Abstract
At critical times in development, cells are able to convert graded signals into discrete developmental outcomes; however, the mechanisms involved are poorly understood. During thymocyte development, cell fate is determined by signals originating from the α β T-cell receptor. Low-affinity/avidity interactions between the T-cell receptor and peptide–MHC complexes direct differentiation to the single-positive stage (positive selection), whereas high-affinity/avidity interactions induce death by apoptosis (negative selection)1,2. Here we show that mice deficient in both calcineurin and nuclear factor of activated T cells (NFAT)c2/c3 lack a population of preselection thymocytes with enhanced ability to activate the mitogen-activated protein kinase (Raf–MEK–ERK) pathway, and fail to undergo positive selection. This defect can be partially rescued with constitutively active Raf, indicating that calcineurin controls MAPK signalling. Analysis of mice deficient in both Bim (which is required for negative selection) and calcineurin revealed that calcineurin-induced ERK (extracellular signal-regulated kinase) sensitization is required for differentiation in response to ‘weak’ positive selecting signals but not in response to ‘strong’ negative selecting signals (which normally induce apoptosis). These results indicate that early calcineurin/NFAT signalling produces a developmental period of ERK hypersensitivity, allowing very weak signals to induce positive selection. This mechanism might be generally useful in the discrimination of graded signals that induce different cell fates.
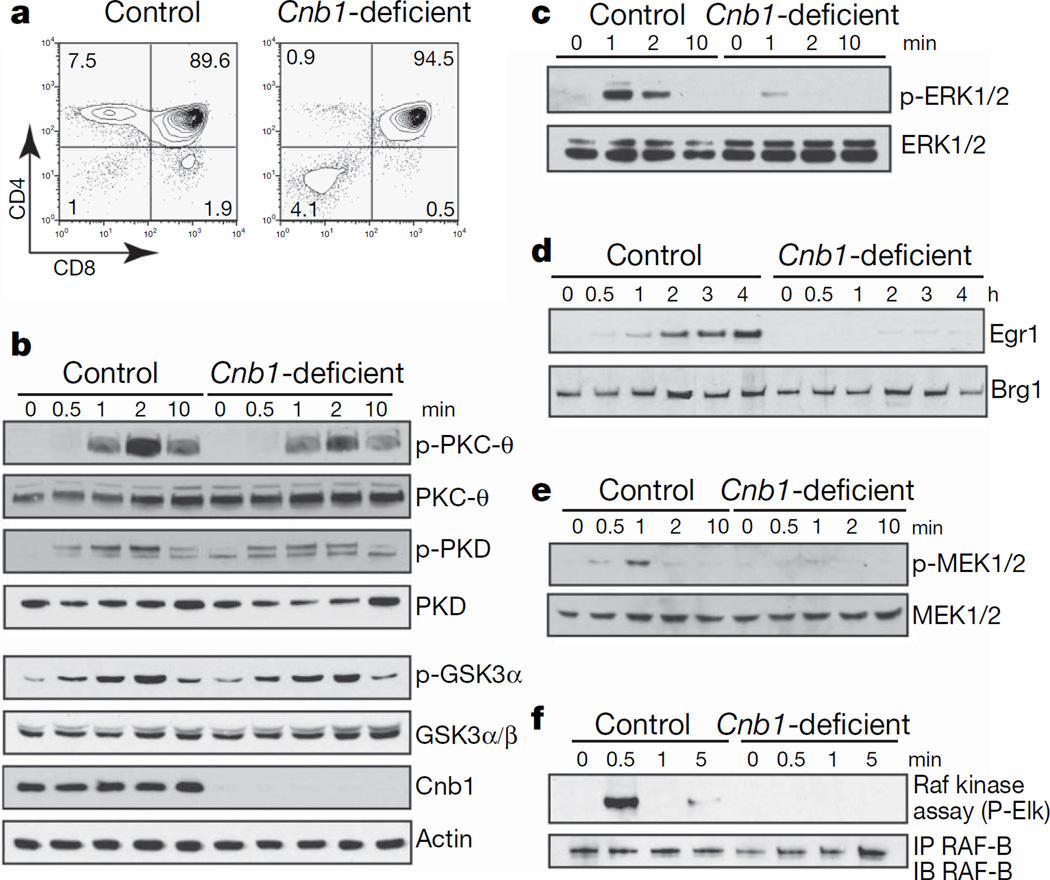
The calcineurin/NFAT3,4 and the Raf–MEK–ERK5–7 pathways have been shown to be required for positive selection of thymocytes but not for their negative selection. Calcineurin B1 (Cnb1)-deficient CD4+ CD8+ double-positive thymocytes lack calcineurin activity, fail to dephosphorylate NFATc transcription factors and are not positively selected (Fig. 1a and ref. 3). Cnb1-deficient thymocytes have normal phosphorylation of JNK (c-Jun N-terminal kinase), p38, protein kinase C-θ, protein kinase D and glycogen synthase kinase 3α after crosslinking of the T-cell receptor (TCR)(Fig.1band ref. 3). Actin polymerization and Ca2+ influx were also normal in these cells (Supplementary Fig. 2a, b). Collectively, these results indicated that Cnb1-deficient thymocytes did not have widespread signalling defects downstream of the TCR. However, Cnb1-deficient thymocytes showed a specific and severe defect in ERK1/2 phosphorylation (Fig. 1c), resulting in almost undetectable induction of the ERK/Elk4 target gene Egr1 (ref. 6) after engagement of the TCR (Fig.1d). In addition, both MAP-kinase kinase (MEK)1/2and Raf activation were defective in Cnb1-deficient double-positive thymocytes (Fig. 1c, e, f) after stimulation by crosslinking of the TCR.
Figure 1. Specific and severe defect in Raf–MEK–ERK activation in Cnb1-deficient thymocytes.
a, Expression of CD4 and CD8 on Cnb1-deficient and control thymocytes. The numbers in the corners of the panels represent the percentage of cells in each quadrant. b, Immunoblot analysis of phosphorylated and total proteins in Cnb1-deficient and control double-positive thymocytes after CD3ε crosslinking. GSK, glycogen synthase kinase; PKC, protein kinase C; PKD, protein kinase D. c, Immunoblot analysis of phosphorylated ERK1/2 in Cnb1-deficient and control double-positive thymocytes after CD3ε crosslinking. d, Immunoblot analysis of Egr1 induction in double-positive thymocytes from Cnb1-deficient and control littermates. Brg1 shows equal loading. e, Immunoblot analysis of phosphorylated MEK1/2 in Cnb1-deficient and control double-positive thymocytes after CD3ε crosslinking. f, Raf-B kinase activity in Cnb1-deficient and control double-positive thymocytes after CD3ε crosslinking. IP, immunoprecipitation; IB, immunoblotting.
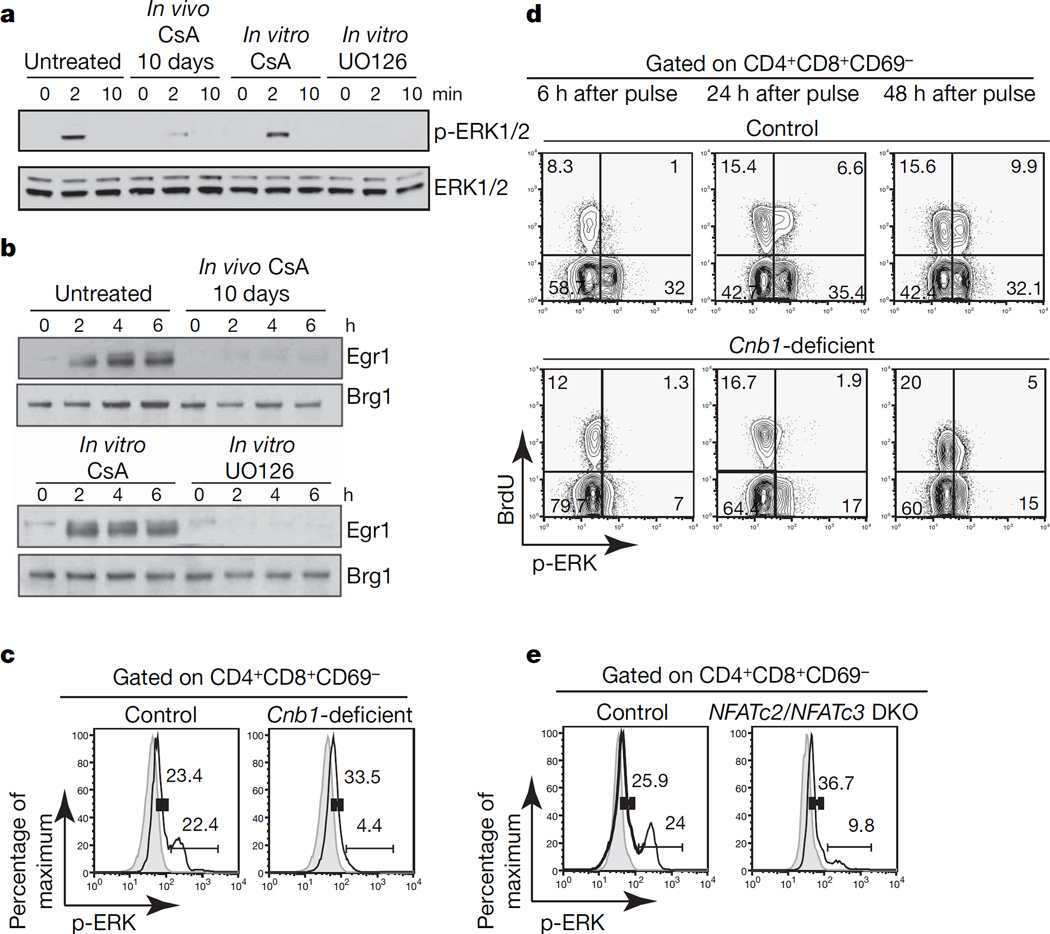
To test whether calcineurin activity was directly required for Raf activation, we stimulated thymocytes in the presence of the calcineurin inhibitor cyclosporin A (CsA) or the MEK1/2 inhibitor UO126. Acute in vitro inhibition of MEK1/2 with UO126 but not inhibition of calcineurin activity with CsA impaired ERK1/2 phosphorylation and Egr1 induction (Fig. 2a, b). In contrast, 10-day treatment of mice in vivo with CsA consistently recapitulated both the block in positive selection and defective ERK1/2 phosphorylation observed in Cnb1-deficient thymocytes (Fig. 2a, band Supplementary Fig. 3a). These data indicated that calcineurin activity was not directly required for ERK1/2 phosphorylation but was instead required during development to acquire the ability to activate ERK1/2 properly in response to subsequent TCR signalling.
Figure 2. Developmental but not direct requirement for calcineurin/NFAT activity for proper activation of ERK.
a, ERK1/2 phosphorylation in double-positive cells from untreated and CsA-treated mice in the presence of CsA (200 ng ml−1) or UO126 (10 µM) after CD3ε crosslinking. b, Erg1 induction in double-positive cells from untreated or CsA-treated mice and in double-positive cells stimulated in the presence of CsA (200 ng ml−1) or UO126 (10 µM) after CD3ε crosslinking. Brg1 shows equal loading. c, ERK1/2 phosphorylation in double-positive CD69-negative Cnb1-deficient and control thymocytes after CD3ε crosslinking for 2 min (solid lines). Grey areas, unstimulated. d, BrdU incorporation and ERK1/2 phosphorylation in double-positive CD69-negative thymocytes from Cnb1-deficient mice and control littermates injected once with BrdU after CD3ε crosslinking for 2 min. The numbers in the corners of the panels represent the percentage of cells in each quadrant. e, ERK1/2 phosphorylation in NFATc2/NFATc3 double knockout (DKO) and control double-positive CD69-negative thymocytes after CD3ε crosslinking for 2 min (solid lines). Grey lines, unstimulated. The numbers in graphs c and e represent the percentage of cells in the indicated interval.
When ERK1/2 phosphorylation was assayed by intracellular staining, two populations with different levels of ERK1/2 phosphorylation were observed in control double-positive CD69-negative thymocytes after crosslinking with anti-CD3ε antibody. The population of thymocytes with the higher level of ERK1/2 phosphorylation was absent in Cnb1-deficient and CsA-treated mice (Fig. 2c and Supplementary Fig. 3b). The specificity of the staining and uniformity of crosslinking was confirmed by pretreating the cells with UO126 and by counterstaining for anti-CD3ε, respectively (Supplementary Fig. 3c, d). We also examined the expression of TCR-β on Cnb1-deficient and control thymocytes and found no difference in the percentage of thymocytes with intermediate levels of TCR-β expression (double-positive TCR-βint), a population that is absent from mice that fail to rearrange the TCR-α chain8–10 (Supplementary Fig. 3e). We concluded that two thymocyte populations existed, within the double-positive CD69-negative population, with different abilities to phosphorylate ERK1/2 and that calcineurin activity was required for the presence of the population with an increased ability to phosphorylate ERK1/2. We refer to these populations as ‘ERK low competence’ and ‘ERK high competence’ populations, respectively.
We used a single bromodeoxyuridine (BrdU) pulse to mark developing thymocytes11 and establish the precursor/progeny relationship of these two populations. At early time points, most BrdU-positive, double-positive thymocytes were in the ‘ERK low competence’ state, with the percentage of BrdU-positive ‘ERK high competence’ double-positive thymocytes increasing over time (Fig. 2d, upper panel). The transition to the ‘ERK high competence’ state was not observed in Cnb1-deficient thymocytes (Fig. 2d, lower panel). These data indicated that the ‘ERK low competence’ state preceded the ‘ERK high competence’ state during normal thymocyte development and that calcineurin activity was required for this transition. The lag time for the development of the ‘ERK high competence’ population was consistent with a requirement for transcription.
Because the calcineurin phosphatase complex regulates the NFATc family of transcription factors12,13, we analysed NFATc2/NFATc3 double-knockout thymocytes, which also have impaired positive selection4. The development of the ‘ERK high competence’ population was impaired in NFATc2/NFATc3 double-knockout thymocytes (Fig. 2e), the incomplete block probably reflecting a partly redundant function of NFATc1. These data indicated that calcineurin/NFAT signalling was required for the transition to the ‘ERK high competence’ state. The developmental requirement for calcineurin and NFAT activity for normal activation of the ERK pathway was specific to thymocytes because short-term or long-term treatment with cyclosporin had no effect on peripheral T and B lymphocytes (Supplementary Fig. 3f and data not shown). Analysis of MHCI/MHCII double-knockout mice revealed that the development of the ‘high ERK competence’ double-positive thymocyte population did not require TCR–MHC (major histocompatibility complex) interaction or positive selection (Supplementary Fig. 4a). Microarray analysis showed that 312 transcripts were differentially expressed in double-positive thymocytes from untreated and CsA-treated MHCI/MHCII double-knockout mice, indicating that calcineurin activity is required for preconditioning of double-positive thymocytes independently of positive selection (Supplementary Fig. 4b, c, and Supplementary Table 1). We speculate that the development of the ‘ERK high competence’ population depends on pre-TCR signalling and/or ligand-independent tonic TCR-α β signalling and that the modulation of Raf activity could be due to a program of gene expression rather than a single modulator working at a single point in the pathway.
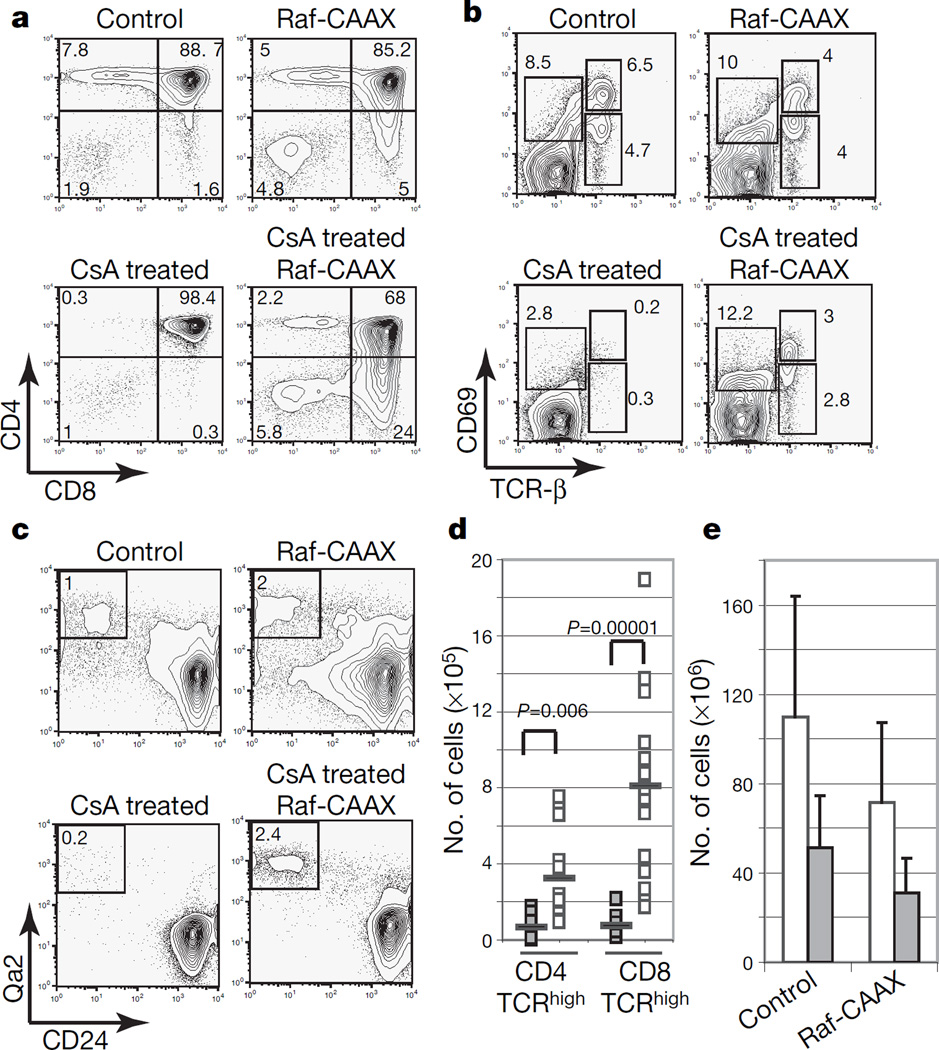
If a main mechanism by which calcineurin controlled positive selection were by modulating the sensitivity of the Raf–MEK–ERK pathway, restoring Raf signalling in the absence of calcineurin activity should lead to at least a partial rescue of positive selection. To test this, we obtained mice whose thymocytes express a constitutively active Raf-1 mutant protein (Raf-CAAX transgenic mice14), which does not induce positive selection in the absence of TCR signalling14. Mice were analysed between four and six weeks of age, before they developed any sign of thymic lymphoma. Long-term treatment with CsA completely blocked positive selection in control mice (Fig. 3a). However, positive selection is rescued in CsA-treated Raf-CAAX mice as assessed by the upregulation of CD69 and TCR-β and the development of mature CD24lowQa2high single-positive CD4 and CD8 thymocytes (Fig. 3a–d). Moreover, CD4 single-positive and CD8 single-positive thymocytes that are rescued in CsA-treated Raf-CAAX mice were able to respond functionally when stimulated with anti-CD3 and anti-CD28 antibodies (Supplementary Fig. 5). Analogous results were obtained when Raf-CAAX was expressed in Cnb1-deficient thymocytes (Supplementary Fig. 6). As expected, the decreased thymic cellularity that is observed in the absence of calcineurin activity as a consequence of impaired transition from double negative to double positive was not rescued by the Raf-CAAX transgene (Fig. 3e and Supplementary Fig. 6d). Raf-CAAX only partly restores ERK phosphorylation in thymocytes from CsA-treated mice (Supplementary Fig. 6e). The fact that the Raf-CAAX transgene does not rescue the double-negative to double-positive developmental block and does not restore appropriate timing or intensity of ERK signalling, together with additional ERK-independent Cnb1/NFAT targets (such as the transcription factor TOX) (ref. 15), might explain the incomplete rescue in Cnb1-deficient Raf-CAAX mice.
Figure 3. Reconstitution of Raf–MEK–ERK signalling partly rescues positive selection in the absence of calcineurin activity.
a, Expression of CD4 and CD8 in Raf-CAAX transgenic and control mice treated with CsA or left untreated. b, Analysis of CD69 and TCR-β expression on thymocytes from Raf-CAAX transgenic and control mice treated with CsA or left untreated. c, Analysis of CD24 and Qa2 expression on thymocytes from Raf-CAAX transgenic and control mice treated or not with CsA. d, Absolute numbers of TCRhigh CD4 and CD8 single-positive cells (n≥8, each square represents an individual mouse; bar equals mean value). P values refer to a one-tailed t-test. Open symbols, CsA, Raf-CAAX; filled symbols, CsA. e, Absolute numbers of thymocytes for mice of indicated genotype (n≥8; error bars show s.d.). Open bars, control; filled bars, CsA-treated. The numbers in the corners of the panels represent the percentage of cells in each quadrant.
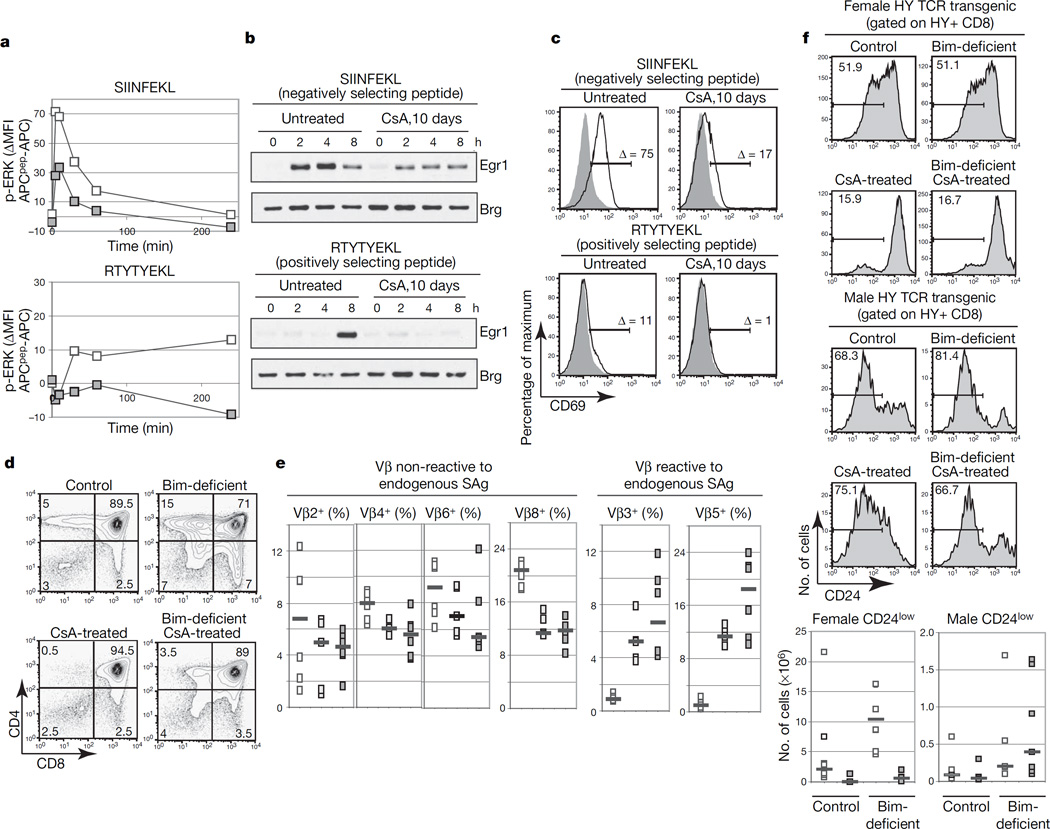
We proposed that this developmental window of ERK hypersensitivity might transiently increase the dynamic range (or bandwidth) of TCR signals, thereby enabling thymocytes to respond to ‘weak’ positively selecting ligands. To test this, we used the OT-I TAP (transporter associated with antigen processing)-null mice mouse model16,17 (OT-I TAP0). We compared the ability of double-positive thymocytes from control and CsA-treated OT-I TAP0 mice to respond to the negatively selecting peptide SIINFEKL and the positively selecting peptide RTYTYEKL (ref. 17). OT-I-positive double-positive thymocytes from control mice responded to both peptides by phosphorylating ERK1/2, inducing Egr1 and upregulating CD69 (Fig. 4a–c and Supplementary Fig. 7). The response to the positively selecting peptide is weaker and delayed, as has been previously reported18,19. In contrast, double-positive thymocytes from CsA-treated mice were able to respond partly to the negatively selecting peptide SIINFEKL but failed to respond to the positively selecting peptide RTYTYEKL (Fig. 4a–c and Supplementary Fig. 7). These data suggested that calcineurin-dependent ERK sensitization is required for a response to weaker positively selecting ligands, whereas stronger negative selecting signals are able to activate the ERK pathway to a certain extent even when the transition to the ‘ERK high competence’ state has not occurred.
Figure 4. Transition to the ‘high ERK competence’ state is required to respond functionally to positively selecting ligands.
a, Phospho-ERK1/2 in OT-I double-positive thymocytes from mice stimulated with SIINFEKL or RTYTYEKL. White symbols, untreated; grey symbols, CsA-treated. b, Egr1 upregulation in OT-I double-positive thymocytes stimulated with SIINFEKL or RTYTYEKL. c, CD69 upregulation in OT-I double-positive thymocytes after 4 h of stimulation. Δ indicates the percentage difference in CD69 thymocytes stimulated by APCs only or by peptide-pulsed APCs. Solid lines, APCs plus peptide; grey areas, APCs only. d, CD4 and CD8 expression in thymocytes from mice of the indicated genotypes. e, Percentage of CD4 single-positive expressing Vβ chains reactive or not to endogenous superantigens (squares show results for individual mice; bars show medians; n≥3). White symbols, untreated control; light grey symbols, Bim-deficient; dark grey symbols, Bim-deficient and CsA-treated. f, Percentages (numbers shown in each panel refer to the percentage of cells in the indicated interval) and absolute numbers of HY+ CD8 single-positive CD24low cells in mice of the indicated genotypes (squares show results for individual mice, bars show means; n≥4). White symbols, untreated; grey symbols, CsA-treated.
If the role of calcineurin were to increase the ‘signalling bandwidth’ and allow effective discrimination of graded signals, one would predict that Cnb1-deficient thymocytes that were prevented from dying should be positively selected in response to stronger signals, which would normally induce cell death. To test this, we used mice deficient in Bim, which is necessary for negative selection20. In control mice, in vivo treatment with CsA completely blocked the development of single-positive thymocytes. However, differentiation occurred in Bim-deficient mice even in the absence of calcineurin activity as assessed by the development of TCR-βhigh CD4 single-positive and CD8 single-positive cells and upregulation of TCR-β, CD69 and CCR7 on double-positive cells (Fig. 4d and Supplementary Fig. 8a–c). The double-negative to double-positive developmental block that is observed in CsA-treated and Cnb1-deficient thymocytes3, which results in an overall decrease in thymus cellularity in these mice, was not rescued by Bim deficiency (Supplementary Fig. 8c). Analogous results were obtained when Bim-deficient mice were crossed with conditional Cnb1-deficient mice (Supplementary Fig. 8d, e). Because Bim deficiency did not rescue development of the ‘ERK high competence’ population itself (Supplementary Fig. 8f), we postulated that in Bim-deficient mice ‘strong’ negatively selecting ligands could trigger the activation of ERK in thymocytes that are in the ‘ERK low competence’ state. According to this hypothesis, in Bim-deficient mice negatively selecting ligands could circumvent the need for the calcineurin-dependent sensitization and could induce the differentiation of single-positive thymocytes that in normal circumstances are negatively selected. Indeed, analysis of Bim-deficient mice in a background (Balb/c) that expresses the MHC class II molecule I-E and allows the superantigen-mediated deletion of T cells2 revealed that in CsA-treated Bim-deficient mice a higher percentage of CD4 single-positive thymocytes expressed TCR Vβ chains that are reactive to endogenous superantigens in comparison with controls, suggesting that single-positive thymocytes that develop in these mice are in fact those that received negatively selecting signals (Fig. 4e).
As a second approach to testing whether the role of ERK competence is to expand the signalling bandwidth, allowing the effective discrimination of weak signals, we examined thymocyte selection in the HYTCR transgenic mouse model. In this model most thymocytes express the HY TCR and are negatively selected by antigens expressed in male but not female mice21. As expected, the deletion of HY thymocytes in male mice (Supplementary Fig. 9a) was impaired by Bim deficiency20. Because CsA treatment resulted in the accumulation of immature CD8 single-positive cells (Supplementary Fig. 9a), we evaluated positive selection by monitoring CD69 upregulation and the development of CD24low and Qa2high CD8 single-positive cells. Treatment with CsA impaired CD69 upregulation and the development of mature CD8 single-positive thymocytes in control and Bim-deficient female mice, whereas it did not block the development of mature CD8 single-positive cells in male mice (Fig. 4f and Supplementary Fig. 9b, c). Similar results were obtained in the HYCD4 model, in which TCR-α β expression is properly timed22 (data not shown). These results suggested that the amount of ERK signalling provided by ‘strong’, negatively selecting signals in CsA-treated mice is able to induce differentiation and that negatively selecting signals do not require the development of ‘ERK competence’.
Cell fate determination often occurs within morphogenic gradients that produce different cell fates at different points in the gradient, apparently as a result of signals of different intensities. A similar analogue-to-digital switch occurs in T-cell development (Supplementary Fig. 1), where signal intensity determines the outcome of TCR–MHC interactions. Our studies indicate that an early calcineurin–NFAT signal sensitizes the Raf–MEK–ERK pathway, allowing responses to weak TCR signals that would otherwise not be detected (Supplementary Fig. 1). In the absence of this calcineurin-dependent preconditioning, the signal intensity needed for positive selection overlaps with that needed for negative selection, and effective discrimination of graded developmental signals cannot occur.
METHODS
Mice
Cnb1 conditional knockout and Raf-CAAX mice have been previously characterized3,14. Lck-cre mice were a gift from C. Wilson. Because no consistent differences were observed among mice of the genotypes Cnb1+/+-lckCre;Cnb1f/+-lckCre, Cnb1Δ/+-lckCre;Cnb1f/f or Cnb1f/Δ, they are collectively referred to as ‘control mice’ throughout this manuscript. In addition, no differences were observed between Cnb1f/f-lckCre and Cnb1f/Δ-lckCre mice, and these animals were used interchangeably for the experiments described in this study and compared with age-matched control mice. OT-I transgenic mice16 on the TAP0 background were a gift from K. A. Hogquist. C57/B6 H2-Ab/β2m double knockout mice, C57BL/6 and HY TCR transgenic mice were purchased from Taconic. Bim-deficient mice were purchased from the Jackson Laboratory. For in vivo treatment with CsA, mice were treated with daily intraperitoneal injections of CsA (30 mg kg−1 d−1) for ten or more days. Mice in which exon 3 of the NFATc3 gene is flanked by loxP sites (NFATc3f/f) were generated in our laboratory4 and crossed to Lck-cre mice and to NFATc2-deficient mice28 to obtain NFATc2/NFATc3-deficient thymocytes.
Phospho-ERK intracellular staining
The staining for phospho-ERK was performed as indicated in the staining protocol provided by Cell Signalling. For co-staining with anti-BrdU and anti-phospho-ERK antibodies, the staining was performed in accordance with instructions from Cell Signalling for the anti-phospho-ERK antibody with the following modifications: first, DNase treatment was performed in accordance with the BD Pharmingen protocol after the blocking step, and second, anti-BrdU antibody was added at the same time as the anti-phospho-ERK antibody. For phospho-ERK staining in OT-I TAP0 thymocytes, T2-H-2Kb APCs were pulsed overnight at a concentration of 106 ml−1 with 2 µM SIINFEKL and 100 µM RTYTYEKL in RPMI medium. APCs were washed twice in PBS and then incubated with thymocytes at a 1:1 ratio at 37 °C for the indicated duration. Reaction was started with a 2-min centrifugation at 300g. Conjugates were disrupted by the addition of ice-cold PBS, 10mM EDTA, 2% paraformaldehyde and by vigorous pipetting. Cells were then left on ice for 5 min and then incubated at 25 °C for 15 min. Staining was performed in accordance with instructions from Cell Signalling, except that the incubation in ice-cold methanol was followed by an additional incubation overnight in methanol at −20 °C.
Biochemical analysis
SDS–PAGE and immunoblotting were conducted with standard procedures. All phospho-specific antibodies and total protein antibody used for immunoblotting were purchased from Cell Signalling with the exception of anti-B-Raf and anti-Egr1 (Santa Cruz Biotechnology) and anti-ERK1/2 (Upstate Biotechnology).
Microarray
The indicated thymocyte populations were sorted on ARIA and the purity was assessed to be at least 98% by reanalysis. Three independent RNA samples from thymocytes sorted from individual mice were analysed for each experimental group. Probe sets were first filtered with DMT software (Affymetrix) by eliminating those that did not have a ‘present call’ in all control samples (for increased calls) or all experimental samples (for decreased calls). Nine pairwise comparisons of the three experimental versus three control samples were performed with DMT software. To be considered significant, probe sets had to receive a ‘change call’ in 100% of comparisons, had to have an absolute log ratio of 1 or more, and had to be considered significantly changed by one-way analysis of variance (ANOVA) (P≤0.01). One-way ANOVA was run with GENESIS software27 on all probe sets, after filtering for absent calls. Probe sets were annotated by submitting them to the Affymetrix analysis website (www.affymetrix.com/analysis/index.affx).
Supplementary Material
Acknowledgments
We thank P. Ebert for helping with calcium flux studies, and K. A. Hogquist and C. Wilson for providing mice and reagents. E.M.G., M.M.W. and A.N.R. were supported by Stanford Graduate Fellowships. M.M.W. was additionally supported by a Howard Hughes Medical Institute predoctoral fellowship. A.N.R. was also supported by a National Science Foundation Graduate Research Fellowship. K.C.B. was supported by the Boehringer Ingelheim Fonds. L.H. was also supported by Agency for Science, Technology and Research Singapore. This work was supported by grants from Howard Hughes Medical Institute and the National Institute of Heath to G.R.C.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions E.M.G., M.M.W. and G.R.C. generated the hypotheses, designed the experiments and wrote the manuscript. E.M.G. performed the experiments and generated the figures. K.C.-B. generated the NFATc3 conditional knockout mice, maintained this line in the NFATc2-null background and contributed to the experiments in Fig. 4. A.N.R. and L.H. contributed to pilot experiments and experiments shown in Fig. 2 and Supplementary Fig. 8. J.R.N. generated the Cnb1 conditional knockout mice, conducted pilot experiments and contributed to experimental rationale. L.M. contributed to experiments shown in Supplementary Fig. 5. B.I. provided the Raf-CAAX transgenic mice.
References
- 1.Palmer E. Negative selection–clearing out the bad apples from the T-cell repertoire. Nature Rev. Immunol. 2003;3:383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 2.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 3.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 4.Cante-Barrett K, Winslow MM, Crabtree GR. Selective role of NFATc3 in positive selection of thymocytes. J. Immunol. 2007;179:103–110. doi: 10.4049/jimmunol.179.1.103. [DOI] [PubMed] [Google Scholar]
- 5.Alberola-Ila J, Forbush KA, Seger R, Krebs EG, Perlmutter RM. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature. 1995;373:620–623. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- 6.Costello PS, Nicolas RH, Watanabe Y, Rosewell I, Treisman R. Ternary complex factor SAP-1 is required for Erk-mediated thymocyte positive selection. Nature Immunol. 2004;5:289–298. doi: 10.1038/ni1038. [DOI] [PubMed] [Google Scholar]
- 7.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Shortman K, Vremec D, Egerton M. The kinetics of T cell antigen receptor expression by subgroups of CD4+8+ thymocytes: delineation of CD4+8+3(2+) thymocytes as post-selection intermediates leading to mature T cells. J. Exp. Med. 1991;173:323–332. doi: 10.1084/jem.173.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor α gene in development of γδ but not γδ T cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 10.Levelt CN, Carsetti R, Eichmann K. Regulation of thymocyte development through CD3. II. Expression of T cell receptor β CD3 epsilon and maturation to the CD4+8+ stage are highly correlated in individual thymocytes. J. Exp. Med. 1993;178:1867–1875. doi: 10.1084/jem.178.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penit C. In vivo thymocyte maturation. BUdR labeling of cycling thymocytes and phenotypic analysis of their progeny support the single lineage model. J. Immunol. 1986;137:2115–2121. [PubMed] [Google Scholar]
- 12.Macian F, et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 13.Gallo EM, Cante-Barrett K, Crabtree GR. Lymphocyte calcium signaling from membrane to nucleus. Nature Immunol. 2006;7:25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]
- 14.Iritani BM, Alberola-Ila J, Forbush KA, Perimutter RM. Distinct signals mediate maturation and allelic exclusion in lymphocyte progenitors. Immunity. 1999;10:713–722. doi: 10.1016/s1074-7613(00)80070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aliahmad P, et al. TOX provides a link between calcineurin activation and CD8 lineage commitment. J. Exp. Med. 2004;199:1089–1099. doi: 10.1084/jem.20040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 17.Hogquist KA, et al. Identification of a naturally occurring ligand for thymic positive selection. Immunity. 1997;6:389–399. doi: 10.1016/s1074-7613(00)80282-4. [DOI] [PubMed] [Google Scholar]
- 18.Werlen G, Hausmann B, Palmer E. A motif in the αβ T-cell receptor controls positive selection by modulating ERK activity. Nature. 2000;406:422–426. doi: 10.1038/35019094. [DOI] [PubMed] [Google Scholar]
- 19.McNeil LK, Starr TK, Hogquist KA. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc. Natl Acad. Sci. USA. 2005;102:13574–13579. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouillet P, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 21.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 22.Baldwin TA, Sandau MM, Jameson SC, Hogquist KA. The timing of TCR α expression critically influences T cell development and selection. J. Exp. Med. 2005;202:111–121. doi: 10.1084/jem.20050359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds LF, et al. Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos, and RasGRP1. J. Biol. Chem. 2004;279:18239–18246. doi: 10.1074/jbc.M400257200. [DOI] [PubMed] [Google Scholar]
- 24.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nature Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 25.Cante-Barrett K, Gallo EM, Winslow MM, Crabtree GR. Thymocyte negative selection is mediated by protein kinase C- and Ca2+-dependent transcriptional induction of bim of cell death. J. Immunol. 2006;176:2299–2306. doi: 10.4049/jimmunol.176.4.2299. [DOI] [PubMed] [Google Scholar]
- 26.Wei ML, Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides. Nature. 1992;356:443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 27.Sturn A, Quackenbush J, Trajanoski Z. Genesis: cluster analysis of microarray data. Bioinformatics. 2002;18:207–208. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 28.Xanthoudakis S, et al. An enhanced immune response in mice lacking the transcription factor NFAT1. Science. 1996;272:892–895. doi: 10.1126/science.272.5263.892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.