Abstract
Oncologic treatments, such as curative radiotherapy and chemoradiation, for head and neck cancer can cause long-term swallowing impairments (dysphagia) that negatively impact quality of life. Radiation-induced dysphagia is comprised of a broad spectrum of structural, mechanical, and neurologic deficits. An understanding of the biomolecular effects of radiation on the time course of wound healing and underlying morphological tissue responses that precede radiation damage will improve options available for dysphagia treatment. The goal of this review is to discuss the pathophysiology of radiation-induced injury and elucidate areas that need further exploration.
Keywords: cancer, radiation, fibrosis, pharynx, swallow, deglutition, deglutition disorders
Introduction
Laryngeal and pharyngeal cancers account for more than 29,000 new diagnoses of malignancies in the United States per year [1]. Radiotherapy and concomitant chemoradiotherapy are widely accepted curative treatment approaches for organ preservation [2]. However, since the upper aerodigestive tract is highly susceptible to radiation-induced damage, treatment-related swallowing impairments continue to represent a significant clinical problem [3]. The use of intensity-modulated radiation therapy (IMRT) has greatly reduced adjacent normal tissue damage through the use of steep dose gradients and the ability to adjust maximum doses to the target shape. However, locoregional control of head and neck squamous cell carcinoma (HNSCC) often requires highly aggressive radiation schemes to reduce tumor repopulation [4]. These accelerated radiation schedules, with high total prescribed doses (> 60–70 Gray [Gy]), result in rapid dose accumulation that is far less tolerable [5].
In attempts to improve swallowing function and quality of life after radiotherapy, it has been advocated that anatomic structures important for swallow be spared or the mean dose be reduced [6,7]. As such, identification of at-risk organs has been the subject of intense focus and controversy in the literature [8,9]. To understand the problem of radiation-induced dysphagia, it is important to consider the unique temporal distinctions of radiation injury, the composition of each anatomical structure involved in swallowing, and the locomotion aspects of swallowing activity. An improved understanding of the biomolecular effects of radiation on the time course of wound healing and of underlying morphological tissue responses that precede radiation damage would also help to improve treatment options for dysphagia.
The goal of this review is to discuss the pathophysiology of radiation-induced injury and elucidate areas that need further exploration. We review the known temporal evolution of radiation-induced injury and physiologic factors that can lead to progressive motor and sensory impairments. We also consider the clinical use of pre-conditioning exercises to develop metabolic reserve capacity in muscles. The biomolecular properties that predict successful post-treatment swallowing outcomes are evaluated by comparing clinical outcomes to muscle physiology and the biomolecular aspects of tissue injury after radiation.
Temporal Differences in Radiation Injury
There is limited information on the pathophysiology of radiation-induced injury in the upper aerodigestive tract. In contrast to normal wound healing from trauma, radiation-induced injuries have an accruing and repetitive nature, which inhibits normal molecular and cellular regulatory processes. It is known that radiation disrupts tissue homeostasis by damaging DNA (deoxyribonucleic acid) in the nucleus of rapidly proliferating cells (i.e., tumor, epithelial, etc) and impeding normal function of organelles in the surrounding cells. These biological events alter molecular pathways involved in cell survival, oxidative stress, and signal transduction [10].
Complicating the study of radiation-induced dysphagia is the wide-range of terminology used to classify temporal effects. Figure 1 compares commonly used terminology across three different medical arenas including: basic radiation literature, dysphagia rehabilitation, and clinical diagnosis. Radiation injuries are clinically classified into acute, subacute, or chronic designations. Early mucosal injuries (acute <3 months or subacute 3–6 months post irradiation) are attributed to cell death and subsequent inflammation [11]; whereas late deeper tissue responses (chronic >6 months post irradiation) are attributed to damage to the vasculature and/or surrounding connective tissue (Table 1). Of note, the severity of early injury is not always an indicator of late reactions (Figure 1, basic radiation literature). Hopewell and colleagues [12,13] and others [14], utilizing models of irradiated rodent and pig skin, indicated that development of late radiation-induced tissue changes can occur irrespective of early epithelial damage. There are a number of biological factors that trigger a diverse repertoire of early or late injuries, including volume administered, dose fractionation, overall treatment time, total dose, and anatomic modifications during treatment [15]. As such, late injuries are further categorized based on their origin (i.e., consequential or generic) (Figure 1). In contrast, dysphagia rehabilitation literature often base outcome studies on broadly defined temporal characteristics (i.e short and long; Figure 1), without consistency as to the patient population and/or period of time from injury.
Figure 1.
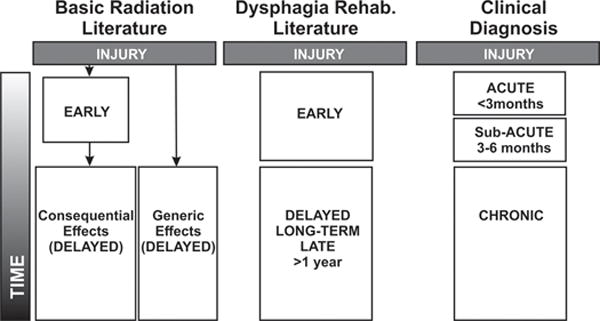
Temporal classifications of radiation-induced injury in head and neck as described in basic radiation, dysphagia rehabilitation, and clinical diagnostic literature. Research findings are complicated by wide-range of terminologies used to describe stages of radiation injury. A more precise classification is necessary to prevent ambiguity and provoke different treatments for each specific injury classification.
Table 1.
Describing biomolecular and pathologic features known to each injury period.
| Time Point | Biomolecular Features | Pathology | Clinical Features |
|---|---|---|---|
| Early ~onset <6 months |
reactive oxygen/nitrogen species, impaired cell proliferation, epithelial denudation | Edema, erythema; leukocyte infiltration; vasodilation; vascular leakage; hypoplasia | Xerostomia, dysgeusia, mucositis, inflammation (i.e., redness, heat, pain, swelling, loss of function) |
|
| |||
| Interval ~ onset 6 months to 5–10 years |
Oxidative damage, genetic changes | Increased fibroblast growth rate, increased collagen content; | Currently unknown |
|
| |||
| Delayed ~ onset >6 months |
Currently unknown | Persistent disorganized matrix, vascular changes | Fibrosis, atrophy |
Characteristics of Early Radiation Injury
In brief, early radiation-induced treatment effects are evident in the epidermis and mucosa. This is mainly due to cell depletion, inflammation, and hypoplasia that can lead to mucositis and desquamation accompanied by edema and erythema (Table 1) [16]. Often acute injuries are transient and resolve within a few months after treatment; however, early injuries can sometimes persist, producing chronic changes that lead to consequential late effects (discussed below). In contrast, there may not be any significant correlations between the severity of acute and chronic radiation-induced injuries. For example, a patient may develop severe fibrosis post radiation treatment despite only suffering a low-grade acute reaction, or a patient with severe acute mucositis (grade 3 or 4) may ultimately experience minimal treatment-related effect 6–9 months later.
The optimum accumulated dose, fraction size, volume, and dose concentrations are aimed to overcome accelerated repopulation of cancer cells after radiotherapy, but can also define the magnitude of inflammatory response and timing of tissue regeneration after exposure [17]. Within minutes after irradiation, bioactive molecules are highly abundant and an inflammatory process is induced in the submucosa. Inflammation induces a cascade of pro-inflammatory cytokines and chemokines (i.e., tumor necrosis factor [TNF], interleukin [IL]-6, IL-1); pro-fibrotic growth factors (i.e., transforming growth factor [TGF]); and increases in vascular permeability, allowing for infiltration of innate immune cells (i.e., neutrophils, macrophages). Stem cell proliferation rate decreases and progressive epithelial breakdown develops as a consequence of accumulated radiation damage [18]. Together, this suppresses the resolution of inflammation and inhibits normal cell repopulation needed for regeneration of the tissue. Previous work has shown that the initiation of reepithelization of normal cells depends strongly on the treatment parameters. For example, in the oral-pharyngeal cavities, epithelial repopulation occurs after a latent period of approximately 7 days after starting irradiation with 5 × 2 Gy/week regimen [19]. Measurements of acute biological effects of radiation (i.e., radiation tolerance) are based upon these epithelial repopulation experiments.
One of the most detrimental effects of radiation exposure is the induction of excessive production of reactive oxygen and nitrogen species (ROS). Reactive species are generated during normal and disease conditions through an oxidative metabolic process within organelles or through an enzymatic source [20]. Under physiologic conditions, ROS acts as a mediator of communication within the cell and facilitate signaling amongst other cells. Activity is highly regulated, because excess production can damage proteins and cell structure, instigating dysfunction or apoptosis. ROS are regulated by controlling any excessive production through enzymatic removal. Mitochondria within a cell are the largest producers of ROS in steady conditions (i.e., superoxide anion), during generation of ATP (adenosine triphosphate) in complex I and III of the electron transport chain. Mitochondria also employ antioxidant defenses (i.e., superoxide dismutase, catalase) that are capable of neutralizing or reducing ROS to a less toxic form [20]. When an imbalance occurs between reactive species and their antioxidant defenses, it can lead to cell stress and eventual tissue destruction, called oxidative damage.
Radiation exposure substantially increases the level of ROS in the microenvironment, because ROS are produced by DNA-damaged cells (i.e., epithelial, tumor). They are also released by phagocytic leukocytes during the resulting inflammatory reaction (i.e., granules produced by infiltrating neutrophils [NADPH oxidases], macrophage phagocytosis), as well as by activation of fibroblasts or myofibroblasts in the tissue. Persistently high levels of ROS are thought to permanently damage mitochondrial DNA, and these injuries can be subsequently passed on to progeny cells, creating an iterative cycle of oxidative stress [21].
Swallow, in many patients during radiotherapy, is within normal functional limits, despite subtle changes to their swallowing physiology and notable diet modifications [22]. In cases where dysphagia is present, it is often attributed to an array of functional characteristics, such as reduced retraction of base of tongue, poor epiglottic retroflexion, reduced laryngeal elevation, delay in pharyngeal transit, and/or poor coordination of swallowing muscles [23,22]. In many cases, these functional characteristics are accompanied by oral mucositis, causing continuous pain, resulting in difficulty with oral eating, malnutrition, and/or weight loss [24–26]; systemic fatigue and nausea may also play a role diminishing motivation to eat [27]. Of note, mucositis is also strongly related to other cofactors including smoking, infection, oral hygiene, and nutritional status [17,28].
Characteristics of Delayed Radiation Injury
Delayed (or late) radiation injuries are distinguished by when the damage originates (Figure 1). A previous study by Jung et al. [29] examined data sets taken from the literature on late morbidities of radiotherapy and determined their kinetics for the occurrence of complications. In the head and neck region, two-component curves were observed with >8 years post follow-up data; a steep initial decline was followed by an exponential decay 12–18 months after treatment.
As previously mentioned, persistent injuries originating from early severe mucosa reactions are due to irradiation and are considered consequential late effects [5]. These responses are highly influenced by overall treatment time but not directly attributed to radiation injury. Consequential late effects are thought to occur because a delay in re-epithelialization after irradiation reduces barrier function, compromising the underlying lamina propria and allowing for subsequent infection or trauma. For example, severe oral mucositis that develops during radiation therapy can persist, resulting in necrosis or delayed ulcer. In contrast, late tissue changes that originate in response to direct radiation to target tissue, are referred to as generic late effects (Figure 1) [17]. Severity of generic late reactions are influenced by fractionation and do not correspond to the intensity of early damage. Disturbance to normal cellular behavior is thought be the primary inducer of nonspecific fibrosis that leads to abnormalities in organ function. Radiation-induced neuropathies are an example of a generic effect within the head and neck region, resulting in muscle or nerve dysfunction [30].
Common pathologic features of late responding injuries include progressive collagen accumulation, permanent fiber disorganization, altered microvasculature, production of pro-fibrotic growth factors (i.e., TGF), and/or eventual loss of elasticity (i.e., atrophy) [31,32]. Late tissue injuries develop when chronic pathologic processes fail to down-regulate fibrogenic deposition; these abnormalities in remodeling can expand across compartments, entrapping underlying muscle and nerves [31]. The absorbed radiation dose and volume of tissue in the radiation field can affect the depth of damage [33]. Several factors have been implicated in the mechanism of permanent cell damage and fibrotic changes, including persistent oxidative stress, microvascular damage, and/or subsequent loss of stem cells required for regenerating damaged tissue [34].
Clinical and translational studies have indicated that radiation may alter restorative cellular behavior by prompting genetic abnormalities in late responding cells (i.e., fibroblast, myocytes, endothelial). In histologic examination of non-ulcerated skin taken from patients with long-term radiation-induced fibrosis (>7 years), Rudolph et al. [35] observed dense collagen networks and irregularities in ultrastructure of fibroblast-like cells. This included degenerating mitochondria, multiple dark vacuoles, and dilated endoplasmic reticulum. Further investigation of the pig skin biopsies harvested from fibrotic tissue 6 and 20 months after irradiation to thigh reported persistent over-activated fibroblast phenotypes after exposure (1–6 Gy/min; total 30–64 Gy). Irradiated fibroblasts appeared to escape early senescence, leading to persistently high proliferative abilities along with atypical morphologic changes [36]. Similar work has also demonstrated that irradiated fibroblasts engage in accelerated extracellular matrix (ECM) synthesis with a predilection for deposition of early immature (randomly organized) collagen type III fibrils as opposed to mature type I [37]. This work demonstrates that radiation induces long-term pathologic changes in fibroblasts. As a result of this finding, recent attempts have been made to characterize the genetic abnormalities within irradiated fibroblasts in an effort to predict radiation-induced fibrosis. Rodningen et al. [38], isolated fibroblasts from non-irradiated skin biopsies taken from 30 different breast cancer patients and exposed them to fractionated radiation schemes (3 × 3.5 Gy). Results demonstrated that irradiation upregulated transcription factors involved in oxidative stress and ECM remodeling. Further work identified unique transcription profiles that could differentiate between patients who were at low- or high-risk of radiation-induced fibrosis and disclosed 14 differentially expressed genes that could be used to predict radiation-induced fibrosis [39].
Onset of post-radiation dysphagia has been linked to both consequential and generic late effects. Early inflammatory damage to mucosa (i.e., xerostomia, mucositis) and/or radiation dose have been significantly correlated with dysphagia at 6–12 months post-treatment [40], indicative of consequential effects. However, dysphagia can also present years (>2) after treatment with no appreciable early symptoms, which may be due to fibrosis [41,42] and/or atrophy [43,8,41,44–46], and is suggestive of a generic effects [17].
The degree of skeletal muscle injury post radiation is dependent on location and variety of tissue types affected. Dose and/or volumetric dose (VD) limits, to the following areas, have been suggested to mitigate dysphagia, including anterior oral cavity (V30 < 65% and V35 < 35%), geniohyoid (< ~60 Gy), glottic and supraglottic larynx (<40–48 Gy; V50 to <21%), superior and middle pharyngeal constrictors (<63Gy; V55 < 80% and V65 < 30%), and inferior pharyngeal constrictors (<54 Gy; V50 to <51%) [47,6,48–52]. Eisbruch et al. [6] postulated that the location of pharyngeal and laryngeal tissue and the architectural design of their compartments may influence the severity of radiation injury compared to peripheral swallowing musculature (i.e., geniohyoid, mylohyoid). The notion was based on proximity of the muscle to the mucosa and its underlying connective tissue since these areas are highly susceptible to early inflammatory damage, which can thicken the ECM and subsequently restrain underlying muscle. Hence, fibrotic changes in or around key musculature reduce their locomotion.
Muscles of the larynx, pharynx, and upper esophagus lie deep to submucosa, which is overlaid with stratified squamous epithelium and the lamina propria of the mucosal layer; seromucinous glands and lymphoid aggregates are present throughout the mucosal layer. Radiation injury in these areas causes noticeable soft tissue deformities (i.e., stricture, stenosis) that alter the contour of the tube-like compartment. These changes can, in turn, profoundly affect pressure differentials and alter the rate of bolus flow requiring need for dilation. Therefore, shielding these critical structures during radiotherapy will limit the total dose, which is thought to reduce the risk of late toxicities (i.e., edema, aspiration, xerostomia, voice dysfunction, pharyngo-esophageal stricture) [51,53–55]. Correlating swallow with dosimetric factors, Caglar et al. [49] and Caudell et al. [48] demonstrated that mean dose to larynx and inferior pharyngeal constrictors related to severity of dysphagia and percutaneous endoscopic gastrostomy (PEG) tube dependence at 1yr respectively. Interestingly, Caudell et al. [48] also examined the effects of laryngeal blocking with IMRT using a matched low anterior neck field and found while it reduced the mean dose to inferior pharyngeal constrictors, the total dose to larynx was not altered and incidence of late-onset dysphagia did not improve. This suggests that although IMRT allows for sparing critical swallow structures outside the target volume, radiation can still spread to surrounding areas closest to primary tumors leading to unavoidable swallow complications. Research is underway studying various radiotherapy techniques (i.e., split-field IMRT, image-guided radiotherapy, brachytherapy, cyberknife) in effort to reduce volumetric doses to larynx and pharynx muscles.
The submental complex (digastric, mylohyoid, and geniohyoid) composes the floor of mouth, deep to the submandibular space, and are covered superficially by the dermis and platysma muscle. As such, external radiation may be partially absorbed by soft tissue within the submandibular space (i.e., submandibular glands and lymph nodes), lessening the radiation dose to the suprahyoid muscles. Two dosimetry studies were recently conducted correlating dysphagia with radiation dose to the genioglossus and submental complex [52,50]. Kumar et al. [52] measured dosimetric characteristics in genioglossus, and found that a minimum dose (30 Gy) strongly correlated with abnormal (≥3) penetration-aspiration scores. Further work demonstrated that doses >60Gy to the geniohyoid correlated with aspiration, and alterations in swallowing kinematics (i.e., hyoid elevation, pharyngeal transit time and cricopharyngeal opening). However, upon closer examination the primary tumor site, sample selection bias, and/or treatment related effects were not controlled. Therefore, the impact of radiation to floor of mouth musculature in comparison to uninvolved swallow muscles is difficult to ascertain from this data. It is plausible that floor of mouth structures are prone to underlying late-responding tissue changes (i.e., fibrotic, atrophy) that develop over a longer period of time. In cross-sectional analysis, Szczesniak et al. [56] examined 226 HNSCC patients 0.5 to 8 years post-radiation to determine prevalence and severity of persistent dysphagia. Of patients with no baseline clinical diagnosis of dysphagia during radiation treatment, 22% self-reported during follow-up evaluation that swallowing problems had arisen after treatment; these patients described various difficulties with clearance of solid foods.
Salivary tissue is also known to be highly vulnerable to radiation damage. In a rodent model, Coppes et al. [57] studied the effects of conventional and accelerated fractionated irradiation (32Gy total) to the parotid and submandibular/sublingual glands. Their results demonstrated that the submandibular gland was more susceptible to late treatment effects compared to the parotid gland, as reflected by: reduced gland salivary flow rate, cell loss, and increased fibrosis ~8–9 months after irradiation.
Primary tumor and/or radiation site might also play a key role determining at-risk patient populations. Levendag et al. [47] and Caglar et al. [49] identified high dose to superior and middle pharyngeal constrictors is predictor of aspiration in those treated for oropharynx primary cancers. Additionally, Schwartz et al. [51] analyzed dose-volume constraints associated with dysphagia in those with oropharynx primary cancers who underwent IMRT using laryngeal block and conventional AP low neck field. Results demonstrated that the anterior oral cavity structures and superior pharyngeal constrictor muscles were most at-risk for radiation-induced dysphagia, as indicated by strong association between radiation dose to these critical areas and poor swallowing outcomes as measured by Oropharyngeal Swallowing Efficiency. In contrast, Szczesniak et al. [56] found that patient characteristics (i.e., tumor site, therapy, age, gender, or use of chemotherapy) were poor predictors of incidence and severity of late-onset dysphagia. Further work is needed to determine the influence of radiation on the morphology and physiology of structures prominently involved in swallowing and on tissue known to be susceptible to late effects of radiation (i.e., muscle, nerves, and stroma).
Characteristics of Latency Period
Persistent biological changes can go undetected for years after radiation, but eventually will result in functional deficits that reduce quality of life and increase risk of mortality. In contrast to the lack of change implied by the term, the latent period between early and delayed radiation treatment effect is not a time of dormancy. Progressive aberrant wound healing is underway, causing fibrosis to form in deep tissue compartments (i.e., muscle and nerves). As discussed previously, permanent ultrastructural changes are likely a result of pathologically-induced deviations mediated at the cellular level (i.e., metabolic, genetic). Fibroblasts are responsible for matrix production and consequently, persistent stressors (i.e., ROS, etc.) can alter their activity, leading to an imbalance between collagen synthesis and degradation.
A persistent annual risk in developing late effects after HNSCC treatment has been reported [29]; however, information about the length of latent time before the first onset of late effects is lacking. One would expect that the rate of cell turnover in the tissue correlates with the sequelae of late radiation effects. However, various intrinsic factors (i.e., age, genetics, etc.) can affect cellular activity after radiation and therefore influence the probability of developing a late effect.
Neuromuscular Radiation Injury
Muscles
Skeletal muscle was previously thought to be radiation resistant, because of latent mitotic activity (cell growth) and limited reporting of adverse side effects [58]. However, functional decline, muscle weakness, and poor range of movement are now commonly reported complications in irradiated HNSCC survivors [41,59,27]. To date, the underlying skeletal muscle pathological changes related to radiation are not clearly understood.
It is now known that a single high dose of radiation can create permanent muscle contracture within a few hours of exposure [60,61]. Studies in the frog, dating back to the 1930’s, demonstrated that high-dose irradiation can result in striated muscle contraction in the hind-leg [61]. Further work by Khan [62] investigated changes in the pectoralis major muscle to a single 10–15 Gy dose of radiation in a rabbit model. Under electron microscopy, subtle changes to the myofibers and microvasculature could be seen as early as 24 hours post irradiation. Furthermore, skeletal muscle had progressive disruption 40 days after exposure, culminating in atrophy of type II fibers and microvascular necrosis.
Schwenen et al. [63], in a rat hindquarter, studied effects of amino acid availability after radiation treatment, which is an important determinant of muscle metabolism. Their results demonstrated that a single 10–15 Gy dose of gamma radiation is associated with muscle proteolysis (protein breakdown) within 4–6 hours of exposure, as indicated by an increase in alanine and glutamine amino acids. Recently, evidence was presented to suggest that radiation-induced disturbances in skeletal muscle regeneration may be attributed to perturbations in metabolic properties. Hardee et al. [64] studied fiber type alterations 2-weeks post-radiation in mouse hind limb comparing single high dose (16Gy) treatment to fractionated doses (4×4). Results showed wide-ranging damage with single high doses but a selective loss of Type IIB myofibers was found irrespective of the dose administered, suggesting that these fibers are more susceptible to radiation injury.
For review, the myofiber is the contractile unit comprising the skeletal muscle, and each unit contains three filaments: myosin, actin, and titin. The sarcoplasm encircles the myofiber providing glycogen and myoglobin needed for energy and oxygen storage respectively. Myosin can present in three different forms (Type I, Type IIA, and Type IIB) with variations in metabolic and contractile properties. Type I (slow-twitch) fibers are involved in tone, stiffness, and fine postural adjustments and have a high oxidative capacity. These functions require not only greater mitochondrial content, but a mechanism that works efficiently to limit oxidative damage by regulating ROS production and removal. Alternatively, Type IIB (fast-twitch) fibers involved in rapid and phasic activity depend heavily on glycolytic metabolism. Therefore, they require less mitochondrial density, and the mitochondria in Type IIB fibers exhibit imperfect ROS removal capacity.
Based on fiber type and response to ROS, muscles with the highest glycolytic capacity (Type IIB) are most at risk for radiation damage. For example, the outer layer of the inferior pharyngeal constrictor muscles in humans is predominately comprised of Type II fibers with a low oxidative capacity compared to inner layer [65]. Therefore, the distinct outer neuromuscular compartment thought to be responsible for coarse movement of bolus through the lumen might be at greater risk of radiation injury. Clinically, irradiated HNSCC survivors often present with difficulties attributed to pharyngeal dysmotility including impaired bolus movement during swallow and post-swallow residue in the posterior pharyngeal wall, laryngeal vestibule and pyriform sinus [66].
There are a number of other swallowing muscles composed of fibers with high glycolytic capacity. Given this knowledge, it is perhaps not surprising that several muscles have been identified as critical structures at risk for causing post-radiation dysphagia. However, studying these muscle fiber types in isolation does not account for differences in other physiologic properties, such as the relative influence that each muscle exerts during swallowing and the spectrum of muscles with heterogeneous compared to homogenous fiber type distribution. Additionally, oxidative damage is known to be highly selective to Type II fibers [67]. This is based upon the fact that mitochondria within each myofiber type have unique intrinsic features that can alter ROS processing [67]. As described earlier, ROS is a byproduct of normal energy production in mitochondria and is also induced during host immune defense and in response to radiation. Oxidative stress can occur when there is an imbalance between ROS and antioxidant defenses. In light of our limited understanding of the pharyngeal muscles that are vital to functional swallowing and airway protection, further work is needed to characterize changes in metabolic and physiologic demands of the varied musculature involved in swallowing, thus permitting further taxonomy of which muscles are most at risk for damage with radiation.
Peripheral Nerves
Perturbations in motor and sensory pathways can occur as result of early or late radiation injuries, influencing airway protection [41]. Peripheral nerves that innervate the swallowing musculature and transmit information to/from the central nervous system are susceptible to damage. This often arises from tumor invasion, cancer treatment(s), or infiltration (compression) of the nerve.
In general, intense radiation can lead to thermal and/or mechanical damage, triggering a cascade of inflammatory mediators (i.e., cytokines), neuropeptides, and glutamate signals (i.e., cell metabolism) in mucosa. Once injury stimuli is detected, nociceptive afferent fibers respond by releasing peptides that cause local tissue dysfunction and transmit neural signaling evoking autonomic reflexes [68]. As discussed earlier, the most common complication of radiation is oral mucositis, which is cytotoxic response that breaks down the epithelium lining causing significant pain and discomfort. Depolarization of nociceptive pain fibers occurs in response to detection of oxidative stress. Specifically, imbalance in ROS activity and subsequent initiation of NF-kB transcription pathways associated with mucositis is detected by sensory receptors, such as transient receptor potential (TRP) family of ion-channel proteins, endothelin-1, tumor necrosis factor, and nerve growth factors [69]. Persistent or uncontrolled pain can result from neuropathic sensory dysfunction. In the rat injury model, Simonyan et al. [70] showed that acute inflammation and trauma to vocal folds can elicit immunoreactive effects to sensory nuclei in the brain stem. Thus, injuries to glottis could upregulate central sensory response, promoting long-term hyper-excitability.
Inflammation and fibrosis that accompanies irradiation can also alter muscle and nerve electrophysiology (i.e intensity, speed, etc). Sensory inputs (i.e., bolus size, consistency, taste, temperature, etc.) are involved in initiation and regulation of cough and swallowing reflexes [71]. For signal processing, minimum thresholds must be met to induce downstream afferent pathways and signal pattern generators. Disruption of these pathways can lead to hyposensitivity, which may be responsible for the increased incidence of silent aspiration found with irradiated HNSCC patients. Further work is needed to determine the late effects to sensory and motor function caused by radiation and subsequent fibrosis.
Permanent injury to lower cranial nerves is a rare progressive complication of radiation therapy, which presents several years after treatment [72]. Bulbar palsy is the most common symptom of radiation-induced neuropathies in HNSCC survivors. This is due to proximity of the cranial nerves with respect to the radiation field and nearness to areas highly susceptible to fibrosis [30]. This type of radiation injury is thought to result as a consequence of late fibrotic changes. Several researchers have referred to the progression as fibroatrophic [73], where muscle fibers are persistently replaced with fibrotic tissue, subsequently reducing their motility as they become weakened and atrophied. Fibrosis is characterized by a loss of vascularity and matrix disorganization, which disrupts well-defined compartmentalized structures. The excessive collagen deposits can eventually entrap nerve trunks or alter the vascular networks between or within the nerve tracts, leading to neurologic deficits (i.e., neuropathy, myopathy) [58]. The mechanism that causes the transformation from fibrosis to atrophy is not well-understood; it is possibly related to fragmentation or degeneration of muscle fibers and/or mechanism of disuse atrophy [73]. It is well known that oral intake declines during radiotherapy for HNSCC [24]. Therefore, muscle disuse likely plays a role in development of swallowing muscle impairments after radiation.
Developing Metabolic Reserve Capacity–Prophylactic Strength Exercises
Throughout the swallowing literature, there is strong support for the use of prophylactic strength-based exercises to prevent or limit the occurrence of radiation-induced dysphagia [74–77]. To date, it is unclear if maintaining oral intake throughout radiation treatment, or if additional swallowing exercises are necessary to reduce dysphagia in irradiated patients. Swallow exercise programs are broadly aimed at preventing restrictions in motion and reducing immobility or disuse atrophy. However, if eating behaviors are not already impaired by the tumor or swallowing therapy is initiated concomitantly with irradiation, the goal to restore “strength” is not entirely applicable.
Many studies that have investigated the impact of prophylactic exercises have considerable methodological heterogeneity in the prescribed exercise regime, onset of implementation (i.e., before, during radiation), and tumor site/stage [78]. However, a recent study by Hutcheson et al. [79] analyzed swallowing activity in 497 patients who underwent radiotherapy or chemoradiation to treat pharyngeal cancer. Their results demonstrated significantly better long-term swallowing outcomes (i.e., 2–4 times more likely to eat a regular diet at ~2 years) when patients adhered to prophylactic swallowing exercise goals and/or maintained full PO intake during radiotherapy.
Beginning exercise interventions before initiation of therapy regime may prove to be beneficial. Other fields have demonstrated positive effects of pre-conditioning. For example, treadmill training 10 weeks prior to radiation (~1 Gy), was shown to upregulate antioxidant enzymes and enhance mitochondrial activity in the mouse hind-limb [80]. Pre-radiation prophylactic swallowing therapy could be used similarly to increase muscle fatigue resistance attributed to alterations in mitochondria biogenesis, myofiber strength, and inhibition of oxidative stress [81–83]. More specifically, active skeletal muscles are predisposed to high constitutive levels of oxidative production, due to rapid increases in ROS that are produced during contraction [84]. As such, it is crucial that myofibers in swallowing muscles have efficient antioxidant capabilities to combat radiation-induced ROS prior to radiation therapy that would otherwise cause irreversible damage.
Atrophy, is another concern, and is a result of alterations in muscle protein turnover resulting in loss of muscle mass [85]. In normal tissue, this process is mediated by disturbances to muscle protein synthesis [86,87]; however, following irradiation, the loss or reduced renewal of stem cells is partly responsible for this phenomenon [73]. Additionally, atrophy is attributed to permanent genetic alterations in local cells, affecting their signaling, protein turnover, and regulation of self-renewal [73]. This knowledge provides some evidence that the initiation of the prescribed prophylactic swallowing exercises (i.e., before, consecutively, or immediately following treatment) may impact therapeutic outcomes.
Although normal muscles display remarkable plasticity with exercise, irradiation can distort the fatigability and metabolism of radiated muscle. Therefore, radiation inhibits the energy capacity and contractile mechanisms necessary to make significant gains from repetitive, strength based exercises. Previous work by Carnaby-Mann et al. [76] evaluated the effectiveness of swallow weight-based training “Pharyngocise” in maintaining oropharyngeal function with 58 HNSCC patients. Exercises were administered consecutively with chemoradiation, and swallowing function was found to improve with exercise compared to controls. However, MRIs of the genioglossus, hyoglossus, and mylohyoid muscles in those patients who were prescribed “Pharyngocise” exhibited reduced T2 relaxation times at end of radiotherapy (6-weeks) compared to baseline, denoting a reduction in muscle size and composition. Further work is needed to determine the influence of the intensity and duration of the exercise challenge in maintaining swallowing function following radiation.
Electrical stimulation (i.e., neuromuscular [NMES], transcutaneous [TENS]) has also been introduced as prophylactic and/or rehabilitation approach for radiation-induced injury. However, the literature is controversial regarding the clinical and physiological effectiveness of this approach for improving muscle recruitment and swallow function [88–90]. Several studies by Ludlow [91,92] and others [93] have described the effect NMES on swallow function. Although these studies are not specific for HNSSC, they highlight possible limitations of this approach in irradiated tissue. First, a portion of the swallowing muscles thought to be most affected by irradiation are located deep beneath the skin (i.e., posterior larynx, pharynx), complicating the argument for its use as a motor-unit recruitment tool. Second, surface musculature have conflicting roles (e.g., sternohyoid and omohyoid covering thyrohyoid), which can cause unnatural simultaneous activation (i.e a dissension of the larynx during swallow) [94,95]. Of note, the therapeutic success of NMES is likely impacted by the timing of therapy, primary location site, purpose of the therapy (disordered movement [motor or sensory], diet, pain), and outcomes being measured (muscle force, movement, swallowing, diet, etc.).
The majority of the work undertaken with electrical stimulation in treating HNSSC patients has focused on treatments to counteract swallowing complications that occur after irradiation, and currently there are two published double-blinded randomized control trials. First, Ryu et al. [90] compared effects of NMES to low-intensity TENS with HNSSC (n = 26) ~16 days post radiation therapy and diagnosed with dysphagia. Both electrical stimulation therapies were given in concurrence with conventional swallow therapy and electrodes were placed above and below thyroid notch. A slight decline in functional dysphagia scale was found with NMES (33.9±13.2 pre to 22.4±13.4 post), denoting an improvement in severity of dysphagia. Interestingly, no significant differences were found in quality of life measures (i.e., M.D. Anderson dysphagia inventory) or clinical dysphagia scale. Secondly, Langmore et al. [88] studied the effects of NMES therapy, in HNCSS patients (n=170) with moderate-to-severe dysphagia, and at least 3 months after completion of radiation. NMES, administered to submental region, was compared to a sham treatment, and both were given congruently with traditional swallow exercises. The penetration-aspiration scores were found to be statistically reduced in sham group compared to those treated with NMES, although effect size reduced overall clinical relevance. However, both groups demonstrated significant improvements in diet and quality of life (Performance Status Scale and Head and Neck Cancer Inventory). These results suggest that NMES had little to no effect on the swallow function of HNSCC patients with chronic cases of radiation-induced dysphagia.
Given that irradiated tissue is plagued with aberrant muscle physiology (i.e., fibrosis, cell function), it is not surprising that aforementioned studies did not find remarkable differences with NMES. The stimulant was delivered at maximum intensity levels, which can lead to a high degree of muscle fatigue and propensity for stimulation-induced metabolic changes. Future clinical research should consider the pathophysiologic differences in radiation-induced injuries that were discussed in previous sections. In order to generate useful conclusions, well controlled studies are needed using stratified random sampling to divide subjects into relevant categories (i.e., primary radiation site, onset of dysphagia, time post-radiation) controlling important characteristics of the irradiated tissue environment that could alter responses to treatment.
Electrical stimulation therapy can also be used to modulate endogenous electrochemical pathways, enhancing wound healing and perfusion, as well as perturbing afferent sensory fibers controlling pain impulses. This application uses low frequencies and/or low intensity levels with no discernible muscle contraction. Although the approach is not directly aimed at improving range of motion, it intends to reduce negative side effects that often inhibit therapy compliance. One particular study by Bhatt et al. [96] tested the effects of low-voltage NMES as a prophylactic dysphagia treatment with advanced staged (TNM stages III and IV) laryngeal and pharyngeal HNSCC undergoing chemoradiation schemes. Although worsening swallowing function was found with both NMES treated [n=41] and control [n=54] groups, the severity of swallowing dysfunction was found to be significantly less with low-voltage NMES as indicated by functional oral intake scale. Of note, this particular subset of HNSCCs is at very high risk for radiation-induced dysphagia [23], so improvements in swallow activity with NMES may lead to greater oral intake, preventing effects of immobility. Although not studied, it would be particularly interesting to know if the electrical stimulation that was provided decreased pain associated with irradiation. Early case studies have shown that administering TENS at low frequencies (0.5Hz) and low amplitudes (50–500μAmp) during the period of radiation treatment of HNC resulted in fewer radiation interruptions [97,98]. Authors attributed the results to reduction of pain. More recently, preliminary findings from ongoing controlled clinical trial (double-blinded) comparing high frequency TENS to placebo (low intensity TENS) and sham treatment observed similar effects in reducing resting pain; however, none of the treatments tested had the effect of reducing functional pain or improving oral function [99,100]. Further research is warranted to ascertain the physiologic effects of prophylactic electrical stimulation treatment during irradiation.
Future Direction
Clinical information regarding the morphology and physiology of radiation-induced late-tissue changes to swallowing musculature is limited. Electrophysiology experiments in a clinical setting are challenging to perform. Additionally, obtaining tissue biopsies of laryngeal and pharyngeal structures require invasive procedures not generally performed unless recurrence is suspected. Lastly, subject availability is also a particular issue, as long-term routine follow-up care is not often conducted >5 years after completion of radiation and follow-up recidivism rates tend to be relatively high. Therefore, animal models are needed to study early and late radiation effects that attribute to dysphagia and compromise the airway. Several experimental rodent models have been used to analyze radiosensitivity of the mandible and salivary glands that can lead to mucositis, xerostomia, or osteoradionecrosis. Fenner et al. [101] analyzed histopathological changes to rat mandible after hyperfractioned stereotactic radiotherapy (15Gy × 4 fractions, over 6 wks). Their results demonstrated signs of fibrosis and necrosis including cell loss, hypovascularity, and increases in pro-fibrotic growth factors (i.e., TGF). Sonstevold et al. [102] studied histological and functional alteration following irradiation to mandible with 15Gy × 5 fractions administered within 63 day period. Results showed decreases in saliva, increase fibril diameter and decreased vasculature in skin, masticatory muscle and submandibular gland.
To date, there is only one animal study investigating radiation changes to swallowing muscle physiology. Russell and Connor [103] studied the structure and contractility after whole-body irradiation (11Gy × 2 fractions, within 2 day period) contoured using lead shielding to anterior digastric and genioglossus muscles in young and aged rats. Although no fibrotic changes where measured 12-weeks after irradiation, results showed physiologic changes in muscle activity as indicated by significant decreases in tongue forces, reduced speed of contraction, and increased fatigue during protrusion. This work indicates that the level of lingual dysfunction may be underestimated if judged solely on the basis of fibrosis. Further work is needed to determine how dysphagia is precipitated by radiation-induced changes within the late-responding tissues in laryngopharynx.
Acknowledgments
Funding: NIH HL111215; Kentucky Spinal Cord and Head Injury Trust and the Commonwealth of Kentucky Challenge for Excellence.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Society AC. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 2.Kuo P, Chen MM, Decker RH, Yarbrough WG, Judson BL. Hypopharyngeal cancer incidence, treatment, and survival: temporal trends in the United States. Laryngoscope. 2014;124(9):2064–2069. doi: 10.1002/lary.24651. [DOI] [PubMed] [Google Scholar]
- 3.Batth SS, Caudell JJ, Chen AM. Practical considerations in reducing swallowing dysfunction following concurrent chemoradiotherapy with intensity-modulated radiotherapy for head and neck cancer. Head Neck. 2014;36(2):291–298. doi: 10.1002/hed.23246. [DOI] [PubMed] [Google Scholar]
- 4.Fenwick JD, Pardo-Montero J, Nahum AE, Malik ZI. Impact of schedule duration on head and neck radiotherapy: accelerated tumor repopulation versus compensatory mucosal proliferation. Int J Radiat Oncol Biol Phys. 2012;82(2):1021–1030. doi: 10.1016/j.ijrobp.2010.12.048. [DOI] [PubMed] [Google Scholar]
- 5.Peters LJ, Ang KK, Thames HD., Jr Accelerated fractionation in the radiation treatment of head and neck cancer. A critical comparison of different strategies. Acta Oncol. 1988;27(2):185–194. doi: 10.3109/02841868809090339. [DOI] [PubMed] [Google Scholar]
- 6.Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, Marsh R, Pameijer FA, Balm AJ. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 7.Roe JW, Carding PN, Dwivedi RC, Kazi RA, Rhys-Evans PH, Harrington KJ, Nutting CM. Swallowing outcomes following Intensity Modulated Radiation Therapy (IMRT) for head & neck cancer - a systematic review. Oral Oncol. 2010;46(10):727–733. doi: 10.1016/j.oraloncology.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Ekberg O, Nylander G. Pharyngeal dysfunction after treatment for pharyngeal cancer with surgery and radiotherapy. Gastrointestinal radiology. 1983;8(2):97–104. doi: 10.1007/BF01948099. [DOI] [PubMed] [Google Scholar]
- 9.Starmer HM. Dysphagia in head and neck cancer: prevention and treatment. Curr Opin Otolaryngol Head Neck Surg. 2014;22(3):195–200. doi: 10.1097/MOO.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 10.Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment - tumorigenesis and therapy. Nature reviews Cancer. 2005;5(11):867–875. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- 11.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nature reviews Cancer. 2006;6(9):702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 12.Hopewell JW. Persistent and late occurring lesions in irradiated feet of rats: their clinical relevance. The British journal of radiology. 1982;55(656):574–578. doi: 10.1259/0007-1285-55-656-574. [DOI] [PubMed] [Google Scholar]
- 13.Hopewell JW, Foster JL, Young CM, Wiernik G. Late radiation damage to pig skin. Radiology. 1979;130(3):783–788. doi: 10.1148/130.3.783. [DOI] [PubMed] [Google Scholar]
- 14.Withers HR, Thames HD, Jr, Flow BL, Mason KA, Hussey DH. The relationship of acute to late skin injury in 2 and 5 fraction/week gamma-ray therapy. Int J Radiat Oncol Biol Phys. 1978;4(7–8):595–601. doi: 10.1016/0360-3016(78)90180-3. [DOI] [PubMed] [Google Scholar]
- 15.Castadot P, Geets X, Lee JA, Gregoire V. Adaptive functional image-guided IMRT in pharyngo-laryngeal squamous cell carcinoma: is the gain in dose distribution worth the effort? Radiother Oncol. 2011;101(3):343–350. doi: 10.1016/j.radonc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Dorr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol. 2001;61(3):223–231. doi: 10.1016/s0167-8140(01)00429-7. [DOI] [PubMed] [Google Scholar]
- 17.Denham JW, Hauer-Jensen M. The radiotherapeutic injury–a complex ‘wound’. Radiother Oncol. 2002;63(2):129–145. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 18.Dörr W. Modulation of repopulation processes in oral mucosa: experimental results. International Journal of Radiation Biology. 2003;79(7):531–537. doi: 10.1080/09553002310001600925. [DOI] [PubMed] [Google Scholar]
- 19.Dörr W, Hamilton CS, Boyd T, Reed B, Denham JW. Radiation-induced changes in cellularity and proliferation in human oral mucosa. International Journal of Radiation Oncology Biology Physics. 2002;52(4):911–917. doi: 10.1016/S0360-3016(01)02721-3. [DOI] [PubMed] [Google Scholar]
- 20.Kam WW, Banati RB. Effects of ionizing radiation on mitochondria. Free radical biology & medicine. 2013;65:607–619. doi: 10.1016/j.freeradbiomed.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Yamamori T, Yasui H, Yamazumi M, Wada Y, Nakamura Y, Nakamura H, Inanami O. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free radical biology & medicine. 2012;53(2):260–270. doi: 10.1016/j.freeradbiomed.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 22.Logemann JA, Pauloski BR, Rademaker AW, Lazarus CL, Gaziano J, Stachowiak L, Newman L, MacCracken E, Santa D, Mittal B. Swallowing disorders in the first year after radiation and chemoradiation. Head Neck. 2008;30(2):148–158. doi: 10.1002/hed.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Logemann JA, Rademaker AW, Pauloski BR, Lazarus CL, Mittal BB, Brockstein B, MacCracken E, Haraf DJ, Vokes EE, Newman LA, Liu D. Site of disease and treatment protocol as correlates of swallowing function in patients with head and neck cancer treated with chemoradiation. Head Neck. 2006;28(1):64–73. doi: 10.1002/hed.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pauloski BR, Rademaker AW, Logemann JA, Lundy D, Bernstein M, McBreen C, Santa D, Campanelli A, Kelchner L, Klaben B, Discekici-Harris M. Relation of mucous membrane alterations to oral intake during the first year after treatment for head and neck cancer. Head Neck. 2011;33(6):774–779. doi: 10.1002/hed.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wygoda A, Rutkowski T, Hutnik M, Składowski K, Goleń M, Pilecki B. Acute mucosal reactions in patients with head and neck cancer. Three patterns of mucositis observed during radiotherapy. Strahlenther Onkol. 2013;189(7):547–551. doi: 10.1007/s00066-013-0311-8. [DOI] [PubMed] [Google Scholar]
- 26.Rademaker AW, Vonesh EF, Logemann JA, Pauloski BR, Liu D, Lazarus CL, Newman LA, May AH, MacCracken E, Gaziano J, Stachowiak L. Eating ability in head and neck cancer patients after treatment with chemoradiation: a 12-month follow-up study accounting for dropout. Head Neck. 2003;25(12):1034–1041. doi: 10.1002/hed.10317. [DOI] [PubMed] [Google Scholar]
- 27.Silver HJ, Dietrich MS, Murphy BA. Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck. 2007;29(10):893–900. doi: 10.1002/hed.20607. [DOI] [PubMed] [Google Scholar]
- 28.Denham JW, Peters LJ, Johansen J, Poulsen M, Lamb DS, Hindley A, O’Brien PC, Spry Na, Penniment M, Krawitz H, Williamson S, Bear J, Tripcony L. Do acute mucosal reactions lead to consequential late reactions in patients with head and neck cancer? Radiotherapy and Oncology. 1999;52(2):157–164. doi: 10.1016/S0167-8140(99)00107-3. [DOI] [PubMed] [Google Scholar]
- 29.Jung H, Beck-Bornholdt HP, Svoboda V, Alberti W, Herrmann T. Quantification of late complications after radiation therapy. Radiother Oncol. 2001;61(3):233–246. doi: 10.1016/s0167-8140(01)00457-1. [DOI] [PubMed] [Google Scholar]
- 30.Lin YS, Jen YM, Lin JC. Radiation-related cranial nerve palsy in patients with nasopharyngeal carcinoma. Cancer. 2002;95(2):404–409. doi: 10.1002/cncr.10668. [DOI] [PubMed] [Google Scholar]
- 31.Remy J, Wegrowski J, Crechet F, Martin M, Daburon F. Long-term overproduction of collagen in radiation-induced fibrosis. Radiat Res. 1991;125(1):14–19. [PubMed] [Google Scholar]
- 32.Martin M, Lefaix JL, Pinton P, Crechet F, Daburon F. Temporal modulation of TGF-beta 1 and beta-actin gene expression in pig skin and muscular fibrosis after ionizing radiation. Radiat Res. 1993;134(1):63–70. [PubMed] [Google Scholar]
- 33.Wegrowski J, Lefaix JL, Lafuma C. Accumulation of glycosaminoglycans in radiation-induced muscular fibrosis. Int J Radiat Biol. 1992;61(5):685–693. doi: 10.1080/09553009214551501. [DOI] [PubMed] [Google Scholar]
- 34.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80(4):251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 35.Rudolph R, Arganese T, Woodward M. The ultrastructure and etiology of chronic radiotherapy damage in human skin. Annals of plastic surgery. 1982;9(4):282–292. doi: 10.1097/00000637-198210000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Martin M, Remy J, Daburon F. Abnormal proliferation and aging of cultured fibroblasts from pigs with subcutaneous fibrosis induced by gamma irradiation. The Journal of investigative dermatology. 1989;93(4):497–500. doi: 10.1111/1523-1747.ep12284053. [DOI] [PubMed] [Google Scholar]
- 37.el Nabout R, Martin M, Remy J, Kern P, Robert L, Lafuma C. Collagen synthesis and deposition in cultured fibroblasts from subcutaneous radiation-induced fibrosis. Modification as a function of cell aging. Matrix (Stuttgart, Germany) 1989;9(5):411–420. doi: 10.1016/s0934-8832(89)80047-2. [DOI] [PubMed] [Google Scholar]
- 38.Rodningen OK, Overgaard J, Alsner J, Hastie T, Borresen-Dale AL. Microarray analysis of the transcriptional response to single or multiple doses of ionizing radiation in human subcutaneous fibroblasts. Radiother Oncol. 2005;77(3):231–240. doi: 10.1016/j.radonc.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Rodningen OK, Borresen-Dale AL, Alsner J, Hastie T, Overgaard J. Radiation-induced gene expression in human subcutaneous fibroblasts is predictive of radiation-induced fibrosis. Radiother Oncol. 2008;86(3):314–320. doi: 10.1016/j.radonc.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 40.van der Laan HP, Bijl HP, Steenbakkers RJ, van der Schaaf A, Chouvalova O, Vemer-van den Hoek JG, Gawryszuk A, van der Laan BF, Oosting SF, Roodenburg JL, Wopken K, Langendijk JA. Acute symptoms during the course of head and neck radiotherapy or chemoradiation are strong predictors of late dysphagia. Radiother Oncol. 2015 doi: 10.1016/j.radonc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 41.Hutcheson KA, Lewin JS, Barringer DA, Lisec A, Gunn GB, Moore MW, Holsinger FC. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer. 2012;118(23):5793–5799. doi: 10.1002/cncr.27631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutcheson KA. Late Radiation-Associated Dysphagia (RAD) in Head and Neck Cancer Survivors. 2015:61–72. doi: 10.1002/hed.23840. [DOI] [PubMed] [Google Scholar]
- 43.Eisele DW, Koch DG, Tarazi AE, Jones B. Case report: aspiration from delayed radiation fibrosis of the neck. Dysphagia. 1991;6(2):120–122. doi: 10.1007/BF02493488. [DOI] [PubMed] [Google Scholar]
- 44.Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer: frequency, intensity and correlation with dose and volume parameters. Radiother Oncol. 2007;85(1):74–82. doi: 10.1016/j.radonc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen NP, Moltz CC, Frank C, Vos P, Smith HJ, Karlsson U, Dutta S, Midyett FA, Barloon J, Sallah S. Dysphagia following chemoradiation for locally advanced head and neck cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2004;15(3):383–388. doi: 10.1093/annonc/mdh101. [DOI] [PubMed] [Google Scholar]
- 46.Wall LR, Ward EC, Cartmill B, Hill AJ. Physiological changes to the swallowing mechanism following (chemo)radiotherapy for head and neck cancer: a systematic review. Dysphagia. 2013;28(4):481–493. doi: 10.1007/s00455-013-9491-8. [DOI] [PubMed] [Google Scholar]
- 47.Levendag PC, Teguh DN, Voet P, van der Est H, Noever I, de Kruijf WJ, Kolkman-Deurloo IK, Prevost JB, Poll J, Schmitz PI, Heijmen BJ. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403–409. doi: 10.1016/j.ijrobp.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 49.Caglar HB, Tishler RB, Othus M, Burke E, Li Y, Goguen L, Wirth LJ, Haddad RI, Norris CM, Court LE, Aninno DJ, Posner MR, Allen AM. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110–1118. doi: 10.1016/j.ijrobp.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 50.Starmer HM, Quon H, Kumar R, Alcorn S, Murano E, Jones B, Humbert I. The Effect of Radiation Dose on Swallowing: Evaluation of Aspiration and Kinematics. Dysphagia. 2015;30(4):430–437. doi: 10.1007/s00455-015-9618-1. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz DL, Hutcheson K, Barringer D, Tucker SL, Kies M, Holsinger FC, Ang KK, Morrison WH, Rosenthal DI, Garden AS, Dong L, Lewin JS. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356–1365. doi: 10.1016/j.ijrobp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar R, Madanikia S, Starmer H, Yang W, Murano E, Alcorn S, McNutt T, Le Y, Quon H. Radiation dose to the floor of mouth muscles predicts swallowing complications following chemoradiation in oropharyngeal squamous cell carcinoma. Oral Oncol. 2014;50(1):65–70. doi: 10.1016/j.oraloncology.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Amdur RJ, Li JG, Liu C, Hinerman RW, Mendenhall WM. Unnecessary laryngeal irradiation in the IMRT era. Head Neck. 2004;26(3):257–263. doi: 10.1002/hed.10379. discussion 263–254. [DOI] [PubMed] [Google Scholar]
- 54.Dornfeld K, Simmons JR, Karnell L, Karnell M, Funk G, Yao M, Wacha J, Zimmerman B, Buatti JM. Radiation doses to structures within and adjacent to the larynx are correlated with long-term diet- and speech-related quality of life. Int J Radiat Oncol Biol Phys. 2007;68(3):750–757. doi: 10.1016/j.ijrobp.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 55.Sanguineti G, Adapala P, Endres EJ, Brack C, Fiorino C, Sormani MP, Parker B. Dosimetric predictors of laryngeal edema. Int J Radiat Oncol Biol Phys. 2007;68(3):741–749. doi: 10.1016/j.ijrobp.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 56.Szczesniak MM, Maclean J, Zhang T, Graham PH, Cook IJ. Persistent dysphagia after head and neck radiotherapy: a common and under-reported complication with significant effect on non-cancer-related mortality. Clin Oncol (R Coll Radiol) 2014;26(11):697–703. doi: 10.1016/j.clon.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Coppes RP, Vissink A, Konings AW. Comparison of radiosensitivity of rat parotid and submandibular glands after different radiation schedules. Radiother Oncol. 2002;63(3):321–328. doi: 10.1016/s0167-8140(02)00129-9. [DOI] [PubMed] [Google Scholar]
- 58.Gillette EL, Mahler PA, Powers BE, Gillette SM, Vujaskovic Z. Late radiation injury to muscle and peripheral nerves. Int J Radiat Oncol Biol Phys. 1995;31(5):1309–1318. doi: 10.1016/0360-3016(94)00422-h. [DOI] [PubMed] [Google Scholar]
- 59.Bleier BS, Levine MS, Mick R, Rubesin SE, Sack SZ, McKinney K, Mirza N. Dysphagia after chemoradiation: analysis by modified barium swallow. Ann Otol Rhinol Laryngol. 2007;116(11):837–841. doi: 10.1177/000348940711601108. [DOI] [PubMed] [Google Scholar]
- 60.Bergstrom RM, Salmi A. Radiation-induced damage in the ultrastructure of striated muscle. Exp Cell Res. 1962;26:226–228. doi: 10.1016/0014-4827(62)90222-7. [DOI] [PubMed] [Google Scholar]
- 61.Bergstrom RM, Blafield R, Salmi A. The effect of x-irradiation on the electrical and mechanical activity of striated frog muscle. Int J Radiat Biol. 1962;4:351–361. doi: 10.1080/09553006214550161. [DOI] [PubMed] [Google Scholar]
- 62.Khan MY. Radiation-induced changes in skeletal muscle. An electron microscopic study. Journal of neuropathology and experimental neurology. 1974;33(1):42–57. doi: 10.1097/00005072-197401000-00004. [DOI] [PubMed] [Google Scholar]
- 63.Schwenen M, Altman KI, Schroder W. Radiation-induced increase in the release of amino acids by isolated, perfused skeletal muscle. Int J Radiat Biol. 1989;55(2):257–269. doi: 10.1080/09553008914550291. [DOI] [PubMed] [Google Scholar]
- 64.Hardee JP, Puppa MJ, Fix DK, Gao S, Hetzler KL, Bateman TA, Carson JA. The effect of radiation dose on mouse skeletal muscle remodeling. Radiol Oncol. 2014;48(3):247–256. doi: 10.2478/raon-2014-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mu L, Sanders I. Neuromuscular compartments and fiber-type regionalization in the human inferior pharyngeal constrictor muscle. Anat Rec. 2001;264(4):367–377. doi: 10.1002/ar.10020. [DOI] [PubMed] [Google Scholar]
- 66.Lazarus CL, Logemann JA, Pauloski BR, Colangelo LA, Kahrilas PJ, Mittal BB, Pierce M. Swallowing disorders in head and neck cancer patients treated with radiotherapy and adjuvant chemotherapy. Laryngoscope. 1996;106(9 Pt 1):1157–1166. doi: 10.1097/00005537-199609000-00021. [DOI] [PubMed] [Google Scholar]
- 67.Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H(2)O(2) generation. American journal of physiology Cell physiology. 2006;290(3):C844–851. doi: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- 68.Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacology & therapeutics. 2011;131(1):142–170. doi: 10.1016/j.pharmthera.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Viet CT, Corby PM, Akinwande A, Schmidt BL. Review of preclinical studies on treatment of mucositis and associated pain. J Dent Res. 2014;93(9):868–875. doi: 10.1177/0022034514540174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simonyan K, Feng X, Henriquez VM, Ludlow CL. Combined laryngeal inflammation and trauma mediate long-lasting immunoreactivity response in the brainstem sensory nuclei in the rat. Frontiers in integrative neuroscience. 2012;6:97. doi: 10.3389/fnint.2012.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia. 2010;25(4):323–333. doi: 10.1007/s00455-010-9301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rong X, Tang Y, Chen M, Lu K, Peng Y. Radiation-induced cranial neuropathy in patients with nasopharyngeal carcinoma. A follow-up study. Strahlenther Onkol. 2012;188(3):282–286. doi: 10.1007/s00066-011-0047-2. [DOI] [PubMed] [Google Scholar]
- 73.Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004;73(2):119–131. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 74.Krisciunas GP, Sokoloff W, Stepas K, Langmore SE. Survey of usual practice: dysphagia therapy in head and neck cancer patients. Dysphagia. 2012;27(4):538–549. doi: 10.1007/s00455-012-9404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kraaijenga SA, van der Molen L, Jacobi I, Hamming-Vrieze O, Hilgers FJ, van den Brekel MW. Prospective clinical study on long-term swallowing function and voice quality in advanced head and neck cancer patients treated with concurrent chemoradiotherapy and preventive swallowing exercises. Eur Arch Otorhinolaryngol. 2015;272(11):3521–3531. doi: 10.1007/s00405-014-3379-6. [DOI] [PubMed] [Google Scholar]
- 76.Carnaby-Mann G, Crary MA, Schmalfuss I, Amdur R. “Pharyngocise”: randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(1):210–219. doi: 10.1016/j.ijrobp.2011.06.1954. [DOI] [PubMed] [Google Scholar]
- 77.Kulbersh BD, Rosenthal EL, McGrew BM, Duncan RD, McColloch NL, Carroll WR, Magnuson JS. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. Laryngoscope. 2006;116(6):883–886. doi: 10.1097/01.mlg.0000217278.96901.fc. [DOI] [PubMed] [Google Scholar]
- 78.Paleri V, Roe JW, Strojan P, Corry J, Gregoire V, Hamoir M, Eisbruch A, Mendenhall WM, Silver CE, Rinaldo A, Takes RP, Ferlito A. Strategies to reduce long-term postchemoradiation dysphagia in patients with head and neck cancer: an evidence-based review. Head Neck. 2014;36(3):431–443. doi: 10.1002/hed.23251. [DOI] [PubMed] [Google Scholar]
- 79.Hutcheson KA, Bhayani MK, Beadle BM, Gold KA, Shinn EH, Lai SY, Lewin J. Eat and exercise during radiotherapy or chemoradiotherapy for pharyngeal cancers: use it or lose it. JAMA otolaryngology– head & neck surgery. 2013;139(11):1127–1134. doi: 10.1001/jamaoto.2013.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Lisio M, Kaczor JJ, Phan N, Tarnopolsky MA, Boreham DR, Parise G. Exercise training enhances the skeletal muscle response to radiation-induced oxidative stress. Muscle Nerve. 2011;43(1):58–64. doi: 10.1002/mus.21797. [DOI] [PubMed] [Google Scholar]
- 81.Hood DA. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol (1985) 2001;90(3):1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- 82.Adhihetty PJ, Irrcher I, Joseph AM, Ljubicic V, Hood DA. Plasticity of skeletal muscle mitochondria in response to contractile activity. Experimental physiology. 2003;88(1):99–107. doi: 10.1113/eph8802505. [DOI] [PubMed] [Google Scholar]
- 83.Scheele C, Nielsen S, Pedersen BK. ROS and myokines promote muscle adaptation to exercise. Trends in endocrinology and metabolism: TEM. 2009;20(3):95–99. doi: 10.1016/j.tem.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 84.Venditti P, Masullo P, Di Meo S. Effect of training on H(2)O(2) release by mitochondria from rat skeletal muscle. Archives of biochemistry and biophysics. 1999;372(2):315–320. doi: 10.1006/abbi.1999.1494. [DOI] [PubMed] [Google Scholar]
- 85.Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol (1985) 2009;107(3):645–654. doi: 10.1152/japplphysiol.00452.2009. [DOI] [PubMed] [Google Scholar]
- 86.Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586(Pt 24):6049–6061. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, Ferrando AA. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. The Journal of clinical endocrinology and metabolism. 2006;91(12):4836–4841. doi: 10.1210/jc.2006-0651. [DOI] [PubMed] [Google Scholar]
- 88.Langmore SE, McCulloch TM, Krisciunas GP, Lazarus CL, Van Daele DJ, Pauloski BR, Rybin D, Doros G. Efficacy of electrical stimulation and exercise for dysphagia in patients with head and neck cancer: A randomized clinical trial. Head Neck. 2015 doi: 10.1002/hed.24197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin PH, Hsiao TY, Chang YC, Ting LL, Chen WS, Chen SC, Wang TG. Effects of functional electrical stimulation on dysphagia caused by radiation therapy in patients with nasopharyngeal carcinoma. Support Care Cancer. 2011;19(1):91–99. doi: 10.1007/s00520-009-0792-2. [DOI] [PubMed] [Google Scholar]
- 90.Ryu JS, Kang JY, Park JY, Nam SY, Choi SH, Roh JL, Kim SY, Choi KH. The effect of electrical stimulation therapy on dysphagia following treatment for head and neck cancer. Oral Oncol. 2009;45(8):665–668. doi: 10.1016/j.oraloncology.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 91.Humbert IA, Poletto CJ, Saxon KG, Kearney PR, Crujido L, Wright-Harp W, Payne J, Jeffries N, Sonies BC, Ludlow CL. The effect of surface electrical stimulation on hyolaryngeal movement in normal individuals at rest and during swallowing. J Appl Physiol (1985) 2006;101(6):1657–1663. doi: 10.1152/japplphysiol.00348.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ludlow CL, Humbert I, Saxon K, Poletto C, Sonies B, Crujido L. Effects of Surface Electrical Stimulation Both at Rest and During Swallowing in Chronic Pharyngeal Dysphagia. Dysphagia. 2007;22(1):1–10. doi: 10.1007/s00455-006-9029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suiter DM, Leder SB, Ruark JL. Effects of neuromuscular electrical stimulation on submental muscle activity. Dysphagia. 2006;21(1):56–60. doi: 10.1007/s00455-005-9010-7. [DOI] [PubMed] [Google Scholar]
- 94.Ludlow CL. Electrical neuromuscular stimulation in dysphagia: current status. Curr Opin Otolaryngol Head Neck Surg. 2010;18(3):159–164. doi: 10.1097/MOO.0b013e3283395dec. [DOI] [PubMed] [Google Scholar]
- 95.Humbert IA, Michou E, MacRae PR, Crujido L. Electrical stimulation and swallowing: how much do we know? Seminars in speech and language. 2012;33(3):203–216. doi: 10.1055/s-0032-1320040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhatt AD, Goodwin N, Cash E, Bhatt G, Silverman CL, Spanos WJ, Bumpous JM, Potts K, Redman R, Allison WA, Dunlap NE. Impact of transcutaneous neuromuscular electrical stimulation on dysphagia in patients with head and neck cancer treated with definitive chemoradiation. Head Neck. 2015;37(7):1051–1056. doi: 10.1002/hed.23708. [DOI] [PubMed] [Google Scholar]
- 97.Bauer W. Electrical treatment of severe head and neck cancer pain. Arch Otolaryngol. 1983;109(6):382–383. doi: 10.1001/archotol.1983.00800200028009. [DOI] [PubMed] [Google Scholar]
- 98.Boswell NS, Bauer W. Noninvasive electrical stimulation for the treatment of radiotherapy side-effects; Presented at the International Conference on Head and Neck Cancer; Baltimore, Maryland. July, 1984; Triologic Society of Otolaryngology; New Orleans, Louisiana. January, 1985; 1985. [Google Scholar]
- 99.Lee J, Rakel B, Dailey D, Vance C, Broderick A, Sleeuwenhoek B, Perkhounkova Y, Sluka K, Anderson C. (375) Transcutaneous Electrical Nerve Stimulation (TENS) reduces head and neck cancer pain: a randomized and placebo-controlled double blind pilot study. The Journal of Pain. 15(4):S69. doi: 10.1016/j.jpain.2014.01.286. [DOI] [Google Scholar]
- 100.Lee J, Rakel B, Dailey D, Vance C, Broderick A, Sluka K, Anderson C. The effectiveness of TENS for head and neck cancer pain and function: a randomized and placebo-controlled double blind pilot study. The Journal of Pain. 13(4):S63. doi: 10.1016/j.jpain.2012.01.265. [DOI] [Google Scholar]
- 101.Fenner M, Park J, Schulz N, Amann K, Grabenbauer GG, Fahrig A, Karg J, Wiltfang J, Neukam FW, Nkenke E. Validation of histologic changes induced by external irradiation in mandibular bone. An experimental animal model. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2010;38(1):47–53. doi: 10.1016/j.jcms.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 102.Sonstevold T, Johannessen AC, Stuhr L. A rat model of radiation injury in the mandibular area. Radiat Oncol. 2015;10:129. doi: 10.1186/s13014-015-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Russell JA, Connor NP. Effects of age and radiation treatment on function of extrinsic tongue muscles. Radiat Oncol. 2014;9:254. doi: 10.1186/s13014-014-0254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


