Abstract
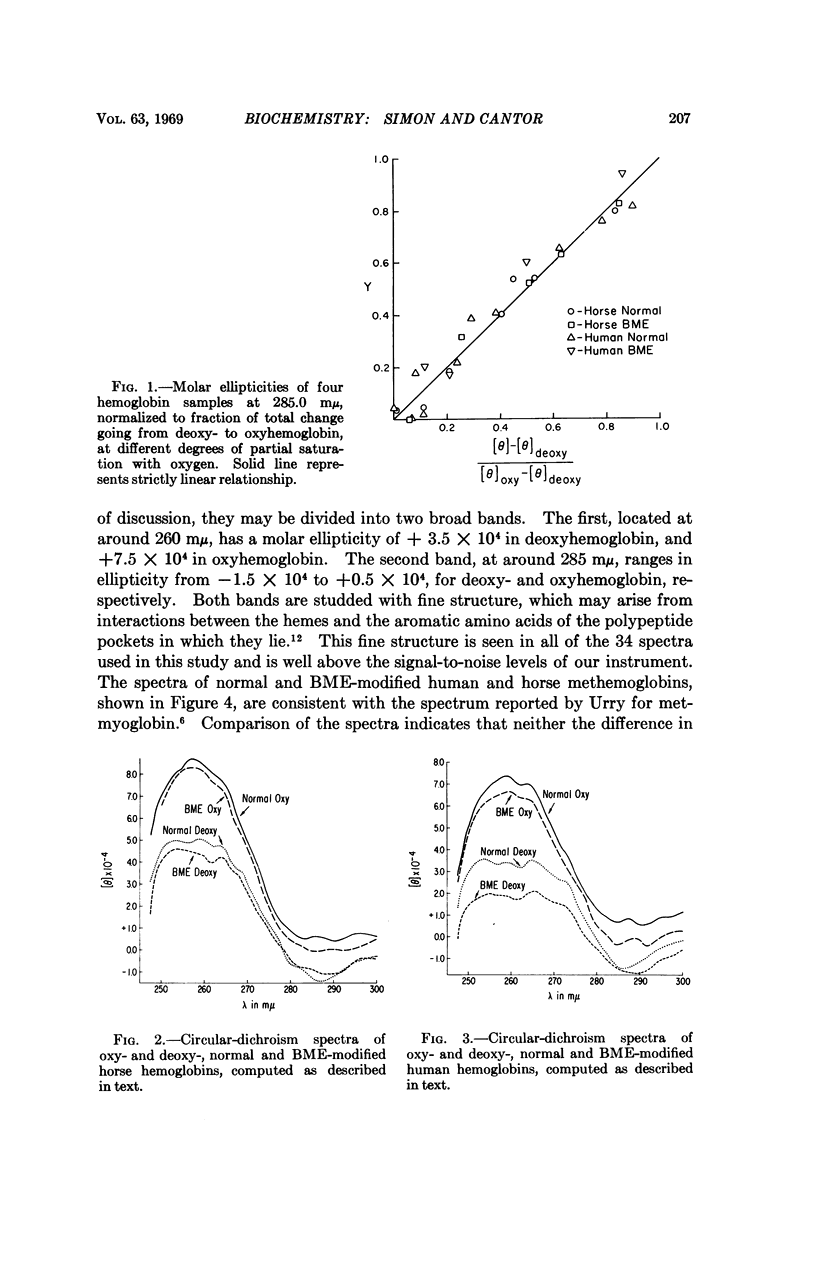
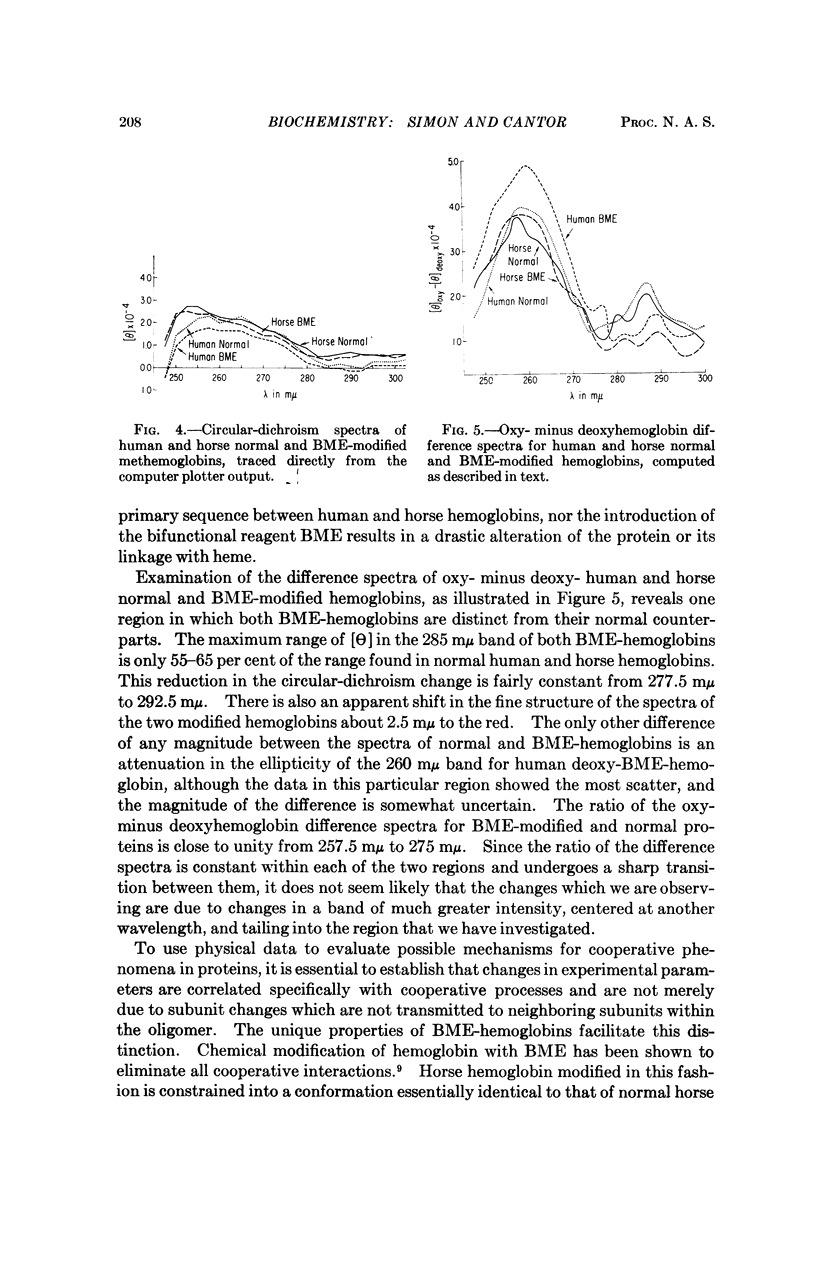
The UV circular-dichroism spectra of human and horse hemoglobins have been determined at various degrees of partial saturation with oxygen. Spectra of the two native hemoglobins were compared with spectra of the corresponding proteins modified with a reagent known to eliminate the conformational rearrangement normally associated with cooperativity. Such comparison indicates that one region, around 260 mμ, is sensitive chiefly to the state of the hemes; changes in another region, around 285 mμ, may be correlated with the conformational transformation linked to cooperative interactions. All circular-dichroism changes are strictly linear with fractional saturation with oxygen. Possible implications of these results to recently proposed mechanisms for cooperativity in proteins are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonini E., Chiancone E., Brunori M. Studies on the relations between molecular and functional properties of hemoglobin. VI. Observations on the kinetics of hemoglobin reactions in concentrated salt solutions. J Biol Chem. 1967 Oct 10;242(19):4360–4366. [PubMed] [Google Scholar]
- BENESCH R., MACDUFF G., BENESCH R. E. DETERMINATION OF OXYGEN EQUILIBRIA WITH A VERSATILE NEW TONOMETER. Anal Biochem. 1965 Apr;11:81–87. doi: 10.1016/0003-2697(65)90045-x. [DOI] [PubMed] [Google Scholar]
- Beychok S., Tyuma I., Benesch R. E., Benesch R. Optically active absorption bands of hemoglobin and its subunits. J Biol Chem. 1967 May 25;242(10):2460–2462. [PubMed] [Google Scholar]
- Brunori M., Engel J., Schuster T. M. The effect of ligand binding on the optical rotatory dispersion of myoglobin, hemoglobin, and isolated hemoglobin subunits. J Biol Chem. 1967 Feb 25;242(4):773–776. [PubMed] [Google Scholar]
- Gibson Q. H., Parkhurst L. J. Kinetic evidence for a tetrameric functional unit in hemoglobin. J Biol Chem. 1968 Oct 25;243(20):5521–5524. [PubMed] [Google Scholar]
- Guidotti G. Studies on the chemistry of hemoglobin. 3. The interactions of the alpha-beta subunits of hemoglobin. J Biol Chem. 1967 Aug 25;242(16):3694–3703. [PubMed] [Google Scholar]
- Javaherian K., Beychok S. Subunit interactions in the conformational change of horse apohemoglobin on binding of hemin. J Mol Biol. 1968 Oct 14;37(1):1–11. doi: 10.1016/0022-2836(68)90069-7. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Miurhead H., Cox J. M., Goaman L. C., Mathews F. S., McGandy E. L., Webb L. E. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: (1) x-ray analysis. Nature. 1968 Jul 6;219(5149):29–32. doi: 10.1038/219029a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Reichlin M., Bucci E., Fronticelli C., Wyman J., Antonini E., Ioppolo C., Rossi-Fanelli A. The properties and interactions of the isolated alpha- and beta-chains of human haemoglobin. IV. Immunological studies involving antibodies against the isolated chains. J Mol Biol. 1966 May;17(1):18–28. doi: 10.1016/s0022-2836(66)80091-8. [DOI] [PubMed] [Google Scholar]
- SPOEK G. L., BAKKER H., WOLVEKAMP H. P. EXPERIMENTS ON THE HAEMOCYANIN-OXYGEN EQUILIBRIUM OF THE BLOOD OF THE EDIBLE SNAIL (HELIX POMATIA L.). Comp Biochem Physiol. 1964 Jun;12:209–221. doi: 10.1016/0010-406x(64)90175-6. [DOI] [PubMed] [Google Scholar]
- Simon S. R., Konigsberg W. H., Bolton W., Perutz M. F. Identity of structure of horse deoxy- and oxyhaemoglobin after reaction with bis(N-maleidomethyl)ether. J Mol Biol. 1967 Sep 28;28(3):451–454. doi: 10.1016/s0022-2836(67)80094-9. [DOI] [PubMed] [Google Scholar]
- Simon S. R., Konigsberg W. H. Chemical modification of hemoglobins: a study of conformation restraint by internal bridging. Proc Natl Acad Sci U S A. 1966 Aug;56(2):749–756. doi: 10.1073/pnas.56.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W. The heme chromophore in the ultraviolet. J Biol Chem. 1967 Oct 10;242(19):4441–4448. [PubMed] [Google Scholar]


