Abstract
BACKGROUND
The National Surgical Adjuvant Breast and Bowel Project B35 and International Breast Cancer Intervention Studies II Ductal carcinoma In Situ (DCIS) trials showed similar treatment effects of anastrozole and tamoxifen in reducing cancer recurrence risk among DCIS patients, but the current body of literature lacks information on the five year adherence rates for these drugs from population-based studies.
METHODS
This study evaluated the initiation and 5-year adherence for women aged 66 to 85 years who had been diagnosed with estrogen receptor(ER)-positive DCIS between 2007 to 2011 according to the Surveillance, Epidemiology, and End Results (SEER) and Texas Cancer Registry databases linked to Medicare claims. Chi-square test, trend test and logistic regression were used to identify factors associated with treatment initiation.
RESULTS
There were 2,871 women with ER-positive DCIS, and approximately 45% began treatment with tamoxifen or aromatase inhibitors (AIs) within 1 year of their DCIS diagnosis. The median age was 73 years for the users and 75 years for the non-users. Women aged 66 to 70 years who underwent lumpectomy and radiation therapy were significantly more likely to initiate hormone therapy. The initiation of therapy was also significantly associated with patients’ geographic location, education, marital status, diagnosis year, and race/ethnicity. Among users, adherence decreased from 67% in the first year to 30% in the fifth year.
CONCLUSIONS
Initiation and adherence levels for tamoxifen or AIs among older women with ER-positive DCIS are low. Future studies should develop methods to ensure that informed discussions take place between health care providers and patients regarding hormonal therapy for cancer prevention.
Keywords: aromatase inhibitor, cancer treatment, chemoprevention, drug adherence, ductal carcinoma in situ (DCIS), tamoxifen
INTRODUCTION
Although breast ductal carcinoma in situ (DCIS) is noninvasive, women with DCIS are at higher risk of developing invasive breast cancer than women without it.1 The standard treatment for DCIS is lumpectomy followed by radiation therapy (LRT). For estrogen receptor positive (ER)-positive tumors, the use of a hormone therapy agent such as tamoxifen for 5 years is also recommended to reduce the risk of second primary breast cancers. Tamoxifen was proven to reduce the risk of invasive breast cancer in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B24 trial and the United Kingdom, Australia, and New Zealand trial. 2-4 Aromatase inhibitors (AIs) are drugs that can block the synthesis of estrogen and inhibit ER-positive breast tumor growth among postmenopausal women. 5 Results from the NSABP B35 trial showed the 10-year breast cancer event rate was 4% lower for patients who used anastrozole versus tamoxifen among postmenopausal women aged 60 years or younger.6 Results from the International Breast Cancer Intervention Studies II Ductal Carcinoma In Situ (IBIS-II DCIS) trial showed that anastrozole had a risk reduction effect similar to that of tamoxifen in DCIS patients. 7 AIs have been used off-label to treat DCIS.
Although tamoxifen and AIs can markedly reduce the risk of invasive breast cancer, their use among ER-positive DCIS patients remains low. Nichols et al. reported a 41% initiation rate for tamoxifen or AIs among ER-positive DCIS patients based on 727 Group Health Cooperative enrollees from 1996 to 2011.8 Virnig et al. reported a 43.9% initiation rate of these drugs among ER-positive DCIS patients diagnosed between 2006 to 2007 based on SEER-Medicare data.9 The literature is void of population-based studies to determine the five year adherence rate among women who initiate treatment. As the optimal preventive effect of these drugs requires five years of treatment, adherence is key for patients to obtain the maximum prevention benefit. The 5-year tamoxifen or AI completion rate among DCIS patients from the NSABP B35 and IBIS-II DCIS clinical trials was 64% and 67%, respectively.6, 7 Factors that have been associated with non-adherence to tamoxifen use include patients’ age, adverse events from tamoxifen or AIs, and physician recommendations among others. 10 Currently, no population-based study exists that evaluates tamoxifen or AI adherence in older women with DCIS. It is important to evaluate adherence among older women to determine whether continued treatment, the current standard of care used to reduce the risk of DCIS progressing to invasive disease, is being appropriately given to this patient population. In this study, we used Medicare Part D data to evaluate tamoxifen or AI initiation and to discover the determinants that influence its use. We hypothesized that patients’ demographic characteristics, tumor characteristics, primary treatment modality, and geographic region influence treatment initiation. We also assessed the five-year adherence. We hypothesized that the five-year adherence rate in this real-world population-based study is lower than that obtained from clinical trials.
MATERIALS AND METHODS
We used SEER–Medicare and TCR–Medicare linked data for this study.11 We selected women ≥66 years with ER-positive DCIS diagnosed between January 2007 and December 2011 who received mastectomy or lumpectomy. Patients had Medicare Parts A and B and were not covered by a health maintenance organization (HMO) for 1 year prior to and 1 year after diagnosis. Patients had a Medicare Part D prescription drug plan for at least 12 months after diagnosis or until death if they died within 12 months. Details of the cohort selection are listed in Supplemental Table 1.
We obtained patients’ demographic, geographic location, socioeconomic status at the census tract level, and tumor characteristics from the cancer registry data. Tumor grade and differentiation information is defined by ICD-O-2 of 1992. From the Part D data, we obtained patients’ tamoxifen or AI prescriptions using brand or generic names of TAMOXIFEN CITRATE, ARIMIDEX, AROMASIN, FEMARA, ANASTROZOLE, EXEMESTANE, or LETROZOLE. 9 A tamoxifen or AI user was defined as a patient who had her first prescription within 12 months after DCIS diagnosis. Using Medicare claims, we calculated each patient's Charlson comorbidity score according to the Klabunde algorithm. 12, 13 Using Medicare claims from diagnosis to 12 months after cancer diagnosis, we identified patients’ primary therapy, including surgery and radiotherapy, according to ICD-9 diagnosis, Current Procedural Terminology (CPT), and Healthcare Common Procedure Coding System (HCPCS) codes (Supplemental Tables 2 & 3).
The initiation of use was evaluated from the DCIS diagnosis until 12 months after the diagnosis. We defined patients’ first treatment date as the first prescription drug date, and the last treatment date as: 1) the end of Medicare Part D continuous coverage, 2) the end of the study period (December 2012), 3) the date of a patient's secondary cancer diagnosis, 4) death, or 5) the end of five year treatment (whichever event occurred first). Adherence was defined as initiating tamoxifen or AI use, and persistence of completing the treatment from one to five years after beginning its use. We measured the adherence by computing the proportion of days covered (PDC) in treatment years 1-5 for women who initiated the treatment. Each year, a patient was considered to be adherent to treatment if she had a PDC ≥ 80%. In a treatment year, the adherence was calculated as: number of patients with PDC ≥80% divided by total eligible patients. Eligible patients were those who had full Medicare Part D coverage by the last treatment date in that year. We adjusted the days of drug supply for early refilled prescriptions by using the algorithm provided by Wang et al.14
Differences in nominal categorical variables were analyzed by the Chi-square test and differences in ordered categorical variables between two groups were analyzed by the Cochrane-Armitage trend test. Binomial distribution was used to compute the confidence intervals for adherence rates. All tests were two-sided and a P-value of less than 0.05 was considered significant. Forward logistic regression selection approach was used to build the multivariable regression model. A variable was selected into the model with a P-value ≤ 0.1. We conducted sensitivity analysis in multiple logistic regression by excluding mastectomy patients to determine if predictors of hormonal therapy were different among women treated with mastectomy versus lumpectomy.
The data were analyzed using SAS 9.4 software developed by SAS Institute in Cary North Carolina. This study received exemption from the Institutional Review Boards at the University of Texas MD Anderson Cancer Center.
RESULTS
We identified 2,871 women aged 66 to 85 years who were diagnosed with ER-positive DCIS from 2007 to 2011. The median age was 73.3 years. Tamoxifen or AI use was started by 1,297 women (45.2%) within one year of their diagnosis. About 72% of them used tamoxifen, 24% used an AI, and 4% switched between these two drugs. The median interval from DCIS diagnosis to first prescription was 3 months.
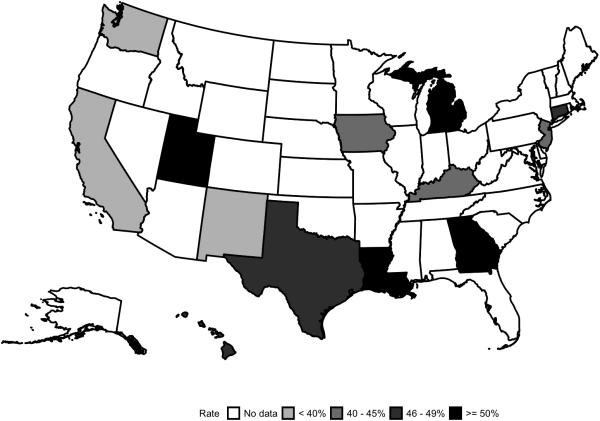
The distribution of patient age, education, treatment with surgery and radiation, and geographic location were significantly different between users and non-users, with a P-value <0.0001 (Table 1). Users were younger than non-users with a median age of 73 vs. 75 years, respectively. Women who underwent LRT were most likely to initiate the treatment, followed by those treated only with lumpectomy or mastectomy with rates of 54.6%, 32.9%, and 33.6%, respectively. Of the 13 cancer registries, the use was lowest in Seattle (28.9%) and highest in Louisiana (59.2%; Figure 1). Other variables such as race/ethnicity, marital status, and median household income level in the census tract of residence were significantly different between users and non-users (P <0.05). The initiation of tamoxifen or AIs was more common in Hispanic and black patients than in white patients (55.6.0%, 51.6%, and 44.1%, respectively; P=0.0003).
Table 1.
Patient Characteristics by User and Non-user
| Variables | N | Percent | Tamoxifen or AI use, No. (%)a | Pb | |
|---|---|---|---|---|---|
| No (%) | Yes (%) | ||||
| Total | 2871 | 100 | 1574 ( 54.8 ) | 1297 ( 45.2 ) | |
| Age, years | |||||
| 66-70 | 984 | 34.3 | 460 ( 46.7 ) | 524 ( 53.3 ) | <.0001 |
| 71-75 | 877 | 30.5 | 467 ( 53.2 ) | 410 ( 46.8 ) | |
| 76-80 | 644 | 22.4 | 403 ( 62.6 ) | 241 ( 37.4 ) | |
| 81-85 | 366 | 12.7 | 244 ( 66.7 ) | 122 ( 33.3 ) | |
| Race/ethnicity | |||||
| White | 2277 | 79.3 | 1273 ( 55.9 ) | 1004 ( 44.1 ) | 0.0003 |
| Hispanic | 207 | 7.2 | 92 ( 44.4 ) | 115 ( 55.6 ) | |
| Black | 248 | 8.6 | 120 ( 48.4 ) | 128 ( 51.6 ) | |
| Other | 139 | 4.8 | 89 ( 64.0 ) | 50 ( 36.0 ) | |
| Marital status | |||||
| Married | 1294 | 45.1 | 669 ( 51.7 ) | 625 ( 48.3 ) | 0.005 |
| Not married | 1370 | 47.7 | 794 ( 58.0 ) | 576 ( 42.0 ) | |
| Unknown | 207 | 7.2 | 111 ( 53.6 ) | 96 ( 46.4 ) | |
| Education level | |||||
| 1st quartilec | 702 | 24.5 | 414 ( 59.0 ) | 288 ( 41.0 ) | <.0001 |
| 2nd quartile | 690 | 24 | 413 ( 59.9 ) | 277 ( 40.1 ) | |
| 3rd quartile | 673 | 23.4 | 361 ( 53.6 ) | 312 ( 46.4 ) | |
| 4th quartile | 769 | 26.8 | 368 ( 47.9 ) | 401 ( 52.1 ) | |
| Unknown | 37 | 1.3 | 18 ( 48.6 ) | 19 ( 51.4 ) | |
| Income level | |||||
| 1st quartiled | 722 | 25.1 | 361 ( 50.0 ) | 361 ( 50.0 ) | 0.004 |
| 2nd quartile | 682 | 23.8 | 379 ( 55.6 ) | 303 ( 44.4 ) | |
| 3rd quartile | 691 | 24.1 | 384 ( 55.6 ) | 307 ( 44.4 ) | |
| 4th quartile | 682 | 23.8 | 406 ( 59.5 ) | 276 ( 40.5 ) | |
| Unknown | 94 | 3.3 | 44 ( 46.8 ) | 50 ( 53.2 ) | |
| Year of cancer diagnosis | |||||
| 2007 | 468 | 16.3 | 234 ( 50.0 ) | 234 ( 50.0 ) | 0.11 |
| 2008 | 553 | 19.3 | 307 ( 55.5 ) | 246 ( 44.5 ) | |
| 2009 | 576 | 20.1 | 311 ( 54.0 ) | 265 ( 46.0 ) | |
| 2010 | 589 | 20.5 | 343 ( 58.2 ) | 246 ( 41.8 ) | |
| 2011 | 685 | 23.9 | 379 ( 55.3 ) | 306 ( 44.7 ) | |
| Tumor gradee | |||||
| I | 381 | 13.3 | 216 ( 56.7 ) | 165 ( 43.3 ) | 0.64 |
| II | 1132 | 39.4 | 606 ( 53.5 ) | 526 ( 46.5 ) | |
| III | 835 | 29.1 | 465 ( 55.7 ) | 370 ( 44.3 ) | |
| IV | 151 | 5.3 | 88 ( 58.3 ) | 63 ( 41.7 ) | |
| Unknown | 372 | 13 | 199 ( 53.5 ) | 173 ( 46.5 ) | |
| Tumor size | |||||
| ≤10 mm | 1130 | 39.4 | 624 ( 55.2 ) | 506 ( 44.8 ) | 0.46 |
| >10mm | 952 | 33.2 | 532 ( 55.9 ) | 420 ( 44.1 ) | |
| Unknown | 789 | 27.5 | 418 ( 53.0 ) | 371 ( 47.0 ) | |
| Surgery and radiation treatment | |||||
| LRT | 1601 | 55.8 | 727 ( 45.4 ) | 874 ( 54.6 ) | <.0001 |
| Lumpectomy | 583 | 20.3 | 391 ( 67.1 ) | 192 ( 32.9 ) | |
| Mastectomy | 687 | 23.9 | 456 ( 66.4 ) | 231 ( 33.6 ) | |
| Charlson comorbidity score | |||||
| 0 | 1810 | 63 | 978 ( 54.0 ) | 832 ( 46.0 ) | 0.28 |
| 1 | 664 | 23.1 | 364 ( 54.8 ) | 300 ( 45.2 ) | |
| >1 | 397 | 13.8 | 232 ( 58.4 ) | 165 ( 41.6 ) | |
| Cancer registry | |||||
| Connecticut | 137 | 4.8 | 70 ( 51.1 ) | 67 ( 48.9 ) | <.0001 |
| Detroit | 123 | 4.3 | 52 ( 42.3 ) | 71 ( 57.7 ) | |
| Hawaii | 23 | 0.8 | 12 ( 52.2 ) | 11 ( 47.8 ) | |
| Iowa | 199 | 6.9 | 114 ( 57.3 ) | 85 ( 42.7 ) | |
| New Mexico | 35 | 1.2 | 23 ( 65.7 ) | 12 ( 34.3 ) | |
| Seattle | 121 | 4.2 | 86 ( 71.1 ) | 35 ( 28.9 ) | |
| Utah | 46 | 1.6 | 22 ( 47.8 ) | 24 ( 52.2 ) | |
| Kentucky | 159 | 5.5 | 88 ( 55.3 ) | 71 ( 44.7 ) | |
| Louisiana | 130 | 4.5 | 53 ( 40.8 ) | 77 ( 59.2 ) | |
| New Jersey | 373 | 13 | 215 ( 57.6 ) | 158 ( 42.4 ) | |
| Texas | 468 | 16.3 | 244 ( 52.1 ) | 224 ( 47.9 ) | |
| Georgia | 318 | 11.1 | 152 ( 47.8 ) | 166 ( 52.2 ) | |
| California | 739 | 25.7 | 443 ( 59.9 ) | 296 ( 40.1 ) | |
Abbreviations: AI, aromatase inhibitor; LRT, lumpectomy followed by radiation therapy.
Row percentages for each stratum are shown.
P values for age, education level, income level, year of cancer diagnosis, tumor grade, and tumor size were determined with the Cochran-Armitage trend test; other P values were determined with the chi-square test.
Highest percentage of residents who graduated from high school
Highest median household income
Grade I is well differentiated, grade II is moderately differentiated, grade III is poorly differentiated, and grade IV is undifferentiated.
Figure 1.
Tamoxifen or AI use in 13 Cancer Registry Regions
In multivariable analysis, the final model includes treatment modality, age, geographic region, education level, race/ethnicity, marital status, and year of diagnosis. Patients who were treated with lumpectomy or mastectomy were less likely to be a user compared to LRT patients (OR, 95% CI 0.48 [0.39, 0.60] and 0.40 [0.33, 0.49], respectively). Overall, patients were more likely to initiate drug use if they were younger than 71 years of age at diagnosis, Hispanic, married, lived in census tracts with low levels of education, underwent LRT, and were diagnosed before 2010 (Table 2). The predictors of use in lumpectomy women were similar to those in Table 2 except that diagnosis year was not significant (Supplemental Table 4).
Table 2.
Associations of Patient Characteristics with Use in Logistic Regression Model
| Variable | Stratum | Odds ratio (95% CI) | Odds ratio (95% CI) | P-value |
|---|---|---|---|---|
| Age, years | 66-70 | Reference | Reference | |
| 71-75 | 0.77 ( 0.64, 0.92 ) | 0.83 ( 0.68, 1.00 ) | 0.05 | |
| 76-80 | 0.52 ( 0.43, 0.64 ) | 0.61 ( 0.49, 0.76 ) | <0.0001 | |
| 81-85 | 0.44 ( 0.34, 0.56 ) | 0.57 ( 0.43, 0.75 ) | <0.0001 | |
| Race/ethnicity | White | Reference | ||
| Hispanic | 1.58 ( 1.19, 2.11 ) | 1.58 ( 1.15, 2.17 ) | 0.005 | |
| Black | 1.35 ( 1.04, 1.76 ) | 1.17 ( 0.87, 1.57 ) | 0.3 | |
| Other | 0.71 ( 0.50, 1.02 ) | 0.81 ( 0.54, 1.21 ) | 0.31 | |
| Marital status | Married | Reference | Reference | |
| Not married | 0.78 ( 0.67, 0.90 ) | 0.79 ( 0.66, 0.93 ) | 0.005 | |
| Unknown | 0.93 ( 0.69, 1.24 ) | 0.94 ( 0.68, 1.29 ) | 0.7 | |
| Education level | 1st quartilea | Reference | Reference | |
| 2nd quartile | 0.96 ( 0.78, 1.19 ) | 0.94 ( 0.75, 1.17 ) | 0.56 | |
| 3rd quartile | 1.24 ( 1.00, 1.54 ) | 1.19 ( 0.95, 1.50 ) | 0.13 | |
| 4th quartile | 1.57 ( 1.27, 1.93 ) | 1.45 ( 1.15, 1.84 ) | 0.002 | |
| Unknown | 1.52 ( 0.78, 2.94 ) | 1.48 ( 0.72, 3.02 ) | 0.29 | |
| Income level | 1st quartileb | Reference | ||
| 2nd quartile | 1.18 ( 0.95, 1.46 ) | |||
| 3rd quartile | 1.18 ( 0.95, 1.46 ) | |||
| 4th quartile | 1.47 ( 1.19,1.82 ) | |||
| Unknown | 1.67 ( 1.08, 2.58 ) | |||
| Year of diagnosis | 2007 | Reference | Reference | |
| 2008 | 0.80 ( 0.63, 1.03 ) | 0.77 ( 0.59, 1.00 ) | 0.05 | |
| 2009 | 0.85 ( 0.67, 1.09 ) | 0.80 ( 0.62, 1.04 ) | 0.09 | |
| 2010 | 0.72 ( 0.56, 0.92 ) | 0.70 ( 0.54, 0.91 ) | 0.007 | |
| 2011 | 0.81 ( 0.64, 1.02 ) | 0.74 ( 0.58, 0.95 ) | 0.02 | |
| Tumor grade | I | Reference | ||
| II | 1.14 ( 0.90, 1.44 ) | |||
| III | 1.04 ( 0.82, 1.33 ) | |||
| IV | 0.94 ( 0.64, 1.37 ) | |||
| Unknown | 1.14 ( 0.85, 1.52 ) | |||
| Tumor size | ≤ 10 mm | Reference | ||
| >10mm | 0.97 ( 0.82, 1.16 ) | |||
| Unknown | 1.09 ( 0.91, 1.31 ) | |||
| Surgery and radiation | LRT | Reference | Reference | |
| Lumpectomy | 0.41 ( 0.33, 0.50 ) | 0.48 ( 0.39, 0.60 ) | <0.0001 | |
| Mastectomy | 0.42 ( 0.35, 0.51 ) | 0.40 ( 0.33, 0.49 ) | <0.0001 | |
| Charlson comorbidity | 0 | Reference | ||
| 1 | 0.97 ( 0.81, 1.16 ) | |||
| >1 | 0.84 ( 0.67, 1.04 ) | |||
| Cancer registry | California | Reference | Reference | |
| Connecticut | 1.43 ( 0.99, 2.07 ) | 1.43 ( 0.98, 2.11 ) | 0.07 | |
| Detroit | 2.04 ( 1.39, 3.01 ) | 2.18 ( 1.44, 3.30 ) | 0.0002 | |
| Georgia | 1.63 ( 1.25, 2.13 ) | 1.52 ( 1.14, 2.03 ) | 0.005 | |
| Hawaii | 1.37 ( 0.60, 3.15 ) | 1.69 ( 0.68, 4.19 ) | 0.26 | |
| Iowa | 1.12 ( 0.81, 1.53 ) | 1.20 ( 0.85, 1.68 ) | 0.3 | |
| Kentucky | 1.21 ( 0.85, 1.71 ) | 1.18 ( 0.81, 1.71 ) | 0.39 | |
| Louisiana | 2.17 ( 1.49, 3.18 ) | 2.24 ( 1.48, 3.37 ) | 0.0001 | |
| New Jersey | 1.10 ( 0.85, 1.42 ) | 1.10 ( 0.84, 1.44 ) | 0.48 | |
| New Mexico | 0.78 ( 0.38, 1.59 ) | 0.84 ( 0.40, 1.76 ) | 0.65 | |
| Seattle | 0.61 ( 0.40, 0.93 ) | 0.62 ( 0.40, 0.97 ) | 0.03 | |
| Texas | 1.37 ( 1.09, 1.74 ) | 1.22 ( 0.94, 1.59 ) | 0.14 | |
| Utah | 1.63 ( 0.90, 2.97 ) | 1.77 ( 0.94, 3.31 ) | 0.08 |
Abbreviations: CI, confidence interval; LRT, lumpectomy followed by radiation therapy.
Bolded values are significant in the multivariable model
highest percentage of residents who graduated from high school
highest median household income
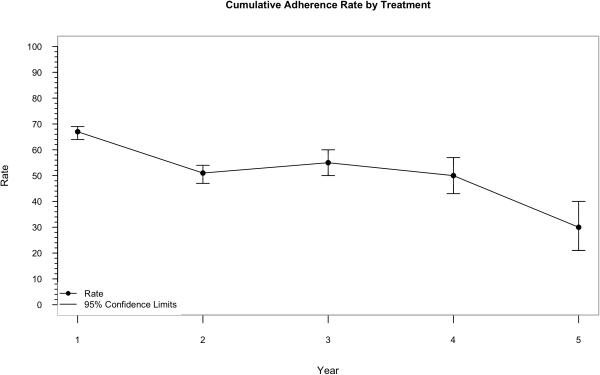
The median Part D coverage for the 1,297 users was 34 months (IQR 19-48). The median days covered by prescribed drugs was 659 days (IQR 300-1135). The adherence rate in the first treatment year was 67%, decreasing to around 50% in the 2nd to 4th treatment years, and further declining to 30% in the fifth year (Table 3, and Figure 2).
Table 3.
Adherence to Use from Years 1 to 5
| Treatment, mo | Na | N adherence | Adherence rate (95% CI) |
|---|---|---|---|
| 1-12 | 1297 | 865 | 0.67 (0.64, 0.69) |
| 13-24 | 814 | 413 | 0.51 (0.47, 0.54) |
| 25-36 | 397 | 218 | 0.55 (0.50, 0.60) |
| 37-48 | 201 | 100 | 0.50 (0.43, 0.57) |
| 49-60 | 92 | 28 | 0.30 (0.21, 0.40) |
Abbreviation: CI, confidence interval.
Number of eligible patients in the specific treatment interval
Figure 2.
Adherence Rate by Treatment Year
DISCUSSION
In this study, we found that 45.2% of women with ER-positive DCIS began therapy with tamoxifen or AIs. Patients treated with lumpectomy or mastectomy without radiation, 71 years of age or older, lived in the Western United States, lived in census tracts with high median levels of education, were white, were unmarried, and diagnosed after 2009 were less likely to use tamoxifen or AI. Among users, only 30% completed 5 years of treatment.
Low tamoxifen or AI uptake and adherence may be attributable to treatment toxicities and small benefit from hormone therapy after lumpectomy and radiation. Both tamoxifen and AI use are associated with side effects. About 29% patients who received tamoxifen or an AI experienced grade 3 or higher toxicities. 6 These adverse events include difficulty with bladder control, gynecological symptoms, thrombosis or embolism, musculoskeletal pain, and vaginal symptoms. 15 In the IBIS-II DCIS trail, about 33% patients discontinued tamoxifen or anastrozole with one of the main reasons being adverse events. 7 In addition to the toxicities, the benefit of using these drugs to prevent invasive breast cancer after LRT seems limited due to the already excellent prognosis for most of these women. The 10 year cumulative rate of invasive ipsilateral breast tumor recurrence after LRT is 10.6% vs. 5.8% by adding five years of tamoxifen treatment. Based on this, 21 women need to take tamoxifen for five years to prevent one case of invasive breast cancer in 10 years. Due to the treatment toxicities and limited benefit of tamoxifen or AI treatment after LRT, the use of these drugs remains low for preventing breast cancer recurrence. In addition, no overall survival benefit has been shown with the use of tamoxifen or AIs after a diagnosis of DCIS.
Our findings of low tamoxifen or AI use are consistent with other studies. We extended the previous work by Virnig et al. by including patients from TCR and adding four years of more recent data from 2009 to 2012.9 Our results were similar to those published by Virnig and colleagues. Nichols et al. identified an overall initiation rate of 20.4% in the Group Health Cooperative database from 2001 to 2011, which is similar to our findings in the Seattle Cancer Registry of 28.9% (Table 1). 8 Flanagan et al. conducted a study of 206,255 DCIS patients diagnosed from 2005 to 2012 in the National Cancer Data Base (NCDB), and found the adjuvant endocrine therapy initiation rate to be 46.4% among patients with ER-positive tumors.16 This rate is similar to our finding of 45.2%. The low rate of use may be attributable to adverse events and excellent prognosis for DCIS patients.
In our study, women with mastectomy had a lower initiation rate compared with LRT women. These finding are not surprising, given that women who have been treated with mastectomy have a lower risk of development of a new breast cancer because only one breast remains at risk. In this situation, the benefit of chemoprevention is smaller and the risk/benefit ratio may not always favor treatment.
We found a significantly higher use of tamoxifen or AIs among Hispanics when compared to non-Hispanic whites; this result is consistent with previous findings.9 A study evaluating hormone therapy among DCIS patients based on six Kaiser Permanente regions from 2001-2011 found that Hispanic women were more likely than white women to receive hormone treatment (OR, 95% CI 1.20 [1.02-1.40]).17 Our findings, which were generated based on a large sample size from diverse US regions, confirm that race/ethnicity is a predictor of hormone therapy use.
To our knowledge, this is the first population-based study to evaluate adherence to tamoxifen or AIs for ER-positive DCIS over five years among older women. Our adherence rate of 30% was much lower than that of the NSAPB B35 trial (64%) and IBIS-II DCIS trial (67%) with p-values <0.0001.6, 7 The higher rates reported from the clinical trials is likely attributable to a highly selected and motivated cohort of patients and detailed follow-up protocols. In contrast, our study is population-based with no follow-up protocol, and the patients were much older than those in the clinical trials. These differences speak to the importance of studying adherence in nationally representative, relatively unselected patient populations rather than relying on data from clinical trials.
Despite our important findings, our study has limitations. Since the median follow-up of our study was 34 months, the estimated adherence rates for the fourth and fifth years were less precise than that of the first three years. Also, information about treatment decision making and the extent to which it was influenced by patients or by health care providers was not available. However, a major strength of our study is that it is population-based, reflecting tamoxifen or AI use among women Medicare beneficiaries who had Medicare Part D coverage. Our study provides useful information on treatment patterns for ER-positive DCIS patients and factors associated with the use among older women. Interestingly, our finding that 23.9% of elderly patients received mastectomy indicates a decreasing trend of mastectomy among older DCIS patients.
Overall, the initiation of tamoxifen or AI among older women with ER-positive DCIS was 45.2%, and the five year adherence was 30%. This low rate should lead to further studies to evaluate the reasons for the low uptake and adherence. The NSABP-B24 clinical trial reported that tamoxifen treatment reduces subsequent breast cancer for ER-positive DCIS patients with a median follow-up time of 14.5 years. 18 With the median follow-up time was only about 3 years for our study, we were not able to evaluate the breast cancer recurrence rate. In the future, with more years of Medicare Part D data are available, we will evaluate whether tamoxifen or AIs use would reduce breast cancer events in population-based studies. Lastly, our study calls for the need to identify biomarkers that can identify patients at high risk of invasive recurrence to maximize the preventive effect, and eliminate unnecessary harm in women with low risk of breast cancer recurrence.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Gary M. Deyter, Kathryn L. Hale and Amy Ninetto for editing the manuscript. The authors acknowledge the Centers for Medicare and Medicaid Services, Information Management Services, Inc., and the SEER and TCR tumor registries in the creation of the SEER and TCR–Medicare databases.
FUNDING SUPPORT
This research was supported in part by a grant (RP140020) from the Cancer Prevention and Research Institute of Texas to The University of Texas MD Anderson Cancer Center, by the Cancer Prevention Fund established at MD Anderson Cancer Center, by the Duncan Family Institute, and by an NCI grant (CA056452) to the Cancer Prevention Research Training Program.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
All authors of this paper declare that they have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Conception and design: H. Zhao, M. Chavez-MacGregor, S.H. Giordano
Development of methodology: H. Zhao, X. Lei, C. Cameron, S. Chang
Analysis and interpretation of data: H. Zhao, N. Hei, Y. Wu, W. Chan
Administrative, technical, or material support: S.H. Giordano
REFERENCES
- 1.Innos K, Horn-Ross PL. Risk of second primary breast cancers among women with ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2008;111:531–540. doi: 10.1007/s10549-007-9807-1. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 3.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362:95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 5.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 6.Margolese RG, Cecchini RS, Julian TB, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)01168-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes JF, Sestak I, Howell A, et al. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet. 2016;387:866–873. doi: 10.1016/S0140-6736(15)01129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols HB, Bowles EJ, Islam J, et al. Tamoxifen Initiation After Ductal Carcinoma In Situ. Oncologist. 2016 doi: 10.1634/theoncologist.2015-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Virnig BA, Torchia MT, Jarosek SL, Durham S, Tuttle TM. Use of endocrine therapy following diagnosis of ductal carcinoma in situ or early invasive breast cancer: Data Points # 14. Data Points Publication Series; Rockville MD: 2011. [PubMed] [Google Scholar]
- 10.Chlebowski RT, Kim J, Haque R. Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res (Phila) 2014;7:378–387. doi: 10.1158/1940-6207.CAPR-13-0389. [DOI] [PubMed] [Google Scholar]
- 11.Chavez-MacGregor M, Niu J, Zhang N, et al. Cardiac Monitoring During Adjuvant Trastuzumab-Based Chemotherapy Among Older Patients With Breast Cancer. J Clin Oncol. 2015;33:2176–2183. doi: 10.1200/JCO.2014.58.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Huang Z, Traubenberg S. [January 26, 2016];Measuring Medication Adherence with Simple Drug Use and Medication Switching. Available from URL: http://support.sas.com/resources/papers/proceedings13/168-2013.pdf.
- 15.Ganz PA, Cecchini RS, Julian TB, et al. Patient-reported outcomes with anastrozole versus tamoxifen for postmenopausal patients with ductal carcinoma in situ treated with lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet. 2015;10021:857–865. doi: 10.1016/S0140-6736(15)01169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flanagan MR, Rendi MH, Gadi VK, Calhoun KE, Gow KW, Javid SH. Adjuvant endocrine therapy i n patients with ductal carcinoma in situ: a population-based retrospective analysis from 2005 to 2012 in the National Cancer Data Base. Ann Surg Oncol. 2015;22:3264–3272. doi: 10.1245/s10434-015-4668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feigelson HS, Carroll NM, Weinmann S, et al. Treatment patterns for ductal carcinoma in situ from 2000-2010 across six integrated health plans. Springerplus. 2015;4:24. doi: 10.1186/s40064-014-0776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. 2012;30:1268–1273. doi: 10.1200/JCO.2010.34.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.