Abstract
Toxoplasma gondii is an obligate intracellular parasite that infects all nucleated cell types in diverse warm-blooded organisms. Many of the surface antigens and effector molecules secreted by the parasite during invasion and intracellular growth are modified by glycans. Glycosylated proteins in the nucleus and cytoplasm have also been reported. Despite their prevalence, the complete inventory and biological significance of glycosylated proteins in Toxoplasma remains unknown. In this study, we aimed to globally profile parasite glycoproteins using a bioorthogonal chemical reporter strategy. This strategy involves the metabolic incorporation of unnatural functional groups (i.e., “chemical reporters”) into Toxoplasma glycans, followed by covalent labeling with visual probes or affinity tags. The two-step approach enables the visualization and identification of newly biosynthesized glycoconjugates in the parasite. Using a buffer that mimics intracellular conditions, extracellular Toxoplasma tachyzoites were found to metabolize and incorporate unnatural sugars (equipped with bioorthogonal functional groups) into diverse proteins. Covalent chemistries were used to visualize and retrieve these labeled structures. Subsequent mass spectrometry analysis revealed 89 unique proteins. This survey identified novel proteins as well as previously characterized proteins from lectin affinity analyses.
Keywords: Apicomplexa, glycobiology, “click” chemistry, metabolic labeling, parasite
1. Introduction
Glycosylation is a ubiquitous post-translational modification that is critically important for diverse cellular functions, including protein folding and sorting, receptor interactions and signal transduction [1, 2]. Glycans are built off polypeptide chains by the actions of numerous glycosyltransferases (enzymes that transfer activated nucleotide sugars to growing glycan chains or underlying protein/lipid units). Glycan structures can be further modified by glycosidases (enzymes that trim sugars from larger structures) or enzymes that add sulfate or acetyl groups to the monosaccharide units. Several proteins synthesized in the rough ER are modified by N-linked glycans (typically attached to asparagine residues), while others are modified via O-linked glycans (attached to serine, threonine, tyrosine, hydroxylysine, or hydroxyproline residues). Some extracellular proteins also comprise glycosylphosphatidylinositol (GPI) anchors, carbohydrate-containing groups that anchor the proteins into the external leaflet of the plasma membrane [3, 4]. In recent years, numerous intracellular proteins relevant to cell signaling, metabolism, and gene expression have been discovered to harbor β-O-GlcNAc residues [5, 6].
The prevalence and central roles of protein glycosylation are only beginning to be appreciated in apicomplexan pathogens such as Toxoplasma gondii. T. gondii is an obligate intracellular parasite, capable of infecting all nucleated cell types in diverse warm-blooded animals and birds [7]. Toxoplasma tachyzoites are the rapidly proliferating form of the parasite that cause acute stage infection. This form and other life cycle stages contain characteristic eukaryotic organelles including a nucleus, endoplasmic reticulum (ER), Golgi apparatus, mitochondrion and plastid, as well as specialized membrane-bound secretory organelles termed micronemes, rhoptries and dense granules. Tachyzoites are bounded by a tri-laminar pellicle consisting of a plasma membrane and underlying inner membrane complex (IMC) and the tachyzoite surface is particularly enriched in GPI-linked surface proteins such as TgSAG1 [8]. In order for Toxoplasma to survive and replicate, extracellular parasites must actively invade host cells in infected organisms, including humans. Invasion of host cells begins with the initial attachment of GPI-anchored surface antigens on Toxoplasma to glycans on the host cell [9]. Secretion from micronemes and rhoptries is required for parasite motility, host cell invasion and establishment of a non-phagosomal membrane-bound parasitophorous vacuole [10, 11]. Within the vacuole, tachyzoites undergo an asexual reproduction by endodyogeny, a process in which two daughter cells are produced inside a mother cell. In the absence of a host cell, the parasite is not able to replicate and will not survive. Trafficking of proteins to the parasite surface and to the microneme, rhoptry and dense granule compartments occurs via post-Golgi sorting [12–14] and is likely to require protein glycosylation.
We have an incomplete understanding of glycan modification in apicomplexan parasites due, in part, to a lack of tools for studying these non-templated biopolymers in their native contexts [15]. Lectins (receptor proteins that bind to glycans) [16, 17] and glycan-specific antibodies [18] are available to survey parasite glycans, but these reagents often bind multiple types of structures and thus provide limited information on the types of glycans present. Additionally, many well-known reagents available for studies of mammalian glycosylation may not recognize unique glycan motifs produced by the parasite. Early reports suggested that GPI anchors are the predominant form of carbohydrate modification of Toxoplasma proteins [18–21]. However, there is now ample evidence that Toxoplasma proteins can be modified by both N- and O-linked pathways [18, 22–27]. Intracellular Toxoplasma proteins decorated with β-O-GlcNAc residues have also been identified [27, 28]. Curiously, the predominant glycans in Toxoplasma tachyzoites are unusual oligomannosidic (Man5–8(GlcNAc)2) and paucimannosidic (Man3–4(GlcNAc)2) sugars, which are rarely present on mature vertebrate glycoproteins [29]. Significantly, tunicamycin-treated parasites exhibited reduced motility, host cell invasion, and growth, suggesting that parasite glycosylation may represent a significant future drug target [17, 27].
Two reports have identified glycosylated proteins in Toxoplasma tachyzoites using lectin affinity chromatography and mass spectrometry (MS). In the first study, a Concanavalin A (ConA) column was used to enrich and identify putative glycoproteins, including cytoskeletal proteins (TgMyoA, TgMyoB/C, TgIMC2, actin, tubulin and TgGAP50), secreted proteins (the microneme proteins TgAMA1 and TgPLP1 and several rhoptry proteins) and likely components of the membrane trafficking machinery (putative sortilin and Sec61 orthologs) [17]. More recently, a set of 132 proteins that are likely modified by glycans was identified by serial lectin affinity chromatography (SLAC) and MS [16]. This glycoproteome included surface antigens, microneme, dense granule and rhoptry components, heat shock proteins, as well as a number of hypothetical proteins. The lectins ConA, wheat germ agglutinin (WGA) and Jacalin were used individually and in serial purification protocols to isolate glycoproteins in this study. While some glycoproteins bound to all three lectins, others were only retained by one or two of the reagents.
As noted above, although lectins can be used to profile some aspects of parasite glycosylation, these reagents do not provide a complete picture of glycan biosynthesis. Complementary information can be captured using the bioorthogonal chemical reporter strategy [30–32]. In this approach, a monosaccharide substrate is modified with a functional group (the reporter) that is chemically inert in biological systems. Upon administration to cells, the modified sugar is processed similarly to its native counterpart and integrated into cellular glycans. Finally, the labeled glycans are reacted with a detectable probe using highly selective, covalent chemistries. Depending on the nature of the probe, this strategy permits both the visualization of glycans and/or their enrichment from complex mixtures for subsequent analyses. In this manuscript, we utilize the bioorthogonal chemical reporter strategy to label and profile proteins in living Toxoplasma tachyzoites. This approach identified both known and novel candidate glycoproteins in the parasite and, importantly, demonstrated that tachyzoites can metabolize and incorporate sugar derivatives into cellular structures in the absence of host cell machinery. Subsets of the identified proteins appear to be modified by O-linked structures, suggesting an important role for these conjugates in the parasite life cycle.
2. Materials and methods
2.1. Culture of host cells and parasites
Toxoplasma (RH and Me49 strains, and Me49-derived lines) were grown in human foreskin fibroblast (HFF) host cells [33]. HFF cells were cultured in DMEM media (GIBCO, Invitrogen) supplemented with 10% fetal bovine serum in a humidified incubator (37 °C, 5.0% CO2).
2.2. Metabolic labeling of parasites
Confluent monolayers of HFF cells were infected with Toxoplasma 24–48 h prior to metabolic labeling. Once the host cells were infected to maximum capacity, they were washed with Endo buffer (44.7 mM K2SO4, 106 mM sucrose, 10 mM MgSO4, 20 mM Tris, 5 mM glucose, 3.5% BSA pH 8.2), a buffer that mimics intracellular conditions [34], and then collected by scraping. Parasites were released from host cells by syringe passage (27-gauge needle) and filtered through a 3-µm mesh. The parasites were pelleted at 1500 ×g for 20 min, aspirated, and resuspended in 5 mL of Endo buffer. The parasites were added to 60-mm plates that had been pre-coated with Ac4GlcNAc [35], Ac4GlcNAz [36], or Ac4GlcNAlk [37].
2.3. Cell lysate preparation
Labeled parasites were collected by centrifugation (1500 ×g for 20 min), washed twice with PBS and resuspended in 75 µL of lysis buffer (1% NP-40, 150 mM NaCl, 50 mM triethanolamine, pH 7.4) containing protease inhibitors (Roche Biosciences). Samples were subjected to five freeze-thaw cycles, pelleted, and the resulting supernatants were collected for analyses. Total protein concentrations were measured using a bicinchoninic acid (BCA) assay (Pierce, ThermoScientific).
2.4. Covalent protein labeling and SDS-PAGE
2.4.a. Cu(I)-catalyzed "click" reactions
Toxoplasma lysates (50 µg) were diluted with lysis buffer to a final concentration of 1 µg/µL. To each sample was added rho-alk, rho-az, biotin-alk [38], or biotin-az [39] (100 µM, from a 10 mM stock solution in DMSO), along with a freshly prepared cocktail of "click" chemistry reagents (sodium ascorbate: 1 mM, from a 50 mM stock solution in water; tris[(1-benzyl-1-H-1,2,3-triazol-4-yl)methyl]amine (TBTA): 100 µM, from a 10 mM stock solution in DMSO); CuSO4•5H2O: 1 mM, from a 50 mM stock solution in water). The final reaction volume was 50 µL in all cases. The reaction mixtures were vortexed and incubated at RT for 1 h. To precipitate the labeled proteins, the samples were treated with ice-cold methanol (1 mL) and placed at −80 °C for 2 h. The precipitates were pelleted at 13,000 ×g for 10 min (at 4 °C), and the supernatants were discarded. The samples were air-dried for 1 h at RT prior to the addition of 15 µL of resuspension buffer (4% SDS, 150 mM NaCl, 50 mM thiethanolamine pH 7.4) and 15 µL of 2× SDS-PAGE loading buffer (20% glycerol, 0.2% bromophenol blue, 1.4% β-mercaptoethanol). The proteins were denatured at 95°C and then resolved on Tris-glycine SDS-PAGE gels. Labeled proteins were detected with antibodies on immunoblots or in-gel fluorescence was used (below).
2.4.b. The Staudinger ligation
Toxoplasma lysates (50 µg) were reacted with phosphine-FLAG-His6 or phosphine-biotin (250 µM, 500 µM stock solution in PBS) at RT for 12 h [40]. The samples were then treated with 4X SDS-PAGE loading buffer (4% SDS, 40% glycerol, 0.4% bromophenol blue, 2.8% β-mercaptoethanol). The samples were then incubated at 95 °C and the proteins were resolved on Tris-glycine SDS-PAGE gels. In some cases, the labeled proteins were detected with antibodies on immunoblots, whereas in other cases, in-gel fluorescence was used (below).
2.5. Visualization of labeled proteins
2.5.a. Immunoblots
Proteins separated by SDS-PAGE were electroblotted to nitrocellulose membranes. Blots with biotinylated proteins were blocked using a solution of 7% BSA in PBS containing 0.1% Tween-20 (PBS-T) for 1 h at RT. FLAG-His6-labeled blots were blocked using a solution of 5% non-fat milk (in PBS-T) for 1 h at RT. The blots were incubated with HRP-α-biotin (Jackson ImmunoResearch, 1:10,000 dilution), IRDye 800 CW streptavidin (Li-Cor, 1:10,000 dilution) or HRP-α-FLAG (Sigma, 1:5,000 dilution) in the appropriate blocking buffer for 1 h at RT, then rinsed with PBS-T (6 × 10 min). Detection of membrane-bound antibodies was accomplished by chemiluminescence (SuperSignal chemiluminescence substrate, Pierce) or near infrared spectroscopy on an Odyssey Infrared Imaging System. Densitometry analyses were performed using ImageJ software. The intensity from each immunoblot lane was divided by the intensity of the corresponding lane from the Ponceau S image. The resulting numbers were normalized to the negative control (background) and plotted.
2.5.b. In-gel fluorescence scanning
Following SDS-PAGE, some gels were incubated with destaining solution (50% methanol/10% acetic acid in water, 10 min), followed by water (10 min). In-gel fluorescent signals were measured on a Typhoon Trio+ scanner. Densitometry analyses were performed using imageJ software. The intensity from each fluorescent lane was divided by the intensity of the corresponding lane from the Coomassie stained gel. The resulting numbers were normalized to the negative control (background) and plotted.
2.6. Immunoprecipitation
Toxoplasma samples were prepared and reacted as previously described. In order to purify TgSAG1, samples were incubated with anti-P30/SAG1 (DG52) [41] at 4 °C, with mixing. The protein-antibody complex was isolated using Pierce Protein A/G Magnetic Beads (Thermo Scientific). Samples were analyzed via immunoblot as above.
2.7. Fluorescence microscopy
Syringe-lysed parasite cultures were purified using a PD-10 desalting column (GE Healthcare) or filter (Millipore, Millex-SV, 5.00 um PVDF membrane) and washed with DMEM media (Corning) supplemented with 3% (vol/vol) heat inactivated FBS (Omega Scientific), penicillin (100 U/mL), and streptomycin (10 µg/mL). The parasites were resuspended in Endo buffer containing Ac4GlcNAz (250–1000 µM) or Ac4GlcNAc (250–1000 µM). After 8 h, the parasites were washed with PBS (3 × 0.5 mL) and DMEM (3 × 0.5 mL). The parasites were then centrifuged onto poly-L lysine coated (0.01%, Sigma) glass coverslips (1500 rpm for 7 min) and fixed with 4% paraformaldehyde in PBS for 15 min at RT. After washing with PBS (3 × 0.25 mL), the parasites were permeabilized with 0.1% Triton-X in PBS for 5 min at RT. The parasites were rinsed with PBS (3 × 0.25 mL) and treated with freshly prepared “click” chemistry cocktail containing biotin-alk as described above. The samples were washed with PBS (3 × 0.25 mL), then blocked for 1 h at RT with PBS + 5% BSA (0.5 mL). The parasite samples were then treated with streptavidin-AlexaFluor594 (Jackson Labs; 1:1000 in PBS) for 30 min at RT, then washed with PBS (3 × 0.25 mL). In some cases streptavidin-AlexaFluor488 (Jackson Labs; 1:1000 in PBS) was used, and the resulting images were false-colored red for consistency. The cover slips were mounted on glass slides with Vectashield mounting media (Vector Laboratories). All samples were prepared in triplicate. Images were acquired on a Nikon Eclipse Ti inverted microscope with NIS-Elements Microscope imaging Software and analyzed with ImageJ.
In some cases, parasites incubated with unnatural sugars in Endo buffer were used to infect HFF cells. HFF cells were grown on glass coverslips submerged in 0.5 mL DMEM media supplemented with 10% FBS (vol/vol), penicillin (100 U/mL), and streptomycin (100 µg/mL). The cells were infected at a multiplicity of infection (MOI) of 5–15 in duplicate, then processed and imaged as above.
2.8. Deglycosylation experiments
2.8.a. PNGaseF treatment
Toxoplasma lysates (40 µg) were diluted with lysis buffer (to total 10 µL), 10% NP-40 (1.3 µL), and reaction buffer (New England Biolabs deglycosylation kit, 1.3 µL). PNGaseF (New England Biolabs 200 U, 0.4 µL) was added to each sample, and the reactions were incubated at 37 °C for 8 h. Proteins were then labeled with biotin-alk and analyzed via immunoblot as above.
2.8.b. Hexosaminidase-f treatment
Toxoplasma lysates (50 µg) were diluted with lysis buffer (to total 54 µL), G2 reaction buffer (New England Biolabs, 6 µL). Hexosaminidase-f (New England Biolabs 5 U, 1.0 µL) was added to each sample, and the reactions were incubated at 37 °C for 16 h. Proteins were then labeled with biotin-alk and analyzed via immunoblot as above. As a control, 45 µL of 0.1 mg/mL 4-nitrophenyl-N-acetyl-β-D-glucosaminide (Sigma) was incubated with 5 µL of G2 reaction buffer and 1 µL of hexosaminidasef (active or heat-killed) or 1 µL of water for 2 h at RT. Released 4-nitrophenol was measured via absorbance at 400 nm.
2.8.c. Tunicamycin treatment
HFF cells were cultured in T-175 flasks for 12 d, then treated with 5 µg/mL tunicamycin for 48 h. The cells were then infected with Toxoplasma tachyzoites. After 24 h growth in tunicamycin-treated host cells, the parasites were isolated as above. Extracellular parasites were then incubated with unnatural sugars in the presence of tunicamycin for an additional 8 h prior to covalent labeling and immunoblot analysis.
2.8.d. OGA treatment
Toxoplasma lysates (40 µg) were diluted with lysis buffer (to total 20 µL) and treated with OGA enzyme as in [42] at 37 °C for 24 h. Proteins were then labeled with biotin-alk and analyzed via immunoblot as above.
2.8.e. β-Elimination
Toxoplasma lysates (40 µg) were diluted with lysis buffer (to total 20 µL) and then labeled with biotin-alk. After the addition of 5 µL of a β-elimination reagent mixture (Sigma), the reaction was incubated at 4 °C for 8–24 h. Proteins were then analyzed via immunoblot as above.
2.9. Enrichment of proteins for MudPIT analysis
Toxoplasma lysate (6 mg total protein in 1 mL of lysis buffer) was treated with 110 µL of 10 mM phosphine-biotin in DMSO (working concentration = 1 mM). The reaction mixture was incubated under an Ar atmosphere for 4 h at 37 °C. Next, 1 mL of ice-cold methanol for each 250 µL of reaction mixture was added, and the samples were incubated at −80 °C for 2 h to precipitate proteins. The precipitates were pelleted at 13,000×g (4 °C, 10 min). The supernatants were discarded, and the samples were re-suspended in ice-cold methanol and precipitated at −80 °C two more times. Precipitates were dissolved in 1% SDS in PBS, and incubated with avidin agarose beads at RT overnight. The beads were washed with 2 column volumes of 1% SDS in PBS (pH 7.4), followed by 2 column volumes of 6 M urea in PBS (pH 7.4), then 2 column volumes of 4 M NaCl in PBS (pH 7.4), and 2 column volumes of 100 mM NH4HCO3 (pH 7.4). The bound species were eluted by boiling the beads in SDS-PAGE loading buffer at 95 °C for 10 min, and then loaded onto Tris-glycine SDS-PAGE gels. The gels were stained with Coomasssie blue and submitted to the UCI Mass Spectrometry Facility for MudPIT analysis. Following in-gel digestion with porcine trypsin, extracted peptides were separated on a C18 column and analyzed by MSE on a SYNAPT G2 instrument with a triziac source (Waters). Data were analyzed using ProteinLynx Global Server software (PLGS 3.0) with the Toxoplasma UniProt database. A spreadsheet of Toxoplasma proteins identified by mass spectrometry using the ToxoDB/uniprot database was further organized using BLASTP and NCBI data to categorize hits into subgroups (Chaperones/stress, Cytoskeleton, Enzymes, Membrane Compartments and Trafficking, Secretory Pathway and Other Cellular Processes). A set of uncharacterized protein hits was also noted and annotated with any available information on protein motifs. Similar data were obtained from two replicate experiments (labeled 1 and 2 in Table 1).
Table 1.
Glycosylated proteins identified by mass spectroscopy
| Protein Name |
Uniprot Number |
Predicted Function |
MS Expt |
Other Evidence |
Lectin MS Surveys |
|---|---|---|---|---|---|
| Chaperones/stress (11) | |||||
| CDC48 (ATPase, proteasome) | B9PFU8 | chaperone | 2 | ||
| Heat shock protein 90 | Q2Y2Q8 | stress response | 1,2 | yes | yes |
| Heat shock protein 90 | F0VBM9 | stress response | 2 | yes | |
| Heat shock protein 90 | F0VEH8 | stress response | 2 | yes | |
| Heat shock protein 70 | Q9U540 | stress response | 1,2 | yes | yes |
| Heat shock protein 70 | Q9UAE9 | stress response | 1 | yes | |
| Heat shock protein 70 | O76274 | stress response | 2 | yes | |
| Heat shock protein 70 | B6KHU4 | stress response | 2 | yes | |
| Heat shock protein 60 | F0VQU9 | stress response | 1 | yes | yes |
| Heat shock protein 20 | B9PGE9 | stress response | 2 | ||
| TCP-1 cpn60 family chaperonin | B6KFE8 | chaperone | 2 | ||
| Cytoskeleton (8) | |||||
| Actin | P53476 | cytoskeleton | 1,2 | yes | |
| α-tubulin1 | B9PJD4 | cytoskeleton | 2 | yes | |
| β-tubulin1 | F0V8J8 | cytoskeleton | 1,2 | ||
| GAP50 (Acid phosphatase) | Q6PQ42 | myosin complex | 1,2 | yes | yes |
| IMC3 | Q6GYB1 | cytoskeleton | 2 | ||
| Myosin A | B9PW84 | myosin complex | 1,2 | yes | yes |
| Myosin light chain 1 | Q95UJ7 | myosin complex | 2 | ||
| Myosin D | MYOD | myosin complex | 2 | ||
| Enzymes (32) | |||||
| Acid phosphatase | B9PQM6 | phosphatase | 1 | yes | |
| Aconitate hydratase (aconitase) | B9PMS3 | TCA | 2 | ||
| ADP-ATP carrier (translocase) | Q9BJ36 | mitochondria | 1,2 | ||
| Asparaginyl tRNA synthetase | B6KCZ4 | tRNA synthetase | 2 | ||
| ATP dependent DNA helicase II | B9PQX8 | helicase | 2 | yes | |
| ATP synthase α-subunit | B9PUY4 | ATP synthesis | 1,2 | yes | |
| ATP synthase β-subunit | Q309Z7 | ATP synthesis | 1,2 | yes | |
| Ca2+-dependent protein kinase 1 | Q3HNM6 | Ca2+ signaling | 2 | yes | |
| Citrate synthase | B6KCK9 | mitochondria | 2 | ||
| Cytochrome P450 | B6K9N1 | mitochondria | 2 | ||
| Cytosol aminopeptidase | B9PTG8 | protein turnover | 2 | yes | |
| Enolase | B9PH46 | glycolysis | 2 | yes | |
| Fructose 1,6-bisphosphatase | Q8MY84 | gluconeogenesis | 1 | ||
| Fumarase | B9Q1R2 | aa metabolism | 2 | ||
| Isocitrate dehydrogenase 2 | B9PW21 | ox decarboxylation | 2 | ||
| Long chain fatty acid CoA ligase | B9PJN2 | FA breakdown | 2 | yes | |
| Long chain fatty acid CoA ligase | B9PJM7 | FA breakdown | 2 | yes | yes |
| Malate quinone oxidoreductase | B6KKB7 | pyruvate metabol | 2 | ||
| Branch ch α-keto acid dehydrogenase | Q1KSF2 | mitochondria | 2 | ||
| Mitochondrial processing peptidase | 1,2 | ||||
| α-subunit | B9PUJ6 | mitochondria | |||
| β-subunit | B9PW21 | mitochondria | 2 | ||
| Peroxiredoxin 3 | Q86GL5 | antioxidant | 2 | ||
| Phosphate carrier protein (TMD) | B9PRN1 | mitochondria | 2 | ||
| Prolyl endopeptidase | B9QH13 | endopeptidase | 2 | yes | |
| Phosphofructokinase | B9Q857 | glycolysis | 2 | yes | |
| Pyridine nucleotide diS oxidoreductase | B9PXF3 | oxidoreductase | 2 | ||
| Pyruvate carboxylase | B9PSZ5 | mitochondrial | 2 | yes | |
| Pyruvate kinase | B6KVA2 | glycolysis | 2 | yes | |
| Succinate CoA synthetase α-subunit | B9PTH6 | mitochondrial | 1,2 | ||
| Succinate CoA synthetase β-subunit | Q1KSE5 | mitochondrial | 1,2 | ||
| Succinate dehydrogenase | B9PZU5 | mitochondrial | 2 | ||
| Tryptophanyl tRNA synthetase | B6KKA3 | tRNA synthetase | 2 | ||
| Other Cellular Processes (13) | |||||
| Histone H2A | F0VGI1 | histone | 1 | yes | |
| AP2 transcription factor (AP2X-9) | B9PZQ1 | transcription factor | 1 | yes | |
| Calmodulin | B9PZ33 | Ca2+ signaling | 2 | ||
| Elongation Factor-1α | B6KN45 | transcription | 2 | yes | yes |
| Elongation Factor-2 | F0VEU2 | transcription | 2 | yes | |
| Elongation factor Tu | B6KC06 | transcription | 2 | yes | |
| Nucleosome assembly protein | B6KAS9 | nucleosomes | 1 | ||
| Prohibitin | B9PP22 | transcription | 2 | yes | |
| Prohibitin | B9PGD2 | transcription | 2 | yes | |
| Ribosomal S3Ae family | F0VIB8 | translation | 2 | ||
| Ribosomal: 60s ribosomal protein | F0VBQ9 | translation | 1 | yes | |
| Thioredoxin | B9PM19 | redox signaling | 2 | ||
| Ubiquitin | F0VPK9 | ubiquitination | 1,2 | yes | |
| Membrane Compartments & Trafficking (10) | |||||
| α-importin (nuclear transport) | B9QIQ9 | transport | 2 | ||
| Gbp1p protein | B9PLQ7 | dynamin superfam | 2 | ||
| Protein disulfide isomerase | Q9BLM8 | ER, diS bonds | 1,2 | yes | yes |
| Rab23 (nuclear transport) | B9PSV9 | transport | 2 | ||
| Ranbp1 domain containing protein | F0V739 | transport | 2 | ||
| Reticulon domain containing protein | B9PMP2 | ER curvature | 2 | ||
| SERCA Calcium ATPase | Q5IH90 | Ca2+ pump | 2 | yes | |
| Signal recognition particle | B9Q211 | rough ER | 2 | ||
| Vacuolar ATP synthase β-subunit | B9PQR4 | H+ pump | 2 | yes | |
| Vacuolar ATP synthase subunit E | F0VAR6 | H+ pump | 2 | yes | |
| Secretory Pathway (11) | |||||
| Apical membrane antigen (AMA1) | B9Q2L9 | secreted, MN | 2 | yes | |
| Mitochondrial association factor | B9Q3S3 | secreted, DG | 2 | yes | |
| Nucleoside triphosphatase 1 (DG) | Q27893 | secreted, DG | 2 | ||
| Nucleoside triphosphatase 2 (DG) | Q27895 | secreted, DG | 1 | ||
| Rhoptry protein ROP5B (rhoptries) | F2YGS5 | secreted, rhoptries | 2 | yes | |
| Rhoptry protein ROP5C (rhoptries) | F2YGS4 | secreted, rhoptries | 1,2 | yes | |
| Rhoptry protein ROP7 (rhoptries) | B6KR07 | secreted, rhoptries | 2 | yes | |
| Rhoptry protein ROP13 (rhoptries) | B9PK73 | secreted, rhoptries | 2 | yes | |
| Rhoptry protein ROP44 (rhoptries) | B6KPH7 | secreted, rhoptries | 1 | ||
| Surface antigen-1 (SAG1, P30) | C7E5U4 | secreted, surface | 1,2 | yes | yes |
| Surface antigen-2 (SAG2) | Q27004 | secreted, surface | 2 | yes | |
| Uncharacterized (4) | |||||
| M protein repeat containing protein | B9PQT9 | No predicted TMD | 2 | N/A | |
| Uncharacterized protein | B6K8Z6 | Borrelia motif, TMD | 1,2 | N/A | |
| Uncharacterized protein | B9Q2P2 | No predicted TMD | 2 | N/A | |
| Uncharacterized protein | F0VB85 | No predicted TMD | 2 | N/A | |
“Other evidence” of glycosylation summarized in [46].
3. Results
3.1. Extracellular Toxoplasma tachyzoites metabolize and incorporate unnatural sugars
We utilized the bioorthogonal chemical reporter strategy to target glycoconjugates in living Toxoplasma tachyzoites. In this technique, peracetylated azido or alkynyl sugars are metabolized by cells and incorporated into glycan structures (Figure 1A). The acetyl groups on each unnatural sugar facilitate cellular uptake, and the free sugars are liberated by esterase activity or hydrolysis. Following installation into proteins or other targets, the unique azido and alkynyl groups can be covalently reacted with probes for visualization or purification [30]. Prior to this report, the chemical reporter strategy had not been used to observe glycosylation or other metabolic processes in Toxoplasma tachyzoites. Labeling parasites within a host cell presented a challenge, as the unnatural sugars had to traverse both the host cell and parasite plasma membranes in order to reach the tachyzoite cytoplasm. Indeed, we were unable to robustly detect the unnatural sugar labels in intracellular tachyzoites even at high doses of sugar. This was likely due to the action of host cell esterases and competition with the host cell metabolic machinery, preventing monosaccharide delivery into the tachyzoite cytoplasm. Since Toxoplasma tachyzoites are most metabolically active when they are intracellular, we labeled extracellular Toxoplasma tachyzoites in established ionic conditions that mimic intracellular cues (Intracellular Endo Buffer) [34].
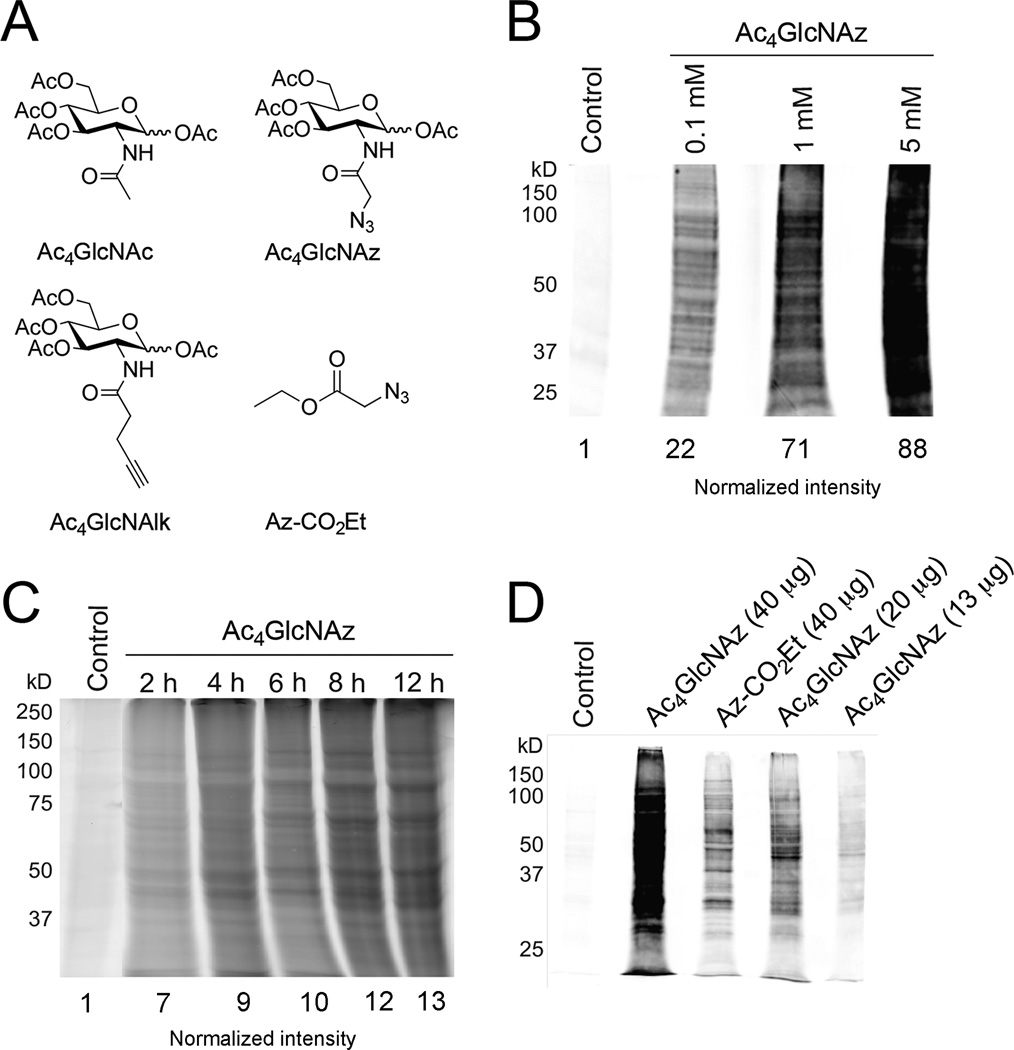
Figure 1. Toxoplasma tachyzoites metabolize unnatural sugars.
(A) Structures of peracetylated N-acetylglucosamine (Ac4GlcNAc) and the corresponding alkynyl (Ac4GlcNAlk) and azido (Ac4GlcNAz) analogs used in metabolic labeling studies. The acetyl groups facilitate sugar uptake into cells and are removed by the action of non-specific esterases in the cytoplasm. An azide-functionalized acyl chain (Az-CO2Et) used in various control experiments is also pictured. The azide and alkyne functional groups can be detected using bioorthogonal “click” chemistries. (B) Toxoplasma tachyzoites incorporate Ac4GlcNAz in a dose-dependent manner. Parasites were incubated with unnatural sugar (0.1–5 mM) or the control sugar Ac4GlcNAc for 8 hours, lysed, and reacted with a biotin-alk tag via “click” chemistry. The labeled proteins were separated via gel electrophoresis and detected via immunoblot with streptavidin. Equivalent protein loading was confirmed via staining with Ponceau S (Figure S1) and normalized intensities are listed. (C) Toxoplasma tachyzoites incorporate Ac4GlcNAz in a time-dependent manner. Parasites were incubated with Ac4GlcNAz (1 mM) or the control sugar Ac4GlcNAc (1 mM) for 2–12 h hours, lysed, and reacted with a rhodamine-alkyne probe. Labeled proteins were separated via gel electrophoresis and analyzed via in-gel fluorescence. Equivalent protein loading was confirmed via staining with Ponceau S (Figure S4) and normalized intensities are listed. (D) Ac4GlcNAz treatment reveals a unique glycoprotein fingerprint. Parasites were incubated with Ac4GlcNAz (1 mM), Ac4GlcNAc (1 h) or a non-sugar probe (Az-CO2Et) for 8 hours, lysed, and reacted with a biotin-alk tag. Labeled proteins were analyzed via immunoblot as in (C). Equivalent protein loading was confirmed via staining with Ponceau S (Figure S7).
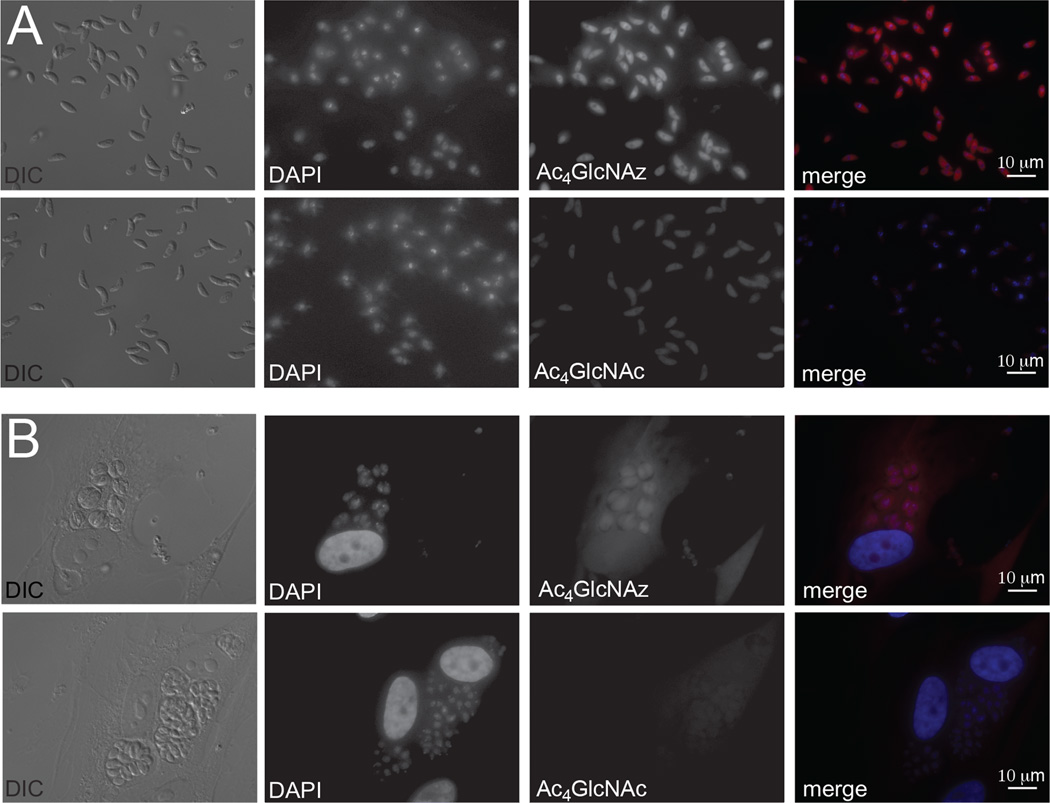
Azido and alkynyl variants of N-acetylglucosamine (Ac4GlcNAz and Ac4GlcNAlk, Figure 1A) have been used to target O-linked and O-GlcNAcylated proteins in mammalian cells and bacteria, and have the potential to target N-linked structures as well [43–46]. When these reagents were administered to extracellular Toxoplasma tachyzoites, they labeled similar subsets of proteins, suggesting that the distinct chemical reporters do not influence metabolism of the monosaccharide (Figures 1B, S1–3). Importantly, tachyzoites incorporate Ac4GlcNAz and Ac4GlcNAlk in a concentration and time dependent manner (Figures 1B–C, S1–6). Previous studies have demonstrated that the N-acyl chain of GlcNAc and related analoggs can be cleaved in cells and subsequently used for protein acetylation [47, 48]. To investigate whether the N-acyl units of Ac4GlcNAz or Ac4GlcNAlk were removed and appended to Toxoplasma proteins, we synthesized N-azido and N-alkynyl acetyl units and examined their incorporation (Figure 1D, S7). Since the labeling patterns were distinct across multiple experiments, we believe that many proteins targeted with Ac4GlcNAz or Ac4GlcNAlk are not simply acetylated. Metabolic incorporation was also visualized using fluorescence microscopy. Ac4GlcNAz-labeled parasites were reacted with biotinylated probes and subsequently stained with streptavidin-conjugated Alexa488 (Figure 2A). Signal was specific to Ac4GlcNAz-treated parasites (likely revealing glycoproteins or glycolipids) and appeared most concentrated in the region around the nucleus, perhaps reflecting ER localization. Importantly, Ac4GlcNAz-treated samples were still able to infect host cells, suggesting that metabolic labeling was tolerated by the parasites (Figures 2B).
Figure 2. Chemical reporters can be visualized in Toxoplasma tachyzoites via fluorescence microscopy.
(A) Parasites were incubated with Ac4GlcNAz (0.5 mM) or Ac4GlcNAc (0.5 mM) for 8 h, prior to fixation with 4% paraformaldehyde in PBS for 15 min at rt. Subsequent reaction with biotin-alk and incubation with streptavidin-AlexaFluor594 enabled fluorescence detection of modified bioconjugates. Exposure times were established with Ac4GlcNAz samples and kept constant for the control samples. (B) Ac4GlcNAz-treated parasites remain viable. Parasites were incubated with Ac4GlcNAz (250 µM) or Ac4GlcNAz (250 µM) for 8 h and then added to HFF monolayers. After 36 h, the samples were fixed, labeled, and imaged as in (A).
3.2 Some proteins appear to be modified by O-linked sugars
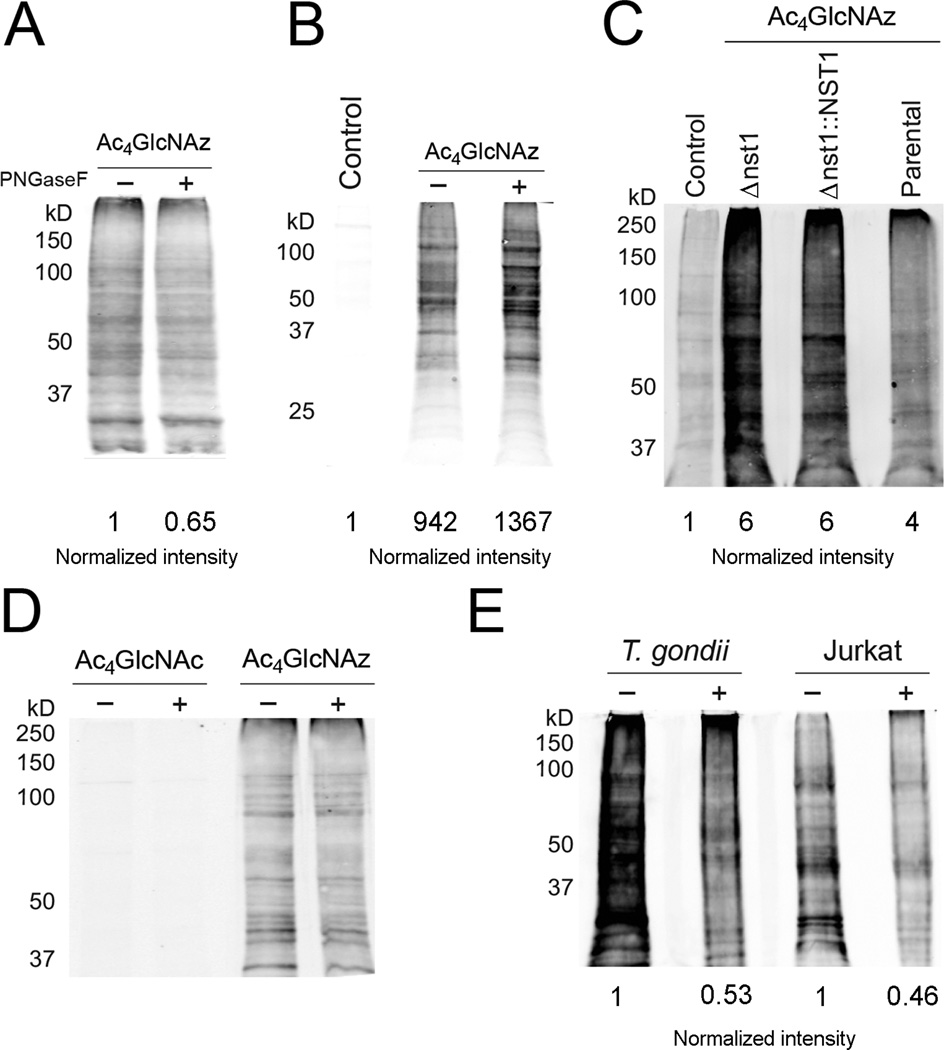
To elucidate the type of labeled structures, we first investigated the common carbohydrate-cleaving enzyme N-glycosidase F (PNGaseF). PNGaseF treatment did not eliminate Ac4GlcNAz-dependent signal in our immunoblot analyses, suggesting that the majority of the label is not present in N-linked glycans or that the enzyme may not recognize features of carbohydrate modification in Toxoplasma (Figures 3A, S8–9). We also examined the effect of tunicamycin treatment on Ac4GlcNAz-labeled parasites. Tunicamycin inhibits N-linked glycosylation in eukaryotes. However, cells must be treated for 24–72 h with the drug to observe any effect, and extracellular parasites are not viable for that length of time. We chose to treat host cells with tunicamycin prior to infection with tachyzoites. Parasites were allowed to replicate in treated cells for 24 h, and then were released and subjected to further tunicamycin treatment (extracellularly) in the presence of the unnatural sugars. Minimal reduction in Ac4GlcNAz-dependent signal was observed in this experiment, indicating that most of the labeled proteins are not susceptible to the effects of tunicamycin, or that insufficient drug entered the parasites (Figures 3B, S10).
Figure 3. Ac4GlcNAz appears to target some O-linked glycans in Toxoplasma tachyzoites.
(A) Parasites were incubated with Ac4GlcNAz (1 mM) or Ac4GlcNAc (1 mM), then lysed and treated with PNGase F. All samples were labeled with biotin-alk and analyzed via immunoblot as in Figure 1B. (B) Parasites were grown in tunicamycin-treated HFFs for 24 h, then harvested and incubated in Endo Buffer with Ac4GlcNAz (1 mM) or Ac4GlcNAc (1 mM) and additional tunicamycin. After 8 h, the parasites were lysed and protein samples were reacted and analyzed via immunoblot as in Figure 1B. (C) Parasites deficient in a UDP-GlcNAc nucleotide sugar transporter (Δnst1) were treated with Ac4GlcNAz (1 mM) or Ac4GlcNAc (1 mM) for 8 h, then lysed and analyzed as in Figure 1B. The complemented strain (Δnst1::NST1) and parental strain (Me49) were similarly processed and analyzed. (D) Parasites were incubated with Ac4GlcNAz (1 mM) or Ac4GlcNAc (1 mM), then lysed and treated with hexosaminidase-f. All samples were labeled with biotin-alk and analyzed via immunoblot as in Figure 1B. (E) Parasites were treated with Ac4GlcNAz (1 mM) or Ac4GlcNAc (1 mM) for 8 h, The samples were then lysed, and proteins were subjected to elimination, labeled with biotin-alk and analyzed as in Figure 1B. As a control, Jurkat cells were labeled with Ac4GlcNAz (0.1 mM) for 48 h, then lysed and subjected to the same conditions as parasite cell lysate.
Ac4GlcNAz can target a second major class of glycans in eukaryotes: O-linked glycans. Boothroyd and coworkers recently reported a Toxoplasma line that is deficient in certain mucin-type O-linked glycans. This strain is null for a nucleotide sugar transporter (NST1) relevant to complex glycan biosynthesis (Figures 3C, S11). When these parasites were treated with Ac4GlcNAz, though, no reduction in labeling was observed compared to the complemented strain. While it is not possible to rule out the incorporation of azido sugar into complex mucins, our data suggest that the majority of Ac4GlcNAz likely targets proteins with simpler O-linked structures, β-O-GlcNAc residues, or other post-translational modifications. Attempts to verify the fate of Ac4GlcNAz by treating lystates with O-GlcNAcase (OGA), an enzyme capable of removing single β-O-GlcNAc residues found on numerous intracellular proteins, were not successful (data not shown). Additionally, no diminishment in signal was observed with a related recombinant lysosomal hexosaminidase (Figures 3D, S12). Beta-elimination conditions reduced Ac4GlcNAz-dependent signal in the parasites, consistent with at least some of the label being localized to O-linked structures (Figures 3E, S13).
3.3. Global profiling reveals both predicted and novel candidate glycosylated proteins
We purified a set of proteins that incorporated the Ac4GlcNAz label using biotinylated reactants and streptavidin beads. The captured and eluted proteins were analyzed via mass spectrometry (MS) and the resulting 89 candidate proteins are listed in Table 1. Importantly, the hits included orthologs of proteins that are known to be O-GlcNAcylated in other species (HSP60, enolase, GAPDH) [49, 50] as well as proteins that were identified in two lectin MS surveys of tachyzoite proteins (myosin A, GAP50) [16, 17]. Some proteins are previously characterized components that are specific to Toxoplasma (SAG1, SAG2) [51, 52] while others are novel hypothetical proteins identified in the Toxoplasma genome. This dataset included orthologs of proteins that are markers of the ER (protein disulfide isomerase, a reticulon domain containing protein and the SERCA calcium ATPase) as well as components that mediate membrane trafficking (BET1, Sec63 and the dynamin family member Gbp1p). Parasite-specific effectors known to be secreted from the rhoptries, micronemes or dense granules (AMA1, NTPase1, NTPase2, ROP5, and ROP7) were also identified. We anticipate that these secreted effectors and membrane compartment markers are likely modified by complex glycan structures. It should also be noted that our MS survey identified known acetylated proteins and processing enzymes. These data suggest that while Ac4GlcNAz is capable of targeting glycan biosynthetic pathways, the probe can be diverted to other metabolic pathways and thus report on other post-translational modifications in Toxoplasma. Simlar results have been observed in cultured human cells [45, 48, 53].
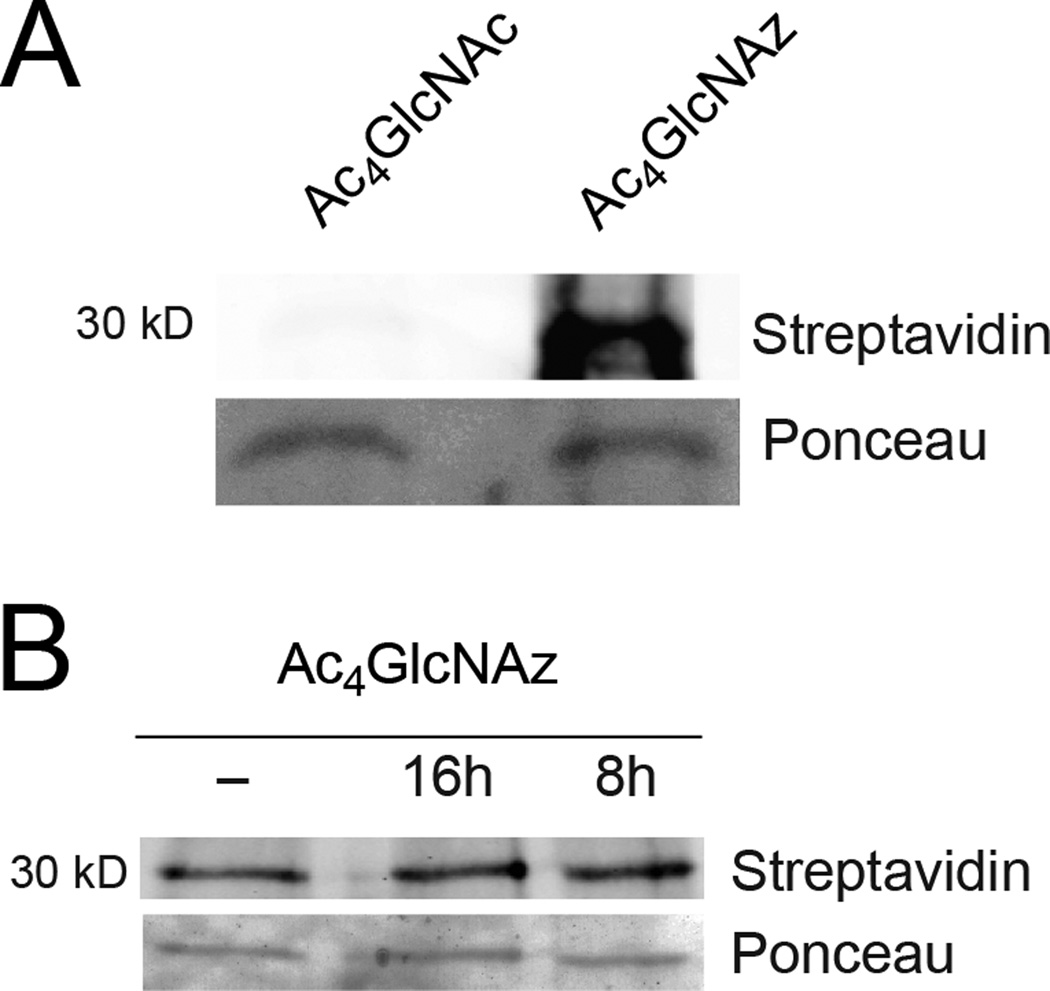
3.4. SAG1 is modified with the metabolic probe
One of the most well-characterized proteins identified by our survey is surface antigen 1 (SAG1). Immunoprecipitation of SAG1 from Ac4GlcNAz-labeled cells verified that it was labeled with the unnatural reporter (Figure 4A). However, we were unable to confirm whether the azido group was present in an O-glycosidic linkage (Figure 4B). SAG1 is a GPI-linked protein [54]. Therefore, the unnatural probe could potentially be associated with the GPI modification or may reflect an N-linked (at N178 and/or N241) site or alternative modification. We also attempted to determine the amino acid site of modification. However, we were unable to detect the altered peptide fragment with MS, most likely because the bulk of SAG1 was synthesized prior to incorporation of the unnatural sugar and the signal from the minor, azide-modified fraction was too low.
Figure 4. Azido label is detected in TgSAG1.
(A) Parasites were labeld with Ac4GlcNAz (1 mM) or Ac4GlcNAc (1 mM, control) for 8 h. The samples were lysed and treated with biotin-alk as above, then incubated with anti-gp30/SAG1 (DG52) antibody overnight at 4°C. The protein-antibody complex was isolated using Pierce Protein A/G Magnetic Beads and analyzed via immunoblot. (B) Parasites were labeled with Ac4GlcNAz (1 mM) or Ac4GlcNAc (1 mM, control) for 8 h. The samples were then lysed, and proteins were subjected to beta-elimination, labeled with biotin-alk as above, then incubated with anti-gp30/SAG1 (DG52) antibody overnight at 4° C. The protein-antibody complex was isolated using Pierce Protein A/G Magnetic Beads and analyzed via immunoblot.
4. Discussion
The details of protein glycosylation in Toxoplasma are less well understood than the process of carbohydrate modification in vertebrate cells, but important features are beginning to emerge from studies that have used a variety of reagents to demonstrate carbohydrate incorporation into modified proteins. Our understanding of glycan biology in Toxoplasma or other apicomplexan parasites is complicated by the need to dissect contributions of the host cell away from parasite-specific processes. This is critically important, as previous results suggest that the specific nature of carbohydrate modification of parasite proteins is influenced by host cell type [55]. However, the Toxoplasma genome has between 14 and 18 annotated glycosyltransferases [56] and the results described here demonstrate that parasites can metabolize and incorporate unnatural sugars in the absence of host cells. Previous studies indicate that the predominant glycans in Toxoplasma are oligomannosidic (Man5–8(GlcNAc)2) and paucimannosidic (Man3–4(GlcNAc)2) sugars, which are rarely present on mature vertebrate glycoproteins [17]. This is consistent with the observation that the Toxoplasma genome lacks annotated glycosidases, suggesting that transferred sugars are not further trimmed.
Unnatural sugars are a promising toolset to begin to elucidate parasite glycosylation machinery. Using these probes, we identified candidate glycoproteins in Toxoplasma tachyzoites complementary to other reports using using lectin affinity chromatography and MS. The first study profiled ConA-purified components while the second survey used ConA, WGA and Jacalin for affinity chromatography. There is a significant overlap of our results with both lectin datasets. In several instances, all three surveys identify the same specific proteins (GAP50, Myosin A, TgAMA1, ROP7, β-tubulin and actin). Our identification of an overlapping protein dataset reinforces the evidence that these components are likely modified by glycosylation. Moreover, we also identified proteins that were not revealed in the previous surveys. This is not surprising, as lectins bind to subsets of glycan structures and may not identify all glycosylated proteins in Toxoplasma. One limitation of our strategy is that the unnatural sugars were only reproducibly incorporated into proteins when we labeled extracellular parasites. This is likely due to the action of esterases in the host cell cytoplasm which may prevent the sugar probes from accessing the parasite cytoplasm. While we were able to identify a number of secreted and surface proteins, these may be less abundantly synthesized in non-replicating parasites. A second limitation is that unnatural sugars can be diverted to other metabolic pathways in Toxoplasma, complicating the assignments of all metabolic end products. Deconvoluting the fates of the unnatural sugars, and the potential crosstalk between metabolic pathways in Toxoplasma, will benefit from additional studies with more refined chemical probes. These reagents, in combination with genetic tools, promise to bolster studies of parasite glycosylation and other post-translational modifications, in additon to the roles of glycoconjugates in mediating essential parasitic processes.
Supplementary Material
Acknowledgments
We are grateful to Melissa Lodoen (UCI), Peter Bradley (UCLA) and John Boothroyd (Stanford) for useful discussions on this work. Research presented in this paper was supported by funds from the UCI School of Physical Sciences to JAP. We also thank members of the Morrissette, Lodoen, and Prescher labs for manuscript edits and helpful discussions.
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
References
- 1.Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, et al. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- 2.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448–442. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomavo S, Dubremetz JF, Schwarz RT. Biosynthesis of glycolipid precursors for glycosylphosphatidylinositol membrane anchors in a Toxoplasma gondii cell-free system. J Biol Chem. 1992;267:21446–21458. [PubMed] [Google Scholar]
- 4.Tomavo S, Dubremetz JF, Schwarz RT. A family of glycolipids from Toxoplasma gondii. Identification of candidate glycolipid precursoRs) for Toxoplasma gondii glycosylphosphatidylinositol membrane anchors. J Biol Chem. 1992;267:11721–11728. [PubMed] [Google Scholar]
- 5.Ma J, Hart GW. O-GlcNAc profiling: from proteins to proteomes. Clin Proteomics. 2014;11:8. doi: 10.1186/1559-0275-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanover JA, Krause MW, Love DC. Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol. 2012;13:312–321. doi: 10.1038/nrm3334. [DOI] [PubMed] [Google Scholar]
- 7.Levine ND. The protozoan phylum Apicomplexa. Boca Raton, FL: CRC Press; 1988. [Google Scholar]
- 8.Lekutis C, Ferguson DJ, Grigg ME, Camps M, Boothroyd JC. Surface antigens of Toxoplasma gondii: variations on a theme. Int J Parasitol. 2001;31:1285–1292. doi: 10.1016/s0020-7519(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 9.Boulanger MJ, Tonkin ML, Crawford J. Apicomplexan parasite adhesins: novel strategies for targeting host cell carbohydrates. Curr Opin Struct Biol. 2010;20:551–559. doi: 10.1016/j.sbi.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Carruthers V, Boothroyd JC. Pulling together: an integrated model of Toxoplasma cell invasion. Curr Opin Microbiol. 2007;10:83–89. doi: 10.1016/j.mib.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Boothroyd JC, Dubremetz J-F. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol. 2008;6:79–88. doi: 10.1038/nrmicro1800. [DOI] [PubMed] [Google Scholar]
- 12.Hager KM, Striepen B, Tilney LG, Roos DS. The nuclear envelope serves as an intermediary between the ER and Golgi complex in the intracellular parasite Toxoplasma gondii. J Cell Sci. 1999;112:2631–2638. doi: 10.1242/jcs.112.16.2631. [DOI] [PubMed] [Google Scholar]
- 13.Stedman TT, Sussmann AR, Joiner KA. Toxoplasma gondii Rab6 mediates a retrograde pathway for sorting of constitutively secreted proteins to the Golgi complex. J Biol Chem. 2003;278:5433–5443. doi: 10.1074/jbc.M209390200. [DOI] [PubMed] [Google Scholar]
- 14.Sheiner L, Dowse TJ, Soldati-Favre D. Identification of trafficking determinants for polytopic rhomboid proteases in Toxoplasma gondii. Traffic. 2008;9:665–677. doi: 10.1111/j.1600-0854.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 15.Prescher JA, Bertozzi CR. Chemical technologies for probing glycans. Cell. 2006;126:851–854. doi: 10.1016/j.cell.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Luo Q, Upadhya R, Zhang H, Madrid-Aliste C, Nieves E, Kim K, et al. Analysis of the glycoproteome of Toxoplasma gondii using lectin affinity chromatography and tandem mass spectrometry. Microbes Infect. 2011;13:1199–1210. doi: 10.1016/j.micinf.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fauquenoy S, Morelle W, Hovasse A, Bednarczyk A, Slomianny C, Schaeffer C, et al. Proteomics and glycomics analyses of N-glycosylated structures involved in Toxoplasma gondii--host cell interactions. Mol Cell Proteomics. 2008;7:891–910. doi: 10.1074/mcp.M700391-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Cervera Y, Harichaux G, Schmidt J, Debierre-Grockiego F, Dehennaut V, Bieker U, et al. Direct evidence of O-GlcNAcylation in the apicomplexan Toxoplasma gondii: a biochemical and bioinformatic study. Amino Acids. 2011;40:847–856. doi: 10.1007/s00726-010-0702-4. [DOI] [PubMed] [Google Scholar]
- 19.Gowda DC, Gupta P, Davidson EA. Glycosylphosphatidylinositol anchors represent the major carbohydrate modification in proteins of intraerythrocytic stage Plasmodium falciparum. J Biol Chem. 1997;272:6428–6439. doi: 10.1074/jbc.272.10.6428. [DOI] [PubMed] [Google Scholar]
- 20.Striepen B, Zinecker CF, Damm JB, Melgers PA, Gerwig GJ, Koolen M, et al. Molecular structure of the "low molecular weight antigen" of Toxoplasma gondii: a glucose alpha 1-4 N-acetylgalactosamine makes free glycosyl-phosphatidylinositols highly immunogenic. J Mol Biol. 1997;266:797–813. doi: 10.1006/jmbi.1996.0806. [DOI] [PubMed] [Google Scholar]
- 21.Zinecker CF, Striepen B, Geyer H, Geyer R, Dubremetz JF, Schwarz RT. Two glycoforms are present in the GPI-membrane anchor of the surface antigen 1 (P30) of Toxoplasma gondii. Mol Biochem Parasitol. 2001;116:127–135. doi: 10.1016/s0166-6851(01)00313-9. [DOI] [PubMed] [Google Scholar]
- 22.Stwora-Wojczyk MM, Dzierszinski F, Roos DS, Spitalnik SL, Wojczyk BS. Functional characterization of a novel Toxoplasma gondii glycosyltransferase: UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase-T3. Arch Biochem Biophys. 2004;426:231–240. doi: 10.1016/j.abb.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Stwora-Wojczyk MM, Kissinger JC, Spitalnik SL, Wojczyk BS. O-glycosylation in Toxoplasma gondii: identification and analysis of a family of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Int J Parasitol. 2004;34:309–322. doi: 10.1016/j.ijpara.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 24.West CM, van der Wel H, Blader IJ. Detection of cytoplasmic glycosylation associated with hydroxyproline. Methods Enzymol. 2006;417:389–404. doi: 10.1016/S0076-6879(06)17023-8. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Brown KM, Wang ZA, van der Wel H, Teygong C, Zhang D, et al. The Skp1 protein from Toxoplasma is modified by a cytoplasmic prolyl 4-hydroxylase associated with oxygen sensing in the social amoeba Dictyostelium. J Biol Chem. 2012;287:25098–25110. doi: 10.1074/jbc.M112.355446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shams-Eldin H, Blaschke T, Anhlan D, Niehus S, Muller J, Azzouz N, et al. High-level expression of the Toxoplasma gondii STT3 gene is required for suppression of the yeast STT3 gene mutation. Mol Biochem Parasitol. 2005;143:6–11. doi: 10.1016/j.molbiopara.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Luk FC, Johnson TM, Beckers CJ. N-linked glycosylation of proteins in the protozoan parasite Toxoplasma gondii. Mol Biochem Parasitol. 2008;157:169–178. doi: 10.1016/j.molbiopara.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz RT, Tomavo S. The current status of the glycobiology of Toxoplasma gondii: glycosylphosphatidylinositols, N- and O-linked glycans. Res Immunol. 1993;144:24–31. doi: 10.1016/s0923-2494(05)80092-6. [DOI] [PubMed] [Google Scholar]
- 29.Fauquenoy S, Hovasse A, Sloves PJ, Morelle W, Dilezitoko Alayi T, Slomianny C, et al. Unusual N-glycan structures required for trafficking Toxoplasma gondii GAP50 to the inner membrane complex regulate host cell entry through parasite motility. Mol Cell Proteomics. 2011;10:M111 008953. doi: 10.1074/mcp.M111.008953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prescher JA, Bertozzi CR. Chemistry in living systems. Nat Chem Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 31.Grammel M, Hang HC. Chemical reporters for biological discovery. Nat Chem Biol. 2013;9:475–484. doi: 10.1038/nchembio.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patterson DM, Nazarova LA, Prescher JA. Finding the right (bioorthogonal) chemistry. ACS chemical biology. 2014;9:592–605. doi: 10.1021/cb400828a. [DOI] [PubMed] [Google Scholar]
- 33.Roos DS, Donald RG, Morrissette NS, Moulton AL. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:27–63. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- 34.Endo T, Tokuda H, Yagita K, Koyama T. Effects of extracellular potassium on acid release and motility initiation in Toxoplasma gondii. J Protozool. 1987;34:291–295. doi: 10.1111/j.1550-7408.1987.tb03177.x. [DOI] [PubMed] [Google Scholar]
- 35.Kretzschmar G, Stahl W. Large scale synthesis of linker-modified sialyl LewisX, LewisX and N-acetyllactosamine. Tetrahedron. 1998;54:6341–6358. [Google Scholar]
- 36.Luchansky SJ, Hang HC, Saxon E, Grunwell JR, Yu C, Dube DH, et al. Constructing azide-labeled cell surfaces using polysaccharide biosynthetic pathways. Methods Enzymol. 2003;362:249–272. doi: 10.1016/S0076-6879(03)01018-8. [DOI] [PubMed] [Google Scholar]
- 37.Gurcel C, Vercoutter-Edouart AS, Fonbonne C, Mortuaire M, Salvador A, Michalski JC, et al. Identification of new O-GlcNAc modified proteins using a click-chemistry-based tagging. Anal Bioanal Chem. 2008;390:2089–2097. doi: 10.1007/s00216-008-1950-y. [DOI] [PubMed] [Google Scholar]
- 38.Charron G, Zhang MM, Yount JS, Wilson J, Raghavan AS, Shamir E, et al. Robust fluorescent detection of protein fatty-acylation with chemical reporters. J Am Chem Soc. 2009;131:4967–4975. doi: 10.1021/ja810122f. [DOI] [PubMed] [Google Scholar]
- 39.Hang HC, Yu C, Pratt MR, Bertozzi CR. Probing glycosyltransferase activities with the Staudinger ligation. J Am Chem Soc. 2004;126:6–7. doi: 10.1021/ja037692m. [DOI] [PubMed] [Google Scholar]
- 40.Laughlin ST, Agard NJ, Baskin JM, Carrico IS, Chang PV, Ganguli AS, et al. Metabolic labeling of glycans with azido sugars for visualization and glycoproteomics. Methods Enzymol. 2006;415:230–250. doi: 10.1016/S0076-6879(06)15015-6. [DOI] [PubMed] [Google Scholar]
- 41.Burg JL, Perelman D, Kasper LH, Ware PL, Boothroyd JC. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol. 1988;141:3584–3591. [PubMed] [Google Scholar]
- 42.Boyce M, Carrico IS, Ganguli AS, Yu SH, Hangauer MJ, Hubbard SC, et al. Metabolic cross-talk allows labeling of O-linked beta-N-acetylglucosamine-modified proteins via the N-acetylgalactosamine salvage pathway. Proc Natl Acad Sci U S A. 2011;108:3141–3146. doi: 10.1073/pnas.1010045108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dube DH, Champasa K, Wang B. Chemical tools to discover and target bacterial glycoproteins. Chem Commun. 2011;47:87–101. doi: 10.1039/c0cc01557a. [DOI] [PubMed] [Google Scholar]
- 44.Breidenbach MA, Gallagher JE, King DS, Smart BP, Wu P, Bertozzi CR. Targeted metabolic labeling of yeast N-glycans with unnatural sugars. Proc Natl Acad Sci U S A. 2010;107:3988–3993. doi: 10.1073/pnas.0911247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaro BW, Yang YY, Hang HC, Pratt MR. Chemical reporters for fluorescent detection and identification of O-GlcNAc-modified proteins reveal glycosylation of the ubiquitin ligase NEDD4-1. Proc Natl Acad Sci U S A. 2011;108:8146–8151. doi: 10.1073/pnas.1102458108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vocadlo DJ, Hang HC, Kim EJ, Hanover JA, Bertozzi CR. A chemical approach for identifying O-GlcNAc-modified proteins in cells. Proc Natl Acad Sci U S A. 2003;100:9116–9121. doi: 10.1073/pnas.1632821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bergfeld AK, Pearce OM, Diaz SL, Pham T, Varki A. Metabolism of vertebrate amino sugars with N-glycolyl groups: elucidating the intracellular fate of the non-human sialic acid N-glycolylneuraminic acid. J Biol Chem. 2012;287:28865–28881. doi: 10.1074/jbc.M112.363549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaro BW, Chuh KN, Pratt MR. Chemical reporter for visualizing metabolic cross-talk between carbohydrate metabolism and protein modification. ACS Chem Biol. 2014;9:1991–1996. doi: 10.1021/cb5005564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayoun D, Kapp T, Edri-Brami M, Ventura T, Cohen M, Avidan A, et al. HSP60 is transported through the secretory pathway of 3-MCA-induced fibrosarcoma tumour cells and undergoes N-glycosylation. FEBS J. 2012;279:2083–2095. doi: 10.1111/j.1742-4658.2012.08594.x. [DOI] [PubMed] [Google Scholar]
- 50.Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the "O-GlcNAc code". Sci STKE. 2005;2005 doi: 10.1126/stke.3122005re13. re13- [DOI] [PubMed] [Google Scholar]
- 51.Manger ID, Hehl AB, Boothroyd JC. The surface of Toxoplasma tachyzoites is dominated by a family of glycosylphosphatidylinositol-anchored antigens related to SAG1. Infect Immun. 1998;66:2237–2244. doi: 10.1128/iai.66.5.2237-2244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pollard AM, Onatolu KN, Hiller L, Haldar K, Knoll LJ. Highly polymorphic family of glycosylphosphatidylinositol-anchored surface antigens with evidence of developmental regulation in Toxoplasma gondii. Infect Immun. 2008;76:103–110. doi: 10.1128/IAI.01170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyce M, Carrico IS, Ganguli AS, Yu SH, Hangauer MJ, Hubbard SC, Kohler JJ, Bertozzi CR. Metabolic cross-talk allows labeling of O-linked beta-N-acetylglucosamine-modified proteins via the N-acetylgalactosamine salvage pathway. Proc Natl Acad Sci U S A. 2011;108:3141–3146. doi: 10.1073/pnas.1010045108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagel SD, Boothroyd JC. The major surface antigen, P30, of Toxoplasma gondii is anchored by a glycolipid. J Biol Chem. 1989;264:5569–5574. [PubMed] [Google Scholar]
- 55.Garenaux E, Shams-Eldin H, Chirat F, Bieker U, Schmidt J, Michalski JC, et al. The dual origin of Toxoplasma gondii N-glycans. Biochemistry. 2008;47:12270–12276. doi: 10.1021/bi801090a. [DOI] [PubMed] [Google Scholar]
- 56.Kissinger JC, Gajria B, Li L, Paulsen IT, Roos DS. ToxoDB: accessing the Toxoplasma gondii genome. Nucleic Acids Res. 2003;31:234–236. doi: 10.1093/nar/gkg072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.