Abstract
In rodents, the medial aspect of the secondary motor cortex (M2) is known by other names including medial agranular cortex, precentral cortex, and frontal orienting field. As a subdivision of the medial prefrontal cortex, M2 can be defined by a distinct set of afferent and efferent connections, microstimulation responses, and lesion outcomes. However, the behavioral role of M2 remains mysterious. Here, we focus on evidence from rodent studies, highlighting recent findings of early and context-dependent choice-related activity in M2 during voluntary behavior. Based on the current understanding, we suggest that a major function for M2 is to flexibly map such antecedent signals as sensory cues to motor actions, thereby enabling adaptive choice behavior.
Keywords: prefrontal cortex, rodents, action selection, sensorimotor transformation, motor planning, voluntary behavior
Introduction – an emerging view of M2 function
The most medial and dorsal portion of the rodent frontal cortex has many names. In the literature, the same location in the brain has been called the shoulder region, medial precentral cortex (PrCm), dorsomedial prefrontal cortex (dmPFC), medial agranular cortex (AGm), second frontal area (Fr2), secondary motor cortex (M2 or MOs), and frontal orienting field (FOF). Moreover, the region may overlap with the vibrissa motor cortex (vM1). The confusing nomenclature has hindered progress to delineate function [1]. As such, the number of names is exceeded by the number of theorized functions, which range from decision-making to action planning, and from motor learning to sensory perception.
In this review, we will refer to the region centering around the shaded area in Fig. 1A as the secondary motor cortex (M2). This notation following the convention of mouse brain atlases [2,3], although unfortunately M2 is a label used for both this medial region and more anterior and lateral locations in the frontal cortex. Demarcating regions in the rodent frontal cortex is challenging because cytoarchitectonic and neurochemical differences are subtle [4–6]. Our choice to focus on the medial portion is motivated by studies of microstimulation responses [7] and cortex-wide connectivity [8], which suggest distinct divisions within the rodent frontal cortex.
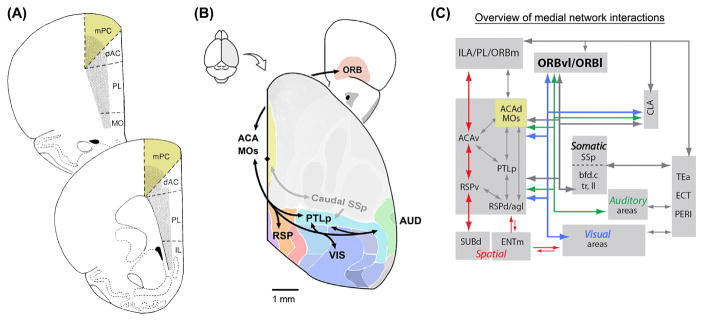
Figure 1. Afferent and efferent connectivity.
(A) Coronal view of rat mPFC subdivisions including M2, labeled as medial precentral cortex (mPC) in this diagram. Other mPFC regions are the dorsal anterior cingulate cortex (dAC), prelimbic cortex (PL), and infralimbic cortex (IL). (B) The medial subnetwork, a cluster of connected cortical regions identified in a study of mouse brain connectivity. Sensory regions including visual cortex (VIS), auditory cortex (AUD) and caudal primary somatosensory cortex (SSp) are connected to association regions including the retrosplenial (RSP), parietal (PTLp), anterior cingulate/secondary motor (ACA, MOs), and orbital areas (ORB). (C) Summary of interactions between the medial subnetwork and prefrontal regions including infralimbic (ILA), and prelimbic (PL) areas, parahippocampal structures including dorsal subiculum (SUBd), medial entorhinal area (ENTm), and other regions including claustrum (CLA), temporal association area (TEa), ectorhinal area (ECT), and perirhinal area (PERI). Part A adapted from [17]. Parts B and C adapted from [8].
What is the behavioral role of M2? On grounds of anatomy and physiology, it has been suggested that M2 is a possible homolog of the premotor cortex, supplementary motor area, or frontal eye field. However, it is difficult to show convincingly a strict one-to-one correspondence between rodent and primate frontal cortical regions, and thus behavioral roles implied through homology are unsatisfying.
Instead, here we will assert that results from rodents alone are sufficient to support a specific behavioral role for M2. To this end, we will summarize evidence from multiple approaches, moving from anatomical connectivity to lesions and inactivations, and then to electrophysiological correlates. The synthesis of old and recent findings leads to this conclusion: M2 is important for linking antecedent conditions, particularly sensory cues, to motor actions. Such a view positions M2 as a critical node in the neural circuitry for the flexible control of voluntary actions.
Afferent connections from diverse cortical and thalamic sources
M2 is a subdivision of the rodent medial prefrontal cortex (mPFC) (Box 1). As expected for an association region, M2 receives inputs from numerous cortical and thalamic sources. Thalamic projections originate from multiple nuclei [9]. Cortical afferents come from visual, somatosensory, auditory, parietal, retrosplenial, and orbital areas [8,10–12]. Multiple types of cortical inputs overlap spatially. Still unknown is whether there exists any topographical organization for the afferents. It has been suggested that rostral M2 receives more somatic sensorimotor inputs, whereas caudal M2 receives more sensory inputs [10,13]. There may also be regional differences: unlike Cg1 which primarily receives afferents from visual areas, M2 has auditory inputs in addition to visual afferents and may therefore be multi-modal [14].
Box 1. M2 is part of the rodent medial prefrontal cortex.
Is M2 a prefrontal or motor region? To answer this question, let’s consider a related question – do rodents have a prefrontal cortex? Knowing what constitutes the rodent mPFC is a logical prerequisite before discussing where M2 belongs. The answer to the second question, unfortunately, is not obvious because homology across species is difficult to ascertain. Arguments may be made at multiple levels, from embryology to cytoarchitecture and from connectivity to neurophysiology. A match at all levels is unlikely. As Leonard wrote in her monograph, one of the earliest studies of the rodent mPFC, “it is perhaps in the nature of association cortex that not all criteria can be satisfied simultaneously [19].” Rodent mPFC clearly differs from primate prefrontal cortex in a number of aspects; it lacks a granular layer, has a different topological organization, and contains a reduced version of the corticostriatal network [79]. Whether the partial similarities are sufficient to draw homology between rodent and primate prefrontal, cingulate, and premotor regions is a matter of debate [80,81].
Although there is no consensus on homology, what has been demonstrated repeatedly is that the rodent mPFC can be considered as a “mediodorsal (MD) thalamic projection cortex”. This is a definition of rodent mPFC that would have to include M2. Rose and Woolsey originally proposed the terminal fields of projections from the mediodorsal nucleus of the thalamus as a criterion for defining the prefrontal cortex [82]. Axons from the lateral division of MD thalamus project to M2 [19,83,84]. On the whole, MD thalamic projections can be found in M2 as well as orbital, prelimbic, infralimbic, anterior cingulate, and agranular insular regions, but they are absent in primary motor and sensory cortices in mice [85]. Therefore, insofar as rodents have a region designated as “prefrontal,” M2 should be considered as part of the mPFC network.
Efferent connections to distinct targets for action control
M2 neurons project to a long list of cortical and subcortical targets. Instead of reproducing the list (see e.g., [15]), it is more illuminating to highlight differences between M2 and its neighboring regions. The primary motor cortex (M1) lacks direct projections to several cortical targets including orbital, insular, parietal, or retrosplenial regions [15,16]. Furthermore, relative to M2, Cg1 has fewer corticocortical projections and connects to different thalamic nuclei [15,17]. Regions targeted by prelimbic and infralimbic cortices, but not M2, include ventral striatum, periaqueductal grey, septum, ventral tegmental area, and a few others [18]. Depending on the efferent target, projection neurons in mPFC reside in different cortical layers [18], and can have distinct long-range axonal collaterals [14].
M2 has several notable efferent connections to brain regions associated with motor control. M2 projects along the corticospinal tract to the spinal cord [4,18]. It also sends axons to the superior colliculus [19] and subcortical nuclei involved in oculomotor control [20,21]. Terminal fields in the striatum are inhomogeneous, centering on the dorsocentral part of the caudate-putamen [9,22]. This is more lateral than terminal fields from Cg1, consistent with the general medial-lateral organization of the rodent mPFC-striatal network [22]. Intriguingly, in the striatum, terminals from M2 overlap with those from the posterior parietal cortex (PPC) in both rats and mice [9,23]. This overlap in corticostriatal targeting is in addition to the direct reciprocal connections between M2 and PPC [8]. To add to the complexity, the retrosplenial cortex connects to both M2 and PPC [12], indicating multiple pathways mediating the interactions between these three association cortical regions.
Summary: drawing homology (or the lack thereof)
A remarkable feature of M2 connectivity is the reciprocal connections to sensory, parietal, and retrosplenial cortices. For this reason, on anatomical grounds it has been speculated that M2 acts as “a key link between multimodal sensory inputs and organized motor output [15].” Although most early studies focused on rats, a large-scale mapping of neocortical networks in mice also reported extensive corticocortical projections in M2 and Cg1, placing them as a component of the “medial subnetwork” (Fig. 1B, C) [8]. For these frontal-to-sensory pathways, both the recipient cell types in sensory areas [24,25] and the information carried by the frontal cortical axons [26] appear to be diverse, suggesting complex mechanisms for top-down control.
Based on anatomical data, various proposals have been put forth relating M2 to the premotor cortex (ventral convexity region of Brodmann area 6), supplementary motor cortex (medial wall of Brodmann area 6), or frontal eye field (Brodmann area 8). Arguments can be made for each homology, particularly if one cherry-picks on features that favor a particular interpretation. Instead, considering all the available evidence, it seems most appropriate to think of M2 as an association area with a combination of characteristics typically ascribed to the aforementioned primate frontal cortical regions.
Electrical microstimulation evokes general orienting movements
In microstimulation studies, an electrical current is injected into the brain tissue to evoke movements. By systematically moving the electrode, a motor map may be generated. In primates, such maps have greatly expanded our understanding of frontal cortex organization [27]. For rat M2, large-amplitude currents are needed to elicit any response, consistent with the presence of a nonprimary motor area [4]. When sufficient current is injected, microstimulation leads to a combination of eye, eyelid, vibrissa, and head movements [4,28]. In particular, for vibrissae, the evoked whisker movements may be ipsilateral, contralateral, or bilateral; moreover, multiple whiskers move in concert, arguing against a topographical representation [7]. A similar combination of vibrissa, neck, and head movements could be evoked by intracortical microstimulation of the medial frontal cortex in C57BL/6 mice [29]. The broad combination of evoked movements may be characterized as orienting.
There are forelimb and hindlimb representations in the motor cortex. Each representation is split into rostral and caudal sites. Relative to the location highlighted in Figure 1, the rostral hindlimb representation is more anterior, and the rostral forelimb representation is more lateral [29]. These rostral representations are smaller than their caudal counterparts, and thus considered as potential homolog of supplementary motor area. However, corticocortical connectivity patterns suggest that the rostral motor areas are anatomically distinct from M2 [8]. Therefore, it remains unclear the extent to which these rostral motor representations may functionally relate to medial M2.
Electrical microstimulation evokes specific vibrissa movements
Conflicting results come from microstimulation studies of vM1. Electrode track reconstructions suggest that vM1 potentially overlaps with M2 [5]. However, opposite to the aforementioned findings for M2, electrical microstimulation of vM1 causes predominantly whisker movements [30,31]. The evoked movements are topographical, showing whisker-by-whisker representation as a function of depth in the cortex. Based solely on the results from vM1, the stimulated regions should be considered as the vibrissa representation of the primary motor cortex, rather than a nonprimary motor area.
Summary: conflicting maps and potential explanations
It is not obvious why microstimulation studies of vM1 and M2 came to conflicting conclusions. One explanation is methodological: microstimulation mapping can yield inconsistent results [32]. This may be partly due to the wide range of stimulation parameters and choice of anesthetic agents. Another possible explanation is that vM1 may have subdivisions, and the anterior portion corresponds to M2. This argument comes from a couple of reports showing that rhythmic whisking may be evoked in posterior vM1, whereas non-rhythmic whisker movements accompanied by complex face, eye, eyelid, and nose movements are associated with stimulating an anterior “retraction-face” subregion of vM1 [33,34]. These may be overlapping subdivisions, rather than distinct modules, in the rodent medial frontal cortex. Finally, although it is generally thought that vM1 is involved in whisking behavior, it is worth noting that there is no consensus on its function [35,36].
Removal of M2 causes neglect, but only transiently
Based on the extensive connections to sensory- and movement-related regions, one may expect M2 lesions to cause perceptual and motor deficits. Indeed, unilateral removal of M2 results in contralateral neglect. Running a T-maze, lesioned rats make fewer contralateral turns [37]. The neglect is due to a choice bias, rather than an inability to turn, because rats can overcome the tendency if reward was removed from the preferred side [37]. Contralateral neglect following M2 lesions can also manifest as increased latency to choice in discrimination, and delayed or loss of orienting responses to visual, auditory, or tactile cue coming from specific directions [38].
Such neglect could be due to deficits in spatial attention, but there are two arguments indicating that the function of M2 is not strictly about the spatial allocation of sensory resources. First, neglect is transient. Most animals recover to original performance 3 weeks after lesion [38]. This suggests that the initial impairments could come from dysfunctions of downstream regions as a result of diaschisis, i.e. the sudden loss of cortical inputs [39]. In support of diaschisis, lesion of M2 is accompanied by changes in activity-dependent gene expression in the striatum, which correlate with the amount of head turning to sensory cues [40]. Moreover, animals recover faster from M2 lesion if they are subjected to visual deprivation at the same time [41]. Second, many studies use sensory-evoked motor responses as the behavioral readout, and thus cannot differentiate sensory impairments from sensorimotor deficits. In an attempt to relate to sensory processing impairment, one study tested whether unilateral eye suture can reproduce behavioral effects of unilateral M2 lesion, and the results were negative [37].
Recently, Erlich et al. investigated the effects of unilateral and bilateral M2 inactivation in rats performing automated orienting tasks [42]. The experimental design allowed for detailed quantification of the behavioral performance because rats made several hundred left or right orienting movements each session. In one task, rats had to orient to the direction signaled by a visual-spatial stimulus – a light on the corresponding side. Contrary to prior reports of neglect, M2-inactivated rats performed at similar levels as control animals. In another task, rats had to perform internally guided actions because there were no external stimuli. Rats with unilateral inactivation of M2 displayed an ipsilateral bias in this free-choice task, suggesting motor neglect. Collectively, these recent results add to the older findings to argue against a deficit in spatial attention.
Removal of M2 impairs actions guided by sensory or motor antecedents
What about sensory cues that are more complex and non-spatial? To answer this question, Passingham et al. taught rats to push or pull a door, with the correct choice indicated by visual cues such as color or room light [43]. Trained rats received bilateral M2 lesions. When tasked to re-apply the visual-motor associations, lesioned rats made more errors, although they eventually reached criterion. Unlike neglect, this was an enduring deficit that persisted more than 21 days after lesion.
Following this first observation, other studies have also found deficits in cue-guided actions. Erlich et al. tested a sensory evidence accumulation task, requiring rats to compare two auditory click trains and then select one of two actions [42]. Behavior associated with M2 inactivation could be described as impairment to the output stage of an accumulator model. Furthermore, deficits in cue-guided actions are more pronounced during moments of behavioral flexibility, when animals have to learn or adapt. Siniscalchi et al. trained head-fixed mice to switch multiple times between multiple non-spatial auditory-motor mappings during a single session [44]. In agreement with sensorimotor deficits, M2-inactivated animals made more perseverative errors when adjusting to perform sound-guided actions. Intriguingly, M2 inactivation actually reduced the number of errors when animals needed to abandon cues in favor of non-conditional responding. Such tendency to repeat and persevere could be the reason why lesioned animals select choices with lower action values [45]. These results indicate that M2 normally biases the subject towards responding based on evidence, which can come from sensory stimuli. When M2 is inactivated, animals use alternative action strategies.
In addition to complex and non-spatial sensory stimuli, M2 is also involved in actions guided by other actions or timing. Specifically, several studies studied the performance of action sequences, in which animals have to press different levers in a specific order. Rats with M2 lesions had trouble initially learning the order, and were impaired subsequently when the sequence was reversed [46]. Interestingly, signatures of goal-directed behavior, including outcome devaluation and contingency degradation, were affected only if the contingent response was an action sequence, and not for single lever presses ([46], but see [47]). These results, together with other studies [48,49], implicate M2 in the learning and use of sequence-level action chunks. The inability to perform organized actions could explain the poor performance in learning more complex skilled movements [50]. At least some of the deficits in organized actions can be reproduced by selective manipulation of corticostriatal projections emanating from M2 [51].
Summary: Executive control on conditional actions
Most lesion and inactivation studies reported diminished task performance, rather than overt changes to specific physical movements. Moreover, behavioral impairments were usually found during learning or adaptation. Following extensive re-training, task performance could return to baseline levels. These observations are in line with M2 exerting executive control on action selection. The emphasis on learning, but not implementation, of motor programs may be a general principle for the cortical control of actions in rodents ([52], but see [53]).
Collectively, the results support a role for M2 in guiding conditional actions, particularly those responses that are preceded by sensory stimuli, timing, or prior actions. This behavioral role is in excellent agreement with the anatomical considerations: motor-related efferent connections to exert control on actions, and sensory-related reciprocal connections to receive contextual inputs. Moving forward, it will be useful to specify the domains of antecedent conditions most dependent on a functional M2. So far, some conditions engage M2 (visual-non spatial [43], auditory click train comparison [42], auditory click train plus a delay period [54], auditory frequency-modulated sweeps [44], prior action [46]), whereas others do not (visual-spatial [42], auditory click train [55]). Do more complex and non-spatial sensory stimuli involve sensory cortices, and thus invoke the use of M2? How does such a sensorimotor role for rodent M2 compare to those previously proposed based on human and non-human primate studies (Box 2)? Furthermore, for the antecedent conditions already identified, which processing steps of the sensorimotor transformation occur in M2, and which are handled elsewhere and then transmitted to M2?
Box 2. Theories of higher-order motor cortex function.
There is a rich history of frontal cortex studies in humans and non-human primates. As early as 1935, Fulton ablated parts of the nonprimary motor cortex and noted “disorganization of the more highly integrated voluntary movements” in his subjects [86]. In this text box, we consider broadly the functions attributed to the premotor cortex, supplementary motor areas, and frontal eye field, and relate them to the rodent data.
Electrophysiological recordings of single units in primate nonprimary motor cortex have uncovered not only a variety of motor preparatory and command activity, but also proprioceptive, gaze, spatial visual, and non-spatial visual signals [87]. These signals are often specific to the task at hand or modulated by the behavioral context [87]. These characteristics mirror the early and task-specific choice activity reported more recently in rodent M2. Furthermore, the preparation and initiation of movements have been associated with distinct population activity dynamics [88]. There also appears to be regional specializations in premotor and supplementary motor areas [89,90]. The extent to which these principles may apply to rodent M2 is unknown.
A number of theories have been put forth regarding the function of the nonprimary motor cortex, particularly in support of skilled movements, motor sequences, and sensory-guided actions [89,91,92]. Mechanistically, the brain region may subserve specific functions within the scheme of action preparation, such as the programming of motor acts, limb stabilization, and suppression of default motor response plans [91]. Specifically for premotor and supplementary motor areas, they may play distinct roles in mediating externally instructed versus internally guided actions, or temporal versus spatial sequences of movements [89]. Aside from motor planning and selection, it has also been postulated that premotor functions could occur concomitantly with the allocation of attention [93,94].
These seemingly disparate functions may be consolidated in a framework of condition-action associations [78,90]. In this view, the nonprimary motor cortex represents how a combination of external stimuli and internal states connects to different possible actions. Some actions involve the evaluation of many competing conditions, and the complexity preferentially invokes the frontal cortex [90]. Such complexity in the antecedent conditions may be formalized as uncertainty in the action selection process, such that the influence on nonprimary motor cortical function can be quantified [95,96]. Building on these ideas, here we propose similar functions for M2 in rodents. In the future, rodents could be an excellent animal model to further dissect the cellular and circuit mechanisms underlying sensorimotor behavior.
In terms of localizing processing steps to specific brain regions, temporally precise perturbation methods such as optogenetics hold great promise. In memory-guided response tasks, silencing the medial frontal cortex during the delay period led to a choice bias consistent with contralateral neglect [55,56]. Response was most impaired for silencing induced near the end of sensory cue, just before motor output [57]. This led to an idea that the function of M2 may be distilled to solving one question: “if the go signal comes now, which choice should I make? [57]” Although transient inactivation is a powerful approach, it should be emphasized that the frontal cortex can recover from perturbations through recurrent connections with other brain regions, thus precludes a straightforward interpretation of null results [58,59].
Choice-related activity in M2 is early
Despite the considerable number of anatomical and inactivation studies of M2, until recently there have been few in vivo recordings from this region. Early recordings in anesthetized rats did not investigate the function of M2 per se, but instead used the preparation as a model to study slow-wave oscillations [60].
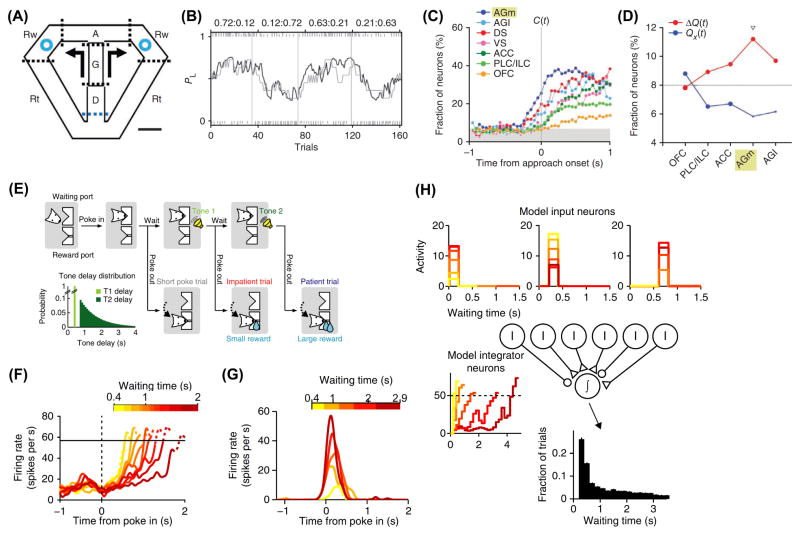
Probably the most remarkable physiological property of M2 neurons is their early choice-related activity. Information about the impending choice could be decoded from M2 neuronal activity about 500 ms before a rat makes its decision in a two-armed-bandit task (Figs. 2A – C) [45]. The result was striking because the neural signal for choice in M2 had the earliest onset among all the frontal and striatal regions examined by the same authors. Namely, choice coding can be measured in M2 before they can be detected in prelimbic, infralimbic, orbitofrontal, anterior cingulate, and primary motor cortices as well as ventral and dorsal striatum (Fig. 2C) [45,61]. Relative to other frontal cortical regions, neural signals for relative, rather than absolute, action values are more prominent in M2 (Fig. 2D). This agrees with the overall theme that M2 is about selecting the impending choice, but not necessarily about computing the values of different options. Such early choice-related activity in M2 has trial-to-trial variations matching those of the upcoming response, suggesting that it contributes causally to action planning [54]. Some cells prefer contralateral choices, whereas others neurons are more active for ipsilateral choices [44,54]. What is the significance of having the earliest choice-related activity in the entire frontal-striatal network? Coherent behavior relies on the selection of a unique action. Early choice-related activity implicates M2 as the original impetus for actions in the rodent frontal cortex.
Figure 2. Choice-related activity in M2 is early.
(A) Onset of choice signals in M2: In each trial, the rat goes through 5 stages: delay (D), go (G), approach to reward (A), reward (Rw), and return (Rt). At the end of go stage, the animal has a choice between two arms. Each arm is assigned to reward with a certain probability. The probabilities change after a block of ~40 trials. (B) Probability of choosing left (gray line) over a behavioral session. Actual performance is compared to a reinforcement-learning model (black line). Top: reward probabilities for the two arms. (C) Time courses of neural signals for the upcoming choice for five frontal cortical and two striatal regions. M2 is denoted as the medial agranular cortex (AGm). AGl, lateral agranular cortex. DS, dorsal striatum. VS, ventral striatum. ACC, anterior cingulate cortex. PLC/ILC, prelimbic/infralimbic cortex. OFC, orbitofrontal cortex. (D) Regional specificity for coding of action value Qx(t) or decision value ΔQx(t) during the last 1 s of the delay stage. The triangle indicates a significant difference between the two fractions (χ2-test, P < 0.05). (E) Cortical circuit mechanisms: The rat initiates a trial with a nose poke. Waiting longer results in a larger reward. Inset, distribution of waiting times. (F) Example M2 neuron with ramp-to-threshold activity. (G) Example M2 neuron with transient predictive activity. (H) An integrator model. Inset, the waiting time histogram generated using the model. Parts (A – D) adapted from [45]. Parts (E – H) adapted from [62].
If M2 drives the selection and planning of actions, what are the mechanisms? Murakami et al. devised a self-paced task in which rats delayed for various waiting times before committing to an action (Fig. 2E) [62]. Two types of M2 neurons have activity patterns predictive of the waiting time of each trial. One type has ramping activity reminiscent of the rise-to-threshold cells postulated to control voluntary movement initiation (Fig. 2F) [63]. The other type has transient activity during the waiting period before movement (Fig. 2G). Through computational modeling, the authors proposed that the two types of neuron act as an integrator and its inputs, therefore endowing M2 with the necessary local circuit elements to time an action (Fig. 2H). The early choice-related activity in rodent M2 likely relates to the readiness potential in human nonprimary motor cortex that precedes self-generated movements [64,65]. Mechanistic studies in rodents can thus provide important insights into the neural processes responsible for the cortical control of voluntary actions.
Choice-related activity in M2 is task-specific
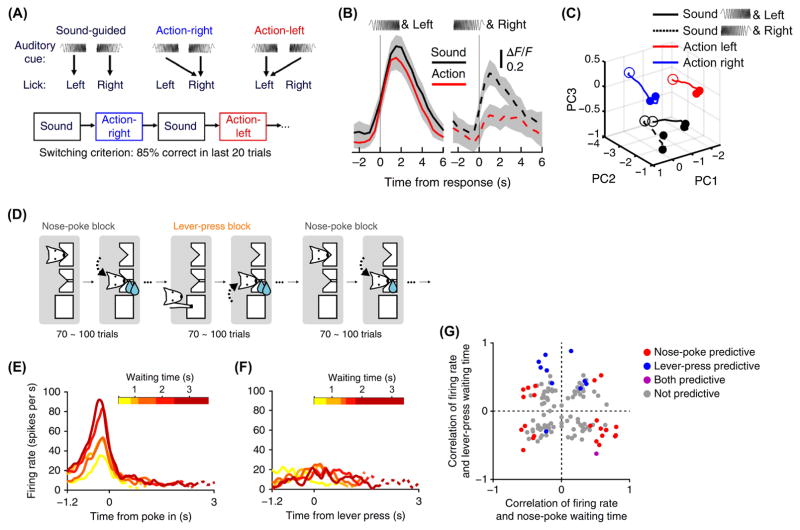
The activity of M2 neurons is modulated by specifics of the task. One factor is the effector. M2 neurons displayed different firing patterns when the operant action was changed from lever-presses to nose-pokes (Figs. 3D – G) [62] or when rotarod was replaced by wheel-running [50]. Another factor is reinforcement. Namely, M2 neurons appear to encode the presence of a reward [45,66]. However, in many tasks, rewards are coupled to consummatory behavior. Studies of M2 that vary the sign and magnitude of reinforcements are lacking. The activity of M2 neurons is also modulated by task engagement. Unlike the categorical neural responses observed in M2 during two alternative-choice behavior [57], preliminary evidence suggests that M2 neurons tune to multiple directions when recorded out of task context [54].
Figure 3. Choice-related activity in M2 is task-specific.
(A) Context specificity: The mouse made left or right licks in response to an auditory cue. Trials were organized into blocks, each with a distinct set of stimulus-response contingencies. When performance reached a criterion, a new block began with different contingencies. (B) Example M2 neuron with context-dependent activity. Gray shading, 95% confidence intervals. (C) Neuronal circuit trajectories were calculated from the trial-averaged activity of a 56-cell ensemble using demixed principal component analysis. PC, principal component. (D) Action specificity: The waiting task (Fig. 2E) implemented with interleaving blocks involving different operant actions: nose-poke and lever-press. (E) Example M2 neuron with nose-poke-specific predictive activity. (F) The cell from (E) during lever-press trials. (G) Summary of nose-poke- and lever-press-specific predictive activities. Each circle represents one neuron. Parts (A – C) adapted from [44]. Parts (D – G) adapted from [62].
To more explicitly determine context dependence, Siniscalchi et al. used two-photon calcium imaging to characterize neural ensemble activity in M2 during flexible sensorimotor behavior (Fig. 3A) [44]. Notably, M2 neurons exhibited distinct activity patterns, both at the single-cell and ensemble levels, for cue-guided versus non-conditional trials (Figs. 3B, C). These trials differed in their sensorimotor contingencies, but were otherwise identical in terms of stimulus, choice, and outcome, indicating that the internal implementation of conditional rules modulated M2 activity. Altogether, the studies indicate task-specific activation, suggesting that the contribution of M2 to action planning may limit to those responses that are behaviorally relevant. The ability to flexibly use antecedents to guide actions could be a central feature of M2.
Summary: Choice-related activity, what is it good for?
Altogether, the early choice-related activity indicates that M2 is an initiator of voluntary actions. The context dependence suggests flexible control to meet behavioral demands. These conclusions add to the previously described anatomical and inactivation data to suggest specific features for the sensorimotor functions carried out by M2.
What is the function of the choice-related activity in M2? Besides the obvious utility in voluntary behavior, a tour-de-force series of studies by Mooney and colleagues have shed light on another potential function in sensory perception [24,26,67]. Their experiments focused on a pathway from M2 to auditory cortex in mice. They found that within the auditory cortex, inputs from M2 primarily have a suppressive effect on firing rates via feedforward inhibition [24]. During locomotion, this pathway was active and contribute to the movement-related suppression of sensory-evoked cortical activity, leading to the idea that M2 provides the corollary discharge to facilitate dynamic adjustment of auditory perception during active behavior [67]. To what extent this potential function of M2 applies to task-specific situation remains to be determined.
Besides the route to sensory cortex, what other brain regions receive choice-related information from M2? For future studies, powerful techniques for dissecting neural circuits in rodents should open up opportunities to understand the action selection process in the brain [68,69]. In studies of frontal cortical circuits, there is exciting progress in elucidating the functional roles of projection neurons [70,71], deciphering the ensemble activity code [72,73], identifying the neural substrates of learning [74,75], delineating the circuit mechanisms underlying decision formation [76,77], and relating the network dysfunctions to mental disorders (Box 3).
Box 3. Potential relevance to stress and depressive-like behavior.
Less appreciated is the potential relevance of M2 to brain disorders. There is thus an opportunity to leverage the expanding knowledge on M2 to study pathophysiological mechanisms in rodent models of neuropsychiatric and neurological disorders. Here, I speculate that M2 may be affected by chronic stress and could contribute to depressive-like behavior.
In studies of chronic stress, much work has focused on the prelimbic and infralimbic sub-regions of mPFC. Does M2 also respond to chronic stress? Whole-brain mapping of neuronal activity provides a bird’s eye view on the affected brain regions in an unbiased manner. One recent study characterized activity-dependent gene expression in the entire mouse brain in the learned helplessness model of depression. Comparisons between susceptible and resilient individuals uncovered significant differential responses in M2, similar to those found in the other mPFC regions [97]. Mappings of metabolic markers showed that subanesthetic ketamine, an agent with psychotomimetic and fast-acting antidepressant properties, leads to elevated activity in both rat and mouse M2 [98,99]. Therefore, both stress manipulations and antidepressant administrations significantly alter neural activity in M2. A core symptom of depression is psychomotor retardation, which manifests as prolonged speech pauses, decisions, and motor responses [100,101]. In light of the role of M2 in health, it is possible that M2 dysfunction contributes to such aspects of neuropsychiatric disturbances in the motor dimension [102].
Because M2 lies on the dorsal surface of the brain, it is amenable to subcellular-resolution optical imaging. As such, M2 seems to be an ideal platform for characterizing structural plasticity in the mPFC. Taking this approach, the turnover of dendritic spines and axonal boutons in the frontal cortex has been studied in response to cocaine administration [103], activation of dopaminergic neurons [104], fear conditioning [105], and rule learning [106]. Specific to stress-related disorders, the fast-acting antidepressant ketamine exerts longitudinal effects on structural plasticity in M2 [107]. Namely, a single, subanesthetic dose of ketamine leads to a prolonged increase in spine density, which is primarily driven by an elevated rate of spine formation. These results demonstrate the potential of using M2 as a platform to study rodent models of mental illnesses.
Concluding Remarks
In summary, M2 is a distinct subdivision of the rodent mPFC, defined by a set of anatomical connections, lesion outcomes, and electrophysiological correlates. Results from the different approaches have converged on a role for M2 in linking such antecedent conditions as sensory information to motor actions. One may argue that condition-action linkages are generally required for many behaviors, and thus the learning and use of linkages must be a common computation in the brain. Indeed, M2 must work in concert with other brain regions including other prefrontal cortical regions, superior colliculus, basal ganglia, and thalamus during associative learning [78].
Nevertheless, M2 stands out in three ways. One, among frontal cortical regions in rodents, M2 and the neighboring Cg1 are unique in receiving an abundance of sensory afferents. They project back to sensory, parietal, and retrosplenial cortices, completing a reciprocally connected network that suggests complex modes of interaction. Two, M2 has the earliest choice-related activity in the frontal-striatal network during adaptive behavior. This timing, as well as the emerging local circuit mechanisms, positions M2 as the source of action signals in the frontal cortex. Three, the activity of M2 neurons depends strongly on context. The highly flexible neural representations are likely to be an important part of adaptive behavior.
Still, there are many gaps remaining in our current understanding (see Outstanding Questions). Unraveling the complexity will provide important insights into the neural circuit mechanisms governing the flexible control of voluntary actions.
Outstanding Questions Box.
What is the relationship between M2 and neighboring frontal cortical regions such as the vibrissa motor cortex, anterior lateral motor cortex, and other mPFC regions? Are they connected and how do they interact? Is there a hierarchy?
There is emerging evidence supporting a topographical organization for sensory cortex connections, linking secondary motor cortex to audition, and cingulate cortex to vision. Does this organization apply to afferents, efferents, or both? Does the potential division of sensory modalities have gradual or sharp boundaries?
What is the function of the inputs from the orbitofrontal cortex?
Why are there multiple direct and indirect pathways connecting M2, posterior parietal cortex, and retrosplenial cortex? What signals do these pathways carry?
What is the precise function of M2 in the executive control of voluntary actions? Does it play a facilitating or permissive role in action initiation? When is it needed?
There are almost an infinite number of antecedent conditions, and only a very small subset is relevant for the task at hand. What are the filtering and gating mechanisms that shape the task-specific signals in M2?
Is there choice-related activity in M2 of naïve animals? What happens during learning?
Dynamical systems theory has provided a fresh perspective for understanding reaching-related activity in the primate dorsal premotor cortex. To what extent can the principles be studied in the rodent frontal cortex?
Loss of voluntary movements, behavioral rigidity, and disorganized thinking are symptoms of a wide range of disorders including schizophrenia, depression, obsessive-compulsive disorder, autism, and Parkinson’s disease. Could M2 dysfunction contribute to the pathophysiology?
Trends Box.
There is rapid progress towards understanding the function of M2. Progress is fueled by accessibility of the region for optical imaging and optogenetics, as well as the development of sophisticated decision-making tasks for rodents.
M2 receives sensory information from reciprocal connections with sensory, parietal, and retrosplenial cortices. It exerts control on actions by projecting to various motor-related subcortical regions.
Removal of M2 causes transient neglect and enduring sensorimotor deficits.
M2 neurons have early and context-dependent choice-related activity, implicating the region as a driver of voluntary actions.
Collectively, the current understanding suggests that M2 maintains a flexible mapping diagram of sensorimotor associations in the service of adaptive choice behavior.
Acknowledgments
We thank Seung-Hee Lee and Daeyeol Lee for comments on an earlier draft of the manuscript. This work was supported by National Institute of Aging center grant P50AG047270 (A.C.K.), National Institute of Mental Health grant R21MH110712 (A.C.K.), NARSAD Young Investigator Award (A.C.K.), and Inscopix DECODE award (A.C.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brecht M. Movement, confusion, and orienting in frontal cortices. Neuron. 2011;72:193–196. doi: 10.1016/j.neuron.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates: compact second edition. San Diego: Academic; 2003. [Google Scholar]
- 3.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2006;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 4.Donoghue JP, Wise SP. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J Comp Neurol. 1982;212:76–88. doi: 10.1002/cne.902120106. [DOI] [PubMed] [Google Scholar]
- 5.Brecht M, et al. Organization of rat vibrissa motor cortex and adjacent areas according to cytoarchitectonics, microstimulation, and intracellular stimulation of identified cells. J Comp Neurol. 2004;479:360–373. doi: 10.1002/cne.20306. [DOI] [PubMed] [Google Scholar]
- 6.Van De Werd HJJM, et al. Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain Struct Funct. 2010;214:339–353. doi: 10.1007/s00429-010-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neafsey EJ, et al. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 2002;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- 8.Zingg B, et al. Neural networks of the mouse neocortex. Cell. 2014;156:1096–1111. doi: 10.1016/j.cell.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reep RL, Corwin JV. Topographic organization of the striatal and thalamic connections of rat medial agranular cortex. Brain Res. 1999;841:43–52. doi: 10.1016/s0006-8993(99)01779-5. [DOI] [PubMed] [Google Scholar]
- 10.Reep RL, et al. Topographic organization in the corticocortical connections of medial agranular cortex in rats. J Comp Neurol. 1990;294:262–280. doi: 10.1002/cne.902940210. [DOI] [PubMed] [Google Scholar]
- 11.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 12.Yamawaki N, et al. A Corticocortical Circuit Directly Links Retrosplenial Cortex to M2 in the Mouse. J Neurosci. 2016;36:9365–9374. doi: 10.1523/JNEUROSCI.1099-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Eden CG, et al. Heterotopic Cortical Afferents to the Medial Prefrontal Cortex in the Rat. A Combined Retrograde and Anterograde Tracer Study. European Journal of Neuroscience. 1992;4:77–97. doi: 10.1111/j.1460-9568.1992.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, et al. Organization of long-range inputs and outputs of frontal cortex for top-down control. Nat Neurosci. 2016 doi: 10.1038/nn.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reep RL, et al. Efferent connections of the rostral portion of medial agranular cortex in rats. Brain Res Bull. 1987;19:203–221. doi: 10.1016/0361-9230(87)90086-4. [DOI] [PubMed] [Google Scholar]
- 16.Jeong M, et al. Comparative three-dimensional connectome map of motor cortical projections in the mouse brain. Sci Rep. 2016;6:20072. doi: 10.1038/srep20072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sesack SR, et al. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 18.Gabbott PLA, et al. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 19.Leonard CM. The prefrontal cortex of the rat. I. Cortical projection of the mediodorsal nucleus. II. Efferent connections. Brain Res. 1969;12:321–343. doi: 10.1016/0006-8993(69)90003-1. [DOI] [PubMed] [Google Scholar]
- 20.Leichnetz GR, et al. Frontal projections to the region of the oculomotor complex in the rat: a retrograde and anterograde HRP study. J Comp Neurol. 1987;263:387–399. doi: 10.1002/cne.902630306. [DOI] [PubMed] [Google Scholar]
- 21.Stuesse SL, Newman DB. Projections from the medial agranular cortex to brain stem visuomotor centers in rats. Exp Brain Res. 1990;80:532–544. doi: 10.1007/BF00227994. [DOI] [PubMed] [Google Scholar]
- 22.Berendse HW, et al. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- 23.Hintiryan H, et al. The mouse cortico-striatal projectome. Nat Neurosci. 2016;19:1100–1114. doi: 10.1038/nn.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson A, et al. A circuit for motor cortical modulation of auditory cortical activity. J Neurosci. 2013;33:14342–14353. doi: 10.1523/JNEUROSCI.2275-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, et al. Long-range and local circuits for top-down modulation of visual cortex processing. Science. 2014;345:660–665. doi: 10.1126/science.1254126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson A, Mooney R. The Basal Forebrain and Motor Cortex Provide Convergent yet Distinct Movement-Related Inputs to the Auditory Cortex. Neuron. 2016;90:635–648. doi: 10.1016/j.neuron.2016.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preuss TM, et al. Movement representation in the dorsal and ventral premotor areas of owl monkeys: a microstimulation study. J Comp Neurol. 1996;371:649–676. doi: 10.1002/(SICI)1096-9861(19960805)371:4<649::AID-CNE12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Hall RD, Lindholm EP. Organization of motor and somatosensory neocortex in the albino rat. Brain Res. 1974;66:23–38. [Google Scholar]
- 29.Tennant KA, et al. The Organization of the Forelimb Representation of the C57BL/6 Mouse Motor Cortex as Defined by Intracortical Microstimulation and Cytoarchitecture. Cereb Cortex. 2011;21:865–876. doi: 10.1093/cercor/bhq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berg RW, Kleinfeld D. Vibrissa movement elicited by rhythmic electrical microstimulation to motor cortex in the aroused rat mimics exploratory whisking. J Neurophysiol. 2003;90:2950–2963. doi: 10.1152/jn.00511.2003. [DOI] [PubMed] [Google Scholar]
- 31.Brecht M, et al. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature. 2004;427:704–710. doi: 10.1038/nature02266. [DOI] [PubMed] [Google Scholar]
- 32.Graziano MSA, Aflalo TN. Rethinking Cortical Organization: Moving Away from Discrete Areas Arranged in Hierarchies. The Neuroscientist. 2007;13:138–147. doi: 10.1177/1073858406295918. [DOI] [PubMed] [Google Scholar]
- 33.Haiss F, Schwarz C. Spatial segregation of different modes of movement control in the whisker representation of rat primary motor cortex. J Neurosci. 2005;25:1579–1587. doi: 10.1523/JNEUROSCI.3760-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferezou I, et al. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Hill DN, et al. Primary motor cortex reports efferent control of vibrissa motion on multiple timescales. Neuron. 2011;72:344–356. doi: 10.1016/j.neuron.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebbesen CL, et al. Vibrissa motor cortex activity suppresses contralateral whisking behavior. Nat Neurosci. 2016 doi: 10.1038/nn.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowey A, Bozek T. Contralateral “neglect” after unilateral dorsomedial prefrontal lesions in rats. Brain Res. 1974;72:53–63. doi: 10.1016/0006-8993(74)90649-0. [DOI] [PubMed] [Google Scholar]
- 38.Crowne DP, Pathria MN. Some attentional effects of unilateral frontal lesions in the rat. Behavioural Brain Research. 1982;6:25–39. doi: 10.1016/0166-4328(82)90079-1. [DOI] [PubMed] [Google Scholar]
- 39.Otchy TM, et al. Acute off-target effects of neural circuit manipulations. Nature. 2015;528:358–363. doi: 10.1038/nature16442. [DOI] [PubMed] [Google Scholar]
- 40.Vargo JM, Marshall JF. Time-dependent changes in dopamine agonist-induced striatal fos immunoreactivity are related to sensory neglect and its recovery after unilateral prefrontal cortex injury. Synapse. 1995;20:305–315. doi: 10.1002/syn.890200404. [DOI] [PubMed] [Google Scholar]
- 41.Crowne DP, et al. Brief deprivation of vision after unilateral lesions of the frontal eye field prevents contralateral inattention. Science. 1983;220:527–530. doi: 10.1126/science.6836298. [DOI] [PubMed] [Google Scholar]
- 42.Erlich JC, et al. Distinct effects of prefrontal and parietal cortex inactivations on an accumulation of evidence task in the rat. eLife. 2015;4:8166. doi: 10.7554/eLife.05457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Passingham RE, et al. Premotor cortex in the rat. Behavioral Neuroscience. 1988;102:101–109. doi: 10.1037//0735-7044.102.1.101. [DOI] [PubMed] [Google Scholar]
- 44.Siniscalchi MJ, et al. Fast and slow transitions in frontal ensemble activity during flexible sensorimotor behavior. Nat Neurosci. 2016;19:1234–1242. doi: 10.1038/nn.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sul JH, et al. Role of rodent secondary motor cortex in value-based action selection. Nat Neurosci. 2011;14:1202–1208. doi: 10.1038/nn.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ostlund SB, et al. Evidence of action sequence chunking in goal-directed instrumental conditioning and its dependence on the dorsomedial prefrontal cortex. J Neurosci. 2009;29:8280–8287. doi: 10.1523/JNEUROSCI.1176-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gremel CM, Costa RM. Premotor cortex is critical for goal-directed actions. Front Comput Neurosci. 2013;7:110. doi: 10.3389/fncom.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bailey KR, Mair RG. Effects of frontal cortex lesions on action sequence learning in the rat. Eur J Neurosci. 2007;25:2905–2915. doi: 10.1111/j.1460-9568.2007.05492.x. [DOI] [PubMed] [Google Scholar]
- 49.Yin HH. The role of the murine motor cortex in action duration and order. Front Integr Neurosci. 2009;3:23. doi: 10.3389/neuro.07.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao VY, et al. Motor Learning Consolidates Arc-Expressing Neuronal Ensembles in Secondary Motor Cortex. Neuron. 2015;86:1385–1392. doi: 10.1016/j.neuron.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothwell PE, et al. Input- and Output-Specific Regulation of Serial Order Performance by Corticostriatal Circuits. Neuron. 2015;88:345–356. doi: 10.1016/j.neuron.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawai R, et al. Motor Cortex Is Required for Learning but Not for Executing a Motor Skill. Neuron. 2015;86:800–812. doi: 10.1016/j.neuron.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo JZ, et al. Cortex commands the performance of skilled movement. eLife. 2015 doi: 10.7554/eLife.10774.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erlich JC, et al. A cortical substrate for memory-guided orienting in the rat. Neuron. 2011;72:330–343. doi: 10.1016/j.neuron.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kopec CD, et al. Cortical and Subcortical Contributions to Short-Term Memory for Orienting Movements. Neuron. 2015;88:367–377. doi: 10.1016/j.neuron.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo ZV, et al. Flow of cortical activity underlying a tactile decision in mice. Neuron. 2014;81:179–194. doi: 10.1016/j.neuron.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanks TD, et al. Distinct relationships of parietal and prefrontal cortices to evidence accumulation. Nature. 2015;520:220–223. doi: 10.1038/nature14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Churchland MM, Shenoy KV. Delay of movement caused by disruption of cortical preparatory activity. J Neurophysiol. 2007;97:348–359. doi: 10.1152/jn.00808.2006. [DOI] [PubMed] [Google Scholar]
- 59.Li N, et al. Robust neuronal dynamics in premotor cortex during motor planning. Nature. 2016;532:459–464. doi: 10.1038/nature17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- 61.Sul JH, et al. Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron. 2010;66:449–460. doi: 10.1016/j.neuron.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murakami M, et al. Neural antecedents of self-initiated actions in secondary motor cortex. Nat Neurosci. 2014;17:1574–1582. doi: 10.1038/nn.3826. [DOI] [PubMed] [Google Scholar]
- 63.Hanes DP, Schall JD. Neural Control of Voluntary Movement Initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- 64.Kornhuber HH, Deecke L. Hirnpotentialänderungen bei Willkürbewegungen und passiven Bewegungen des Menschen: Bereitschaftspotential und reafferente Potentiale. Pflügers Arch. 1965;284:1–17. [PubMed] [Google Scholar]
- 65.Fried I, et al. Internally generated preactivation of single neurons in human medial frontal cortex predicts volition. Neuron. 2011;69:548–562. doi: 10.1016/j.neuron.2010.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kargo WJ, et al. Adaptation of prefrontal cortical firing patterns and their fidelity to changes in action-reward contingencies. J Neurosci. 2007;27:3548–3559. doi: 10.1523/JNEUROSCI.3604-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schneider DM, et al. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature. 2014;513:189–194. doi: 10.1038/nature13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murakami M, Mainen ZF. Preparing and selecting actions with neural populations: toward cortical circuit mechanisms. Current Opinion in Neurobiology. 2015;33:40–46. doi: 10.1016/j.conb.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 69.Brody CD, Hanks TD. Neural underpinnings of the evidence accumulator. Current Opinion in Neurobiology. 2016;37:149–157. doi: 10.1016/j.conb.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li N, et al. A motor cortex circuit for motor planning and movement. Nature. 2015;519:51–56. doi: 10.1038/nature14178. [DOI] [PubMed] [Google Scholar]
- 71.Manita S, et al. A Top-Down Cortical Circuit for Accurate Sensory Perception. Neuron. 2015;86:1304–1316. doi: 10.1016/j.neuron.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Durstewitz D, et al. Abrupt transitions between prefrontal neural ensemble states accompany behavioral transitions during rule learning. Neuron. 2010;66:438–448. doi: 10.1016/j.neuron.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 73.Karlsson MP, et al. Network resets in medial prefrontal cortex mark the onset of behavioral uncertainty. Science. 2012;338:135–139. doi: 10.1126/science.1226518. [DOI] [PubMed] [Google Scholar]
- 74.Peters AJ, et al. Emergence of reproducible spatiotemporal activity during motor learning. Nature. 2014;510:263–267. doi: 10.1038/nature13235. [DOI] [PubMed] [Google Scholar]
- 75.Huber D, et al. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484:473–478. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Narayanan NS, et al. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat Neurosci. 2013;16:1888–1895. doi: 10.1038/nn.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goard MJ, et al. Distinct roles of visual, parietal, and frontal motor cortices in memory-guided sensorimotor decisions. eLife. 2016;5:471. doi: 10.7554/eLife.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wise SP, Murray EA. Arbitrary associations between antecedents and actions. Trends in Neurosciences. 2000;23:271–276. doi: 10.1016/s0166-2236(00)01570-8. [DOI] [PubMed] [Google Scholar]
- 79.Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends in Neurosciences. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 81.Uylings HBM, et al. Do rats have a prefrontal cortex? Behavioural Brain Research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 82.Rose JE, Woolsey CN. The orbitofrontal cortex and its connections with the mediodorsal nucleus in rabbit, sheep and cat. Res Publ Assoc Res Nerv Ment Dis. 1948;27:210–232. [PubMed] [Google Scholar]
- 83.Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- 84.Kuramoto E, et al. Individual mediodorsal thalamic neurons project to multiple areas of the rat prefrontal cortex: A single neuron-tracing study using virus vectors. J Comp Neurol. 2017;525:166–185. doi: 10.1002/cne.24054. [DOI] [PubMed] [Google Scholar]
- 85.Oh SW, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fulton JF. A note on the definition of the “motor” and ‘premotor’ areas. Brain. 1935;58:311–316. [Google Scholar]
- 87.Wise SP, et al. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- 88.Shenoy KV, et al. Cortical control of arm movements: a dynamical systems perspective. Annu Rev Neurosci. 2013;36:337–359. doi: 10.1146/annurev-neuro-062111-150509. [DOI] [PubMed] [Google Scholar]
- 89.Tanji J. Sequential organization of multiple movements: involvement of cortical motor areas. Annu Rev Neurosci. 2001;24:631–651. doi: 10.1146/annurev.neuro.24.1.631. [DOI] [PubMed] [Google Scholar]
- 90.Nachev P, et al. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 91.Wise SP. The primate premotor cortex: past, present, and preparatory. Annu Rev Neurosci. 1985;8:1–19. doi: 10.1146/annurev.ne.08.030185.000245. [DOI] [PubMed] [Google Scholar]
- 92.Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu Rev Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- 93.Rizzolatti G, et al. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- 94.Moore T, et al. Visuomotor origins of covert spatial attention. Neuron. 2003;40:671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 95.Cisek P, Kalaska JF. Neural Mechanisms for Interacting with a World Full of Action Choices. Annu Rev Neurosci. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- 96.Dekleva BM, et al. Uncertainty leads to persistent effects on reach representations in dorsal premotor cortex. eLife. 2016;5:257. doi: 10.7554/eLife.14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim Y, et al. Whole-Brain Mapping of Neuronal Activity in the Learned Helplessness Model of Depression. Front Neural Circuits. 2016;10:3. doi: 10.3389/fncir.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duncan GE, et al. Comparison of brain metabolic activity patterns induced by ketamine, MK-801 and amphetamine in rats: support for NMDA receptor involvement in responses to subanesthetic dose of ketamine. Brain Res. 1999;843:171–183. doi: 10.1016/s0006-8993(99)01776-x. [DOI] [PubMed] [Google Scholar]
- 99.Miyamoto S, et al. Effects of ketamine, MK-801, and amphetamine on regional brain 2-deoxyglucose uptake in freely moving mice. Neuropsychopharmacology. 2000;22:400–412. doi: 10.1016/S0893-133X(99)00127-X. [DOI] [PubMed] [Google Scholar]
- 100.Sobin C, Sackeim HA. Psychomotor symptoms of depression. Am J Psychiatry. 1997;154:4–17. doi: 10.1176/ajp.154.1.4. [DOI] [PubMed] [Google Scholar]
- 101.Buyukdura JS, et al. Psychomotor retardation in depression: biological underpinnings, measurement, and treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:395–409. doi: 10.1016/j.pnpbp.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bernard JA, Mittal VA. Updating the research domain criteria: the utility of a motor dimension. Psychol Med. 2015;45:2685–2689. doi: 10.1017/S0033291715000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Muñoz-Cuevas FJ, et al. Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nat Neurosci. 2013;16:1367–1369. doi: 10.1038/nn.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mastwal S, et al. Phasic dopamine neuron activity elicits unique mesofrontal plasticity in adolescence. J Neurosci. 2014;34:9484–9496. doi: 10.1523/JNEUROSCI.1114-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lai CSW, et al. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2013;482:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- 106.Johnson CM, et al. Rule learning enhances structural plasticity of long-range axons in frontal cortex. Nature Communications. 2016;7:1–14. doi: 10.1038/ncomms10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Phoumthipphavong V, et al. Longitudinal Effects of Ketamine on Dendritic Architecture In Vivo in the Mouse Medial Frontal Cortex. eNeuro. 2016:3. doi: 10.1523/ENEURO.0133-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]