Abstract
Expression of cellular receptors determines viral tropism and limits gene delivery by viral vectors. Protein transduction domains (PTDs) have been shown to deliver proteins, antisense oligonucleotides, liposomes, or plasmid DNA into cells. In our study, we investigated the role of several PTD motifs in adenoviral infection. When physiologically expressed, a PTD from human immunodeficiency virus transactivator of transcription (Tat) did not improve adenoviral infection. We therefore fused PTDs to the ectodomain of the coxsackievirus-adenovirus receptor (CARex) to attach PTDs to adenoviral fiber knobs. CARex-Tat and CARex-VP22 allowed efficient adenoviral infection in nonpermissive cells and significantly improved viral uptake rates in permissive cells. Dose-dependent competition of CARex-PTD-mediated infection using CARex and inhibition experiments with heparin showed that binding of CARex-PTD to both adenoviral fiber and cellular glycosaminoglycans is essential for the improvement of infection. CARex-PTD-treated adenoviruses retained their properties after density gradient ultracentrifugation, indicating stable binding of CARex-PTD to adenoviral particles. Consequently, the mechanism of CARex-PTD-mediated infection involves coating of the viral fiber knobs by CARex-PTD, rather than placement of CARex domains on cell surfaces. Expression of CARex-PTDs led to enhanced lysis of permissive and nonpermissive tumor cells by replicating adenoviruses, indicating that CARex-PTDs are valuable tools to improve the efficacy of oncolytic therapy. Together, our study shows that CARex-PTDs facilitate gene transfer in nonpermissive cells and improve viral uptake at reduced titers and infection times. The data suggest that PTDs fused to virus binding receptors may be a valuable tool to overcome natural tropism of vectors and could be of great interest for gene therapeutic approaches.
Viral vectors are the most efficient means for gene delivery in mammalian cells. Adenoviruses have been widely used for gene therapy applications in vivo and in vitro, as they are able to accommodate large transgenes, can be propagated to high titers and transduce cells independently of their replicative state. However, applications for adenoviral vectors have been limited by the native tropism of the virus. Viral infection first requires efficient binding of viral particles to the plasma membrane prior to cellular uptake. In human epithelial and other permissive cells, entry of species C adenoviruses, including the currently used adenovirus types 2 and 5, depends on the presence of the coxsackievirus B adenovirus receptor (CAR) and integrin coreceptors. The CAR cytoplasmic and transmembrane domains are dispensable for viral entry, indicating that CAR functions solely as an attachment molecule (26, 45, 46). Once the adenovirus has attached to CAR, subsequent events resulting in viral entry are mediated entirely by integrins, such as avβ3 and avβ5 (48), a5β1 (7), and avβ1 (8, 27). Although complex mechanisms are involved, the adenoviral CAR recognition and interaction represent the rate-limiting step of viral infection (15, 21, 52). Human and murine genes for CAR have been identified, and both proteins enable adenoviral infection when corresponding constructs are transfected into nonpermissive CAR-negative tumor or primary cells (2, 3, 26, 39, 40, 46).
In CAR-expressing cells, the receptor is abundant at tight junctions and functions primarily as an adhesion factor by dimerizing with CAR molecules on adjacent cells. CAR sequestered within tight junctions is inaccessible to adenovirus, thus limiting viral spread across epithelial surfaces (4). In the course of adenoviral replication, fiber protein is excessively produced and secreted. Binding to CAR then results in disruption of junctional integrity, facilitating viral escape across the epithelial barrier (44). Integrity and polarity disorders of epithelial cells by changes in the microenvironment, including early malignant transformation, lead to upregulation of CAR expression (1). It has been described that the transmembrane and cytoplasmic domains of CAR mediate growth-inhibitory signals in tumor cells (36). Therefore, CAR-negative cancer cells are selected in later stages of tumor progression. Reduced expression of CAR in advanced tumor stages is one of the major hurdles of adenoviral cancer gene therapy (28).
Some proteins, including Tat (transactivator of transcription) of human immunodeficiency virus (HIV) (17, 19), VP22 of herpes simplex virus (HSV) (13), and antennapedia homeodomain proteins (AntPs) of Drosophila melanogaster and other species (9, 24) are taken up by mammalian cells via an unknown receptor-independent pathway. The identified domains responsible for this property were referred to as protein transduction domains (PTDs) (9, 13, 43). Sequence analysis revealed that most PTDs are 10 to 30 amino acid residues in length and enriched in basic amino acids, e.g., arginine and lysine. PTDs have been reported to deliver therapeutic proteins (37, 53), antisense oligonucleotides (35), liposomes (41, 42), and plasmid DNA (11, 23, 38) into mammalian cells. However, the therapeutic relevance of PTD-mediated cellular uptake is still controversially discussed. There is evidence that the strong cytosolic and nuclear staining of PTD-fusion proteins in target cells may be due to a fixation artifact (29). Furthermore, intracellular translocation by PTD-fusion proteins is in some cases not efficient enough to mediate significant biologic or therapeutic effects in target cells (14, 25).
It was described that pretreatment of adenoviral particles with short chemically synthesized oligopeptides corresponding to the PTDs of Tat or AntP improved adenoviral infection efficacy in vivo and in vitro (18). However, high concentrations of these peptides had to be applied to obtain significant effects. In our study, PTDs were fused to the ectodomain of the CAR receptor to serve as molecular adapters between the adenoviral fiber knob protein and negatively charged surface structures. Application of these adapter molecules strongly increased the affinity of adenoviral particles to CAR-negative nonpermissive cells, allowing efficient infection. Interestingly, CARex-PTDs not only enabled adenoviral infection of nonpermissive tumor cells but also significantly increased the viral uptake rates in permissive tumor cells. We therefore developed recombinant molecular adapters to broaden the spectrum of adenoviral tropism to CAR-negative cells independent of their receptor expression pattern.
MATERIALS AND METHODS
Cell lines and plasmids.
HepG2, Huh7, HT1080, HT29, SAOS-2, MCF-7, U2-OS, SKLU-1, H4IIE, BNL, RT101, T-36274, HeLa, KLN 205, Jurkat-T, P388D.1, RAW264.7, COS-1, and 293 cell lines were obtained from the American Type Culture Collection (Rockville, Md.). Rat ISC cells were kindly provided by K. Wewetzer (Hanover, Germany). Cells were maintained in growth medium (Dulbecco's modified Eagle's medium plus Glutamax; Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Life Technologies), 100 U of penicillin/ml, and 100 μg of streptomycin (Seromed)/ml at 37°C in 5% CO2. MCF-7 cells were additionally supplied with 5 μg of insulin/ml plus 5 μg of transferrin/ml and 5 ng of sodium selenite (Roche Diagnostics)/ml. DC2.4 cells were kindly provided by K. L. Rock (Worcester, Mass.). DC2.4 cells were maintained in HCM medium (10% FBS, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 mM l-glutamine, 1% MEM nonessential amino acids [Life Technologies], 10 mM HEPES, and 50 μM β-mercaptoethanol).
The plasmid LXSN-hCAR encoding the human CAR (hCAR) was a gift from J. DeGregori (Denver, Colo.). The vector pVP22mycHis 2 was purchased from Invitrogen.
Plasmid construction.
For the generation of CARex-VP22, a fragment coding for signal sequence and extracellular domain of the human CAR receptor (CARex) (amino acids [aa]1 to 235) was generated via PCR using the plasmid LXSN-hCAR as a template. The oligonucleotides 5′-GTGGTACC ATG GCG CTC CTG CTG TGC TTC GTG C-3′ (the KpnI site is in italics and the natural start codon of hCAR is in boldface type) and 5′-TAGCGGCCGCC TTT ATT TGA AGG AGG GAC AAC GTT TAG ACG C-3′ were used as primers (the NotI site is in italics; bases printed in boldface are complementary to the 3′ terminus of the hCAR extracellular domain). The resulting fragment was digested with KpnI and NotI and subcloned into the corresponding restriction sites of pVP22mycHis 2 to create an N-terminal CAR fusion protein (plasmid name, pCARex-VP22). The plasmids encoding fusion proteins of CARex and the putative cellular adhesion domains of the HIV Tat protein and AntP, an oligopeptide consisting of nine arginine residues, and an oligopeptide consisting of Gly/Ser/Thr residues as a competitor were generated with synthetic oligonucleotides. All oligonucleotides described below were hybridized with a respective antisense strand, digested with NotI/XbaI, and inserted into the corresponding sites of pCARex-VP22, thereby replacing the VP22 coding fragment. All of the following oligonucleotides contained a sequence for additional glycine and serine residues for spacing the cellular adhesion domain from CARex; the NotI and XbaI sites are given in italics. For cloning of pCARex-Tat48-57, the oligonucleotide 5′ AAAGC GGC CGC GGA GGA GGA AGT GGA GGA GGA GGA GGC AGG AAG AAG CGG AGA CAG CGA CGA AGA GGT CTA GAAA-3′ (coding for aa 48 to 57 of the HIV-Tat protein,; sequence GRKKRRQRRR) was used. The insertion of the sequence 5′AAAGC GGC CGC GGA GGA GGA AGT GGA GGA GGA GGA CGT CGC CGA CGG AGA AGG AGA CGT AGA GGT CTA GAAA-3′ (the coding sequence for RRRRRRRRR is in boldface type) resulted in the plasmid pCARex-9xArg. The fusion of CARex with AntP was achieved by inserting the oligonucleotide 5′AAGC GGC CGC GGA GGA GGA GGA AGA CAG ATC AAA ATA TGG TTC CAA AAC CGG CGC ATG AAA TGG AAG AAA GGT CTA GAAA-3′ (encoding RQIKIWFQNRRMKWKK, representing aa 62 to 77 of Euprymna scolopes AntP), therefore resulting in the plasmid pCARex-AntP62-77. For the generation of a CARex version lacking a functional cellular adhesion domain, the Tat48-57 coding sequence of pCARex-Tat48-57 was replaced by the sequence 5′-AAAGCGGCCGC GGA GGA GGA AGT GGA TCT GGA TCT GGT TCT GGA TCA ACT GGA TCA ACA TCT GGA GGTCTAGAAA-3′, encoding Gly/Ser/Thr residues.
A Tat48-57 control peptide able to be secreted into the extracellular space was generated by deleting large internal parts of the CARex domain in CARex-Tat, thereby leaving the signal sequence of CAR for proper secretion. pCARex-Tat48-57 was digested with EcoRV and NotI (Klenow fill in), and the vector fragment was then religated in-frame with the linker d(pCGGGATCCCG) (NEB), resulting in the plasmid pCAR1-58-Tat48-57. For the expression of VP22 protein, the vector pVP22mycHis 2 (Invitrogen) was applied.
All plasmids were sequenced and tested for expression of CAR fusion proteins and control proteins by Western blot analysis. A mouse anti-myc antibody (Invitrogen) was used as a primary antibody. Plasmids were prepared with the EndoFree Maxi kit (QIAGEN).
Construction and preparation of recombinant adenovirus.
For the production of larger quantities of purified CARex-VP22 and CARex-Tat proteins, PTD-expressing adenoviruses were constructed. AdCARex-VP22 was generated using an in vitro ligation method (Adeno-X expression system; BD Biosciences). pHM3/pBK-CMV, a modified version of pHM3 which contained the eukaryotic expression cassette of pBK-CMV (Stratagene), was used as a shuttle vector. The cDNA fragment was excised from pCARex-VP22 by digestion with HindIII (Klenow fill in) and PmeI and inserted into the SmaI site of pHM3/pBK-CMV. The PI-Sce/I-CeuI fragment of the resulting plasmid was then cloned into the corresponding sites of the adenoviral backbone plasmid pAdHM4, resulting in the plasmid pAdCARex-VP22. AdCARex-Tat was constructed with the Ad-Easy recombination system according to the manufacturer's instructions (QBiogene). The CARex-Tat fragment was isolated from pCARex-Tat by HindIII/PmeI digestion and ligated into the HindIII/EcoRV sites of pShuttle-CMV. The resulting vector was linearized with PmeI, and homologous recombination with the adenoviral backbone plasmid AdEasy 1 was performed with BJ5183 Escherichia coli (resulting plasmid, pAdCARex-Tat).
For the generation of infectious particles, adenoviral plasmids were linearized with PacI and transfected into permissive 293 cells. After occurrence of a cytopathic effect, cells were harvested and infectious particles were released by three freeze-thaw cycles. Cell debris was removed by centrifugation, and the supernatant was subjected to high-titer preparations. For this purpose, 2 × 108 subconfluently grown 293 cells were infected at a multiplicity of infection (MOI) of 5 to 10. Infected cells were harvested when a strong cytopathic effect could be observed, and approximately 50% of the cells were detached. Cells were collected by centrifugation, and viral particles were released by four freeze-thaw cycles. The crude virus suspension was then purified twice by CsCl density gradient centrifugation. Virus preparations were stored at −20°C in 25% glycerol, 10 mM Tris-HCl (pH 7.4), and 1 mM MgCl2. Viral titer was determined by absorbance at an optical density of 260 nm, and the titer of infectious particles was measured by limiting dilution methods or by using the Rapid Titer kit assay (BD Biosciences) according to the manufacturer's instructions.
Construction and generation of the telomerase-dependent replicating adenovirus (hTERT-Ad) was recently described (50).
Recombinant protein preparation and purification.
Limited amounts of recombinant CARex-PTD fusion proteins were produced in transfected 293 cells. Cells were transfected by calcium phosphate precipitation with 1 μg of expression plasmid for CARex fusion proteins, 0.2 μg of pCMV-luciferase for determination of transfection efficacy, and 3.8 μg of pBluescript (Stratagene). After 36 h, the supernatant of transfected cells was harvested, centrifuged for the removal of detached cells, and subjected to adenovirus infection assays (see below). Luciferase activity was measured in extracts of the cell layer according to standard protocols. The results of the measurements were used to adjust the supernatant application in subsequent infection assays.
Larger quantities of purified recombinant protein were prepared by Ni affinity chromatography. Subconfluent COS-1 cells were infected with AdCARex-Tat or AdCARex-VP22 at an MOI of 25 in DMEM supplemented with 2% FBS and incubated for 48 h at 37°C and 5% CO2. Following incubation, the cells were supplemented with a 1/10 volume of 10× equilibration buffer (500 mM NaH2PO4, 3 M NaCl, 100 mM imidazol) and incubated for an additional 2 min. After removal of the supernatant, the cells were refed with fresh medium for a further incubation period to increase the yield. Combined supernatants were cleared by centrifugation at 1,000 × g for 10 min and subsequent filtration (0.22-μm-pore-size filters), and then subjected to a Ni-nitrilotriacetic acid-agarose (QIAGEN)-containing column equilibrated with 50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazol. The column was washed with 50 mM NaH2PO4, 300 mM NaCl, and 20 mM imidazol and eluted with a buffer containing 50 mM NaH2PO4, 300 mM NaCl, and 50 mM l-histidine. The concentration of CARex-PTD fusion proteins in the eluate was determined by the Bio-Rad protein assay and Sypro-Orange-stained sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Function was controlled by adenoviral infection assays (see below). Eluates were dialyzed against 25% glycerol in DMEM, shock frozen in liquid N2, and stored at −80°C.
Western blot analysis.
CARex-PTD and control proteins were detected in cell lysates and supernatants from transfected cells by Western blot analysis. To generate whole-cell lysates from transfected cells, the medium was removed and the cells were washed with phosphate-buffered saline (PBS). The cells were harvested in ice-cold RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and 0.02% protease inhibitor cocktail σ in PBS) and resuspended with a syringe (0.8-mm-diameter cannula). After a 60-min incubation on ice, cell debris was pelleted by centrifugation (10,000 × g; 4°C for 5 min), and the supernatant was retained as a whole-cell lysate. For the preparation of freeze-thaw lysates, transfected cells were first washed with PBS and then scraped off in 500 μl of PBS per 6-mm dish. The cells were then resuspended by intensive vortexing and subjected to four freeze-thaw cycles in liquid nitrogen. The cell debris was removed as described above. Protein content was measured by using the Bio-Rad protein assay concentrate. Supernatants from transfected cells intended for Western blot analysis were obtained directly from the cell dish, and detached cells were removed by centrifugation (1,000 × g; 5 min). For Western blot analysis, 10 μg of protein extract or 20 μl of supernatant was separated by SDS-polyacrylamide gel electrophoresis, and proteins were blotted onto a polyvinylidene difluoride membrane (Immobilon; Millipore). CARex-PTD or control proteins were detected with a mouse anti-myc antibody (Invitrogen) and a goat horseradish peroxidase-coupled anti-mouse immunoglobulin G antibody (Chemicon). Protein bands were visualized with the Western Lightning Chemiluminescent Reagent Plus according to the manufacturer's protocol (Perkin-Elmer Life Sciences).
Adenoviral infection assays.
The efficacy of adenoviral infection was assessed with a LacZ transgenic adenovirus (AdLacZ, a kind gift from D. Brenner, Chapel Hill, N.C.). Furthermore, an adenoviral vector expressing green fluorescent protein (GFP) (AdGFP, generated by homologous recombination of pTrack-CMV with pAdEasy1) was used for fluorescence microscopy detection or fluorescence-activated cell sorter (FACS) analysis of viral infection. For infection assays, target cells were seeded in 6-cm dishes at a density of 5 × 105 cells/dish and grown to subconfluency on the day of infection. The growth medium was removed, and cells were overlaid with infection medium containing CARex fusion proteins. For this purpose, either supernatants from transfected 293 cells or affinity-purified protein resolved in fresh DMEM (2% FBS) was applied. When affinity-purified protein was used, protein concentration in these assays was generally 2 nM in a total volume of 3 ml unless otherwise noted in the figure legends. Protein-covered cells were incubated for a further 30 min prior to the addition of AdLacZ or AdGFP resolved in 1 ml of medium. Cells were infected for 30 min to 4 h, and the medium was exchanged to terminate the infection. Cell layers were then incubated for transgene expression for 48 h. Alternatively, purified protein or supernatants were directly added to the virus in a total volume of 4 ml and mixed for 10 min in an overhead shaker prior to the infection of target cells. Following infection, cells were treated as described above. Variations of this protocol or additional treatments are indicated in the figure legends.
To visualize the degree of infection, the cell layer was fixed in ethanol, rehydrated with PBS, and stained with 1 mg of X-Gal substrate (5-bromo-4-chloro-3-indolyl-β-d-galactoside)/ml, 5 mM Fe3+, 5 mM Fe2+, and 2 mM MgCl2 in PBS. Infection efficacy was assessed by measuring β-galactosidase activity in cell extracts. For this purpose, cells were treated in an appropriate amount of lysis buffer (25 mM Tris-phosphate [pH 7.8], 2 mM EDTA, 2 mM dithiothreitol, 10% glycerol, and 1% Triton X-100) and incubated for 10 min. Cells were scraped off and centrifuged for 5 min at 10,000 × g to remove cell debris. β-Galactosidase activity was measured by adding lysate samples to a reaction buffer containing 1 mg of o-nitrophenyl-β-d-galactopyranoside/ml, 60 mM Na2HPO4, 39 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, and 2 mM dithiothreitol. The reaction was carried out at 37°C and terminated by the addition of a 0.5 volume of 1 M Na2CO3. Substrate turnover was measured with a spectrophotometer at a 405-nm wavelength. To determine the infection degree by means of GFP fluorescence, FACS analysis was performed. Infected cells were detached with EDTA and transferred to FACS tubes. After a centrifugation step (200 × g; 5 min) cells were rinsed with 1 ml of FACS buffer (10 mM HEPES, 1% bovine serum albumin, and 0.1% NaN3 in PBS) and centrifuged again. The cell pellet was resuspended in 500 μl of FACS buffer or an appropriate volume, and a minimum of 50,000 events was counted with a FACScalibur (Beckton Dickinson) with CellQuest software.
Cytolysis assays.
Tumor cells were seeded in 24-well plates at a density of 105 cells/well. After 12 h, cells were infected with hTERT-Ad and the adenovirus wild type (Ad-wt) as replication competent vectors and AdGFP as a nonreplicating control at an MOI of 1 or 0.1. After 4 h of infection, the medium was removed and washed with PBS. Subsequently, cells were infected with AdCARex-VP22, AdCARex-Tat, and AdGFP (control) at an MOI of 2 (for permissive cell lines Huh7 and HepG2) or an MOI of 25 (for low-permissive cell lines SAOS-2, HT1080, and MCF-7), respectively. At 4 h after infection, the medium was exchanged, and cells were further incubated until the occurrence of cytopathic effects. Crystal violet staining was used to demonstrate replication-associated cytopathic effects and subsequent destruction of the cell layer. Six days after infection, the medium was removed, and the cell layer was rinsed with PBS. The wash medium was aspirated, and cells were fixed for 10 min with 10% buffered formaline. Cells were rinsed again with PBS and stained for 30 min with 0.1% crystal violet in 10% ethanol.
RESULTS
PTDs fused to the extracellular domain of CAR facilitate adenoviral infection of nonpermissive carcinoma cells.
Expression of the coxsackievirus adenovirus receptor (CAR) on target cell membranes determines susceptibility to adenoviral infection, thus limiting adenovirus-mediated gene transfer. This leads to a restricted access to tumor cells by these vectors, as tumor cells tend to downregulate CAR expression in the course of malignant progression. Since it has been shown that the transmembrane and cytoplasmic domains of CAR are dispensable for adenoviral infection, we hypothesized that fusion proteins consisting of CARex and PTDs could overcome the obstacle of insufficient target cell recognition by adenovirus. PTDs are known to attach to cellular membranes independent from a single specific receptor molecule. The usage of adapter-like properties of these molecules should lead to adenoviral target cell binding regardless of the CAR expression status of the respective cell. In initial experiments, we used the entire ectodomain of CAR (aa 1 to 235) to construct fusion proteins with PTDs corresponding to nine arginine residues or natural PTD motifs derived from HIV-Tat and AntP. Also, the whole VP22 protein from HSV was included (see Fig. 1 for a summary of genetic construction). The chosen CAR sequence also contained the natural N-terminal signal sequence (Ld) for proper secretion of recombinant CARex-PTD proteins. In the experiments, we also included a construct for testing the effects of Tat-PTD alone on adenoviral infection. This was achieved by deleting large parts of the CARex domain of CARex-Tat but leaving the CAR signal sequence. Effects on adenoviral infection mediated by VP22 alone were controlled by the commercially available vector expressing VP22. VP22 is known to leave the producer cell, subsequently adhering and transducing neighboring cells. The CARex domain linked to 10 Gly/Ser/Thr residues as a nonfunctional PTD was used as an additional negative control (termed CARex) or as competitor in later experiments. Expression and secretion of the constructed proteins were investigated by Western blot analysis (Fig. 2A) of whole-cell extracts and supernatants of transfected 293 cells. Though all proteins were correctly expressed as shown by whole-cell extract analysis, CARex-9xR and VP22 could not be detected in the supernatant of producer cells (an additional band different from the expected size occurred in VP22 supernatants). There is evidence that a 9-mer of arginine mediates cellular uptake of coupled agents much more efficiently than AntP or Tat (47), suggesting a higher affinity to the respective target structures. Thus, either an improper release and/or efficient rebinding to the producer cells might be considered reasons for the absence of soluble CARex-9xR.
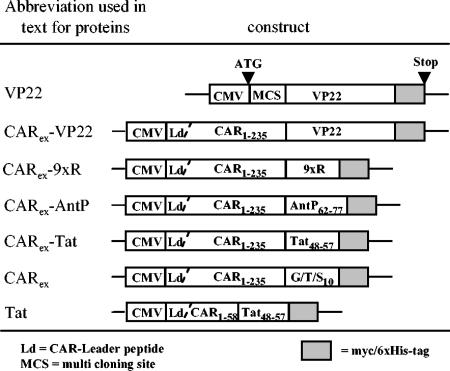
FIG. 1.
Plasmid construction overview. Names of the corresponding plasmids are (from top to bottom): pVP22mycHis 2, pCARex-VP22, pCARex-9xArg, pCARex-AntP62-77, pCARex-Tat48-57, pCARex-G/T/S10, and pCAR1-58-Tat48-57.
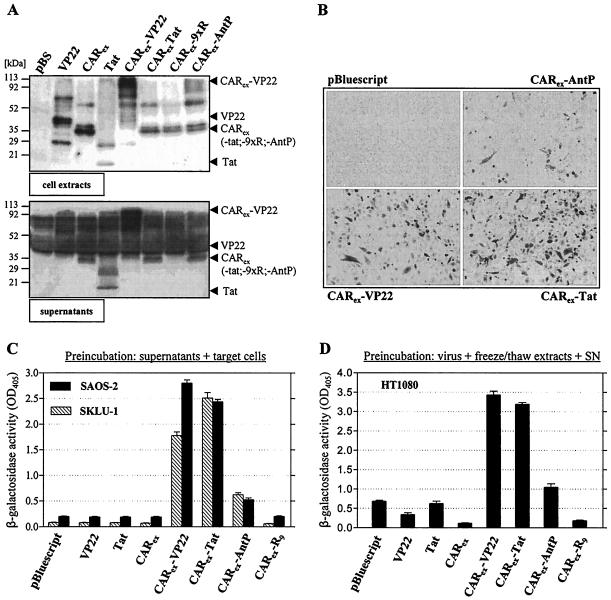
FIG. 2.
Soluble recombinant fusion proteins consisting of the entire ectodomain of hCAR and cell adhesion peptides derived from Tat, VP22, and AntP enable adenoviral infection of SKLU-1, SAOS-2, and HT1080 cells. 293 cells were transfected with expression vectors for CARex-PTDs and control proteins as indicated in the figure. (A) Samples of whole-cell extracts (top) and supernatants (bottom) of producer cells were analyzed for CARex-PTD presence by Western blotting. (B) SKLU-1 cells were treated with equivalent amounts of supernatants from CARex-PTD-transfected 293 cells. AdLacZ was added at an MOI of 10, and the infection was carried out for 4 h. After 48 h, infected SKLU-1 cells were visualized by X-Gal staining. (C) SKLU-1 and SAOS-2 cells were treated as described in the legend to panel B. Infection efficacy was determined by measuring β-galactosidase activity in cell extracts. (D) CARex-PTD-transfected 293 cells were lysed by freeze-thaw cycles. Freeze-thaw lysates were diluted in their respective supernatants, combined with AdLacZ (MOI, 10), incubated for 10 min, and then applied to HT1080 target cells for 4 h. Infection efficacy was determined by a β-galactosidase assay.
To investigate whether application of Tat or chimeric CARex-PTD proteins is able to improve viral uptake, we pretreated nonpermissive SKLU-1 and SAOS-2 cells with supernatants of transfected 293 cells. To monitor successful adenoviral infection, an adenovirus encoding LacZ was applied (AdLacZ). Subsequently, efficacy of viral infection was assessed by β-galactosidase staining and β-galactosidase activity measurements. Treatment of nonpermissive SKLU-1 cells with CARex-VP22, CARex-Tat, and CARex-AntP allowed for efficient adenoviral infection (Fig. 2B). However, the CAR fusion of AntP was less effective than fusion proteins of Tat and VP22. In contrast to the chimeric CARex-PTD proteins, neither CARex nor Tat alone improved adenoviral uptake, as these proteins lack either affinity to target cells or the adenoviral particle, respectively (Fig. 2C). The results could be confirmed with SAOS-2 cells, which possess a low but detectable susceptibility to viral infection. To include putative effects of VP22 and CARex-9xR (which are not secreted or improperly secreted), we combined freeze-thaw extracts of producer cells and the corresponding supernatants for pretreatment of the reporter virus (Fig. 2D). Subsequent infection of HT1080 cells confirmed the efficacy of CARex-VP22, CARex-Tat and, at least in part, CARex-AntP-mediated adenoviral infection, whereas Tat alone had no effect compared to the control (Fig. 2D). Furthermore, the results revealed an inhibitory effect on adenoviral infection when the CAR ectodomain was applied, showing that this protein can act as a competitor for natural CAR-mediated adenoviral infection. Interestingly, for unknown reasons, the low susceptibility of HT1080 cells was further decreased after the addition of CARex-9xR. Together, the results showed that CARex-VP22 and CARex-Tat represent functional adapter molecules for adenoviral infection. These proteins can be physiologically expressed and obtained in a soluble form, which is an important prerequisite for future in vivo applications. For further studies, we therefore focused on CARex-VP22 and CARex-Tat proteins and constructed corresponding transgenic adenoviruses for efficient expression and subsequent purification of both fusion proteins. Purified recombinant CARex-PTD was then subjected to experiments to assess improvement of viral infection in a large panel of nonpermissive or low-permissive tumor cell lines. The study included rat hepatoma (H4IIE), mouse hepatoma (BNL), mouse skin epidermal (RT-101 and T-36274), human colon carcinoma (RKO), human osteosarcoma (SAOS-2), human lung adenocarcinoma (SKLU-1), human breast carcinoma (MCF-7), and human fibrosarcoma (HT1080) cell lines. In all nonpermissive cancer cells investigated, pretreatment with 2 nM recombinant CARex-VP22 and CARex-Tat allowed efficient adenoviral infection at low virus titers (Fig. 3A and B). Adenoviral gene transfer was most enhanced in SKLU-1, BNL, RT-101, and T36274 cells, as these cell lines are completely nonpermissive for untreated virus. Other cell lines tested were at least partially permissive to untreated virus after long-time exposure, suggesting that they can be infected to an acceptable degree when very high virus titers are used. With CARex-PTD- treated virus, these cells could be efficiently infected at an MOI of 10. However, as the given cell population was not completely infected under these experimental conditions, we enhanced protein application and virus MOI within a modest range. Figure 3C demonstrates that MCF-7, H4IIE, HT1080, and RKO cells can be almost completely transduced (80 to 100%) using 8 nM adapter protein and AdGFP at an MOI of 30. Together, these results confirmed the broad and effective applicability of CARex-PTD-mediated adenoviral gene transfer.
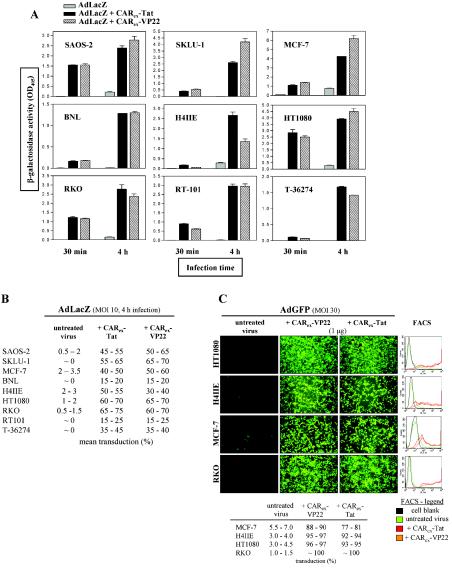
FIG. 3.
Determination of CARex-VP22- and CARex-Tat-mediated adenoviral infection in nonpermissive or partially permissive tumor cell lines. (A) Different target cell types were overlaid with medium containing purified CARex-VP22 or CARex-Tat at a concentration of 2 nM. AdLacZ (MOI, 10) was then added, and infection was carried out for 30 min or 4 h as indicated. After 48 h of incubation, infection efficacy was determined by measuring β-galactosidase activity in extracts from infected cells. (B) The total degree of infection was determined by X-Gal staining. (C) A total of 1 μg of recombinant protein was dissolved in medium and combined with AdGFP (MOI, 30) and incubated for 10 min prior to a 4-h infection of target cells. After 48 h, GFP expression was monitored by fluorescence microscopy, and infection efficacy was determined by FACS analysis. The results indicate that adenovirus treatment with CARex-VP22 and CARex-Tat facilitates effective infection of nonpermissive cells.
Application of CARex-PTDs improves virus uptake rate in permissive carcinoma cell lines.
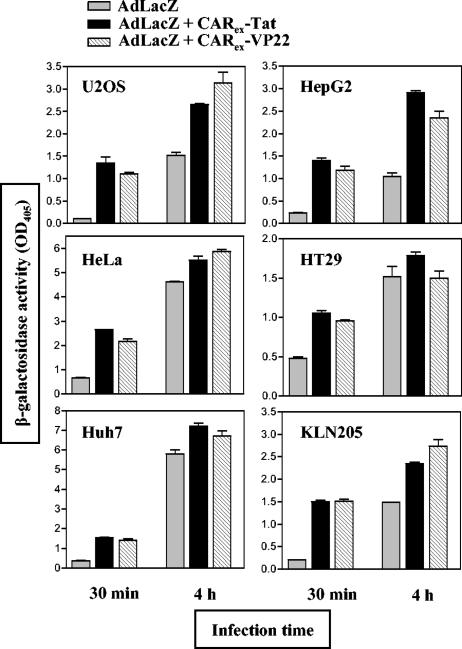
The binding of CAR to the adenoviral fiber knob protein represents a typical receptor-ligand interaction whose affinity is comparable to CAR-CAR interactions. CAR dimerization is essential for physiological CAR functions as adhesion factor and CAR-CAR binding is disrupted in the presence of fiber knob proteins. As PTDs do not bind to a single specific receptor, it is difficult to estimate their affinity to potential target structures in vivo. Assuming that PTDs have perhaps less affinity to their target structures than fiber knob has to CAR, we considered that CARex-PTD binding to adenoviruses might result in reduced infection of permissive tumor cells. Permissive cell lines, including human hepatoma (HepG2 and Huh7), colon carcinoma (HT29), cervix carcinoma (HeLa), osteosarcoma (U2OS), and mouse lung squamous carcinoma (KLN205) cell lines were investigated. Interestingly, a competitive CARex-PTD-mediated inhibition of adenoviral infection could not be observed. Moreover, application of CARex-VP22 and CARex-Tat significantly improved the virus uptake rate in all permissive tumor cells tested when the target cells were exposed to the virus for only a short period of time (Fig. 4). When the exposure time was prolonged to 4 h, the effect was diminished or disappeared in several cell lines, because transduction efficacy in permissive cells under these conditions is mainly determined by the given MOI. These observations indicate that the broadened tropism of CARex-PTD-treated adenovirus does not result in reduction, but in improvement of viral uptake rate in permissive CAR-expressing cells.
FIG. 4.
CARex-VP22 and CARex-Tat improve adenoviral infection of permissive tumor cell lines. Different target cell types were overlaid with medium containing purified CARex-VP22 or CARex-Tat, respectively, at a concentration of 2 nM. AdLacZ (MOI, 10) was then added, and infection was carried out for 30 min or 4 h as indicated. After 48 h of incubation, infection efficacy was determined by β-galactosidase assays of extracts from infected cells.
Binding of CARex-PTD both to adenovirus fiber and to cellular proteoglycans is essential for improvement of adenoviral infection.
Our results showed that neither CARex nor PTD alone improves uptake of adenovirus in cells. In contrast, CARex-PTD improved adenoviral infection efficiently, suggesting that CARex-PTD indeed functions as a molecular adapter between the adenoviral fiber knob protein and PTD-affinity target structures on cellular membranes. To confirm that specific binding of PTD to adenoviral particles is essential for PTD-mediated viral infection, binding of CARex-VP22 and CARex-Tat to adenoviral particles was competed for by CARex prior to adenoviral infection. Blocking of adenoviral fiber knobs with CARex inhibited CARex-PTD-mediated adenoviral infection (Fig. 5A), consistent with our hypothesis that specific binding of PTD to viral particles via the CARex domain is important for the improvement of infection.
FIG. 5.
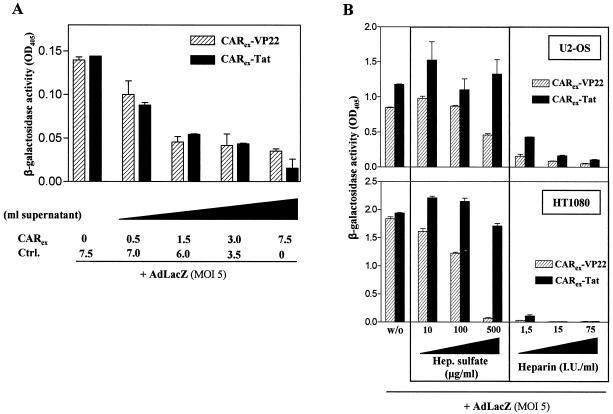
CARex-VP22- or CARex-Tat-mediated adenoviral infection can be competed by recombinant CARex lacking a functional PTD and is effectively inhibited by negatively charged heparin and/or heparan sulfate. (A) To block fiber knobs of AdLacZ, the virus was treated for 15 min with various amounts of supernatant from 293 cells expressing CARex. Then, purified CARex-VP22 or CARex-Tat was added at a final concentration of 1 nM, and the mixture was subjected to SKLU-1 cells for an infection period of 30 min. Infected cells were maintained for 48 h and infection efficacy was determined by measuring β-galactosidase activity in cellular extracts. (B) U2-OS (top) and HT1080 (bottom) cells were covered with medium containing 2 nM purified CARex-PTD fusion proteins and increasing amounts of heparan sulfate or heparin, as indicated. AdLacZ (MOI, 5) was added, and infection was carried out for 30 min. Infected cells were maintained for 48 h, and infection efficacy was determined by β-galactosidase assays of cellular extracts.
It has been shown that positively charged PTDs adhere to heparan sulfate-glycosaminoglycans on cell surfaces and that this interaction can be inhibited by negatively charged heparin and soluble heparan sulfate (5). To investigate the role of CARex-PTD protein binding to glycosaminoglycans in PTD-mediated viral infection, we treated cells with increasing doses of heparan sulfate or heparin. Heparan sulfate and heparin inhibited CARex-VP22-mediated viral infection in a dose dependent manner. CARex-Tat-mediated viral infection was also significantly inhibited by heparin but not by heparan sulfate (Fig. 3B), suggesting differential binding of CARex-Tat and CARex-VP22 to surface glycosaminoglycans. Taken together, the inhibition of PTD-mediated viral infection by CARex and heparin confirms that binding of CARex-PTD to adenovirus fiber and to cellular glycosaminoglycans are both essential for CARex-PTD-mediated adenoviral infection.
CARex-PTD and adenovirus form stable complexes.
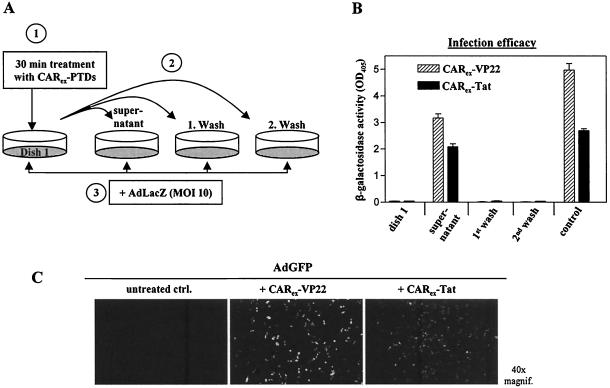
Competition experiments using CARex as described above (Fig. 2D and 5A) pointed to a mechanism involving the coating of virus particles by CARex-PTD binding to adenoviral fiber knobs. To clarify this issue, we next investigated whether CARex-PTD proteins are stably attached to adenoviral particles by binding to fiber knob proteins. Alternatively, we considered that the cell surface is covered by CARex-PTDs through PTD-glycosaminoglycan interaction, thus exposing the CAR domain to viral particles. To estimate binding of CARex-PTD proteins to cell membranes, SKLU-1 cells were treated with CARex-VP22 or CARex-Tat for 30 min (Fig. 6A, dish 1). Subsequently, the supernatant of these cells and subsequent wash fractions were transferred to separate cell dishes, which were then infected with AdLacZ. Only cells that received the supernatant of the initially treated cell layer showed an enhanced susceptibility for adenoviral infection, suggesting low binding strength of CARex-PTD to cell surfaces. Like cells from dish 1 (Fig. 6A), both cell layers treated with the wash fractions did not show any adenoviral infection, indicating that CARex-PTD proteins were almost completely removed simply by transferring the supernatant.
FIG. 6.
CARex-PTD fusion proteins form stable complexes with adenoviral vectors. SKLU-1 cells (dish 1) were treated with CARex fusion proteins (2 nM) and incubated for 30 min. The supernatant and two wash fractions (medium) were then transferred to separate SKLU-1 dishes. For the control sample, the supernatant was left on a dish. Subsequently, all cells were infected with AdLacZ (MOI, 10) for 4 h and incubated for transgene expression. (A) Experimental setup. (B) Results of the corresponding β-galactosidase activity measurements. (C) AdGFP was dialyzed against DMEM and treated with purified recombinant CARex-VP22 or CARex-Tat in a 10-fold molar excess relative to adenoviral fiber knob molecules of AdGFP. As a control, AdGFP received DMEM alone. The virus preparations were then subjected to a CsCl density gradient ultracentrifugation for separation of unbound protein. The virus band was extracted, dialyzed against DMEM, and quantified by determination of optical density at 260 nm. Infection of SKLU-1 cells was carried out for 4 h at an MOI of 50.
To investigate the stability of CARex-PTD attachment to adenoviral fiber knobs, AdGFP was treated with purified recombinant CARex-VP22 or CARex-Tat in a 10-fold molar excess regarding the fiber knob molecules. To separate unbound proteins from viral particles, the adenovirus preparations were subjected to a CsCl density gradient ultracentrifugation. CARex-PTD-treated adenoviruses still infected nonpermissive tumor cells effectively, even after a density gradient ultracentrifugation (Fig. 6C), indicating the formation of stable complexes of adenoviral particles with CARex-PTDs. As a consequence of these observations together with the competition results described above, we concluded that the molecular mechanism of CARex-PTD-mediated viral infection preferably involves stable complexing of viral particles with CARex-PTDs prior to cell surface binding, rather than CARex-PTD coating of cell surfaces prior to recognition by adenoviral particles.
CARex-VP22 is more stable than CARex-Tat.
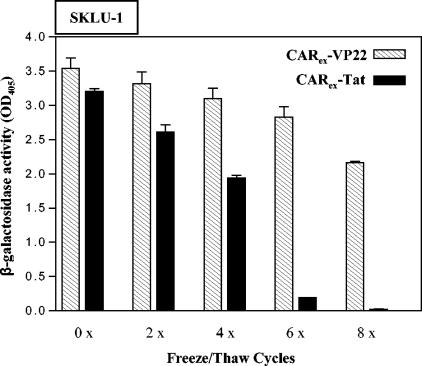
Recombinant CARex-PTD proteins might serve as effective agents to improve adenoviral infection and to broaden the application spectrum of adenoviral vectors. For wide applicability, the stability of protein isolates is an important requirement. We therefore investigated stability of CARex-PTD by treating protein isolates with repeated freeze-thaw cycles. As shown in Fig. 7, both CARex-PTD proteins retained most of their capability to improve adenoviral infection following at least four freeze-thaw cycles. However, the activity of CARex-Tat was largely destroyed after six freeze-thaw cycles, whereas CARex-VP22 was almost stable. Consistent with these findings, CARex-VP22 was also more resistant to heat than CARex-Tat (data not shown).
FIG. 7.
Purified recombinant CARex-VP22 reveals higher stability against thermal influence than CARex-Tat. Purified recombinant CARex-VP22 and CARex-Tat preparations were subjected to freeze-thaw cycles as indicated, mixed with medium, and added to SKLU-1 target cells at a final concentration of 2 nM. Subsequently, cells were infected with AdLacZ (MOI, 10). Infection efficacy was determined by β-galactosidase assays of cellular extracts from infected cells.
CARex-PTDs improve adenoviral infection of nonepithelial cells.
Over the past several years, a number of gene therapeutic approaches have been investigated with adenoviruses in therapeutically relevant nonepithelial cell types, such as T cells (34), macrophages (16, 51), dendritic cells (22, 30, 33), or neuronal cells (31). As nonepithelial cells express little or no CAR, an acceptable degree of viral infection usually requires very high adenovirus doses, which often impair target cell specific functions and/or result in cytopathic effects due to fiber knob-related toxicity.
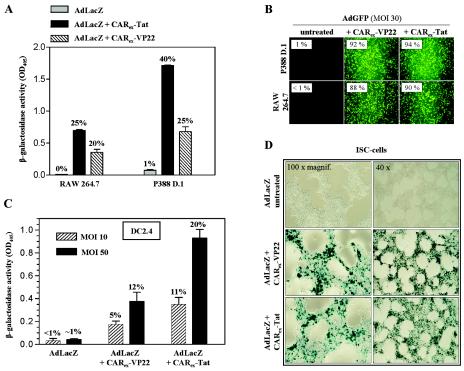
Therefore, we investigated the utility of CARex-PTD proteins for improvement of adenoviral infection of cell lines representing macrophages, Schwann cells, and dendritic cells. As shown in Fig. 8A, CARex-Tat and CARex-VP22 strongly improved adenoviral uptake at a low MOI in RAW264.7 and P388 D.1 cells. These cell types could be almost completely transduced with an MOI of 30 and 8 nM recombinant CARex-PTD (Fig. 8B). CARex-PTD application improved transduction of DC2.4 dendritic cells (Fig. 8C) and Jurkat T-cells (data not shown). Furthermore, CARex-PTD-mediated adenoviral infection was highly effective in rat immortalized Schwann cells (Fig. 8D). The results underline that CARex-PTD proteins are valuable tools for efficient and nontoxic adenoviral gene transfer in a large variety of cell types.
FIG. 8.
Application of CARex fusion proteins increases adenoviral infection in cell lines derived from immune cells and the nervous system. (A) RAW264.7 macrophages and P388D.1 monocyte/macrophage cells were treated with 2 nM purified recombinant CARex-VP22 or CARex-Tat, respectively, and infected with AdLacZ (MOI of 10; 4 h). Infection efficacy was determined by β-galactosidase assay and X-Gal staining (degree of infection is indicated on top of the bars). (B) AdGFP (MOI, 30) was preincubated with CARex-VP22 or CARex-Tat at a final concentration of 8 nM and then added to RAW264.7 and p388D.1 target cells. Infection efficacy was determined by fluorescence microscopy and FACS. (C) AdLacZ treated with 2 nM CARex-VP22 or CARex-Tat was applied to DC2.4 dendritic cells at MOIs of 10 and 50 for an infection time of 30 min. Infection was quantified by β-galactosidase assay or FACS analysis (AdGFP was applied under the same conditions). (D) Immortalized Schwann (ISC) cells were treated as described in the legend to panel A. The degree of infected ISC cells was visualized by X-Gal staining.
Receptor-independent infection by CARex-PTD enhances lysis of tumor cells by conditionally replicating vectors.
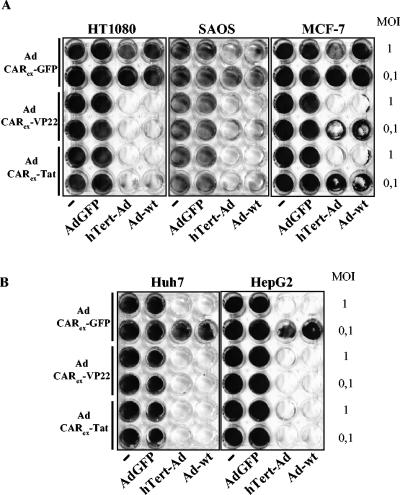
Virotherapy of solid tumors is often impaired by the lack of virus spreading in virus-receptor negative tumors. Several attempts have been made to enhance the oncolytic activity of conditionally replicating viruses by retargeting of vectors to tumor-specific receptors, such as epidermal growth factor receptor. However, receptor-mediated infection is a general limitation of virotherapy because of the heterogeneity of receptor expression in human tumors. The accelerated receptor-independent viral infection by CARex-PTDs may be a potential tool to overcome a major bottleneck in the field of oncolytic virotherapy, namely the lack of efficient viral spreading caused by barriers of receptor-negative tumor cells. To investigate whether physiological expression of CARex-PTD is suitable to improve infection and viral spreading of replicating adenoviruses, we treated tumor cells with Ad-wt or with hTERT-Ad. Expression of CARex-PTDs by nonreplicating adenoviruses facilitated efficient lysis of nonpermissive tumor cells (Fig. 9A). The same results were obtained when the CARex-PTD-expressing and control viruses were precoated with purified CARex-VP22 (in a 10-fold molar excess according to the fiber knob) and when the viruses were subsequently applied at a lowered MOI of 2 (data not shown). This procedure should exclude the possibility that the results can be attributed to a CARex-PTD-mediated changing of the effective MOI of the initial infection.
FIG. 9.
Receptor-independent infection mediated by CARex-PTD enhances lysis of tumor cell layers by conditionally and nonconditionally replicating Ad vectors. Tumor cell layers were infected with hTERT-Ad, Ad-wt, and Ad-GFP (nonreplicating control) at the MOI indicated. Furthermore, the cells were infected with AdCARex-VP22, AdCARex-Tat, and AdGFP (control) at an MOI of 25 (for low-permissive cell lines SAOS-2, HT1080, and MCF-7) (A) or an MOI of 2 (for permissive cell lines Huh7 and HepG2) (B). After 6 days, replication-associated cell layer destruction was visualized by crystal violet staining.
Additionally, CARex-PTD-mediated viral infection enhanced the oncolytic activity of hTERT-Ad and Ad-wt in permissive tumor cells as well, indicating an improved uptake of vectors mediated by CARex-PTDs compared to CAR-receptor-mediated infection (Fig. 9B).
DISCUSSION
Viral infection requires binding of viral particles to the plasma membrane. As a consequence, expression of viral receptors determines viral tropism and frequently limits effective gene delivery by viral vectors. It has recently been shown that application of small synthetic Tat- and AntP-PTD peptides may increase viral entry and that these molecules could improve gene expression at reduced viral titers to circumvent cytotoxicity (18). Regarding our physiological experimental conditions, only PTDs fused to the viral receptor domain CARex, which facilitated adenoviral infection of nonpermissive cells at low titers. There was no evidence for an infection-improving effect mediated by Tat-PTD alone, indicating that specific binding of the PTD to the viral particle via the CARex domain is necessary for efficient PTD-mediated viral infection. However, the discrepancy of results may be explained by different experimental conditions. Whereas Gratton et al. (18) preincubated adenoviral particles with up to 0.5 mM synthetic AntP-PTD or Tat-PTD peptides, we used either supernatants containing physiologically expressed CARex-Tat or purified CARex-Tat at concentrations of 2 nM to obtain significant effects. Assuming that the protein in our preparations was fully active, the concentrations differed by a calculated factor of 2.5 × 105 fold, comparing both approaches. This underlines the efficacy and physiological relevance of CARex-PTD application for adenoviral gene transfer. The importance of PTD binding to viral particles via CARex was further confirmed by blocking of adenoviral fiber knobs through CARex, which resulted in a dose-dependent competitive inhibition of CARex-PTD-mediated adenoviral infection. Furthermore, we were able to stably complex adenoviral particles with CARex-PTDs. CARex-PTD binding to adenoviral particles was resistant to density gradient ultracentrifugation. As we found no evidence of strong binding of CARex-PTDs to cellular surfaces, we postulate a mechanism for CARex-PTD-mediated adenoviral infection including CARex-PTD covering of the viral fiber knobs, subsequently leading to cell surface attachment via PTD. However, the exact mechanisms of subsequent particle internalization following CARex-PTD-mediated cell surface recognition have to be elucidated and await future experiments. Primarily, the role of integrins is of significant interest. Furthermore, CARex-PTD-mediated adenoviral infection can serve as a suitable experimental model to identify and characterize further target structures for HIV Tat and HSV VP22.
Since many cancer cells downregulate CAR expression, several attempts have been made to retarget adenoviral vectors to tumors to achieve effective infection. To overcome low affinity of adenoviral vectors to CAR-negative tumor cells, bispecific antibodies or fusion proteins which bind the adenoviral fiber knob and target receptors overrepresented on tumor cell surfaces, such as epidermal growth factor receptor, have been used to mediate viral attachment and infection (6, 10, 12, 20, 32). Another strategy to enhance the affinity of adenoviruses to cells includes mutations of the fiber knob by inserting arginine-glycine-aspartic acid (RGD)-charged or negatively charged polylysine motifs (49). However, incorporating mutations into the fiber knob can depress fiber protein synthesis, which results in significantly decreased viral titers and impaired viral spreading within a tumor tissue.
Our results showed that receptor-specific targeting is not essential for efficient viral infection. PTDs linked to viral particles sufficiently mediate viral infection in all nonpermissive cells investigated. Additionally, CARex-PTD application significantly enhances viral uptake in permissive cancer cells, demonstrating that CARex-PTD-mediated infection is equivalent to or, under certain conditions, even more effective than receptor-specific viral uptake. Retargeting of viral vectors to receptors overexpressed by tumors may lead to a reduction of undesirable side effects in vivo but may also strongly limit the efficacy of gene delivery, considering heterogeneous receptor expression of tumor subpopulations within tumor entities. Tumor-restricted replication of viral vectors combined with CARex-PTD-mediated spreading of the replicating vectors within tumor tissue may be a solution to this dilemma. We therefore investigated whether expression of CARex-PTD improves the oncolytic activity of a telomerase-dependent conditionally replicating adenovirus. Interestingly, PTD-mediated viral infection enhanced not only oncolysis of nonpermissive tumor cells but also highly permissive cancer cells, indicating that receptor-independent infection by PTD is an attractive strategy for virotherapy of tumors. The CARex-PTD-mediated adenoviral infection retargets the virus to nonspecific cell surface structures also abundant on normal cells. Hypothetically, this could raise safety and toxicity problems, which certainly have to be addressed in future in vivo experiments. However, if CARex-PTD is encoded by a tumor-specific conditionally replicating virus (such as hTERT-Ad), high concentrations of extracellular CARex-PTD should be restricted to regions where the virus productively replicates.
Gene transfer with adenoviral vectors in nonepithelial cells, such as T cells (34), macrophages (16, 51), or dendritic cells (22, 30, 33), has been reported. However, high virus doses and prolonged exposure of target cells to virus were required, and cytopathic effects and changes of the target cell properties are considerable shortcomings of this approach. In contrast, CARex-PTDs facilitate efficient adenoviral infection at low titers and short exposure times, avoiding vector-related toxicity. Since there was no evidence of cytotoxicity of recombinant CARex-PTD fusion proteins in our experiments (Fig. 2B and 8B and data not shown), PTDs fused to viral receptors may be valuable tools to improve and broaden the applicability of virus-mediated gene transfer.
Acknowledgments
Research was supported by a grant from the Deutsche Forschungsgemeinschaft (KU 12/13 3/2).
We thank Kenneth Rock (Dana Farber Cancer Institute, Worcester, Mass.) for kindly providing the dendritic cell line DC2.4.
REFERENCES
- 1.Anders, M., R. Hansen, R. X. Ding, K. A. Rauen, M. J. Bissell, and W. M. Korn. 2003. Disruption of 3D tissue integrity facilitates adenovirus infection by deregulating the coxsackievirus and adenovirus receptor. Proc. Natl. Acad. Sci. USA 100:1943-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson, J. M., A. Krithivas, L. Celi, G. Droguett, M. S. Horwitz, T. Wickham, R. L. Crowell, and R. W. Finberg. 1998. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J. Virol. 72:415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, C. J., J. T. Shieh, R. J. Pickles, T. Okegawa, J. T. Hsieh, and J. M. Bergelson. 2001. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 98:15191-15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Console, S., C. Marty, C. Garcia-Echeverria, R. Schwendener, and K. Ballmer-Hofer. 2003. Antennapedia and HIV transactivator of transcription (TAT) “protein transduction domains” promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J. Biol. Chem. 278:35109-35114. [DOI] [PubMed] [Google Scholar]
- 6.Cripe, T. P., E. J. Dunphy, A. D. Holub, A. Saini, N. H. Vasi, Y. Y. Mahller, M. H. Collins, J. D. Snyder, V. Krasnykh, D. T. Curiel, T. J. Wickham, J. DeGregori, J. M. Bergelson, and M. A. Currier. 2001. Fiber knob modifications overcome low, heterogeneous expression of the coxsackievirus-adenovirus receptor that limits adenovirus gene transfer and oncolysis for human rhabdomyosarcoma cells. Cancer Res. 61:2953-2960. [PubMed] [Google Scholar]
- 7.Davison, E., R. M. Diaz, I. R. Hart, G. Santis, and J. F. Marshall. 1997. Integrin α5β1-mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. J. Virol. 71:6204-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davison, E., I. Kirby, J. Whitehouse, I. Hart, J. F. Marshall, and G. Santis. 2001. Adenovirus type 5 uptake by lung adenocarcinoma cells in culture correlates with Ad5 fibre binding is mediated by α(v)β1 integrin and can be modulated by changes in β1 integrin function. J. Gene Med. 3:550-559. [DOI] [PubMed] [Google Scholar]
- 9.Derossi, D., A. H. Joliot, G. Chassaing, and A. Prochiantz. 1994. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 269:10444-10450. [PubMed] [Google Scholar]
- 10.Dmitriev, I., E. Kashentseva, B. E. Rogers, V. Krasnykh, and D. T. Curiel. 2000. Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J. Virol. 74:6875-6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eguchi, A., T. Akuta, H. Okuyama, T. Senda, H. Yokoi, H. Inokuchi, S. Fujita, T. Hayakawa, K. Takeda, M. Hasegawa, and M. Nakanishi. 2001. Protein transduction domain of HIV-1 Tat protein promotes efficient delivery of DNA into mammalian cells. J. Biol. Chem. 276:26204-26210. [DOI] [PubMed] [Google Scholar]
- 12.Einfeld, D. A., D. E. Brough, P. W. Roelvink, I. Kovesdi, and T. J. Wickham. 1999. Construction of a pseudoreceptor that mediates transduction by adenoviruses expressing a ligand in fiber or penton base. J. Virol. 73:9130-9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott, G., and P. O'Hare. 1997. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell 88:223-233. [DOI] [PubMed] [Google Scholar]
- 14.Falnes, P. O., J. Wesche, and S. Olsnes. 2001. Ability of the Tat basic domain and VP22 to mediate cell binding, but not membrane translocation of the diphtheria toxin A-fragment. Biochemistry 40:4349-4358. [DOI] [PubMed] [Google Scholar]
- 15.Fechner, H., X. Wang, H. Wang, A. Jansen, M. Pauschinger, H. Scherubl, J. M. Bergelson, H. P. Schultheiss, and W. Poller. 2000. Trans-complementation of vector replication versus coxsackie-adenovirus-receptor overexpression to improve transgene expression in poorly permissive cancer cells. Gene Ther. 7:1954-1968. [DOI] [PubMed] [Google Scholar]
- 16.Foxwell, B., K. Browne, J. Bondeson, C. Clarke, R. de Martin, F. Brennan, and M. Feldmann. 1998. Efficient adenoviral infection with IκB α reveals that macrophage tumor necrosis factor alpha production in rheumatoid arthritis is NF-κB dependent. Proc. Natl. Acad. Sci. USA 95:8211-8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frankel, A. D., and C. O. Pabo. 1988. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55:1189-1193. [DOI] [PubMed] [Google Scholar]
- 18.Gratton, J. P., J. Yu, J. W. Griffith, R. W. Babbitt, R. S. Scotland, R. Hickey, F. J. Giordano, and W. C. Sessa. 2003. Cell-permeable peptides improve cellular uptake and therapeutic gene delivery of replication-deficient viruses in cells and in vivo. Nat. Med. 9:357-362. [DOI] [PubMed] [Google Scholar]
- 19.Green, M., and P. M. Loewenstein. 1988. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 55:1179-1188. [DOI] [PubMed] [Google Scholar]
- 20.Hemminki, A., I. Dmitriev, B. Liu, R. A. Desmond, R. Alemany, and D. T. Curiel. 2001. Targeting oncolytic adenoviral agents to the epidermal growth factor pathway with a secretory fusion molecule. Cancer Res. 61:6377-6381. [PubMed] [Google Scholar]
- 21.Hidaka, C., E. Milano, P. L. Leopold, J. M. Bergelson, N. R. Hackett, R. W. Finberg, T. J. Wickham, I. Kovesdi, P. Roelvink, and R. G. Crystal. 1999. CAR-dependent and CAR-independent pathways of adenovirus vector-mediated gene transfer and expression in human fibroblasts. J. Clin. Investig. 103:579-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirschowitz, E. A., J. D. Weaver, G. E. Hidalgo, and D. E. Doherty. 2000. Murine dendritic cells infected with adenovirus vectors show signs of activation. Gene Ther. 7:1112-1120. [DOI] [PubMed] [Google Scholar]
- 23.Ignatovich, I. A., E. B. Dizhe, A. V. Pavlotskaya, B. N. Akifiev, S. V. Burov, S. V. Orlov, and A. P. Perevozchikov. 2003. Complexes of plasmid DNA with basic domain 47-57 of the HIV-1 Tat protein are transferred to mammalian cells by endocytosis-mediated pathways. J. Biol. Chem. 278:42625-42636. [DOI] [PubMed] [Google Scholar]
- 24.Joliot, A., C. Pernelle, H. Deagostini-Bazin, and A. Prochiantz. 1991. Antennapedia homeobox peptide regulates neural morphogenesis. Proc. Natl. Acad. Sci. USA 88:1864-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leifert, J. A., S. Harkins, and J. L. Whitton. 2002. Full-length proteins attached to the HIV tat protein transduction domain are neither transduced between cells, nor exhibit enhanced immunogenicity. Gene Ther. 9:1422-1428. [DOI] [PubMed] [Google Scholar]
- 26.Leon, R. P., T. Hedlund, S. J. Meech, S. Li, J. Schaack, S. P. Hunger, R. C. Duke, and J. DeGregori. 1998. Adenoviral-mediated gene transfer in lymphocytes. Proc. Natl. Acad. Sci. USA 95:13159-13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, E., S. L. Brown, D. G. Stupack, X. S. Puente, D. A. Cheresh, and G. R. Nemerow. 2001. Integrin αvβ1 is an adenovirus coreceptor. J. Virol. 75:5405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, Y., R. C. Pong, J. M. Bergelson, M. C. Hall, A. I. Sagalowsky, C. P. Tseng, Z. Wang, and J. T. Hsieh. 1999. Loss of adenoviral receptor expression in human bladder cancer cells: a potential impact on the efficacy of gene therapy. Cancer Res. 59:325-330. [PubMed] [Google Scholar]
- 29.Lundberg, M., and M. Johansson. 2002. Positively charged DNA-binding proteins cause apparent cell membrane translocation. Biochem. Biophys. Res. Commun. 291:367-371. [DOI] [PubMed] [Google Scholar]
- 30.Mercier, S., H. Gahery-Segard, M. Monteil, R. Lengagne, J. G. Guillet, M. Eloit, and C. Denesvre. 2002. Distinct roles of adenovirus vector-transduced dendritic cells, myoblasts, and endothelial cells in mediating an immune response against a transgene product. J. Virol. 76:2899-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millecamps, S., H. Kiefer, V. Navarro, M. C. Geoffroy, J. J. Robert, F. Finiels, J. Mallet, and M. Barkats. 1999. Neuron-restrictive silencer elements mediate neuron specificity of adenoviral gene expression. Nat. Biotechnol. 17:865-869. [DOI] [PubMed] [Google Scholar]
- 32.Miller, C. R., D. J. Buchsbaum, P. N. Reynolds, J. T. Douglas, G. Y. Gillespie, M. S. Mayo, D. Raben, and D. T. Curiel. 1998. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Res. 58:5738-5748. [PubMed] [Google Scholar]
- 33.Miller, G., S. Lahrs, V. G. Pillarisetty, A. B. Shah, and R. P. DeMatteo. 2002. Adenovirus infection enhances dendritic cell immunostimulatory properties and induces natural killer and T-cell-mediated tumor protection. Cancer Res. 62:5260-5266. [PubMed] [Google Scholar]
- 34.Nakamura, Y., H. Wakimoto, J. Abe, Y. Kanegae, I. Saito, M. Aoyagi, K. Hirakawa, and H. Hamada. 1994. Adoptive immunotherapy with murine tumor-specific T lymphocytes engineered to secrete interleukin 2. Cancer Res. 54:5757-5760. [PubMed] [Google Scholar]
- 35.Normand, N., H. van Leeuwen, and P. O'Hare. 2001. Particle formation by a conserved domain of the herpes simplex virus protein VP22 facilitating protein and nucleic acid delivery. J. Biol. Chem. 276:15042-15050. [DOI] [PubMed] [Google Scholar]
- 36.Okegawa, T., R. C. Pong, Y. Li, J. M. Bergelson, A. I. Sagalowsky, and J. T. Hsieh. 2001. The mechanism of the growth-inhibitory effect of coxsackie and adenovirus receptor (CAR) on human bladder cancer: a functional analysis of car protein structure. Cancer Res. 61:6592-6600. [PubMed] [Google Scholar]
- 37.Phelan, A., G. Elliott, and P. O'Hare. 1998. Intercellular delivery of functional p53 by the herpesvirus protein VP22. Nat. Biotechnol. 16:440-443. [DOI] [PubMed] [Google Scholar]
- 38.Rudolph, C., C. Plank, J. Lausier, U. Schillinger, R. H. Muller, and J. Rosenecker. 2003. Oligomers of the arginine-rich motif of the HIV-1 TAT protein are capable of transferring plasmid DNA into cells. J. Biol. Chem. 278:11411-11418. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt, M. R., B. Piekos, M. S. Cabatingan, and R. T. Woodland. 2000. Expression of a human coxsackie/adenovirus receptor transgene permits adenovirus infection of primary lymphocytes. J. Immunol. 165:4112-4119. [DOI] [PubMed] [Google Scholar]
- 40.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torchilin, V. P., T. S. Levchenko, R. Rammohan, N. Volodina, B. Papahadjopoulos-Sternberg, and G. G. D'Souza. 2003. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc. Natl. Acad. Sci. USA 100:1972-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torchilin, V. P., R. Rammohan, V. Weissig, and T. S. Levchenko. 2001. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. USA 98:8786-8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vives, E., P. Brodin, and B. Lebleu. 1997. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 272:16010-16017. [DOI] [PubMed] [Google Scholar]
- 44.Walters, R. W., P. Freimuth, T. O. Moninger, I. Ganske, J. Zabner, and M. J. Welsh. 2002. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 110:789-799. [DOI] [PubMed] [Google Scholar]
- 45.Wan, Y. Y., R. P. Leon, R. Marks, C. M. Cham, J. Schaack, T. F. Gajewski, and J. DeGregori. 2000. Transgenic expression of the coxsackie/adenovirus receptor enables adenoviral-mediated gene delivery in naive T cells. Proc. Natl. Acad. Sci. USA 97:13784-13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, X., and J. M. Bergelson. 1999. Coxsackievirus and adenovirus receptor cytoplasmic and transmembrane domains are not essential for coxsackievirus and adenovirus infection. J. Virol. 73:2559-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wender, P. A., D. J. Mitchell, K. Pattabiraman, E. T. Pelkey, L. Steinman, and J. B. Rothbard. 2000. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc. Natl. Acad. Sci. USA 97:13003-13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 49.Wickham, T. J., E. Tzeng, L. L. Shears, P. W. Roelvink, Y. Li, G. M. Lee, D. E. Brough, A. Lizonova, and I. Kovesdi. 1997. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J. Virol. 71:8221-8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wirth, T., L. Zender, B. Schulte, B. Mundt, R. Plentz, K. L. Rudolph, M. Manns, S. Kubicka, and F. Kuhnel. 2003. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Res. 63:3181-3188. [PubMed] [Google Scholar]
- 51.Wirtz, S., C. Becker, R. Blumberg, P. R. Galle, and M. F. Neurath. 2002. Treatment of T cell-dependent experimental colitis in SCID mice by local administration of an adenovirus expressing IL-18 antisense mRNA. J. Immunol. 168:411-420. [DOI] [PubMed] [Google Scholar]
- 52.You, Z., D. C. Fischer, X. Tong, A. Hasenburg, E. Aguilar-Cordova, and D. G. Kieback. 2001. Coxsackievirus-adenovirus receptor expression in ovarian cancer cell lines is associated with increased adenovirus transduction efficiency and transgene expression. Cancer Gene Ther. 8:168-175. [DOI] [PubMed] [Google Scholar]
- 53.Zender, L., R. Kock, M. Eckhard, B. Frericks, T. Gosling, T. Gebhardt, S. Drobek, M. Galanski, F. Kuhnel, M. Manns, and S. Kubicka. 2002. Gene therapy by intrahepatic and intratumoral trafficking of p53-VP22 induces regression of liver tumors. Gastroenterology 123:608-618. [DOI] [PubMed] [Google Scholar]