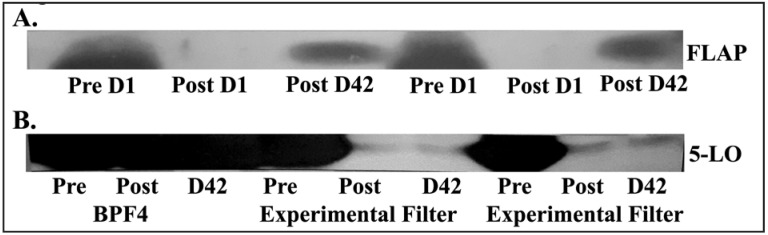
Experimental filtration of RBC units, as discussed above, not only removes 2 logs of IgG but it also significantly decreases the priming activity and obviated stored RBCs as the second event in a 2-event animal model of TRALI1. In addition, the measured concentrations of AA and 5-HETE were also decreased in the units that underwent experimental filtration vs those that were just leucoreduced using the Haemonetics BPF4 filter1. As stated previously, active Prdx6 accumulates during RBC storage. When inhibitors of phospholipase activity were added (aristocholic acid and MJ33, a specific inhibitor of the Prdx6 phospholipase), the generation of lipid priming activity was significantly decreased by 25±3% and 26±2%. In addition, when the structure of 5-lipoxygenase activating protein (FLAP) and 5-lipoxygenase were investigated, they demonstrated more than 10% homology with IgG and thus may be removed by the experimental filters. To investigate this removal, immunoblots from pre-filtration supernatants, and supernatants from both the leucoreduced (control) or experimentally filtered units, demonstrated that the FLAP and 5-LO immunoreactivity, present in pre-filtration and in leucoreduced supernatants, was removed by the experimental filters (Figure 1). These data demonstrate that these experimental filters not only remove the immunoglobulins implicated in TRALI, but also the enzymes required to generate the neutral lipids during storage, which have been implicated in both TRALI and post-injury ALI1.
Figure 1.
Pre-storage experimental filtration removes 5-lipoxygenase activating protein (FLAP) and 5-lipoxygenase (5-LO) from red blood cell (RBC) units.
(A) There is significant FLAP immunoreactivity prior to filtration in day 1 (Pre D1) supernatants that is removed by filtration (Post D1) which re-accumulates during routine storage by day 42, the end of storage (Post D42). The supernatants from two different RBC units were used. (B) There is significant reactivity for 5-lipoxygenase (5-LO) both before (Pre) and after (Post) leucoreduction and at the end of storage (D42) with the Haemonetics BPF4 filter. The 5-LO immunoreactivity is present prior to experimental filtration (Pre) that is removed from the supernatant of 2 separate RBC units that does not re-accumulate during routine storage D42. Figure representative of 3 separate experiments which demonstrated similar results.
Conclusions
Pre-storage leucoreduction of RBCs results in fewer febrile transfusion reactions, decreased HLA alloimmunisations, decreased exposure to CMV, and decreased amounts of pro-inflammatory molecules including leucotrienes, lyso-PCs, and sCD40L. It also decreases the release of proteins from contaminating leucocytes and platelets. The non-polar lipids which do accumulate may be obviated by the use of a new leucoreduction filtration system, and possibly by the use of additive solution-3 (AS-3) and other novel storage methods. While transfusions of LR-RBCs has saved countless lives, further work is needed to continue to improve efficacy.
Footnotes
Funding and resources
This work was supported by Bonfils Blood Center and grants P50 GM049222 and UM1 HL1008771 from NIGMS and NHLBI, NIH, respectively.
The Authors declare no conflicts of interest.
References
- 1.Silliman CC, Kelher MR, Khan SY, et al. Experimental prestorage filtration removes antibodies and decreases lipids in RBC supernatants mitigating TRALI in vivo. Blood. 2014;123:3488–95. doi: 10.1182/blood-2013-10-532424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hebert PC, Fergusson D, Blajchman MA, et al. Clinical outcomes following institution of the Canadian universal leukoreduction program for red blood cell transfusions. JAMA. 2003;289:1941–9. doi: 10.1001/jama.289.15.1941. [DOI] [PubMed] [Google Scholar]
- 3.Khan SY, Kelher MR, Heal JM, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–62. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McFaul SJ, Corley JB, Mester CW, Nath J. Packed blood cells stored in AS-5 become proinflammatory during storage. Transfusion. 2009;49:1451–60. doi: 10.1111/j.1537-2995.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 5.Silliman CC, Moore EE, Kelher MR, et al. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–54. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyman TH, Dinarello CA, Banerjee A, et al. Physiological levels of interleukin-18 stimulate multiple neutrophil functions through p38 MAP kinase activation. J Leukoc Biol. 2002;72:401–9. [PubMed] [Google Scholar]
- 7.Bianchi M, Vaglio S, Pupella S, et al. Leucoreduction of blood components: an effective way to increase blood safety? Blood Transfus. 2016;14:214–27. doi: 10.2450/2015.0154-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Alessandro A. Leucoreduction of blood components: clinical and molecular evidence. Blood Transfus. 2016;14:212–3. doi: 10.2450/2015.0291-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bock A, Tucker N, Kelher MR, et al. Alpha-enolase causes proinflammatory activation of pulmonary microvascular endothelial cells and primes neutrophils through plasmin activation of protease-activated receptor 2. Shock. 2015;44:137–42. doi: 10.1097/SHK.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silliman CC, Paterson AJ, Dickey WO, et al. The association of biologically active lipids with the development of transfusion-related acute lung injury: a retrospective study. Transfusion. 1997;37:719–26. doi: 10.1046/j.1537-2995.1997.37797369448.x. [DOI] [PubMed] [Google Scholar]
- 11.Silliman CC, Voelkel NF, Allard JD, et al. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–67. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silliman CC. The two-event model of transfusion-related acute lung injury. Crit Care Med. 2006;34( 5 Suppl):S124–31. doi: 10.1097/01.CCM.0000214292.62276.8E. [DOI] [PubMed] [Google Scholar]
- 13.Silliman CC, Clay KL, Thurman GW, et al. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124:684–94. [PMC free article] [PubMed] [Google Scholar]
- 14.D’Alessandro A, Hansen KC, Silliman CC, et al. Metabolomics of AS-5 RBC supernatants following routine storage. Vox Sang. 2015;108:131–40. doi: 10.1111/vox.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Alessandro A, Nemkov T, Kelher M, et al. Routine storage of red blood cell (RBC) units in additive solution-3: a comprehensive investigation of the RBC metabolome. Transfusion. 2015;55:1155–68. doi: 10.1111/trf.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Alessandro A, Nemkov T, Hansen KC, et al. Red blood cell storage in additive solution-7 preserves energy and redox metabolism: a metabolomics approach. Transfusion. 2015;55:2955–66. doi: 10.1111/trf.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Amici GM, Mirasole C, D’Alessandro A, et al. Red blood cell storage in SAGM and AS3: a comparison through the membrane two-dimensional electrophoresis proteome. Blood Transfus. 2012;10:s46–54. doi: 10.2450/2012.008S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu X, Felcyn JR, Odem-Davis K, Zimring JC. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: a targeted metabolomics study. Transfusion. 2016;56:2560–70. doi: 10.1111/trf.13748. [DOI] [PubMed] [Google Scholar]
- 19.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012;76:168–80. doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Roback JD, Josephson CD, Waller EK, et al. Metabolomics of ADSOL (AS-1) red blood cell storage. Transfus Med Rev. 2014;28:41–55. doi: 10.1016/j.tmrv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silliman CC, Boshkov LK, Mehdizadehkashi Z, et al. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–62. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 22.Kelher MR, Masuno T, Moore EE, et al. Plasma from stored packed red blood cells and MHC class I antibodies causes acute lung injury in a 2-event in vivo rat model. Blood. 2009;113:2079–87. doi: 10.1182/blood-2008-09-177857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimring JC, Spitalnik SL, Odem-Davis K. Lost in translation: signal and frequency amplification in animal modeling. Transfusion. 2016;56:773–4. doi: 10.1111/trf.13457. [DOI] [PubMed] [Google Scholar]
- 24.Vlaar AP, Kulik W, Nieuwland R, et al. Accumulation of bioactive lipids during storage of blood products is not cell but plasma derived and temperature dependent. Transfusion. 2011;51:2358–66. doi: 10.1111/j.1537-2995.2011.03177.x. [DOI] [PubMed] [Google Scholar]
- 25.Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119:1757–67. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49:440–52. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 27.Eder AF, Herron RM, Jr, Strupp A, et al. Effective reduction of transfusion-related acute lung injury risk with male-predominant plasma strategy in the American Red Cross (2006–2008) Transfusion. 2010;50:1732–42. doi: 10.1111/j.1537-2995.2010.02652.x. [DOI] [PubMed] [Google Scholar]
- 28.Eder AF, Dy BA, Perez JM, et al. The residual risk of transfusion-related acute lung injury at the American Red Cross (2008–2011): limitations of a predominantly male-donor plasma mitigation strategy. Transfusion. 2013;53:1442–9. doi: 10.1111/j.1537-2995.2012.03935.x. [DOI] [PubMed] [Google Scholar]
- 29.Eder AF, Herron R, Strupp A, et al. Transfusion-related acute lung injury surveillance (2003–2005) and the potential impact of the selective use of plasma from male donors in the American Red Cross. Transfusion. 2007;47:599–607. doi: 10.1111/j.1537-2995.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 30.Silliman CC, Moore EE, Le T, Land KJ. One to one to what? Transfusion. 2010;50:2066–7. doi: 10.1111/j.1537-2995.2010.02740.x. [DOI] [PubMed] [Google Scholar]
- 31.Win N, Chapman CE, Bowles KM, et al. How much residual plasma may cause TRALI? Transfus Med. 2008;18:276–80. doi: 10.1111/j.1365-3148.2008.00885.x. [DOI] [PubMed] [Google Scholar]
- 32.Peters AL, van Hezel ME, Cortjens B, et al. Transfusion of 35-day stored RBCs in the presence of endotoxemia does not result in lung injury in humans. Crit Care Med. 2016;44:e412–9. doi: 10.1097/CCM.0000000000001614. [DOI] [PubMed] [Google Scholar]
- 33.Peters AL, Vervaart MA, van Bruggen R, et al. Non-polar lipids accumulate during storage of transfusion products and do not contribute to the onset of transfusion-realted acute lung injury. Vox Sang. 2017;112:25–32. doi: 10.1111/vox.12453. [DOI] [PubMed] [Google Scholar]
- 34.Chang SW, Feddersen CO, Henson PM, Voelkel NF. Platelet-activating factor mediates hemodynamic changes and lung injury in endotoxin-treated rats. J Clin Invest. 1987;79:1498–509. doi: 10.1172/JCI112980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang SW, Sakai A, Voelkel NF. Dibutyryl-cAMP blocks endotoxin-induced lung injury in rats. Am Rev Respir Dis. 1989;140:1814–7. doi: 10.1164/ajrccm/140.6.1814. [DOI] [PubMed] [Google Scholar]
- 36.Chang SW, Fernyak S, Voelkel NF. Beneficial effect of a platelet-activating factor antagonist, WEB 2086, on endotoxin-induced lung injury. Am J Physiol. 1990;258:H153–8. doi: 10.1152/ajpheart.1990.258.1.H153. [DOI] [PubMed] [Google Scholar]
- 37.Silliman CC, Khan SY, Ball JB, et al. Mirasol Pathogen Reduction Technology treatment does not affect acute lung injury in a two-event in vivo model caused by stored blood components. Vox Sang. 2010;98:525–30. doi: 10.1111/j.1423-0410.2009.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurley JC. Self-administration of Salmonella endotoxin. N Engl J Med. 1993;329:1426–7. [PubMed] [Google Scholar]
- 39.Sauter C, Wolfensberger C. Interferon in human serum after injection of endotoxin. Lancet. 1980;2:852–3. doi: 10.1016/s0140-6736(80)90189-0. [DOI] [PubMed] [Google Scholar]
- 40.Taveira da Silva AM, Kaulbach HC, Chuidian FS, et al. Brief report: shock and multiple-organ dysfunction after self-administration of Salmonella endotoxin. N Engl J Med. 1993;328:1457–60. doi: 10.1056/NEJM199305203282005. [DOI] [PubMed] [Google Scholar]
- 41.Dudek SM, Munoz NM, Desai A, et al. Group V phospholipase A2 mediates barrier disruption of human pulmonary endothelial cells caused by LPS in vitro. Am J Respir Cell Mol Biol. 2011;44:361–8. doi: 10.1165/rcmb.2009-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyman TH, Bjornsen AJ, Elzi DJ, et al. A two-insult in vitro model of PMN-mediated pulmonary endothelial damage: requirements for adherence and chemokine release. Am J Physiol Cell Physiol. 2002;283:C1592–603. doi: 10.1152/ajpcell.00540.2001. [DOI] [PubMed] [Google Scholar]
- 43.Warner AE, DeCamp MM, Jr, Molina RM, Brain JD. Pulmonary removal of circulating endotoxin results in acute lung injury in sheep. Lab Invest. 1988;59:219–30. [PubMed] [Google Scholar]
- 44.Weppler A, Issekutz AC. Alveolar epithelium down-modulates endotoxin-but not tumor necrosis factor alpha-induced activation of endothelium and selectively inhibits neutrophil transendothelial migration. Exp Lung Res. 2008;34:425–53. doi: 10.1080/01902140802130105. [DOI] [PubMed] [Google Scholar]
- 45.Yao Z, Mates JM, Cheplowitz AM, et al. Blood-borne lipopolysaccharide is rapidly eliminated by liver sinusoidal endothelial cells via high-density lipoprotein. J Immunol. 2016;197:2390–9. doi: 10.4049/jimmunol.1600702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dzieciatkowska M, Silliman CC, Moore EE, et al. Proteomic analysis of the supernatant of red blood cell units: the effects of storage and leucoreduction. Vox Sang. 2013;105:210–8. doi: 10.1111/vox.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chatterjee S, Feinstein SI, Dodia C, et al. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J Biol Chem. 2011;286:11696–706. doi: 10.1074/jbc.M110.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leavey PJ, Sellins KS, Thurman G, et al. In vivo treatment with granulocyte colony-stimulating factor results in divergent effects on neutrophil functions measured in vitro. Blood. 1998;92:4366–74. [PubMed] [Google Scholar]
- 49.Liang Y, Jansen M, Aronow B, et al. The quantitative trait gene latexin influences the size of the hematopoietic stem cell population in mice. Nat Genet. 2007;39:178–88. doi: 10.1038/ng1938. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Feinstein SI, Manevich Y, et al. Mitogen-activated protein kinase-mediated phosphorylation of peroxiredoxin 6 regulates its phospholipase A(2) activity. Biochem J. 2009;419:669–79. doi: 10.1042/BJ20082061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdul-Hussien H, Soekhoe RG, Weber E, et al. Collagen degradation in the abdominal aneurysm: a conspiracy of matrix metalloproteinase and cysteine collagenases. Am J Pathol. 2007;170:809–17. doi: 10.2353/ajpath.2007.060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jackson PL, Xu X, Wilson L, et al. Human neutrophil elastase-mediated cleavage sites of MMP-9 and TIMP-1: implications to cystic fibrosis proteolytic dysfunction. Mol Med. 2010;16:159–66. doi: 10.2119/molmed.2009.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sauaia A, Moore FA, Moore EE, et al. Early predictors of postinjury multiple organ failure. Arch Surg. 1994;129:39–45. doi: 10.1001/archsurg.1994.01420250051006. [DOI] [PubMed] [Google Scholar]
- 54.Sauaia A, Moore FA, Moore EE, et al. Multiple organ failure can be predicted as early as 12 hours after injury. J Trauma. 1998;45:291–301. doi: 10.1097/00005373-199808000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Weinberg JA, McGwin G, Jr, Vandromme MJ, et al. Duration of red cell storage influences mortality after trauma. J Trauma. 2010;69:1427–31. doi: 10.1097/TA.0b013e3181fa0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–2. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 57.Ciesla DJ, Moore EE, Johnson JL, et al. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg. 2005;140:432–8. doi: 10.1001/archsurg.140.5.432. [DOI] [PubMed] [Google Scholar]
- 58.Sauaia A, Moore EE, Johnson JL, et al. Temporal trends of postinjury multiple-organ failure: still resource intensive, morbid, and lethal. J Trauma Acute Care Surg. 2014;76:582–92. doi: 10.1097/TA.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77:811–7. doi: 10.1097/TA.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore HB, Moore EE, Gonzalez E, et al. Hemolysis exacerbates hyperfibrinolysis, whereas platelolysis shuts down fibrinolysis: evolving concepts of the spectrum of fibrinolysis in response to severe injury. Shock. 2015;43:39–46. doi: 10.1097/SHK.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]