Abstract
Diverse pathological insults trigger a cardiac remodeling process during which myocytes undergo hypertrophy, with consequent decline in cardiac function and eventual heart failure. Multiple transcriptional regulators of pathological cardiac hypertrophy are controlled at the level of subcellular distribution. For example, prohypertrophic transcription factors belonging to the nuclear factor of activated T cells (NFAT) and GATA families are subject to CRM1-dependent nuclear export but are rapidly relocalized to the nucleus in response to cues for hypertrophic growth. Here, we demonstrate that the antihypertrophic chromatin-modifying enzyme histone deacetylase 5 (HDAC5) is shuttled out of the cardiomyocyte nucleus via a CRM1-mediated pathway in response to diverse signals for hypertrophy. CRM1 antagonists block the agonist-mediated nuclear export of HDAC 5 and repress pathological gene expression and associated hypertrophy of cultured cardiomyocytes. Conversely, CRM1 activity is dispensable for nonpathological cardiac gene activation mediated by thyroid hormone and insulin-like growth factor 1, agonists that fail to trigger the nuclear export of HDAC5. These results suggest a selective role for CRM1 in derepression of pathological cardiac genes via its neutralizing effects on antihypertrophic factors such as HDAC5. Pharmacological approaches targeting CRM1-dependent nuclear export in heart muscle may have salutary effects on cardiac function by suppressing maladaptive changes in gene expression evoked by stress signals.
A common mechanism controlling gene expression involves altering the subcellular distribution of transcriptional regulators. A multitude of transcription factors and cofactors possess nuclear localization sequences (NLSs) and nuclear export signals (NESs) that mediate entry into and exit from the nucleus, respectively. Frequently, signal transduction pathways that impinge on transcriptional regulators function by positively or negatively affecting the activities of these intrinsic targeting domains.
For proteins over ∼40 kDa, passage into and out of the nucleus is governed by the nuclear pore complex (NPC), a multisubunit structure embedded in the nuclear envelope (27). Positively charged NLSs are bound by importins α and β, which tether cargo to the cytosolic face of the NPC and facilitate translocation of proteins into the nucleus. The CRM1 protein, also referred to as exportin, mediates the transit of proteins out of the nucleus (16), although CRM1-independent mechanisms for nuclear export exist (25, 33). CRM1 binds hydrophobic NESs together with the small GTP binding protein Ran, and these ternary complexes are shuttled out of the nucleus through a series of interactions with the NPC.
The capacity of nuclear import and export machinery to access an NLS or NES is often dictated by signaling events that culminate in exposure or masking of these regulatory sequences (12). This may occur through direct modification of the target protein or via modification of an associated factor. Phosphorylation has been most commonly implicated in this mode of control, although roles for other types of posttranslational modifications (e.g., acetylation) in the regulation of protein localization have recently been revealed (9).
Cardiac myocytes lose the ability to divide after birth but “remodel” in response to stress signals that arise from a variety of cardiovascular disorders, including myocardial infarction and hypertension. A common outcome of stress in the heart is cardiomyocyte hypertrophy, a growth response during which individual myocytes increase in size without dividing, assemble additional contractile units (sarcomeres) to maximize force generation, and reactivate a fetal program of gene expression (37). While there may initially be beneficial elements to this type of cardiac growth, for example the normalization of wall stress, prolonged hypertrophy in response to pathological signals is associated with an increase in morbidity and mortality due to heart failure (17).
Importantly, cardiac hypertrophy is not always deleterious. Cardiac hypertrophy that occurs during postnatal development and in endurance athletes, referred to as physiological hypertrophy, is clearly salutary and phenotypically distinct from the pathological hypertrophy seen in individuals with cardiovascular disease (10). Molecular distinctions between pathological and physiological cardiac hypertrophy can be made at the levels of apoptotic gene regulation (28) and the fetal gene program (4). For example, signals for pathological hypertrophy stimulate the expression of embryonic beta-myosin heavy chain (β-MyHC) and reduce the expression of adult α-MyHC, with the net outcome of diminished myofibrillar ATPase activity and impaired contractility (43). The gene encoding sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) is also downregulated during pathological cardiac hypertrophy, which results in altered cardiac calcium handling (52). In contrast, cues for physiological hypertrophy do not repress the expression of α-MyHC or SERCA and instead have been shown to block the downregulation of these genes mediated by pathological signals (49, 58). The counterregulatory effects of exercise on α-MyHC and SERCA expression can be mimicked by thyroid hormone (7, 31). In addition, insulin-like growth factor 1 (IGF-1) signaling has been shown to maintain α-MyHC levels in stressed myocardium (34).
Roles for several transcriptional regulators in the control of pathological cardiac hypertrophy have now been validated by in vitro and in vivo studies. Sequence-specific DNA binding factors that positively regulate cardiac hypertrophy include nuclear factor of activated T cells (NFAT) (44), myocyte enhancer factor 2 (MEF2) (47, 50), serum response factor (SRF) (66), and GATA4 (35, 44, 45). Recently, chromatin-modifying enzymes that govern the access of transcriptional machinery to DNA templates have also emerged as key regulators of cardiac growth. The p300 coactivator, which possesses histone acetyltransferase activity, promotes hypertrophic growth by acetylating core histones in cardiac gene regulatory regions, resulting in relaxation of local chromatin and consequent transcriptional activation (21, 63). In contrast, class II histone deacetylases (HDACs) 5 and 9 suppress cardiac hypertrophy by removing acetyl groups from nucleosomal histones (8, 65).
Many transcriptional regulators involved in cardiac hypertrophy are subject to control at the level of subcellular localization. For example, phosphorylation of serine residues on NFAT results in intramolecular masking of two distinct NLSs, with consequent cytoplasmic sequestration of the transcription factor (2). Retention of NFAT in the cytoplasm is reinforced through CRM1-mediated nuclear export of the phosphorylated form of the transcription factor (67). In response to signals that increase intracellular calcium, NFAT is dephosphorylated by the calcium- and calmodulin-dependent phosphatase calcineurin, resulting in exposure of these NLSs and nuclear import of the transcription factor. Overexpression of constitutively active calcineurin or nonphosphorylatable, constitutively nuclear NFAT is sufficient to induce cardiac hypertrophy in transgenic mice (44).
The prohypertrophic transcription factors GATA4 and SRF are also subject to signal-dependent nucleo-cytoplasmic shuttling (6, 45). In the case of GATA4, phosphorylation by glycogen synthase kinase 3β (GSK-3β) appears to stimulate CRM1-dependent nuclear export of the transcription factor, and signals for cardiac hypertrophy counteract this process by neutralizing GSK-3β function (45).
Class II HDACs 5 and 9 suppress cardiac hypertrophy through interactions with MEF2 and possibly other prohypertrophic transcription factors (38). Studies of noncardiac cells have defined a CRM1-dependent pathway that relieves MEF2 from the repressive effects of HDACs. Phosphorylation of class II HDACs at two conserved serine residues promotes their association with the 14-3-3 chaperone protein, which results in the exposure of a cryptic CRM1-dependent NES in the carboxy termini of the transcriptional repressors (19, 40). We have proposed that signal-dependent nuclear export of class II HDACs serves as a final common endpoint for cardiac hypertrophic signaling cascades, since signal-resistant forms of HDAC5 or 9 block hypertrophy in response to diverse pathological signals, and targeted disruption of these transcriptional repressors results in spontaneous cardiac hypertrophy (5, 8, 60, 65).
Despite our understanding of the importance of nuclear export in the control of transcriptional regulators of cardiac hypertrophy, the phenotypic consequences of CRM1 antagonism on cardiac myocytes has remained unknown. We sought to test the hypothesis that agonist-mediated cardiac hypertrophy can be blocked by inhibiting the nuclear export of negative regulators of this growth response, such as HDAC5. Here, we demonstrate that CRM1 antagonists dose-dependently suppress pathological gene expression and the growth of cultured cardiomyocytes exposed to hypertrophic agonists, such as the α1-adrenergic receptor ligand phenylephrine (PE). In contrast, the beneficial induction of α-MyHC and SERCA expression mediated by thyroid hormone or IGF-1 occurs independently of CRM1. Inhibition of cardiac hypertrophy by CRM1 antagonists is associated with the blockade of HDAC5 nuclear export and repression of NFAT and MEF2 target gene expression. These results define a novel and selective role for CRM1 in derepression of pathological cardiac genes and suggest a potential therapeutic use for pharmacological inhibitors of nuclear export.
MATERIALS AND METHODS
Neonatal rat ventricular myocyte (NRVM) preparation and culture.
Hearts were dissected from 1- to 3-day-old Sprague-Dawley rats, minced, and digested with collagenase (600 μg/ml; Worthington) and pancreatin (full-strength-activity equivalent; Sigma) in 1× Ads buffer (NaCl [116 mM], HEPES [20 mM; pH 7.4], NaH2PO4 [4.8 mM], KCl [5 mM], MgSO4 [400 μM], and glucose [5.5 mM]). Cells were centrifuged through a step gradient of Percoll (Pharmacia) to separate myocytes from fibroblasts, and the myocyte pool was further enriched by preplating the cells for 2 h to remove adherent fibroblasts from the cell population. Cells were cultured overnight on dishes coated with gelatin (0.2%; Sigma) in Dulbecco's modified Eagle's medium (DMEM) containing fetal bovine serum (FBS) (10%), l-glutamine (2 mM), and penicillin-streptomycin. After overnight culture, cells were washed with serum-free medium and maintained in DMEM supplemented with Neutridoma-SP (Roche Applied Science), which contains albumin, insulin, transferrin, and other defined organic and inorganic compounds, at either 0.1 or 0.3% (vol/vol), as indicated below.
Adenovirus production and analysis of GFP-HDAC5.
cDNA for full-length human HDAC5 (encoding 1,122 amino acids) was fused to sequences encoding enhanced GFP (Clontech) in pcDNA3.1+ (Invitrogen). The resultant construct encodes GFP fused in frame to the amino terminus of HDAC5. A construct encoding GFP fused to HDAC5 containing alanines in place of serines at positions 259 and 498 was generated in the same manner. For adenovirus production, GFP-HDAC5 cDNAs were subcloned into pAC-CMV (3) and constructs were cotransfected into 293 cells with pJM17 by employing Fugene 6 (Roche Molecular Biochemicals). Primary lysates were used to reinfect 293 cells, and viral plaques were obtained by the agar overlay method. Clonal populations of adenovirus were amplified upon reinfection of 293 cells. For analysis of GFP-HDAC5 localization in NRVMs, cells were plated in the presence of adenovirus (multiplicity of infection, ∼50) on gelatin-coated 96-well dishes (104 cells/well; Costar) in DMEM containing FBS (10%), l-glutamine (2 mM), and penicillin-streptomycin. After overnight culture, cells were washed with serum-free medium and maintained in DMEM (100 μl) supplemented with Neutridoma-SP (0.1%) for 4 h prior to treatment with agonists and leptomycin B (LMB). Images were captured at a ×40 magnification with a fluorescence microscope (Nikon Eclipse TS100) equipped with a digital camera (Photometrics CoolSNAP HQ) and MetaMorph imaging software.
Indirect immunofluorescence.
NRVMs (2.5 × 105) were plated on gelatin-coated six-well dishes. Following the treatments indicated below, cells were fixed with formalin (10%) in phosphate-buffered saline (PBS), permeabilized, blocked with PBS containing NP-40 (0.1%) and bovine serum albumin (BSA) (3%), and incubated in the same solution, but with primary antibodies for sarcomeric α-actinin (mouse monoclonal antibody, 1:200 dilution; Sigma) or atrial natriuretic factor (ANF) (rabbit polyclonal antibody, 1:200 dilution; Peninsula Laboratories). Cells were washed five times with PBS and incubated with fluorescein- or cy3-conjugated secondary antibodies (goat polyclonal antibodies, 1:200 and 1:1000 dilutions, respectively; Jackson Laboratories), followed by a brief incubation with Hoechst dye (Molecular Probes). Cells were washed five times in PBS, washed one time with water, and sequentially covered with mounting solution (SlowFade; Molecular Probes) and glass coverslips. Proteins were visualized with an inverted fluorescence microscope (Olympus model BH-2) at a ×40 magnification, and images were captured using a digital camera (Photometrics; Roper Scientific).
ANF competition enzyme-linked immunosorbent assay (ELISA) and total protein measurements.
NRVMs were plated on gelatin-coated 96-well dishes (104/well). After overnight incubation in the presence of FBS (10%), cells were cultured in serum-free DMEM (200 μl) containing Neutridoma-SP (0.1%) in the absence or presence of PE (20 μM; Sigma) or FBS (10%) and the concentrations of LMB (LC Laboratories) indicated below. After 72 h of stimulation, medium supernatants (50 μl) were removed and added to 96-well Immulon II plates (Dynex) that had previously been coated with goat anti-mouse Fc fragments (1 μg/ml; Jackson Laboratories). Some wells received ANF peptide standards (Phoenix Peptide) in place of culture supernatant. Monoclonal anti-ANF antibody (50 ng/ml in Tris-buffered saline with 0.1% Tween 20 [TBS-T]; Biodesign) and biotinylated ANF peptide (1 ng/ml in TBS-T; Phoenix Peptide) were added to wells (25 μl each) and incubated for 2 h at room temperature. Some wells were treated with TBS-T lacking medium supernatant or peptide to provide background controls. Wells were washed five times with TBS-T and exposed to horseradish peroxidase (HRP)-conjugated streptavidin (100 μl of a 1:10,000 dilution per well in TBS-T; Jackson Laboratories) for 1 h at room temperature. Wells were washed five times with TBS-T and exposed to TMB substrate (100 μl; Kirkegaard & Perry), which provides a colorimetric readout, for 30 min at room temperature. Reactions were terminated with H2SO4 (2 N, 100 μl/well), and absorbance at 450 nm was detected with a Fusion plate reader (Perkin Elmer/Packard). For total protein measurements, cells were washed five times with PBS, and Bradford reagent (Bio-Rad) was added to each well of the plate (100 μl/well). Color development was allowed to proceed for 30 min prior to measurement at 595 nm with a microtiter plate spectrophotometer (Molecular Devices).
α- and β-MyHC cytoblot assay.
NRVMs were plated on gelatin-coated 96-well dishes (104 cells/well) in DMEM (200 μl) containing l-glutamine (2 mM), penicillin-streptomycin, and charcoal-stripped FBS (10%) lacking thyroid hormone, which is known to induce α-MyHC expression and coordinately downregulate β-MyHC expression. Following overnight culture, growth medium was replaced with serum-free DMEM supplemented with Neutridoma-SP (0.3%, vol/vol) in the absence or presence of LMB (18.5 nM) and the hypertrophic agonist indicated below. Cells were washed two times with PBS and fixed with methanol (30 min on ice). Cells were rinsed two times with PBS, blocked with PBS containing BSA (0.1%; 60 min at room temperature), and incubated with anti-rat cardiac α-MyHC antibody (mouse monoclonal hybridoma supernatant BA-G5; American Type Culture Collection) or anti-rat β-MyHC antibody (mouse monoclonal hybridoma supernatant, 200 μl/well, 1 h at room temperature; University of Iowa Developmental Studies Hybridoma Bank). Cells were washed three times with PBS containing BSA (1%) and incubated with the same solution but containing HRP-conjugated anti-mouse secondary antibody (1:500 dilution, 1 h at room temperature; Southern Biotech). Cells were washed three times with PBS containing BSA (1%), luminol (Pierce) was added, and luminescence was detected with a Fusion plate reader (Perkin Elmer/Packard). Triiodothyronine (T3) and IGF-1 were obtained from Calbiochem and were used at 3 and 100 nM, respectively.
Cell volume measurements.
Three-dimensional measurements of isolated NRVMs were conducted using a Coulter model Z2 instrument (Beckman), as previously described (54). Briefly, cells in six-well dishes (2.5 × 105/well) were washed one time with PBS and trypsinized (200 μl trypsin, 2 min at room temperature). DMEM containing 10% FBS (2 ml) was added to trypsinized cells, and cells were dispersed by vigorous pipetting. Cells were washed one time with PBS and transferred to cuvettes containing Isoflow buffer (10 ml; Coulter). Volume measurements were obtained for 104 cells, with Coulter aperture diameter limits between 6 and 26 μm. Cell volumes are expressed in cubic micrometers.
Toxicity measurements.
Cell viability was assessed with a LIVE/DEAD viability-cytotoxicity kit (Molecular Probes). This system employs calcein AM fluorescent dye, which is retained by viable cells, and an ethidium homodimer, which enters only cells with damaged membranes. Adenylate kinase (AK) was measured in culture supernatants by using the bioluminescence ToxiLight kit (Cambrex) according to the manufacturer's instructions. Changes in intracellular ADP/ATP ratios were measured with the bioluminescence ApoGlow BioAssay system (Cambrex), according to the manufacturer's instructions. AK and ATP assays were run in a 96-well format, and values were measured with a Fusion plate reader (Perkin Elmer/Packard).
RNA analysis.
NRVMs were plated on gelatin-coated 10-cm-diameter dishes (2 × 106 cells/dish). Following the treatments indicated in the figure legends, RNA was isolated from cardiomyocytes with Trizol reagent (Gibco/BRL). Total RNA (2 μg) was vacuum blotted onto nitrocellulose membranes (Bio-Rad) with a 96-well format dot blotter (Bio-Rad). Membranes were blocked in 4× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing sodium dodecyl sulfate (SDS; 1%), 5× Denhardt's reagent, sodium pyrophosphate (0.05%), and 100 μg of sonicated salmon sperm DNA per ml (4 h at 50°C) and incubated with 32P-end-labeled oligonucleotide probes (106 cpm/ml, 14 h at 50°C). Oligonucleotides were as follows: ANF (5′-AATGTGACCAAGCTGCGTGACACACCACAAGGGCTTAGGATCTTTTGCGATCTGCTCAAG-3), α-SK-actin (5′-TGGAGCAAAACAGAATGGCTGGCTTTAATGCTTCAAGTTTTCCATTTCCTTTCCACAGGG-3′), α-MHC (5′-CGAACGTTTATGTTTATTGTGGATTGGCCACAGCGAGGGTCTGCTGGAGAGG-3′), β-MHC (5′-GCTTTATTCTGCTTCCACCTAAAGGGCTGTTGCAAAGGCTCCAGGTCTGAGGGCTTC-3′), and GAPDH (5′-GGAACATGTAGACCATGTAGTTGAGGTCAATGAAG-3′). Blots were washed twice with 0.5× SSC containing SDS (0.1%, 10 min at 50°C) and analyzed by autoradiography.
Immunoblot and immunoprecipitation analyses.
Whole-cell protein extracts were prepared from NRVMs by using Tris buffer (50 mM, pH 7.5) containing EDTA (5 mM), Triton X-100 (1%), protease inhibitor cocktail (Complete; Roche), phenylmethylsulfonyl fluoride (1 mM), and phosphatase inhibitors (sodium pyrophosphate [1 mM], sodium fluoride [2 mM], β-glycerol phosphate [10 mM], sodium molybdate [1 mM], and sodium orthovanadate [1 mM]). Lysates were sonicated briefly and clarified by centrifugation. Protein concentrations were determined by Bradford assay (Bio-Rad), and 20 μg of total protein was resolved by SDS-polyacrylamide gel electrophoresis (PAGE) with gradient gels (4 to 20% polyacrylamide; Invitrogen). Proteins were transferred to nitrocellulose membranes (Bio-Rad) and immunoblotted with anti-SERCA2 antibody (mouse monoclonal antibody, 1:1,000 dilution; Affinity Bioreagents) or a peptide antibody directed against the carboxy terminus of murine modulatory calcineurin-interacting protein 1 (MCIP-1) (5) (rabbit polyclonal antibody, 1:5,000 dilution). After being washed with TBS-T, membranes were incubated with HRP-conjugated anti-mouse or anti-rabbit secondary antibodies (4050, 1:10,000 dilution; Southern Biotechnology), and proteins were visualized using an enhanced chemiluminescence system (Pierce). Blots were reprobed with anti-calnexin rabbit polyclonal antibody to control for protein loading (5). For immunoprecipitation, protein lysates were exposed to HDAC5-specific antiserum (39) and protein G Sepharose beads (Amersham Biosciences). Immunoprecipitates were washed five times with lysis buffer, resolved by SDS-PAGE, and immunoblotted with mouse monoclonal antibodies specific for either GFP (1:2,500 dilution; BD Biosciences) or 14-3-3 (H-8, 1:1,000 dilution; Santa Cruz).
RESULTS
CRM1- and 14-3-3-mediated nuclear export of HDAC 5 in cardiac myocytes.
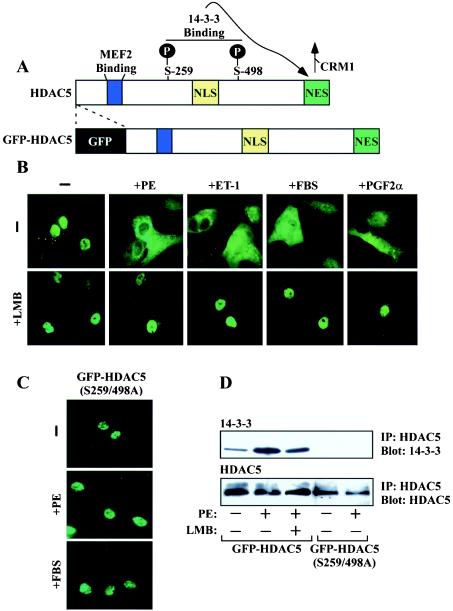
Class II HDACs associate with MEF2 via an 18-amino-acid motif that resembles the peptide-binding cleft of major histocompatibility complex (22). Upon phosphorylation of two conserved serines that flank the NLS, class II HDACs are bound by the 14-3-3 chaperone protein. Binding of 14-3-3 leads to exposure of a cryptic class II HDAC NES and subsequent CRM1-dependent nuclear export of the transcriptional repressors, thereby freeing MEF2 to activate downstream target genes (Fig. 1A).
FIG. 1.
Signal-dependent nuclear export of HDAC5 in cardiomyocytes. (A) HDAC5 harbors an 18-amino-acid MEF2 binding domain (blue), a nuclear localization signal rich in basic residues (yellow), and a carboxy-terminal NES that is required for CRM1-mediated nucleo-cytoplasmic shuttling (green). Upon phosphorylation at two serine residues (S259 and S498 in human HDAC5), HDAC5 is bound by the intracellular chaperone protein 14-3-3, which results in exposure of the otherwise cryptic NES and the consequent nuclear export of the transcriptional repressor. Sequences encoding GFP were fused in frame to the amino terminus of HDAC5. (B) NRVMs were infected with adenovirus encodingGFP-HDAC5. Cells were treated for 1 h with PE (20 μM), ET-1 (50 nM), FBS (10%), or prostaglandin F2α (PGF2α; 10 μM) in the absence or presence of LMB (18.5 nM), and live-cell images were captured with an inverted fluorescence microscope. (C) NRVMs were infected with adenovirus encoding GFP fused to HDAC5 containing alanines in place of the 14-3-3 target sites [GFP-HDAC5 (S259/498A)] and treated with the indicated agonists, as described for panel B. (D) NRVMs were infected with adenovirus encoding GFP-HDAC5 or GFP-HDAC5 (S259/498A) and cultured overnight. Cells were washed and cultured in serum-free medium for 4 h prior to stimulation with PE (20 μM) for 1 h. HDAC5 was immunoprecipitated (IP) from cell lysates with HDAC5-specific antibody, and associated, endogenous 14-3-3 was detected by immunoblotting (top panel). Blots were reprobed with anti-GFP antibody to reveal total amounts of immunoprecipitated, ectopic HDAC5 (bottom panel).
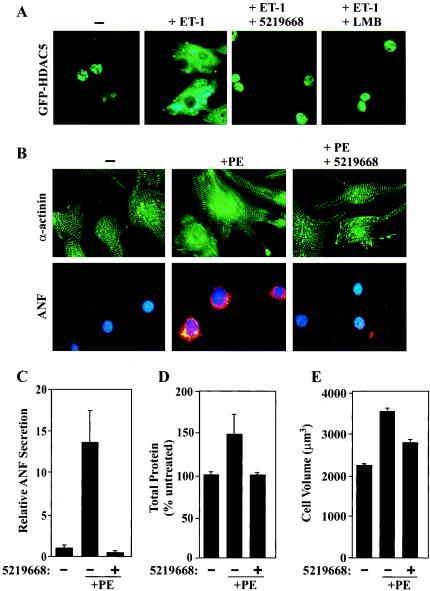
Whether or not this pathway for HDAC neutralization functions to control cardiac hypertrophy has remained unclear. To address this issue, we employed primary NRVMs, which provide a tractable in vitro model of cardiac hypertrophy (55, 56), and adenovirus encoding HDAC5 fused to GFP (Fig. 1A). As shown in Fig. 1B, GFP-HDAC5 was predominantly nuclear in serum-starved NRVMs. However, HDAC5 rapidly relocalized to the cytoplasm in response to four independent hypertrophic stimuli, the α1-adrenergic agonist PE, endothelin-1 (ET-1), FBS, and prostaglandin F2α. Agonist-dependent nuclear export of HDAC5 was abolished by a highly specific CRM1 antagonist, LMB (Fig. 1B, bottom panels), or by conversion of the 14-3-3 binding sites in HDAC5 to nonphosphorylatable alanine residues (Fig. 1C).
Experiments were performed to confirm that inhibition of HDAC5 nuclear export by LMB was a specific outcome of CRM1 antagonism, rather than a nonspecific effect on HDAC5-directed signaling event(s). Phosphorylation-dependent binding of 14-3-3 to HDAC5 was assessed by coimmunoprecipitation analysis. NRVMs were infected with GFP-HDAC5-encoding adenovirus and treated with PE in the absence or presence of LMB. HDAC5 was immunoprecipitated from cell lysates, and associated, endogenous 14-3-3 was detected by immunoblotting. As shown in Fig. 1D, PE stimulated the binding of 14-3-3 to the HDAC5 whose phosphorylation was dependent on Ser-259 and Ser-498. LMB had little effect on the phosphorylation-dependent binding of 14-3-3 to HDAC5, while a protein kinase C (PKC) inhibitor attenuated this association (data not shown), consistent with our prior results demonstrating a role for PKC and PKD in the control of cardiac HDAC5 phosphorylation (60). Furthermore, LMB did not alter the activation of PKC or PKD in response to PE (data not shown). Together, these findings suggest that LMB retains HDAC5 in the cardiomyocyte nucleus by blocking nuclear export per se and support the hypothesis that nucleo-cytoplasmic shuttling of HDAC5 is coupled to the cardiac hypertrophic growth program.
A CRM1 antagonist suppresses fetal cardiac gene induction.
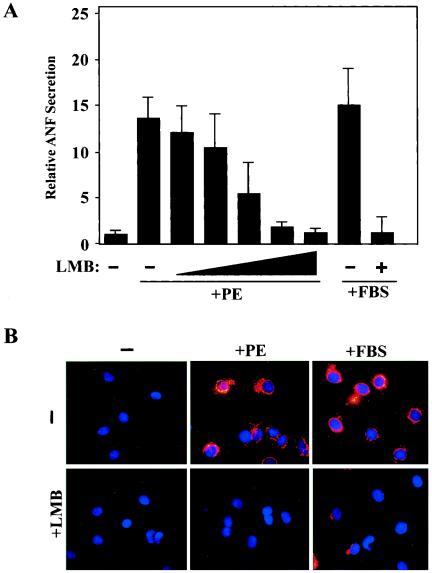
Stress signaling in cardiomyocytes results in upregulation of the pathological fetal gene program, which is normally silenced in the adult heart. The gene encoding secreted atrial natriuretic factor (ANF) is a prototypical component of this program that is potently stimulated in ventricular myocytes exposed to cues for pathological cardiac hypertrophy. To begin to investigate the role of CRM1-dependent nuclear export in cardiac hypertrophy, we measured ANF protein in culture supernatants from NRVMs treated with hypertrophic agonists in the absence or presence of LMB. As shown in Fig. 2A, ANF expression was dramatically induced in NRVMs treated with PE or FBS, both of which trigger profound hypertrophy. Remarkably, low nanomolar concentrations of LMB efficiently blocked ANF induction by these hypertrophic agonists.
FIG. 2.
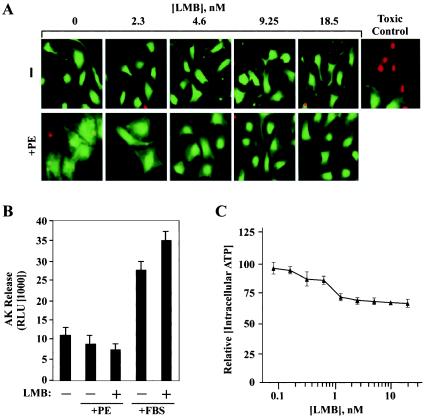
Effects of a CRM1 inhibitor on ANF expression in cardiomyocytes. NRVMs were treated for 48 h with PE (20 μM) or FBS (5%) in the absence or presence of LMB (2.3, 4.6, 9.25, or 18.5 nM). ELISA was employed to measure concentrations of secreted ANF in culture supernatants. ANF values are presented relative to those of untreated controls (set at 1) ± standard deviations from eight independent samples. (B) NRVMs were treated with PE (20 μM) or FBS (10%) in the absence or presence of LMB (18.5 nM), and ANF protein was detected by indirect immunofluorescence with anti-ANF antibodies (red). Nuclei were stained with Hoechst dye (blue). Agonist-induced perinuclear expression of ANF was inhibited by LMB (lower panels).
Agonist-dependent elevation of ANF expression can also be examined by immunostaining cardiomyocytes with ANF-specific antibodies. Following stimulation with PE or FBS, prominent ANF protein expression is observed within the secretory pathway, as evidenced by its perinuclear localization (Fig. 2B). Consistent with the reduction in ANF secretion mediated by LMB (Fig. 2A), ANF immunostaining was markedly reduced in the presence of this CRM1 inhibitor. LMB was moderately less effective at blocking ANF expression mediated by FBS relative to PE, perhaps owing to the presence of LMB binding proteins in serum. LMB also blocked ANF expression induced by prostaglandin F2α and ET-1, which trigger cardiac hypertrophy through distinct receptors (data not shown).
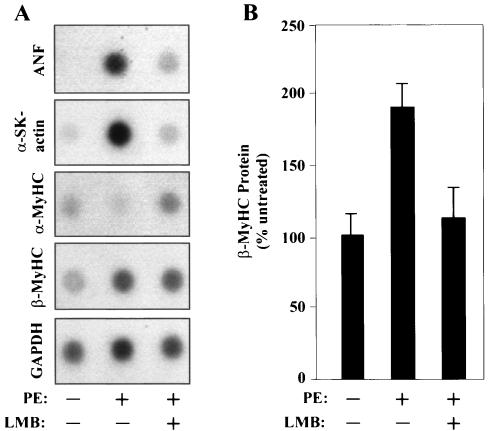
To rule out possible effects of LMB treatment on ANF protein stability, we examined mRNA levels for ANF and other fetal gene markers in cells treated with this CRM1 antagonist. As shown in Fig. 3A, LMB blocked the PE-mediated induction of ANF transcripts, as well as those for another classical fetal cardiac gene marker, alpha-skeletal actin.
FIG. 3.
A CRM1 inhibitor blocks the induction of the fetal cardiac gene program by PE. (A) NRVMs were treated for 48 h with PE (20 μM) in the absence or presence of LMB (18.5 nM), and the indicated transcripts were detected by RNA dot blot analysis. α-SK-actin, alpha-skeletal actin. (B) NRVMs were cultured for 48 h with LMB (18.5 nM) in the absence or presence of PE (20 μM). Levels of β-MyHC protein were measured by cytoblot analysis and are graphed as percentages of expression relative to the expression of untreated controls (set at 100%). Values are means ± standard deviations from eight independent samples.
Stress signaling in the heart enhances the expression of embryonic β-MyHC and reduces the expression of adult α-MyHC. Since β-MyHC has slower ATPase activity than α-MyHC, the net outcome of this isoform switch is impaired contractility. Antagonism of CRM1 with LMB effectively blocked MyHC isoform switching mediated by PE. LMB repressed PE-induced β-MyHC mRNA and protein expression (Fig. 3) and inhibited the downregulation of α-MyHC transcripts (Fig. 3A). LMB consistently reduced β-MyHC protein levels to a greater extent than mRNA levels. Together, the results suggest a role for CRM1-dependent nuclear export in the activation of fetal cardiac genes in response to cues for pathological hypertrophy.
LMB blocks morphological features of cardiac hypertrophy.
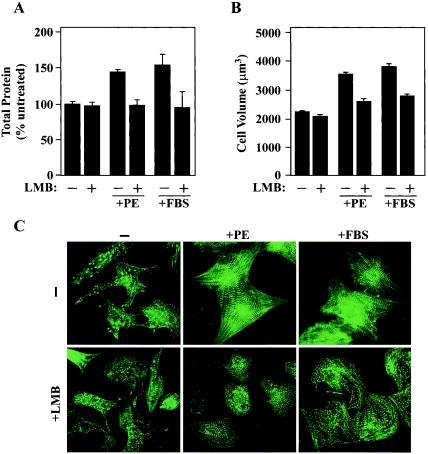
In addition to fetal gene induction, cardiac hypertrophy is associated with increased cell size due to enhanced protein synthesis and with elevated assembly and organization of sarcomeres. LMB was employed to assess the role of CRM1 in the regulation of these features of cardiac hypertrophy. Treatment of NRVMs for 48 h with PE or FBS resulted in 44%- and 54%-increased total cellular protein contents, which was effectively blocked by LMB (Fig. 4A). As shown in Fig. 4B, the reduction in protein synthesis mediated by LMB correlated with diminished cell size, as determined with a Coulter counter.
FIG. 4.
A CRM1 inhibitor blocks agonist-induced increases in cardiomyocyte size and sarcomere organization. (A) NRVMs were treated with PE (20 μM) or FBS (5%) for 48 h in the absence or presence of LMB (18.5 nM). Total cellular protein was quantified by the Bradford assay and is presented as a percentage of that in untreated cells. Values are averages from eight independent samples ± standard deviations. (B) NRVMs were treated as described for panel A and trypsinized for Coulter Counter analysis. Average cell volumes (in cubic micrometers) were determined for 104 cells. Error bars represent standard deviations of results from three independent experiments. (C) NRVMs were treated for 48 h with PE (20 μM) or FBS (5%) in the absence or presence of LMB (18.5 nM). Cells were fixed and stained for α-actinin to reveal sarcomeres by indirect immunofluorescence. LMB prevents the enhanced organization of the sarcomeres in response to hypertrophic agonists.
NRVMs sarcomeres were visualized by indirect immunofluorescence with an antibody directed to sarcomeric α-actinin. Treatment of cells with PE or FBS led to parallel assembly of highly ordered sarcomeres, while sarcomeres from unstimulated cells were disorganized (Fig. 4C). CRM1 inhibition rendered NRVMs unresponsive to the sarcomere-altering effects of the hypertrophic agonists.
LMB is nontoxic to cardiomyocytes.
It remained possible that cytotoxicity induced by LMB was being mistaken for antihypertrophic activity. To rule out this possibility, we examined whether LMB was toxic to cardiomyocytes by staining cells with calcein AM, which is retained by and fluoresces in viable cells, and ethidium bromide, which enters only cells with compromised membranes. As shown in Fig. 5A, LMB-treated cells retained calcein dye as efficiently as control cells and were not significantly stained by ethidium bromide.
FIG. 5.
CRM1 inhibition does not alter cardiomyocyte viability. (A) NRVMs were left untreated or stimulated with PE (20 μM) for 48 h in the absence or presence of the indicated concentrations of LMB. Cells were stained with calcein AM dye, which is retained by and fluoresces in viable cells (green), and ethidium bromide (red), which enters only cells with compromised membranes. (B) AK was detected in culture medium of NRVMs stimulated for 48 h with PE (20 μM) or FBS (5%) in the absence or presence of LMB (18.5 nM). Values represent the means ± standard deviations of results from eight independent samples. The higher values from FBS-treated cells are a consequence of the AK present in serum. (C) NRVMs were cultured in 96-well dishes and treated with the indicated concentrations of LMB for 48 h in the absence of hypertrophic stimulus. Intracellular ATP concentrations were determined for eight independent samples from each treatment group and are graphed relative to values for untreated cells (set at 100). Standard deviations are shown.
As an independent measure of toxicity, we assayed for release of AK into the culture medium to assess cell membrane integrity. As shown in Fig. 5B, LMB did not trigger AK release from NRVMs treated with PE or FBS. Of note, the higher values from FBS-treated cells were a consequence of exogenous AK present in serum. Finally, intracellular ATP concentrations were quantified to further assess possible effects of LMB on cardiomyocyte viability. Increasing concentrations of LMB led to a modest reduction in intracellular ATP (Fig. 5C), whereas a toxic control reduced cardiomyocyte ATP levels by ∼100-fold (data not shown). Of note, we have observed increased intracellular ATP concentrations in cells treated with hypertrophic agonists. Thus, given our findings with AK and calcein, we propose that the effect of LMB on intracellular ATP is a reflection of the antihypertrophic action of this compound. These results further support the notion that LMB blocks cardiac hypertrophy by impinging on cellular components that are required for the growth process and not by nonspecifically inducing cellular demise.
Antihypertrophic action of a newly identified CRM1 inhibitor.
To rule out the possibility that inhibition of cardiac hypertrophy by LMB was mediated independently of CRM1 repression, we employed a recently discovered small-molecule inhibitor of CRM1. This compound, termed 5219668, is structurally distinct from LMB and was identified during screening of a small molecule library (ChemBridge) for agents that block nuclear export of the FOXO1a transcription factor (29). Similar to LMB, 5219668 covalently modifies CRM1 and acts as a general inhibitor of CRM1-mediated nuclear export (see Discussion). As shown in Fig. 6A, 5219668 potently repressed agonist-mediated nuclear export of HDAC5. Consistent with this finding, 5219668 blocked PE-induced sarcomere assembly and ANF expression (Fig. 6B and C). In addition, 5219668 repressed the increases in total cellular protein and cell volume normally induced by PE (Fig. 6D and E). Of note, 5219668 was cytotoxic only at doses well above those required to block cardiac hypertrophy (data not shown). These findings further suggest that CRM1 activity is required for agonist-mediated pathological gene expression and cardiac hypertrophy.
FIG. 6.
A newly discovered CRM1 inhibitor blocks cardiac hypertrophy. (A) NRVMs were infected with adenovirus encoding GFP-HDAC5 and treated for 2 h with ET-1 in the absence or presence of 5219668 (1.25 μM; 5219668 is the ChemBridge Corporation catalog number) or LMB (18.5 nM). (B) NRVMs were treated with PE (20 μM) for 48 h in the absence or presence of 5219668 (1.25 μM). Cells were fixed and analyzed by indirect immunofluorescence to reveal sarcomeres (green, top panels) and ANF (red, bottom panels). Nuclei were stained with Hoechst dye (blue, bottom panels). (C) NRVMs were treated for 48 h with PE (20 μM) in the absence or presence of 5219668 (1.25 μM). ELISA was employed to measure concentrations of secreted ANF in culture supernatants. ANF values are presented relative to those of untreated cells (set at 1) and were derived from eight independent samples for each condition. Standard deviations are shown. (D) The Bradford assay was employed to quantifytotal cellular protein from cells analyzed for the results shown in panel C. Values were averaged for eight independent samples for each condition and are presented as percentages of levels in untreated cells (100%) ± standard deviations. (E) NRVMs were treated for 48 h with PE (20 μM) in the absence or presence of 5219668 (1.25 μM). Average cell volumes for 104 cells per condition were measured using a Coulter Counter. Error bars represent standard deviations from three independent experiments.
CRM1 inhibitors do not block the expression of α-MyHC and SERCA.
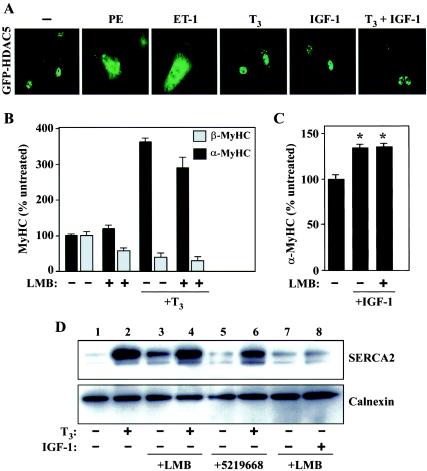
During pathological cardiac hypertrophy, the genes encoding α-MyHC and SERCA2 are repressed. Beneficial effects of exercise on cardiac function have been linked to upregulation of these genes and can be mimicked in vitro and in vivo by the bioactive form of thyroid hormone, T3, or by IGF-1. To begin to address the potential role of HDAC5 and CRM1 in the control of α-MyHC and SERCA levels, we determined the effects of T3 and IGF-1 on HDAC5 localization in cultured NRVMs. Unlike PE and ET-1, which rapidly trigger nuclear export of HDAC5 in cardiomyocytes, the subcellular distribution of HDAC5 was unaffected by treatment with T3 and/or IGF-1 (Fig. 7A).
FIG. 7.
Agonist-mediated induction of α-MyHC and SERCA expression are unaffected by CRM1 inhibition. (A) NRVMs were infected with adenovirus encoding GFP-HDAC5 and stimulated for 2 h with PE (20 μM), ET-1 (50 nM), T3 (3 nM), IGF-1 (100 nM), or T3 (3 nM) plus IGF-1 (100 nM). (B) NRVMs were treated for 48 h with T3 (3 nM) in the absence or presence of LMB (18.5 nM). Effects of LMB on T3-mediated regulation of α- and β-MyHC protein expression were assessed by cytoblot analysis. Average values were determined from at least eight independent wells per condition and are depicted relative to levels in untreated controls (set at 100%). Standard deviations are shown. (C) NRVMs were treated for 48 h with IGF-1 (100 nM) in the absence or presence of LMB (18.5 nM). Levels of α-MyHC protein expression were assessed by cytoblot analysis, as described for panel B. *, P < 0.0001 versus values for untreated cells. (D) NRVMs were cultured for 48 h with T3 (3 nM) or IGF-1 (100 nM) in the absence or presence of LMB (18.5 nM) or 5219668 (1.25 μM). Effects of LMB on T3- and IGF-1-induced expression of SERCA2 were determined by immunoblotting with anti-SERCA antibodies. The blot was reprobed with antibodies to the calnexin chaperone protein to control for protein loading.
On the basis of these findings, we hypothesized that agonist-mediated upregulation of α-MyHC and SERCA expression occurs in a CRM1-independent manner. Indeed, as shown in Fig. 7B, T3 potently stimulated α-MyHC expression while concomitantly repressing expression of embryonic β-MyHC. Remarkably, LMB had no effect on this coordinate regulation of MyHC expression in response to T3, nor did it alter the increase in α-MyHC expression mediated by IGF-1 (Fig. 7C). Likewise, CRM1 inhibition failed to alter T3-induced SERCA2 expression (Fig. 7D) and appeared to modestly stimulate SERCA expression in the absence of T3 (compare lanes 1, 3, and 5). These results suggest that CRM1 is selectively involved in the regulation of pathological cardiac gene expression.
LMB represses NFAT-dependent transcription.
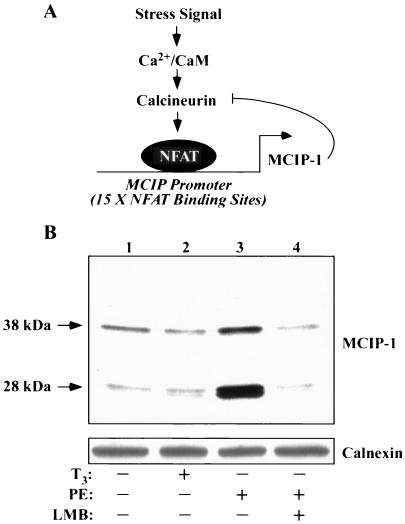
A recent study suggests a role for NFAT in the control of pathological, but not physiological, cardiac hypertrophy (61). We hypothesized that the selective antihypertrophic action of CRM1 inhibitors may in part be mediated through this transcription factor. To begin to address this possibility, we assessed potential effects of LMB on the expression of MCIP-1, also known as Down syndrome critical region 1, which is a negative-feedback regulator of calcineurin signaling (Fig. 8A) (59). MCIP-1 is expressed as 28- and 38-kDa isoforms (5). Expression of 28-kDa MCIP-1 is regulated by an alternative promoter that harbors 15 NFAT binding sites, and thus this lower-molecular-mass form of the protein serves as a sensitive marker of NFAT activity (64). As shown in Fig. 8B, PE stimulated the expression of NFAT-dependent 28-kDa MCIP-1, while T3 failed to alter MCIP levels. LMB completely blocked the agonist-mediated induction of MCIP-1 expression. These results suggest that CRM1 activity is required for stimulation of NFAT target gene expression. Consistent with this conclusion, we have found that LMB potently represses a luciferase reporter gene under the control of the MCIP promoter (data not shown).
FIG. 8.
Effects of a CRM1 inhibition on NFAT-dependent gene expression. (A) MCIP-1 functions in a negative-feedback loop to control calcineurin signaling. The Ca2+-calmodulin (CaM)-dependent phosphatase calcineurin is activated in response to stress signals that increase intracellular Ca2+. Calcineurin dephosphorylates NFAT, promoting its import into the nucleus. Calcineurin signaling stimulates the expression of a 28-kDa isoform of MCIP-1 by virtue of an alternative promoter harboring 15 NFAT binding sites. Newly expressed MCIP-1 protein binds to and negatively regulates calcineurin. (B) NRVMs were cultured in the absence or presence of LMB (18.5 nM), as indicated. Cells were stimulated with either T3 (3 nM) or PE (20 μM) for 48 h prior to preparation of protein lysates for immunoblot analysis with anti-MCIP1 antibodies. Induction of the calcineurin-responsive form of MCIP-1 (28 kDa) is blocked by LMB. Blots were reprobed with antibodies to the calnexin chaperone protein to control for protein loading (bottom panel).
DISCUSSION
The findings of this study reveal a novel role for the CRM1 nuclear export receptor in the control of pathological gene expression in postnatal cardiac myocytes. The findings place CRM1 antagonists on a short list of small molecules capable of repressing this gene program at the level of transcription. Although LMB is a potent and highly specific inhibitor of CRM1, toxic side effects have prohibited its use in the clinic to treat cancer (48). Nonetheless, the data presented here point to the potential utility of alternative therapeutic approaches that target nuclear export in the heart.
Mechanism of action and functional analogs of LMB.
LMB is a Streptomyces metabolite that covalently attaches to the sulfhydryl group of cysteine at position 529 in CRM1 and thereby prohibits CRM1 from associating with NES-containing cargo (32). Although modification of CRM1 by LMB is a highly efficient and selective process, the irreversibility of the reaction is pharmacologically undesirable and may have contributed to the extreme toxicity encountered during the clinical trial for cancer (48). A movement exists to identify novel CRM1 inhibitors that are less toxic and better suited for use as therapeutics (30). To date, this effort has focused on CRM1 antagonism in the treatment of patients with cancer or human immunodeficiency virus infection. However, our data highlight an unforeseen potential for such inhibitors as therapeutics for heart failure.
Recently, a synthetic small molecule, PKF050-638, and valtrate, from roots of the medicinal plant, Valerianae Radix, were identified as inhibitors of CRM1 function by virtue of their ability to block the nuclear export of the human immunodeficiency virus Rev protein (13, 46). Additionally, a screen for molecules that block nuclear export of the FOXO1a transcription factor yielded 19 novel CRM1 antagonists (29). The most potent CRM1 inhibitor of the 19 compounds was 5219668, which we show here functions as an efficient repressor of cardiac hypertrophy (Fig. 6). Remarkably, like LMB, each of these newly identified nuclear export inhibitors appears to function via covalent modification of the cysteine at position 529 in CRM1. As a result, extended efforts to identify novel, reversible CRM1 antagonists are warranted.
Selective repression of a subset of cardiac genes by CRM1 inhibitors.
A striking finding of the present study was that inhibition of CRM1 activity resulted in selective repression of pathological cardiac gene expression, while beneficial gene programs elicited by T3 and IGF-1 were spared. Perez-Terzic and coworkers reported global downregulation of nuclear import in cardiomyocytes undergoing pathological hypertrophy in response to PE and proposed this as a mechanism to accommodate increased demands for nuclear export of mRNA and protein during hypertrophy (51). Given these elegant findings, it was possible that CRM1 inhibition would result in the repression of growth-related cardiac genes in response not only to PE, but also to thyroid hormone and IGF-1, which are implicated in physiological cardiac growth (7, 14, 31, 42). Our results to the contrary suggest that LMB selectively represses pathological cardiac gene expression by blocking CRM1-mediated neutralization of a factor(s) that specifically controls this deleterious gene program. We propose that HDAC5 is one such factor, a hypothesis consistent with results demonstrating that HDAC5 is driven out of the nucleus in response to PE but not IGF-1 or T3 (Fig. 7A).
Targeting class II HDACs to control cardiac hypertrophy.
Signal-dependent nuclear export of class II HDACs, HDAC5 and HDAC9 in particular, appears to be a final common step required to unlock the fetal gene program in stressed myocardium. In support of this hypothesis, expression of signal-resistant mutants of either HDAC5 or HDAC9, which chronically reside in the nucleus, results in inhibition of agonist-mediated cardiomyocyte hypertrophy (5, 60, 65).
Class II HDACs likely contribute to the antihypertrophic action of LMB. By preventing nuclear export of these transcriptional repressors in the face of stress signals, LMB is predicted to promote nuclear MEF2-HDAC interactions and thus suppress genes under the control of MEF2 and other prohypertrophic transcription factors that associate with MEF2, including NFAT (Fig. 9). Of note, we have previously shown that 14-3-3-bound HDAC5 fails to efficiently associate with MEF2 (40). Thus, it is predicted that a portion of the LMB-trapped nuclear pool of HDAC5 remains unphosphorylated or becomes dephosphorylated despite the presence of active upstream signaling. Future chromatin immunoprecipitation experiments will test this hypothesis by addressing the ability of HDAC5 to engage promoter-bound MEF2 in LMB-treated cells.
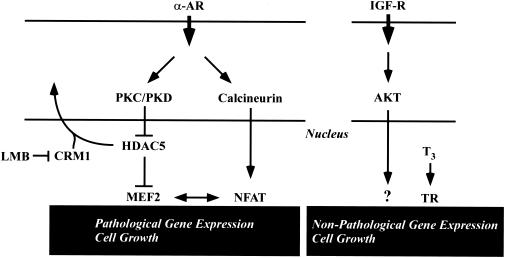
FIG. 9.
Model for repression of pathological cardiac gene expression by CRM1. Pathological cardiac hypertrophy can be triggered by a number of stress stimuli, including agonists that stimulate the α-adrenergic receptor (α-AR). Hypertrophic stimuli activate the Ca2+-calmodulin-dependent phosphatase calcineurin. Calcineurin dephosphorylates the NFAT transcription factor, driving it to the nucleus, where it stimulates pathological gene expression. Stress signals also stimulate the PKC/PKD pathway, resulting in phosphorylation of HDAC5, which normally binds to repress the function of the MEF2 transcription factor. Phospho-HDAC5 is escorted from the nucleus via a CRM1-dependent pathway, freeing MEF2 to activate downstream target genes that promote pathological hypertrophy. CRM1 inhibitors such as LMB retain HDAC5 in the nucleus, thereby repressing MEF2-dependent transcription. Since MEF2 interacts with NFAT, nuclear retention of HDAC5 is also predicted to suppress NFAT target gene expression. Binding of the bioactive form of thyroid hormone (T3) to its receptor (TR) triggers expression of genes that are associated with increased cardiac contractility and nonpathological cardiac growth, including α-MyHC and SERCA. Nonpathological cardiac gene expression and growth can also be triggered by signaling via IGF receptor (IGF-R) and its downstream effectors, including the AKT kinase. These pathways for cardiac gene expression function independently of CRM1.
Reversible inhibitors of CRM1 or small molecules that selectively repress nuclear export of class II HDACs should suppress pathological cardiac gene expression and may be better tolerated in vivo than LMB. Class II HDAC-directed small molecules may function either at the levels of HDAC kinases, such as PKC and PKD, as-yet-unidentified HDAC phosphatases, or at subsequent steps that are required for engagement of class II HDACs with nuclear export machinery. With regard to the latter point, class II HDACs harbor a conserved carboxy-terminal NES (consensus sequence, Val-X-X-X-X- X-Leu-X-Val) that diverges from classical CRM1 docking domains (consensus sequence, Leu-X-X-X-Leu-X-X-Leu-X-Leu), raising the possibility of selective disruption of class II HDAC-CRM1 interactions (41).
Non-HDAC targets of CRM1.
A plethora of protein targets for CRM1 have been identified, and CRM1 has also been implicated in the control of RNA transport (57). Thus, it is likely that the antihypertrophic activity of LMB is governed by factors in addition to class II HDACs. In this regard, recent studies have revealed the existence of complex counterregulatory pathways that repress cardiac hypertrophic growth. Components of these antihypertrophic networks include the genes encoding glycogen-synthase kinase 3β (1, 23), A20 (11), phospholipase A2 (24), cyclic-GMP-dependent protein kinase (15, 26, 62), and c-Jun N-terminal kinase (36, 53). Notably, GSK-3β, phospholipase A2 and cyclic-GMP-dependent protein kinase have all been shown to shuttle between the nucleus and the cytoplasm (18, 20, 23). Thus, it is conceivable that LMB-mediated nuclear retention of these signaling molecules contributes to the repression of pathological cardiac gene expression.
As mentioned previously, prohypertrophic transcription factors, including NFAT and GATA4, are negatively regulated by CRM1-dependent nuclear export. As such, it was possible that CRM1 inhibition in cardiac myocytes would result in spontaneous or enhanced agonist-dependent cardiac hypertrophy due to increased nuclear expression of these transcription factors. However, our data suggest that LMB blocks the nuclear export of negative regulators of cardiac hypertrophy that function dominantly over these prohypertrophic factors. We propose that one such dominantly acting factor is HDAC5, a hypothesis that is consistent with the ability of the constitutively nuclear form of this chromatin-modifying enzyme to block hypertrophy in response to multiple pathological cues (5, 60, 65).
Conclusions.
The results presented here suggest that HDAC5 lies at a nodal point in cardiac myocytes, serving to couple multiple signaling pathways for hypertrophy to a common pathological gene program. The data also suggest that CRM1-mediated nuclear export of HDAC5 and as-yet-unidentified negative regulators is specifically involved in the control of pathological cardiac gene expression but not the expression of genes associated with beneficial cardiac performance, including α-MyHC and SERCA. Thus, it is intriguing to speculate that novel inhibitors of nuclear export with pharmacological properties more desirable than LMB will provide benefit in the context of cardiovascular disease. Expanded high-throughput screening efforts and follow-up testing in animal models of cardiac hypertrophy and heart failure should shed light on this issue.
Acknowledgments
We thank R. Plichta, L. Hollingsworth, and E. Isakson for cardiomyocyte preparation, M. Drietz for assistance with the Coulter Counter measurements, N. Pagratis and P. Papst for advice, and E. Olson and R. Gorczynski for critical reading of the manuscript.
REFERENCES
- 1.Antos, C. L., T. A. McKinsey, N. Frey, W. Kutschke, J. McAnally, J. M. Shelton, J. A. Richardson, J. A. Hill, and E. N. Olson. 2002. Activated glycogen synthase kinase-3β suppresses cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 99:907-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beals, C. R., N. A. Clipstone, S. N. Ho, and G. R. Crabtree. 1997. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev. 11:824-834. [DOI] [PubMed] [Google Scholar]
- 3.Becker, T. C., R. J. Noel, W. S. Coats, A. M. Gomez-Foix, T. Alam, R. D. Gerard, and C. B. Newgard. 1994. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 43:161-189. [DOI] [PubMed] [Google Scholar]
- 4.Braunwald, E., and M. R. Bristow. 2000. Congestive heart failure: fifty years of progress. Circulation 102:IV14-IV23. [DOI] [PubMed] [Google Scholar]
- 5.Bush, E., J. Fielitz, L. Melvin, M. Martinez-Arnold, T. A. McKinsey, R. Plichta, and E. N. Olson. 2004. A small molecular activator of cardiac hypertrophy uncovered in a chemical screen for modifiers of the calcineurin signaling pathway. Proc. Natl. Acad. Sci. USA 101:2870-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camoretti-Mercado, B., H. W. Liu, A. J. Halayko, S. M. Forsythe, J. W. Kyle, B. Li, Y. Fu, J. McConville, P. Kogut, J. E. Vieira, N. M. Patel, M. B. Hershenson, E. Fuchs, S. Sinha, J. M. Miano, M. S. Parmacek, J. K. Burkhardt, and J. Solway. 2000. Physiological control of smooth muscle-specific gene expression through regulated nuclear translocation of serum response factor J. Biol. Chem. 275:30387-30393. [DOI] [PubMed] [Google Scholar]
- 7.Chang, K. C., V. M. Figueredo, J. H. Schreur, K. Kariya, M. W. Weiner, P. C. Simpson, and S. A. Camacho. 1997. Thyroid hormone improves function and Ca2+ handling in pressure overload hypertrophy. Association with increased sarcoplasmic reticulum Ca2+-ATPase and alpha-myosin heavy chain in rat hearts. J. Clin. Investig. 100:1742-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, S., T. A. McKinsey, C. L. Zhang, J. A. Richardson, J. Hill, and E. N. Olson. 2004. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol. Cell. Biol. 24:8467-8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, L. F., W. Fischle, E. Verdin, and W. C. Greene. 2001. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 293:1653-1657. [DOI] [PubMed] [Google Scholar]
- 10.Colan, S. D. 1997. Mechanics of left ventricular systolic and diastolic function in physiologic hypertrophy of the athlete's heart. Cardiol. Clin. 15:355-372. [DOI] [PubMed] [Google Scholar]
- 11.Cook, S. A., M. S. Novikov, Y. Ahn, T. Matsui, and A. Rosenzweig. 2003. A20 is dynamically regulated in the heart and inhibits the hypertrophic response. Circulation 108:664-667. [DOI] [PubMed] [Google Scholar]
- 12.Cyert, M. S. 2001. Regulation of nuclear localization during signaling. J. Biol. Chem. 276:20805-20808. [DOI] [PubMed] [Google Scholar]
- 13.Daelemans, D., E. Afonina, J. Nilsson, G. Werner, J. Kjems, E. De Clercq, G. N. Pavlakis, and A. M. Vandamme. 2002. A synthetic HIV-1 Rev inhibitor interfering with the CRM1-mediated nuclear export. Proc. Natl. Acad. Sci. USA 99:14440-14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delaughter, M. C., G. E. Taffet, M. L. Fiorotto, M. L. Entman, and R. J. Schwartz. 1999. Local insulin-like growth factor I expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice. FASEB J. 13:1923-1929. [DOI] [PubMed] [Google Scholar]
- 15.Fiedler, B., S. M. Lohmann, A. Smolenski, S. Linnemuller, B. Pieske, F. Schroder, J. D. Molkentin, H. Drexler, and K. C. Wollert. 2002. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc. Natl. Acad. Sci. USA 99:11363-11368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 17.Frey, N., and E. N. Olson. 2003. Cardiac hypertrophy: the good, the bad, and the ugly. Annu. Rev. Physiol. 65:45-79. [DOI] [PubMed] [Google Scholar]
- 18.Grewal, S., E. E. Morrison, S. Ponnambalam, and J. H. Walker. 2002. Nuclear localisation of cytosolic phospholipase A2-alpha in the EA.hy. 926 human endothelial cell line is proliferation dependent and modulated by phosphorylation. J. Cell Sci. 115:4533-4543. [DOI] [PubMed] [Google Scholar]
- 19.Grozinger, C. M., and S. L. Schreiber. 2000. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc. Natl. Acad. Sci. USA 97:7835-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gudi, T., S. M. Lohmann, and R. B. Pilz. 1997. Regulation of gene expression by cyclic GMP-dependent protein kinase requires nuclear translocation of the kinase: identification of a nuclear localization signal. Mol. Cell. Biol. 17:5244-5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gusterson, R. J., E. Jazrawi, I. M. Adcock, and D. S. Latchman. 2003. The transcriptional co-activators CREB-binding protein (CBP) and p300 play a critical role in cardiac hypertrophy that is dependent on their histone acetyltransferase activity. J. Biol. Chem. 278:6838-6847. [DOI] [PubMed] [Google Scholar]
- 22.Han, A., F. Pan, J. C. Stroud, H. D. Youn, J. O. Liu, and L. Chen. 2003. Sequence-specific recruitment of transcriptional co-repressor Cabin1 by myocyte enhancer factor-2. Nature 422:730-734. [DOI] [PubMed] [Google Scholar]
- 23.Haq, S., G. Choukroun, Z. B. Kang, H. Ranu, T. Matsui, A. Rosenzweig, J. D. Molkentin, A. Alessandrini, J. Woodgett, R. Hajjar, A. Michael, and T. Force. 2000. Glycogen synthase kinase-3β is a negative regulator of cardiomyocyte hypertrophy. J. Cell Biol. 151:117-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haq, S., H. Kilter, A. Michael, J. Tao, E. O'Leary, X. M. Sun, B. Walters, K. Bhattacharya, X. Chen, L. Cui, M. Andreucci, A. Rosenzweig, J. L. Guerrero, R. Patten, R. Liao, J. Molkentin, M. Picard, J. V. Bonventre, and T. Force. 2003. Deletion of cytosolic phospholipase A2 promotes striated muscle growth. Nat. Med. 9:944-951. [DOI] [PubMed] [Google Scholar]
- 25.Holaska, J. M., B. E. Black, D. C. Love, J. A. Hanover, J. Leszyk, and B. M. Paschal. 2001. Calreticulin is a receptor for nuclear export. J. Cell Biol. 152:127-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holtwick, R., M. van Eickels, B. V. Skryabin, H. A. Baba, A. Bubikat, F. Begrow, M. D. Schneider, D. L. Garbers, and M. Kuhn. 2003. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J. Clin. Investig. 111:1399-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jans, D. A., C. Y. Xiao, and M. H. Lam. 2000. Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays 22:532-544. [DOI] [PubMed] [Google Scholar]
- 28.Kang, P. M., P. Yue, Z. Liu, O. Tarnavski, N. Bodyak, and S. Izumo. 2004. Alterations in apoptosis regulatory factors during hypertrophy and heart failure. Am. J. Physiol. Heart Circ. Physiol. 287:H72-H80. [DOI] [PubMed] [Google Scholar]
- 29.Kau, T. R., F. Schroeder, S. Ramaswamy, C. L. Wojciechowski, J. J. Zhao, T. M. Roberts, J. Clardy, W. R. Sellers, and P. A. Silver. 2003. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell 4:463-476. [DOI] [PubMed] [Google Scholar]
- 30.Kau, T. R., and P. A. Silver. 2003. Nuclear transport as a target for cell growth. Drug Discov. Today 8:78-85. [DOI] [PubMed] [Google Scholar]
- 31.Kinugawa, K., K. Yonekura, R. C. Ribeiro, Y. Eto, T. Aoyagi, J. D. Baxter, S. A. Camacho, M. R. Bristow, C. S. Long, and P. C. Simpson. 2001. Regulation of thyroid hormone receptor isoforms in physiological and pathological cardiac hypertrophy. Circ. Res. 89:591-598. [DOI] [PubMed] [Google Scholar]
- 32.Kudo, N., N. Matsumori, H. Taoka, D. Fujiwara, E. P. Schreiner, B. Wolff, M. Yoshida, and S. Horinouchi. 1999. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 96:9112-9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kutay, U., F. R. Bischoff, S. Kostka, R. Kraft, and D. Gorlich. 1997. Export of importin alpha from the nucleus is mediated by a specific nuclear transport factor. Cell 90:1061-1071. [DOI] [PubMed] [Google Scholar]
- 34.Li, B., M. Setoguchi, X. Wang, A. M. Andreoli, A. Leri, A. Malhotra, J. Kajstura, and P. Anversa. 1999. Insulin-like growth factor-1 attenuates the detrimental impact of nonocclusive coronary artery constriction on the heart. Circ. Res. 84:1007-1019. [DOI] [PubMed] [Google Scholar]
- 35.Liang, Q., and J. D. Molkentin. 2002. Divergent signaling pathways converge on GATA4 to regulate cardiac hypertrophic gene expression. J. Mol. Cell. Cardiol. 34:611-616. [DOI] [PubMed] [Google Scholar]
- 36.Liang, Q., O. F. Bueno, B. J. Wilkins, C. Y. Kuan, Y. Xia, and J. D. Molkentin. 2003. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J. 22:5079-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacLellan, W. R., and M. D. Schneider. 2000. Genetic dissection of cardiac growth control pathways. Annu. Rev. Physiol. 62:289-319. [DOI] [PubMed] [Google Scholar]
- 38.McKinsey, T. A., and E. N. Olson. 2004. Cardiac histone acetylation—therapeutic opportunities abound. Trends Genet. 20:206-213. [DOI] [PubMed] [Google Scholar]
- 39.McKinsey, T. A., C. L. Zhang, J. Lu, and E. N. Olson. 2000. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408:106-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKinsey, T. A., C. L. Zhang, and E. N. Olson. 2000. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc. Natl. Acad. Sci. USA 97:14400-14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKinsey, T. A., C. L. Zhang, and E. N. Olson. 2001. Identification of a signal-responsive nuclear export sequence in class II histone deacetylases Mol. Cell. Biol. 21:6312-6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMullen, J. R., T. Shioi, W. Y. Huang, L. Zhang, O. Tarnavski, E. Bisping, M. Schinke, S. Kong, M. C. Sherwood, J. Brown, L. Riggi, P. M. Kang, and S. Izumo. 2004. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J. Biol. Chem. 279:4782-4793. [DOI] [PubMed] [Google Scholar]
- 43.Mercadier, J. J., A. M. Lompre, C. Wisnewsky, J. L. Samuel, J. Bercovici, B. Swynghedauw, and K. Schwartz. 1981. Myosin isoenzyme changes in several models of rat cardiac hypertrophy. Circ. Res. 49:525-532. [DOI] [PubMed] [Google Scholar]
- 44.Molkentin, J. D., J. R. Lu, C. L. Antos, B. Markham, J. Richardson, J. Robbins, S. R. Grant, and E. N. Olson. 1998. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morisco, C., K. Seta, S. E. Hardt, Y. Lee, S. F. Vatner, and J. Sadoshima. 2001. Glycogen synthase kinase 3beta regulates GATA4 in cardiac myocytes. J. Biol. Chem. 276:28586-28597. [DOI] [PubMed] [Google Scholar]
- 46.Murakami, N., Y. Ye, M. Kawanishi, S. Aoki, N. Kudo, M. Yoshida, E. E. Nakayama, T. Shioda, and M. Kobayashi. 2002. New Rev-transport inhibitor with anti-HIV activity from Valerianae Radix. Bioorg. Med. Chem. Lett. 12:2807-2810. [DOI] [PubMed] [Google Scholar]
- 47.Naya, F. J., C. Wu, J. A. Richardson, P. Overbeek, and E. N. Olson. 1999. Transcriptional activity of MEF2 during mouse embryogenesis monitored with a MEF2-dependent transgene. Development 126:2045-2052. [DOI] [PubMed] [Google Scholar]
- 48.Newlands, E. S., G. J. Rustin, and M. H. Brampton. 1996. Phase I trial of elactocin. Br. J. Cancer 74:648-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orenstein, T. L., T. G. Parker, J. W. Butany, J. M. Goodmanm, F. Dawood, W. H. Wen, L. Wee, T. Martino, P. R. McLaughlin, and P. P. Liu. 1995. Favorable left ventricular remodeling following large myocardial infarction by exercise training. Effect on ventricular morphology and gene expression. J. Clin. Investig. 96:858-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Passier, R., H. Zeng, N. Frey, F. J. Naya, R. L. Nicol, T. A. McKinsey, P. Overbeek, J. A. Richardson, S. R. Grant, and E. N. Olson. 2000. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J. Clin. Investig. 105:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez-Terzic, C., A. M. Gacy, R. Bortolon, P. P. Dzeja, M. Puceat, M. Jaconi, F. G. Prendergast, and A. Terzic. 2001. Directed inhibition of nuclear import in cellular hypertrophy J. Biol. Chem. 276:20566-20571. [DOI] [PubMed] [Google Scholar]
- 52.Qi, M., T. R. Shannon, D. E. Euler, D. M. Bers, and A. M. Samarel. 1997. Downregulation of sarcoplasmic reticulum Ca(2+)-ATPase during progression of left ventricular hypertrophy. Am. J. Physiol. 272:H2416-H2424. [DOI] [PubMed] [Google Scholar]
- 53.Sadoshima, J., O. Montagne, Q. Wang, G. Yang, J. Warden, J. Liu, G. Takagi, V. Karoor, C. Hong, G. L. Johnson, D. E. Vatner, and S. F. Vatner. 2002. The MEKK1-JNK pathway plays a protective role in pressure overload but does not mediate cardiac hypertrophy. J. Clin. Investig. 110:271-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Said, S., T. Tamura, and A. M. Gerdes. 1998. Measurement of isolated myocyte volume using the Coulter models Z2 and ZM/C256: a comparison of instrument function. BioTechniques 25:522-525. [DOI] [PubMed] [Google Scholar]
- 55.Simpson, P., A. McGrath, and S. Savion. 1982. Myocyte hypertrophy in neonatal rat heart cultures and its regulation by serum and by catecholamines. Circ. Res. 51:787-801. [DOI] [PubMed] [Google Scholar]
- 56.Simpson, P., and S. Savion. 1982. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ. Res. 50:101-116. [DOI] [PubMed] [Google Scholar]
- 57.Stutz, F., and M. Rosbash. 1998. Nuclear RNA export. Genes Dev. 12:3303-3319. [DOI] [PubMed] [Google Scholar]
- 58.Tate, C. A., T. Helgason, M. F. Hyek, R. P. McBride, M. Chen, M. A. Richardson, and G. E. Taffet. 1996. SERCA2a and mitochondrial cytochrome oxidase expression are increased in hearts of exercise-trained old rats. Am. J. Physiol. Heart Circ. Physiol. 271:H68-H72. [DOI] [PubMed] [Google Scholar]
- 59.Vega, R. B., R. Bassel-Duby, and E. N. Olson. 2003. Control of cardiac growth and function by calcineurin signaling. J. Biol. Chem. 278:36981-36984. [DOI] [PubMed] [Google Scholar]
- 60.Vega, R. B., B. C. Harrison, E. Meadows, C. R. Roberts, P. J. Papst, E. N. Olson, and T. A. McKinsey. 2004. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol. Cell. Biol. 24:8374-8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilkins, B. J., Y. S. Dai, O. F. Bueno, S. A. Parsons, J. Xu, D. M. Plank, F. Jones, T. R. Kimball, and J. D. Molkentin. 2004. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ. Res. 94:110-118. [DOI] [PubMed] [Google Scholar]
- 62.Wollert, K. C., B. Fiedler, S. Gambaryan, A. Smolenski, J. Heineke, E. Butt, C. Trautwein, S. M. Lohmann, and H. Drexler. 2002. Gene transfer of cGMP-dependent protein kinase I enhances the antihypertrophic effects of nitric oxide in cardiomyocytes. Hypertension 39:87-92. [DOI] [PubMed] [Google Scholar]
- 63.Yanazume, T., K. Hasegawa, T. Morimoto, T. Kawamura, H. Wada, A. Matsumori, Y. Kawase, M. Hirai, and T. Kita. 2003. Cardiac p300 is involved in myocyte growth with decompensated heart failure. Mol. Cell. Biol. 23:3593-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang, J., B. Rothermel, R. B. Vega, N. Frey, T. A. McKinsey, E. N. Olson, R. Bassel-Duby, and R. S. Williams. 2000. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ. Res. 87:E61-E68. [DOI] [PubMed] [Google Scholar]
- 65.Zhang, C. L., T. A. McKinsey, S. Chang, C. L. Antos, J. A. Hill, and E. N. Olson. 2002. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110:479-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang, X., G. Azhar, J. Chai, P. Sheridan, K. Nagano, T. Brown, J. Yang, K. Khrapko, A. M. Borras, J. Lawitts, R. P. Misra, and J. Y. Wei. 2001. Cardiomyopathy in transgenic mice with cardiac-specific overexpression of serum response factor. Am. J. Physiol. Heart Circ. Physiol. 280:H1782-H1792. [DOI] [PubMed] [Google Scholar]
- 67.Zhu, J., and F. McKeon. 1999. NF-AT activation requires suppression of Crm1 dependent export by calcineurin. Nature 398:256-260. [DOI] [PubMed] [Google Scholar]