In the coastal Rabai region of southeast Kenya, two populations of mosquitoes live within a half mile of each other but lead separate and curiously different lifestyles. Entomologists in the mid-20th century found that color distinguished the two subspecies of Aedes aegypti (Aedes aegypti aegypti are brown and Aedes aegypti formosa are black) as well as the hosts they chose for blood meals. The “domestic” brown form frequented people’s homes, preferentially feeding on people and laying their eggs in indoor water receptacles. Meanwhile, the black-colored “forest” form roamed the nearby woods preferring to bite nonhuman animals and lay its eggs in tree trunk holes.

This light sheet microscope image of an adult female Anopheles gambiae mosquito includes genetic labeling to show olfactory neurons (green). The antennae, maxillary palp, and labella on the proboscis contain many such neurons. Image courtesy of Olena Riabinina, Courtney Akitake, and Chris Potter (Johns Hopkins University School of Medicine, Baltimore).
Following up on these reports some 50 years later as a postdoctoral fellow at The Rockefeller University in New York, Lindy McBride saw an opportunity to examine this natural case study with modern genetic tools. The feeding habits of Aedes aegypti aegypti make it a dangerously efficient vector for spreading yellow fever, dengue, Zika, and other diseases between humans. The other subspecies poses much less of a threat.
To understand why, McBride traveled to Rabai to collect mosquito larvae and pupae from people’s homes and the nearby woods. She wanted to compare the two mosquitoes genetically and discover what changes had led the more recently evolved domestic form to hunt humans.
Back at Rockefeller in the laboratory of her then-postdoctorate advisor Leslie Vosshall, McBride raised 30 strains of mosquitoes from the samples and used RNA sequencing to compare the genes expressed in their antennae (a major olfactory organ). In 2014, the group reported 14 genes that were expressed differently between the two subspecies and that strongly correlated with a preference for humans (1).
That project kicked off a line of investigation that McBride has continued as a professor at Princeton University in New Jersey. Such work is part of a growing movement, buoyed by gene-editing advances, to detail the molecular and neural mechanisms by which mosquitoes sense their hosts. The ultimate aim: stemming mosquito-borne disease. “Understanding this process has profound implications for global health: by using this information to intervene, to repel mosquitoes or otherwise confuse them, and reduce close encounters between mosquitoes and humans,” says Laurence Zwiebel, a professor at Vanderbilt University in Nashville, Tennessee. The mosquito’s sense of smell, many believe, may be a prime target for disruption.
Anatomy of a Smell
Most of the time, mosquitoes dine on plant nectars and honeydew. But when the time comes to lay eggs, female mosquitoes go for blood, which is loaded with proteins they need for egg development. Aedes aegypti and Anopheles gambiae (also known as the malaria mosquito) seek out human blood, in particular. “There’s been a lot of research to try to find one smoking gun that would actually prevent the mosquitoes from finding humans. But there are so many sensory systems that are tied in,” says Jeff Riffell, an olfactory neuroscientist at the University of Washington in Seattle.
As early as the 1950s and 1960s, scientists had identified carbon dioxide, heat, and moisture as key attractants. Over the years, researchers have added to that list human foot odor, lactic acid, and even high-contrast visual cues in the environment (2). But many of these cues appear to interact with each other; researchers are still disentangling the myriad mechanisms by which mosquitoes locate and recognize their hosts.
Humans send hundreds of odorous molecules into the air, some fraction of which are thought to interact with receptors (around 100 or more, depending on mosquito species) on each of the insect’s three “noses”: the antennae, a mound near the mouth called the maxillary palp, and the proboscis. Signals from those receptors activate still-unmapped networks of neurons to somehow guide the insect’s feeding.
Elucidating and learning to hack this complex system could pay off in a big way, as traditional insecticidal approaches have proven to be a “zero sum game,” Vosshall explained last November at the Society for Neuroscience annual meeting in San Diego. Multiple efforts since the 1960s have shown that under such extreme selective pressure, the animals rapidly evolve resistance to different pesticides.
Among the 14 genes that McBride and her colleagues flagged in comparing the two subspecies of Aedes aegypti, the team focused on one encoding an odorant receptor gene, called Or4, that was present at much higher levels in the domestic females’ antennae. To figure out Or4’s role, the researchers ran an extract of human odor through a gas chromatograph, separating the substance into its component chemicals. They found that Or4 responded strongly to a molecule called sulcatone, which is enriched in the scent of humans, compared with that of guinea pigs and many other animals. Furthermore, genetic variants of Or4 carried by the human-seeking mosquitoes were significantly more sensitive to sulcatone than the variants found in the animal-biting mosquitoes (1).
But sulcatone-sensing by Or4 is only one part of the puzzle. Vosshall’s group is working to pinpoint other key players by introducing mutations into select genes, a strategy that became feasible only recently.
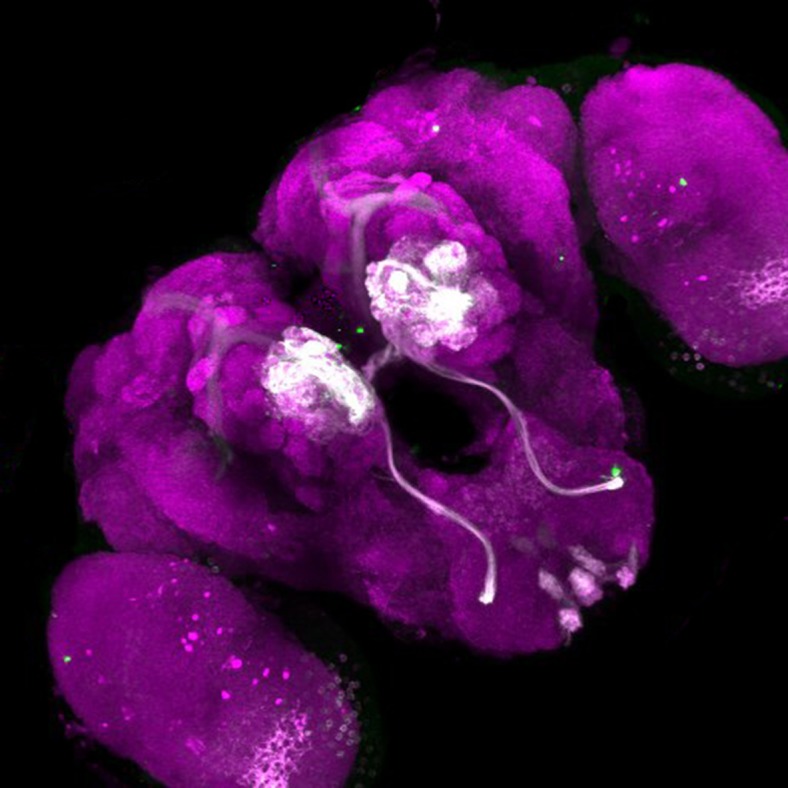
Fluorescent labeling shows how olfactory neurons innervate different parts of the mosquito brain, offering clues as to how the insects process smells and pursue blood meals. Reproduced with permission from ref. 8.
Precision Engineering
Making precise mutations in mosquitoes has traditionally been extremely difficult. Unlike fruit flies, mosquitoes can be tricky to breed in the laboratory. And many of the conventional genetic tools and screening techniques, optimized over decades for fruit flies, have not translated well to mosquitoes. But in recent years, researchers have begun to make headway with new, more efficient tools for manipulating specific genes.
In 2013, Vosshall’s team published one of the first uses of zinc-finger nucleases (a recently devised gene-editing technique, and a precursor to CRISPR-Cas9 technology) in Aedes aegypti (3). The researchers disrupted expression of orco (odorant receptor co-receptor). Although orco mutants lost their strong preference for people, they were still able to locate humans, indicating the involvement of still other proteins and receptors.
In a separate gene-editing study, the team disabled Gr3, a gene required for mosquitoes to sense carbon dioxide, thought to be a critical host-seeking cue. Comparisons of the mutants with unaltered mosquitoes showed that carbon dioxide detection triggered mosquitoes to seek out heat, and enhanced the insects’ attraction to either lactic acid or human scent alone. But when postdoctoral fellow Conor McMeniman sat in a large closed arena and counted the mosquitoes that landed on him, Gr3 mutants were only slightly impaired in finding him (4). Again, it
“We don't really know what repellants are doing to the mosquito nose and brain that tells the mosquito to stay away.”
—Chris Potter
seemed that the mosquito nervous system had workarounds built in, using other sensory cues and alternate biological pathways. Many predict that the rise of the highly efficient CRISPR-Cas9 gene-editing technology will further accelerate efforts to dissect this complex process gene by gene.
Multi-Pronged Attack
In his laboratory at Vanderbilt, Zwiebel is taking a different approach against this highly redundant system. Instead of trying to isolate and disable just the right components, Zwiebel is studying a molecule that he hopes can overwhelm the insect’s olfactory system. “When you get on an elevator with someone who’s put on too much perfume, you want to get out of that elevator,” he explains. “Overstimulation is much worse than anything else.”
Zwiebel’s laboratory has developed a high-throughput chemical screening technique, similar to those used by pharmaceutical companies to test drugs (5). By expressing various combinations of Anopheles gambiae odorant receptors and coreceptors in cultured cells, the researchers can efficiently characterize their responses to a battery of chemicals.
In 2011, the group uncovered the first substance known to directly activate the Orco coreceptor (6). Orco is not itself an odorant receptor, but works together with nearly 80 odorant receptors in Anopheles gambiae. Zwiebel hopes that the molecule they named VUAA1 (for Vanderbilt University Allosteric Agonist 1) can lead to an “excitorepellant” that simultaneously activates wide swaths of receptors (each tuned to different odors), thus flooding the olfactory system and disrupting the animals’ ability to recognize humans. The team’s initial behavioral tests suggest that mosquitoes are extremely averse to the molecule. Zwiebel’s group has since modified VUAA1 to produce even more potent and effective formulations (7).
In pursuit of additional molecular targets for repellants, Chris Potter at Johns Hopkins University in Baltimore, Maryland, is working to map the mosquito’s neural circuits. “We don’t really know what repellants are doing to the mosquito nose and brain that tells the mosquito to stay away,” he says. Last year, Potter’s group introduced a new technique for expressing genes of interest in specific mosquito cells (8); he’s now using it to make fluorescent calcium indicators flash when a neuron gets excited. By identifying the neurons that respond to different repellants, and studying the receptors they express, Potter hopes he can find or design more chemicals to foil mosquitoes.
“It’s an exciting time,” says Potter. “To figure out what’s going on inside the mosquito’s mind to allow it to navigate to a human host—that’s something that 10 years ago was not even possible. Now with the ability to start readily manipulating the genome, we actually have the potential to make inroads.”
References
- 1.McBride CS, et al. Evolution of mosquito preference for humans linked to an odorant receptor. Nature. 2014;515(7526):222–227. doi: 10.1038/nature13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Breugel F, Riffell J, Fairhall A, Dickinson MH. Mosquitoes use vision to associate odor plumes with thermal targets. Curr Biol. 2015;25(16):2123–2129. doi: 10.1016/j.cub.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeGennaro M, et al. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 2013;498(7455):487–491. doi: 10.1038/nature12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell. 2014;156(5):1060–1071. doi: 10.1016/j.cell.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinker DC, et al. Novel high-throughput screens of Anopheles gambiae odorant receptors reveal candidate behavior-modifying chemicals for mosquitoes. Physiol Entomol. 2012;37:33–41. doi: 10.1111/j.1365-3032.2011.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones PL, Pask GM, Rinker DC, Zwiebel LJ. Functional agonism of insect odorant receptor ion channels. Proc Natl Acad Sci USA. 2011;108(21):8821–8825. doi: 10.1073/pnas.1102425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor RW, et al. Structure-activity relationship of a broad-spectrum insect odorant receptor agonist. ACS Chem Biol. 2012;7(10):1647–1652. doi: 10.1021/cb300331z. [DOI] [PubMed] [Google Scholar]
- 8.Riabinina O, et al. Organization of olfactory centres in the malaria mosquito Anopheles gambiae. Nat Commun. 2016;7:13010. doi: 10.1038/ncomms13010. [DOI] [PMC free article] [PubMed] [Google Scholar]


