Abstract
Electronic excitation energy can be transferred between chromophores separated by distances of the order of 30 Å. Förster proposed that the transfer occurs by a dipole-dipole resonance interaction which depends on certain spectroscopic and geometric properties of the donor-acceptor pair. His prediction that the rate of transfer depends on the inverse sixth power of the distance between the chromophores was verified previously. In this work, we tested a second prediction of Förster's theory, namely, that the transfer rate is proportional to J, the magnitude of the overlap between the emission spectrum of the energy donor and the absorption spectrum of the energy acceptor.
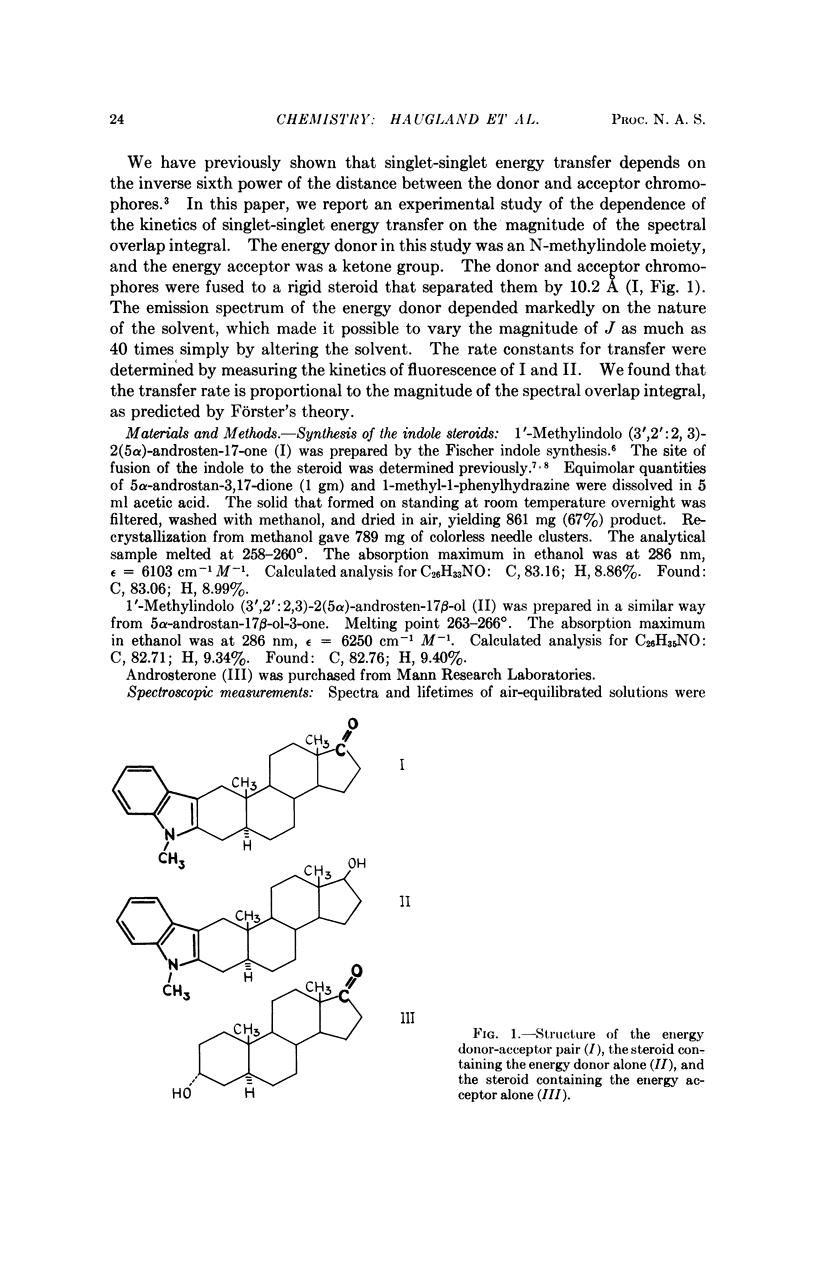
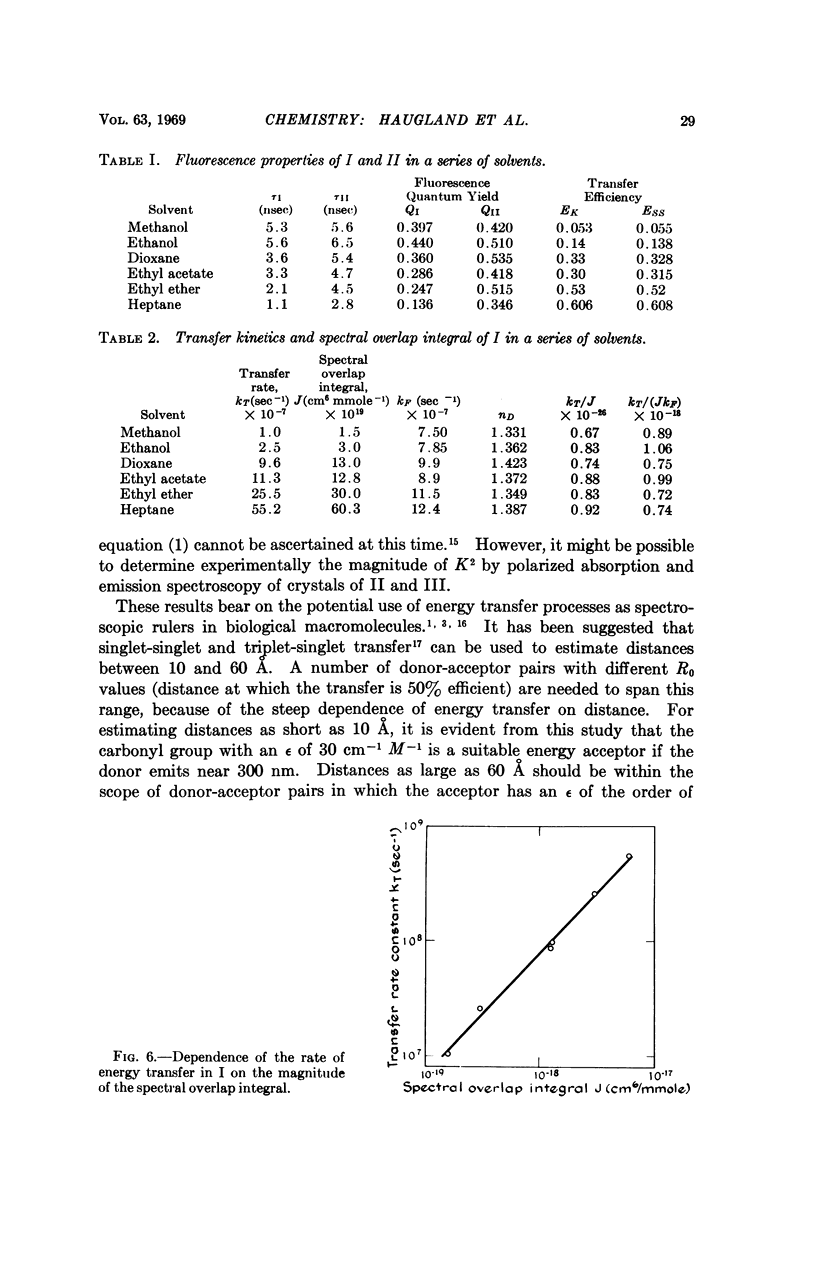
The energy donor was an N-methylindole moiety, and the acceptor was a ketone. These chromophores were fused to a rigid steroid that separated them by 10.2 Å. Rate constants for singlet-singlet energy transfer in this system were obtained by nanosecond flash spectroscopy. J was varied over a 40-fold range simply by altering the solvent. We found that the transfer rate is proportional to J, as predicted by Förster's theory. The results bear on the potential use of this energy transfer process to measure distances in biological macromolecules. It is evident that the length of such a spectroscopic ruler can readily be controlled by varying the magnitude of the spectral overlap integral of the energy donor-acceptor pair.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ban Y., Sato Y. Studies on the structures of some cholestanoindoles. Chem Pharm Bull (Tokyo) 1965 Sep;13(9):1073–1077. doi: 10.1248/cpb.13.1073. [DOI] [PubMed] [Google Scholar]
- Galley W. C. On the triplet states of polynucleotide--acridine complexes. I. Triplet energy delocalization in the 9-aminoacridine-DNA complex. Biopolymers. 1968;6(9):1279–1296. doi: 10.1002/bip.1968.360060905. [DOI] [PubMed] [Google Scholar]
- Galley W. C., Stryer L. Triplet-triplet energy transfer in proteins as a criterion of proximity. Proc Natl Acad Sci U S A. 1968 May;60(1):108–114. doi: 10.1073/pnas.60.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundley L., Coburn T., Garwin E., Stryer L. Nanosecond fluorimeter. Rev Sci Instrum. 1967 Apr;38(4):488–492. doi: 10.1063/1.1720743. [DOI] [PubMed] [Google Scholar]
- LATT S. A., CHEUNG H. T., BLOUT E. R. ENERGY TRANSFER. A SYSTEM WITH RELATIVELY FIXED DONOR-ACCEPTOR SEPARATION. J Am Chem Soc. 1965 Mar 5;87:995–1003. doi: 10.1021/ja01083a011. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence spectroscopy of proteins. Science. 1968 Nov 1;162(3853):526–533. doi: 10.1126/science.162.3853.526. [DOI] [PubMed] [Google Scholar]
- Stryer L., Haugland R. P. Energy transfer: a spectroscopic ruler. Proc Natl Acad Sci U S A. 1967 Aug;58(2):719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J Mol Biol. 1965 Sep;13(2):482–495. doi: 10.1016/s0022-2836(65)80111-5. [DOI] [PubMed] [Google Scholar]


