Abstract
The cis-acting elements for Brome mosaic virus (BMV) RNA synthesis have been characterized primarily for RNA3. To identify additional replicase-binding elements, nested fragments of all three of the BMV RNAs, both plus- and minus-sense fragments, were constructed and tested for binding enriched BMV replicase in a template competition assay. Ten RNA fragments containing replicase-binding sites were identified; eight were characterized further because they were more effective competitors. All eight mapped to noncoding regions of BMV RNAs, and the positions of seven localized to sequences containing previously characterized core promoter elements (C. C. Kao, Mol. Plant Pathol. 3:55-62, 2001), thus suggesting the identities of the replicase-binding sites. Three contained the tRNA-like structures that direct minus-strand RNA synthesis, three were within the 3′ region of each minus-strand RNA that contained the core promoter for genomic plus-strand initiation, and one was in the core subgenomic promoter. Single-nucleotide mutations known previously to abolish RNA synthesis in vitro prevented replicase binding. When tested in the context of the respective full-length RNAs, the same mutations abolished BMV RNA synthesis in transfected barley protoplasts. The eighth site was within the intercistronic region (ICR) of plus-strand RNA3. Further mapping showed that a sequence of 22 consecutive adenylates was responsible for binding the replicase, with 16 being the minimal required length. Deletion of the poly(A) sequence was previously shown to severely debilitate BMV RNA replication in plants (E. Smirnyagina, Y. H. Hsu, N. Chua, and P. Ahlquist, Virology 198:427-436, 1994). Interestingly, the B box motif in the ICR of RNA3, which has previously been determined to bind the 1a protein, does not bind the replicase. These results identify the replicase-binding sites in all of the BMV RNAs and suggest that the recognition of RNA3 is different from that of RNA1 and RNA2.
The replication of positive-strand RNA viruses is essential for pathogenesis. The responsible enzyme, the viral replicase, is a complex that includes the virus-encoded RNA-dependent RNA polymerase, additional viral replicase proteins, and host factors (6, 32). In addition, specific interactions between the viral genomic RNAs and viral replicase are required for replication and/or transcription of the viral RNAs (1, 6). The interaction process is likely to be quite complex because an RNA virus expresses different classes of RNAs at programmed levels and times.
Brome mosaic virus (BMV) belongs to the alphavirus-like superfamily of plant and animal positive-strand RNA viruses. The BMV genome is divided into three capped RNAs, designated RNA1, RNA2, and RNA3 (3, 26). RNA1 and RNA2 encode replication-associated proteins, while RNA3 encodes two proteins required for systemic movement of the virus in plants and encapsidation of viral RNAs. Due to the dicistronic nature of RNA3, the second cistron encoding the capsid is translated from subgenomic RNA that is transcribed from the subgenomic promoter. In all, three classes of RNAs must be produced during successful BMV replication: genomic minus-strand, genomic plus-strand, and subgenomic RNAs.
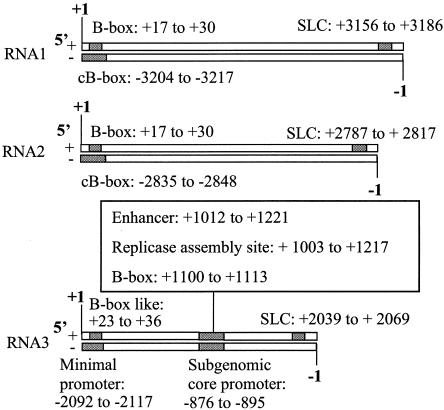
cis-acting elements for efficient genomic plus-strand, minus-strand, and subgenomic BMV RNA synthesis have been characterized by a combination of approaches, including RNA synthesis by the BMV replicase in vitro (37), transfection of BMV RNAs into protoplasts (34), analysis in plants (7), and replication in Saccharomyces cerevisiae, which is permissive for BMV replication and transcription (23). Each study generally focused on one cis-acting element and on RNA3, which does not contain functions directly required for replication. Nonetheless, a number of required elements have been identified (Fig. 1).
FIG. 1.
Summary of previously reported cis-acting sequences in BMV RNAs. Locations of the cis-acting sequences are denoted by shaded rectangles. Near each rectangle is a brief description of the name of each motif and the nucleotide positions at which the motif resides. Plus- and minus-strand RNAs are denoted with “+” or “−. ” The first nucleotide at the 5′ end of the plus- and minus-strand RNAs are designated, respectively, with +1 and −1. The amount of area shaded denotes the approximate location within the RNA and is not to scale.
The 3′ noncoding regions (NCR) of BMV genomic plus-strand RNAs form a tRNA-like structure that directs the initiation of minus-strand RNA synthesis in vitro (10, 15) and in vivo (38, 46). Stem-loop C (SLC) within the tRNA-like structure of RNA3 binds the BMV replicase through an RNA structure called a clamped adenine motif (29). Given the highly similar structures predicted for RNA1 and RNA2, it is likely that the same structures are required to direct their minus-strand replication (Fig. 1). A mutation in SLC in RNA2 was shown to affect RNA replication in barley protoplasts (39).
A 26-nucleotide (nt) sequence at the 3′ end of minus-strand RNA2 was capable of directing genomic plus-strand RNA2 initiation (49). One feature of this sequence is a sequence called the cB box. The cB box is also found in minus-strand RNA1 and in the intercistronic region (ICR), but not at the 3′ region, of minus-strand RNA3. The core of the cB box is a 4-nt sequence, CCAA, which is conserved at comparable positions in RNA1 and RNA2 of other members of the Bromoviridae (48).
The cB box is the sequence complementary to a previously identified regulatory element, called the B box (16, 25, 33, 35). Of course, the B box is present at the position complementary to the locations of the cB boxes (Fig. 1). In barley cells, the deletion of the B box resulted in defects in BMV replication and transcription (35, 50). In yeasts the B box in RNA2 binds the 1a protein in a process postulated to be required for translocating RNA2 into membranes, presumably the location of the BMV replicase and translation (11, 12).
The ∼200-nt ICR in RNA3 also contains a number of other regulatory sequences in addition to the B box. The ICR contains an enhancer that was involved in regulating RNA replication and transcription (Fig. 1) (51). It also contains a cis-acting sequence required for the assembly of a functional BMV replicase complex when BMV RNAs are expressed in yeast (Fig. 1) (23, 37, 40, 52). The complement of the ICR contains the promoter for subgenomic RNA4 transcription, which consists of an upstream A/U-rich sequence, a poly(U) tract, and the 20-nt core promoter sequence recognized by the BMV replicase in vivo and in vitro (16, 17, 42, 45).
For this study, we sought to identify the replicase-binding sites in all of the BMV RNAs. We expected to encounter sequences known to direct BMV RNA synthesis and possibly other regulatory sequences that have not been linked to replicase binding. Eight such sequences were identified in vitro and characterized.
MATERIALS AND METHODS
Molecular manipulations.
DNAs that can direct the transcription of the desired portion of the RNAs were generated by PCR using the Vent DNA polymerase (New England Biolabs); cDNA clones of BMV RNAs; pB1TP3, pB2TP5 and pB3TP8 as templates (24); and appropriate primers (DNA Genesys, Sigma Inc.). The forward primer contained a T7 promoter with an initiation guanylate that can direct plus-strand RNA transcription. The reverse primer contains an SP6 promoter with an initiation guanylate that can direct the synthesis of minus-strand RNAs (Fig. 2A). The DNA products for transcription were purified by phenol-chloroform extraction and ethanol precipitation. For in vitro transcription, we used the protocol described in the work of Kao et al. (27) and transcripts were purified from denaturing gels after excision of bands of the correct size and elution of crushed gel fragments with 0.3 M sodium acetate.
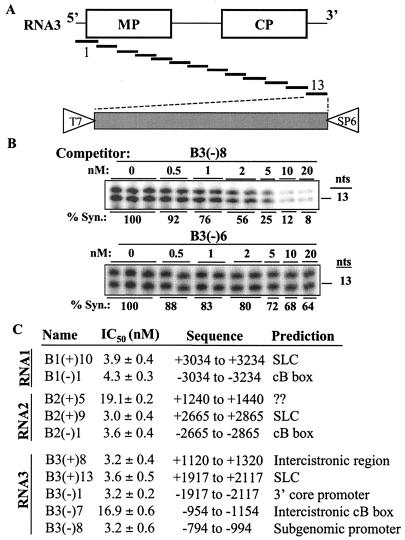
FIG. 2.
Regions in BMV RNAs that confer higher-affinity binding to the BMV replicase in vitro. (A) Schema used to generate desired competitor RNAs. Overlapping cDNA fragments flanked by T7 and SP6 promoters were produced by PCR using appropriate primers and BMV plasmids from the work of Janda and Ahlquist (23). (B) Results from a typical template competition assay used to determine the IC50 for each competitor RNA. The gel image shows the effects of a competitor, B3(−)8, that binds the replicase and of B3(−)6, which does not. Template −20/13 at 2 nM served as the reference RNA in these assays. The amount of product from −20/13 is quantified against each concentration of competitor to derive the IC50. % Syn., percent synthesized. (C) Summary of the 10 RNA fragments that we detected to bind the BMV replicase, their IC50s, the location of the BMV that they encode, and the motif that may bind the replicase. Eight of the binders for which IC50s were lower than 5 nM are considered effective and are characterized further in this work. The motifs within these eight were predicted based on previous characterizations detailed in the introduction. The weak replicase-binding motif within B2(+)5 was not previously identified, and its relevance in BMV replication remains to be determined.
Mutations within BMV cDNAs were made by use of a site-directed mutagenesis kit (Stratagene, San Diego, Calif.) and appropriate DNA oligonucleotides. The existence of each mutation and the absence of spurious changes were confirmed by DNA sequencing using a Big Dye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Inc.)
Homopolyadenylates of various lengths were generated by alkaline hydrolysis of high-molecular-weight poly(A) (Sigma Aldrich Inc., St. Louis, Mo.). Treatment with 0.2 M NaOH at 37°C for 30 min generated a ladder of fragments that were individually purified from a denaturing gel.
Construction of chimeric BMV RNAs.
Chimeric BMV RNAs were made to examine whether the replicase-binding motifs function together with other cis-acting elements. Plasmids encoding chimeric BMV cDNAs were made by use of a combination of PCR, site-directed mutagenesis, and swapping of restriction fragments into the appropriate background according to the protocol of Higuchi et al. (21). All of the cDNAs were confirmed to have only the intended changes by DNA sequencing. The specifics for the construction of the plasmids are quite involved. Details and maps will be provided upon request.
RNA replicase assay.
BMV replicase was prepared from infected barley as previously described (53). Standard replicase assays were carried out as described by Adkins et al. (2). Template competition assays were performed with a 2 nM concentration of −20/13, which directs robust synthesis of a 13-nt product and a 14-nt product. The latter results from nontemplated addition due to the terminal transferase activity of the BMV replicase (42, 43). Increasing concentrations of each competitor RNA were tested, and the effects on the accumulation of both products were quantified. All quantifications of radiolabel were performed with a PhosphorImager and Molecular Dynamics software. Each competition assay tested six RNA concentrations, and RNAs that were effective competitors were tested at least three times to determine the standard error for the concentration of the competitor required to reduce synthesis from the reference RNA to 50% (IC50).
BMV replication in barley protoplasts.
Mutants and infectious transcripts used for transfection of barley protoplasts were produced as previously described (19). Protoplasts were isolated from 5-day-old primary barley leaves as described by Kroner et al. (31) and were usually inoculated with 0.5 μg each of capped transcripts of the desired combination of the three BMV RNAs. Protoplasts were harvested at specific times after transfection (usually 12 h) for total RNA extraction. The RNA was analyzed by Northern blot hybridization that used probes specific for the 3′-terminal 200 nt of the minus- or plus-strand BMV RNA3 (31). Images of rRNAs were photographed from the nylon membrane to which RNAs were transferred. The RNAs were stained with ethidium bromide.
RESULTS
Mapping of replicase-binding sites.
To identify sites in BMV RNAs required in vitro for efficient replicase binding, a series of cDNA fragments was synthesized by PCR. Each fragment contained a T7 promoter on the sense strand and a SP6 promoter on the antisense strand to allow the production of plus- and minus-sense transcripts, respectively (Fig. 2A). Each RNA fragment was ca. 200- or 400-nt long and overlapped its neighboring fragments by 40 nt. The nesting of the RNA fragments was designed to decrease the possibility of deleting an RNA element required for replicase binding. For nomenclature, each RNA fragment was named by using the BMV RNA from which it was derived, a symbol indicating whether it was positive (+) or negative (−) sense, and a number indicating the order of the DNA template in the nested set. For example, B1(+)1 indicates that the fragment was derived from BMV RNA1, was positive sense, and was the 5′-most fragment in a series of nested fragments. The nucleotide positions for each RNA fragment are listed in Table 1. The positions of the nucleotides are named from 5′ to 3′.
TABLE 1.
Summary of the competitor RNAs used in the template competition assays
| RNA1
|
RNA2
|
RNA3
|
|||
|---|---|---|---|---|---|
| Competitor | Sequence (nt) | Competitor | Sequence (nt) | Competitor | Sequence (nt) |
| B1(+)1 | +1 to +200 | B2(+)1 | +1 to +200 | B3(+)1 | +1 to +200 |
| B1(+)2 | +160 to +560 | B2(+)2 | +160 to +560 | B3(+)2 | +160 to +360 |
| B1(+)3 | +520 to +920 | B2(+)3 | +520 to +920 | B3(+)3 | +320 to +520 |
| B1(+)4 | +880 to +1280 | B2(+)4 | +880 to +1280 | B3(+)4 | +480 to +680 |
| B1(+)5 | +1240 to +1640 | B2(+)5 | +1240 to +1640 | B3(+)5 | +640 to +840 |
| B1(+)6 | +1600 to +2000 | B2(+)6 | +1600 to +2000 | B3(+)6 | +800 to +1000 |
| B1(+)7 | +1960 to +2360 | B2(+)7 | +1960 to +2360 | B3(+)7 | +960 to +1160 |
| B1(+)8 | +2320 to +2720 | B2(+)8 | +2320 to +2720 | B3(+)8 | +1120 to +1320 |
| B1(+)9 | +2680 to +3080 | B2(+)9 | +2665 to +2865 | B3(+)9 | +1280 to +1480 |
| B1(+)10 | +3034 to +3234 | B3(+)10 | +1440 to +1640 | ||
| B3(+)11 | +1600 to +1800 | ||||
| B3(+)12 | +1760 to +1960 | ||||
| B3(+)13 | +1917 to +2117 | ||||
| B1(−)1 | −3034 to −3234 | B2(−)1 | −2665 to −2865 | B3(−)1 | −1917 to −2117 |
| B1(−)2 | −2674 to −3074 | B2(−)2 | −2305 to −2705 | B3(−)2 | −1754 to −1954 |
| B1(−)3 | −2314 to −2714 | B2(−)3 | −1945 to −2345 | B3(−)3 | −1594 to −1794 |
| B1(−)4 | −1954 to −2354 | B2(−)4 | −1585 to −1985 | B3(−)4 | −1434 to −1634 |
| B1(−)5 | −1594 to −1994 | B2(−)5 | −1225 to −1625 | B3(−)5 | −1274 to −1474 |
| B1(−)6 | −1234 to −1634 | B2(−)6 | −865 to −1265 | B3(−)6 | −1114 to −1314 |
| B1(−)7 | −874 to −1274 | B2(−)7 | −505 to −905 | B3(−)7 | −954 to −1154 |
| B1(−)8 | −514 to −914 | B2(−)8 | −145 to −545 | B3(−)8 | −794 to −994 |
| B1(−)9 | −154 to −554 | B2(−)9 | −1 to −200 | B3(−)9 | −634 to −834 |
| B1(−)10 | −1 to −200 | B3(−)10 | −477 to −677 | ||
| B3(−)11 | −317 to −517 | ||||
| B3(−)12 | −157 to −357 | ||||
| B3(−)13 | −1 to −200 | ||||
Each RNA fragment was used to compete with the BMV replicase from at least one reference RNA that is normally capable of directing specific RNA synthesis by the replicase (42). A reference RNA named −20/13 that was used in all template competition assays was the functional core promoter template for subgenomic RNA synthesis. A second RNA that could direct genomic minus-strand RNA, named SLC + 8 (29), was used as an independent reference template for the competition assays with RNAs derived from RNA3. However, we found that the IC50s observed for −20/13 and SLC + 8 showed no significant differences from one another, and we stopped using SLC + 8 for the assays of fragments from RNA1 and RNA2.
Three general classes of competitors were identified. First, the majority had IC50s that did not reach 50% of all of the concentrations tested. Based on the highest concentration of competitor tested, these were assigned an IC50 of >20 nM. The vast majority of these RNAs were not expected to reach the IC50 even if much higher competitor concentrations were tested. A second class of competitor contained two RNA fragments, B2(+)5 and B3(−)7, which had IC50s of 19.1 and 16.9 nM, respectively. These represent weak binders that are not emphasized in this study. A third class of eight fragments had IC50s of between 3 and 5 nM. We shall refer to these eight as effective binders. Representative results from an effective binder and a nonbinding RNA fragment are represented in Fig. 2B.
The eight effective binders are located in both plus- and minus-strand RNAs of the three BMV RNAs. Their locations in BMV RNAs and IC50s are summarized in Fig. 2C. Two effective binders were from plus-strand RNA3 and were located, respectively, in the ICR [B3(+)8] and in the 3′ tRNA-like structure region [B3(+)13]. For minus-strand BMV RNA3, B3(−)1, the 3′-most fragment, and B3(−)8, which contains the complement of the ICR, were effective replicase binders (Fig. 2C). Plus-strand RNA1 and RNA2 each had one effective binder that mapped to their respective 3′-terminal fragments, B1(+)10 and B2(+)9. The respective 3′-terminal fragments of minus-strand RNA1 and RNA2, B1(−)1 and B2(−)1, were also effective binders. Based on the previous characterizations of the cis-acting sequences within BMV RNAs presented in the introduction, we could predict the majority of the motifs within each of the eight effective binders. The predictions are listed in Fig. 2C.
Stem-loop C is a major binding site in plus-strand BMV RNAs.
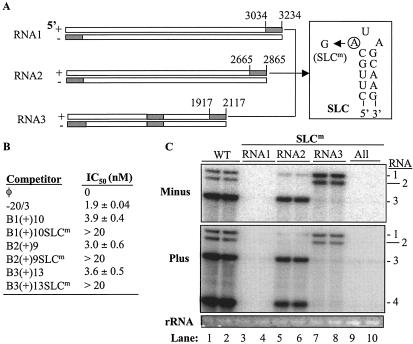
Within the tRNA-like structure of each of the plus-strand BMV RNAs, the clamped adenine motif (CAM) in SLC is expected to be the replicase-binding site that directs minus-strand initiation (10, 29). RNA fragments B1(+)10, B2(+)9, and B3(+)13 contain the 3′ tRNA-like sequences that was previously characterized for RNA synthesis (15) and for replicase binding (10). We hypothesize that mutations in the CAM within these three fragments would affect replicase binding (Fig. 3A). Changing the clamped adenine to a guanine within RNA fragments B1(+)10, B2(+)9, and B3(+)13 increased the IC50 for replicase binding to >20 nM (Fig. 3B), in support of the hypothesis that the SLC in BMV RNAs is required for replicase binding in these fragments. To examine whether disruption of replicase binding in vitro is correlated with an effect on RNA replication in cells, the same mutations were built into full-length BMV RNAs and the replication of the resultant RNAs were tested in barley protoplasts in combination with the other two wild-type RNAs. A combination wherein all three RNAs contained the clamped adenine mutation was also tested (Fig. 3C, lanes 3 to 4 and 9 to 10), as was a combination of all three wild-type transcripts (Fig. 3C, lanes 1 to 2). Consistent with in vitro binding results, mutations in the CAM decreased minus-strand RNA accumulation in barley protoplasts (Fig. 3C), likely accounting for a concomitant decrease in plus-strand RNA3 accumulation (Fig. 3C). A mutation in RNA1 or in all three RNAs reduced all RNA accumulation to background levels (Fig. 3C, lanes 3 to 4 and 9 to 10), likely due to RNA1 or its encoded product being required for the formation of active replicase (40). In contrast, while a mutation in RNA2 decreased replication of RNA2 to near background levels, it still allowed a reduced level of RNA4 transcription, suggesting that the amount of 2a protein translated from the replication-defective RNA2 was sufficient for some level of RNA synthesis.
FIG. 3.
Roles of SLC in replicase binding and BMV RNA accumulation in barley protoplasts. (A) Schematic representation of the BMV RNAs and the locations of the eight effective replicase-binding sequences. The shaded regions containing SLC are flanked with numbers denoting the nucleotide positions of the RNA fragment. The bars represent BMV RNAs, and the portion of SLC containing the clamped adenine (circled) is shown in the box. The term “SLCm” designates an RNA with a mutation in the clamped adenine. (B) IC50s of a number of RNAs that were used in a template competition assay. RNA −20/3 served as a positive control, and its replicase-binding properties were characterized by Sivakumaran et al. (45). The other RNAs contain the 3′ NCR of the three BMV RNAs (Table 1). SLCm indicates that the clamped adenine was changed to a guanine. (C) Effects of SLC mutations in transfected barley protoplasts. The gel image shows the effect of SLC mutations on genomic minus-strand and genomic plus-strand accumulation. The identities of the most relevant RNAs used in the transfections are shown above the image. “All” indicates that the RNAs were from protoplasts transfected with all three BMV RNAs that had mutated SLCs. The gel image of the 18S rRNAs is intended to show an internal loading control. WT, wild type.
Subgenomic core promoter confers binding to BMV replicase.
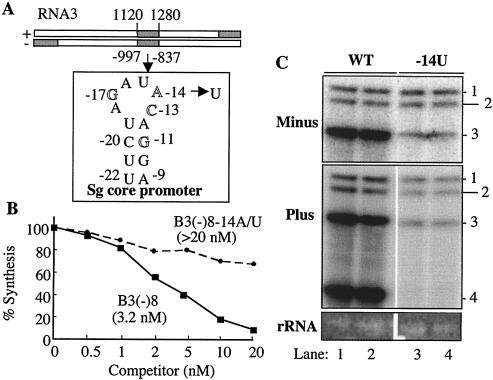
B3(−)8 contains stretches that are A/U rich, homouridylates, and the core promoter for subgenomic initiation. The subgenomic core promoter of 20 nt with a short template was sufficient for replicase-binding RNA synthesis in vitro (42). Four key residues at positions −17, −14, −13, and −11 relative to the initiation site are required for these activities (Fig. 4A) (43, 45). Therefore, we hypothesize that the core promoter within B3(−)8 is responsible for replicase binding. A nucleotide substitution changing −14A to a U in the context of B3(−)8 caused the IC50 of the resultant RNA to become >20 nM (Fig. 4B). The same mutation in the context of full-length RNA3 abolished subgenomic RNA4 synthesis and reduced the levels of the other BMV RNAs (Fig. 4C). This result is consistent with those from our recent characterizations of the BMV core promoter (45).
FIG. 4.
Effects of the subgenomic core promoter on replicase binding and BMV RNA accumulation in cells. (A) Schematic of plus- and minus-strand BMV RNA3s, with the eight identified replicase-binding sites (shaded) and the location of the subgenomic core promoter. The secondary structure of the subgenomic core promoter sequences was predicted by the MFOLD program (55) and validated by mutational analysis performed as described in the work of Sivakumaran et al. (45). The locations of the key residues in the core promoter are indicated by their positions relative to the initiation cytidylate (+1). The mutation at position −14A (mutated to a U) is shown by the arrow. (B) Quantitative results from competition assays in which the amounts of products synthesized from a 2 nM concentration of −20/13 are plotted against each concentration of B3(−)8 and B3(−)8-14A/U. The IC50s of the two RNAs were from two independent assays that were highly similar. (C) Effects of the −14U mutation that abolished replicase binding on BMV RNA accumulation in barley protoplast. The identities of the most-relevant RNAs in the transfected mixture are indicated above the gel image. 18S rRNA serves as an internal loading control. WT, wild type.
The cB box in minus-strand RNA1 and RNA2 is required for efficient BMV replicase binding.
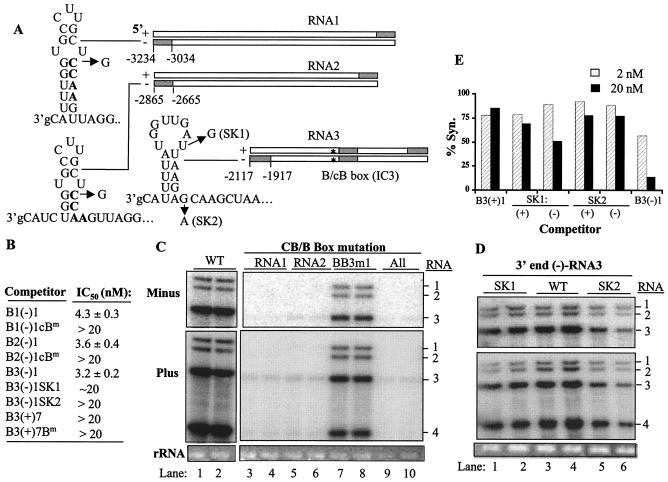
The 3′ ends of minus-strand BMV RNAs contain the core promoter for genomic plus-strand RNA synthesis (47). The minimal requirement for RNA2 was found to be a cB box approximately 16 nt from +1C (19, 47, 49). The cB box inhibited RNA synthesis when fused to the promoter template for minus-strand RNA synthesis, suggesting that it may bind the BMV replicase (48). The key feature of the cB box is the motif CCAA since mutations in this sequence had the most-severe effects on RNA synthesis by the BMV replicase in vitro (48). Notably, the cB box is present near the end of minus-strand RNA1 and RNA2. A comparable sequence is present in the complement of the ICR, not at the 3′ end of the minus-strand RNA3.
We hypothesize that B1(−)1 and B2(−)1 bind to the replicase through the cB box. Changing the cB box from CCAA to GCAA in B1(−)1 and B2(−)1 caused the IC50 to change from 4 to >20 nM (Fig. 5B), indicating that the cB box plays a significant role in binding the BMV replicase. RNA1 and RNA2 with the same nucleotide changes were unable to produce either minus- or plus-strand RNAs in transfected protoplasts. The effect on genomic plus-strand accumulation was expected since the mutations affect the core promoter for genomic plus-strand RNA initiation. However, the effect on minus-strand initiation was unexpected, given that the cB boxes would not have been made before minus-strand initiation. It is possible that the concomitant change in the B box in the plus-strand RNAs affected minus-strand synthesis.
FIG. 5.
Role of the cB box on replicase binding and on BMV RNA accumulation in barley protoplasts. (A) Schematic of the RNAs examined in this set of experiments. The shaded regions denote the positions of the eight replicase-binding sites. The three sites that were analyzed in this set of experiments have numbers that denote the nucleotide positions flanking the fragments. The intercistronic B site, denoted with an asterisk, is also examined in this figure. The RNA secondary structures are the predicted structures that contain a cB box in minus-strand RNA1 and RNA2 or the AAUU-box in RNA3 (47). Arrows identify single-nucleotide changes in the cB box motif in RNA1 and RNA2 and in the cB-like sequence of RNA3. For RNA1 and RNA2, the mutations are simply named cB/B box mutations. In minus-strand RNA3, two mutations were made, and the name of each is given in parentheses. Locations of the cB box and B box in the RNA3 ICR are denoted by asterisks. (B) Results from template competition assays. RNA fragments with mutations are denoted by an addition to the names of the RNA. (C) Northern blot analysis of mutated RNA accumulated in barley protoplasts. The most-relevant RNA in the transfected mixture is noted above the gel image. The identities of wild-type (WT) and mutant RNAs are shown to the side of the image. (D) Effects of mutations in the 3′ end of BMV RNA3 on RNA accumulation in barley protoplasts. (E) Results from competition assay using B3(−)1 or B3(+)1 with the SK1 or SK2 mutation. The reference template is −20/13. The results were tested twice, yielding consistent results. % Syn., percent synthesis.
Next, we examined the role of the cB and/or B box in the ICR of RNA3. In the template competition assay, B3(+)7 (containing the wild-type B box) had an IC50 of >20 nM, while B3(−)7 (containing the cB box) had an IC50 of 16.9 nM. Changing this cB box from 3′ CCAA 5′ to 3′ GCAA 5′ in the context of B3(−)7 decreased replicase binding to >20 nM (Fig. 2C and data not shown), indicating that the cB box in the ICR of RNA3 did bind the BMV replicase, albeit not with the same effectiveness as the comparable sequences in minus-strand RNA1 and RNA2. The same mutation (BB3m1) in the context of BMV RNA3 causes minus- and plus-strand RNA3 levels to be approximately 40% of wild-type levels. Again, the effect on RNA replication is less than that of comparable sequences in RNA1 and RNA2 (Fig. 3C). We have confirmed the ca. twofold effect of mutations in the intercistronic cB/B box with five additional single-nucleotide substitutions, and the mutations have at most a two- to threefold effect on the stability of RNA3 (data not shown). For the sake of focusing on the eight effective binding sites, the ICR cB/B box will not be addressed further in this work.
RNA B3(−)1 is an effective replicase binder despite the absence of a recognizable cB box. There are two candidates for a sequence comparable to the cB box. First, the sequence 3′ UUAA 5′ exists in a position similar to the position of the 3′ CCAA 5′ sequences in minus-strand RNA1 and RNA2. Second, Sullivan and Ahlquist (51) proposed that the sequence from nt 20 to 29 in plus-strand RNA3 forms a B box-like motif (named the BBL motif). We first mutated the UUAA sequence to GUAA in a mutant RNA named SK1. The SK1 mutation decreased minus- or plus-strand RNA3 accumulation minimally, to 68 and 65% of that of the wild type (Fig. 5D, lanes 1 to 2). In the template competition, the SK1 mutation was tested in both plus- and minus-strand RNAs in the context of B3(+)1 and B3(−)1, respectively. B3(+)1 did not bind the BMV replicase, and the SK1 mutation did not alter this result (Fig. 5E). In the context of minus-strand RNA, B3(−)1 had an IC50 of 3.2 nM and the SK1 mutation changed the IC50 to 20 nM but did not abolish binding. The UUAA sequence thus appears to have some, but perhaps not a major, role in RNA3 replication. A mutation in the BBL motif was made, changing the 3′ GCAA 5′ sequence to 3′ ACAA 5′ (Fig. 5A). This mutation, named SK2, reduced RNA binding (IC50 > 20 nM) (Fig. 5A and E) and RNA3 accumulation in barley protoplasts to 20% of that of the wild type (Fig. 5D, lanes 5 to 6). The effective binding site within B3(−)1 thus appears to involve the complement to the BBL motif identified by Sullivan and Ahlquist (51), with some contribution of neighboring sequences.
Intercistronic poly(A) tract.
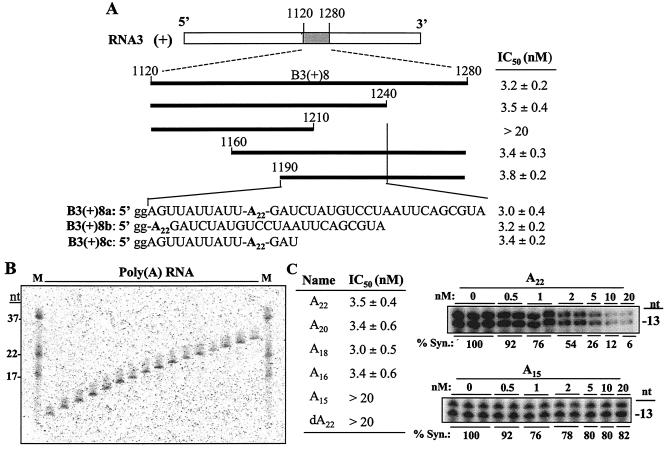
Thus far, we have identified seven of the eight effective replicase-binding sites. The eighth maps within B3(+)8, which spans nt 1120 to 1280 in the ICR, adjacent to the fragment containing the cB/B box. Possible replicase interaction motifs within this sequence include a replicase assembly site (37), a replication enhancer (17), and a homopolyadenylate sequence (17, 50). Given a number of reasonable candidates that can bind the replicase, we decided to more systematically locate the responsible sequence with a series of RNA fragments derived from B3(+)8 (Fig. 6A). In template competition assays, the IC50s of RNAs containing sequence from nt 1120 to 1210 were all >20 nM while those of RNAs containing nt 1190 to 1240 were lower than 3.8 nM (Fig. 6A). The most prominent feature within this sequence is the homopolyadenylate. To further define the site in the segment responsible for replicase binding, we made and tested RNAs B3(+)8a, B3(+)8b, and B3(+)8c, which contain overlapping sequences derived from nt 1190 to 1240. All three were effective binders, again with the homopolyadenylate being common to the three RNAs (Fig. 6A).
FIG. 6.
The poly(A) sequence in the ICR can bind the BMV replicase. (A) Mapped sequences in B3(+)8 that contained at least one replicase-binding sequence. The IC50s of RNAs tested in template competition assays are summarized to the right of the bars that denote the portion of the RNA derived from B3(+)8. The initial mapping led to the identification of the sequence between nt 1190 and 1240 as the one encoding a replicase-binding sequence. RNAs B3(+)8a, B3(+)8b, and B3(+)8c were made to further address the responsible sequence. In the sequences shown, lowercase g's represents nucleotides that were added to the BMV sequence (in uppercase letters) to allow transcription by the T7 RNA polymerase. (B) Preparation of poly(A) molecules of various lengths for use in template competition assays. The gel contains the purified RNAs separated in a 20% denaturing polyacrylamide gel and stained with toluidine blue. The nucleotide lengths of several marker RNAs are shown to the left side of the gel. (C) Summary of the results from template competition assays for poly(A) molecules of different lengths or a chemically synthesized molecule of 22 deoxyadenylates. The two gel images are from template competition assays performed with competitors of 22 or 15 adenylates. % Syn., percent synthesis.
To examine whether the poly(A) sequence is required and sufficient to bind the BMV replicase, poly(A) molecules of differing lengths were prepared by alkaline hydrolysis of poly(A). Molecules of 22 to 16 adenylates had IC50s lower than 3.5 nM (Fig. 6C). However, A15 had an IC50 of >20 nM (Fig. 6C). These results indicate that at least 16 polyadenylates are required for efficient replicase binding. A DNA version of A22 had an IC50 of >20 nM, indicating that the BMV replicase specifically recognized the RNA version of the poly(A).
Additional intramolecular RNA interactions.
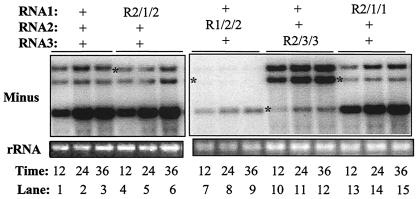
The observation that there is only one replicase-binding site each in plus- and minus-strand RNA1 and RNA2 does not indicate that interactions between the ends of BMV RNAs are dispensable for RNA replication. To determine whether these interactions do contribute to BMV replication, we made a series of chimeric RNAs in which the 5′ and 3′ NCR of BMV RNAs were precisely exchanged without the introduction of extraneous nucleotide changes. The names of the chimeric RNAs are in three parts that are separated by slashes. The first part denotes the origin of the 5′ NCR, the middle portion denotes the protein-coding region, and the third part denotes the origin of the 3′ NCR. For example, R2/1/2 will have the 5′ and 3′ NCR from RNA2, respectively, and the coding sequence for 1a.
When the heterologous 5′ NCR was fused to RNA2, RNA1, or RNA3, as in constructs R1/2/2, R2/3/3, and R2/1/1, the chimeras were debilitated in minus-strand RNA replication (Fig. 7, lanes 7 to 15). However, when both the 5′ and 3′ ends came from RNA2, as in R2/1/2, wild-type levels of RNA replication were observed (Fig. 7, lanes 4 to 6). Parallel results were observed for the plus-strand versions of all of these RNAs (data not shown). These results indicate that compatible pairing of the 5′ and 3′ NCR in RNA2 is required for efficient replication, even though there is only one detectable replicase-binding site in plus- and minus-strand RNA2. A similar chimera where the 2a coding sequence fused to the 5′ and 3′ NCR of RNA1 named R1/2/1 did not yield a replication-competent RNA (data not shown). This negative result suggests that there may be sequences in addition to the RNA1 NCR that are required to function together for RNA replication.
FIG. 7.
Requirement for compatible interaction between 5′ and 3′ NCR in BMV RNA2. A Northern blot examining the accumulation of the minus-strand replication products from several chimeric BMV RNAs is shown. The names of the RNAs denote the origin of the 5′ NCR, the protein-coding region, and the 3′ NCR of a BMV RNA. For example, R2/1/2 indicates that the 5′ and 3′ NCR came from RNA2 but that the protein-coding region was from RNA1. Plus-strand RNA products are not shown but exhibited the same trends seen with the minus-strand RNAs. The combinations of the RNAs transfected into protoplasts are shown above the gel image. Total RNAs were purified from protoplasts at the times (in hours) indicated below the gel images. Asterisks identify the positions expected of the chimeric RNAs. All of the samples in this experiment were on one blot but were cropped to present the results in a more logical manner.
DISCUSSION
In this report, we used an in vitro replicase-binding assay to identify replicase-binding sites in all plus- and minus-strand BMV RNAs. This approach has the benefit of identifying a potential function in BMV RNA without having to fulfill all of the requirements for RNA-dependent RNA synthesis in vitro or in vivo. The competition assay can distinguish three classes of competitor RNAs (Fig. 2): apparent nonbinders, ineffective binders with IC50s from 15 to 20 nM, and effective binders with IC50s lower than 5 nM. The eight effective binding sites are positioned in the NCR of both RNA strands and in the intercistronic region of RNA3 (Fig. 2C and Table 1). Except within the polyadenylate sequence, which we did not test, single-nucleotide substitutions in the other seven sites decreased both replicase-binding in vitro and BMV RNA accumulation in transfected protoplasts. For the poly(A) sequence, Smirnyagina et al. (50) demonstrated that a deletion of the polyadenylate tract resulted in debilitated RNA replication. Progeny viruses recovered from this input mutant were either revertants or had second-site mutations. Therefore, all eight effective binding sites identified in vitro have roles in the infected cell.
B box versus the cB box.
The roles of the SLC and subgenomic core promoter have been characterized extensively in vitro and in vivo, and their functions in directing RNA synthesis appear to be more straightforward. However, the B box and cB boxes represent a more complex situation. The B box was shown to be involved in BMV RNA replication (16, 35, 36). The intercistronic B box in RNA3 was reported to bind to the 1a protein and to form the membrane-associated structure where the BMV replicase is located in yeast (40). The B box of plus-strand RNA2 also binds 1a (11). Pogue and Hall (34) have tried to distinguish whether the B box functions in plus- or minus-strand RNA, and they determined that the most likely effect is through the plus-strand RNA. However, it is often difficult to distinguish intricately linked effects in the cell. We demonstrate in this work that the cB box in minus-sense RNA is more important for replicase binding than the B box. Previously, we showed that the cB box in minus-strand RNA is required for the initiation of genomic plus-strand RNA synthesis in vitro (47, 48). Taken together, both the B and cB boxes appear to have independent and required roles in BMV RNA replication. More specifically, the B box binds 1a and regulates translation and replicase assembly while the cB box binds the replicase for the initiation of genomic plus-strand RNA synthesis. One prediction of this model is that a 1a molecule(s) associated with the BMV replicase has a role distinct from that of free replicase-independent 1a molecules.
In yeast, mutations in the B box in the RNA3 ICR decreased the half-life of the mutant RNA by more than 20-fold, while in barley, we have found that RNAs with B box mutations reduce RNA3 replication by only twofold, with about comparable decreases in the RNA half-lives (data not shown). The results with both yeast and barley show a role for the ICR B and/or cB box in BMV infections, but there is a significant difference in the magnitudes of the effects of mutations. While RNA decay rates may differ significantly in yeast and barley, there may be a trivial explanation for the observed differences: the mutations examined in yeasts were deletions of multiple nucleotides, while our results are from single-nucleotide substitutions.
A different mode of BMV RNA3 recognition?
Results from our analysis confirmed the preexisting notion that the BMV replicase recognized RNA1 and RNA2 by similar mechanisms but that RNA3 was recognized in a different way. A demonstration of this difference was provided by effects of the mutations in the cB/B boxes in the three BMV RNAs. Mutations in RNA1 and RNA2 had more-severe effects on replicase binding and on RNA replication than did mutations in the intercistronic cB box in RNA3 (Fig. 5). Another striking difference between RNA1 and RNA2 was the presence of only one effective binding site apiece in plus- and minus-strand RNA1 and RNA2 (near the initiation site for RNA synthesis), while both plus- and minus-strand RNA3 had two effective binding sites. It is likely that RNA3 requires additional sequences for specific recognition by the BMV replicase since it is recognized by the replicase in trans. Characterizations of viral cis-acting elements, including those in BMV, have generally focused on RNAs whose replication was not coupled to the production of the replicase components. Such an analysis may miss some requirements that are unique to the RNA(s) whose replication is linked to the production of replication proteins.
The identification of only one effective binding site in each plus- and minus-strand RNA1 and RNA2 does not mean that additional interactions, such as ones that involve the 5′-end-3′-end interactions or intermolecular interactions, are unimportant for BMV replication. Such interactions have been demonstrated for a number of systems to provide the means to fine tune the regulation of viral replication or transcription against the needs of other required processes, such as translation (14, 18, 20, 22, 28, 30, 44). For BMV, the 5′ portion of RNA3 was shown to help the initiation of minus-strand RNAs at the 3′ end of the BMV RNA (9). Furthermore, we have found that compatible 5′ and 3′ NCR of BMV RNA2 were able to drive higher levels of a chimeric RNA, R2/1/2 (Fig. 7), suggesting that additional interactions will prove important for regulating BMV RNA levels. Our mapping results do suggest, however, that not all of the participating sequences that interact will necessarily bind the replicase.
The replicase for phage Qβ is the only other viral genome analyzed systematically for replicase-binding sites. Qβ is a monopartite RNA virus (54). Binding to the plus- and minus-strand Qβ RNAs was mapped by electron microscopy and by using an RNase protection assay and hybridization of the protected fragments to restriction fragments of the Qβ genome (4). Interaction with the plus-strand involved two internal sites (M and S) (reference 4 and references therein). However, binding to the 3′ end was detected only in the presence of the host factor after the Qβ RdRp initiated RNA synthesis. Binding to the minus strand was primarily at the 3′ end and occurred with apparently lower affinity. In comparison, the BMV replicase binds to the 3′ regions of all plus- and minus-strand RNAs; internal binding occurred only with RNA3.
Poly(A) tract.
The intercistronic poly(A) tract has previously been demonstrated to be required for BMV RNA replication (17). We have found that the intercistronic poly(A) sequence binds the BMV replicase specifically (Fig. 6). The BMV replicase was shown to not bind to homocytidylates (2). At present, we can only speculate about the component in the replicase that binds poly(A). An obvious candidate is the cellular poly(A)-binding protein, which requires a stretch of ∼12 adenylates for stable binding. We have found that 16 nt of the typically 22-nt-long BMV poly(A) tract was sufficient to effectively bind the BMV replicase. Another candidate is the eukaryotic homologue of the Qβ host factor, which also binds poly(A) with high affinity (8, 41).
Viral poly(A) tracts are usually found, like cellular mRNAs, at the 3′ ends of viral RNAs. In other viral systems, the poly(A) tail may regulate RNA replication and the timing of replication and translation (5, 13, 20). It will be interesting to determine further how an intercistronic poly(A) tract regulates processes needed for BMV infection.
In summary, our analysis of the replicase-binding sites has confirmed the role of many of the motifs that were previously found to participate in BMV RNA replication and helps to clarify the functions of these sequences. Results from this analysis will be useful for a more integrated view of the requirements for BMV replication and transcription.
Acknowledgments
We acknowledge the helpful discussion and editing of the Texas A&M University Cereal Killers.
S.-K.C. acknowledges a fellowship by the Korean Science & Engineering Foundation (KOSEF). Funding was provided by the National Science Foundation.
REFERENCES
- 1.Adkins, S., and C. C. Kao. 1998. Subgenomic RNA promoters dictate the mode of recognition by bromoviral RNA-dependent RNA polymerases. Virology 252:1-8. [DOI] [PubMed] [Google Scholar]
- 2.Adkins, S., R. W. Siegel, J.-H. Sun, and C. C. Kao. 1997. Minimal templates directing accurate initiation of subgenomic RNA synthesis in vitro by the brome mosaic virus RNA-dependent RNA polymerase. RNA 3:634-647. [PMC free article] [PubMed] [Google Scholar]
- 3.Ahlquist, P. 1992. Bromovirus RNA replication and transcription. Curr. Opin. Genet. Dev. 2:271-276. [DOI] [PubMed] [Google Scholar]
- 4.Barrera, I., D. Schuppli, J. M. Sogo, and H. Weber. 1993. Different mechanisms of recognition of bacteriophage Qβ plus and minus strand RNAs by Qβ replicase. J. Mol. Biol. 232:512-521. [DOI] [PubMed] [Google Scholar]
- 5.Barton D. J., B. J. O'Donnell, and J. B. Flanegan. 2001. 5′ cloverleaf in poliovirus RNA is a cis-acting replication element required for negative-strand synthesis. EMBO J. 20:1439-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck, K. W. 1996. Comparison of the replication of positive-strand RNA viruses of plants and animals. Adv. Virus Res. 47:159-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bujarski, J. J., P. Ahlquist, T. C. Hall, T. W. Dreher, and P. Kaesberg. 1986. Modulation of replication, aminoacylation, and adenylation in vitro and infectivity in vivo of brome mosaic virus RNAs containing deletions within the multifunctional 3′ end. EMBO J. 5:1769-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmichael, G. G., K. Weber, A. Niveleau, and A. J. Wahba. 1975. The host factor required for RNA phage Qβ RNA replication in vitro. J. Biol. Chem. 250:3607-3612. [PubMed] [Google Scholar]
- 9.Chapman, M., A. L. N. Rao, and C. C. Kao. 1998. The 5′ end of BMV RNAs promotes initiation of (−)-strand RNA synthesis in vitro and in vivo. Virology 252:458-467. [DOI] [PubMed] [Google Scholar]
- 10.Chapman, M. R., and C. C. Kao. 1999. A minimal RNA promoter for minus-strand RNA synthesis by the brome mosaic virus polymerase complex. J. Mol. Biol. 286:709-720. [DOI] [PubMed] [Google Scholar]
- 11.Chen, J., A. Noueir, and P. Ahlquist. 2001. Brome mosaic virus protein 1a recruits viral RNA2 to RNA replication through a 5′ proximal RNA2 signal. J. Virol. 75:3207-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, J., A. Noueir, and P. Ahlquist. 2003. An alternate pathway for recruiting template RNA to the brome mosaic virus RNA replication complex. J. Virol. 77:2568-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng, J. H., C. W. Peng, Y. H. Hsu, and C. H. Tsai. 2002. The synthesis of minus-strand RNA of bamboo mosaic potexvirus initiates from multiple sites within the poly(A) tail. J. Virol. 76:6114-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi, I. R., and K. A. White. 2002. An RNA activator of subgenomic mRNA1 transcription in tomato bushy stunt virus. J. Biol. Chem. 277:3760-3766. [DOI] [PubMed] [Google Scholar]
- 15.Dreher, T. W., and T. C. Hall. 1988. Mutational analysis of the sequence and structural requirements in brome mosaic virus RNA for minus strand promoter activity. J. Mol. Biol. 201:31-40. [DOI] [PubMed] [Google Scholar]
- 16.French, R., and P. Ahlquist. 1987. Intercistronic as well as terminal sequences are required for efficient amplification of brome mosaic virus RNA3. J. Virol. 61:1457-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French, R., and P. Ahlquist. 1988. Characterization and engineering of sequences controlling in vivo synthesis of brome mosaic virus subgenomic RNA. J. Virol. 62:2411-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallie, D. R. 2002. Protein-protein interactions required during translation. Plant Mol. Biol. 50:949-970. [DOI] [PubMed] [Google Scholar]
- 19.Hema, M., and C. C. Kao. 2004. Template sequence near initiation nucleotide can modulate brome mosaic virus RNA accumulation in plant protoplasts. J. Virol. 78:1169-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herold, J., and R. Andino. 2001. Poliovirus RNA replication requires genome circularization through a protein-protein bridge. Mol. Cell 7:581-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higuchi, R., B. Krummel, and R. K. Saiki. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 16:7351-7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isken, O., C. W. Grassmann, R. T. Sarisky, M. Kann, S. Zhang, F. Grosse, P. N. Kao, and S. E. Behrens. 2003. Members of the NF90/NFAR protein group are involved in the life cycle of a positive-strand RNA virus. EMBO J. 22:5655-5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janda, M., and P. Ahlquist. 1993. RNA-dependent replication, transcription, and persistence of brome mosaic virus RNA replicons in S. cerevisiae. Cell 72:961-970. [DOI] [PubMed] [Google Scholar]
- 24.Janda, M., R. French, and P. Ahlquist. 1987. High efficiency T7 polymerase synthesis of infectious RNA from cloned brome mosaic virus cDNA and effects of 5′ extensions on transcript infectivity. Virology 158:259-262. [DOI] [PubMed] [Google Scholar]
- 25.Kao, C. C. 2002. Lessons learned from the core RNA promoters of Brome mosaic virus and Cucumber mosaic virus. Mol. Plant Pathol. 3:53-59. [DOI] [PubMed] [Google Scholar]
- 26.Kao, C. C., and K. Sivakumaran. 2000. Brome mosaic virus, good for an RNA virologist's basic needs. Mol. Plant Pathol. 1:91-98. [DOI] [PubMed] [Google Scholar]
- 27.Kao, C. C., M. Zheng, and S. Ruedisser. 1999. A simple and efficient method to reduce nontemplated nucleotide addition at the 3′ terminus of RNAs transcribed by T7 RNA polymerase. RNA 5:1268-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kean, K. M. 2003. The role of mRNA 5′-noncoding and 3′-end sequences on 40S ribosomal subunit recruitment, and how RNA viruses successfully compete with cellular mRNAs to ensure their own protein synthesis. Biol. Cell 95:129-139. [DOI] [PubMed] [Google Scholar]
- 29.Kim, C.-H., C. C. Kao, and I. Tinoco. 2000. RNA motifs that determine specificity between a viral replicase and its promoter. Nat. Struct. Biol. 7:415-423. [DOI] [PubMed] [Google Scholar]
- 30.Kim, K. H., and C. L. Hemenway. 1999. The 5′ nontranslated region of potato virus X RNA affects both genomic and subgenomic RNA synthesis. J. Virol. 70:5533-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroner, P. A., B. M. Young, and P. Ahlquist. 1990. Analysis of the role of brome mosaic virus 1a protein domains in RNA replication, using linker insertion mutagenesis. J. Virol. 64:6110-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai, M. M. 1998. Cellular factors in the transcription and replication of viral RNA genomes: a parallel to DNA-dependent RNA transcription. Virology 244:1-12. [DOI] [PubMed] [Google Scholar]
- 33.Marsh, L. E., and T. C. Hall. 1987. Evidence implicating a tRNA heritage for the promoters of positive-strand RNA synthesis in brome mosaic and related viruses. Cold Spring Harbor Symp. Quant. Biol. 52:331-341. [DOI] [PubMed] [Google Scholar]
- 34.Pogue, G. P., and T. C. Hall. 1992. The requirements for 5′ stem-loop structure in brome mosaic virus replication supports a new model for viral positive-strand RNA initiation. J. Virol. 66:674-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pogue, G. P., L. E. March, J. P. Connell, and T. C. Hall. 1992. The requirement for ICR-like sequences in the replication of brome mosaic virus genomic RNA. Virology 188:742-753. [DOI] [PubMed] [Google Scholar]
- 36.Pogue, G. P., L. E. March, and T. C. Hall. 1990. Point mutations in the ICR2 motif of brome mosaic virus RNAs debilitate (+)-strand replication. Virology 178:152-160. [DOI] [PubMed] [Google Scholar]
- 37.Quadt, R., M. Ishikawa, M. Janda, and P. Ahlquist. 1995. Formation of brome mosaic virus RNA-dependent RNA polymerase in yeast requires coexpression of viral proteins and viral RNA. Proc. Natl. Acad. Sci. USA 92:4892-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao, A. L. N., T. W. Dreher, L. E. Marsh, and T. C. Hall. 1989. Telomeric function of the tRNA-like structure of brome mosaic virus RNA. Proc. Natl. Acad. Sci. USA 86:5335-5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao, A. L. N., B. P. Sullivan, and T. C. Hall. 1990. Use of Chenopodium hybridum facilitates isolation of brome mosaic virus RNA recombinants. J. Gen. Virol. 71:1403-1407. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz, M., J. Chen, M. Janda, M. Sullivan, J. den Boon, and P. Ahlquist. 2002. A positive-strand RNA virus replication complex parallels form and function of retrovirus capsids. Mol. Cell 9:505-514. [DOI] [PubMed] [Google Scholar]
- 41.Senear, A. W., and J. A. Steitz. 1976. Site-specific interaction of Qb host factor and ribosomal protein S1 with Qb and R17 bacteriophage RNAs. J. Biol. Chem. 251:1902-1912. [PubMed] [Google Scholar]
- 42.Siegel, R. W., S. Adkins, and C. C. Kao. 1997. Sequence-specific recognition of a subgenomic promoter by a viral RNA polymerase. Proc. Natl. Acad. Sci. USA 94:11238-11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siegel, R. W., L. Bellon, L. Beigelman, and C. C. Kao. 1998. Moieties in an RNA promoter specifically recognized by a viral RNA-dependent RNA polymerase. Proc. Natl. Acad. Sci. USA 95:11613-11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sit, T. L., A. A. Vaewhongs, and S. A. Lommel. 1998. RNA-mediated trans-activation of transcription from a viral RNA. Science 281:829-832. [DOI] [PubMed] [Google Scholar]
- 45.Sivakumaran, K., S. K. Choi, M. Hema, and C. C. Kao. 2004. Requirements for brome mosaic virus subgenomic RNA synthesis in vivo and replicase-core promoter interactions in vitro. J. Virol. 78:6091-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sivakumaran, K., M. Hema, and C. C. Kao. 2003. Brome mosaic virus RNA syntheses in vitro and in barley protoplasts. J. Virol. 77:5703-5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sivakumaran, K., and C. C. Kao. 1999. Initiation of genomic plus-strand RNA synthesis from DNA and RNA templates by a viral RNA-dependent RNA polymerase. J. Virol. 73:6415-6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sivakumaran, K., and C. C. Kao. 2000. Genomic plus-strand RNA synthesis by brome mosaic virus (BMV) RNA replicase requires a sequence that is complementary to the binding site of the BMV helicase-like protein. Mol. Plant Pathol. 1:337-346. [DOI] [PubMed] [Google Scholar]
- 49.Sivakumaran, K., C. H. Kim, R. Tayon, and C. C. Kao. 1999. RNA sequence and secondary structural determinants in a minimal viral promoter that directs replicase recognition and initiation of genomic plus-strand RNA synthesis. J. Mol. Biol. 294:667-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smirnyagina, E., Y. H. Hsu, N. Chua, and P. Ahlquist. 1994. Second-site mutations ion the brome mosaic virus RNA3 intercistronic region partially suppress a defect in coat protein mRNA transcription. Virology 198:427-436. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan, M., and P. Ahlquist. 1997. Cis-acting signals in bromovirus RNA replication and gene expression: networking with viral proteins and host factors. Semin. Virol. 8:221-230. [Google Scholar]
- 52.Sullivan, M., and P. Ahlquist. 1999. A brome mosaic virus intergenic RNA3 replication signal functions with viral replication protein 1a to dramatically stabilize RNA in vivo. J. Virol. 73:2622-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun, J. H., S. Adkins, G. Faurote, and C. C. Kao. 1996. Initiation of (−)-strand RNA synthesis catalyzed by the BMV RNA-dependent RNA polymerase: synthesis of oligonucleotides. Virology 226:1-12. [DOI] [PubMed] [Google Scholar]
- 54.Van Duin, J. 1988. Single-stranded RNA bacteriophages, p. 117-167. In R. Calendar (ed.), The bacteriophages. Plenum Press, New York, N.Y.
- 55.Zuker, M., D. H. Mathews, and D. H. Turner. 1999. Algorithms and thermodynamics for RNA secondary structure prediction: a practical guide in RNA biochemistry and biotechnology, p. 11-43. In J. Barciszewski and B. F. C. Clark (ed.), NATO ASI series. Kluwer Academic Publisher, Dordrecht, The Netherlands.