Abstract
Background
Regulatory T (Treg) cells, a subset of CD4+ T lymphocytes, are mediators of immunosuppression in cancer, and, thus, variants in genes encoding Treg cell immune molecules could be associated with ovarian cancer.
Methods
In a population of 15,596 epithelial ovarian cancer (EOC) cases and 23,236 controls, we measured genetic associations of 1,351 SNPs in Treg cell pathway genes with odds of ovarian cancer and tested pathway and gene-level associations, overall and by histotype, for the 25 genes, using the admixture likelihood (AML) method. The most significant single SNP associations were tested for correlation with expression levels in 44 ovarian cancer patients.
Results
The most significant global associations for all genes in the pathway were seen in endometrioid (p = 0.082) and clear cell (p = 0.083), with the most significant gene level association seen with (p = 0.001) and clear cell EOC. Gene associations with histotypes at< 0.05 included:(p = 0.005 and = 0.008, serous and high-grade serous, respectively), (p = 0.035, endometrioid and mucinous), (p = 0.03, mucinous), (p = 0.022, clear cell), (p = 0.021 endometrioid) and (p = 0.017 and = 0.025, endometrioid and mucinous, respectively).
Conclusions
Common inherited gene variation in Treg cell pathways shows some evidence of germline genetic contribution to odds of EOC that varies by histologic subtype and may be associated with mRNA expression of immune-complex receptor in EOC patients.
Keywords: ovarian cancer, immunosuppression, biomarkers, genetic variation, TGFBR2
INTRODUCTION
Ovarian cancer is the leading cause of death due to gynecological cancers in the United States [1]. Although two-thirds of ovarian cancer patients initially respond to surgical debulking and chemotherapy [2], a majority eventually relapse [3, 4]. The five-year survival rate of ovarian cancer varies significantly across clinical stages, with almost 90% of stage I patients surviving, to just a little over 20% of advanced-stage patients surviving [5].
In recent years, host tumor immunosuppression has attracted research in ovarian cancer in hopes of identifying underlying biological mechanisms that determine the development and progression of ovarian cancer. Ovarian tumors have been found to induce migration of immunosuppressive cells into tumor tissue [6]. Thus, exploring molecular pathways underlying suppression of immune responses in ovarian cancer to identify novel targets for immunotherapy and/or to identify markers that can predict the risk of ovarian cancer may be a route to both treating this deadly disease and/or earlier identification.
An important pathway to consider in immune function is suppression of host immune response by regulatory T (Treg) cells, a subset of CD4+ T cells that maintain immune tolerance and inhibit the development of an antitumor immune response. In fact, higher prevalence of Treg cells has been found in various cancers [7–12], including ovarian cancer [13–16], compared to controls. Treg cells have been detected in ovarian tumors [15], as well as in malignant ascites [13] and peripheral blood [16] of ovarian cancer patients. Further, an association of ovarian cancer outcomes with genetic variation in Treg-related genes specific to induction, trafficking, or immunosuppressive function of Treg cells, also suggests a role for the Treg cell phenotype in ovarian cancer [17]. Given the importance of inherited factors in both ovarian cancer and Treg cells, we sought to characterize their role in ovarian cancer etiology. We conducted a comprehensive epidemiological study in which we investigated the significance of single nucleotide polymorphisms (SNPs) in the Treg cell pathway and mRNA expression profiles in epithelial ovarian cancer (EOC) etiology.
RESULTS
The descriptive characteristics of the study population are presented in Table 1. The majority of EOC patients (n = 9,330) were of the serous histology. Compared to controls, cases were significantly older and more likely to report a family history of breast or ovarian cancer and a personal history of endometriosis. Conversely, pregnancy, tubal ligation, breastfeeding, and use of oral contraceptives (OCs) were more likely to be reported by controls.
Table 1. Descriptive characteristics of 15,596 ovarian cancer cases and 23,236 controls from the Ovarian Cancer Association Consortium (OCAC).
| Variable | Case N = 15596 | Control N = 23236 | P value |
|---|---|---|---|
| Age1 | 57.36 (11.70) | 55.61 (11.90) | <0.0001 |
| Ethnicity2 | |||
| Non-Hispanic | 13847 (99.6) | 21539 (99.7) | 0.03 |
| Hispanic | 56 (.4) | 59 (.3) | |
| Missing | 1693 | 1638 | |
| Family history of ovarian cancer2 | |||
| No | 5891 (91.6) | 7643 (95.7) | <0.0001 |
| Yes | 543 (8.4) | 343 (4.3) | |
| Missing | 9162 | 15250 | |
| Height1 | 1.64 (0.07) | 1.63 (0.06) | <0.0001 |
| Missing | 4571 | 6596 | |
| Weight1 | 57.2 (9.80) | 56.4 (8.71) | |
| Missing | 6600 | 9277 | <0.0001 |
| Body Mass Index (BMI)1 | 21.29 (3.43) | 21.18 (3.06) | 0.01 |
| Missing | 6642 | 9311 | |
| Age at menarche1 | 12.8 (1.60) | 12.9 (1.68) | 0.02 |
| Missing | 4914 | 7195 | |
| Total number of pregnancies1 | 2.37(1.80) | 2.63(1.74) | <0.0001 |
| Missing | 4879 | 7066 | |
| Breast feeding2 | |||
| No | 3070 (40.5) | 4078 (30.2) | <0.0001 |
| Yes | 4502 (59.5) | 9426 (69.8) | |
| Missing | 8024 | 9732 | |
| Menopausal status2 | |||
| Pre/perimenopausal | 3585 (32.4) | 4519 (28) | <0.0001 |
| Post-menopausal | 7491 (67.6) | 11640 (72.0) | |
| Missing | 4520 | 7077 | |
| HRT2 | |||
| No | 2675 (44.3) | 3237 (44.6) | 0.73 |
| Yes | 3366 (55.7) | 4025 (55.4) | |
| Missing | 9555 | 15974 | |
| OC use2 | |||
| Never | 4465 (41.9) | 6054 (37.4) | <0.0001 |
| Ever | 6191 (58.1) | 10152 (62.6) | |
| Missing | 4940 | 7030 | |
| OC use in months1 | 38.21 (59.83) | 49.40(69.27) | <0.0001 |
| Missing | 5164 | 7209 | |
| Tubal ligation2 | |||
| No | 8420 (84.4) | 8278 (76.7) | <0.0001 |
| Yes | 1562 (15.7) | 2514 (23.3) | |
| Missing | 5614 | 12444 | |
| Endometriosis2 | |||
| No | 7435 (90.8) | 10030 (93.2) | <0.0001 |
| Yes | 755 (9.2) | 731 (6.8) | |
| Missing | 7406 | 12475 | |
| Hysterectomy2 | |||
| No | 7352 (68.3) | 13103 (81.2) | <0.0001 |
| Yes | 3413 (31.7) | 3025 (18.8) | |
| Missing | 4831 | 7108 | |
| Clinical characteristics Histology2 | |||
| Serous | 9330 (59.8) | ||
| Mucinous | 1592 (10.2) | ||
| Endometrioid | 2099 (13.5) | ||
| Clear cell | 1033 (6.6) | ||
| Mixed Cell | 505 (3.2) | ||
| Other | 1037 (6.7) | ||
| Behavior2 | |||
| LMP | 1724 (11.1) | ||
| Invasive | 13872 (88.9) | ||
| FIGO stage2 | |||
| 1 | 3488 (31.7) | ||
| 2 | 1147 (10.4) | ||
| 3 | 5412 (49.2) | ||
| 4 | 954 (8.7) | ||
| Grade2 | |||
| Well differentiated | 1240 (12.5) | ||
| Moderately differentiated | 2427 (24.4) | ||
| Poorly differentiated | 5591 (56.2) | ||
| Undifferentiated | 699 (7.0) | ||
| Missing | 5639 | ||
Mean (standard deviation),
N(%), CI = Confidence interval, BMI = body mass index, HRT = hormone replacement therapy, OC = oral contraceptive, LMP = low malignant potential
Association of genetic variation by histotype
P-values for the gene burden test for each gene in the pathway and the Treg cell pathway (all SNPs analyzed together) by histotype (serous, high-grade serous, endometrioid, clear cell, invasive mucinous) are presented in Table 2. The most significant burden test (p = 0.001) was seen with TGFBR2 and clear cell EOC. Other gene associations with histotypes at p < 0.05 included: IL12B (p = 0.005 and p = 0.008, serous and high-grade serous, respectively), IL8RA (p = 0.035, endometrioid and invasive mucinous), LGALS1 (p = 0.03, invasive mucinous), STAT5B (p = 0.022, clear cell), TGFBR1 (p = 0.021, endometrioid) and TGFBR2 (p = 0.017 and p = 0.025, endometrioid and invasive mucinous, respectively). The most significant global associations for all genes in the Treg cell pathway were seen in endometrioid (p = 0.082) and clear cell (p = 0.083) EOC.
Table 2. Admixture maximum likelihood gene burden p-values for each gene in the Treg cell pathway and overall considering all genes.
| Gene | Serous (n = 9,330) | High-grade serous (n = 5,792) | Endometrioid (n = 2,060) | Clear cell (n = 1,021) | Invasive Mucinous (n = 933) |
|---|---|---|---|---|---|
| CTLA4 | 0.612 | 0.984 | 0.337 | 0.471 | 0.178 |
| FCRL3 | 0.426 | 0.388 | 0.464 | 0.546 | 0.110 |
| FOXP3 | 0.362 | 0.254 | 0.630 | 0.525 | 0.287 |
| GZMB | 0.484 | 0.203 | 0.220 | 0.931 | 0.847 |
| HDAC9 | 0.679 | 0.864 | 0.212 | 0.398 | 0.990 |
| IL12B | 0.005 | 0.008 | 0.127 | 0.915 | 0.088 |
| IL17RA | 0.269 | 0.243 | 0.974 | 0.831 | 0.652 |
| IL23A | 0.137 | 0.111 | 0.990 | 0.431 | 0.561 |
| IL23R | 0.423 | 0.903 | 0.470 | 0.101 | 0.221 |
| IL2RA | 0.948 | 0.960 | 0.153 | 0.281 | 0.148 |
| IL7 | 0.915 | 0.933 | 0.339 | 0.822 | 0.670 |
| IL7R | 0.558 | 0.562 | 0.296 | 0.459 | 0.670 |
| IL8RA | 0.118 | 0.084 | 0.035 | 0.344 | 0.035 |
| LGALS1 | 0.222 | 0.054 | 0.841 | 0.520 | 0.030 |
| LGALS9 | 0.958 | 0.949 | 0.649 | 0.885 | 0.081 |
| PRKCQ | 0.511 | 0.862 | 0.879 | 0.528 | 0.729 |
| STAT5A | 0.283 | 0.463 | 0.556 | 0.117 | 0.442 |
| STAT5B | 0.721 | 0.873 | 0.412 | 0.022 | 0.297 |
| TGFB1 | 0.864 | 0.908 | 0.864 | 0.966 | 0.168 |
| TGFB2 | 0.739 | 0.418 | 0.481 | 0.087 | 0.672 |
| TGFB3 | 0.335 | 0.250 | 0.139 | 0.354 | 0.438 |
| TGFBR1 | 0.378 | 0.398 | 0.021 | 0.504 | 0.493 |
| TGFBR2 | 0.644 | 0.242 | 0.017 | 0.001 | 0.025 |
| TGFBR3 | 0.068 | 0.256 | 0.446 | 0.295 | 0.366 |
| TNFSF14 | 0.742 | 0.521 | 0.964 | 0.981 | 0.848 |
| Treg cell gene pathway | 0.444 | 0.719 | 0.082 | 0.083 | 0.632 |
Single SNP associations for each gene are shown in Supplemental Table 1. The effective number of independent SNPs tested was 370; applying a bonferroni correction for testing 370 SNPs across 5 groups, yields p < 2.7 × 10−5 as the significance threshold. No single SNPs remains significant after correction for multiple testing within histotype. The most single SNP association was seen with TGFBR2 and clear cell; the T allele in rs3773636 was associated with a 21% increased risk of clear cell ovarian cancer (OR = 1.21, 95% CI = 1.10-1.33, p = 0.0001).
eQTL in TGFBR2 associate with FCGR2B expression
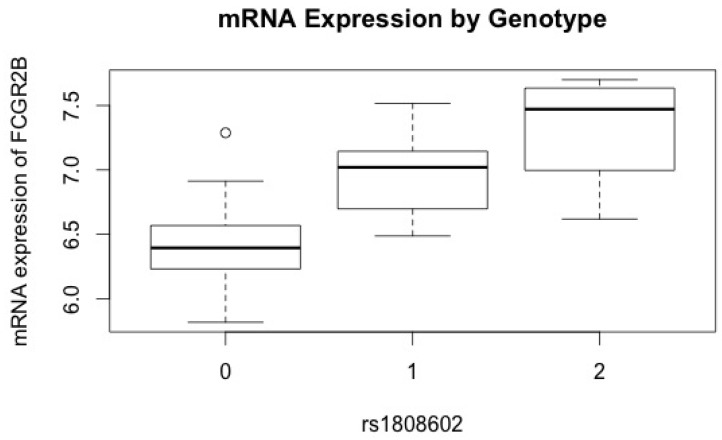
TGFBR2 contained the SNP with the most significant association with risk of clear cell EOC and also contained several additional SNPs with suggestive associations with clear cell and mucinous EOC. Thus, SNPs in TGFBR2 were correlated with mRNA expression levels as measured by the 9,634 probes passing quality control (QC) and showing expression above the background in at least 25% of the samples [18]. Regression analyses showed the most significant association between rs1808602 and FCGR2B (PFDR < .05) with an adjusted r2 = 0.51 for a model including both SNP and histology; the variation attributable to the SNP alone was r2 = 0.45. Each additional copy of the minor (G) allele (minor allele frequency (MAF) = 42.4%) was associated with an increase in mRNA expression level of 0.51 in FCGR2B (Figure 1). This SNP-gene association was the only association significant after correction for multiple testing.
Figure 1. Association of variant alleles in TGFBR2 with circulating mRNA expression levels in FCGR2B.
FCGR2B mRNA expression levels (y-axis) versus rs1808602 (x-axis). Each additional copy of the variant allele (G) in rs1808602 was associated with a significant increase in mRNA expression level after adjusting for age and histology.
DISCUSSION
Treg cells have been shown to suppress tumor antigen specific immunity in ovarian cancer, in vitro and in vivo [13]. However, the role of Treg cells in the etiology of ovarian cancer is not well established. We attempted to evaluate robust genetic biomarkers associated with Treg cells in relation to EOC in a large sample pooled from the Ovarian Cancer Association Consortium (OCAC). We hypothesized that SNPs in genes that regulate the function of Treg cells could potentially be associated with variation in immune response to ovarian tumors. Hence, in this study we evaluated SNPs in 25 genes thought to govern the function of Treg cells to determine their association to EOC. We found a modest association between TGFBR2 and invasive clear cell EOC. SNPs in this gene have been found to be associated with other pathological conditions, including gastric and colorectal cancer [19, 20]. The TGF-β family of cytokines plays an important role in proliferation, differentiation, and apoptosis of many cell types [21]. However, some tumors, such as ovarian tumors, evade the anti-proliferative effects of TGF-β by acquiring mutations in TGF-β signaling pathway [22]. Furthermore, the TGF-β signaling pathway plays a paradoxical role in tumorigenesis, initially suppressing and later promoting tumor growth and metastasis [23].
The significant association of rs1808602 in TGFBR2 with lymphoblastoid cell line (LCL) mRNA expression of FCGR2B (FcγRIIB) adds evidence for an immune component in ovarian carcinogenesis. FCGR2B binds to the Fc component of the antigen-IgG immune complex, suppressing immune response through several mechanisms, including inhibition of antigen presentation to T lymphocytes as well as reduced phagocytosis by neutrophils [24]. The only inhibitory receptor among members of the FcGR family in humans, FCGR2B, expressed on B lymphocytes [25] and follicular dendritic cells, is thought to be critical for maintenance of humoral immune response [26, 27]. The modest correlation between the TGFBR2 polymorphism and mRNA expression of FCGR2B observed suggests that TGF-β cytokine signaling pathway may, directly or indirectly through Treg cells, regulate the expression of FcGR, thereby potentially altering the balance between pro-inflammatory and anti-inflammatory immune response. Furthermore, the downstream inhibitory effect of FCGR2B expression is not limited to immune cells. Experimental models have demonstrated the potential of FCGR2B to promote tumorigenesis when expressed on non-lymphoid tumor cells [28, 29]. FCGR2B expression is thought to be a mechanism of immune escape by tumor cells [30]. Thus, our findings indicate that polymorphisms in TGFBR2 may potentially affect inter-individual variation in anti-tumor immune response through FcG receptor modulation. Additional evidence for Treg-cell-related eQTL SNPs has been seen with survival in ovarian cancer [31, 32]. Specifically, genetic variation in CD80 was associated with poorer survival of endometrioid cases and with increased tumor CD80 expression. The above findings suggest that inherited factors contributing to ovarian cancer etiology and outcome may, in part, drive the expression of important immune-related genes.
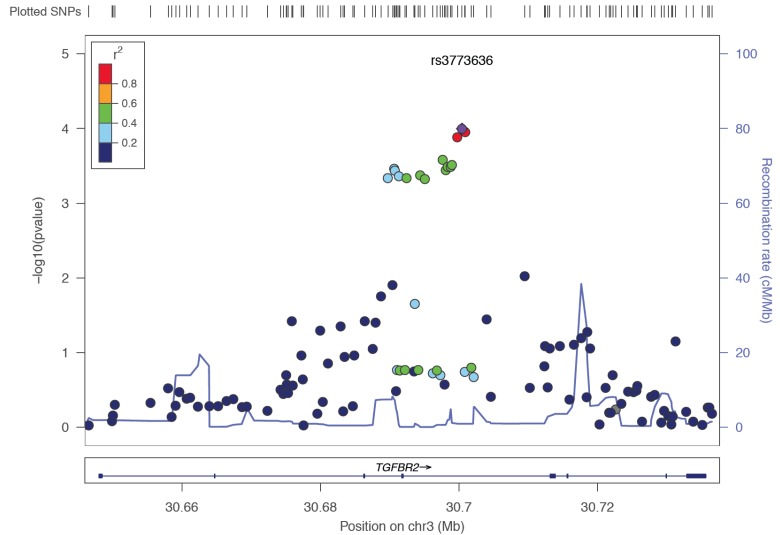
Further evaluation of the structure of TGFBR2 showed that the rs3773636 SNP is in strong linkage disequilibrium (r2 = 1) with a SNP (rs995435) that is thought to likely affect binding of proteins such as HNF4A, EP300, and GATA2, all associated with the balance of cell differentiation [33] (Figure 2). This SNP resides in SMAD4 and ELF5 (an ETS-related transcription factor) motifs in a relatively important position. In addition, we find that rs1463535 in TGFBR2, ~2 Mb from rs3773636 and independent of rs3773636, is associated (p < 8e-05) with expression of TGFBR2 in lymphoblastoid cell lines (p < 8e-05) [34].
Figure 2. Linkage disequilibrium structure and regional association map of TGFBR2 with risk of clear cell ovarian cancer.
Each dot indicates a SNP, with the corresponding region on Chromosome 3 (x axis) and negative log10 p-value (y axis) associated with the SNP; color-coding reflects pairwise linkage disequilibrium. The purple dot is rs3773636, the most significant genetic association with clear cell ovarian cancer (p = 0.0001). It is located on Chromosome 3 at 30,690,658 bp (hg19) in TGFBR2.
Although we find relatively weak associations between SNPs in the Treg cell pathway and EOC etiology, we do see modest evidence that TGFBR2 contains an eQTL that is perhaps modulating expression of inhibitory immune-complex receptor genes. Thus, the Treg cell genetic hypothesis perhaps merits further investigation in a larger, more diverse population.
MATERIALS AND METHODS
SNP selection
An extensive literature review of studies examining the role of regulatory T cells in immune response was conducted in 2010, and genes relevant to the function of Treg cells were identified. Tag SNPs in 25 genes (MAF ≥ 0.05),were selected using the SNP database on Genome Variation Server [35]. SNP selection parameters included an r2 > = 0.8 and the Centre d'Etude du Polymorphisme Humain (CEPH) reference population. The genomic region was expanded upstream and downstream (5 Kb) of each gene using linkage disequilibrium block structure to capture tag SNPs in regulatory regions. Tag SNPs were then assessed for design scores using Illumina's Assay Design Tool for Infinium, and SNPs with a design score < 0.4 were excluded. SNPs were also excluded if the call rate was < 95%, if the test for deviation from Hardy Weinberg equilibrium proportions in controls was p < 10−4, or if greater than 2% discordance in duplicate pairs was observed. Of the 1,358 SNPs from the Treg cell pathway that were included for genotyping, a total of 1,351 passed QC and were included in the analysis presented in this paper (Supplemental Table 2).
Study population, genotyping, and quality control
Germline DNA (250 ng genomic or 750 ng whole-genome amplified) from a total of 15,596 ovarian cancer cases and 23,236 controls from 40 studies in the OCAC (Supplemental Table 3) was genotyped on a custom Illumina iSelect BeadArray. OCAC is an international, multidisciplinary consortium, comprising population-based, hospital-based and nested case-control, and case-only studies of ovarian cancer, conducted in the United States, Europe, Asia, and Australia. Genotype calling and quality control procedures were described previously [36, 37]. Samples with a genotype call rate of < 95% were excluded. Hap Map samples from European (CEU, N = 60), African (YRI, N = 53), and Asian (JPT+CHB, N = 88) populations were used to estimate intercontinental ancestry for each individual using the Local Ancestry in Admixed Population (LAMP) program [38], and variation in population substructure was estimated using principal components (PCs). Only individuals with a LAMP score greater than 90% European ancestry were included in the present analyses.
Statistical analyses
Logistic regression analyses in PLINK were used to test for evidence of additive associations of SNPs by histotype and restricted to invasive tumor behavior [39]. Evaluation of the scree plot of eigenvectors, derived using Eigenstrat, revealed that five PCs explained most of the variation in population substructure; the logistic regression models were adjusted accordingly for PCs, along with age. PC analysis was done using an in-house program written in C++ using the Intel MKL libraries for eigenvectors (available at http://ccge.medschl.cam.ac.uk/software/) [40]. We used the approach of Li et al. to calculate the effective number of independent SNPs tested, and this value was then used in a Bonferroni correction to determine single SNP significance [41, 42]. Regional association plots for SNPs with significant associations were constructed using LocusZoom software [43].
Both gene-level tests of association and global Treg cell pathway analyses by ovarian cancer histotypes were conducted using the admixture likelihood (AML) method [40, 44]. The AML method assumes a proportion of variants in each gene or pathway (α) is associated with outcome. The effect size of each SNP is assumed to be on a non-central χ2 distribution with non-centrality parameter η, which approximately captures that SNP's contribution to the total genetic variance of the outcome. To accommodate the correlation between SNPs in each gene, AML uses a pseudo-maximum likelihood method to estimate the α and η. For each gene-level and pathway-level test, we performed 1,000 simulations, assuming that the maximum proportion of associated SNPs in each gene or pathway was 0.20. We report p-values for the AML trend test.
Expression quantitative trait loci (eQTL) analysis in ovarian cancer patients
We measured trans and cis genotype associations with mRNA expression levels in LCL collected pre-treatment from unrelated EOC cases enrolled in the Gilda Radner Ovarian Family Cancer Registry (GRR) at Roswell Park Cancer Institute (RPCI), a part of the larger OCAC study described above. Microarray-based gene expression was assayed using the Illumina HumanHT-12v3 Gene Expression Beadchip, with almost 50,000 probes derived from the National Center for Biotechnology Information Reference Sequence (NCBI) RefSeq (Build 36.2, Rel 22) and the UniGene (Build 199) databases [45]. Beadscan was used to scan and extract the raw intensity and the data corrected by local background subtraction in GenomeStudio module. A quantile normalization algorithm in the lumi package in the R-based Bioconductor Package was used to normalize the log2 transformed intensity data. For data QC, we excluded the probes with detection P value > 0.05 (the P values were generated in BeadStudio software) in at least 25% of the samples, yielding 9,634 genes (18). Both LCL mRNA levels and genotype data were available on 44 patients with EOC from the GRR. Genes containing the SNPs most significantly associated with risk of EOC were selected for SNP-mRNA expression level analyses using linear regression adjusted for patient age and histotype. All analyses were corrected for multiple testing [46].
SUPPLEMENTARY MATERIAL
Acknowledgments
The Australian Ovarian Cancer Study Management Group (D. Bowtell, G. Chenevix-Trench, A. deFazio, D. Gertig, A. Green, P. Webb) and ACS Investigators (A. Green, P. Parsons, N. Hayward, P. Webb, D. Whiteman) thank all the clinical and scientific collaborators (see http://www.aocstudy.org/) and the women for their contribution. The Belgian Ovarium Cancer Study wished to thank Gilian Peuteman, Thomas Van Brussel and Dominiek Smeets for technical assistance. The German Ovarian Cancer Study (GER) thanks Ursula Eilber and Tanja Koehler for competent technical assistance. The Helsinki Ovarian Cancer Study study was supported by the Helsinki University Central Hospital Reseaarch Fund. The Mayo Clinic Ovarian Cancer Case-Control Study, for iCOGS thanks C. Hilker, S. Windebank, and J. Vollenweider for iSelect genotyping. The Nurses Health Study and Nurses Health Study II thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. The Study of Epidemiology and Risk Factors in Cancer Heredity (SEARCH) thanks the SEARCH team, Craig Luccarini, Caroline Baynes, Don Conroy. Thanks to all members of Scottish Gynaecological Clinical Trails group and SCOTROC1 investigators. United Kingdom Ovarian cancer Population Study thanks I. Jacobs, M.Widschwendter, E. Wozniak, A. Ryan, J. Ford and N. Balogun for their contribution to the study. The UK Familial Ovarian Cancer Registry thanks Carole Pye.
Footnotes
CONFLICTS OF INTEREST
D. Cramer reports a financial relationship with Beasley Allen Crow. E. Goode reports a relationship with Johnson & Johnson. M.T. Goodman is a consultant/advisory board member for Johnson & Johnson. No additional conflicts of interest were reported.
FINANCIAL SUPPORT
This study was supported by funding from several sources including the Ovarian Cancer Research Fund thanks to donations by the family and friends of Kathryn Sladek Smith (PPD/RPCI.07); the Genetic Associations and Mechanisms in Oncology (GAME-ON): a NCI Cancer Post-GWAS Initiative (U19-CA148112 and U19-CA148537); the European Community's Seventh Framework Programme under grant agreement n° 223175 (HEALTH-F2-2009-223175); the Canadian Institutes for Health Research (CIHR) MOP-86727 and the CIHR Team in Familial Risks of Breast Cancer; the American Cancer Society (CRTG-00-196-01-CCE); the California Cancer Research Program (00-01389V-20170, N01-CN25403, 2II0200); Cancer Council Victoria; Cancer Council Queensland; Cancer Council New South Wales; Cancer Council South Australia; Cancer Council Tasmania; Cancer Foundation of Western Australia; the Cancer Institute of New Jersey; Cancer Research UK (C490/A6187, C490/A10119, C490/A10124, C536/A13086, C536/A6689, C1287/A10118, C1287/A 10710, C12292/A11174, C5047/A8384, C5047/A15007, C5047/A10692); the Celma Mastry Ovarian Cancer Foundation; the Danish Cancer Society (94-222-52); the ELAN Program of the University of Erlangen-Nuremberg; the Eve Appeal; the Helsinki University Central Hospital Research Fund; Helse Vest; Imperial Experimental Cancer Research Centre (C1312/A15589); the Norwegian Cancer Society; the Norwegian Research Council; the Ovarian Cancer Research Fund; Nationaal Kankerplan of Belgium; the L. & S. Milken Foundation; the Polish Ministry of Science and Higher Education; the US National Institutes of Health/National Center for Research Resources/General Clinical Research Center grant MO1-RR000056; the US National Cancer Institute (RPCI-UPCI Ovarian Cancer SPORE P50CA159981-01A1, K07-CA095666, K07-K07-CA80668, CA143047, K22-CA138563, N01-CN55424, N01-PC067010, N01-PC035137, P01-CA017054, P01-CA087696, P20-GM103418, P30-CA072720, P30-CA15083, P30-CA168524, P30-CA008748, P50-CA105009, P50-CA136393, R01-CA014089, R01-CA016056, R01-CA017054, R01-CA049449, R01-CA050385, R01-CA054419, R01-CA058598, R01-CA058860, R01-CA061107, R01-CA061132, R01-CA063682, R01-CA064277, R01-CA067262, R01-CA071766, R01-CA074850, R01-CA076016, R01-CA080742, R01-CA080978, R01-CA128978, R01-CA083918, R01-CA087538, R01-CA092044, R01-095023, R01-CA106414, R01-CA122443, R01-CA61107, R01-CA112523, R01-CA114343, R01-CA126841, R01-CA136924, R01-CA149429, R03-CA113148, R03-CA115195, R21-GM86689, R37-CA070867, R37-CA70867, U01-CA069417, U01-CA071966, CA58860, CA92044, PSA042205, UM1-CA186107, P01-CA87969, R01-CA49449, UM1-CA176726, R01-CA67262 and Intramural research funds); National Institute of Environmental Health Sciences (T32ES013678); the US Department of Defense Ovarian Cancer Research Program (W81XWH-07-0449); the US Army Medical Research and Material Command (DAMD17-98-1-8659, DAMD17-01-1-0729, DAMD17-02-1-0666, DAMD17-02-1-0669, W81XWH-10-1-0280, W81XWH-10-1-0341); the National Health and Medical Research Council of Australia (199600, 400413, and 400281); the German Federal Ministry of Education and Research of Germany Programme of Clinical Biomedical Research (01 GB 9401); the state of Baden-Wu?rttemberg through Medical Faculty of the University of Ulm (P.685); the Minnesota Ovarian Cancer Alliance; the Mayo Foundation; the Fred C. and Katherine B. Andersen Foundation; the Lon V. Smith Foundation (LVS-39420); the Polish Committee for Scientific Research (4P05C 028 14 and 2P05A 068 27); the Oak Foundation; the OHSU Foundation; the Mermaid I project; the Rudolf-Bartling Foundation; the UK National Institute for Health Research Biomedical Research Centres at the University of Cambridge; Imperial College London; University College Hospital “Womens Health Theme” and the Royal Marsden Hospital; WorkSafeBC; Komen Foundation for the Cure; and the Breast Cancer Research Foundation; the Lon V Smith Foundation grant LVS-39420.
G. Chenevix-Trench and P.M. Webb are supported by the Australian National Health and Medical Research Council; B. Karlan holds an American Cancer Society Early Detection Professorship (SIOP-06-258-01-COUN); and A. Berchuck holds the Barbara Thomason Ovarian Cancer Research Professorship from the American Cancer Society (SIOP-06-090-06). Cytometry services were provided by the Flow and Image Cytometry Core facility at the Roswell Park Cancer Institute which is supported in part by the NCI Cancer Center Support Grant 5P30 CA016056.
REFERENCES
- 1.Liu Y, Zheng P. FOXP3 and breast cancer: implications for therapy and diagnosis. Pharmacogenomics. 2007;8:1485–1487. doi: 10.2217/14622416.8.11.1485. [DOI] [PubMed] [Google Scholar]
- 2.Gavalas NG, Karadimou A, Dimopoulos MA, Bamias A. Immune response in ovarian cancer: how is the immune system involved in prognosis and therapy: potential for treatment utilization. Clin Dev Immunol. 2010;2010:791603. doi: 10.1155/2010/791603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yigit R, Massuger LFAG, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol. 2010;117:366–372. doi: 10.1016/j.ygyno.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R, Gynecologic Oncology G. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 5.Surveillance, Epidemiology, End Results Program: National Cancer Institute http://seer.cancer.gov/
- 6.Zou W, Machelon V, Coulomb-L'Hermin A, Borvak J, Nome F, Isaeva T, Wei S, Krzysiek R, Durand-Gasselin I, Gordon A, Pustilnik T, Curiel DT, Galanaud P, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 7.Liyanage UK, Moore TT, Joo H-G, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 8.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 9.Perez SA, Karamouzis MV, Skarlos DV, Ardavanis A, Sotiriadou NN, Iliopoulou EG, Salagianni ML, Orphanos G, Baxevanis CN, Rigatos G, Papamichail M. CD4+CD25+ regulatory T-cell frequency in HER-2/neu (HER)-positive and HER-negative advanced-stage breast cancer patients. Clin Cancer Res. 2007;13:2714–2721. doi: 10.1158/1078-0432.CCR-06-2347. [DOI] [PubMed] [Google Scholar]
- 10.Chikamatsu K, Sakakura K, Whiteside TL, Furuya N. Relationships between regulatory T cells and CD8+ effector populations in patients with squamous cell carcinoma of the head and neck. Head Neck. 2007;29:120–127. doi: 10.1002/hed.20490. [DOI] [PubMed] [Google Scholar]
- 11.Karagoz B, Bilgi O, Gumus M, Erikci AA, Sayan O, Turken O, Kandemir EG, Ozturk A, Yaylaci M. CD8+CD28-cells and CD4+CD25+ regulatory T cells in the peripheral blood of advanced stage lung cancer patients. Med Oncol. 2010;27:29–33. doi: 10.1007/s12032-008-9165-9. [DOI] [PubMed] [Google Scholar]
- 12.Okita R, Saeki T, Takashima S, Yamaguchi Y, Toge T. CD4+CD25+ regulatory T cells in the peripheral blood of patients with breast cancer and non-small cell lung cancer. Oncol Rep. 2005;14:1269–1273. [PubMed] [Google Scholar]
- 13.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 14.Barnett B, Kryczek I, Cheng P, Zou W, Curiel TJ. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol. 2005;54:369–377. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 15.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 16.Li X, Ye DF, Xie X, Chen HZ, Lu WG. Proportion of CD4+CD25+ regulatory T cell is increased in the patients with ovarian carcinoma. Cancer Invest. 2005;23:399–403. [PubMed] [Google Scholar]
- 17.Derycke MS, Charbonneau B, Preston CC, Kalli KR, Knutson KL, Rider DN, Goode EL. Toward understanding the genetics of regulatory T cells in ovarian cancer. Oncoimmunology. 2013;2:16. doi: 10.4161/onci.24535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H, Shen J, Wang D, Guo Y, Gregory S, Medico L, Hu Q, Yan L, Odunsi K, Lele S, Liu S. Associations between gene expression variations and ovarian cancer risk alleles identified from genome wide association studies. PLoS One. 2012;7:2. doi: 10.1371/journal.pone.0047962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin G, Wang L, Chen W, Hu Z, Zhou Y, Tan Y, Wang J, Hua Z, Ding W, Shen J, Zhang Z, Wang X, Xu Y, et al. Variant alleles of TGFB1 and TGFBR2 are associated with a decreased risk of gastric cancer in a Chinese population. International Journal of Cancer. 2007;120:1330–1335. doi: 10.1002/ijc.22443. [DOI] [PubMed] [Google Scholar]
- 20.Ogino S, Kawasaki T, Ogawa A, Kirkner GJ, Loda M, Fuchs CS. TGFBR2 mutation is correlated with CpG island methylator phenotype in microsatellite instability-high colorectal cancer. Human pathology. 2007;38:614–620. doi: 10.1016/j.humpath.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Kang Y. Pro-metastasis function of TGFbeta mediated by the Smad pathway. J Cell Biochem. 2006;98:1380–1390. doi: 10.1002/jcb.20928. [DOI] [PubMed] [Google Scholar]
- 22.Antony ML, Nair R, Sebastian P, Karunagaran D. Changes in expression, and/or mutations in TGF-beta receptors (TGF-beta RI and TGF-beta RII) and Smad 4 in human ovarian tumors. J Cancer Res Clin Oncol. 2010;136:351–361. doi: 10.1007/s00432-009-0703-4. [DOI] [PubMed] [Google Scholar]
- 23.Hempel N, How T, Cooper SJ, Green TR, Dong M, Copland JA, Wood CG, Blobe GC. Expression of the type III TGF-receptor is negatively regulated by TGF- Carcinogenesis. 2008;29:905–912. doi: 10.1093/carcin/bgn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith KGC, Clatworthy MR. Fc[gamma]RIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010;10:328–343. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amigorena S, Bonnerot C, Choquet D, Fridman WH, Teillaud JL. Fc gamma RII expression in resting and activated B lymphocytes. Eur J Immunol. 1989;19:1379–1385. doi: 10.1002/eji.1830190805. [DOI] [PubMed] [Google Scholar]
- 26.Qin D, Wu J, Vora KA, Ravetch JV, Szakal AK, Manser T, Tew JG. Fc gamma receptor IIB on follicular dendritic cells regulates the B cell recall response. J Immunol. 2000;164:6268–6275. doi: 10.4049/jimmunol.164.12.6268. [DOI] [PubMed] [Google Scholar]
- 27.Barrington RA, Pozdnyakova O, Zafari MR, Benjamin CD, Carroll MC. B lymphocyte memory: role of stromal cell complement and FcgammaRIIB receptors. Journal of Experimental Medicine. 2002;196:1189–1199. doi: 10.1084/jem.20021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zusman T, Gohar O, Eliassi H, Avivi Y, Lisansky E, Sautes C, Even J, Bonnerot C, Fridman WH, Witz IP, Ran M. The murine Fc-gamma (Fc gamma) receptor type II B1 is a tumorigenicity-enhancing factor in polyoma-virus-transformed 3T3 cells. International Journal of Cancer. 1996;65:221–229. doi: 10.1002/(SICI)1097-0215(19960117)65:2<221::AID-IJC16>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 29.Zusman T, Lisansky E, Arons E, Anavi R, Bonnerot C, Sautes C, Fridman WH, Witz IP, Ran M. Contribution of the intracellular domain of murine Fc-gamma receptor type IIB1 to its tumor-enhancing potential. International Journal of Cancer. 1996;68:219–227. doi: 10.1002/(SICI)1097-0215(19961009)68:2<219::AID-IJC14>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Callanan MB, Le Baccon P, Mossuz P, Duley S, Bastard C, Hamoudi R, Dyer MJ, Klobeck G, Rimokh R, Sotto JJ, Leroux D. The IgG Fc receptor, FcgammaRIIB, is a target for deregulation by chromosomal translocation in malignant lymphoma. Proc Natl Acad Sci U S A. 2000;97:309–314. doi: 10.1073/pnas.97.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goode EL, DeRycke M, Kalli KR, Oberg AL, Cunningham JM, Maurer MJ, Fridley BL, Armasu SM, Serie DJ, Ramar P, Goergen K, Vierkant RA, Rider DN, et al. Inherited variants in regulatory T cell genes and outcome of ovarian cancer. PLoS One. 2013;8:30. doi: 10.1371/journal.pone.0053903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charbonneau B, Moysich KB, Kalli KR, Oberg AL, Vierkant RA, Fogarty ZC, Block MS, Maurer MJ, Goergen KM, Fridley BL, Cunningham JM, Rider DN, Preston C, et al. Large-scale evaluation of common variation in regulatory T cell-related genes and ovarian cancer outcome. Cancer Immunol Res. 2014;2:332–340. doi: 10.1158/2326-6066.CIR-13-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, Karczewski KJ, Park J, Hitz BC, Weng S, Cherry JM, Snyder M. Annotation of functional variation in personal genomes using RegulomeDB. Genome research. 2012;22:1790–1797. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duan S, Huang RS, Zhang W, Bleibel WK, Roe CA, Clark TA, Chen TX, Schweitzer AC, Blume JE, Cox NJ, Dolan ME. Genetic architecture of transcript-level variation in humans. Am J Hum Genet. 2008;82:1101–1113. doi: 10.1016/j.ajhg.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Genome Variation Server (GVS) http://gvs.gs.washington.edu/GVS144/
- 36.Pharoah PD, Tsai YY, Ramus SJ, Phelan CM, Goode EL, Lawrenson K, Buckley M, Fridley BL, Tyrer JP, Shen H, Weber R, Karevan R, Larson MC, et al. GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nature genetics. 2013;45:362–370. doi: 10.1038/ng.2564. 370e361-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White KL, Vierkant RA, Fogarty ZC, Charbonneau B, Block MS, Pharoah PD, Chenevix-Trench G, for AACSg, Rossing MA, Cramer DW, Pearce CL, Schildkraut JM, Menon U, et al. Analysis of over 10,000 Cases finds no association between previously reported candidate polymorphisms and ovarian cancer outcome. Cancer epidemiology, biomarkers & prevention. 2013;22:987–992. doi: 10.1158/1055-9965.EPI-13-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sankararaman S, Sridhar S, Kimmel G, Halperin E. Estimating local ancestry in admixed populations. American journal of human genetics. 2008;82:290–303. doi: 10.1016/j.ajhg.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee AW, Tyrer JP, Doherty JA, Stram DA, Kupryjanczyk J, Dansonka-Mieszkowska A, Plisiecka-Halasa J, Spiewankiewicz B, Myers EJ, Chenevix-Trench G, Fasching PA, Beckmann MW, Ekici AB, et al. Evaluating the ovarian cancer gonadotropin hypothesis: a candidate gene study. Gynecol Oncol. 2015;136:542–548. doi: 10.1016/j.ygyno.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hendricks AE, Dupuis J, Logue MW, Myers RH, Lunetta KL. Correction for multiple testing in a gene region. European journal of human genetics : EJHG. 2014;22:414–418. doi: 10.1038/ejhg.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005;95:221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- 43.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyrer J, Pharoah PD, Easton DF. The admixture maximum likelihood test: a novel experiment-wise test of association between disease and multiple SNPs. Genetic epidemiology. 2006;30:636–643. doi: 10.1002/gepi.20175. [DOI] [PubMed] [Google Scholar]
- 45.Werness BA, Ramus SJ, DiCioccio RA, Whittemore AS, Garlinghouse-Jones K, Oakley-Girvan I, Tsukada Y, Harrington P, Gayther SA, Ponder BA, Piver MS. Histopathology, FIGO stage, and BRCA mutation status of ovarian cancers from the Gilda Radner Familial Ovarian Cancer Registry. Int J Gynecol Pathol. 2004;23:29–34. doi: 10.1097/01.pgp.0000101083.35393.cd. [DOI] [PubMed] [Google Scholar]
- 46.Huang T, Cai YD. An information-theoretic machine learning approach to expression QTL analysis. PLoS One. 2013:8. doi: 10.1371/journal.pone.0067899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.