Abstract
Tunneling nanotubes (TNTs) are membranous conduits for direct cell-to-cell communication. Until the past decade, little had been known about their composite structure, function, and mechanisms of action in both normal physiologic conditions as well as in disease states. Now TNTs are attracting increasing interest for their key role(s) in the pathogenesis of disease, including neurodegenerative disorders, inflammatory and infectious diseases, and cancer. The field of TNT biology is still in its infancy, but inroads have been made in determining potential mechanisms and function of these remarkable structures. For example, TNTs function as critical conduits for cellular exchange of information; thus, in cancer, they may play an important role in critical pathophysiologic features of the disease, including cellular invasion, metastasis, and emergence of chemotherapy drug resistance. Although the TNT field is still in a nascent stage, we propose that TNTs can be investigated as novel targets for drug-based treatment of cancer and other diseases.
Keywords: Tunneling nanotubes, intercellular transfer, intercellular communication, in vivo imaging, 3D confocal imaging, 3D cell biology, membrane tubes
Brief Caption
Tunneling nanotubes (TNTs) are long actin-based membranous extensions that can facilitate direct intercellular trafficking of signals and cargo between cells. The study of TNTs represents a relatively new and exciting field of biology. In this Viewpoint article, we provide perspective on how TNTs may play a critical role in intercellular communication in a spectrum of disease processes, especially in cancer.
Introduction
The role of intercellular communication has been studied extensively for decades in both normal and abnormal tissue environments. Investigation of endogenous nano-sized delivery vehicles such as exosomes and microvesicles has further expanded our knowledge of delivery mechanisms for intercellular cross-talk [1–7]. Gap junctions and soluble diffusible signals such as cytokines are effective and sufficient for messages that need to be conveyed over relatively short distances [8]. However, for cells physically separated within a biological microenvironment, long-range forms of communication are necessary to literally ‘bridge the gap’ for such cross-talk to take place. For example, as cancer cells are dispersed throughout the tumor matrix with stromal components, the distance between them may significantly reduce their communication via chemokines, cytokines, or exosomes/microvesicles, especially in dense tumors with high interstitial pressures.
Elucidating modes of intercellular communication: tunneling nanotubes as a new and distinct player in the field
Tunneling nanotubes (TNTs) represent a unique and highly efficient candidate to explain how intercellular communication takes places in a spectrum of disease processes, especially in cancer. Structurally, TNTs are membranous cellular extensions that vary in width from 50–1000 nm, and in length from a few to several hundred microns [9, 10] (Figure 1). However, these parameters may differ significantly between cell types. The function of TNTs is to act as intercellular conduits for exchange of cargo, including mitochondria, proteins, Golgi vesicles, viruses, microRNAs (miRNAs), and exosomes [9, 11–25]. As such, TNTs serve as cellular ‘nano-highways’ for mediating transport of cellular organelles and cargo between connected but non-adjacent cells. Extensive use of inverted microscopic and confocal microscopic fluorescent time-lapse imaging has emerged as a staple for laboratories in identifying movement of cargo via TNTs and direct cell-to-cell transfer. Both unidirectional and bidirectional transport have been observed, and may depend on the width and length of TNTs in addition to cell types and cargo being transported [9, 26–28].
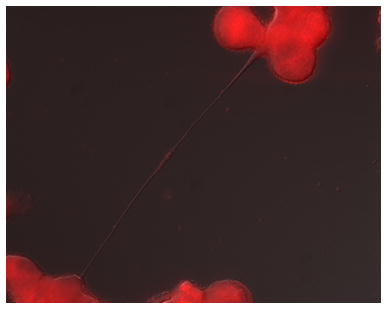
Figure 1. Representative example of tunneling nanotubes connecting cancer cells (malignant pleural mesothelioma).
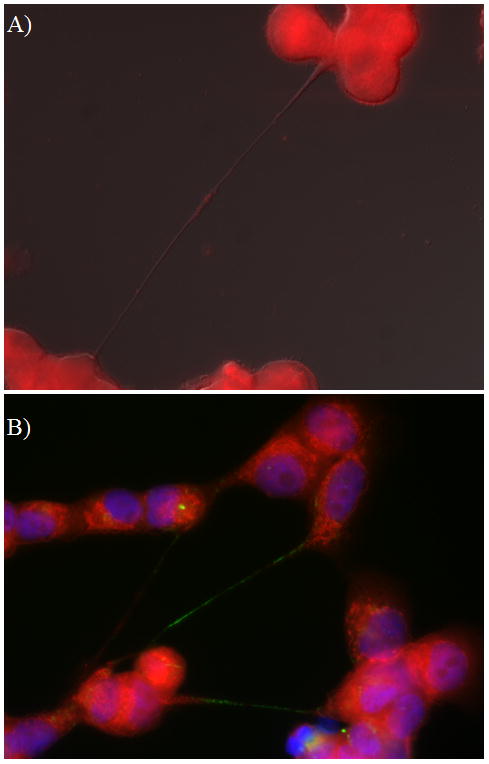
Images were taken using a Leica TCS SP5 microscope with 40x/1.25 NA oil objective lenses.
A) Two malignant mesothelioma cells (MSTO-211H biphasic mesothelioma cell line) connected by a long thin tunneling nanotube that is transporting mitochondria. The mitochondrial cargo is noted halfway along the nanotube as a visible bulge, stained using fluorescent MitoTracker Red.
B) Tunneling nanotubes connecting malignant mesothelioma cells and transporting mitochondria (red stain; MitoTracker Red) as well as Golgi vesicles (green; GM130 antibody). Blue = DAPI staining of nuclei).
Whether TNTs form between different cells may ultimately depend on cell types, combinations thereof, and in what context. For example, in our early work, we co-cultured cancerous cells from mesothelioma (a malignancy of the pleural lining of the lung cavity) with non-cancerous mesothelial cells, and found no communication via TNTs between the two different cell populations (although each cell type formed numerous TNTs among themselves) [9]. However, our subsequent studies did detect TNT formation between different cell types, including co-cultures of osteosarcoma (bone cancer) and osteoblasts, and between ovarian cancer and ovarian epithelial cells [29]. Numerous studies in non-cancer biology have readily detected TNT formation between different cell types, including cardiomyocyte and mesenchymal stem cells that led to rescue of damaged cells via transfer of mitochondria [30–32]. This finding has been reproduced in several studies and serves as one of the prime examples in the field of how TNT interactions may be time- and context-dependent. Interestingly, while TNTs may form transiently in cell culture over minutes to hours, their stability is variable. In fact, they are highly sensitive to stress factors such as prolonged exposure to light, excessive movement (e.g. shaking of culture plates), and other mechanical stresses [9, 26, 33]. This sensitivity highlights the difficulty in evaluating the effects of intercellular transfer of cargo mediated by these cellular extensions.
It is probable that future studies will more clearly elucidate the role of TNTs as critical effectors in the pathophysiology of disease(s). Thus, it is reasonable to postulate that disrupting or preventing TNT-mediated communication by targeted drugs or other means will present a novel therapeutic strategy [22, 34, 35]. However, it would also be critical to identify mechanisms and underlying genetics that underlie TNT biogenesis. To date there have been few studies which have been reported in the literature.
The importance of cell cycle in the genesis of TNTs has yet to be explored. Work from our group investigating how TNTs form between cancer cells has shown that factors stimulating or suppressing TNT formation are not entirely dependent on the rate of cellular proliferation [9, 36]. Subtypes of TNTs have been described, including most commonly those that extend between cells located at short or long distances. One type forms between cells that are already adjacent or in very close proximity to each other; these cells then track in different directions but leave TNTs (or TNT-like intercellular bridges) behind and intact for a finite amount of time [37]. This is not unlike the abscission that occurs during the process of cytokinesis, which results in intercellular bridges between dividing cells, and/or long connections formed during early cellular development. To distinguish TNTs from these processes, the use of time-lapse imaging is particularly helpful for confirming the provenance of TNTs that form at long-range between distant cells or groups of cells in culture.
Making the transition: moving toward evaluating TNTs in vivo
TNTs are novel candidates to explain how direct cell-to-cell communication process occurs [22]. Initial experiments from our group consistently demonstrated TNTs in vitro, and also in freshly resected and intact malignant mesothelioma and lung adenocarcinoma tumors from human patients [9]. We and others have subsequently demonstrated that TNTs are not exclusive to these two malignancies, but can form between malignant cells from a wide variety of histologic cancers, regardless of the site of origin. Key supportive evidence for TNTs in vivo comes also from the field of immunology, where TNTs were imaged in an inflammatory cornea animal model [38].
Current approaches to assessing tumors for clinical evaluation depend on 2D histopathologic evaluation. One of the main questions in the field of TNTs is the physiologic and pathological relevance of these structures. Despite increasing data supporting a role for TNTs as conduits for intercellular transfer of molecular cargo, many existing studies have only visualized TNTs in vitro. To demonstrate their presence in vivo, we developed detailed protocols for imaging of putative TNTs in human tumors, based in part on our main research focus in the cell biology of highly invasive cancers. The approach fundamentally rested on the need to image these extracellular protrusions as a 3-dimensional structure. We propose that reproducible and consistent imaging techniques will be essential to characterizing the role of these structures in cancer, as well as normal tissue.
History, perspectives, and future directions on 3D imaging of TNTs and TNT-like cellular protrusions: progress since identification of TNTs in 2004
Following the identification and report of TNTs in isolated PC12 (pheochromocytoma) cells by Rustom et al. more than a decade ago [26], nearly all published studies of TNTs have involved examination of their function in cell culture. Chinnery et al. provided the first evidence of membrane nanotubes by using an animal model of inflammation to image dendritic cell nanotubes in an ex vivo model of corneal disease [38]. To that point, nanotubes had not been imaged in tumors or in any other context other than in cell culture. Thus, we sought to confirm our in vitro findings of TNT formation by imaging these cellular connections in malignant tumors. In 2012, we reported the first evidence of nanotube connections in tumors from human patients with cancer, with initial studies focusing on cancers of the lung and of pleural lining (mesothelioma) [9].
We subsequently detected TNTs in tumors from patients with ovarian cancer [25] and also identified and reported TNTs in murine models of osteosarcoma [25]. Other groups have adopted these imaging techniques to successfully image TNTs in tumors, including laryngeal carcinomas and ovarian tumors, further broadening our understanding of the potential relevance of these structures in vivo [39, 40]. A recent and elegant study provided the first demonstration of membrane tubes (termed tumor microtubes due to their increased thickness and width compared to nanotubes) in a live, in vivo animal model of malignant brain tumors [41]. Furthermore, they also provided clear evidence linking the finding of tumor microtubes to clinical prognosis, as they correlated microtubes to established prognostic factors in gliomas such as the presence or absence of deletions of chromosomes 1p and 19q. A subsequent study used similar confocal techniques for visualizing membrane nanotubes connecting macrophages in muscle tissue in vivo [42]. Collectively, these studies provided a body of growing evidence that confocal techniques for visualizing TNTs can be adopted for investigations into the function of TNT-mediated intercellular communication in vivo and in real time.
TNTs in cancer: Understanding the niche for TNTs in the complex and heterogeneous tumor-stromal matrix
Intercellular communication between cancer cells is crucial to the progression of invasive cancers, but the mechanisms by which communication occurs between distant and proximal cells in a tumor matrix remain poorly understood. Stromal cells may comprise as much as 90% of a malignant tumor, underscoring the need to identify modes of transport between malignant cells not located in immediate proximity [43, 44]. Furthermore, cell-cell junctions are disrupted upon epithelial-mesenchymal transition, a potential precursor to metastasis [45, 46], (presumably) making intercellular communication via these junctions impossible for separated cells. Thus, in the context of cancer, there is a major gap in knowledge of how intercellular communication occurs between distant and proximal cells in the tumor matrix of malignant tumors [47, 48]. Exosomes, microvesicles, and microparticles are increasingly explored as purveyors of long-distance messages, but reliance on diffusion does not allow for a high rate of specificity and certainty in reaching potential target cells. This all occurs in the physiologic context of a 3D tumor matrix that is dynamic and constantly changing. Performing confocal imaging of tumors allows for a ‘snapshot’ of this dynamic process and serves as a foundation for using similar techniques for in vivo imaging to observe intercellular communication occurring in real time. The tools and techniques published to date and reviewed briefly here provide proof-of-principle evidence that nanotubes can be imaged in intact tumors. One next step to determining the pathophysiologic role of TNTs in disease is to determine how to correlate these 3D findings to etiology, progression, and response of these diseases to current modalities of treatment.
Establishing the role of TNTs in human pathophysiology and other diseases
Globally, research on TNTs has encompassed a wide variety of non-cancer cell types, including immune-derived cells (dendritic cells and monocytes [49, 50], mature macrophages [51, 52], T cells [53–55], B cells [27], and neutrophils [56]), other cells of mesodermal origin (kidney cells [57], mesothelial cells [58]), endothelial progenitor cells [21], mesenchymal stromal cells [19, 59], cardiomyocytes [17]) and cells of ectodermal origin (neuronal cells [28]), among others. Thus while TNTs may play a natural role in cell physiology and are thus not specific to cancer, TNTs also represent a newly recognized form of intercellular communication and transfer of signals in cancer [9, 22]. Indeed, there has been a steep rise in the number of research and review articles on these structures over the past several years, indicating a growing level of interest in investigating and elucidating their mechanisms, functions, and their general role(s) in the pathogenesis of human disease as well as normal human physiology (Figure 2).
Figure 2.
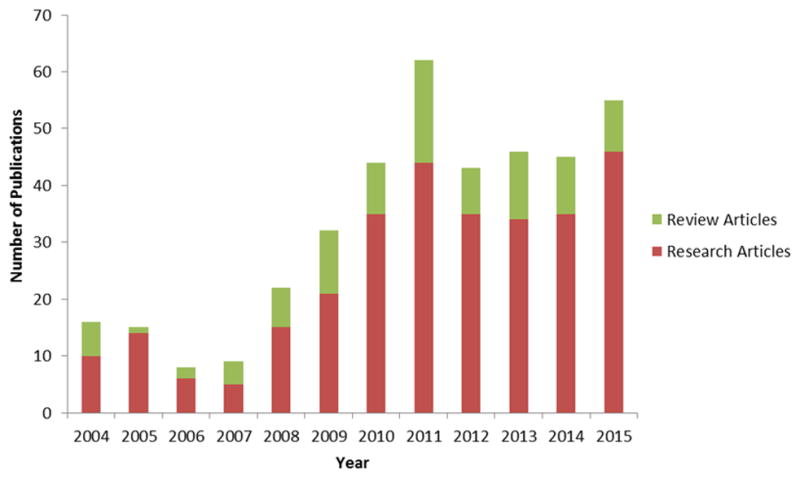
Number of TNT or related publications (research and review articles) published since the initial identification of TNTs by Rustom et al (Science, 2004).
Investigating roles and therapeutic targeting of TNTs in cancer and other diseases in the context of clinically relevant scenarios
It is now well-established that 2-dimensional in vitro evaluation of cancer cells is inadequate for accurately assessing cellular behavior in tumors, whether for early stages of cancer, later stages of metastatic cell seeding, and even for testing response during drug screening. Spheroid, organoid and other 3D models of tumors are under active investigation, and it is imperative that TNT researchers also adapt and develop methods for both in vitro and in vivo assessment of TNTs. Nonetheless, despite the relative inadequacies of standard 2D in vitro culture, critical information can in fact be gleaned regarding TNT formation and behavior in cancer. As basic and simple as it is, the classic scratch or wound-healing assay can be used not only to assess cancer cell invasion, but also reveal that TNT formation takes place along the leading invasive edge of malignant cells. Our work using time-lapse imaging discovered this form of cell-cell communication as malignant mesothelioma cells migrated toward each other and proliferated while moving to fill an empty space (Lou et al, PLoS ONE 2012, Supplemental Movie S1)[9]. Extending that to real-life clinical scenarios to cancer, we can imagine that the 3D version of this would in fact be microscopic remnant and scattered cells left behind following surgical resection of a malignant tumor. Thus the findings from a simple scratch assay allowed us to propose 3 hypotheses: First, TNTs may facilitate progression at the leading edge of invasive tumors. Second, residual scattered malignant cells that remain following definitive surgical resection and chemotherapy treatment may produce TNTs that coordinate cell activity and stimulate recurrence of the treated tumor. Third, malignant cells may harness TNTs as conduits for propagation of resistance to chemotherapeutic agents, via transfer of cellular chemoresistance factors or cargo that increases drug efflux (e.g. p-glycoprotein[60]). Thus with this perspective in mind, we postulate that one strategy for disrupting TNT-mediated intercellular communication would be to use TNT-specific inhibitors in the peri-operative period (i.e. for a short period leading up to, during, and immediately following surgical resection of a malignant tumor) [34]. At the present time, the use of continuous peri-operative drug-based treatment is not a routine standard of care.
The maturation of methods for studying TNTs has broad implication outside of cancer research as well. Indeed, it is important to acknowledge that while there are likely heterogeneity and differences in TNTs across various cell types, information gleaned from one form of cells or disease type can potentially be applied to TNTs in other settings. As one example TNTs have been shown to induce antibiotic resistance in bacteria [61] via acquisition of conjugated plasmids. This finding has a parallel scenario in cancer: cancer cells may also be capable of acquiring genes that induce resistance to chemotherapy through this same, or a very similar, form of TNT-mediated horizontal transfer. An additional example comes from the formation of membrane nanotubes in natural killer (NK) cells, from studies which were done nearly a decade ago. In this case, nanotubes connected NK cells to target cells, facilitating distant target cell lysis via facilitation of contact and movement of target cells toward NK cells [62]. Studies, which have demonstrated the propagation of signals for apoptosis, such as caspase proteins [63], via TNTs support the idea that other similar-sized signals could be transmitted as well.
Tunneling nanotubes: a novel drug target for cancer-directed therapy
TNTs are not exclusive to cancer, and are a cellular entity in ‘normal’, non-malignant cells. Just as cellular proliferation is increased in cancer – perversion of a normal cellular and physiologic process – TNTs may be significantly upregulated in the setting of malignant tumors [9, 23, 36]. Thus it will be important to establish standard procedures and methods for quantitating TNTs in conditions that are similar to the tumor microenvironment in vivo. For the purposes of in vitro examination of TNTs, we discovered that a low-serum, hyperglycemic medium was useful for stimulating increased formation of TNTs, up to 5-fold compared to standard passage medium for the cells we were using (10% FCS, 25 mM glucose RPMI). While TNTs are not specific nor unique to cancer, and while non-malignant cells also form TNTs, the potential formation of TNTs – and establishment of TNTs between malignant and non-malignant cells—is an active area of exploration in our laboratory. While we initially observed that malignant mesothelioma did not form TNTs to mesothelial cells when co-cultured, we have found other instances of malignant-stromal cell interaction via TNTs in other cancers (unpublished data and [29]).
To this end, determining a higher ratio of TNTs in cancer cells compared to stromal cells in the microenvironment that would make them a viable target (a ‘therapeutic index’, so to speak). It would be important to determine how increased ‘intercellular trafficking’ of cellular cargo via TNTs lead to or promotes cell transformation and tumor formation. As an example, previous work from our lab established the concept of a “nanotube index,” in which ratios could be calculated quantifiably demonstrating differences in rate of TNT formation over time among various cancer and stromal cell lines [10, 36]. For example, we showed that malignant mesothelioma cells formed significantly more TNTs than non-cancerous cells [23]. However, with ovarian cancer cells, there was notable heterogeneity in the rate of TNT formation among cancer cell lines and benign ovarian epithelial cells. Although platinum-chemoresistant cells (C200 and SKOV3 cell lines) were increased over chemosensitive and normal epithelial cells (A2780 and IOSE, respectively), the mean number of TNTs formed per cell was also highest in these latter cell populations [36]. This result could have been due to the sheer number of chemoresistant cells, leading to crowding in culture conditions that simply did not provide enough room for TNT growth. Most notably, TNTs proliferate most readily in subconfluent cultures. In addition to forming TNTs in co-culture, IOSE cells when cultured alone showed significantly more TNTs/cell than SKOV3 cells [36]. These findings supported our hypothesis that TNTs initiate distal tumor-stroma cell interactions, with potential variability as to the source of the initiating cell. Additional studies are needed to identify the underlying mechanism responsible for TNT formation and how it is regulated between cell types.
A novel strategy for cancer therapy and inflammatory diseases: Severing the lines of TNT-mediated communication
It will be imperative to identify structural components associated with, or more ideally specific to TNTs in order to identify pharmacologic inhibitors of those components. To date, however, investigation has relied on actin-destabilizing agents as a fundamental structural backbone of TNTs. For in vitro study of TNT disruption, commonly available agents such as cytochalasin D or B, latrunculin A and others have been used [55, 64, 65]. However, none of these agents is TNT-specific and it will be important to identify potential TNT-specific inhibitors that make pharmacologic sense in the context of each disease. The development of a specific inhibitor that could target TNTs across all cell types with high affinity would, of course, be optimal. For suppression of cancer TNTs, our studies have demonstrated that blocking the mTOR pathway with direct pathway (everolimus) or indirect pathway (metformin) inhibitors was an effective approach in both mesothelioma and ovarian cancer in vitro [9, 36]. Another pharmacologic approach that efficaciously suppressed TNT formation in mesothelioma was inhibiting the actin-bundling protein fascin with a derivative of the drug migrastatin[9, 10, 36]. The use of these drugs highlight just a few examples of medically and clinically relevant therapeutics that could be used to block TNT-mediated communication in disease, but which would require further extensive investigation in vivo. Agents that disrupt TNT-mediated communication would play a potential role in adjunct treatment of cancer, such as in the post-surgical setting when microscopic remnant cells reconnect with each other in order to reform the malignant tumor(s) [34]. Scenarios in which interruption of such communication would be critical to success include tumor invasion, tumor recurrence, and development of chemotherapy resistance [34].
Conclusions and perspectives
The mechanisms by which cells communicate with one another in the tumor microenvironment are not well understood [2, 66, 67]. Published and ongoing work in this emerging field challenge the paradigm that gap junctions, exosomes, or cytokines, and other diffusible chemical signals are exclusive modes by which cells mediate intercellular communication. Tunneling nanotubes are a natural biologic conduit for intercellular signaling and transport of cellular cargo. At this time, there appear to be more questions than answers in terms of what the mechanisms and functions of these unique cellular protrusions are in various cell types. However, the maturation of methods for studying TNTs has broad implications for cancer research and also other fields of medical biology. In oncology, it is worth asking the question: if cells can harness nanotubes for apoptosis, why not for proliferation of malignancies? Future work in this field may examine transfer of genetic materials, such as DNA, mRNA, and small RNAs. In at least one published study, mRNA-containing organelles were documented to transfer via what seem to be TNTs (stable cytoplasmic bridges, also called ring canals) joining rat spermatids [68]. Blockade of such transfer would have potential implications for cancer therapy [22]. Our group has demonstrated TNT-mediated intercellular transfer of miRNAs implicated in chemoresistance, including transfer between malignant and stromal cells as well as between malignant cells [25]. Thus, blockade of such transfer would have strong potential implications as a new and likely complementary approach to targeted cancer therapy at the cellular level [22]. The role of TNTs in the pathogenesis of cancer is both exciting and notably will be investigated as the next generation of prognostic markers and novel targets for cancer treatment. We anticipate that the level of enthusiasm for investigation of TNTs will be matched by researchers investigating other forms of disease as well.
Acknowledgments
We thank Michael Franklin for excellent editorial suggestions and critical review of the manuscript. We especially wish to thank and acknowledge the contributions of Katia Manova-Todorova, Sho Fujisawa, and Yevgeniy Romin for their expert advice and assistance with microscopy imaging of TNTs over the years.
Research Funding
Research reported and discussed in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114 (KL2 award to E.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. In addition, this research has also been supported by Minnesota Masonic Charities; the Minnesota Medical Foundation/University of Minnesota Foundation; the Masonic Cancer Center and Department of Medicine, Division of Hematology, Oncology and Transplantation, University of Minnesota; Institutional Research Grant #118198-IRG-58-001-52-IRG94 from the American Cancer Society; the National Pancreas Foundation; the Mezin-Koats Colon Cancer Research Award; The Randy Shaver Cancer Research and Community Fund; the Litman Family Fund for Cancer Research; the Baker Street Foundation; and the University of Minnesota Deborah E. Powell Center for Women’s Health Interdisciplinary Seed Grant support (Grant #PCWH-2013-002).
Abbreviations
- TNTs
Tunneling nanotubes
Footnotes
Author Contributions
E.L. wrote the initial draft of the manuscript. All authors edited and approved the manuscript prior to submission.
References
- 1.Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol. 2004;164:1807–15. doi: 10.1016/S0002-9440(10)63739-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cottin S, Ghani K, de Campos-Lima PO, Caruso M. Gemcitabine intercellular diffusion mediated by gap junctions: new implications for cancer therapy. Mol Cancer. 2010;9:141. doi: 10.1186/1476-4598-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naus CC, Laird DW. Implications and challenges of connexin connections to cancer. Nat Rev Cancer. 2010;10:435–41. doi: 10.1038/nrc2841. [DOI] [PubMed] [Google Scholar]
- 5.Strassburg S, Hodson NW, Hill PI, Richardson SM, Hoyland JA. Bi-Directional Exchange of Membrane Components Occurs during Co-Culture of Mesenchymal Stem Cells and Nucleus Pulposus Cells. PLoS One. 2012;7:e33739. doi: 10.1371/journal.pone.0033739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–68. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 7.Pap E, Pallinger E, Falus A. The role of membrane vesicles in tumorigenesis. Crit Rev Oncol Hematol. 2011;79:213–23. doi: 10.1016/j.critrevonc.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Francis K, Palsson BO. Effective intercellular communication distances are determined by the relative time constants for cyto/chemokine secretion and diffusion. Proc Natl Acad Sci U S A. 1997;94:12258–62. doi: 10.1073/pnas.94.23.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lou E, Fujisawa S, Morozov A, Barlas A, Romin Y, Dogan Y, Gholami S, Moreira AL, Manova-Todorova K, Moore MA. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS One. 2012;7:e33093. doi: 10.1371/journal.pone.0033093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ady JW, Desir S, Thayanithy V, Vogel RI, Moreira AL, Downey RJ, Fong Y, Manova-Todorova K, Moore MA, Lou E. Intercellular communication in malignant pleural mesothelioma: properties of tunneling nanotubes. Front Physiol. 2014;5:400. doi: 10.3389/fphys.2014.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9:431–6. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- 12.Kadiu I, Gendelman HE. Human immunodeficiency virus type 1 endocytic trafficking through macrophage bridging conduits facilitates spread of infection. J Neuroimmune Pharmacol. 2011;6:658–75. doi: 10.1007/s11481-011-9298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onfelt B, Purbhoo MA, Nedvetzki S, Sowinski S, Davis DM. Long-distance calls between cells connected by tunneling nanotubules. Sci STKE. 2005;2005:pe55. doi: 10.1126/stke.3132005pe55. [DOI] [PubMed] [Google Scholar]
- 14.Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat Cell Biol. 2007;9:310–5. doi: 10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherer NM, Mothes W. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends Cell Biol. 2008;18:414–20. doi: 10.1016/j.tcb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He K, Shi X, Zhang X, Dang S, Ma X, Liu F, Xu M, Lv Z, Han D, Fang X, Zhang Y. Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovasc Res. 2011;92:39–47. doi: 10.1093/cvr/cvr189. [DOI] [PubMed] [Google Scholar]
- 17.Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–41. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 18.Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, Seabra MC, Neil MA, French PM, Davis DM. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–83. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 19.Plotnikov EY, Khryapenkova TG, Galkina SI, Sukhikh GT, Zorov DB. Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp Cell Res. 2010;316:2447–55. doi: 10.1016/j.yexcr.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Veruki ML, Bukoreshtliev NV, Hartveit E, Gerdes HH. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc Natl Acad Sci U S A. 2010;107:17194–9. doi: 10.1073/pnas.1006785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasuda K, Park HC, Ratliff B, Addabbo F, Hatzopoulos AK, Chander P, Goligorsky MS. Adriamycin nephropathy: a failure of endothelial progenitor cell-induced repair. Am J Pathol. 2010;176:1685–95. doi: 10.2353/ajpath.2010.091071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lou E, Fujisawa S, Barlas A, Romin Y, Manova-Todorova K, Moore MAS, Subramanian S. Tunneling nanotubes: A new paradigm for studying intercellular communication and therapeutics in cancer. Commun Integr Biol. 2012;5 doi: 10.4161/cib.20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ady JW, Desir S, Thayanithy V, Vogel RI, Moreira AL, Downey RJ, Fong Y, Manova-Todorova K, Moore MAS, Lou E. Intercellular Communication in Malignant Pleural Mesothelioma: Properties of Tunneling Nanotubes. Frontiers in Physiology. 2014;5 doi: 10.3389/fphys.2014.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thayanithy V, Babatunde V, Dickson EL, Wong P, Oh S, Ke X, Barlas A, Fujisawa S, Romin Y, Moreira AL, Downey RJ, Steer CJ, Subramanian S, Manova-Todorova K, Moore MAS, Lou E. Tumor exosomes induce tunneling nanotubes in lipid raft-enriched regions of human mesothelioma cells. Exp Cell Res. 2014;323:178–188. doi: 10.1016/j.yexcr.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thayanithy V, Dickson EL, Steer C, Subramanian S, Lou E. Tumor-stromal cross talk: direct cell-to-cell transfer of oncogenic microRNAs via tunneling nanotubes. Transl Res. 2014 doi: 10.1016/j.trsl.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–10. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 27.Xu W, Santini PA, Sullivan JS, He B, Shan M, Ball SC, Dyer WB, Ketas TJ, Chadburn A, Cohen-Gould L, Knowles DM, Chiu A, Sanders RW, Chen K, Cerutti A. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10:1008–17. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, Chenouard N, de Chaumont F, Martino A, Enninga J, Olivo-Marin JC, Mannel D, Zurzolo C. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–36. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 29.Thayanithy V, Dickson EL, Steer C, Subramanian S, Lou E. Tumor-stromal cross talk: direct cell-to-cell transfer of oncogenic microRNAs via tunneling nanotubes. Transl Res. 2014;164:359–65. doi: 10.1016/j.trsl.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–65. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–8. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallabhaneni KC, Haller H, Dumler I. Vascular Smooth Muscle Cells Initiate Proliferation of Mesenchymal Stem Cells by Mitochondrial Transfer via Tunneling Nanotubes. Stem Cells and Development. 2012;21:3104–3113. doi: 10.1089/scd.2011.0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polak R, de Rooij B, Pieters R, den Boer ML. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood. 2015;126:2404–14. doi: 10.1182/blood-2015-03-634238. [DOI] [PubMed] [Google Scholar]
- 34.Lou E. Intercellular conduits in tumours: the new social network. Trends Cancer. 2016;2:3–5. doi: 10.1016/j.trecan.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lou E, Subramanian S. Tunneling Nanotubes: Intercellular conduits for direct cell-to-cell communication in cancer. Springer Berlin Heidelberg; New York, NY: 2016. [Google Scholar]
- 36.Desir S, Dickson EL, Vogel RI, Thayanithy V, Wong P, Teoh D, Geller MA, Steer CJ, Subramanian S, Lou E. Tunneling nanotube formation is stimulated by hypoxia in ovarian cancer cells. Oncotarget. 2016 doi: 10.18632/oncotarget.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veranic P, Lokar M, Schutz GJ, Weghuber J, Wieser S, Hagerstrand H, Kralj-Iglic V, Iglic A. Different types of cell-to-cell connections mediated by nanotubular structures. Biophys J. 2008;95:4416–25. doi: 10.1529/biophysj.108.131375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: Membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180:5779–83. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antanaviciute I, Rysevaite K, Liutkevicius V, Marandykina A, Rimkute L, Sveikatiene R, Uloza V, Skeberdis VA. Long-Distance Communication between Laryngeal Carcinoma Cells. PLoS One. 2014;9:e99196. doi: 10.1371/journal.pone.0099196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasquier J, Guerrouahen BS, Al Thawadi H, Ghiabi P, Maleki M, Abu-Kaoud N, Jacob A, Mirshahi M, Galas L, Rafii S, Le Foll F, Rafii A. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med. 2013;11:94. doi: 10.1186/1479-5876-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M, Huang L, Ratliff M, Karimian Jazi K, Kurz FT, Schmenger T, Lemke D, Gommel M, Pauli M, Liao Y, Haring P, Pusch S, Herl V, Steinhauser C, Krunic D, Jarahian M, Miletic H, Berghoff AS, Griesbeck O, Kalamakis G, Garaschuk O, Preusser M, Weiss S, Liu H, Heiland S, Platten M, Huber PE, Kuner T, von Deimling A, Wick W, Winkler F. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528:93–8. doi: 10.1038/nature16071. [DOI] [PubMed] [Google Scholar]
- 42.Rehberg M, Nekolla K, Sellner S, Praetner M, Mildner K, Zeuschner D, Krombach F. Intercellular Transport of Nanomaterials is Mediated by Membrane Nanotubes In Vivo. Small. 2016 doi: 10.1002/smll.201503606. [DOI] [PubMed] [Google Scholar]
- 43.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 44.Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;264:169–84. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- 45.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Lamorte L, Royal I, Naujokas M, Park M. Crk adapter proteins promote an epithelial-mesenchymal-like transition and are required for HGF-mediated cell spreading and breakdown of epithelial adherens junctions. Mol Biol Cell. 2002;13:1449–61. doi: 10.1091/mbc.01-10-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruckert F, Grutzmann R, Pilarsky C. Feedback within the inter-cellular communication and tumorigenesis in carcinomas. PLoS One. 2012;7:e36719. doi: 10.1371/journal.pone.0036719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Axelrod R, Axelrod DE, Pienta KJ. Evolution of cooperation among tumor cells. Proc Natl Acad Sci U S A. 2006;103:13474–9. doi: 10.1073/pnas.0606053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salter RD, Watkins SC. Dynamic properties of antigen uptake and communication between dendritic cells. Immunol Res. 2006;36:211–20. doi: 10.1385/IR:36:1:211. [DOI] [PubMed] [Google Scholar]
- 50.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–18. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, Kitamura H, Ito M, Watarai H, Hazelett CC, Yeaman C, Ohno H. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat Cell Biol. 2009;11:1427–32. doi: 10.1038/ncb1990. [DOI] [PubMed] [Google Scholar]
- 52.Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell Immunol. 2009;254:142–8. doi: 10.1016/j.cellimm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sowinski S, Alakoskela JM, Jolly C, Davis DM. Optimized methods for imaging membrane nanotubes between T cells and trafficking of HIV-1. Methods. 2011;53:27–33. doi: 10.1016/j.ymeth.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–9. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 55.Rudnicka D, Feldmann J, Porrot F, Wietgrefe S, Guadagnini S, Prevost MC, Estaquier J, Haase AT, Sol-Foulon N, Schwartz O. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J Virol. 2009;83:6234–46. doi: 10.1128/JVI.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galkina SI, Stadnichuk VI, Molotkovsky JG, Romanova JM, Sud’ina GF, Klein T. Microbial alkaloid staurosporine induces formation of nanometer-wide membrane tubular extensions (cytonemes, membrane tethers) in human neutrophils. Cell Adh Migr. 2010;4:32–8. doi: 10.4161/cam.4.1.10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gurke S, Barroso JF, Hodneland E, Bukoreshtliev NV, Schlicker O, Gerdes HH. Tunneling nanotube (TNT)-like structures facilitate a constitutive, actomyosin-dependent exchange of endocytic organelles between normal rat kidney cells. Exp Cell Res. 2008;314:3669–83. doi: 10.1016/j.yexcr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 58.Ranzinger J, Rustom A, Abel M, Leyh J, Kihm L, Witkowski M, Scheurich P, Zeier M, Schwenger V. Nanotube action between human mesothelial cells reveals novel aspects of inflammatory responses. PLoS One. 2011;6:e29537. doi: 10.1371/journal.pone.0029537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cselenyak A, Pankotai E, Horvath EM, Kiss L, Lacza Z. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol. 2010;11:29. doi: 10.1186/1471-2121-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pasquier J, Galas L, Boulange-Lecomte C, Rioult D, Bultelle F, Magal P, Webb G, Le Foll F. Different modalities of intercellular membrane exchanges mediate cell-to-cell p-glycoprotein transfers in MCF-7 breast cancer cells. J Biol Chem. 2012;287:7374–87. doi: 10.1074/jbc.M111.312157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dubey GP, Ben-Yehuda S. Intercellular nanotubes mediate bacterial communication. Cell. 2011;144:590–600. doi: 10.1016/j.cell.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 62.Chauveau A, Aucher A, Eissmann P, Vivier E, Davis DM. Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells. Proc Natl Acad Sci U S A. 2010;107:5545–50. doi: 10.1073/pnas.0910074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arkwright PD, Luchetti F, Tour J, Roberts C, Ayub R, Morales AP, Rodriguez JJ, Gilmore A, Canonico B, Papa S, Esposti MD. Fas stimulation of T lymphocytes promotes rapid intercellular exchange of death signals via membrane nanotubes. Cell Res. 2010;20:72–88. doi: 10.1038/cr.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bukoreshtliev NV, Wang X, Hodneland E, Gurke S, Barroso JF, Gerdes HH. Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells. FEBS Lett. 2009;583:1481–8. doi: 10.1016/j.febslet.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 65.Jung S, Park J-Y, Joo J-H, Kim Y-M, Ha K-S. Extracellular ultrathin fibers sensitive to intracellular reactive oxygen species: Formation of intercellular membrane bridges. Exp Cell Res. 2011 doi: 10.1016/j.yexcr.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 66.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–9. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–31. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 68.Ventela S, Toppari J, Parvinen M. Intercellular organelle traffic through cytoplasmic bridges in early spermatids of the rat: mechanisms of haploid gene product sharing. Mol Biol Cell. 2003;14:2768–80. doi: 10.1091/mbc.E02-10-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]


