Abstract
Amyloid precursor protein (APP) is evolutionary conserved protein expressed in endothelial cells of cerebral and peripheral arteries. In this review, we discuss mechanisms responsible for expression and proteolytic cleavage of APP in endothelial cells. We focus on physiological and pathological implications of APP expression in vascular endothelium.
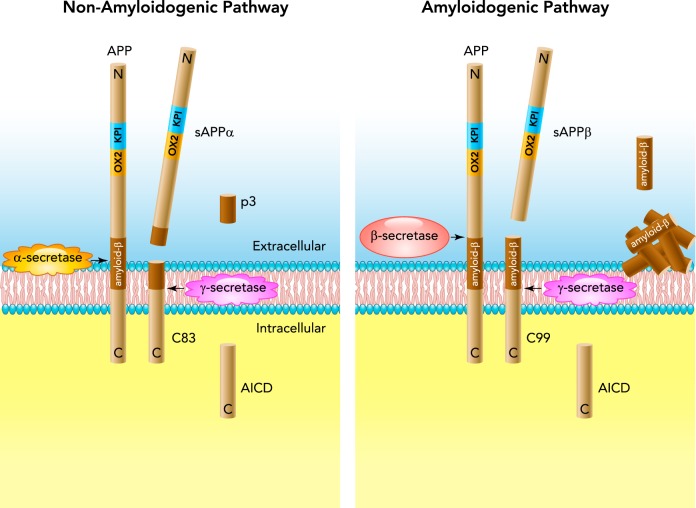
Amyloid precursor protein (APP) together with amyloid precursor-like proteins (APLPs) APLP1 and APLP2 constitute the family of transmembrane glycoproteins highly expressed in the human brain, kidney, and platelets (64, 87, 110). APP is the only member of the family encoding amyloid-β (Aβ) peptides, which are considered major culprits in pathogenesis of Alzheimer's disease (AD) (73, 159, 162). The biological properties of APP have been the subject of intense investigation for almost 30 years (65, 87, 153, 172). Strong conservation of APP during evolution from invertebrates to humans implies that it serves an important cellular function(s) (128). Relevant to this review, APP is expressed in vascular endothelium of cerebral, coronary, and peripheral blood vessels (6, 42, 64, 94). In addition, expression and activity of enzymes responsible for proteolytic cleavage of APP (FIGURE 1) has been detected in endothelial cells of large conduit arteries, resistance arteries, and microvessels. However, vascular function of APP and its cleavage products is incompletely understood.
FIGURE 1.
Schematic diagram of non-amyloidogenic and amyloidogenic processing of APP770 in endothelial cells
Left: in the non-amyloidogenic pathway, APP770 is cleaved by α-secretase, yielding secretion of sAPPα ectodomain into the lumen of blood vessel wall and release of carboxyl-terminal fragment C83. C83 is further cleaved by γ-secretase within the transmembrane domain, yielding p3 and APP intracellular domain (AICD) peptide. Right: during amyloidogenic processing β-secretase cleaves APP770 at the NH2 terminus of the amyloid-β (Aβ) domain, thus generating sAPPβ and carboxyl-terminal fragment C99. The latter is cleaved by γ-secretase, thus producing Aβ peptides and AICD. Please note that APP770 is a full-length protein containing Kunitz-type serine protease inhibitor (KPI) domain in tandem with an OX-2 antigen (OX2) domain in its extracellular region.
APP in Endothelial Cells
Three isoforms of APPs generated by alternative splicing of exons 7 and 8 have been identified: APP695, APP751, and APP770 (number refers to the number of amino acids). APP695 is predominantly expressed in neurons and is lacking two exons (87). APP751 and APP770 possess Kunitz-type serine protease inhibitor (KPI) domain encoded by exon 7, whereas APP770 contains an additional immunoregulatory OX-2 antigen domain (OX2) encoded by exon 8 in their extracellular region and are expressed in brain and peripheral tissues (64, 147, 173). Endothelial cells express APP751 and APP770 (61, 94). Intriguingly, expression of these isoforms is higher in endothelial cells of cerebral blood vessels compared with peripheral arteries (94). Most importantly, in endothelial cells, APP could be processed by non-amyloidogenic and amyloidogenic pathway (FIGURE 1) (7, 61, 74, 94, 95, 199).
Soluble APPα
Under physiological conditions, APP is predominantly cleaved at the cell surface by α-secretase via non-amyloidogenic processing, thereby resulting in the secretion of soluble APPα (sAPPα) ectodomain into the lumen of blood vessel wall and abluminal release of carboxyl-terminal fragment C83 (95) (FIGURE 1; d'Uscio LV, Katusic ZS, unpublished observations). Within the transmembrane domain, C83 is further cleaved by the γ-secretase complex consisting of at least four proteins [presenilin-1 (PS1), nicastrin, anterior pharynx-defective 1, and presenilin enhancer 2], yielding an APP intracellular domain (AICD) peptide and a 3-kDa carboxyl-terminal fragment known as p3 (FIGURE 1). Thus α-secretase cleaves APP within the Aβ sequence, thereby preventing generation of Aβ (58). Several isoforms of a disintegrin and metalloproteinase (ADAM) are responsible for α-secretase activity: ADAM9, ADAM10, and ADAM17. In human cerebrovascular endothelium, ADAM10 (but not ADAM9 or ADAM17) appears to be the most relevant constitutively active α-secretase, even though all three isoforms are expressed in endothelial cells (78, 100).
It has been suggested that sAPPα possesses physiological functions in neuronal cells such as neuroprotection and synaptogenesis (96). The exact receptor(s) activated by sAPPα are not known, but existing evidence suggests that APP family members as well as putative cell surface receptors such as insulin-like growth factor 1 and/or insulin receptors may interact with sAPPα (67, 86, 121). Furthermore, sAPPα can induce production of cyclic guanosine 3′,5′-monophosphate (cGMP) in neuronal cells (10, 84); however, the exact signaling pathway responsible for this effect is unknown. Inadequate sAPPα levels can lead to increased amyloidogenic processing of APP, thus increasing susceptibility to oxidative stress in AD (86, 98, 132, 135). Indeed, overexpression of sAPPα protects the cells from excessive amyloidogenic processing of APP and accumulation of cytotoxic Aβ peptides (132). To our knowledge, there is no information available in current literature regarding the effects of sAPPα in endothelial cells.
Soluble APPβ
β-Site APP-cleaving enzyme 1 (BACE1 or β-secretase) is widely expressed in neuronal and nonneuronal cells, including endothelial cells (7, 52). During amyloidogenic APP processing, the BACE1 cleaves APP at the NH2 terminus of the Aβ domain, thus generating soluble APPβ (sAPPβ) and carboxyl-terminal fragment C99 (129) (FIGURE 1). The latter is further cleaved by the γ-secretase generating Aβ peptides, most commonly Aβ1-40 and Aβ1–42, and APP intracellular domain AICD (93, 115). Under ischemic conditions, increase in cholesterol levels in the lipid membrane enhances enzymatic activity of BACE1 in brain endothelial cells (16, 17, 107). Although sAPPβ shares the same sequence as sAPPα, apart from the last 16 COOH-terminal amino acids, its neuroprotective effect is much less potent (27). In some studies, however, it has been reported that sAPPβ exerts a pro-apoptotic effect in neuronal cells (129). The functional relevance of sAPPβ production by vascular endothelium is unknown.
Aβ
Aβ is a short 4-KDa hydrophobic peptide consisting of 36–43 amino acids, and it is generated by amyloidogenic processing of APP. The main species of Aβ released from endothelial cells and platelets is Aβ1–40, which contributes to formation of vascular Aβ deposits, whereas the predominant form in neuronal plaques is Aβ1–42 (79, 154, 165). Moreover, elevated local concentrations of Aβ1–40 and Aβ1–42 are reported to be similarly neurotoxic, but they clearly differ in their toxicity to endothelial cells. Aβ1–40 was found to be less toxic to human aortic endothelial cells compared with Aβ1–42 or Aβ25–35 (44, 169).
AICD
AICD is generated by both non-amyloidogenic and amyloidogenic processing of APP (FIGURE 1) and plays an important role as a transcriptional regulator (136, 163). During evolution, this small fragment (6.5 kDa) was highly conserved from fly to human, suggesting a significant role in physiology and/or preservation of homeostasis under pathological conditions (11). In neurons, AICD is very labile and has a rapid turnover, with a half-life of ∼5–30 min (39, 92). However, AICD can be stabilized through binding to several adaptor proteins, e.g., Fe65, and subsequently translocate to the nucleus and modulate gene expression (127). Whether AICD serves an important physiological function in endothelial cells remains to be determined in future studies.
p3
The p3 peptide produced exclusively during non-amyloidogenic processing of APP corresponds to residues 17–42 at the carboxyl end of Aβ. There is no evidence for the physiological vascular function of p3. Of note, studies in zebrafish indicate that p3 does not contribute to vascular defects detected in APP-deficient embryos (113).
Functions of APP in the Cardiovascular System
APP as Coagulation Inhibitor
Several reports have shown that APP is abundantly expressed in platelets and that it is secreted in the circulation upon platelet activation, thus contributing to ∼90% of circulating sAPPα/sAPPβ (19, 36, 62, 95, 110, 183). The predominant forms of APP in platelets are APP751 and APP770, whereas APP695, which is the most abundant in neuronal tissue, is nearly undetectable in platelets (19, 62, 95, 111). The KPI domain present in both APP751 and APP770 isoforms has been reported to be an inhibitor of activated coagulation enzymes such as platelet coagulation factors IXa and XIa, which are known to be involved in prothrombin activation (158, 166). Upon platelet activation, sAPP is secreted from α-granules and inhibits coagulation factors, thus preventing thrombosis in cerebral, coronary, and peripheral arteries (105, 191, 193). Indeed, studies in APP-deficient mice revealed that thrombosis was accelerated by genetic inactivation of APP (193). Of note, activation of protein kinase C stimulates sAPPα release by platelets (164). Thus sAPPα released from platelets may function in both cerebral and peripheral vascular system as an anticoagulant and hence makes an important contribution to the regulation of hemostasis (35, 105, 164, 165). Production and release of sAPPα from endothelium also contributes to protection against coagulation (see below) (31, 78, 157).
APP in Angiogenesis
Angiogenesis is dependent on proliferation, migration, growth, and differentiation of endothelial cells. APP is highly expressed in the endothelium during fetal life, suggesting an important role for APP and/or its metabolites in early angiogenesis (134). The residues 1–16 of Aβ are essential for vascular activity of Aβ because impaired angiogenesis observed in APP-deficient zebrafish could be restored by Aβ treatment (113). Additionally, angiogenesis in the brain is reduced by β- or γ-secretase inhibitors (138). Several studies reported that low nanomolar concentrations of either Aβ1–40 or Aβ1–42 promote angiogenesis by increase of growth, migration, and tube branching in cultured cerebral and peripheral endothelial cells (15, 22, 23). In contrast, supraphysiological concentrations (micromolar) of Aβ1–40 and Aβ1–42 impair angiogenesis and accelerate senescence of endothelial cells in vitro (54, 77, 117, 145).
It is well established that vascular endothelial growth factor (VEGF) plays a fundamental role in normal and abnormal angiogenesis (60). Indeed, increased expression of VEGF-A and increased cerebral microvessel density have been detected in the brain of different APP transgenic mouse models overexpressing mutated APP and in brains of AD patients (13, 32, 176). Increased local concentration of Aβ appears to contribute to increased angiogenesis in murine models of AD and in patients suffering from the disease. In contrast, angiotensin II-induced hypertension in APP/PS1 transgenic mice overexpressing mutated human APP and PS1 proteins further increased cerebral vascular Aβ deposits accompanied by a reduction in cerebral microvessel density and a decrease in VEGF-A expression in the brain (32). Moreover, cell culture studies reveal that Aβ also inhibits VEGF-induced tyrosine phosphorylation of VEGF receptor 2 as well as VEGF-stimulated phosphorylations of Akt and endothelial nitric oxide (NO) synthase (eNOS) in endothelial cells, indicating that high concentrations of Aβ act as a VEGF antagonist (32, 77, 145).
Role of NO and Prostacyclin in Expression and Processing of Endothelial APP
Role of NO
There are only few studies regarding regulation of APP processing in endothelial cells (summarized in Table 1). Recently, it was recognized that, in human brain microvessel endothelial cells, a complete loss of endothelial NO increases protein expressions of APP and BACE1 as well as production and release of Aβ peptides (7). These observations were confirmed in cerebral microvessels of young eNOS-deficient mice (7) as well as in cultured neurons (103). Moreover, NO-mediated suppression of APP and BACE1 protein expression is mediated by the cGMP signaling pathway (7). Supplementation of NO increases cGMP levels and in turn attenuates increased expression of APP and BACE1 in cerebral microvessels of eNOS-deficient mice, suggesting that the NO/cGMP pathway plays an important role in modulating expression and processing of APP (8). Importantly, cerebral blood flow is unchanged in eNOS-deficient mice (4), indicating that changes in NO rather than alteration in blood flow is responsible for increased expression and amyloidogenic processing of APP. Consistent with this conclusion, Aβ level is also increased in cerebral arteries and arterioles in aged heterozygous eNOS-deficient mice (171). These observations may help to explain the contribution of endothelial dysfunction caused by the loss of endothelial NO production to development of AD pathology, including cerebral amyloid angiopathy (9, 171).
Table 1.
Modulators of APP processing in endothelial cells
| APP Processing Enzymes and Products |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effectors/Stimuli | Cell Types | APP | Aβ | Mixed sAPPα/β | sAPPα | sAPPβ | α-secretase | β-secretase | Molecular Mechanisms | References |
| Nitric oxide | HBMEC | ↓ | ↓ | ↓ | eNOS/cGMP activation | 7, 8 | ||||
| Iloprost | HBMEC | ↑ | ↔ | ↑ | ↑ | ↔ | Via cAMP and PPARδ activation | 78 | ||
| Cicaprost, forskolin | HBMEC | ↑ | ↑ | cAMP activation | 78 | |||||
| GW501516 | HBMEC | ↔ | ↑ | ↑ | ↔ | SIRT1 activation | 78 | |||
| Thrombin | HUVEC | ↑ | PKC activation | 31 | ||||||
| Interleukin-1 | HBMEC, MBEC, HUVEC | ↑ | ↑ | Increased APP mRNA via PKC activation | 20, 61, 66, 95 | |||||
| Glucose | HUVEC | ↑ | ↑ | ? | 26 | |||||
| TNF-α | MBEC | ↓ (intra-cellular) | Decreased mRNA and protein synthesis of cellular prion protein | 196 | ||||||
| Heat shock | HUVEC | ↑ | Increased mRNA and protein synthesis | 30 | ||||||
| Oxygen-glucose deprivation | RBMEC | ↑ | ↑ | ↑ | Via hypoxia inducible factor-1 | 16, 17 | ||||
| Hypoxia | MBEC | ↑ | ↑ | ↑ | ↑↑ | Increased protein synthesis of APP | 126 | |||
HBMEC, human brain microvessel endothelial cells; HUVEC, human umbilical vein endothelial cells; MBEC, mouse brain endothelial cell line; RBMEC, rat brain microvessel endothelial cells; GW501516, peroxisome proliferator-activated receptors δ (PPARδ) agonist; eNOS, endothelial nitric oxide synthase; cGMP, cyclic guanosine 3′,5′-monophosphate; cAMP, cyclic adenosine 3′,5′-monophosphate; SIRT1, sirtuin 1; PKC, protein kinase C; TNF-α, tumor necrosis factor-α; ↑, upregulation; ↓, downregulation; ↔, no effect.
Blank fields in this table represent that this parameter was not tested. No data are available regarding the effects of effectors/stimuli on APP-processing products AICD and p3 in endothelial cells.
Given the anti-inflammatory effect of NO under physiological conditions (46), decreased bioavailability of NO can increase local concentration of inflammatory factors and thrombin, which are present in eNOS-deficient mice brains and in microvessels isolated from AD patient brain cortexes (9, 68, 70, 171). Thrombin and interleukin-1 can, in turn, further exacerbate expression of APP in endothelial cells via activation of protein kinase C (31, 66) (Table 1). Moreover, tumor necrosis factor-α decreases expression of cellular prion protein in brain microvessel endothelial cells and thus reduces Aβ clearance from the brain (196).
Role of Prostacyclin
Endothelium-derived prostacyclin (PGI2) is a potent vasodilator and anticoagulant and plays an essential role in preservation of cerebrovascular homeostasis under pathological conditions (59, 124, 160, 186). The actions of PGI2 are mediated by activation of PGI2 receptor (IP receptor) as well as peroxisome proliferator-activated receptor δ (PPARδ). A recent study shows that activation of IP receptor and cyclic adenosine 3′,5′-monophosphate (cAMP) signaling pathway by PGI2 stimulates expression of APP in brain microvessel endothelial cells (78) (Table 1). Remarkably, PGI2 increases expression and activity of non-amyloidogenic processing enzyme α-secretase ADAM10 and secretion of sAPPα. The amyloidogenic processing pathway is unaffected by PGI2 in brain microvessel endothelial cells (Table 1). Furthermore, deletion of PPARδ abolished the iloprost-induced ADAM10 activation and release of sAPPα in brain microvessel endothelial cells, thus demonstrating that PGI2-induced generation of sAPPα is also dependent on activation of PPARδ (78).
Collectively, cell culture and knockout mice studies indicate that both NO and PGI2 possess differential roles in expression and processing of APP in cerebral arteries. NO suppresses amyloidogenic processing of APP, whereas activation of PGI2 stimulates non-amyloidogenic processing of APP. It appears that both NO and PGI2 exert vasoprotective effects on APP metabolism by minimizing production of Aβ peptides and by promoting generation of sAPPα, respectively. Of note, enhanced production of PGI2 in eNOS-deficient mice is considered a compensatory mechanism designed to maintain normal vascular function during NO deficiency (146, 168). Consistent with this concept, it appears likely that higher concentrations of PGI2 promote non-amyloidogenic processing of APP, thereby antagonizing excessive production of Aβ generated by elevated activity of β-secretase in eNOS-deficient mice.
APP Processing and Endothelial Dysfunction
Effects of Overexpression of Wild-Type APP
Increased APP expression and Aβ production in neuronal cells has been shown to be induced by high levels of stress hormones such as catecholamines, glucocorticoids, and central administration of angiotensin II (71, 106, 200). In endothelial cells, high glucose increases expression and amyloidogenic processing of APP (26). In addition, cell surface localization of APP is increased during ischemia, cellular stress, or inflammation in endothelial cells, indicating that increased APP expression may serve as a homeostatic response to stress (17, 30, 125, 126) (Table 1). It is interesting to note that shear stress induced by unidirectional pulsatile laminar flow can increase transcription of APP in human vascular endothelial cells, further supporting the idea that APP is a stress response protein (51). In addition, overexpression of wild-type APP695 in neuronal tissue exerts a neuroprotective effect during ischemic injury induced by middle cerebral artery occlusion (34). In contrast, APP knockout mice experience high mortality in response to cerebral ischemia, suggesting that loss of APP or APP cleavage fragments increases vulnerability to ischemia (97). The exact mechanisms underlying protective function of APP are incompletely understood and remain to be determined.
Effects of Overexpression of Mutated APP
A number of transgenic mouse lines overexpressing APP have been developed to study the pathogenesis of AD and associated endothelial function. For example, transgenic mice overexpressing double Swedish mutation of human APP at K670N/M671L (Tg2576) display elevated Aβ levels both in brain and in plasma (42, 89). An increased oxidative stress has been observed in these young mice, thereby causing endothelial dysfunction and reduced bioavailability of NO in cerebral and peripheral arteries (42, 139–141, 156). This effect is mediated by the increased production of superoxide anion via the activation of NADPH oxidase (42, 75, 140). Furthermore, scavenger receptor CD36, an endothelial membrane glycoprotein that binds Aβ, is required for the oxidative stress induced by Aβ (120, 141). Indeed, genetic deletion of CD36 rescues endothelial function from oxidative stress observed in Tg2576 mice cerebral arteries, demonstrating that CD36 inactivation is vasoprotective (141).
Intracarotid administration of Aβ1–40 (but not Aβ1–42) inhibits the increase in cerebral blood flow caused by endothelium-dependent vasodilators in wild-type mice, indicating that elevated Aβ1–40 levels alone are sufficient to impair endothelial function observed in Tg2576 (130, 141, 142). On the other hand, endothelial dysfunction is less pronounced in transgenic mice expressing human neuronal APP harboring the Swedish K670N/M671L and Dutch/Iowa E693Q/D694N mutations (Tg-SwDI) that display elevated Aβ levels in brain but not in plasma (45). Interestingly, administration of exogenous Aβ1–40 further worsens endothelial dysfunction in Tg-SwDI but not in Tg2576 mice (which already have high circulating Aβ1–40 levels), again suggesting that circulating Aβ1–40 levels contribute to the alterations of endothelial function in cerebral arteries (142).
Increased formation of peroxynitrite, which is generated from the reaction of superoxide anion with NO, can easily oxidize tetrahydrobiopterin, an essential cofactor for enzymatic activity of eNOS (122). The consequences of high concentration of peroxynitrite are decreased levels of tetrahydrobiopterin in cerebral and peripheral arteries and consequent uncoupling of eNOS increase eNOS-derived superoxide anion production leading to impairment of endothelium-dependent relaxations (41, 42, 139, 155, 156, 181). In vivo treatment with tetrahydrobiopterin prevents eNOS uncoupling and decreases superoxide anion concentration in cerebral microvessels of Tg2576 mice (156), thereby suggesting that uncoupling of eNOS contributes to oxidative stress induced by Aβ peptides.
Effects of Exogenous Aβ on Cultured Endothelial Cells
Aβ possess both pro-oxidant and antioxidant properties (5). Aβ generation at lower physiological concentrations (∼0.1 nM) has been shown to exhibit antioxidant properties by prevention of autoxidation of lipoproteins in CSF and low-density lipoprotein in plasma (99). Aβ1–40 possesses the highest antioxidant capacity compared with other Aβ isoforms. Neuroprotective properties at low nanomolar concentrations have been described in prior studies (5, 99, 188).
In vitro studies in endothelial cell culture employed supraphysiological concentrations of Aβ (micromolar range; summarized in Table 2). For example, long-term exposure to high Aβ concentrations promotes Ca2+ influx into endothelial cells, thereby causing injury and apoptosis (194). Furthermore, long-term Aβ treatment can increase generation of reactive oxygen species, thus causing oxidative modifications of proteins and lipid membranes, leading to reduced endothelial function (Table 2). Aβ peptides can also cause expression of inflammatory genes and proteins (68, 184), which in turn can increase thrombin expression in cerebral endothelial cells (69, 198). Interestingly, one study shows that short-term treatment with low concentration of Aβ1–40 stimulated production of kinins and cGMP in endothelial cells (190) (Table 2). There is evidence that Aβ interacts with the receptor for advanced glycation end products (RAGE) that critically regulates Aβ transport into brain vascular endothelial cells and can trigger intracellular signaling (24, 25, 109). Indeed, low concentration of Aβ increases intracellular production of hydrogen peroxide (25), which is known to activate soluble guanylate cyclase and production of cGMP in endothelial cells (18, 43, 91).
Table 2.
Effects of Aβ on cultured endothelial cells
| Cell Types | Aβ Type | Pre-Aggregated Aβ Used | Concentration Range Tested | Incubation Time | Minimum Concentration Required | Molecular Mechanisms of Aβ | References |
|---|---|---|---|---|---|---|---|
| HBMEC | Aβ1–42 | Yes | 0.0125–1.25 μM | 1 h | 0.125 μM | Upregulation of C-C chemokine receptor type 5 via JNK, ERK, PI3K activation through RAGE | 109 |
| HBMEC | Aβ1–42 | Yes | 0.001–10 μM | 24 h | 0.01 μM | Increased production of hydrogen peroxide through RAGE | 25 |
| HBEC | Aβ1–40 | Yes | 1–20 μM | 2, 4, 8, 12 h | 5 μM | Increase in inflammatory markers MCP-1, IL-8, IL-6, GRO via AP-1 transcription | 184 |
| MCEC | Aβ25–35 | Yes | 2.5–40 μM | 24 h | 20 μM | Decreased heat shock protein 90, p-Akt, telomerase reverse transcriptase, cyclin-dependent kinase 4 | 29 |
| Aβ1–40 | |||||||
| MCEC | Aβ25–35 | — | 25 μM | 24 h | 25 μM | Activation of AP-1/pro-apoptotic protein Bim and Smac release | 197 |
| MCEC | Aβ25–35 | — | 0.01–50 μM | 24 h | 1 μM | Apoptosis via activation of caspase-8/caspase-3, mitochondrial dysfunction | 194 |
| MCEC | Aβ25–35 | Yes | 20 μM | 24 h | 20 μM | Activation of apoptosis signal-regulating kinase 1 leading Bax increase via phosphorylations of p53 and p38-MAPK | 80 |
| Rat brain EC | Aβ25–35 | — | 47–380 μM | 72 h | 188 μM | Cell toxicity via increased LDH release and decreased glucose consumption | 149 |
| Mouse brain EC line | Aβ1–40 | — | 0.3 μM | 30 min | 0.3 μM | PARP activation via oxidative-nitrosative stress, increase in intracellular Ca2+ | 144 |
| BAEC | Aβ1–42 | No | 0.05–2.2 μM | 1–5 min | 0.22 μM | Increased Ca2+-influx | 12 |
| BAEC, RMCEC | Aβ1–40 | — | 0.1–1 μM | 30 min | 0.1 μM | Stimulation of cGMP production, release of kinins | 190 |
| BAEC | Aβ25–35 | — | 1 μM | 24 h | 1 μM | Impaired activation of K+-channels and reduced nitrite production | 28 |
| BAEC | Aβ25–35 | — | 0.1–1000 nM | 24 h | 1 nM | Reduced nitrite production | 150 |
| Aβ1–40 | — | 0.1–1000 nM | 24 h | 10 nM | |||
| BAEC | Aβ25–35 | Yes | 1 μM | 24 h | 1 μM | Production of reactive oxygen species, blockade of agonist-stimulated eNOS phosphorylation at Ser1179, decreased nitric oxide production | 104 |
| Aβ1–42 | 5 μM | 24 h | 5 μM | ||||
| HAEC | Aβ25–35 | — | Up to 40 μM | 30 min to 24 h | 5 μM | Apoptosis, necrosis via reactive oxygen species, increase in intracellular Ca2+ | 169 |
| Aβ1–42 | Up to 32 μM | 4 μM | |||||
| Porcine PAEC | Aβ25–35 | Yes | 1–50 μM | 18 h | 20 μM | Apoptosis, increased free radicals production, dysregulation of Ca2+ homeostasis, impaired glucose uptake | 14 |
| 24 h | 5 μM |
EC, endothelial cells; HBMEC, human brain microvessel endothelial cells; HBEC, human brain endothelial cells; MCEC, mouse cerebral endothelial cells; BAEC, bovine aortic endothelial cells; RMCEC, rat microvascular coronary endothelial cells; HAEC, human aortic endothelial cells; PAEC, pulmonary aortic endothelial cells; Aβ, amyloid-β; RAGE, receptor for advanced glycation end products; Akt, protein kinase B; MAPK, mitogen-activated protein kinase; AP-1, activator protein 1; JNK, c-Jun NH2-terminal kinase; ERK, extracellular signal-regulated kinase; PI3K, phosphoinositide 3-kinases; MCP-1, monocyte chemoattractant protein-1; IL-6, interleukin-6; IL-8, interleukin-8; GRO, growth-regulated oncogene; LDH, lactate dehydrogenase; eNOS, endothelial nitric oxide synthase; cGMP, cyclic guanosine 3′,5′-monophosphate; -, unknown or not tested.
In brain endothelial cells, mitochondria are very important sensors of oxidative stress because they are the primary site of reactive oxygen species formation (133). In vitro experiments indicate that stimulation of brain endothelial cells with Aβ peptide causes nitration of the manganese form of superoxide dismutase and mitochondrial dysfunction, resulting in the loss of their ability to support their antioxidant function (2, 3, 55, 194) (Table 2).
Aβ can exist as monomer, dimer, oligomer, protofibril, and/or fibrillar aggregates. However, mouse Aβ is less aggregable and toxic than human Aβ (171). It is important to note that low concentrations of Aβ do not form oligomer or fibrils, and freshly prepared Aβ also contains only a very small percentage of short fibrils (12, 195). In contrast, elevated Aβ concentrations in solutions (≥1 μM) can easily form oligomers and fibrils that have been shown to exert cytotoxicity in vitro and in vivo (44, 82, 83, 194, 197) (Table 2). In addition, both Aβ peptides Aβ1–40 and Aβ1–42 can form dimmers or octamers after 24 h of incubation at 37°C (90). In summary, the vascular effects of Aβ are dependent on type of Aβ peptide, aggregation status, incubation time, and concentration of Aβ (Table 2).
Effects of Exogenous Aβ on Isolated Arteries
In vitro vasomotor studies regarding direct effects of Aβ in isolated cerebral and peripheral arteries are summarized in Table 3. Short-term incubation of arteries with high concentrations (micromolar) of Aβ peptides increases the vasoconstrictions to phenylephrine, serotonin, and endothelin-1 (38, 72, 137, 177). The molecular mechanisms responsible for these observations include increased production of prostaglandins via activation of cyclooxygenase (COX-2) as well as increased production of reactive oxygen species. Furthermore, in most studies, incubation of cerebral and peripheral arteries with soluble Aβ1–40 impairs endothelium-dependent relaxations. Increased reactive oxygen species and decreased eNOS activity are most likely explanations for endothelial dysfunction since free-radical scavenger superoxide dismutase is able to normalize Aβ-induced endothelial dysfunction (53, 130, 177, 179) (Table 3). In contrast, one study was unable to demonstrate increased production of reactive oxygen species induced by Aβ1–40, suggesting that oxidative stress is not involved in endothelial dysfunction induced by Aβ1–40 (63). The reason for the discrepancy may be due to the different Aβ preparations. The limitations of short-term in vitro studies (Table 3) are that the concentrations of exogenous Aβ are much higher than circulating Aβ levels reported in transgenic mice overexpressing mutated human APP or in patients with AD.
Table 3.
Effects of Aβ on isolated blood vessels
| Blood Vessel Types | Aβ Types | Concentration Range Studied | Incubation Time | Minimal Concentration Required | Cellular and/or Molecular Mechanisms of Aβ | References |
|---|---|---|---|---|---|---|
| Rat cerebral artery | Aβ1–40 | 0.0001–1 μM | 15 min | 0.1 μM | Impaired endothelium-dependent relaxation to ACh | 150 |
| Aβ25–35 | 0.0001–1 μM | 15 min | 0.01 μM | |||
| Mouse cerebral artery | Aβ1–40 | 0.01–10 μM | 30 min | 1 μM | Decreased cerebral blood flow to ACh | 130, 131 |
| Aβ1–42 | 0.01–10 μM | 30 min | n/a | No change | ||
| Rat basilar artery | Aβ1–40 | 1 μM | 30 min | 1 μM | Impaired endothelium-dependent relaxation to substance P | 63 |
| Rat penetrating cerebral arterioles | Aβ1–40 | 0.001–1 μM | — | 0.1 μM | Reduction of tone diameter by increased contraction; decreased endothelium-dependent relaxation to ATP | 53 |
| Aβ1–42 | ||||||
| Human middle cerebral artery | Aβ1–40 | 1 μM | 5 min | 1 μM | Increased contractions to endothelin-1 via COX-2 and p38 mitogen-activated protein kinase activation | 137 |
| Human middle cerebral artery | Aβ1–40 | 2 μM | 2–6 h | 2 μM | Increased production of PGF2α and PGE2 | 137 |
| Bovine middle cerebral artery | Aβ1–40 | 1 μM | 15 min | 1 μM | Impaired endothelium-dependent relaxation to bradykinin. | 179 |
| Rat coronary artery | Aβ1–40 | 1 μM | 15 min | 1 μM | Increased production of reactive oxygen species; impaired endothelium-dependent relaxation to ACh | 178 |
| Mouse aorta | Aβ25–35 | 1 μM | 24 h | 1 μM | Increased vasoconstriction to serotonin; activation of α1-adrenergic receptor | 72 |
| Rat aorta | Aβ1–40, Aβ1–42 | 1 μM | 10 min | 1 μM | Increased contractions to endothelin-1 | 38 |
| Rat aorta | Mixed (?) Aβ1–39, Aβ1–40, Aβ1–42 | 1 μM | 15 min | 1 μM | Increased production of reactive oxygen species; impaired endothelium-dependent relaxation to ACh; increased contractions to phenylephrine | 177 |
| Rat aorta | Aβ1–40 | 1 μM | 30 min | 1 μM | Impaired endothelium-dependent relaxation to ACh, decreased eNOS phosphorylation at Ser1177; decreased eNOS activity; increased PKC phosphorylation at Ser660 | 63 |
Aβ, amyloid-β; ACh, acetylcholine; PKC, protein kinase C; COX-2, cycloogygenase-2, PGF2α, prostaglandin F2α; PGE2, prostaglandin E2.
Clearance of Aβ
In healthy humans, the average circulating levels of Aβ are in the picomolar range (85, 154). Moreover, the concentrations of Aβ are about 10-fold higher in cerebrospinal fluid (CSF) than in mice and human plasma (85, 89). Circulating Aβ levels decline in APP transgenic mice as well as in control individuals with aging and AD. This phenomenon is caused by increased Aβ deposition in the brain (85, 89). Therefore, the highest circulating concentration of Aβ measured in AD patient is ∼50 nM (101, 102).
It is important to note that steady-state levels of Aβ peptides are not only regulated by APP processing but also by the clearance of Aβ through the blood-brain barrier (BBB) (89, 118, 151, 161). The BBB is mainly formed by the cerebral vascular endothelial cells that line the capillaries of the brain. The unique phenotype of the BBB is characterized by intercellular tight junctions, adherens junctions, and numerous membrane receptors and transporters allowing trade of substances between systemic circulation and extracellular fluid compartment of the brain (57). Aβ peptide is cleared from the brain by receptor-mediated transport across the endothelium, by enzymatic degradation, and by periarterial drainage along vascular basement membranes (50, 76, 185). Three BBB-related transport proteins for Aβ peptide have been identified: lipoprotein receptor protein-1 (LRP-1), P-glycoprotein (P-gp), and RAGE (33, 49, 195). Reduction of endothelial expression of either LRP-1 or P-gp in BBB is associated with impaired clearance of Aβ peptide across the BBB into the circulation (33, 161). Under inflammatory conditions, antioxidant treatment preserves LRP-1 function and Aβ efflux, suggesting that LRP-1 can operate independently of P-gp in BBB (56). On the other hand, RAGE binds and transports circulating Aβ peptide toxins across the BBB into the brain (49). Chronic vascular insult, such as hypertension and/or inflammation, can activate RAGE in brain vascular endothelial cells, favoring Aβ deposition (24, 109). Moreover, there is recent evidence that Aβ peptide is also catabolized in peripheral tissues and organs such as liver, kidney, gastrointestinal tract, and skin (192), suggesting that brain-derived Aβ peptide is physiologically cleared through the capillary network of these organs.
Implications for Atherosclerosis and Cerebral Amyloid Angiopathy
APP and Atherosclerosis
Atherosclerosis is considered a chronic inflammatory disease, which can be triggered by cardiovascular risk factors such as hypercholesterolemia, aging, and hypertension (112). Endothelium-derived vasoactive factors play an important regulatory role in vascular homeostasis due to the strategic position of the endothelium between the vascular smooth muscle cells (VSMC) and the circulating blood (40, 114). Several studies have demonstrated that APP and Aβ are increased in apolipoprotein E (ApoE)-deficient mice atherosclerotic aortas, in advanced human carotid artery plaques, and in plasma of patients with coronary heart disease (6, 48, 167). The exact cause for increased amyloidogenic processing of APP during progression of atherosclerosis is unknown; however, inflammatory cytokines such as interleukin-1 have been reported to stimulate APP expression in endothelial cells (30, 61, 66). Overexpression of mutated human APP stimulates development of fatty streak lesions (early atherosclerosis) and endothelial dysfunction in the aorta of Tg2576 mice (42, 108). The presence of APP in endothelium is also important for monocytes adhesion to endothelial cells (6). Consistent with these observations, overexpression of mutated human APP accelerated aortic atherosclerotic development in ApoE-deficient mice (180). Conversely, genetic deletion of the APP gene attenuated atherogenesis in ApoE-deficient mice without affecting cholesterol levels (182). However, the major limitation of the study in double APP/ApoE knockout mice is that only histological studies were performed, and plaque formation was reduced only in distal thoracic and abdominal aorta but not in the aortic valves, root, and arch (182). Furthermore, existing literature suggests that atherosclerosis develops much slower in intracranial arteries compared with peripheral arteries (152). A number of reasons could be responsible for vascular heterogeneity in atherosclerotic pathology (reviewed in Ref. 152). In aggregate, these studies suggest that excessive amyloidogenic progressing of APP is pro-atherosclerotic. However, more precise analysis employing endothelial-specific overexpression or deletion of APP is required to define the exact role of endothelial APP in pathogenesis of atherosclerosis.
APP and Cerebral Amyloid Angiopathy
Cerebral amyloid angiopathy (CAA) is manifested by increased deposition of Aβ peptides in the wall of cerebral arteries, and it is a common pathological feature of AD. Indeed, abnormally high local levels of Aβ pep tide can damage the cerebral arteries by deposition and aggregation in the vasculature, thus leading to vascular disintegration and micro-hemorrhage (47, 81, 148, 170). Existing evidence suggests that neuronal cells are a dominant source of Aβ (21, 45); however, endothelial cells and VSMC are also able to generate Aβ (37, 52). In this regard, it is important to note that BACE-1 is predominantly localized on the abluminal membrane of brain endothelial cells, thereby suggesting that Aβ generation in endothelium may considerably contribute to amyloid formation in the blood vessel wall (52). As already mentioned, VSMC also expresses APP protein and all required enzymes (α-, β-, and γ-secretases) for non-amyloidogenic and amyloidogenic processing of APP (37). Oxidative stress can increase BACE1 expression in VSMC and, in turn, release of Aβ1–40 and Aβ1–42 (37). There is evidence that Aβ is normally eliminated by the perivascular drainage, thereby preventing deposition of Aβ in the vascular wall (187). Nevertheless, excessively high concentration of Aβ in cerebral blood vessels can lead to a loss of VSMCs, resulting in the vascular distention and weakening of the vascular wall (88, 116, 119, 189). Recent research using animal models suggests that Aβ decreases adhesion of VSMC to the basement membrane and that increased deposition of Aβ in cerebral capillaries may be associated with occlusion and disturbance of cerebral blood flow (123, 175). Genetic deletion of CD36 or treatment with anti-Aβ antibody reduces CAA and micro-hemorrhages in Tg2576 mice cerebral arteries (143, 174). Furthermore, cerebrovascular protection by antioxidant therapy is dependent on a decrease in CAA formation as well as a direct reduction in CAA-induced vasomotor impairment (1, 75). Reduction of Aβ load in endothelial cells (and VSMC) appears to be a promising strategy in the prevention and treatment of CAA.
Future Directions
A number questions regarding expression and function of APP in blood vessels remain to be answered. First, vascular function of APP has to be better defined. Second, cellular signaling and the roles of APP cleavage products under physiological and pathological conditions have to be identified. Third, signal transduction mechanisms responsible for control of APP expression and processing have to be identified. Fourth, heterogeneity in vascular expression and function of APP in different brain regions and different size vessels deserve additional attention. Fifth, contribution of endothelial APP to pathogenesis of vascular and neuronal diseases (including AD) remains to be established. In aggregate, these advances may provide new targets for development of badly needed therapies for prevention and treatment of cerebrovascular disease and dementia.
Footnotes
This work was supported by National Heart, Lung, and Blood Institute R01 Grants HL-111062 and HL-131515, and by the Mayo Foundation.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: L.V.d. and Z.S.K. prepared figures; L.V.d. and Z.S.K. drafted manuscript; L.V.d., T.H., and Z.S.K. edited and revised manuscript; L.V.d., T.H., and Z.S.K. approved final version of manuscript.
References
- 1.Agyare EK, Leonard SR, Curran GL, Yu CC, Lowe VJ, Paravastu AK, Poduslo JF, Kandimalla KK. Traffic jam at the blood-brain barrier promotes greater accumulation of Alzheimer's disease amyloid-β proteins in the cerebral vasculature. Mol Pharm 10: 1557–1565, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aliev G, Smith MA, Obrenovich ME, de la Torre JC, Perry G. Role of vascular hypoperfusion-induced oxidative stress and mitochondria failure in the pathogenesis of Azheimer disease. Neurotox Res 5: 491–504, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Anantharaman M, Tangpong J, Keller JN, Murphy MP, Markesbery WR, Kiningham KK, St Clair DK. β-Amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLH/NLH X PS-1P264L/P264L double knock-in mouse model of Alzheimer's disease. Am J Pathol 168: 1608–1618, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atochin DN, Demchenko IT, Astern J, Boso AE, Piantadosi CA, Huang PL. Contributions of endothelial and neuronal nitric oxide synthases to cerebrovascular responses to hyperoxia. J Cereb Blood Flow Metab 23: 1219–1226, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Atwood CS, Obrenovich ME, Liu T, Chan H, Perry G, Smith MA, Martins RN. Amyloid-β: a chameleon walking in two worlds: a review of the trophic and toxic properties of amyloid-β. Brain Res Brain Res Rev 43: 1–16, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Austin SA, Sens MA, Combs CK. Amyloid precursor protein mediates a tyrosine kinase-dependent activation response in endothelial cells. J Neurosci 29: 14451–14462, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin SA, Santhanam AV, Katusic ZS. Endothelial nitric oxide modulates expression and processing of amyloid precursor protein. Circ Res 107: 1498–1502, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austin SA, d'Uscio LV, Katusic ZS. Supplementation of nitric oxide attenuates AβPP and BACE1 protein in cerebral microcirculation of eNOS-deficient mice J Alzheimers Dis 33: 29–33, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin SA, Santhanam AV, Hinton DJ, Choi DS, Katusic ZS. Endothelial nitric oxide deficiency promotes Alzheimer's disease pathology. J Neurochem 127: 691–700, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barger SW, Fiscus RR, Ruth P, Hofmann F, Mattson MP. Role of cyclic GMP in the regulation of neuronal calcium and survival by secreted forms of β-amyloid precursor. J Neurochem 64: 2087–2096, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Beckett C, Nalivaeva NN, Belyaev ND, Turner AJ. Nuclear signalling by membrane protein intracellular domains: the AICD enigma. Cell Signal 24: 402–409, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia R, Lin H, Lal R. Fresh and globular amyloid β protein (1–42) induces rapid cellular degeneration: evidence for AβP channel-mediated cellular toxicity. FASEB J 14: 1233–1243, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Biron KE, Dickstein DL, Gopaul R, Jefferies WA. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer's disease. PLos One 6: e23789, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanc EM, Toborek M, Mark RJ, Hennig B, Mattson MP. Amyloid β-peptide induces cell monolayer albumin permeability, impairs glucose transport, and induces apoptosis in vascular endothelial cells. J Neurochem 68: 1870–1881, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Boscolo E, Folin M, Nico B, Grandi C, Mangieri D, Longo V, Scienza R, Zampieri P, Conconi MT, Parnigotto PP, Ribatti D. β Amyloid angiogenic activity in vitro and in vivo. Int J Mol Med 19: 581–587, 2007. [PubMed] [Google Scholar]
- 16.Brambillaa A, Lonati E, Milani C, Rizzo AM, Farina F, Botto L, Masserini M, Palestini P, Bulbarelli A. Ischemic conditions and β-secretase activation: the impact of membrane cholesterol enrichment as triggering factor in rat brain endothelial cells. Int J Biochem Cell Biol 69: 95–104, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Bulbarelli A, Lonati E, Brambilla A, Orlando A, Cazzaniga E, Piazza F, Ferrarese C, Masserini M, Sancini G. Aβ42 production in brain capillary endothelial cells after oxygen and glucose deprivation. Mol Cell Neurosci 49: 415–422, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Burke-Wolin T, Abate CJ, Wolin MS, Gurtner GH. Hydrogen peroxide-induced pulmonary vasodilation: role of guanosine 3′,5′-cyclic monophosphate. Am J Physiol Lung Cell Mol Physiol 261: L393–L398, 1991. [DOI] [PubMed] [Google Scholar]
- 19.Bush AI, Martins RN, Rumble B, Moir R, Fuller S, Milward E, Currie J, Ames D, Weidemann A, Fischer P, et al. The amyloid precursor protein of Alzheimer's disease is released by human platelets. J Biol Chem 265: 15977–15983, 1990. [PubMed] [Google Scholar]
- 20.Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE, Greengard P. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer β/A4 amyloid protein precursor. Proc Natl Acad Sci USA 89: 10075–10078, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calhoun ME, Burgermeister P, Phinney AL, Stalder M, Tolnay M, Wiederhold KH, Abramowski D, Sturchler-Pierrat C, Sommer B, Staufenbiel M, Jucker M. Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid. Proc Natl Acad Sci USA 96: 14088–14093, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron DJ, Galvin C, Alkam T, Sidhu H, Ellison J, Luna S, Ethell DW. Alzheimer's-related peptide amyloid-β plays a conserved role in angiogenesis. PLos One 7: e39598, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantara S, Donnini S, Morbidelli L, Giachetti A, Schulz R, Memo M, Ziche M. Physiological levels of amyloid peptides stimulate the angiogenic response through FGF-2. FASEB J 18: 1943–1945, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Carnevale D, Mascio G, D'Andrea I, Fardella V, Bell RD, Branchi I, Pallante F, Zlokovic B, Yan SS, Lembo G. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 60: 188–197, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrano A, Hoozemans JJ, van der Vies SM, Rozemuller AJ, van Horssen J, de Vries HE. Amyloid beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid Redox Signal 15: 1167–1178, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Chao AC, Lee TC, Juo SH, Yang DI. Hyperglycemia increases the production of amyloid β-peptide leading to decreased endothelial tight junction. CNS Neurosci Ther 22: 291–297, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chasseigneaux S, Allinquant B. Functions of Aβ, sAPPα and sAPPβ: similarities and differences. J Neurochem 120, Suppl 1: 99–108, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Chi X, Sutton ET, Hellermann G, Price JM. Potassium channel openers prevent β-amyloid toxicity in bovine vascular endothelial cells. Neurosci Lett 290: 9–12, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Chiu WT, Shen SC, Yang LY, Chow JM, Wu CY, Chen YC. Inhibition of HSP90-dependent telomerase activity in amyloid β-induced apoptosis of cerebral endothelial cells. J Cell Physiol 226: 2041–2051, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Ciallella JR, Rangnekar VV, McGillis JP. Heat shock alters Alzheimer's β amyloid precursor protein expression in human endothelial cells. J Neurosci Res 37: 769–776, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Ciallella JR, Figueiredo H, Smith-Swintosky V, McGillis JP. Thrombin induces surface and intracellular secretion of amyloid precursor protein from human endothelial cells. Thromb Haemost 81: 630–637, 1999. [PubMed] [Google Scholar]
- 32.Cifuentes D, Poittevin M, Dere E, Broqueres-You D, Bonnin P, Benessiano J, Pocard M, Mariani J, Kubis N, Merkulova-Rainon T, Levy BI. Hypertension accelerates the progression of Alzheimer-like pathology in a mouse model of the disease. Hypertension 65: 218–224, 2015. [DOI] [PubMed] [Google Scholar]
- 33.Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-β deposition in an Alzheimer disease mouse model. J Clin Invest 115: 3285–3290, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke J, Thornell A, Corbett D, Soininen H, Hiltunen M, Jolkkonen J. Overexpression of APP provides neuroprotection in the absence of functional benefit following middle cerebral artery occlusion in rats. Eur J Neurosci 26: 1845–1852, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Colciaghi F, Borroni B, Pastorino L, Marcello E, Zimmermann M, Cattabeni F, Padovani A, Di Luca M. α-Secretase ADAM10 as well as αAPPs is reduced in platelets and CSF of Alzheimer disease patients. Mol Med 8: 67–74, 2002. [PMC free article] [PubMed] [Google Scholar]
- 36.Cole GM, Galasko D, Shapiro IP, Saitoh T. Stimulated platelets release amyloid β-protein precursor. Biochem Biophys Res Commun 170: 288–295, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coma M, Guix FX, Ill-Raga G, Uribesalgo I, Alameda F, Valverde MA, Munoz FJ. Oxidative stress triggers the amyloidogenic pathway in human vascular smooth muscle cells. Neurobiol Aging 29: 969–980, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Crawford F, Suo ZM, Fang CH, Mullan M. Characteristics of the in vitro vasoactivity of β-amyloid peptides. Exp Neurol 150: 159–168, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Cupers P, Orlans I, Craessaerts K, Annaert W, De Strooper B. The amyloid precursor protein (APP)-cytoplasmic fragment generated by γ-secretase is rapidly degraded but distributes partially in a nuclear fraction of neurones in culture. J Neurochem 78: 1168–1178, 2001. [DOI] [PubMed] [Google Scholar]
- 40.d'Uscio LV, Baker TA, Mantilla CB, Smith L, Weiler D, Sieck GC, Katusic ZS. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 21: 1017–1022, 2001. [DOI] [PubMed] [Google Scholar]
- 41.d'Uscio LV, Smith LA, Katusic ZS. Differential effects of eNOS uncoupling on conduit and small arteries in GTP-cyclohydrolase I-deficient hph-1 mice. Am J Physiol Heart Circ Physiol 301: H2227–H2234, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.d'Uscio LV, Das P, Santhanam AV, He T, Younkin SG, Katusic ZS. Activation of PPARδ prevents endothelial dysfunction induced by overexpression of amyloid-β precursor protein. Cardiovasc Res 96: 504–512, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.d'Uscio LV, He T, Santhanam AV, Tai LJ, Evans RM, Katusic ZS. Mechanisms of vascular dysfunction in mice with endothelium-specific deletion of the PPAR-δ gene. Am J Physiol Heart Circ Physiol 306: H1001–H1010, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dahlgren KN, Manelli AM, Stine WB Jr,. Baker LK, Krafft GA, and LaDu MJ. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J Biol Chem 277: 32046–32053, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, Zlokovic BV, Van Nostrand WE. Early-onset and robust cerebral microvascular accumulation of amyloid β-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem 279: 20296–20306, 2004. [DOI] [PubMed] [Google Scholar]
- 46.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 96: 60–68, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de la Torre JC. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol 3: 184–190, 2004. [DOI] [PubMed] [Google Scholar]
- 48.De Meyer GR, De Cleen DM, Cooper S, Knaapen MW, Jans DM, Martinet W, Herman AG, Bult H, Kockx MM. Platelet phagocytosis and processing of β-amyloid precursor protein as a mechanism of macrophage activation in atherosclerosis. Circ Res 90: 1197–1204, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 9: 907–913, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-β peptide across the blood-brain barrier: implication for therapies in Alzheimer's disease. CNS Neurol Disord Drug Targets 8: 16–30, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 100: 1689–1698, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Devraj K, Poznanovic S, Spahn C, Schwall G, Harter PN, Mittelbronn M, Antoniello K, Paganetti P, Muhs A, Heilemann M, Hawkins RA, Schrattenholz A, Liebner S. BACE-1 is expressed in the blood-brain barrier endothelium and is upregulated in a murine model of Alzheimer's disease. J Cereb Blood Flow Metab 36: 1281–1294, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dietrich HH, Xiang C, Han BH, Zipfel GJ, Holtzman DM. Soluble amyloid-beta, effect on cerebral arteriolar regulation and vascular cells. Mol Neurodegener 5: 15, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donnini S, Solito R, Cetti E, Corti F, Giachetti A, Carra S, Beltrame M, Cotelli F, Ziche M. Aβ peptides accelerate the senescence of endothelial cells in vitro and in vivo, impairing angiogenesis. FASEB J 24: 2385–2395, 2010. [DOI] [PubMed] [Google Scholar]
- 55.Eckert A, Hauptmann S, Scherping I, Rhein V, Muller-Spahn F, Gotz J, Muller WE. Soluble β-amyloid leads to mitochondrial defects in amyloid precursor protein and tau transgenic mice. Neurodegener Dis 5: 157–159, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Erickson MA, Hansen K, Banks WA. Inflammation-induced dysfunction of the low-density lipoprotein receptor-related protein-1 at the blood-brain barrier: protection by the antioxidant N-acetylcysteine. Brain Behav Immun 26: 1085–1094, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erickson MA, Banks WA. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer's disease. J Cereb Blood Flow Metab 33: 1500–1513, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D, Ward PJ. Cleavage of amyloid β peptide during constitutive processing of its precursor. Science 248: 1122–1124, 1990. [DOI] [PubMed] [Google Scholar]
- 59.Fang YC, Wu JS, Chen JJ, Cheung WM, Tseng PH, Tam KB, Shyue SK, Lin TN. Induction of prostacyclin/PGI2 synthase expression after cerebral ischemia-reperfusion. J Cereb Blood Flow Metab 26: 491–501, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 280: C1358–C1366, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Forloni G, Demicheli F, Giorgi S, Bendotti C, Angeretti N. Expression of amyloid precursor protein mRNAs in endothelial, neuronal and glial cells: modulation by interleukin-1. Brain Res Mol Brain Res 16: 128–134, 1992. [DOI] [PubMed] [Google Scholar]
- 62.Gardella JE, Ghiso J, Gorgone GA, Marratta D, Kaplan AP, Frangione B, Gorevic PD. Intact Alzheimer amyloid precursor protein (APP) is present in platelet membranes and is encoded by platelet mRNA. Biochem Biophys Res Commun 173: 129 2–1298, 1990. [DOI] [PubMed] [Google Scholar]
- 63.Gentile MT, Vecchione C, Maffei A, Aretini A, Marino G, Poulet R, Capobianco L, Selvetella G, Lembo G. Mechanisms of soluble β-amyloid impairment of endothelial function. J Biol Chem 279: 48135–48142, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Golde TE, Estus S, Usiak M, Younkin LH, Younkin SG. Expression of β amyloid protein precursor mRNAs: recognition of a novel alternatively spliced form and quantitation in Alzheimer's disease using PCR. Neuron 4: 253–267, 1990. [DOI] [PubMed] [Google Scholar]
- 65.Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science 235: 877–880, 1987. [DOI] [PubMed] [Google Scholar]
- 66.Goldgaber D, Harris HW, Hla T, Maciag T, Donnelly RJ, Jacobsen JS, Vitek MP, Gajdusek DC. Interleukin 1 regulates synthesis of amyloid β-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci USA 86: 7606–7610, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gralle M, Botelho MG, Wouters FS. Neuroprotective secreted amyloid precursor protein acts by disrupting amyloid precursor protein dimers. J Biol Chem 284: 15016–15025, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer's disease. Neurobiol Aging 22: 837–842, 2001. [DOI] [PubMed] [Google Scholar]
- 69.Grammas P, Ottman T, Reimann-Philipp U, Larabee J, Weigel PH. Injured brain endothelial cells release neurotoxic thrombin. J Alzheimers Dis 6: 275–281, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Grammas P, Samany PG, Thirumangalakudi L. Thrombin and inflammatory proteins are elevated in Alzheimer's disease microvessels: implications for disease pathogenesis. J Alzheimers Dis 9: 51–58, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-β and tau pathology in a mouse model of Alzheimer's disease. J Neurosci 26: 9047–9056, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haase N, Herse F, Spallek B, Haase H, Morano I, Qadri F, Szijarto IA, Rohm I, Yilmaz A, Warrington JP, Ryan MJ, Gollasch M, Muller DN, Dechend R, Wallukat G. Amyloid-β peptides activate α1-adrenergic cardiovascular receptors. Hypertension 62: 966–972, 2013. [DOI] [PubMed] [Google Scholar]
- 73.Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, Selkoe DJ. Amyloid β-petide is produced by cultured cells during normal metabolism. Nature 259: 322–325, 1992. [DOI] [PubMed] [Google Scholar]
- 74.Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ. Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature 357: 500–503, 1992. [DOI] [PubMed] [Google Scholar]
- 75.Han BH, Zhou ML, Johnson AW, Singh I, Liao F, Vellimana AK, Nelson JW, Milner E, Cirrito JR, Basak J, Yoo M, Dietrich HH, Holtzman DM, Zipfel GJ. Contribution of reactive oxygen species to cerebral amyloid angiopathy, vasomotor dysfunction, and microhemorrhage in aged Tg2576 mice. Proc Natl Acad Sci USA 112: 881–890, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hawkes CA, Gatherer M, Sharp MM, Dorr A, Yuen HM, Kalaria R, Weller RO, Carare RO. Regional differences in the morphological and functional effects of aging on cerebral basement membranes and perivascular drainage of amyloid-beta from the mouse brain. Aging Cell 12: 224–236, 2013. [DOI] [PubMed] [Google Scholar]
- 77.Hayashi S, Sato N, Yamamoto A, Ikegame Y, Nakashima S, Ogihara T, Morishita R. Alzheimer disease-associated peptide, amyloid β40, inhibits vascular regeneration with induction of endothelial autophagy. Arterioscler Thromb Vasc Biol 29: 1909–1915, 2009. [DOI] [PubMed] [Google Scholar]
- 78.He T, Santhanam AV, Lu T, d'Uscio LV, Katusic ZS. Role of prostacyclin signaling in endothelial production of soluble amyloid precursor protein-α in cerebral microvessels. J Cereb Blood Flow Metab. In press. [DOI] [PMC free article] [PubMed]
- 79.Herzig MC, Winkler DT, Burgermeister P, Pfeifer M, Kohler E, Schmidt SD, Danner S, Abramowski D, Sturchler-Pierrat C, Burki K, van Duinen SG, Maat-Schieman ML, Staufenbiel M, Mathews PM, Jucker M. Aβ is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci 7: 954–960, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Hsu MJ, Hsu CY, Chen BC, Chen MC, Ou G, Lin CH. Apoptosis signal-regulating kinase 1 in amyloid β peptide-induced cerebral endothelial cell apoptosis. J Neurosci 27: 5719–5729, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu X, Crick SL, Bu G, Frieden C, Pappu RV, Lee JM. Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-beta peptide. Proc Natl Acad Sci USA 106: 20324–20329, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iadecola C. Cerebrovascular effects of amyloid-β peptides: mechanisms and implications for Alzheimer's dementia. Cell Mol Neurobiol 23: 681–689, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iadecola C. The pathobiology of vascular dementia. Neuron 80: 844–866, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ishida A, Furukawa K, Keller JN, Mattson MP. Secreted form of β-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. Neuroreport 8: 2133–2137, 1997. [DOI] [PubMed] [Google Scholar]
- 85.Janelidze S, Stomrud E, Palmqvist S, Zetterberg H, van Westen D, Jeromin A, Song L, Hanlon D, Tan Hehir CA, Baker D, Blennow K, Hansson O. Plasma β-amyloid in Alzheimer's disease and vascular disease. Sci Rep 6: 26801, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jimenez S, Torres M, Vizuete M, Sanchez-Varo R, Sanchez-Mejias E, Trujillo-Estrada L, Carmona-Cuenca I, Caballero C, Ruano D, Gutierrez A, Vitorica J. Age-dependent accumulation of soluble amyloid beta (Aβ) oligomers reverses the neuroprotective effect of soluble amyloid precursor protein-alpha (sAPPα) by modulating phosphatidylinositol 3-kinase (PI3K)/Akt-GSK-3β pathway in Alzheimer mouse model. J Biol Chem 286: 18414–18425, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736, 1987. [DOI] [PubMed] [Google Scholar]
- 88.Kawai M, Kalaria RN, Cras P, Siedlak SL, Velasco ME, Shelton ER, Chan HW, Greenberg BD, Perry G. Degeneration of vascular muscle cells in cerebral amyloid angiopathy of Alzheimer disease. Brain Res 623: 142–146, 1993. [DOI] [PubMed] [Google Scholar]
- 89.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci 21: 372–381, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kerr ML, Gasperini R, Gibbs ME, Hou X, Shepherd CE, Strickland DK, Foa L, Lawen A, Small DH. Inhibition of Aβ aggregation and neurotoxicity by the 39-kDa receptor-associated protein. J Neurochem 112: 1199–1209, 2010. [DOI] [PubMed] [Google Scholar]
- 91.Kim SM, Byun JS, Jung YD, Kang IC, Choi SY, Lee KY. The effects of oxygen radicals on the activity of nitric oxide synthase and guanylate cyclase. Exp Mol Med 30: 221–226, 1998. [DOI] [PubMed] [Google Scholar]
- 92.Kimberly WT, Zheng JB, Guenette SY, Selkoe DJ. The intracellular domain of the β-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem 276: 40288–40292, 2001. [DOI] [PubMed] [Google Scholar]
- 93.Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. γ-Secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci USA 100: 6382–6387, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kitazume S, Tachida Y, Kato M, Yamaguchi Y, Honda T, Hashimoto Y, Wada Y, Saito T, Iwata N, Saido T, Taniguchi N. Brain endothelial cells produce amyloid β from amyloid precursor protein 770 and preferentially secrete the O-glycosylated form. J Biol Chem 285: 40097–40103, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kitazume S, Yoshihisa A, Yamaki T, Oikawa M, Tachida Y, Ogawa K, Imamaki R, Hagiwara Y, Kinoshita N, Takeishi Y, Furukawa K, Tomita N, Arai H, Iwata N, Saido T, Yamamoto N, Taniguchi N. Soluble amyloid precursor protein 770 is released from inflamed endothelial cells and activated platelets. A novel biomarker for acute coronary syndrome. J Biol Chem 287: 40817–40825, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kögel D, Deller T, Behl C. Roles of amyloid precursor protein family members in neuroprotection, stress signaling and aging. Exp Brain Res 217: 471–479, 2012. [DOI] [PubMed] [Google Scholar]
- 97.Koike MA, Lin AJ, Pham J, Nguyen E, Yeh JJ, Rahimian R, Tromberg BJ, Choi B, Green KN, LaFerla FM. APP knockout mice experience acute mortality as the result of ischemia. PLos One 7: e42665, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the α-secretase ADAM 10. Proc Natl Acad Sci USA 98: 5815–5820, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kontush A, Berndt C, Weber W, Akopyan V, Arlt S, Schippling S, Beisiegel U. Amyloid-β is an antioxidant for lipoproteins in cerebrospinal fluid and plasma. Free Radic Biol Med 30: 119–128, 2001. [DOI] [PubMed] [Google Scholar]
- 100.Kuhn PH, Wang H, Dislich B, Colombo A, Zeitschel U, Ellwart JW, Kremmer E, Rossner S, Lichtenthaler SF. ADAM10 is the physiologically relevant, constitutive α-secretase of the amyloid precursor protein in primary neurons. EMBO J 29: 3020–3032, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kuo YM, Emmerling MR, Lampert HC, Hempelman SR, Kokjohn TA, Woods AS, Cotter RJ, Roher AE. High levels of circulating Aβ42 are sequestered by plasma proteins in Alzheimer's disease. Biochem Biophys Res Commun 257: 787–791, 1999. [DOI] [PubMed] [Google Scholar]
- 102.Kuo YM, Kokjohn TA, Watson MD, Woods AS, Cotter RJ, Sue LI, Kalback WM, Emmerling MR, Beach TG, Roher AE. Elevated Aβ42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AβPP metabolism. Am J Pathol 156: 797–805, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kwak YD, Wang R, Li JJ, Zhang YW, Xu H, Liao FF. Differential regulation of BACE1 expression by oxidative and nitrosative signals. Mol Neurodegener 6: 17, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lamoke F, Mazzone V, Persichini T, Maraschi A, Harris MB, Venema RC, Colasanti M, Gliozzi M, Muscoli C, Bartoli M, Mollace V. Amyloid β peptide-induced inhibition of endothelial nitric oxide production involves oxidative stress-mediated constitutive eNOS/HSP90 interaction and disruption of agonist-mediated Akt activation. J Neuroinflamm 12: 84, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lang IM, Moser KM, Schleef RR. Expression of Kunitz protease inhibitor: containing forms of amyloid β-protein precursor within vascular thrombi. Circulation 94: 2728–2734, 1996. [DOI] [PubMed] [Google Scholar]
- 106.Lee RK, Araki W, Wurtman RJ. Stimulation of amyloid precursor protein synthesis by adrenergic receptors coupled to cAMP formation. Proc Natl Acad Sci USA 94: 5422–5426, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee SJ, Liyanage U, Bickel PE, Xia W, Lansbury PT Jr, Kosik KS. A detergent-insoluble membrane compartment contains Aβ in vivo. Nat Med 4: 730–734, 1998. [DOI] [PubMed] [Google Scholar]
- 108.Li L, Cao D, Garber DW, Kim H, Fukuchi K. Association of aortic atherosclerosis with cerebral beta-amyloidosis and learning deficits in a mouse model of Alzheimer's disease. Am J Pathol 163: 2155–2164, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li M, Shang DS, Zhao WD, Tian L, Li B, Fang WG, Zhu L, Man SM, Chen YH. Amyloid β interaction with receptor for advanced glycation end products up-regulates brain endothelial CCR5 expression and promotes T cells crossing the blood-brain barrier. J Immunol 182: 5778–5788, 2009. [DOI] [PubMed] [Google Scholar]
- 110.Li QX, Berndt MC, Bush AI, Rumble B, Mackenzie I, Friedhuber A, Beyreuther K, Masters CL. Membrane-associated forms of the βA4 amyloid protein precursor of Alzheimer's disease in human platelet and brain: surface expression on the activated human platelet. Blood 84: 133–142, 1994. [PubMed] [Google Scholar]
- 111.Li QX, Evin G, Small DH, Multhaup G, Beyreuther K, Masters CL. Proteolytic processing of Alzheimer's disease βA4 amyloid precursor protein in human platelets. J Biol Chem 270: 14140–14147, 1995. [DOI] [PubMed] [Google Scholar]
- 112.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 105: 1135–1143, 2002. [DOI] [PubMed] [Google Scholar]
- 113.Luna S, Cameron DJ, Ethell DW. Amyloid-β and APP deficiencies cause severe cerebrovascular defects: important work for an old villain. PLos One 8: e75052, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lüscher TF, Vanhoutte PM. The Endothelium: Modulator of Cardiovascular Function. Boca Raton, FL: CRC Press, 1990, p. 1–215. [Google Scholar]
- 115.Ma JF, Wang HM, Li QY, Zhang Y, Pan J, Qiang Q, Xin XY, Tang HD, Ding JQ, Chen SD. Starvation triggers Aβ42 generation from human umbilical vascular endothelial cells. FEBS Lett 584: 3101–3106, 2010. [DOI] [PubMed] [Google Scholar]
- 116.Maeda A, Yamada M, Itoh Y, Otomo E, Hayakawa M, Miyatake T. Computer-assisted three-dimensional image analysis of cerebral amyloid angiopathy. Stroke 24: 1857–1864, 1993. [DOI] [PubMed] [Google Scholar]
- 117.Magrane J, Christensen RA, Rosen KM, Veereshwarayya V, Querfurth HW. Dissociation of ERK and Akt signaling in endothelial cell angiogenic responses to β-amyloid. Exp Cell Res 312: 996–1010, 2006. [DOI] [PubMed] [Google Scholar]
- 118.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science 330: 1774, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miao J, Xu F, Davis J, Otte-Holler I, Verbeek MM, Van Nostrand WE. Cerebral microvascular amyloid β protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid β precursor protein. Am J Pathol 167: 505–515, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miller TW, Isenberg JS, Shih HB, Wang Y, Roberts DD. Amyloid-β inhibits No-cGMP signaling in a CD36- and CD47-dependent manner. PLos One 5: e15686, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Milosch N, Tanriover G, Kundu A, Rami A, Francois JC, Baumkotter F, Weyer SW, Samanta A, Jaschke A, Brod F, Buchholz CJ, Kins S, Behl C, Muller UC, Kogel D. Holo-APP and G-protein-mediated signaling are required for sAPPα-induced activation of the Akt survival pathway. Cell Death Dis 5: e1391, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun 263: 681–684, 1999. [DOI] [PubMed] [Google Scholar]
- 123.Mok SS, Losic D, Barrow CJ, Turner BJ, Masters CL, Martin LL, Small DH. The β-amyloid peptide of Alzheimer's disease decreases adhesion of vascular smooth muscle cells to the basement membrane. J Neurochem 96: 53–64, 2006. [DOI] [PubMed] [Google Scholar]
- 124.Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature 263: 663–665, 1976. [DOI] [PubMed] [Google Scholar]
- 125.Moreira PI, Smith MA, Zhu X, Santos MS, Oliveira CR, Perry G. Therapeutic potential of oxidant mechanisms in Alzheimer's disease. Expert Rev Neurother 4: 995–1004, 2004. [DOI] [PubMed] [Google Scholar]
- 126.Muche A, Burger S, Arendt T, Schliebs R. Hypoxic stress, brain vascular system, and β-amyloid: a primary cell culture study. Nutr Neurosci 18: 1–11, 2015. [DOI] [PubMed] [Google Scholar]
- 127.Müller T, Meyer HE, Egensperger R, Marcus K. The amyloid precursor protein intracellular domain (AICD) as modulator of gene expression, apoptosis, and cytoskeletal dynamics-relevance for A lzheimer's disease. Prog Neurobiol 85: 393–406, 2008. [DOI] [PubMed] [Google Scholar]
- 128.Nicolas M, Hassan BA. Amyloid precursor protein and neural development. Development 141: 2543–2548, 2014. [DOI] [PubMed] [Google Scholar]
- 129.Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature 457: 981–989, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 130.Niwa K, Carlson GA, Iadecola C. Exogenous Aβ1–40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab 20: 1659–1668, 2000. [DOI] [PubMed] [Google Scholar]
- 131.Niwa K, Porter VA, Kazama K, Cornfield D, Carlson GA, Iadecola C. Aβ-peptides enhance vasoconstriction in cerebral circulation. Am J Physiol Heart Circ Physiol 281: H2417–H2424, 2001. [DOI] [PubMed] [Google Scholar]
- 132.Obregon D, Hou H, Deng J, Giunta B, Tian J, Darlington D, Shahaduzzaman M, Zhu Y, Mori T, Mattson MP, Tan J. Soluble amyloid precursor protein-α modulates β-secretase activity and amyloid-β generation. Nat Commun 3: 777, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol 1: 409–417, 1977. [DOI] [PubMed] [Google Scholar]
- 134.Ott MO, Bullock SL. A gene trap insertion reveals that amyloid precursor protein expression is a very early event in murine embryogenesis. Dev Genes Evol 211: 355–357, 2001. [DOI] [PubMed] [Google Scholar]
- 135.Palmert MR, Usiak M, Mayeux R, Raskind M, Tourtellotte WW, Younkin SG. Soluble derivatives of the β amyloid protein precursor in cerebrospinal fluid: alterations in normal aging and in Alzheimer's disease. Neurology 40: 1028–1034, 1990. [DOI] [PubMed] [Google Scholar]
- 136.Pardossi-Piquard R, Checler F. The physiology of the β-amyloid precursor protein intracellular domain AICD. J Neurochem 120, Suppl 1: 109–124, 2012. [DOI] [PubMed] [Google Scholar]
- 137.Paris D, Humphrey J, Quadros A, Patel N, Crescentini R, Crawford F, Mullan M. Vasoactive effects of Aβ in isolated human cerebrovessels and in a transgenic mouse model of Alzheimer's disease: role of inflammation. Neurol Res 25: 642–651, 2003. [DOI] [PubMed] [Google Scholar]
- 138.Paris D, Quadros A, Patel N, DelleDonne A, Humphrey J, Mullan M. Inhibition of angiogenesis and tumor growth by beta and gamma-secretase inhibitors. Eur J Pharmacol 514: 1–15, 2005. [DOI] [PubMed] [Google Scholar]
- 139.Park L, Anrather J, Forster C, Kazama K, Carlson GA, Iadecola C. Aβ-induced vascular oxidative stress and attenuation of functional hyperemia in mouse somatosensory cortex. J Cereb Blood Flow Metab 24: 334–342, 2004. [DOI] [PubMed] [Google Scholar]
- 140.Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid β peptide. J Neurosci 25: 1769–1777, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park L, Wang G, Zhou P, Zhou J, Pitstick R, Previti ML, Younkin L, Younkin SG, Van Nostrand WE, Cho S, Anrather J, Carlson GA, Iadecola C. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-β. Proc Natl Acad Sci USA 108: 5063–5068, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Park L, Zhou P, Koizumi K, El Jamal S, Previti ML, Van Nostrand WE, Carlson G, Iadecola C. Brain and circulating levels of Aβ1–40 differentially contribute to vasomotor dysfunction in the mouse brain. Stroke 44: 198–204, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park L, Zhou J, Zhou P, Pistick R, El Jamal S, Younkin L, Pierce J, Arreguin A, Anrather J, Younkin SG, Carlson GA, McEwen BS, Iadecola C. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc Natl Acad Sci USA 110: 3089–3094, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park L, Wang G, Moore J, Girouard H, Zhou P, Anrather J, Iadecola C. The key role of transient receptor potential melastatin-2 channels in amyloid-β-induced neurovascular dysfunction. Nat Commun 5: 5318, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patel NS, Mathura VS, Bachmeier C, Beaulieu-Abdelahad D, Laporte V, Weeks O, Mullan M, Paris D. Alzheimer's β-amyloid peptide blocks vascular endothelial growth factor mediated signaling via direct interaction with VEGFR-2. J Neurochem 112: 66–76, 2010. [DOI] [PubMed] [Google Scholar]
- 146.Pickard JD. Role of prostaglandins and arachidonic acid derivatives in the coupling of cerebral blood flow to cerebral metabolism. J Cereb Blood Flow Metab 1: 361–384, 1981. [DOI] [PubMed] [Google Scholar]
- 147.Ponte P, Gonzalez-DeWhitt P, Schilling J, Miller J, Hsu D, Greenberg B, Davis K, Wallace W, Lieberburg I, Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature 331: 525–527, 1988. [DOI] [PubMed] [Google Scholar]
- 148.Prelli F, Castano E, Glenner GG, Frangione B. Differences between vascular and plaque core amyloid in Alzheimer's disease. J Neurochem 51: 648–651, 1988. [DOI] [PubMed] [Google Scholar]
- 149.Preston JE, Hipkiss AR, Himsworth DT, Romero IA, Abbott JN. Toxic effects of β-amyloid(25–35) on immortalised rat brain endothelial cell: protection by carnosine, homocarnosine and β-alanine. Neurosci Lett 242: 105–108, 1998. [DOI] [PubMed] [Google Scholar]
- 150.Price JM, Chi X, Hellermann G, Sutton ET. Physiological levels of β-amyloid induce cerebral vessel dysfunction and reduce endothelial nitric oxide production. Neurol Res 23: 506–512, 2001. [DOI] [PubMed] [Google Scholar]
- 151.Qosa H, Abuasal BS, Romero IA, Weksler B, Couraud PO, Keller JN, Kaddoumi A. Differences in amyloid-beta clearance across mouse and human blood-brain barrier models: kinetic analysis and mechanistic modeling. Neuropharmacology 79: 668–678, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ritz K, Denswil NP, Stam OC, van Lieshout JJ, Daemen MJ. Cause and mechanisms of intracranial atherosclerosis. Circulation 130: 1407–1414, 2014. [DOI] [PubMed] [Google Scholar]
- 153.Robakis NK, Ramakrishna N, Wolfe G, Wisniewski HM. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci USA 84: 4190–4194, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Roher AE, Esh CL, Kokjohn TA, Castano EM, Van Vickle GD, Kalback WM, Patton RL, Luehrs DC, Daugs ID, Kuo YM, Emmerling MR, Soares H, Quinn JF, Kaye J, Connor DJ, Silverberg NB, Adler CH, Seward JD, Beach TG, Sabbagh MN. Amyloid β peptides in human plasma and tissues and their significance for Alzheimer's disease. Alzheimers Dement 5: 18–29, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Santhanam AV, d'Uscio LV, Smith LA, Katusic ZS. Uncoupling of eNOS causes superoxide anion production and impairs NO signaling in the cerebral microvessels of hph-1 mice. J Neurochem 122: 1211–1218, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Santhanam AVR, d'Uscio LV, He TR, Das P, Younkin SG, Katusic ZS. Uncoupling of endothelial nitric oxide synthase in cerebral vasculature of Tg2576 mice. J Neurochem 134: 1129–1138, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sato T, Sawada S, Tsuda Y, Komatsu S, Akamatsu N, Kono Y, Higaki T, Imamura H, Tada Y, Yamasaki S, Tamagaki T, Nakagawa K, Tsuji H, Nakagawa M. The mechanism of thrombin-induced prostacyclin synthesis in human endothelial cells with reference to the gene transcription of prostacyclin-related enzymes and Ca2+ kinetics. J Pharmacol Toxicol Methods 41: 173–182, 1999. [DOI] [PubMed] [Google Scholar]
- 158.Schmaier AH, Dahl LD, Rozemuller AJ, Roos RA, Wagner SL, Chung R, Van Nostrand WE. Protease nexin-2/amyloid β protein precursor. A tight-binding inhibitor of coagulation factor IXa. J Clin Invest 92: 2540–2545, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Selkoe DJ, Podlisny MB, Joachim CL, Vickers EA, Lee G, Fritz LC, Oltersdorf T. β-Amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci USA 85: 7341–7345, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shakil H, Saleem S. Genetic deletion of prostacyclin IP receptor exacerbates transient global cerebral ischemia in aging mice. Brain Sci 3: 1095–1108, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer's amyloid-β(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 106: 1489–1499, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, McKay DM, Tintner R, Frangione B, Younkin SG. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 258: 126–129, 1992. [DOI] [PubMed] [Google Scholar]
- 163.Simons ER, Marshall DC, Long HJ, Otto K, Billingslea A, Tibbles H, Wells J, Eisenhauer P, Fine RE, Cribbs DH, Davies TA, Abraham CR. Blood brain barrier endothelial cells express candidate amyloid precursor protein-cleaving secretases. Amyloid 5: 153–162, 1998. [DOI] [PubMed] [Google Scholar]
- 164.Skovronsky DM, Lee VM, Pratico D. Amyloid precursor protein and amyloid beta peptide in human platelets. Role of cyclooxygenase and protein kinase C. J Biol Chem 276: 17036–17043, 2001. [DOI] [PubMed] [Google Scholar]
- 165.Smirnov A, Trupp A, Henkel AW, Bloch E, Reulbach U, Lewczuk P, Riggert J, Kornhuber J, Wiltfang J. Differential processing and secretion of Aβ peptides and sAPPα in human platelets is regulated by thrombin and prostaglandine 2. Neurobiol Aging 30: 1552–1562, 2009. [DOI] [PubMed] [Google Scholar]
- 166.Smith RP, Higuchi DA, Broze GJ Jr. Platelet coagulation factor XIa-inhibitor, a form of Alzheimer amyloid precursor protein. Science 248: 1126–1128, 1990. [DOI] [PubMed] [Google Scholar]
- 167.Stamatelopoulos K, Sibbing D, Rallidis LS, Georgiopoulos G, Stakos D, Braun S, Gatsiou A, Sopova K, Kotakos C, Varounis C, Tellis CC, Kastritis E, Alevizaki M, Tselepis AD, Alexopoulos P, Laske C, Keller T, Kastrati A, Dimmeler S, Zeiher AM, Stellos K. Amyloid-beta (1–40) and the risk of death from cardiovascular causes in patients with coronary heart disease. J Am Coll Cardiol 65: 904–916, 2015. [DOI] [PubMed] [Google Scholar]
- 168.Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, Koller A, Kaley G. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res 85: 288–293, 1999. [DOI] [PubMed] [Google Scholar]
- 169.Suo ZM, Fang CH, Crawford F, Mullan M. Superoxide free radical and intracellular calcium mediate Aβ(1–42) induced endothelial toxicity. Brain Res 762: 144–152, 1997. [DOI] [PubMed] [Google Scholar]
- 170.Suzuki N, Iwatsubo T, Odaka A, Ishibashi Y, Kitada C, Ihara Y. High tissue content of soluble beta 1–40 is linked to cerebral amyloid angiopathy. Am J Pathol 145: 452–460, 1994. [PMC free article] [PubMed] [Google Scholar]
- 171.Tan XL, Xue YQ, Ma T, Wang X, Li JJ, Lan L, Malik KU, McDonald MP, Dopico AM, Liao FF. Partial eNOS deficiency causes spontaneous thrombotic cerebral infarction, amyloid angiopathy and cognitive impairment. Mol Neurodegener 10: 24, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tanzi RE, Gusella JF, Watkins PC, Bruns GA, St George-Hyslop P, Van Keuren ML, Patterson D, Pagan S, Kurnit DM, Neve RL. Amyloid β protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science 235: 880–884, 1987. [DOI] [PubMed] [Google Scholar]
- 173.Tanzi RE, McClatchey AI, Lamperti ED, Villa-Komaroff L, Gusella JF, Neve RL. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature 331: 528–530, 1988. [DOI] [PubMed] [Google Scholar]
- 174.Thakker DR, Weatherspoon MR, Harrison J, Keene TE, Lane DS, Kaemmerer WF, Stewart GR, Shafer LL. Intracerebroventricular amyloid-β antibodies reduce cerebral amyloid angiopathy and associated micro-hemorrhages in aged Tg2576 mice. Proc Natl Acad Sci USA 106: 4501–4506, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Thal DR, Capetillo-Zarate E, Larionov S, Staufenbiel M, Zurbruegg S, Beckmann N. Capillary cerebral amyloid angiopathy is associated with vessel occlusion and cerebral blood flow disturbances. Neurobiol Aging 30: 1936–1948, 2009. [DOI] [PubMed] [Google Scholar]
- 176.Thirumangalakudi L, Samany PG, Owoso A, Wiskar B, Grammas P. Angiogenic proteins are expressed by brain blood vessels in Alzheimer's disease. J Alzheimers Dis 10: 111–118, 2006. [DOI] [PubMed] [Google Scholar]
- 177.Thomas T, Thomas G, McLendon C, Sutton T, Mullan M . β-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature 380: 168–171, 1996. [DOI] [PubMed] [Google Scholar]
- 178.Thomas T, Sutton ET, Hellermann A, Price JM. β-Amyloid-induced coronary artery vasoactivity and endothelial damage J Cardiovasc Pharm 30: 517–522, 1997. [DOI] [PubMed] [Google Scholar]
- 179.Thomas T, McLendon C, Sutton ET, Thomas G. Cerebrovascular endothelial dysfunction mediated by β-amyloid. Neuroreport 8: 1387–1391, 1997. [DOI] [PubMed] [Google Scholar]
- 180.Tibolla G, Norata GD, Meda C, Arnaboldi L, Uboldi P, Piazza F, Ferrarese C, Corsini A, Maggi A, Vegeto E, Catapano AL. Increased atherosclerosis and vascular inflammation in APP transgenic mice with apolipoprotein E deficiency. Atherosclerosis 210: 78–87, 2010. [DOI] [PubMed] [Google Scholar]
- 181.Tong XK, Nicolakakis N, Kocharyan A, Hamel E. Vascular remodeling versus amyloid β-induced oxidative stress in the cerebrovascular dysfunctions associated with Alzheimer's disease. J Neurosci 25: 11165–11174, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Van De Parre TJ, Guns PJ, Fransen P, Martinet W, Bult H, Herman AG, De Meyer GR. Attenuated atherogenesis in apolipoprotein E-deficient mice lacking amyloid precursor protein. Atherosclerosis 216: 54–58, 2011. [DOI] [PubMed] [Google Scholar]
- 183.Van Nostrand WE, Schmaier AH, Farrow JS, Cunningham DD. Protease nexin-II (amyloid β-protein precursor): a platelet α-granule protein. Science 248: 745–748, 1990. [DOI] [PubMed] [Google Scholar]
- 184.Vukic V, Callaghan D, Walker D, Lue LF, Liu QY, Couraud PO, Romero IA, Weksler B, Stanimirovic DB, Zhang W. Expression of inflammatory genes induced by β-amyloid peptides in human brain endothelial cells and in Alzheimer's brain is mediated by the JNK-AP1 signaling pathway. Neurobiol Dis 34: 95–106, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang DS, Dickson DW, Malter JS. β-Amyloid degradation and Alzheimer's disease. J Biomed Biotechnol 2006: 58406, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Weksler BB, Marcus AJ, Jaffe EA. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci USA 74: 3922–3926, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-β peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol 18: 253–266, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Whitson JS, Selkoe DJ, Cotman CW. Amyloid β protein enhances the survival of hippocampal neurons in vitro. Science 243: 1488–1490, 1989. [DOI] [PubMed] [Google Scholar]
- 189.Winkler DT, Bondolfi L, Herzig MC, Jann L, Calhoun ME, Wiederhold KH, Tolnay M, Staufenbiel M, Jucker M. Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J Neurosci 21: 1619–1627, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wirth KJ, Fink E, Rudolphi K, Heitsch H, Deutschlander N, Wiemer G. Amyloid β-(1–40) stimulates cyclic GMP production via release of kinins in primary cultured endothelial cells. Eur J Pharmacol 382: 27–33, 1999. [DOI] [PubMed] [Google Scholar]
- 191.Wu W, Li H, Navaneetham D, Reichenbach ZW, Tuma RF, Walsh PN. The kunitz protease inhibitor domain of protease nexin-2 inhibits factor XIa and murine carotid artery and middle cerebral artery thrombosis. Blood 120: 671–677, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xiang Y, Bu XL, Liu YH, Zhu C, Shen LL, Jiao SS, Zhu XY, Giunta B, Tan J, Song WH, Zhou HD, Zhou XF, Wang YJ. Physiological amyloid-beta clearance in the periphery and its therapeutic potential for Alzheimer's disease. Acta Neuropathol (Berl) 130: 487–499, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xu F, Davis J, Miao J, Previti ML, Romanov G, Ziegler K, Van Nostrand WE. Protease nexin-2/amyloid β-protein precursor limits cerebral thrombosis. Proc Natl Acad Sci USA 102: 18135–18140, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu J, Chen SW, Ku G, Ahmed SH, Xu JM, Chen H, Hsu CY. Amyloid beta peptide-induced cerebral endothelial cell death involves mitochondrial dysfunction and caspase activation. J Cereb Blood Flow Metab 21: 702–710, 2001. [DOI] [PubMed] [Google Scholar]
- 195.Yamada K, Hashimoto T, Yabuki C, Nagae Y, Tachikawa M, Strickland DK, Liu Q, Bu G, Basak JM, Holtzman DM, Ohtsuki S, Terasaki T, Iwatsubo T. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid β peptides in an in vitro model of the blood-brain barrier cells. J Biol Chem 283: 34554–34562, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yasutaka Y, Watanabe T, Nakashima A, Matsumoto J, Futagami K, Yamauchi A, Kataoka Y. Tumor necrosis factor-α reduces β-amyloid accumulation primarily by lowering cellular prion protein levels in a brain endothelial cell line. FEBS Lett 589: 263–268, 2015. [DOI] [PubMed] [Google Scholar]
- 197.Yin KJ, Lee JM, Chen SD, Xu J, Hsu CY. Amyloid-β induces Smc release via AP-1/Bim activation in cerebral endothelial cells. J Neurosci 22: 9764–9770, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yin X, Wright J, Wall T, Grammas P. Brain endothelial cells synthesize neurotoxic thrombin in Alzheimer's disease. Am J Pathol 176: 1600–1606, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhao B, Sisodia SS, Kusiak JW. Altered processing of a mutant amyloid precursor protein in neuronal and endothelial cells. J Neurosci Res 40: 261–268, 1995. [DOI] [PubMed] [Google Scholar]
- 200.Zhu D, Shi J, Zhang Y, Wang B, Liu W, Chen Z, Tong Q. Central angiotensin II stimulation promotes β amyloid production in Sprague Dawley rats. PLos One 6: e16037, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]