Significance
Metronomic dosing has been proposed as an alternative to maximum tolerated doses of chemotherapy. Even though this strategy has shown promise in improving therapeutic outcomes in some cases, the underlying mechanisms of action are not fully understood. In this study, using mathematical modeling, we show that metronomic chemotherapy could be beneficial by normalizing tumor blood vessel function and improving tumor perfusion. Improved perfusion enhances the delivery of drugs to solid tumors and alleviates hypoxia. These effects also result in an improved immune response, contributing to increased killing of cancer cells, including more resistant cancer stem-like cells.
Keywords: thrompospondin-1, tumor perfusion, oxygenation, immune response, drug delivery
Abstract
Metronomic dosing of chemotherapy—defined as frequent administration at lower doses—has been shown to be more efficacious than maximum tolerated dose treatment in preclinical studies, and is currently being tested in the clinic. Although multiple mechanisms of benefit from metronomic chemotherapy have been proposed, how these mechanisms are related to one another and which one is dominant for a given tumor–drug combination is not known. To this end, we have developed a mathematical model that incorporates various proposed mechanisms, and report here that improved function of tumor vessels is a key determinant of benefit from metronomic chemotherapy. In our analysis, we used multiple dosage schedules and incorporated interactions among cancer cells, stem-like cancer cells, immune cells, and the tumor vasculature. We found that metronomic chemotherapy induces functional normalization of tumor blood vessels, resulting in improved tumor perfusion. Improved perfusion alleviates hypoxia, which reprograms the immunosuppressive tumor microenvironment toward immunostimulation and improves drug delivery and therapeutic outcomes. Indeed, in our model, improved vessel function enhanced the delivery of oxygen and drugs, increased the number of effector immune cells, and decreased the number of regulatory T cells, which in turn killed a larger number of cancer cells, including cancer stem-like cells. Vessel function was further improved owing to decompression of intratumoral vessels as a result of increased killing of cancer cells, setting up a positive feedback loop. Our model enables evaluation of the relative importance of these mechanisms, and suggests guidelines for the optimal use of metronomic therapy.
Metronomic chemotherapy is based on the frequent administration of chemotherapeutic drugs at lower doses than the maximum tolerated dose (MTD) and with minimal drug-free breaks (1–3). Although metronomic chemotherapy was initially thought to work as an antiangiogenic therapy (4, 5), it also has been shown to affect the immune system and to directly affect cancer cells, including the more chemoresistant stem-like cancer cells. The antiangiogenic effect of metronomic chemotherapy on the tumor vasculature may be mediated via increased levels of the endogenous angiogenesis inhibitor thrombospondin-1 (TSP-1) (6). TSP-1 is produced not only by stromal cells, but also by cancer cells (7), and induces apoptosis in circulating endothelial cells (8–11). Other negative regulators of angiogenesis (e.g., endostatin, soluble VEGF receptors) also can affect vascular function (6).
In earlier work, we hypothesized that the benefit of metronomic therapy derived from vascular normalization (12). Later studies showed that metronomic chemotherapy can indeed normalize tumor blood vessels (13, 14). Vessel normalization repairs the structure and function of the tumor vessel wall and tumor vascular network, and can improve tumor perfusion and oxygenation (12, 15). Alleviation of hypoxia is known to decrease the number of cancer stem-like cells (CSCs), enhance the efficacy of various therapeutic regimens, decrease invasion, and promote an immunostimulatory microenvironment in tumors (6, 12, 16).
Consistent with the effect of vascular normalization on the immune system, metronomic chemotherapy also has been shown to reprogram the tumor microenvironment from immunosuppressive to immunostimulatory (6, 17). Increased delivery and/or cytotoxic activity of immune effector cells and the selective depletion of regulatory T cells (Tregs), among other factors, can improve immune response (18–20). Finally, metronomic chemotherapy might increase the killing of cancer cells and CSCs via improved delivery of cytotoxic drugs (21, 22). Cancer cells, immune cells, and the tumor vasculature interact with one another in multiple ways and at different levels.
Given these complex interactions, predicting the outcomes of preclinical and clinical studies a priori is difficult (2, 3, 23–25). We hypothesize that a mathematical model that incorporates the known mechanisms of the benefit of metronomic chemotherapy may explain these diverse outcomes reported in the literature, and provide guidelines for its optimal use and future experiments.
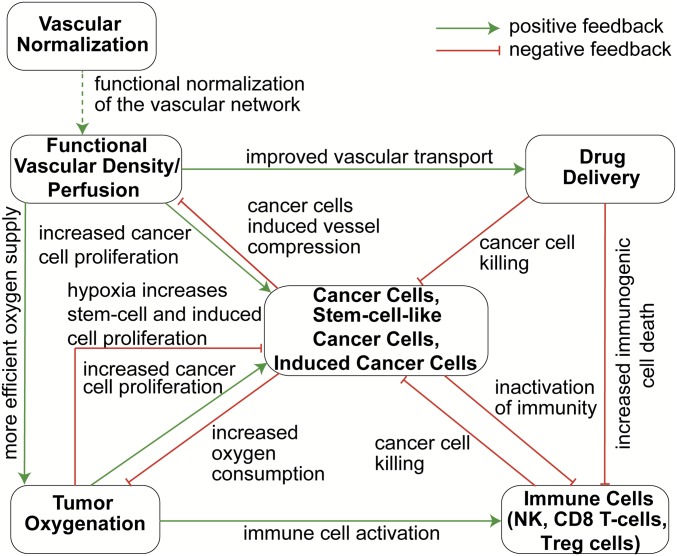
To date, only a few mathematical models of metronomic therapy have been published, none of which accounts for interactions among cancer cells, CSCs, immune cells, and tumor blood vessels (26–32). To this end, we have developed a mathematical model for tumor growth that accounts for three different phenotypes of cancer cells—nonstem cancer cells (CCs), CSCs (which are more resistant to drugs, hypoxia, and the immune system), and CCs that are induced by chemotherapy to acquire a more stem-like phenotype, which we refer to as treatment-induced cancer cells (ICCs) (33), as well as immune cells [natural killer (NK) cells, CD8+ T cells, and Tregs], tumor vasculature, and their interactions (Fig. 1). The model also accounts for the delivery and cell killing potential of chemotherapy based on the dose and schedule, and for transformations among CCs, CSCs, and ICCs (33). Specifically, as shown in Fig. 1, drug delivery and tumor oxygenation depend on the functional vascular density (i.e., tumor perfusion). Tissue oxygenation favors the activity of immune cells and the proliferation rate of CCs, whereas hypoxia promotes CSC and ICC phenotypes (34).
Fig. 1.
Schematic of interactions among model components. An increase in vascular density improves drug and oxygen delivery (54). Increases in drug levels result in more efficient killing of CCs, CSCs, and ICCs (57, 58). Higher oxygen concentrations accelerate the proliferation rates of CCs and the activity of immune cells, whereas hypoxia favors CSC and ICC proliferation (33, 34, 61). An increase in immune response results in more efficient killing of all cancer cells (53). Increased proliferation of all cancer cell types leads to increased oxygen consumption, inactivation of immune cells, and decrease vessel diameters due to compression (51, 52). Vascular normalization improves functionality of the vascular network, leading to an increase in functional vascular density (7, 37), which enhances cancer cell proliferation, but increased drug delivery kills proliferating cancer and immune cells (12, 35).
Along with oxygen supply, malignant cell density may be affected by drug delivery and the killing potential of immune cells. In turn, malignant cell density could directly affect vascular function through compression of blood vessels. The function of tumor vessels can be improved by vascular normalization that repairs the abnormal structure and hyperpermeability of tumor blood vessel walls. The resulting improvement in vascular function may increase malignant cell proliferation, but also can improve drug delivery, which increases cell killing (12, 35).
Here we used multiple protocols for MTD and metronomic chemotherapies and compared model predictions with three independent sets of preclinical data to test the model’s validity. The model offers insight into the relative contribution of various mechanisms of action of metronomic chemotherapy and potential strategies to use it more effectively. It also can guide further experimental studies to probe the link between vascular and immune responses to treatment.
Results
TSP-1–Induced Vascular Normalization Can Significantly Inhibit Tumor Growth.
Our mathematical model incorporates interrelations among components that play key roles in tumor progression. Specification of these components, as well as their interactions, was performed independently for each component based on existing experimental data (details provided in Methods and SI Appendix, Table S1). Then, when assembled as a system, the model is capable of rendering predictions that are not intuitive when the elements of the model are viewed in isolation.
Given the crucial contribution of TSP-1 to the normalization of tumor blood vessels and the subsequent increase in functional vessel density, we first performed a parametric analysis to investigate how relative increases in the amount of TSP-1 affect cancer and immune cell populations, oxygen and drug concentration, and finally overall tumor growth. Based on experimental protocols, we simulated a murine tumor that grows within a period of 30 d, with chemotherapy administered once weekly (4, 14, 36).
The results of our analysis are presented in Table 1 and SI Appendix, Figs. S1 and S2. Vascular normalization through an increase in TSP-1 level increases tumor oxygen supply and intratumoral drug concentration (SI Appendix, Fig. S1 B and C). Increased drug concentration leads to decreases in all cancer cell types, which in combination with improved tissue oxygenation increases the number and activation of NK cells (SI Appendix, Fig. S1D). Increased NK cell activity complements the action of chemotherapy to reduce cancer cell densities of all three phenotypes, as well as the overall volume of the tumor (SI Appendix, Fig. S2). Finally, the increase in CD8+ T cell level is moderate (SI Appendix, Fig. S1E) and less dramatic than that of NK cell level, suggesting that NK cells dominate the immune response under our assumptions. Furthermore, higher drug concentrations decrease the population of Tregs (SI Appendix, Fig. S1F). The spatial distribution of all quantities is presented in SI Appendix, Fig. S3, and the temporal variation of all cell types in shown SI Appendix, Fig. S4. Taken together, our results suggest that a prerequisite for the efficacy of metronomic chemotherapy is normalization of the structure and function of the tumor vasculature.
Table 1.
Effect of TSP-1 level on model variables at the end of the simulation
| Relative TSP-1 level | Oxygen, mol/m3 | Drug delivery* | NK cells | CD8+ T cells | Tregs | Cancer cells | Stem cell-like cancer cells | Induced cancer cells | Tumor volume, mm3 |
| 1 | 0.0252 | 0.476 | 18.1 | 3.34 | 1.07 | 33.9 | 3.39 | 32.5 | 6,914.9 |
| 2 | 0.0308 | 0.539 | 22.6 | 3.89 | 1.07 | 27.8 | 3.20 | 31.9 | 5,936.8 |
| 3 | 0.0412 | 0.642 | 27.3 | 4.18 | 1.06 | 24.3 | 2.80 | 28.4 | 4,110.9 |
| 4 | 0.0576 | 0.910 | 32.2 | 4.30 | 1.06 | 17.9 | 2.27 | 23.5 | 2,516.4 |
All cell concentrations are normalized to the initial cancer cell concentration. The parameters were calculated in the middle distance of the tumor center and periphery.
Drug concentration taken up by all three types of cancer cells relative to vascular concentration.
Dose Scheduling and Time for Tumor Relapse Determine Treatment Efficacy.
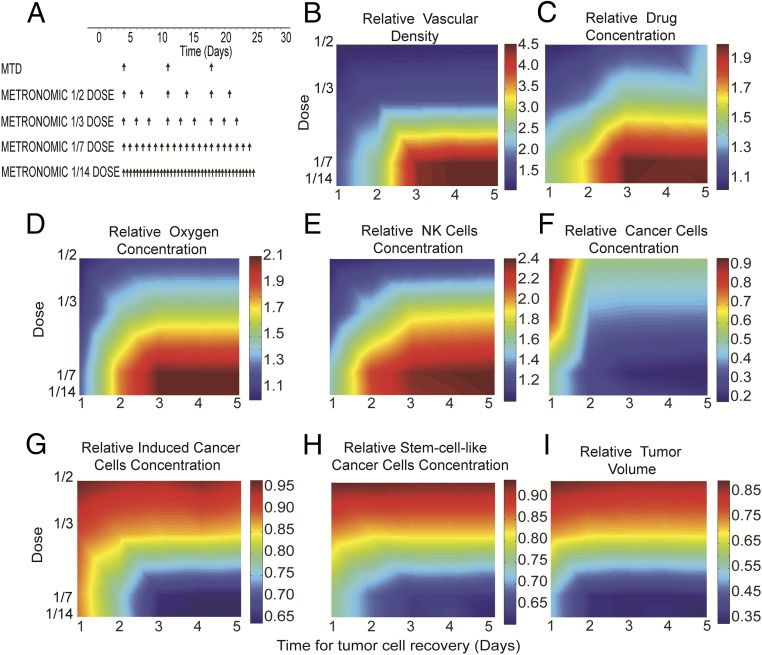
We next investigated the dose and schedule of metronomic chemotherapy that can lead to the most effective outcomes. For this analysis, we tested the impact of different dose fractions of TSP-1 levels (SI Appendix, Fig. S5) (7, 37). We simulated tumor response over 30 d using the different dose scenarios shown in Fig. 2A. Note that even though the amount of drug administered at each dose cycle was different, the total amount of drug administered within the 30-d period was the same in all cases. Furthermore, the therapeutic outcomes for the different scenarios of the total dose and frequency of drug administration also depended on the time required for CCs, CSCs, and ICCs to recover following chemotherapy. Tumor relapse is more likely to occur with a longer drug-free period or a shorter time needed for cancer cells to recover after a cycle of chemotherapy. This tumor recovery time can vary from 1 to 5 d (38, 39), and thus simulations were repeated for a combination of different dosage schedules and recovery times. The MTD chemotherapy was administered once every week, whereas four different protocols for metronomic chemotherapy were used, with the dose varying from 1/2 to 1/14 of the MTD. In the metronomic chemotherapy simulations, the drug was administered more frequently, so that the total drug amount remained the same (Fig. 2A).
Fig. 2.
Effects of dose scheduling and time for tumor relapse on the efficacy of metronomic therapy. (A) Five different protocols were used for a 30-d period; MTD was given once per week, and metronomic chemotherapy was administered in smaller, more frequent doses, with the total amount of drug administered kept constant. (B–I) Phase diagrams for the effects of dose scheduling and tumor relapse/cell recovery on tumor oxygenation, drug delivery, immune cell and cancer cell populations, and tumor volume. Relative values of metronomic therapy with respect to the corresponding values of the MTD protocol are presented. Values of model parameters shown in the figure were calculated in the middle distance between the tumor center and periphery.
The results of the simulations are summarized as phase diagrams in Fig. 2 B–I and SI Appendix, Fig. S6. In these diagrams, the relative changes in the quantities for the metronomic chemotherapy protocols are plotted with respect to the corresponding values of the MTD. As discussed previously, for metronomic chemotherapy to be effective, it should improve the function of the tumor vasculature. Fig. 2B shows that the functional vascular density increased with administration of frequent low doses and for tumor recovery times exceeding 2.5 d. For shorter recovery times, the efficacy of treatment was compromised by allowing too much time for the tumor to relapse. SI Appendix, Fig. S7 depicts the temporal evolution of the total number of cancer cells for different times for tumor recovery. These model predictions are in agreement with previously reported experimental data (38, 39).
Validation of Model Predictions.
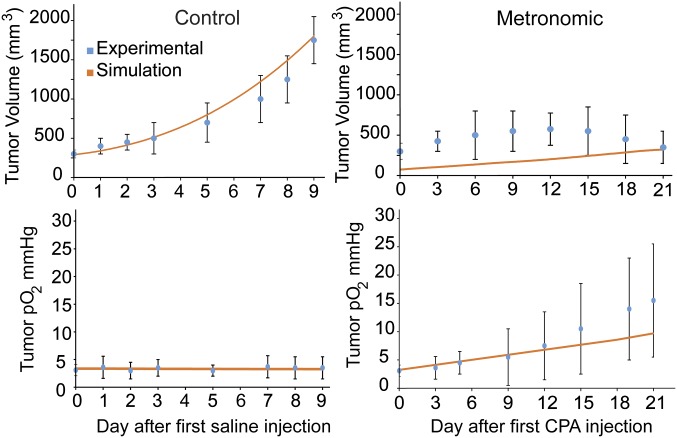
To assess the validity of our mathematical model and to justify the values of the model parameters used, we compared model predictions with published experimental data. The model was tested against the results of three independent studies (13, 14, 40). In those studies, in vivo experiments in murine tumor models were performed and the efficacy of metronomic chemotherapy was compared with no treatment (control group) or with an MTD treatment. To simulate each experiment in our model, we adjusted the amount and frequency of the dose according to the experimental protocol of each study. Subsequently, the sole model parameter that was varied for the evaluation of the model was k1, which describes the dependence of CC proliferation on oxygen (SI Appendix, Table S2). The value of k1 was assigned to match model predictions with the final tumor volume reported in the experimental data. Note, however, that the same value of the parameter was used for each metronomic/control or metronomic/MTD set of experiments; therefore, despite the large number of parameters in the model, only one parameter was varied for each set of experiments, which, as shown in Fig. 3 and SI Appendix, Figs. S8 and S9, was sufficient for the model predictions to match the results of all of the experiments.
Fig. 3.
Comparison of model predictions with previously reported experimental data (13).
In the first study, model predictions were compared with data reported by Doloff et al. (13) from a weekly low-dose cyclophosphamide (CPA) regimen used as a metronomic protocol, which induced increased tumor oxygenation in 9L gliosarcoma tumors (Fig. 3). Our model predictions agree with the data on tumor growth and partial oxygen pressure for both the control and the CPA-treated groups. In a second study, patient-derived pancreatic tumor xenografts were treated with metronomic gemcitabine and MTD (14), and changes in tumor volume, percentage of hypoxia, and functional vascular density were compared with the model predictions (SI Appendix, Fig. S8). The model captured the general response of metronomic chemotherapy for both tumor types and for all experimental protocols. In the third study (40), patient-derived xenografts of pancreatic cancer were treated with metronomic gemcitabine or saline, and again changes in tumor volume, hypoxic fraction, and functional vascular density were compared with model predictions (SI Appendix, Fig. S9). In these studies, hypoxia was measured using a hypoxia marker (EF5+), whereas in the model any concentration below the normal oxygen level was considered hypoxic. In addition, functional vascular density was measured via CD31+/Hoechst+ staining, which cannot be directly related to the corresponding model parameter. In those cases, relative changes in the vascular density of the metronomic chemotherapy group to the MTD group (for the second study) and the control group (for the third study) were used for comparison.
Parametric Analysis of Critical Model Variables.
This being a multivariate mathematical framework, we performed a parametric analysis to determine which parameters have the strongest effects on therapeutic outcomes. Drug delivery is associated with the functional vascular density and the pore size (permeability) of the tumor vessel wall. In SI Appendix, Fig. S10, the concentration of the drug is plotted as a function of these two parameters. Drug delivery is optimized for high vascular densities and for pore sizes on the order of 150 nm, the pore size value that we used in our simulations for vascular normalization. For the parameters associated with the immune response, the activity of both NK cells and CD8+ T cells in the model was taken to be a linear function of tumor oxygenation. A nonlinear expression would change the results only quantitatively, but the general conclusions of this study would remain unaffected. Assuming that the activity of immune cells was not affected by vascular normalization, the efficacy of metronomic chemotherapy would be lower, but still better than that of MTD owing to increased drug delivery (SI Appendix, Fig. S11). Furthermore, assuming that vascular normalization did not occur in metronomic chemotherapy, the efficacy of treatment would be better than that of MTD owing to reduced immunogenic cell death and the decreased number of Tregs as a result of metronomic dose scheduling (SI Appendix, Fig. S12) (41).
Another parameter that might affect the population/function of NK cells and CD8+ T cells is the inhibition term from Tregs. SI Appendix, Fig. S13 depicts the volume of a tumor using the baseline value of the model parameters (SI Appendix, Table S1), as well as the predicted tumor volume when varying the value of the Treg inhibition term in SI Appendix, Eq. S5. Again, the results are qualitatively similar.
Discussion
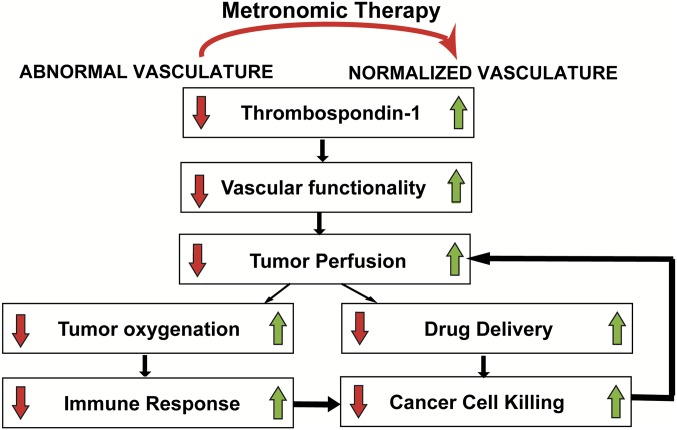
Metronomic chemotherapy has been proposed as an alternative to MTD treatment. The changes imparted by metronomic chemotherapy on cancer cells and the tumor microenvironment have been well studied, but how these multiple mechanisms of action contribute to reducing tumor growth is not intuitive and is difficult to elucidate experimentally. The goal of the present study was to develop a mathematical model incorporating changes induced by chemotherapy in the tumor vasculature, cells of the immune system, and cancer cells to predict under what conditions metronomic dose/scheduling can be more beneficial than MTD. After comparing data from the literature and that derived from our model, we conclude that metronomic chemotherapy is effective when it induces normalization of the tumor vasculature. Vascular normalization sets up a sequence of events that include reduced vessel leakiness, improved tumor functional vascular density and perfusion, increased oxygen supply and alleviation of hypoxia, enhanced immune system activation, and improved drug delivery (Fig. 4).
Fig. 4.
Schematic of the proposed mechanism of action of metronomic therapy. Metronomic therapy increases TSP-1 levels, inducing normalization of the tumor vessels. Vascular normalization increases the functionality of the tumor vessels, thereby improving tumor perfusion and oxygenation. Improved perfusion increases cancer cell proliferation owing to improved nutrient and oxygen supply. In addition, improved perfusion enhances the delivery of chemotherapy, whereas improved oxygenation increases the number and killing efficacy of immune cells. Consequently, more cancer cells are killed, which decompresses collapsed tumor blood vessels, further increasing tumor perfusion and causing a positive feedback loop.
Importantly, tumor perfusion improves during metronomic chemotherapy via both direct and indirect mechanisms. The direct mechanism is the normalization of tumor vasculature via increased levels of TSP-1, and the indirect mechanism is the decompression of tumor vessels owing to a reduction in the mechanical forces exerted on the vessels by the killing of all types of cancer cells. Therefore, as more malignant cells are killed, the less compression is applied on the vessels, allowing collapsed vessels to open up and become capable of carrying blood, resulting in a positive feedback loop (Fig. 4).
Experimental studies have shown that low and more frequent doses of chemotherapy have the ability to increase TSP-1 levels, which, according to our results, should improve treatment outcomes. Optimal dosage scheduling should be based on both the chemotherapy regimen used and the tumor type being treated. In a clinical situation, the optimal dose can be determined by the changes that chemotherapy induces in tumor vessel permeability and perfusion. Both vessel permeability and perfusion can be measured clinically with magnetic resonance imaging (MRI) (42, 43); thus, the optimal dose and schedule to reduce vessel permeability and improve perfusion can be inferred using MRI. Given the continuously evolving nature of each cancer, as well as differences among tumor types, among tumors of the same type, and between a primary tumor and its metastases, the selection of an optimal dosage schedule must be patient-specific. Furthermore, our model suggests that the time required for cancer cells to recover from a dose of chemotherapy is a key parameter in optimizing metronomic chemotherapy. Our calculations based on daily treatments show that to be most effective, the time for tumor recovery should be at least 2.5 times longer than the interval between treatments.
This model has several limitations. It accounts only for increased TSP-1 as the mechanism of vascular normalization. Modulation of other molecules, such as a decrease in VEGF level, also can induce vascular normalization (6, 44). Various cells, including cancer and stromal cells, produce VEGF in hypoxic conditions. Judicious doses of anti-VEGF treatment can restore the structure and function of abnormal tumor vessels. Indeed, anti-VEGF therapy not only decreases vessel wall pore size (permeability), but also “normalizes” the structure of the vasculature (6, 44). In our model, we consider only pore size changes, and not the vessel morphology explicitly. Regarding metronomic therapy, the increased killing of cancer and stromal cells due to TSP-1–induced vascular normalization decreases the number of cells. Fewer cells means less production of VEGF, which in turn should further normalize vessels and improve vascular function. In addition, TSP-1 levels are not likely to increase instantaneously after the first injection of low-dose chemotherapy, as we assumed in our model. In reality, there should be a sigmoidal shape with one or multiple time constant(s) to reach a steady state. Furthermore, our model does not account for the direct effects of the chemotherapeutic agent on functional vascular density. It also does not account for the fact that CSCs reside not only in hypoxic regions, but also in the perivascular niche. Moreover, alleviation of hypoxia also can convert protumor macrophages into antitumor macrophages, a phenomenon not currently incorporated into the model. This change alone can cause tumor regression without changes in NK or CD8+ cells (6). Other cells that are important in immune regulation, such as macrophages, B cells, CD4+ cells, and dendritic cells, are not considered. Furthermore, the model is limited in that it does not account for the fact that improved vascular support resulting from vessel normalization can increase the recruitment of immune cells into the tumors (16). Incorporation of increased infiltration of immune cells owing to better vascular function would further improve the effect of metronomic chemotherapy. Finally, the model does not account for the effect of metronomic chemotherapy on the activation of cancer-associated fibroblasts (45).
The foregoing limitations are expected to affect model predictions only quantitatively, leaving the basic conclusion of this study unaffected. In the meantime, the model can serve as a foundation for further experimental studies to examine the link between vascular and immune responses to treatment. Out of necessity, the present model oversimplifies some important features that are as-yet insufficiently well characterized for incorporation into a mathematical model, but the framework developed here can be readily adapted as more data become available.
Given its complexity and comprehensiveness, our model includes a large number of parameters (SI Appendix, Table S1) that are associated with tumor growth, proliferation and death rate of various cancer cells, drug delivery, tumor properties, and other functions. Baseline values of the model parameters were determined independently based on our previous work, as well as the work by others. Nevertheless, model predictions are in good agreement with a large set of experimental data. Deviations from the experiments are relatively large in some cases, presumably owing to certain assumptions in the model and the use of values of parameters taken from other studies and for other tumor types. Consequently, we performed an analysis to identify the most critical parameters by varying their values over the ranges reported in the literature. The qualitative predictions of the model were found to be robust with respect to the values of the parameters used.
Methods
Mathematical Model.
A detailed description of the model and its assumptions, along with model equations, are provided in SI Appendix. The model accounts for the spatiotemporal distribution of CCs, CSCs, and ICCs (with CSCs and ICCs considered more resistant to cytotoxic drugs, hypoxia, and the immune system compared with CCs), cells of the immune system, the tumor vasculature, tissue oxygenation, drug delivery, and interconversion among CCs, CSCs, and ICSs (Fig. 1). Tumor growth is determined by the combined proliferation of all cancer cell types, as well as their killing rate by immune cells and chemotherapy. The proliferation of malignant cells depends on vessel functionality (i.e., tumor perfusion), through its capacity to supply oxygen and nutrients to the tissue. We made the distinction, however, that whereas CC proliferation increases with oxygen levels, CSC and ICC proliferation increases under hypoxic conditions. Therefore, we assume that in hypoxic conditions, the proliferation of CSCs and ICCs increases inversely with oxygen concentration, so that as oxygen concentration approaches 0, the proliferation rate is twice the rate at normal oxygen concentration (34).
In the model, vessel functionality is assumed to be affected by two parameters: the hyperpermeability and compression of tumor vessels (37). Other parameters affecting vessel functionality, such as intravascular thrombosis and the formation of vascular shunts via the dysregulation of vessel diameter, tortuosity, and connectivity, are not considered in the current formulation. Functional shunting refers to perturbations of the mechanisms of structural adaption in tumors that can give rise to a maldistribution of blood flow (46, 47). Before treatment, tumor vessels are considered hyperpermeable, and a vessel wall pore size of 400 nm was chosen as the baseline value for the simulations (48). Vessel hyperpermeability reduces perfusion, because a very leaky upstream vessel might reduce the blood supply to downstream vessels (6, 49, 50). Furthermore, malignant cells exert mechanical forces on blood vessels, and as cell density increases might cause compression of intratumoral vessels, drastically reducing tumor perfusion and oxygenation (51, 52). An analysis of published experimental data (51, 52) reveals that the relative decrease in the diameter of tumor blood vessels is inversely proportional to cell density (SI Appendix, Fig. S14). In our previous studies, we related vessel functionality to the permeability and compression of tumor vessels (37, 48, 51). In the present study, we applied this relationship to account for how rapid cancer cell proliferation can slow tumor growth owing to blood vessel compression and oxygen deprivation.
Apart from cancer cell populations, our model accounts for the immune system, particularly for three types of immune cells: NK cells, CD8+ T cells, and Tregs. de Pillis et al. (53) investigated the effects of NK and CD8+ T cells on tumor growth, deriving the values of the model parameters through a comparison with preclinical and clinical data. Our model takes into consideration the growth/death rate of the cells, their inactivation by cancer cells, and their activation by oxygen, which increases their killing potential. Transport of oxygen and chemotherapy was modeled based on previous work (50, 54). Fluid transport across the tumor vessel wall is described using Starling's approximation. Transport of oxygen or drug molecules then depends on the permeability of the vessel wall, as well as on functional vessel density. In the tumor interstitial space, transport and cellular uptake of oxygen and drugs are described by convection-diffusion-reaction equations (55, 56). The numbers of CCs, CSCs, and ICCs killed by chemotherapy are functions of drug uptake, taking into account that CSCs and ICCs are more chemoresistant than CCs (57, 58). The interrelations among the different model components are shown in Fig. 1, and values of the model parameters and domain are presented in SI Appendix, Tables S1 and S2 and Fig. S15.
Model Specification.
According to previously published experimental data (59, 60), low-dose metronomic therapy increases TSP-1 levels compared with MTD. In our model, we assume a sigmoidal increase of TSP-1 with a decrease in dose, as shown in SI Appendix, Fig. S5. Even though a different function could be used for the dependence of TSP-1 level on dosage schedule, the results would change only quantitatively, leaving the main conclusion of our study unaffected. Furthermore, in previous work (7) we showed that an increase in TSP-1 led to normalization of the tumor vessels, characterized by decreases in vascular permeability and diameter accompanied by an increase in functional vessel density (12, 15, 44). Changes in vascular permeability are modeled as variations of the vessel wall pore size (54). For the lowest doses of metronomic chemotherapy, the vessel wall pore size decreased from 400 nm (pore size in MTD) to 150 nm (i.e., a 2.5-fold change), which corresponds to the decrease in vascular permeability that we observed experimentally (7). In addition, Izumi et al. (7) reported an increase in functional vessel density (referred to as Svo in SI Appendix, Eq. S21) following vessel normalization, and Stylianopoulos and Jain (37) correlated the fraction of functional vessels with vessel wall pore size (SI Appendix, Fig. S5 B and C). The effects of TSP-1 level on vessel wall pore size and functional vascular density are expected to be time-dependent, but in our model we used the final/posttreatment value from the beginning, owing to a lack of pertinent experimental data. The functional vessel density is further subjected to vessel compression by cancer cells, such that as the cell density increases, the number of functional vessels decreases ( SI Appendix, Fig. S14). Finally, we assumed that the activity of immune cells is affected by tumor oxygenation (6, 34, 61). Owing to a lack of studies that directly relate oxygen levels to the data on the activity of NK or CD8+ T cells incorporated in our model, we used values from the range reported by de Pillis et al. (53), whose model focuses on the action of NK and CD8+ T cells, with values of the model parameters specified by comparing model predictions with preclinical and clinical data. Under complete hypoxic conditions, we used the lowest value for the activity of NK cells and CD8+ T cells reported by de Pillis et al. (53), which increased linearly to the highest value for normal oxygenation conditions.
Supplementary Material
Acknowledgments
This work was supported by the European Research Council Grant 336839 (to T.S.); the National Cancer Institute Grants P01 CA080124, R01 CA126642, R01 CA115767, R01 CA096915, R01 CA085140, and R01 CA098706 and Outstanding Investigator Award R35-CA197743; and the US Department of Defense Breast Cancer Research Innovator Award W81XWH-10-1-0016 (to R.K.J.) and Grant R01 HL128168 (to J.W.B.).
Footnotes
Conflict of interest statement: R.K.J. received consultant fees from Ophthotech, SPARC, and SynDevRx. R.K.J. owns equity in Enlight, Ophthotech, SynDevRx, and XTuit and serves on the Board of Directors of XTuit and the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, and Tekla Healthcare Opportunities Fund. No reagents or funding from these companies were used in these studies.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700340114/-/DCSupplemental.
References
- 1.Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: New rationale for new directions. Nat Rev Clin Oncol. 2010;7(8):455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 2.André N, Carré M, Pasquier E. Metronomics: Towards personalized chemotherapy? Nat Rev Clin Oncol. 2014;11(7):413–431. doi: 10.1038/nrclinonc.2014.89. [DOI] [PubMed] [Google Scholar]
- 3.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4(6):423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 4.Browder T, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60(7):1878–1886. [PubMed] [Google Scholar]
- 5.Klement G, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105(8):R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RK. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: Herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416(6878):279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 8.Bertolini F, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63(15):4342–4346. [PubMed] [Google Scholar]
- 9.Shaked Y, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313(5794):1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 10.Mancuso P, et al. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006;108(2):452–459. doi: 10.1182/blood-2005-11-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasquier E, et al. Concentration- and schedule-dependent effects of chemotherapy on the angiogenic potential and drug sensitivity of vascular endothelial cells. Angiogenesis. 2013;16(2):373–386. doi: 10.1007/s10456-012-9321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 13.Doloff JC, et al. Increased tumor oxygenation and drug uptake during anti-angiogenic weekly low dose cyclophosphamide enhances the anti-tumor effect of weekly tirapazamine. Curr Cancer Drug Targets. 2009;9(6):777–788. doi: 10.2174/156800909789271503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cham KK, et al. Metronomic gemcitabine suppresses tumour growth, improves perfusion, and reduces hypoxia in human pancreatic ductal adenocarcinoma. Br J Cancer. 2010;103(1):52–60. doi: 10.1038/sj.bjc.6605727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med. 2001;7(9):987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA. 2012;109(43):17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73(10):2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghiringhelli F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneno R, et al. Chemotherapeutic agents in low noncytotoxic concentrations increase immunogenicity of human colon cancer cells. Cell Oncol (Dordr) 2011;34(2):97–106. doi: 10.1007/s13402-010-0005-5. [DOI] [PubMed] [Google Scholar]
- 20.Kan S, et al. Suppressive effects of cyclophosphamide and gemcitabine on regulatory T-cell induction in vitro. Anticancer Res. 2012;32(12):5363–5369. [PubMed] [Google Scholar]
- 21.Folkins C, et al. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67(8):3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 22.Vives M, et al. Metronomic chemotherapy following the maximum tolerated dose is an effective anti-tumour therapy affecting angiogenesis, tumour dissemination and cancer stem cells. Int J Cancer. 2013;133(10):2464–2472. doi: 10.1002/ijc.28259. [DOI] [PubMed] [Google Scholar]
- 23.Kerbel RS. Strategies for improving the clinical benefit of antiangiogenic drug-based therapies for breast cancer. J Mammary Gland Biol Neoplasia. 2012;17(3-4):229–239. doi: 10.1007/s10911-012-9266-0. [DOI] [PubMed] [Google Scholar]
- 24.Kerbel RS, Grothey A. Gastrointestinal cancer: Rationale for metronomic chemotherapy in phase III trials. Nat Rev Clin Oncol. 2015;12(6):313–314. doi: 10.1038/nrclinonc.2015.89. [DOI] [PubMed] [Google Scholar]
- 25.Jedeszko C, et al. Postsurgical adjuvant or metastatic renal cell carcinoma therapy models reveal potent antitumor activity of metronomic oral topotecan with pazopanib. Sci Transl Med. 2015;7(282):282ra50. doi: 10.1126/scitranslmed.3010722. [DOI] [PubMed] [Google Scholar]
- 26.Faivre C, Barbolosi D, Pasquier E, André N. A mathematical model for the administration of temozolomide: Comparative analysis of conventional and metronomic chemotherapy regimens. Cancer Chemother Pharmacol. 2013;71(4):1013–1019. doi: 10.1007/s00280-013-2095-z. [DOI] [PubMed] [Google Scholar]
- 27.Benzekry S, Hahnfeldt P. Maximum tolerated dose versus metronomic scheduling in the treatment of metastatic cancers. J Theor Biol. 2013;335:235–244. doi: 10.1016/j.jtbi.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 28.Panetta JC, Schaiquevich P, Santana VM, Stewart CF. Using pharmacokinetic and pharmacodynamic modeling and simulation to evaluate importance of schedule in topotecan therapy for pediatric neuroblastoma. Clin Cancer Res. 2008;14(1):318–325. doi: 10.1158/1078-0432.CCR-07-1243. [DOI] [PubMed] [Google Scholar]
- 29.Liao D, Estévez-Salmerón L, Tlsty TD. Generalized principles of stochasticity can be used to control dynamic heterogeneity. Phys Biol. 2012;9(6):065006. doi: 10.1088/1478-3975/9/6/065006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahnfeldt P, Hlatky L, Klement GL. Center of Cancer Systems Biology second annual workshop—tumor metronomics: Timing and dose level dynamics. Cancer Res. 2013;73(10):2949–2954. doi: 10.1158/0008-5472.CAN-12-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGuire MF, et al. Formalizing an integrative, multidisciplinary cancer therapy discovery workflow. Cancer Res. 2013;73(20):6111–6117. doi: 10.1158/0008-5472.CAN-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schättler H, Ledzewicz U, Amini B. Dynamical properties of a minimally parameterized mathematical model for metronomic chemotherapy. J Math Biol. 2016;72(5):1255–1280. doi: 10.1007/s00285-015-0907-y. [DOI] [PubMed] [Google Scholar]
- 33.Goldman A, et al. Temporally sequenced anticancer drugs overcome adaptive resistance by targeting a vulnerable chemotherapy-induced phenotypic transition. Nat Commun. 2015;6:6139. doi: 10.1038/ncomms7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conley SJ, et al. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci USA. 2012;109(8):2784–2789. doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8(4):309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 36.Mupparaju S, et al. Repeated tumor oximetry to identify therapeutic window during metronomic cyclophosphamide treatment of 9L gliomas. Oncol Rep. 2011;26(1):281–286. doi: 10.3892/or.2011.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stylianopoulos T, Jain RK. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc Natl Acad Sci USA. 2013;110(46):18632–18637. doi: 10.1073/pnas.1318415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephens TC, Peacock JH. Tumour volume response, initial cell kill and cellular repopulation in B16 melanoma treated with cyclophosphamide and 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea. Br J Cancer. 1977;36(3):313–321. doi: 10.1038/bjc.1977.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis AJ, Tannock JF. Repopulation of tumour cells between cycles of chemotherapy: A neglected factor. Lancet Oncol. 2000;1:86–93. doi: 10.1016/s1470-2045(00)00019-x. [DOI] [PubMed] [Google Scholar]
- 40.Yapp DT, et al. The differential effects of metronomic gemcitabine and antiangiogenic treatment in patient-derived xenografts of pancreatic cancer: Treatment effects on metabolism, vascular function, cell proliferation, and tumor growth. Angiogenesis. 2016;19(2):229–244. doi: 10.1007/s10456-016-9503-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfirschke C, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. 2016;44(2):343–354. doi: 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorensen AG, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69(13):5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batchelor TT, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci USA. 2013;110(47):19059–64. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain RK. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerbel RS, Shaked Y. Therapy-activated stromal cells can dictate tumor fate. J Exp Med. 2016;213(13):2831–2833. doi: 10.1084/jem.20161845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pries AR, Höpfner M, le Noble F, Dewhirst MW, Secomb TW. The shunt problem: Control of functional shunting in normal and tumour vasculature. Nat Rev Cancer. 2010;10(8):587–593. doi: 10.1038/nrc2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pries AR, Reglin B, Secomb TW. Structural adaptation of microvascular networks: Functional roles of adaptive responses. Am J Physiol Heart Circ Physiol. 2001;281(3):H1015–H1025. doi: 10.1152/ajpheart.2001.281.3.H1015. [DOI] [PubMed] [Google Scholar]
- 48.Hobbs SK, et al. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95(8):4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Netti PA, Roberge S, Boucher Y, Baxter LT, Jain RK. Effect of transvascular fluid exchange on pressure-flow relationship in tumors: A proposed mechanism for tumor blood flow heterogeneity. Microvasc Res. 1996;52(1):27–46. doi: 10.1006/mvre.1996.0041. [DOI] [PubMed] [Google Scholar]
- 50.Baish JW, Netti PA, Jain RK. Transmural coupling of fluid flow in microcirculatory network and interstitium in tumors. Microvasc Res. 1997;53(2):128–141. doi: 10.1006/mvre.1996.2005. [DOI] [PubMed] [Google Scholar]
- 51.Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: Clinical implications. Cancer Res. 1999;59(15):3776–3782. [PubMed] [Google Scholar]
- 52.Padera TP, et al. Pathology: Cancer cells compress intratumour vessels. Nature. 2004;427(6976):695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 53.de Pillis LG, Radunskaya AE, Wiseman CL. A validated mathematical model of cell-mediated immune response to tumor growth. Cancer Res. 2005;65(17):7950–7958. doi: 10.1158/0008-5472.CAN-05-0564. [DOI] [PubMed] [Google Scholar]
- 54.Stylianopoulos T, Economides EA, Baish JW, Fukumura D, Jain RK. Towards optimal design of cancer nanomedicines: Multi-stage nanoparticles for the treatment of solid tumors. Ann Biomed Eng. 2015;43(9):2291–2300. doi: 10.1007/s10439-015-1276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: Transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. 2008;60(12):1421–1434. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGuire BJ, Secomb TW. A theoretical model for oxygen transport in skeletal muscle under conditions of high oxygen demand. J Appl Physiol (1985) 2001;91(5):2255–2265. doi: 10.1152/jappl.2001.91.5.2255. [DOI] [PubMed] [Google Scholar]
- 57.Eikenberry S. A tumor cord model for doxorubicin delivery and dose optimization in solid tumors. Theor Biol Med Model. 2009;6:16. doi: 10.1186/1742-4682-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu G, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci USA. 2003;100(22):12917–12922. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamano Y, et al. Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res. 2004;64(5):1570–1574. doi: 10.1158/0008-5472.can-03-3126. [DOI] [PubMed] [Google Scholar]
- 61.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74(3):665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.