Abstract
Background
Cardiac arrests are a major public health concern worldwide. The extent and types of randomized controlled trials (RCTs) – our most reliable source of clinical evidence – conducted in these high-risk patients over recent years are largely unknown.
Methods and Results
We performed a systematic review, identifying all RCTs published in PubMed, EMBASE, Scopus, Web of Science, and the Cochrane Library from 1995 to 2014 that focused on acute treatment of non-traumatic cardiac arrest in adults. We then extracted data on the setting of study populations, types and timing of interventions studied, risk of bias, outcomes reported and how these factors have changed over time. Over this twenty-year period, 92 RCTs were published containing 64,309 patients (median, 225.5 per trial). Of these, 81 RCTs (88.0%) involved out-of-hospital cardiac arrest whereas 4 (4.3%) involved in-hospital cardiac arrest and 7 (7.6%) included both. Eighteen RCTs (19.6%) were performed in the U.S., 68 (73.9%) were performed outside the U.S., and 6 (6.5%) were performed in both settings. Thirty-eight RCTs (41.3%) evaluated drug therapy, 39 (42.4%) evaluated device therapy, and 15 (16.3%) evaluated protocol improvements. Seventy-four RCTs (80.4%) examined interventions during the cardiac arrest, 15 (16.3%) examined post-cardiac arrest treatment, and 3 (3.3%) studied both. Overall, reporting of risk of bias was limited. The most common outcome reported was ROSC: 86 (93.5%) with only 22 (23.9%) reporting survival beyond 6 months. Fifty-three RCTs (57.6%) reported global ordinal outcomes whereas 15 (16.3%) reported quality-of-life. RCTs in the last 5 years were more likely to be focused on protocol improvement and post-cardiac arrest care.
Conclusions
Important gaps in RCTs of cardiac arrest treatments exist, especially those examining in-hospital cardiac arrest, protocol improvement, post-cardiac arrest care, and long-term or quality-of-life outcomes.
Keywords: cardiopulmonary resuscitation, heart arrest, health services research, outcomes research, resuscitation
Introduction
Cardiac arrests are a serious public health concern worldwide1, 2. Approximately 347,000 out-of-hospital cardiac arrests (OHCAs) and 209,000 in-hospital cardiac arrests (IHCAs) occur in adults each year in the United States1, with millions more occurring across the rest of North America, Europe and Asia3. While survival has increased significantly in the past decade4, 5, it remains unacceptably low6, 7 despite considerable attention devoted toward enhancing emergency response systems, high-quality and bystander cardiopulmonary resuscitation, immediate defibrillation, and post-arrest care therapies like therapeutic hypothermia8, 9. This lack of improvement is striking in contrast to other cardiovascular diseases like acute myocardial infarction, which have seen dramatic improvements in early and late mortality10.
A potential reason for limited progress in cardiac arrest treatments may result from a lack of randomized controlled trials (RCTs) – traditionally the most reliable source of clinical evidence for medical treatments11. Indeed, a recent expert opinion piece12 and Institute of Medicine report2 have cited minimal investment in research and infrastructure for RCTs to study cardiac arrest treatments relative to its high disease burden in the general population. Yet, despite this potential “mismatch” between the published science and public health burden of cardiac arrest, there is little objective information that exists to guide where exactly contemporary RCTs may be most deficient or where specific opportunities for advancement with future trials may be greatest. In particular, the focus, design, and quality of RCTs that target treatments in cardiac arrest are largely unknown but could vary significantly, as the condition covers broad populations and heterogeneous therapies.
Accordingly, we performed a systematic review of RCTs in cardiac arrest treatment performed over the last 20 years, focusing on key aspects of their design, including the setting of study populations (OHCA, IHCA), the types of interventions studied (i.e., drug, device or protocol improvement), timing (i.e., during the cardiac arrest or after return of spontaneous circulation [ROSC]), risk of bias and outcomes reported (i.e., process measure, ROSC, survival to discharge, long-term survival, global ordinal outcomes, and quality-of-life). Our findings have implications for both the current management of cardiac arrests and prioritization of future work.
Methods
Data Sources, Study Identification and Selection
We performed a systematic review using guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, or PRISMA13. A comprehensive, computerized literature search of the following electronic databases was conducted: PubMed, EMBASE, Scopus, Web of Science, and the Cochrane Library. We identified relevant English-language studies published from January 1, 1995 to December 31, 2014 utilizing keywords and controlled vocabulary terms (MeSH and EMTREE) related to cardiac arrest. We included medical subject headings (MeSH) terms heart arrest, cardiac arrest, and cardiopulmonary resuscitation, with the PubMed Clinical Queries Narrow Therapy filter limiting the search to primarily randomized or other controlled clinical trials. Full details of the replicable search strategies for each of the databases and a PRISMA checklist are available in Supplemental Appendices C and D, respectively. Abstracts from conferences, proceedings or clinical trial registries were not included, as we were interested in RCTs that were ultimately published in peer-reviewed literature. We also manually reviewed bibliographies of included RCTs as well as recent Cochrane reviews to identify references we may have missed during our primary search.
Titles and abstracts from all initially retrieved articles were independently reviewed by three investigators (S.S.S., D.S., J.J.L.) for potential inclusion in the study. We included a study if it involved adult human subjects with non-traumatic cardiac arrest where treatments were applied either during the arrest or immediately post-cardiac arrest (within 24 hours of ROSC). We excluded RCTs of public health interventions; primary or secondary prevention of cardiac arrest in high-risk patients (e.g., implantable cardioverter defibrillators [ICDs]); animal studies; studies that exclusively included neonatal or pediatric patients; simulation studies; and studies of provoked cardioplegic arrest (e.g., cardiac surgery). Trials were also excluded if the primary population included patients with conditions in addition to cardiac arrest (e.g. sepsis, cardiogenic shock or ST elevation myocardial infarction). Lastly, our primary analysis eliminated RCTs that primarily piloted the feasibility of new, highly exploratory treatments by restricting our cohort to those with at least 50 patients. We did extract several data elements from these smaller reports and provide them in the Supplemental Appendix. If multiple reports shared the same cohort (i.e. interim analyses, prespecified substudies or studies published in multiple journals), we only included the report with the largest study population in our primary analysis (although data from the additional reports were included in our evaluation of outcome assessments when relevant).
After retrieving full articles of potentially relevant trials, two reviewers (S.S.S., D.S.) independently assessed each study's eligibility on the basis of these inclusion and exclusion criteria. Any discrepant opinions were resolved through consensus or consultation with a third investigator (B.K.N.).
Data Extraction and Risk of Bias Assessment
Information from each RCT was extracted independently by at least two of three reviewers (S.S.S., D.S., J.J.L.) using a standardized form. The following variables were collected: author, title, journal and year of publication, location of arrest (OHCAs, IHCAs, or both), initial cardiac arrest rhythm (pulseless ventricular tachycardia/ventricular fibrillation, asystole/pulseless electrical activity, or both), size (number randomized to primary analysis) and patient characteristics of the study population (age, gender, witnessed arrest, provision of bystander CPR), type of intervention (drug, device, protocol improvement), and the timing of intervention (during cardiac arrest [i.e., pre-ROSC] or immediately post-cardiac arrest [i.e., post-ROSC]). We extracted the mean (and if not available, median) age of both intervention and control/placebo arms of each RCT. For those trials with multiple intervention arms or multiple sites represented, we calculated a weighted average based on reported data. For example, if a given study had an intervention A with sample size 100 with average age 60, and intervention B with sample size 200 with average age 65, the weighted mean would be: (60 * 100) + (65 * 200) / 300 = 63.3. For the purposes of this systematic review, our interest was at the level of randomized controlled trials and not patients. Thus, we did not account for differential study-specific sample sizes. We also extracted study design features such as single versus multicenter trial, geographic location (U.S., non-U.S., both), and source of funding (government, industry, hospital/institutional, none or not reported). Missing data were extracted as unavailable.
By necessity, we relied on each RCT's definitions for several key variables, which were consistent across studies for most but not all variables. For example, bystander CPR was typically defined as any attempt at CPR initiated by a person other than the EMS or first responder team regardless of whether the event was witnessed or not. Our assessment of whether an RCT studied a protocol improvement was defined by us as an intervention that examined a change in timing or approach for implementing a treatment (e.g., pre-hospital therapeutic hypothermia versus routine care with hospital-initiated therapeutic hypothermia) and not if the treatment was given or not.
We also extracted data on outcome assessments in each RCT and how these outcomes were reported in the article (“positive” if the primary null hypothesis defined by the authors was rejected, “negative” if not). For consistency, we categorized outcomes into the following groups: process measures, outcome measures, ROSC, survival to hospital discharge, 30-day to 6-month survival, long-term survival (defined as greater than 6 months), neurological outcomes, global ordinal outcomes or quality-of-life. Although no single measurement has been validated to completely characterize neurological status following acute cardiac arrest, neurological assessments included the National Institutes of Health Stroke Scale (NIHSS) and Full Outcome of Responsiveness (FOUR) score for comatose patients. Similarly, global ordinal outcomes, adopted from the taxonomy developed by the 2011 AHA Consensus Statement on “Primary Outcomes for Resuscitation Science Studies,”14 included the following functional outcome measures: cerebral performance category (CPC); overall performance category (OPC); Glasgow Coma Scale (GCS); Glasgow-Pittsburgh Coma Scale (GPCS); GOS, Glasgow Outcome Scale; mRS, modified Rankin Scale; or other functional outcome measures. Cognitive measures such as the mini-mental status exam (MMSE) were grouped under “other functional outcome measure.” Quality-of-life measures included any assessments, such as those evaluating physical and psychological perceived health status, functional status (i.e., activities of daily living, occupational status and discharge destination), or other relevant measures.
Finally, two reviewers (S.S.S., D.S.) assessed the risk of bias using the Cochrane assessment tool15, modified to focus on the following domains most relevant to RCTs in cardiac arrest: sequence generation, allocation concealment, blinding of primary personnel, blinding of primary outcome assessors, and blinding of global ordinal/quality-of-life outcome assessors. We did not consider blinding of subjects to be a key element of study design, as subjects suffering from cardiac arrest are unaware of the intervention(s). Furthermore, blinding of personnel and providers is not feasible in many cardiac arrest trials due to the type(s) of interventions studied (i.e. active compression-decompression devices, timing of chest compressions and defibrillation during CPR, etc.). Nevertheless, these studies were deemed to be “high risk” for this domain. Any disagreements in assessment between reviewers were resolved through discussions or consultation between investigators (S.S.S., D.S., B.K.N.).
Statistical Analysis
Characteristics of RCTs were reported in absolute values and percentages. As we were particularly interested in study characteristics of recent clinical trials, we compared the prevalence of these in the last 5 years of the study period (2010-2014) relative to earlier periods (1995-2009) using simple logistic regression models with the last 5 years of the study period as the dependent variable. The characteristics we independently evaluated included: number of subjects, location of arrest (OHCA, IHCA), single versus multicenter trial, geographic location, source of funding, type and timing of intervention, and outcomes assessment. We considered a p-value of less than 0.05 as indicating statistical significance. All analyses were performed using Stata 14.0. (Stata Corporation, College Station, Texas).
Results
Study Characteristics
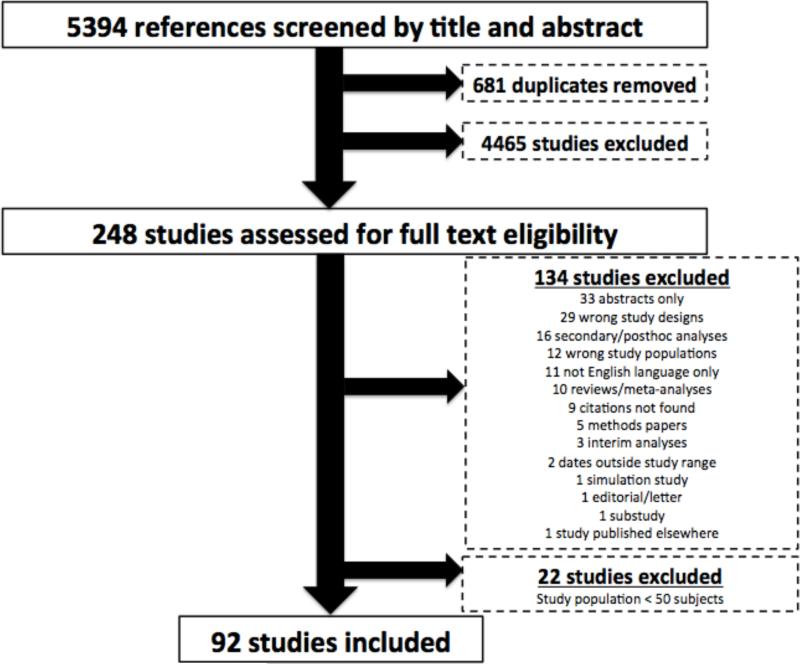
Our initial search returned 5394 citations published between January 1, 1995 and December 31, 2014. We identified 248 studies for full-text review, with a total of 114 RCTs identified. Twenty-two of these studies enrolled less than 50 patients as they were largely piloting the feasibility of highly exploratory treatments and were eliminated. Thus, 92 RCTs of cardiac arrest containing 64,309 subjects met final eligibility criteria for inclusion (Supplemental Appendix Table A). The final selection of studies for inclusion is displayed in Figure 1. The mean and median study population was 699 (SD, 1482) and 225.5 (IQR, 119-703.75), respectively, with mean age of 65.6 (SD, 3.2) and 69.7% (SD, 8.6%) men. Supplemental Appendix Table A lists the 92 RCTs individually by their journal publication and year, along with select study characteristics.
Figure 1.
PRISMA Flow Diagram for Selection of RCTs.
A total of 81 RCTs with 60,447 subjects involved OHCA exclusively, whereas 4 RCTs with 724 subjects involved IHCA exclusively and 7 RCTs with 3,138 subjects involved both locations of arrest. Eighteen trials (19.6%) containing 7,687 patients were performed in the U.S., 68 (73.9%) comprised of 30,400 subjects were performed outside the U.S., and 6 (6.5%) containing 26,222 subjects were performed in both settings. Of note, 5 out of the 6 studies performed in both locations (i.e., U.S. and abroad) were performed within the last 5 years. Table 1 provides summary statistics of several study characteristics for the 92 RCTs, stratified by location of arrest as OHCA, IHCA, or both. More studies were performed in the last 5-year period between 2010 and 2014, as compared with earlier years. Fifty-two (56.5%) RCTs were industry-sponsored whereas sixteen (17.4%) were government-funded. Thirty-eight (41.3%) RCTs studied drugs, an additional 39 (42.4%) studied devices and 15 (16.3%) studied protocol improvements. None of the four IHCA studies examined device or protocol interventions but focused entirely on evaluating drugs. Seventy-four (80.4%) RCTs examined interventions during the cardiac arrest, 15 (16.3%) examined post-cardiac arrest care and 3 (3.3%) studied both.
Table 1.
General Characteristics of Contemporary RCTs for Cardiac Arrest Stratified by Type. CPR, cardiopulmonary resuscitation; IHCA, in-hospital cardiac arrest; IQR, interquartile range; OHCA, out-of-hospital cardiac arrest; PEA, pulseless electrical activity; RCT, randomized controlled trial; VF, ventricular fibrillation; VT, ventricular tachycardia.
| Variable | Total (N=92) | OHCA (N=81) | IHCA (N=4) | OHCA/THCA (N=7) |
|---|---|---|---|---|
| Number of subjects | 64,309 | 60,447 | 724 | 3138 |
| Median/study (IQR) | 225.5 (119-703.75) | 234 (120-750) | 178 (142-217) | 145 (104-460.5) |
| Average Patient Age [μ (σ)] | 65.6 (3.2) | 65.5 (3.0) | 66.2 (3.5) | 66.2 (4.6) |
| Percent Male [%(σ)] | 69.7 (8.6) | 70.6 (8.4) | 65.0 (5.5) | 62.2 (8.9) |
| Witnessed Arrest [% (σ)] | 74.0 (17.3) | 72.7 (17.6) | 81.0 (8.3) | 86.4 (14.7) |
| Bystander CPR [% (σ)] | 41.1 (21.9) | 40.1 (20.3) | N/A | 45.3 (34.8) |
| Multicenter | 61 (66.3) | 55 (67.9) | 2 (50.0) | 4 (57.1) |
| U.S. only | 18 (19.6) | 17 (21.0) | 1 (25.0) | 0 |
| Positive Outcome | 25 (27.2) | 23 (28.4) | 2 (50.0) | 0 |
| Industry-sponsored | 52 (56.5) | 47 (58.0) | 2 (50.0) | 3 (42.9) |
| Publication Year | ||||
| 1995-1999 | 19 (20.7) | 15 (18.5) | 1 (25.0) | 3 (42.9) |
| 2000-2004 | 20 (21.7) | 19 (23.5) | 1 (25.0) | 0 |
| 2005-2009 | 23 (25.0) | 22 (27.2) | 1 (25.0) | 0 |
| 2010-2014 | 30 (32.6) | 25 (30.9) | 1 (25.0) | 4 (57.1) |
| Type of Intervention | ||||
| Drug | 38 (41.3) | 31 (38.3) | 4 (100.0) | 3 (42.9) |
| Device | 39 (42.4) | 36 (44.4) | 0 | 3 (42.9) |
| Process Improvement | 15 (16.3) | 14 (17.3) | 0 | 1 (14.3) |
| Timing of Intervention | ||||
| During Cardiac Arrest | 74 (80.4) | 68 (84.0) | 1 (25.0) | 5 (71.4) |
| Post Cardiac Arrest | 15 (16.3) | 13 (16.0) | 0 | 2 (28.6) |
| During/Post Cardiac Arrest | 3 (3.3) | 0 | 3 (75.0) | 0 |
| Initial Arrest Rhythm | ||||
| Pulseless VT/VF | 19 (20.7) | 19 (23.5) | 0 | 0 |
| PEA | 7 (7.6) | 6 (7.4) | 0 | 1 (14.3) |
| Both | 66 (71.7) | 56 (69.1) | 4 (100.0) | 6 (85.7) |
Continuous Variables are reported as [μ (σ)] and categorical variables are reported as [n (%)]. Most variables are categorical. Data for population characteristics provided for intervention group of RCTs.
Risk of Bias and Outcomes Assessed
A review of risk of bias of the RCTs revealed significant heterogeneity with an overall limited reporting of specific criteria (Table 2). Specifically, risk of bias was often difficult to assess due to the frequent absence of detailed reporting, often resulting in the attribution of “unclear” risk of bias. This was most frequently observed when reporting sequence generation (59.8%), allocation concealment (47.8%), and adequate blinding of global ordinal/quality-of-life outcome assessors (52.2%) (Table 2). The blinding of primary personnel was “high risk” in the majority of studies (58.7%), likely due to the large number of trials focusing on device or protocol improvement interventions where blinding was expected to be challenging. Lastly, the blinding of primary outcome assessors was “low risk” in the vast majority of trials (91.3%), due to the fact that most trials assessed relatively objective endpoints such as ROSC or survival, which are unlikely to be misattributed regardless of outcome assessor blinding. Supplemental Appendix Table B lists the risk of bias assessment for the 92 studies.
Table 2.
Risk of Bias Assessment of RCTs for Cardiac Arrests
| Risk of Bias | |||
|---|---|---|---|
| Domain | Low | Unclear | High |
| Sequence generation (n=92) | 32 (34.8) | 55 (59.8) | 5 (5.4) |
| Allocation concealment (n=92) | 30 (32.6) | 44 (47.8) | 18 (19.6) |
| Blinding of primary personnel (n=92) | 36 (39.1) | 2 (2.2) | 54 (58.7) |
| Blinding of primary outcome assessors (n=92) | 84 (91.3) | 8 (8.7) | 0 (0) |
| Blinding of global ordinal/QOL outcome assessors (n=67) | 29 (43.3) | 35 (52.2) | 3 (4.5) |
Categorical variables are reported as [n (%)]
Twenty-five studies did not report a global ordinal and/or quality-of-life (QOL) outcome.
The most common outcome reported was ROSC (86 trials, 93.5%); 16 (17.4%) reported process measures, 77 (83.7%) reported survival to discharge, 20 (21.7%) reported 30-day to 6-month survival, and 22 (23.9%) reported survival beyond 6 months (Table 3). Fifty-three (57.6%) RCTs reported global ordinal outcomes whereas 15 (16.3%) reported quality-of-life measures. The most common tool for measurement of global ordinal outcomes was the CPC (or OPC) (43 of 53 [81.1%]). Eighteen (19.6%) studies reported other functional outcome measures, such as the modified MMSE. Notably, neurological assessments such as the NIHSS or FOUR score for comatose patients were not utilized in any of the RCTs in our systematic review. Twenty-one (25.9%) RCTs in OHCA evaluated survival beyond 6 months. None of the RCTs in IHCA and one of the mixed studies evaluated survival beyond 6 months. Twenty-three (28.4%) RCTs in OHCA reported a positive study outcome whereas two (50.0%) of the four RCTs in IHCA and none of the mixed RCTs with both OHCA and IHCA achieved statistical significance with respect to their primary endpoint.
Table 3.
Measurement of Survival, Global Ordinal and Quality-of-Life Outcomes in RCTs for Cardiac Arrests
| Variable | Total (N=92) | OHCA (N=81) | IHCA (N=4) | OHCA/IHCA (N=7) |
|---|---|---|---|---|
| ROSC | 86 (93.5) | 75 (92.6) | 4 (100.0) | 7 (100.0) |
| Survival to Hospital Discharge | 77 (83.7) | 68 (84.0) | 4 (100.0) | 5 (71.4) |
| 30-day-to-6-month Survival | 20 (21.7) | 15 (18.5) | 3 (75.0) | 2 (28.6) |
| Long-Term Survival (> 6 months) | 22 (23.9) | 21 (25.9) | 0 | 1 (14.3) |
| Neurologically Intact Survival | 60 (65.9) | 52 (65.0) | 4 (100.0) | 4 (57.1) |
| Quality-of-Life Outcomes | 15 (16.3) | 14 (17.3) | 1 (25.0) | 0 |
| Global Ordinal Outcomes | 53 (57.6) | 45 (55.5) | 4 (100.0) | 4 (57.1) |
| CPC/OPC | 43 (46.7) | 38 (46.9) | 2 (50.0) | 3 (42.9) |
| GCS/GPCS | 18 (19.6) | 13 (16.0) | 3 (75.0) | 2 (28.6) |
| mRS | 5 (5.4) | 5 (6.2) | 0 | 0 |
| Other Functional Outcome | 18 (19.6) | 16 (19.8) | 1 (25.0) | 1 (14.3) |
Categorical variables are reported as [n (%)]. Abbreviations: CPC, cerebral performance category; OPC, overall performance category; GCS, Glasgow Coma Scale; GPCS, Glasgow-Pittsburgh Coma Scale; mRS, modified Rankin Scale.
Factors Associated with RCTs performed in the last 5 years
We found a total of 30 (32.6%) RCTs were performed in the last 5 years. We found several factors that were correlated with these more contemporary RCTs as compared with RCTs performed in the preceding 15 years; these are displayed in Table 4. Overall, we found a non-significant trend toward larger, multicenter studies in the last 5 years. Studies that focus on protocol improvements and post-arrest care were significantly more common. However, we did not find an increase in RCTs of IHCA over time, nor did we find significant differences in other factors we evaluated, including industry funding, U.S.-based studies, and survival assessments beyond ROSC.
Table 4.
Trends in Select Characteristics of Cardiac Arrest RCTs from 1995-2014
| Variable | 1995-1999 | 2000-2004 | 2005-2009 | 2010-2014 | p-value |
|---|---|---|---|---|---|
| Total number of studies | 19 (20.7) | 20 (21.7) | 23 (25.0) | 30 (32.6) | |
| Any IHCA | 4 (21.1) | 1 (5.0) | 1 (4.3) | 5 (16.7) | 0.338 |
| Subjects* | 10,347 (16.1) | 4,796 (7.5) | 10,953 (17.0) | 38,213 (59.4) | 0.055 |
| Multicenter | 7 (36.8) | 16 (80.0) | 14 (60.9) | 24 (80.0) | 0.058 |
| Geography (U.S.) | 5 (26.3) | 4 (20.0) | 5 (21.7) | 4 (13.3) | 0.300 |
| Industry Funding | 9 (47.4) | 13 (65.0) | 16 (69.6) | 14 (46.7) | 0.187 |
| Survival Assessment Beyond ROSC | 19 (100.0) | 18 (90.0) | 20 (87.0) | 26 (86.7) | 0.430 |
| Drug | 10 (52.6) | 11 (55.0) | 11 (47.8) | 6 (20.0) | 0.005 |
| Device | 9 (47.4) | 7 (35.0) | 9 (39.1) | 14 (46.7) | 0.564 |
| Process Improvement | 0 | 2 (10.0) | 3 (13.0) | 10 (33.3) | 0.004 |
| Timing (During Cardiac Arrest) | 18 (94.7) | 17 (85.0) | 19 (82.6) | 20 (66.7) | 0.025 |
Categorical variables are reported as [n (% total)]. p-value reported for logistic regression comparing variables during last 5 years (2010-2014) relative to values in prior intervals (1995-2009).
p-value for subjects reported for comparison per 100 trial participants [% total out of 64,309].
Discussion
This systematic review brings together twenty years of resuscitation research on RCTs. It includes more than 90 RCTs with nearly 65,000 patients in total, making it to our knowledge the largest and most comprehensive systematic review of randomized investigations involving acute treatments studied in cardiac arrest. It highlights an overall paucity of RCTs in cardiac arrest, as well as wide variation in their study design, settings, interventions, and reporting of outcomes. Overall, we found particularly important gaps in RCTs examining IHCA, protocol improvement interventions, post-cardiac arrest care, and long-term survival and health status outcomes. Future RCTs could better target these knowledge gaps to improve our understanding of optimal management strategies for these high-risk patients.
The overall paucity of RCTs relative to the burden of disease in cardiac arrest is striking. In our systematic review, we found an average of 4.6 RCTs published annually representing just over 3200 patients enrolled each year. This could be considered a relatively modest investment in this disease process relative to its estimated burden in the general U.S. population (approximately 535,000 combined OHCA and IHCA events occur in the U.S. annually with significant mortality)1. For instance, this estimate represents approximately 2.5 cardiac arrest RCTs performed per 10,000 cardiac arrest deaths annually for OHCA (and just 0.5 cardiac arrest RCTs performed per 10,000 cardiac arrest deaths annually for IHCA). This value lies within the same order of magnitude (6 published RCTs per 10,000 deaths per year) as described in a “back-of-the-envelope” calculation by Ornato and colleagues in a recent expert opinion piece evaluating the public health burden of cardiac arrest12. This statistic becomes better placed in context when one considers that there are 25 to 86 times the number of published RCTs per 10,000 deaths per year for heart failure, stroke and myocardial infarction. Our findings reinforce the call by Ornato and colleagues for further clinical research into cardiac arrest with a particular emphasis on RCTs, as an opportunity exists to align research prioritization with the public health need12.
Our systematic review identified several gaps that may help better define priorities in resuscitation research in order to fully realize this opportunity. For example, we discovered a striking paucity of studies examining IHCA. Only four studies in the last 20 years have focused on an exclusive IHCA cohort, and just an additional seven studies examined both IHCA and OHCA (with most enrolling more patients with OHCA). Thus, RCTs in IHCA are clearly a fertile area for investigation given that IHCAs may make up as much as 40% of all cardiac arrests and there are prominent etiologic differences that distinguish it from OHCA5. The need for greater evidence for IHCA should be balanced against some evidence to suggest temporal progress in IHCA outcomes16, as compared to more modest improvements in OHCA over the same interval. This suggests that progress has been made in IHCA without the investment in RCTs perhaps through better patient selection or implementation of resuscitation care. Further investigation is needed related to the setting or type of intervention in cardiac arrest and the role of RCTs in improving the evidence base of therapies. Another prominent area that our review identified was a significant gap in the study of protocol interventions that evaluate the impact of systems changes in the management of cardiac arrest. Yet while the vast majority of RCTs have examined drugs and devices, we did note that many more recent RCTs have begun to explore protocol interventions in the last 5 years. Finally, our study showed a dearth of interventions examining post-cardiac arrest care. Yet this area has also changed in recent years with a push toward increased evaluation of treatments instituted after ROSC has been achieved, likely driven by greater interest in therapeutic hypothermia.
Clinical trials in cardiac arrest treatments pose major logistical challenges due to the acuity and unexpected nature of its presentation, as well as heterogeneity of its patients, etiologies and settings, including perceived barriers to informed consent. In 2011, the American Heart Association published a consensus statement specifically detailing the challenges for RCTs for cardiac arrest with respect to selection of a meaningful primary outcome14. After extensive deliberation, it was clear that no single primary outcome would be appropriate for all studies of cardiac arrest with recommendations for pairing a time point and physiological condition to a specific question14. These recommendations highlight challenges in the design and performance of RCTs in this area and the potential for great variation17.
An additional key finding from our study is that outcomes assessment continues to be limited. These results are consistent with a previously published systematic review demonstrating the heterogeneity and lack of consistency in outcomes reporting in studies of cardiac arrest17. Like Whitehead and colleagues in prior work, we found no single outcome measure was universally or consistently assessed17. Our data and these earlier reports continue to support the development of a standardized core outcome set through the COSCA: Core Outcome Set for Cardiac Arrest initiative (http://www.comet-initiative.org/studies/details/284). Our study also shows a striking lack of non-mortality related patient outcomes, like quality-of-life, and long-term assessments18. Even when assessed, the measurement was not optimal. We found that the most common non-survival assessment was measurement of neurological outcomes using the CPC and done in the hospital setting. While the CPC may be simple and easy to use, it is not patient-centered and serves as a coarse functional assessment at best.
A few pragmatic and financial concerns also merit consideration, particularly in regards to sample selection bias and the assessment of outcomes. A very high proportion of witnessed arrest was reported in our systematic review (74%) as compared to contemporary epidemiologic data from the 2014 Cardiac Arrest Registry to Enhance Survival (38% witnessed by bystander; 12% by EMS provider)1. This highlights the ongoing challenges for RCTs in cardiac arrest to improve external validity through recruitment of a more representative population. Moreover, as we strive to generate common outcome measures, some degree of outcome heterogeneity across studies may be expected. Distinct study-specific outcomes may be justified based on patient characteristics, study design or trial intervention. In addition, comprehensive systematic assessment of long-term functional or quality-of-life outcomes is likely to be expensive and labor-intensive. As shown in a recent substudy of the Resuscitation Outcomes Consortium (ROC PRIMED), less than half of the OHCA survivors who were discharged could provide consent and be interviewed for a telephone assessment of neurologic function, cognitive impairment, health-related quality of life, and depression up to 6 months after discharge19. The process proved to be not only tedious and expensive, but also marked differences were observed in the cohort that was surveyed as compared to the cohort that could not provide consent due to death or loss to follow-up. Critically examining these sobering realities with regard to outcome and attempting to develop new approaches that address these challenges will be an important step as resuscitation science moves forward.
Our study should be interpreted with the following limitations. First, we did not have individual-level patient data for each study and could not address potential heterogeneity of treatment effects (drug, device, protocol interventions). However, our goal was not to summarize such diverse types of treatments, which is beyond the capability of this type of review. Second, we chose to focus our attention on the acute treatment of cardiac arrests in individuals, and therefore, excluded large public health interventions, such as the Public Access Defibrillation Trial20. Third, the substantial heterogeneity of study designs is partly reflected by the complex nature of resuscitation research and the different types of treatments evaluated. This limited our ability to systematically measure the “quality” of RCTs, and so we independently collected aspects of study design shown to be useful in prior work for different medical conditions and elected not to provide a summary score to avoid confusion15, 21, 22. Finally, there is the possibility of publication bias in this field due to selective reporting, as we identified several ‘small’ studies that were published and the rate of “positive” outcomes was remarkably high at 27%. We speculate that many negative studies are likely to have remained unreported in the literature but the significant heterogeneity of interventions we assessed made it difficult to formally assess for this possibility. Although these types of RCTs are important to recognize as initial steps for evaluating highly exploratory treatments, they are unlikely to clinically impact most patients in any substantial way.
Despite these limitations, we believe our findings have important implications for how best to prioritize future work in cardiac arrest. We have noted that RCTs themselves are changing in size and scope with the emergence of a non-significant trend toward larger trials that involve multiple centers. Although recent data suggest RCTs may be increasingly targeting protocol interventions and post-cardiac arrest care, there is no shift toward RCTs for IHCA or change in outcomes assessments. Finally, we found a limited number of new therapies proven effective in RCTs during the 20-year study period, suggesting that recent gains, however modest, may have been predominantly attributed to system-of-care optimization rather than new treatments.
Conclusions
Although cardiac arrests are a major public health concern worldwide, the extent and types of RCTs conducted in these high-risk patients has been largely unknown. We identified important gaps in research related to cardiac arrest treatments, with a paucity of RCTs with respect to the overall burden of disease in the general population. Particularly striking gaps in knowledge include RCTs examining IHCA, protocol improvement, post-cardiac arrest care, and long-term and quality-of-life outcomes. Although some of these characteristics are changing over time, greater knowledge of these gaps in research may help prioritize future work to improve care for these high-risk patients.
Supplementary Material
What is Known:
Approximately 347,000 out-of-hospital cardiac arrests (OHCAs) and 209,000 in-hospital cardiac arrests (IHCAs) occur in adults each year in the United States, with millions more occurring across the rest of North America, Europe and Asia.
Although survival has increased significantly in the past decade, it remains unacceptably low.
What the Study Adds:
In this systematic review, we found 92 RCTs with 64,309 patients published between 1995 and 2014.
There is an overall lack of RCTs in adult cardiac arrest relative to its disease burden.
Several important gaps were identified in RCTs including the infrequent focus on in-hospital cardiac arrest, protocol improvements, post-cardiac arrest care, and long-term or quality-of-life outcomes.
Acknowledgments
The authors wish to gratefully acknowledge the expert assistance of Whitney Townsend, our reference librarian. We also wish to acknowledge the tools available on Covidence.org to facilitate citation selection, including independent screening of titles and abstracts, full text reviews, and risk of bias assessment. Dr. Sinha had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Sources: Dr. Sinha is supported by the National Institutes of Health T32 postdoctoral research training grant (5T32HL007853-17). Drs. Chan and Nallamothu are supported by a research grant from the National Heart, Lung, and Blood Institute (NHLBI, 1R01HL123980). Dr. Nallamothu is also supported by a research grant from the VA Health Services Research & Development Program (IIR 13-079-2). Dr. Neumar is supported by research grants from the American Heart Association Grant-in-Aid program (15GRNT25890002) and the NHLBI (R44 HL091606-05, R01HL123227-03). Dr. Sukul is also supported by the same NIH T32 postdoctoral research training grant as Dr. Sinha (5T32HL007853).
Footnotes
Disclosures: None.
References
- 1.Writing Group M. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. American Heart Association Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Graham R, McCoy MA, Schultz AM, editors. Strategies to Improve Cardiac Arrest Survival: A Time to Act. National Academies Press; Washington (DC): 2015. [PubMed] [Google Scholar]
- 3.Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation. 2010;81:1479–87. doi: 10.1016/j.resuscitation.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Daya MR, Schmicker RH, Zive DM, Rea TD, Nichol G, Buick JE, Brooks S, Christenson J, MacPhee R, Craig A, Rittenberger JC, Davis DP, May S, Wigginton J, Wang H, Resuscitation Outcomes Consortium I Out-of-hospital cardiac arrest survival improving over time: Results from the Resuscitation Outcomes Consortium (ROC). Resuscitation. 2015;91:108–15. doi: 10.1016/j.resuscitation.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girotra S, Cram P, Spertus JA, Nallamothu BK, Li Y, Jones PG, Chan PS, American Heart Association's Get With the Guidelines-Resuscitation I Hospital variation in survival trends for in-hospital cardiac arrest. J Am Heart Assoc. 2014;3:e000871. doi: 10.1161/JAHA.114.000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hazinski MF, Nolan JP, Aickin R, Bhanji F, Billi JE, Callaway CW, Castren M, de Caen AR, Ferrer JM, Finn JC, Gent LM, Griffin RE, Iverson S, Lang E, Lim SH, Maconochie IK, Montgomery WH, Morley PT, Nadkarni VM, Neumar RW, Nikolaou NI, Perkins GD, Perlman JM, Singletary EM, Soar J, Travers AH, Welsford M, Wyllie J, Zideman DA. Part 1: Executive Summary: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015;132:S2–S39. doi: 10.1161/CIR.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 7.Neumar RW, Shuster M, Callaway CW, Gent LM, Atkins DL, Bhanji F, Brooks SC, de Caen AR, Donnino MW, Ferrer JM, Kleinman ME, Kronick SL, Lavonas EJ, Link MS, Mancini ME, Morrison LJ, O'Connor RE, Samson RA, Schexnayder SM, Singletary EM, Sinz EH, Travers AH, Wyckoff MH, Hazinski MF. Part 1: Executive Summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S315–67. doi: 10.1161/CIR.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 8.Becker LB, Aufderheide TP, Graham R. Strategies to Improve Survival From Cardiac Arrest: A Report From the Institute of Medicine. JAMA. 2015;314:223–4. doi: 10.1001/jama.2015.8454. [DOI] [PubMed] [Google Scholar]
- 9.Neumar RW, Eigel B, Callaway CW, Estes NA, 3rd, Jollis JG, Kleinman ME, Morrison LJ, Peberdy MA, Rabinstein A, Rea TD, Sendelbach S. American Heart Association Response to the 2015 Institute of Medicine Report on Strategies to Improve Cardiac Arrest Survival. Circulation. 2015;132:1049–70. doi: 10.1161/CIR.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 10.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 11.Nallamothu BK, Hayward RA, Bates ER. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008;118:1294–303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- 12.Ornato JP, Becker LB, Weisfeldt ML, Wright BA. Cardiac arrest and resuscitation: an opportunity to align research prioritization and public health need. Circulation. 2010;122:1876–9. doi: 10.1161/CIRCULATIONAHA.110.963991. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker LB, Aufderheide TP, Geocadin RG, Callaway CW, Lazar RM, Donnino MW, Nadkarni VM, Abella BS, Adrie C, Berg RA, Merchant RM, O'Connor RE, Meltzer DO, Holm MB, Longstreth WT, Halperin HR, American Heart Association Emergency Cardiovascular Care C, Council on Cardiopulmonary CCP and Resuscitation Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation. 2011;124:2158–77. doi: 10.1161/CIR.0b013e3182340239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundh A, Gotzsche PC. Recommendations by Cochrane Review Groups for assessment of the risk of bias in studies. BMC Med Res Methodol. 2008;8:22. doi: 10.1186/1471-2288-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS, American Heart Association Get with the Guidelines-Resuscitation I Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367:1912–20. doi: 10.1056/NEJMoa1109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitehead L, Perkins GD, Clarey A, Haywood KL. A systematic review of the outcomes reported in cardiac arrest clinical trials: the need for a core outcome set. Resuscitation. 2015;88:150–7. doi: 10.1016/j.resuscitation.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Elliott VJ, Rodgers DL, Brett SJ. Systematic review of quality of life and other patient-centred outcomes after cardiac arrest survival. Resuscitation. 2011;82:247–56. doi: 10.1016/j.resuscitation.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 19.Nichol G, Guffey D, Stiell IG, Leroux B, Cheskes S, Idris A, Kudenchuk PJ, Macphee RS, Wittwer L, Rittenberger JC, Rea TD, Sheehan K, Rac VE, Raina K, Gorman K, Aufderheide T, Resuscitation Outcomes Consortium I Post-discharge outcomes after resuscitation from out-of-hospital cardiac arrest: A ROC PRIMED substudy. Resuscitation. 2015;93:74–81. doi: 10.1016/j.resuscitation.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Hallstrom AP, Ornato JP, Weisfeldt M, Travers A, Christenson J, McBurnie MA, Zalenski R, Becker LB, Schron EB, Proschan M, Public Access Defibrillation Trial I. Public-access defibrillation and survival after out-of-hospital cardiac arrest. N Engl J Med. 2004;351:637–46. doi: 10.1056/NEJMoa040566. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G and Cochrane Statistical Methods G. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hrobjartsson A, Boutron I, Turner L, Altman DG, Moher D. Assessing risk of bias in randomised clinical trials included in Cochrane Reviews: the why is easy, the how is a challenge. Cochrane Database Syst Rev. 2013;4:ED000058. doi: 10.1002/14651858.ED000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.