Abstract
Objectives
Identification of a biomarker for acute recanalization could have significant clinical impact.
Methods
We prospectively collected baseline, 24‐h, and 90‐day clinical and imaging data from consecutive ischemic stroke patients who fulfilled standard clinical eligibility criteria for treatment with intravenous recombinant tissue plasminogen activator (rtPA). Computed tomography angiography was acquired at 24 h and assessed using the thrombolysis is myocardial infarction (TIMI) scale with a score of 2b/3 indicating recanalization. Blood samples collected at 24 h after stroke symptom onset were used to measure the inflammatory biomarkers of glycoprotein IIb (CD41) expressing microparticles (MP), C‐reactive protein (CRP), COX 2, APOE, and Angiopoietin 1. Analysis was performed using linear regression and Pearson's correlation coefficient.
Results
A total of 57 patients met study eligibility criteria and had sufficient data and sample quality to be analyzed. Circulating levels of platelet derived CD41 + MP were significantly related to reperfusion (Pearson correlation, PC: 0.554, P < 0.001) and recanalization (PC: 0.495, P < 0.001) as well as related with 3‐month modified Rankin Score (PC: 0.483, P < 0.001). CRP was significantly negatively correlated with recanalization on 24 h CTA (PC: −0.292, P = 0.041). Backward logistic regression with CRP and CD41 + MP increased the association with reperfusion (r 2 = 0.357 P < 0.001).
Interpretation
There is a significant relationship between the inflammatory biomarkers CD41 + MP and CRP and recanalization.
Introduction
Ischemic stroke is the most common stroke subtype and makes up 70–80% of strokes worldwide and is caused by a cerebral blood vessel obstruction. Current treatment of ischemic stroke aims to open, recanalization of a blood vessel to restore blood flow, or reperfuse ischemic tissue and prevent that issue from infarcting. Rapid recanalization of an occluded intracranial artery is the primary clinical objective following an acute ischemic event and is strongly associated with improved functional outcomes and reduced mortality in stroke victims.1 Restoration of blood flow with reperfusion therapy brings with it immune cells from the periphery with neutrophils being considered the first responders, entering the central nervous system (CNS) within 30 min.2 Neutrophils will clear necrotic tissue and are essential for the modulation of CNS immunity, but also contributes to tissue injury.3 Clinical research has observed that subacute elevated numbers of peripheral innate immune cells, mostly neutrophils, coupled with reduced number of lymphocytes, is associated with poor outcome at hospital discharge and months after stroke.4 However, stroke is a highly heterogeneous disease resulting in a wide range of immune and patient responses that are related to symptom severity, patient comorbidity status, and disease treatment. Therefore, the range of patient responses may be proportional to the therapy success or failure.5
Intravenous thrombolysis with recombinant tissue plasminogen activator (rtPA) is currently the first‐line therapy for acute brain ischemia. However, the treatment is only clinically effective in approximately one third of treated patients, prompting the search for more effective approaches. Recent trials of endovascular reperfusion therapy demonstrate superior recanalization and clinical outcome compared to intravenous thrombolysis alone due to a superior rate of vessel recanalization.6 The ability to quickly and easily identify successful recanalization after intravenous therapy would provide a potentially useful tool for patient triage for endovascular therapy.
Blood‐based biomarkers of ischemic stroke may offer a rapid form of individual patient assessment for intravenous treatment response or prediction of patient outcome. Platelet activity and inflammatory markers are potential indicators of rapid pathology changes in ischemic stroke patients as they are centrally activated, are first responders to tissue damage and have been associated with patient outcome.7 In humans, small membrane‐bound particles (<1 μm) known as microvesicles or microparticles (MP) are shed from platelets in response to activation or apoptosis.8 Cardiac studies indicate that recanalization may result in exposure of blood‐bound platelets to acutely ischemic tissue which stimulates platelet MP release and initiates an inflammatory reaction. Currently, there are limited published data on the occurrence of platelet MP activity in acute stroke or any possible relationship between MP and recanalization. We hypothesized that following intravenous thrombolysis in acute ischemic stroke patients, the levels of circulating MP and inflammatory markers will vary depending on the individual patient recanalization status.
Methods
We studied consecutive acute ischemic stroke patients treated with intravenous rt‐PA, Alteplase, within 4.5 h of stroke onset. During the recruitment period (January 2013 to December 2014), the standard protocol for all patients presenting with stroke‐like symptoms to the John Hunter Hospital, NSW, Australia, included a baseline noncontrast computed tomography (CT) to rule out hemorrhage and then a multimodal CT assessment. At 24 h post symptom onset, all patients underwent a follow‐up magnetic resonance imaging (MRI) assessment. Intravenous rtPA was administered to all eligible patients with the diagnosis of ischemic stroke following baseline imaging in accordance with local guidelines. Patients with a small vessel occlusion were excluded from the study. The National Institutes of Health Stroke Scale (NIHSS) was used to assess clinical stroke severity at baseline and 24 h, and the modified Rankin score (mRS) was recorded at 3 months as a measure of functional outcome. Ethics approval for this study was granted by the Hunter New England Human Research Ethics Committee, and written informed consent was obtained from all participants.
Multimodal CT protocol
Acute CT imaging included whole‐brain noncontrast CT (NCCT), CT perfusion (CTP), and CT angiography (CTA) using a 320 slice scanner (Toshiba Aquilion One). A whole‐brain noncontrast CT was performed in one rotation (detector width 16 cm). Next, a four‐dimensional time‐resolved whole‐brain CTA and whole‐brain perfusion were acquired simultaneously. For the CTA‐CTP, 40 mL of contrast agent (ultravist 370; Bayer HealthCare, Berlin, Germany) was injected at 6 mL sec−1 followed by 30 mL of saline9. A continuous scan with a total scan time of 65 sec (56.0 mGy; DLP 896.3 mGy cm) was used.
24‐h imaging protocol
As close as possible to 24 h after acute imaging, all patients underwent a stroke magnetic resonance imaging (MRI) protocol on a 1.5T or 3T scanner (Siemens Avanto or Verio). The MR protocol included: diffusion‐weighted imaging (DWI), perfusion‐weighted imaging (PWI), MR time of flight angiography (MRA), and fluid attenuated inversion recovery (FLAIR) imaging.
CT analysis
All imaging postprocessing was undertaken with commercial software (MiStar, Melbourne, Australia, version 3.2.63). Perfusion data were processed using a single value deconvolution algorithm with delay and dispersion correction.10 Previously validated thresholds were applied in order to measure the volume of the acute perfusion lesion (relative delay time, DT >3 sec) and acute infarct core (relative CBF <30%). Acute and 24‐h vessel occlusion status on baseline CTA were performed by two stroke neurologists, with any disagreement resolved by consensus with a third neurologist using the thrombolysis is myocardial infarction (TIMI) scale with recanalization defined as 2b or 3.
Biomarker sample processing
Peripheral blood was collected into sodium citrate tubes from all patients within 24 h after stroke unit admission and processed within 2 h of collection. Plasma was separated from cells by centrifugation for 15 min at 400g, then platelet‐free plasma was prepared by double serial centrifugation at 2100g for 15 min before being aliquoted and stored at −80°C. Commercially available enzyme‐linked immunosorbent assays were performed in duplicate on freshly thawed aliquots according to the manufacturer's instructions for detection of Angiopoietin‐1, IGF‐1, hCRP (RayBiotech Inc; Norcross, GA), and Apolipoprotein E (Abcam; Cambridge, UK), with individual minimal detection limits of 74 pg/mL, 768 pg/mL, 22.2 pg/mL, and 31 ng/mL, respectively. The intra‐assay coefficients of variation were between 2.5% and 11.5%. Eight random patients had a second aliquot analyzed as a reproducibility assessment.
Analysis of microparticles (MP) by flow cytometry
Staining and analysis of MPs were performed essentially as previously described11 and according to guidelines established by the International Society on Thrombosis and Haemostasis Vascular Biology scientific and standardization committee incorporating modifications suggested for the BD FACS Canto (BD Biosciences, San Jose, CA). To calibrate the cytometer, a blend of 2:1:1 0.5, 0.9, and 3–μm‐diameter fluorescent beads (Megamix, Biocytex, Marseille, France) was used to ensure adequate Fourier shell correlation resolution and set the lower MP detection limit according to the manufacturer's instructions. A 10‐μL aliquot of platelet‐free plasma was incubated at room temperature for 30 min with an antibody against platelet CD41 (CD41‐PE; clone PL2‐49, Biocytex) or an isotype control (IgG1‐PE; clone 2DNP‐2H11/2H12, Biocytex). All assays were diluted 50‐fold in calcium‐rich binding buffer as supplied by the manufacturer (eBioscience) or PBS (without Ca+2 and Mg+2) for negative controls. CountBright absolute counting beads (Molecular Probes, Inc, Eugene, OR) were added to enable absolute MP quantification and events were collected for 60 sec at low flow rate on a BD FACS Canto (BD Biosciences) prior to analysis using FACS Diva software (BD Biosciences). The absolute number of MPs in each plasma sample was calculated using the formula: MPs/μL = (MP count/bead count) ×(total beads in tube/ test volume).
Analysis
Univariate analysis was performed using linear regression and Spearman's correlation statistics to compare biomarker concentrations to pathophysiological characteristics identified on advanced imaging, which included acute infarct core volume, 24‐h infarct core volume, acute penumbra volume, the volume of penumbra salvaged, recanalization, collateral circulation quality, acute severe hypoperfusion volume, and 24‐h hemorrhage. Interaction testing was performed on clinical variables to assess any potential relationships between the biological variables and patient recanalization status. A backwards logistic regression analysis was undertaken using the variables with a P < 0.01 from the univariate analysis. All calculations were performed using SPSS (IBM, version 22) with an alpha of 0.05.
Results
During the study period, 65 patients were screened and had their blood samples taken. Eight patients were excluded from the analysis due to poor quality imaging (n = 4), damaged or missing blood sample (n = 1), or thrombolysis contraindication (n = 3). The remaining 57 patients met study criteria for analysis. There were 36 patients with a middle cerebral artery (MCA) occlusion, 18 with an anterior cerebral artery (ACA) occlusion, and five with a posterior cerebral artery (PCA) occlusion. Patients with an MCA occlusion had similar rates of recanalization as those with an ACA or PCA occlusion (50% vs. 55% P = 0.181). Average time from stroke onset to thrombolysis was 2.8 h (SD = 1.1 h), from stroke onset to blood sampling was 20.2 h (SD = 6.4 h), and from thrombolysis to blood sampling was 17.4 h (SD = 5.9 h). Patient clinical and imaging demographics are reported in Table 1.
Table 1.
Study patient characteristics
| Clinical characteristics | Patients with recanalization (n = 29) | Patients without recanalization (n = 28) | P |
|---|---|---|---|
| Mean Age (Standard deviation) | 62 (12) | 67 (17) | 0.397 |
| Male Sex, N (%) | 11 (38%) | 15 (53%) | 0.247 |
| Hypertension, N (%) | 18 (62%) | 17 (61%) | 0.269 |
| Hyperlipidemia, N (%) | 16 (55%) | 19 (68%) | 0.678 |
| Mean acute NIHSS (standard deviation) | 15 (5) | 13 (5) | 0.571 |
| Mean 24 h NIHSS (standard deviation) | 8 (3) | 11 (4) | <0.05 |
| Current smoker, N (%) | 6 (21%) | 6 (21%) | 0.469 |
| Diabetes, N (%) | 4 (14%) | 6 (21%) | 0.124 |
| Imaging characteristics | Patients with recanalization (n = 29) | Patients without recanalization (n = 28) | P |
|---|---|---|---|
| Mean acute infarct core volume (mL, standard deviation) | 25 (7) | 34 (15) | 0.419 |
| Mean acute penumbra volume (mL, standard deviation) | 69 (51) | 61 (49) | 0.592 |
| Mean 24‐h Infarct core volume (mL, standard deviation) | 27 (22) | 57 (46) | <0.001 |
Total numbers of CD41 + MP were significantly related to reperfusion (Pearson's correlation, PC: 0.554, P < 0.001, Table 2) and recanalization at 24 h (PC: 0.495, P < 0.001, Figure 1) as well as related to 3 month mRS (PC: 0.483, P < 0.001). CRP was significantly negatively correlated with recanalization on 24 h CTA (PC: −0.292, P = 0.041). Other markers such as COX‐2, ANGPT1, or APOE did not significantly associate with recanalization at 24 h. CD 41 + MP were not significantly related to the concentrations of CRP (PC: 0.074, P = 0.596), COX‐2 (PC: −0.124, P = 0.477), APOE (PC: 0.135, P = 0.329), or ANGPT1 (PC: 0.065, P = 0.65).
Table 2.
Comparison of biomarker and clinical outcomes of patients with and without recanalization at 24 h
| Patients with recanalization (n = 29) | Patients without recanalization (n = 28) | P | |
|---|---|---|---|
| Platelet derived CD41 + MP/μL−1(mean, 95% CI) | 896 (679–933) | 539 (359–619) | <0.001 |
| 90 day mRS (median, range) | 1 (0–2) | 4 (2–5) | <0.001 |
Figure 1.
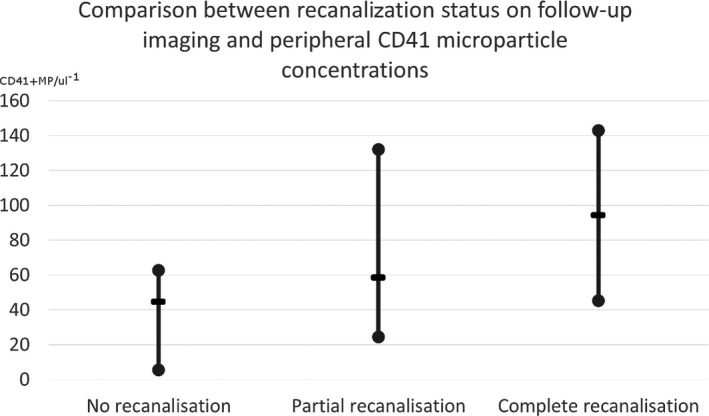
A comparison between recanalization status and CD41 microparticles concentrations. The middle line represents the median value.
Using linear regression, CD41 + MP was significantly related with extent of reperfusion (r 2 = 0.306, P < 0.001) but CRP was not (r 2 = 0.066 P = 0.061). Patient age, sex, and acute NIHSS did not have a significant interaction with CD41 + MP results and recanalization or reperfusion (P > 0.05). Backward logistic regression identified that CRP and CD41 + MP were strongly related to reperfusion at 24 h (r 2 = 0.357 P < 0.001).
Discussion
We have identified that in ischemic stroke patients with partial or complete recanalization following treatment with intravenous rtPA, a significant increase in the levels of CD41 + MP is seen compared to patients with poorer recanalization status. Given recanalization is such a strong predictor of clinical outcome, the level of CD41 + MP was also significantly associated with favorable long‐term patient outcome. The increase in CD41 + MP may occur due to restoration of blood flow to ischemic tissue which causes platelets to shed their microparticles. However, this study is not able to identify if the increase in MP levels is immediate after recanalization and a further study with immediate postrecanalization blood sampling is warranted. Previous studies have identified that micropartical shedding is related to the volume of the infarct core12 and was maintained for up to 90 days. However, our study did not identify as strong a link between infarct core volume and micropartical release, but instead recanalization status, which has a major influence on infarct volume in human stroke. Furthermore, previous studies have compared micropartical activation to atherosclerosis13 and large‐artery atherothrombosis etiology14, which are stroke subtypes with reduced recanalization rates with intravenous rtPA and subsequent worse clinical outcomes. A larger study aimed at assessing stroke etiology and advanced imaging biomarkers may identify if micropartical release is associated with a more specific cause or stroke severity in general.
The GPIIb/IIIa (CD41) pathway is a crucial mediator of irreversible platelet aggregation. Previous animal studies have identified that inhibition of the GPIIb/IIIa pathway did not reduce infarct volume or improve functional outcome.15 However, these studies did demonstrate that GPIIb/IIIa blockade resulted in higher rates of intracerebral hemorrhages and higher mortality. Other studies using GPIIb/IIIa antagonists report an improved functional outcome after experimental stroke, but these were also accompanied by a dose‐dependent increase in intracranial bleeding16. Similarly, bleeding problems were also observed during the AbEST‐II trial, studying the efficacy of the GPIIb/IIIa antagonist Abciximab in patients with acute ischemic stroke17. It is postulated that GPIIb/IIIa stimulated inflammation may increase matrix metalloproteinase (MMP) activity leading to blood brain barrier breakdown and a subsequent risk of hemorrhage as were seen in the clinical trials.18, 19
Some of limitations of our study must be acknowledged. All biomarkers were measured only at one time point with a single blood draw approximately 24 h post stroke onset which is in the same time frame in which recanalization was assessed on follow‐up imaging. Single measurements can be influenced by many factors such as infection, stress, and timing of measurement. Additionally, we did not take a prethrombolysis blood draw and cannot be certain that the levels of CD41 + MPs were not already elevated in patients who would go on to be recanalized.
In conclusion, we have demonstrated a significant relationship between inflammatory biomarkers and both recanalization and reperfusion after treatment with intravenous rtPA. CD 41 + MP released from platelets may be a biomarker of reperfusion therapy effectiveness, however, further studies are needed to clarify the temporal relationships between CD 41 + MP levels, timing of reperfusion therapy, and recanalization. A marker of recanalization with intravenous therapy could provide a clinically useful guide for triage to endovascular therapy.
Conflicts of Interest
No authors have any relevant conflicts of interest to declare.
All coauthors have seen and agree with the contents of the manuscript.
References
- 1. Rha JK, Saver J. The Impact of Recanalization on Ischemic Stroke Outcome: A Meta‐Analysis. Stroke. 2007;38:967–973. [DOI] [PubMed] [Google Scholar]
- 2. Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 2010;87:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol 2011;10(5):471–480. [DOI] [PubMed] [Google Scholar]
- 4. Akopov SE, Simonian NA, Grigorian GS. Dynamics of polymorphonuclear eukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke 1996;27:1739–1743. [DOI] [PubMed] [Google Scholar]
- 5. Pagram H, Bivard A, Lincz L, Levi C. Immunity and stroke, the hurdles of stroke research translation. Int J Stroke 2016; pii: 1747493016676622. [DOI] [PubMed] [Google Scholar]
- 6. Dicula TT, Brogger J, Naess H, et al. Admission C – reactive protein after acute ischemic stroke is associated with stroke severity and mortality: The ‘Bergen stroke study’. BMC Neurol. 2009;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Del Zoppo GJ, Schmid‐Schonbein GW, Mori E, et al. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke 1991;22:1276–1283. [DOI] [PubMed] [Google Scholar]
- 8. Burnier L, Fontana P, Kwak BR, Angelillo‐Scherrer A. Cell‐derived microparticles in haemostasis and vascular medicine. Thromb Haemost 2009;101: 439–451. [PubMed] [Google Scholar]
- 9. Christensen H, Boysen G. C‐reactive protein and white blood cell count increases in the first 24 hours after acute stroke. Cerebrovasc Dis 2004;18:214–219. [DOI] [PubMed] [Google Scholar]
- 10. Bivard A, Levi C, Spratt N, Parsons M. Perfusion CT in acute stroke: A comprehensive analysis of infarct and penumbra. Radiology 2013;267:543–550. [DOI] [PubMed] [Google Scholar]
- 11. Alkhatatbeh MJ, Mhaidat NM, Enjeti AK, et al. The putative diabetic plasma marker, soluble CD36, is non‐cleaved, non‐soluble and entirely associated with microparticles. J Thromb Haemost 2011;9:844–851. [DOI] [PubMed] [Google Scholar]
- 12. Chiva‐Blanch G, Suades R, Crespo J, et al. Badimon. Microparticle Shedding from Neural Progenitor Cells and Vascular Compartment Cells Is Increased in Ischemic Stroke. PLoS ONE 2016;11(1):e0148176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cherian P, Hankey GJ, Eikelboom JW, et al. Endothelial and platelet activation in acute ischemic stroke and its etiological subtypes. Stroke 2003. Sep;34:2132–2137. [DOI] [PubMed] [Google Scholar]
- 14. Lukasik M, Rozalski M, Luzak B, et al. Platelet activation and reactivity in the convalescent phase of ischaemic stroke. Thromb Haemost 2010. Mar;103:644–650. [DOI] [PubMed] [Google Scholar]
- 15. Kleinschnitz C, Pozgajova M, Pham M, et al. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation 2007;115:2323–2330. [DOI] [PubMed] [Google Scholar]
- 16. Choudhri TF, Hoh BL, Prestigiacomo CJ, et al. , et al. Targeted inhibition of intrinsic coagulation limits cerebral injury in stroke without increasing intracerebral hemorrhage. J Exp Med 1999;190:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adams HP, Effron MB, Torner J, et al. , et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT‐II). Stroke 2008;39:87–99. [DOI] [PubMed] [Google Scholar]
- 18. Zhao Y, Li Z, Wang R, et al. Angiopoietin 1 counteracts vascular endothelial growth factor‐induced blood‐brain barrier permeability and alleviates ischemic injury in the early stages of transient focal cerebral ischemia in rats. Neurol Res 2010; 32:748–755. [DOI] [PubMed] [Google Scholar]
- 19. Bhatt DL, Topol EJ. Scientific and therapeutic advances in antiplatelet therapy. Nat. Rev. Drug Discov. 2003;2:15–28. [DOI] [PubMed] [Google Scholar]


