Abstract
Cancer growth and metastasis depends on the availability of energy. Energy-sensing systems are critical in maintaining a balance between the energy supply and utilization of energy for tumor growth. A central regulator in this process is AMP-activated protein kinase (AMPK). In times of energy deficit, AMPK is allosterically modified by the binding of increased levels of AMP and ADP, making it a target of specific AMPK kinases (AMPKKs). AMPK signaling prompts cells to produce energy at the expense of growth and motility, opposing the actions of insulin and growth factors. Increasing AMPK activity may thus prevent the proliferation and metastasis of tumor cells. Activated AMPK also suppresses aromatase, which lowers estrogen formation and prevents breast cancer growth. Biguanides can be used to activate AMPK, but AMPK activity is modified by many different interacting factors; understanding these factors is important in order to control the abnormal growth processes that lead to breast cancer neoplasia. Fatty acids, estrogens, androgens, adipokines, and another energy sensor, sirtuin-1, alter the phosphorylation and activation of AMPK. Isoforms of AMPK differ among tissues and may serve specific functions. Targeting AMPK regulatory processes at points other than the upstream AMPKKs may provide additional approaches for prevention of breast cancer neoplasia, growth, and metastasis.
Keywords: Breast cancer, AMP-dependent protein kinase, biguanides
Much attention is currently being paid to altered metabolism as it relates to progression of breast cancer [1]. Observations of responses to metformin in diabetic patients has been an instigator of this approach [2,3]. As one of the primary points in the triage of energy flow, AMP-activated protein kinase (AMPK) is the focus of attention. AMPK responds allosterically to increases in the ratio of AMP/ATP, enhancing AMPK activation by AMPK kinases (AMPKKs) [4]. AMPK is also modified indirectly by hormones, adipokines, sirtuin-1, and metabolic products. The effect of AMPK activation on aromatase within the breast is of particular interest in postmenopausal women with breast cancer [5]. Synergism of biguanides with tamoxifen, aromatase inhibitors or trastuzumab/lapatinib may have particular application in breast cancer. This review is focused on the regulation of metabolism by AMPK in breast cancer and the optimal application of biguanides for treatment.
Structure of AMPK
AMPK plays an important role in energy balance at both cellular and whole body levels by balancing nutrient supply and demand. It is a heterotrimeric complex that consists of a catalytic (α) and two regulatory (β and γ) subunits [4,6,7]. There are two isoforms of the AMPKα subunit (AMPKα1 and AMPKα2), two isoforms of the β subunit (AMPKβ1 and AMPKβ2), and three isoforms of the γ subunit (AMPKγ1, AMPKγ2, and AMPKγ3), each of which is encoded by a separate gene [6]. The catalytic α-subunit is composed of a serine/threonine kinase domain containing a threonine residue (Thr172) that is phosphorylated by upstream kinases, an auto-inhibitory domain (AID) with a negative effect on kinase activity, a linker region, and an α-subunit carboxy-terminal domain (α-CTD). A flexible serine-threonine-rich loop (ST loop) within the α-CTD can be phosphorylated by Akt. The AMPKβ subunit contains a carbohydrate-binding module (CBM) that binds glycogen and a β-CTD region that serves as a bridge between the α- and γ-subunits. The AMPKγ subunit contains variable NH2-terminal regions followed by a short sequence involved in β-subunit binding, then four cystathionine β-synthase (CBS) domains that are paired to form two Bateman domains. These domains are the sites to which phosphorylated adenosines (AMP and ADP) bind to activate AMPK signaling [4] (Figure 1).
Figure 1.
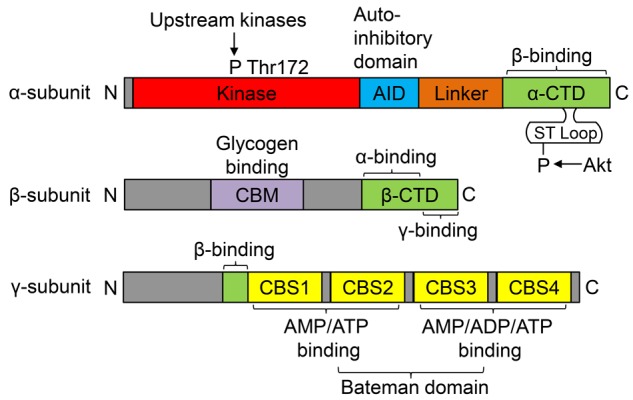
Domain structure of typical mammalian AMPK. AMPK complexes are heterotrimers composed of α-, β- and γ-subunits in a 1:1:1 ratio. The β-subunit carboxy-terminal domain (β-CTD) forms the core of the AMPK complex, binding to the α-CTD sequence in the α-subunit and the β-binding sequence in the amino terminus of the γ-subunit (green). The β-subunit also contains a carbohydrate-binding module (CBM) that is a binding site for glycogen (purple). The catalytic α-subunit contains conventional serine/threonine kinase domains with a threonine residue (Thr172) that is phosphorylated by upstream kinases (red). The kinase domain is followed by an auto-inhibitory domain (AID; blue) that has a negative effect on kinase activity. The AID is connected to the α-CTD by a less well conserved linker (orange). A flexible serine-threonine-rich loop (ST loop) within the α-CTD can be phosphorylated by Akt. The γ-subunit contains variable NH2-terminal regions followed by the short β-subunit binding sequence, then four tandem repeats of cystathionine β-synthase (CBS) motifs that act in pairs to form the binding sites for adenine nucleotides in mammalian AMPK (yellow). There is one binding site between CBS1 and CBS2 and two binding sites between CBS3 and CBS4. Four CBS domains are paired to form two Bateman domains.
It is likely that all cells in the body express AMPK. The AMPKα1 isoform is widely distributed among organ systems, whereas the AMPKα2 isoform is limited to muscle, liver, and adipose tissue [8]. The heart, skeletal muscle, and liver are the most studied organs in terms of AMPK activity, but brain and adipose tissue have also been studied intensively. Human skeletal muscle expresses primarily the α2, β2, and γ3 isoforms [9]; however, after prolonged exercise, the AMPKα1 isoform is specifically increased [10]. Higher levels of AMPK are expressed in subcutaneous adipose tissue than in visceral fat [11], and subcutaneous but not visceral adipose tissue is negatively correlated with serum adiponectin levels in obese individuals [12].
Activation of AMPK
Activation of AMPK is accomplished by competitive replacement of ATP on the AMPKγ subunit by ADP or AMP (Figure 1). Saturation of the AMP binding site on the AMPKγ subunit leads to a thousand-fold activation of AMPK by upstream AMPKKs [13]. Although ADP has significantly lower affinity for AMPK, its much greater concentration in stressed cells makes it an important regulator of AMPK [7]. Binding of the first AMP or ADP to the CBS domain in the AMPKγ subunit triggers a conformational change, opens the heterotrimeric complex, and exposes the catalytic domain of the AMPKα subunit to AMPKK phosphorylation on Thr172. AMP or ADP binding to AMPK also attenuates the deactivation of AMPKα by protein phosphatase 2Cα and increases the duration of Thr172 phosphorylation of the AMPKα subunit [13]. Binding of the first AMP or ADP additionally increases the affinity of the binding site in the CBS domain of the AMPKγ subunit for a second phosphorylated adenosine (AMP or ADP) [14]. Three isoforms of the AMPKγ subunit have been identified, and the binding capacity of AMP and ADP differ among them [15]. The activation of AMPK leads to shift towards energy production and storage (anabolism) and away from energy utilization (catabolism) and cell growth and proliferation.
Activation of AMPK requires phosphorylation of the Thr172 in the AMPKα subunit by calcium-calmodulin protein kinase kinases (CaMKKs) and/or another AMPKK consisting of LKB1 (STK11), a scaffolding protein called mouse protein 25, and the STE-related adapter [16-18]. Formation of this LKB1 complex is facilitated by deacetylation of LKB1 via sirtuin-1 and, thus, there is an interdependency between the distinct energy sensing regulators AMPK and sirtuin-1 [16,19]. The LKB1 complex phosphorylates at least 13 different AMPK-related protein kinases, although none of the AMPK-related kinases are activated by AMPK-activating metformin or AMP analogs [20]. In addition, Oakhill et al. [14] have shown that myristoylation of the AMPKβ subunit is required for activation of AMPK. Loss of β-subunit myristoylation abolishes AMPK activation by AMP. In the human (but not in the mouse), stress upregulates transcription of the β1- and β2-subunits of AMPK via a p53-dependent process, increasing the activity of AMPK in skeletal muscle, heart, white fat, and liver [21]. Alternatively, oxidative stress may activate AMPK by increasing cellular AMP and ADP [22]. Many other factors have significant allosteric effects on activity of AMPK, as reviewed by Hardie et al. [4].
Interactions of AMPK with endogenous hormonal and metabolic factors
AMPK is recognized as a master sensor of energy homeostasis and is a hub for the convergence of endocrine signals, including estradiol, androgens, inflammatory factors, leptin, and adiponectin, that modulate the energy balance [23,24] (Figure 2, Table 3).
Figure 2.
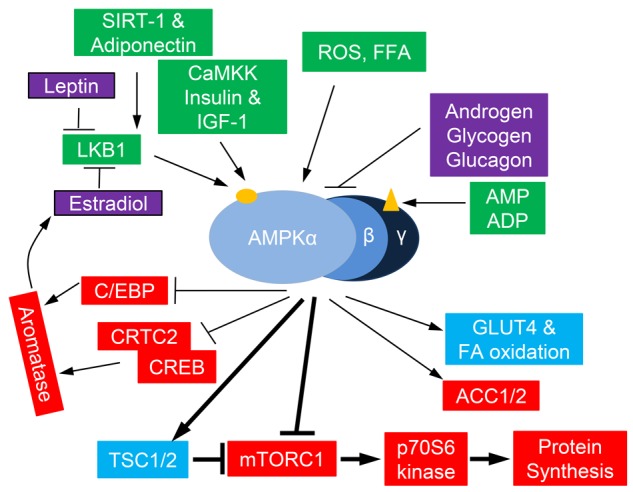
Activation of AMPK by endogenous hormonal and metabolic factors. Pathways that activate AMPK are shown in green and pathways that inhibit AMPK are shown in purple. Within the AMPK heterotrimer, the AMP/ADP binding site(s) are shown on the γ-subunit (triangle); the phosphorylation site (Thr172) is shown on the α-subunit (oval). Downstream targets of AMPK are shown in blue and red, leading to stimulation of energy storing pathways (blue) and inhibition of energy utilization pathways (red), as well as inhibition of aromatase.
Table 3.
Recent publications on AMPK
| Interaction of AMPK with endogenous hormonal and metabolic factors |
| Toyama et al., 2016: AMP-activated protein kinase mediates mitochondrial fission in response to energy stress [94]. Activation of AMPK promotes mitochondrial fragmentation through an identified mitochondrial fission factor. |
| Shrestha et al., 2016: Critical role of AMPK/FoxO3 in globular adiponectin-induced cell cycle arrest and apoptosis in cancer cells [39]. Suppression of AMPK blocked adiponectin-induced cell cycle arrest in both HepG2 and MCF-7 cells. |
| Mauro et al., 2015: Estrogen receptor-alpha drives adiponectin effects on cyclin D1 expression in breast cancer cells [38]. |
| Guo, et al., 2015: Synergistic antitumor activity of vitamin D3 combined with metformin in human breast carcinoma MDA-MB-231 cells involves m-TOR related signaling pathways [62]. |
|
|
| Exogenous AMPK activators and inhibitors |
| de Queiroz et al., 2015: Metformin reduces the Walker-256 tumor development in obese-MSG rats via AMPK and FOXO3a [36]. |
| Liu et al., 2015: Phenformin induces cell cycle change, apoptosis, and mesenchymal-epithelial transition and regulates the AMPK/mTOR/p70s6k and MAPK/ERK pathways in breast cancer cells [88]. |
| Fan et al., 2015: Metformin exerts anticancer effects through the inhibition of the Sonic hedgehog signaling pathway in breast cancer [90]. |
| Rice et al., 2015: Dual effect of metformin on growth inhibition and oestradiol production in breast cancer cells [57]. |
|
|
| Expression of AMPK subunits in tumor tissue |
| Ross et al., 2016: Differential regulation of AMP and ADP of AMPK complexes containing different gamma subunit isoforms [15]. Activities of AMP, ADP, CaMKKβ, and A769662 on AMPK isoforms. |
|
|
| Mechanism of AMPK activity in the proliferation, apoptosis, and migration of breast cancer cell lines |
| Gollavilli et al., 2015: AMPK inhibits MTDH expression via GSK3beta and SIRT1 activation: potential role in triple negative breast cancer cell proliferation [82]. Metformin downregulated the oncogenic protein metadherin. |
| Tanaka et al., 2015: Mild glucose starvation induces KDM2A-mediated H3K36me2 demethylation through AMPK to reduce rRNA transcription and cell proliferation [83]. |
| Jhaveri et al., 2015: AMP-activated kinase (AMPK) regulates activity of HER2 and EGFR in breast cancer [89]. The growth factors have sequences that are potential substrates for AMPK. |
| Domenech et al., 2015: AMPK and PFKFB3 mediate glycolysis and survival in response to mitophagy during mitotic arrest [99]. |
| Li et al., 2015: p53 is required for metformin-induced growth inhibition, senescence and apoptosis in breast cancer cells [93]. |
| Chou et al., 2014: AMPK reverses the mesenchymal phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a signaling axis [81]. |
|
|
| AMPK and human breast cancer |
| Zadra et al., 2015: Dissecting the dual role of AMPK in cancer: From experimental to human studies [1]. |
| Hadad et al., 2015: Evidence for biological effects of metformin in operable breast cancer: biomarker analysis in a pre-operative window of opportunity randomized trial [113]. |
| Dowling et al., 2015: Changes in insulin receptor signaling underlie neoadjuvant metformin administration in breast cancer: a prospective window of opportunity neoadjuvant study [114]. |
| et al., 2104: Metformin-mode of action and clinical implications for diabetes and cancer [51]. |
| Lega et al., 2014: The effect of metformin on mortality following cancer among patients with diabetes [54]. |
| Thompson, 2014: Molecular pathways: preclinical models and clinical trials with metformin in breast cancer [24]. |
Sex steroid hormones
Estrogens play a major role in the modulation of energy balance through central and peripheral actions. Estradiol inhibits AMPK through estrogen receptor α (ERα) in the ventromedial nucleus of the hypothalamus, leading to stimulation of thermogenesis in brown adipose tissue and weight loss through activation of the sympathetic nervous system [25]. On the other hand, both estrogen [26,27] and leptin [28,29] inhibit AMPK phosphorylation in the liver and adipose tissue by inhibiting LKB1 activity. Androgens have a similar effect in adipocytes. Both testosterone and dihydrotestosterone (DHT) inhibit AMPK activation [30] and increase food intake [31].
Insulin, growth factors, and adipokines
Insulin- and insulin-like growth factor-1-induced Akt phosphorylation suppresses phosphorylation of the AMPKα subunit at Thr172, decreasing activation of AMPK and increasing energy utilization [32,33]. While leptin, produced by adipose tissue, has inhibitory effects in the liver and muscle, adiponectin has stimulatory effects in both liver and muscle [34]. Exercise [33] and adiponectin [35] increase AMPK activation in both adipose tissue and muscle. In obesity, the ratio of leptin to adiponectin in adipose tissue is elevated, with a net effect of decreasing AMPK activation; however, this effect can be partially overcome by treatment with biguanides such as metformin [36]. Adiponectin activation of AMPK is also described in studies of various breast cancer cell lines. Treatment of MCF-7, T47D, and MDA-MB-231 breast cancer cells with adiponectin results in increased cytoplasmic LKB1 accumulation. This increases the activity of AMPK by stimulating of AMPKα phosphorylation at Thr172 without changing total AMPK protein expression levels [37]. Differential effects of adiponectin in breast tumors may be dependent on the presence or absence of ERα. Adiponectin promotes growth of ERα-positive (ERα+) MCF-7 breast tumor cells and inhibits growth of ERα-negative (ERα-) MDA-MB-231 breast tumor cell xenografts [38]. AMPK is necessary for the action of adiponectin on suppression of cyclin D1 and cell cycle arrest in both HepG2 (liver cancer cells) and MCF-7 cells [39]. FoxO3a is an intermediate in this process.
Metabolic intermediates
Essential fatty acids, except for oleic acid [40] and 3-phosphoglycerate [16,41], increase the activity of AMPK. Glycogen has been shown to suppress the activation of AMPK by binding to the specific CBM on the AMPKβ subunit [28,42] and glucagon inhibits AMPK via increased PKA-mediated inhibitory phosphorylation of serine 173 and reduced phosphorylation at Thr172 on the AMPKα subunit [43].
Other factors
Accumulation of NAD or NADH in cells is not a stimulus for activation of AMPK [13]. Based on nutrient availability, the other main sensor of energy balance, sirtuin-1, a histone deacetylase, is stimulated by NAD and inhibited by NADH and nicotinamide to increase energy availability [16]. In addition, the relationship between energy needs and activation of AMPK is not strict. For example, AMPK activation in response to exercise was suppressed in a study of trained subjects compared with sedentary individuals [10]. Exercise training markedly increased LKB1 and MO25 but did not increase AMPK [44]. Caloric restriction in human subjects had very little effect on AMPKα1 or AMPKα2 activities in heart or skeletal muscle, whereas plasma leptin increased by 8-fold [45].
Exogenous AMPK activators and inhibitors
Biguanides
The biguanides (metformin and phenformin) activate the AMPK pathway, inhibit protein and lipid synthesis, and elicit anti-tumor effects in breast tissue (Table 3). The biguanides act, in part, through facilitating LKB1 phosphorylation of AMPKα at Thr172 [46,47]. Metformin and phenformin also activate AMPK by inhibiting complex 1 of the mitochondrial respiratory chain, causing a decrease in formation of ATP and an increase in AMP accumulation [42,48]. Metformin is the most commonly used anti-diabetic drug and has been associated with decreased risk of breast cancer [49], probably due, at least in part, to activation of the AMPK pathway in the liver, which decreases circulating levels of insulin and glucose [50,51]. Although phenformin has been withdrawn by the FDA for treatment of diabetes because of its major side effect of lactic acidosis, it may have an advantage over metformin in breast cancer treatment and prevention. First, phenformin, unlike metformin, does not require the presence of the organic cation transporter 1 (OCT-1/SLC22A1), which is highly expressed only in hepatocytes [51], has a more ubiquitous uptake in tissues, and may be useful as a drug for activating AMPK directly in cancer cells [52]. Second, phenformin is a 50-fold more potent inhibitor of mitochondrial complex 1 than metformin and further increases the AMP/ATP ratio that promotes AMPK activation and signaling [48,53]. In addition, some population-based studies have suggested that metformin reduces cancer risk when it is used in patients with diabetes compared to a control group [2,3]. However, a recent meta-analysis found no significant benefit from metformin on mortality from breast cancer in patients with diabetes [54].
Other AMPK activators
An AMP mimetic, 5-aminoimidazole-4-carboxamide-1-b-D-ribofuranoside (AICAR), acts exclusively by facilitating an increase in AMPKα subunit phosphorylation at Thr172 by LKB1. The thiazolidinedione family of PPARγ agonists (rosiglitazone and troglitazone) stimulates AMPK by increasing the ratio of AMP/ATP in muscle cells [55], although this may not apply to MCF-10A non-malignant breast cells. Metformin and AICAR, but not thiazolidinediones, suppress estradiol-induced cellular growth [56,57]. Resveratrol, at least in the brain, activates AMPK via CaMKK [58]. Honokiol is a polyphenol extracted from seed cones of magnolia species [59] that has been shown to increase cytoplasmic accumulation of LKB1 and AMPK activity, thereby inhibiting cell proliferation [60]. The tea polyphenols also promote activation of AMPK. Treatment of MDA-MB-231 breast cancer cell lines with the green tea polyphenol epigallocatechin gallate (EGCG) analogs 4 and 6 activate the AMPK pathway, resulting in a decrease in mammosphere formation, cancer stem cell population, and breast cancer proliferation in a dose-dependent fashion [61]. Recently, vitamin D has been shown to promote the anti-tumor effects of metformin in ERα- MDA-MB-231 breast cancer cells, possibly by activating the AMPK/mTOR-related pathway [62]. Proteasome inhibitors such as bortezomib have also been shown to activate AMPK [63]. The mechanism is indirect, via an increase in the AMPK activator CaMKKβ. The active metabolite of aspirin (salicylate) was recently identified as an activator of AMPK [64]. A-769662, a direct activator of AMPK, binds to the same site on the AMPKβ1 subunit as salicylate. Although A-769662 mimics the actions of AMP, it has no effect on binding of AMP to the Bateman domains of the AMPKγ subunit. Many other pharmacologic agonists of AMPK have been investigated [65] and have been described elsewhere [40].
AMPK inhibitors
Effective pharmacologic antagonists of AMPK include compound C (dorsomorphin), a small molecule, cell-permeable pyrazolopyrimidine derivative that acts as a reversible ATP competitive inhibitor of AMPK [66]. Adenine 9β-arabinofuranoside acts in a similar manner to inhibit AMPK activation [40].
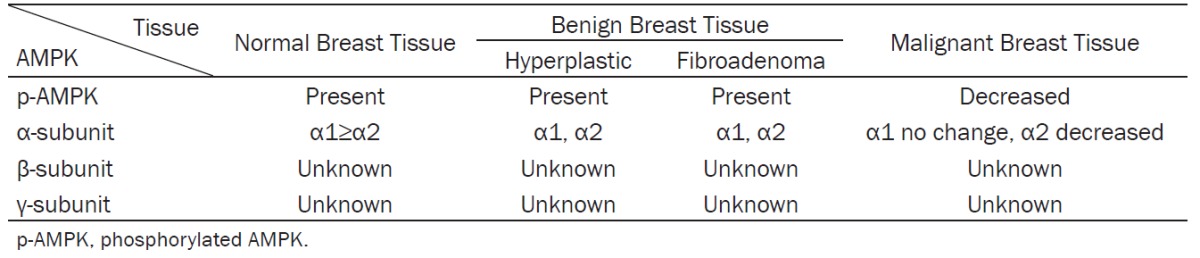
Differential expression of AMPK subunits in normal/benign breast tissue
Both humans and mice contain two AMPKα isoforms (α1 and α2) [67]. AMPKα1 is present at much higher levels than AMPKα2 in human breast tissue. We observed that AMPKα1 mRNA levels are 10-fold higher than those of AMPKα2 in mouse mammary tissue (unpublished data). Both isoforms are also expressed in hyperplastic breast tissues and human fibroadenoma [68]. Thus far, no publications have reported the expression of the several isoforms of the β and γ subunits of AMPK in normal or benign breast tissues (Table 1).
Table 1.
Phosphorylation of AMPK and the distribution of AMPK subunits in normal, benign, and malignant breast tissue
|
Mammary tissue is composed of the epithelial, myoepithelial, and stromal (connective/adipose tissue) cells [69]. The epithelial cells of ducts and alveoli are embedded in the stroma, the main components of which are adipocytes and fibroblasts (preadipocytes) [5,70,71]. We found that phosphorylated AMPKα subunit is present in both mammary epithelial and stromal cells in mice (unpublished data). Phosphorylated AMPK (pAMPK) has also been observed in primary human breast epithelial cells and human preadipocytes from fresh breast tissues [72]. One study showed that AMPKα1, but not AMPKα2, is present in the cytoplasm of epithelial cells of human breast tissue by immunohistochemistry (IHC) [73]. In contrast, another study showed that normal human breast tissue contained a strong, uniform expression of both AMPKα1 and AMPKα2 at the same levels within the ductal epithelia [68]. The expression patterns of different isoforms of the AMPKβ- and γ-subunits have not been established in epithelial cells or preadipocytes. AMPK expression and localization in myoepithelial cells also has not been determined. Since AMPK is expressed in the cytoplasm and the majority of components in the cytoplasm is lost in mature adipocytes during tissue processing for IHC, it is unclear whether α, β and γ subunits of AMPK are expressed in mature adipocytes of the breast adipose tissue based on IHC.
The expression of AMPK subunits in breast tumor tissue
AMPKα and pAMPKα (Thr172) expression was assessed in human breast cancers by Zhang et al. [74]. Both were present with a mixture of diffuse and granular cytoplasmic staining. Heterogeneous staining was shown between, as well as within, certain tumor cores for both markers, varying from weak to intense staining. High AMPKα expression was associated with improved local recurrence-free (P = 0.019), relapse-free (P = 0.016), and breast cancer-specific survival (P < 0.001). The phosphorylated form of AMPKα was not associated with survival outcomes [74]. However, other studies have shown that pAMPK was decreased by about 90% in tumor tissue of two cohorts of 354 primary breast cancer patients compared to normal breast epithelial cells. Phosphorylation of acetyl-CoA carboxylase, a direct target of AMPK, was significantly positively associated with pAMPK [68,75]. In both cohorts, reduced pAMPK was also significantly associated with higher histological grade and axillary node metastasis. These findings show that AMPK is dysfunctional in primary breast cancer [68,75]. Reduced AMPK signaling and the inverse relationship with histological grade and axillary node metastasis suggest that AMPK reactivation could have preventive and therapeutic potential in breast cancer. The lack of association of pAMPK with breast cancer in the Zhang et al. [74] study is inconsistent with other studies.
The expression levels of AMPKα1 and AMPKα2 in breast cancer tissue are different from those in benign tissues. In one study, AMPKα1 protein was expressed at the same levels in patient-matched adjacent normal epithelium (ADJ) and tumor tissue; however, AMPKα2 protein immunostaining by IHC was significantly lower, by 27%, in tumor samples compared to patient-matched ADJ samples, and by 37% compared to normal mammary epithelium [75]. Furthermore, AMPKα2 but not AMPKα1 expression was found to be significantly suppressed in all cancer grades compared to normal, hyperplastic, or fibroadenoma tissues [68]. Together, these data reveal a significant and widespread reduction of AMPKα2 expression in mammary epithelial carcinoma (Table 1). Exogenous expression of AMPKα2 subunit in MCF-7 xenografts restored their growth control mechanisms, reduced proliferation, and increased apoptosis. Clearly, AMPKα2 activity plays a crucial role in modulating cell growth signals and tumor development (45).
Mechanism of AMPK activity in the proliferation, apoptosis, and migration of breast cancer cell lines
Anti-proliferative effects of AMPK activation
Activated AMPK phosphorylates TSC1/TSC2 and Raptor, causing the inhibition of mTOR; as a consequence, the translation of many macromolecules and lipids required for cell growth is inhibited [42], including p70S6 kinase for protein synthesis and enzymes for the synthesis of fatty acids, triglycerides, cholesterol, and glycogen [4]. Inhibition of tumor suppressor LKB1 reduces AMPK phosphorylation and lifts the inhibition of TSC2 on mTOR activity, thereby upregulating its downstream kinases and enzymes; this leads to increased protein and lipid synthesis and increased cellular growth and proliferation without cell cycle regulation [76,77]. AMPK activation can also inhibit the cell cycle by downregulating TSC2-mTOR and upregulating the p53-p21WAF1 axis via phosphorylation of p53 and the cyclin-dependent kinase inhibitor p21WAF1 [78,79]. Moreover, AMPK tumor suppressive activity is increased by suppressing HIF1α and its downstream glycolytic genes, reversing the Warburg effect [80]. pAMPK may also act to suppress tumor growth and metastasis by decreasing expression of FOXO3a and E-cadherin via inhibition of Akt signaling [81], and by inducing GSK3β- and sirtuin-1-mediated downregulation of the oncogenic protein metadherin [82]. This is accomplished by overcoming c-MYC upregulation of metadherin. In addition, under mild glucose starvation, AMPK has been shown to suppress rRNA transcription and cell proliferation by upregulation of the histone demethylase KDM2A [83]. Cancer metabolism itself may suppress the activity of AMPK because Akt, which is frequently overexpressed in cancer, is capable of phosphorylating AMPK at Ser485, reducing its activity. Thus, activation of PI3K or inhibition of PTEN will suppress AMPK by increasing Akt. This results in increased anabolic activity of tumor cells.
A possible protective effect of AMPK activation via metformin is related to the inhibition of aromatase activity, which decreases the conversion of androstenedione and testosterone to estradiol. However, the conversion of estrone and estrone sulfate to estradiol via 17β-hydroxysteroid dehydrogenase/steroid sulfatase is not affected by metformin [84]. In addition, overexpression of CYP1A1, which is common in breast cancer cells, may promote cancer proliferation in part through suppression of pAMPK [85]. Other cancer-specific mechanisms may also be involved in reducing the availability of AMPK [1].
AMPK activation by metformin exhibits its effects on cell proliferation in a dosage- and cell line-dependent manner [86]. MCF-7 and T47D (ERα+/PR+), BT474 (ER+, PR+, and HER2+), and BT20 and MDA-MB-453 (ER-, PR-, and HER2-) breast cancer cell lines showed a decrease in cell proliferation in response to metformin treatment. Metformin caused a decrease in cyclin D1, which led to release of sequestered p27Kip1 and p21Cip1 and cell cycle arrest. On the other hand, the very aggressive triple-negative breast cancer (TNBC) cell line MDA-MB-231 (ER-, PR-, and HER2-) was resistant to metformin’s effect due to low levels of cyclin D1 [86]. Another study also showed that in vitro metformin treatment reduced the growth of ERα+/PR+ MCF-7 cells by 82% and T47D cells by 96% when compared with untreated controls. However, the growth of TNBC cells (MDA-MB-435 and MDA-MB-231) showed only partial repression, by 40% and 29%, respectively. Clearly, TNBC cells are not as sensitive as ERα+/PR+ cells to metformin treatment with respect to limiting cell proliferation [87].
Biguanides or other activators of AMPK may have an additional function in breast cancer cell lines that express elevated levels of HER2 (HCC1419 and SKBR3) or epidermal growth factor receptor (EGFR; HCC1806 and BT549) [88]. Jhaveri et al. showed that AICAR administration leads to decreased phosphorylation of HER2 and EGFR in such cells and it is 2- to 5-fold more effective in suppressing cell growth than in cells lacking HER2 or EGFR overexpression [89]. They identified amino acid sequences in HER2 and EGFR that are potential substrates for AMPK. The sonic hedgehog (Shh) pathway may also be involved in the effects of metformin on breast cancer growth [90]. Metformin inhibited recombinant human Shh-induced cell migration, invasion, and stemness, and reduced cell proliferation in a xenograft model. The effects were reversed by siRNA-mediated suppression of AMPK. Thus, metformin inhibits growth of breast cancer cells via several mechanisms, primarily the AMPK/mTOR signaling pathway. Anti-proliferative effects by biguanides in breast cancer cell lines occur via not only cell cycle arrest but also a pro-apoptotic effect [91,92].
AMPK activation in breast cancer cell apoptosis
Activation of AMPK by phenformin or AICAR not only has dose- and cell type-dependent anti-proliferative effects in breast cancer cell lines, but also has p53-dependent apoptotic effects [91,93]. Activation of AMPK is essential for mitochondrial fragmentation [94], a process that promotes timely apoptosis. Mitochondrial fission factor (MFF) is a mitochondrial outer membrane receptor for DRP1, the cytoplasmic guanosine triphosphatase that catalyzes mitochondrial fission, and a substrate of AMPK [94]. In MCF-7, T47D, and MDA-MB-231 breast cancer cell lines, typical apoptotic morphological features (e.g., fragmentation into apoptotic bodies) were seen after AMPK activation, though these features were less pronounced in T47D cells compared to the other two lines. AMPK activation induced mitochondrial membrane depolarization, indicating apoptosis in all cell lines; phenformin had the strongest and AICAR had the weakest depolarization effect (phenformin > metformin > AICAR) [91]. It is apparent that the pro-apoptotic action of AMPK is partially p53-dependent since it is seen, even if to a lesser some degree, in p53 mutated MDA-MB-231 cell lines, and it appears that growth inhibition of T47D cells is more related to cell cycle arrest than to apoptosis [91]. On the contrary, phenformin treatment of xenografts of ERα+/PR+ MCF-7 and TNBC MDA-MB-231 cells caused a decrease in the number of mitotic figures without changing the number of apoptotic cells, suggesting an AMPK-induced cell cycle arrest leading to inhibition of the development and growth of these breast cancer xenografts [95]. Cell proliferation marker Ki67 showed no significant decline in phenformin-treated xenograft models [95], indicating that the Ki67 and mitotic activity index were discrepant in these xenograft models [96].
AMPK activation in breast cancer cell migration
AMPK indirectly inhibits the activity of p70S6 kinase, resulting in inhibition of actin cytoskeleton reorganization and inhibition of cell migration. Treatment of MCF-7 and MDA-MB-231 xenografts with Honokiol led to a dose-dependent inhibition of migration and a significantly decreased rate of tumor growth, tumor size, and weight compared with the control group [60]. The growth of HCC1806 (EGFR+) breast cancer cells with an LKB1 homozygous mutation was not inhibited by Honokiol, which demonstrates that LKB1 is necessary for Honokiol modulation of AMPK-mediated growth inhibition [60]. Honokiol treatment also inhibited FAK phosphorylation and associated cell motility in breast cancer cell lines [60].
Oncogenic activity of AMPK activation
Recently, the oncogenic activity of AMPK was studied in relation to metabolic stress (hypoxia, nutrient deprivation, and matrix detachment). Under conditions of nutrient deficiency, pAMPK can provide necessary ATP by increasing fatty acid oxidation. Fatty acid oxidation produces increased NADPH levels, and utilization of NADPH is decreased by AMPK phosphorylation of ACC. The resulting increased levels of NADPH reduce the formation of cytotoxic ROS and increase cancer cell survival [17]. Another important mechanism by which AMPK may promote cancer progression involves the activation of ULK1, the autophagy initiating kinase, under conditions of nutrient restriction [97]. Some level of autophagy allows the cells to persist in a dormant state until nutrients are available [98,99]. Autophagy is regulated by opposing effects of AMPK and mTOR: active mTOR inhibits ULK1 in nutrient-replete conditions and active AMPK stimulates ULK1 in nutrient-deficient conditions. When ATP is deficient, pAMPK predominates and autophagy will be initiated, allowing the cancer cell to survive. If the expression of LKB1 or AMPK is very low in a cancer cell, energy levels may be insufficient for survival. In this case, AMPK activation and autophagy would help cancer cell survival. Therefore, AMPK inhibitors, rather than activators, may work well with drugs that increase metabolic stress to inhibit tumor growth [1]. Although salicylate is a direct activator of AMPK, in the absence of AMPK gene expression, salicylate has a negative effect on cell survival through increased CYP2E1 activity and ROS formation [100]. In MYC-driven cancers such as TNBC, MYC-dependent growth strains cellular energy resources and stimulates AMP-activated kinase to provide energy [101]. MYC promotes lipid, nucleotide, and protein synthesis; these biosynthetic processes deplete cellular resources and lead to activation of AMPK. This enhances the ability of the tumor cells to meet energy demands.
In summary, at early stages of tumor growth, when adequate energy is available, the beneficial effects of biguanides on tumor growth inhibition are likely dominant. After biguanide treatment, the balance of energy availability and protein and lipid biosynthesis is tilted in favor of energy availability and against biosynthetic processes needed for growth. Thus, a metabolic imbalance is created to suppress tumor growth. However, as the tumor grows and energy sources and nutrients become limited, biguanides may support tumor maintenance by increasing the availability of energy sources and promoting autophagy.
AMPK and human breast cancer
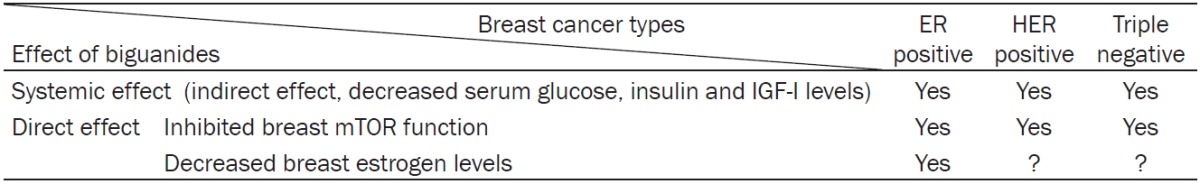
Breast cancer is the most common cancer among women in the United States (other than skin cancer). About 1 in 8 (12%) women in the US will develop invasive breast cancer during their lifetime. Breast cancer can be divided into four main molecular subtypes based on the presence or absence of biological markers: hormone (estrogen or progesterone) receptors (HR+/HR-) and excess levels of human epidermal growth factor receptor 2 (HER2+/HER2-), a protein promoting breast epithelial cell growth [102,103]. These four subtypes are luminal A (HR+/HER2-, 74%), luminal B (HR+/HER2+, 10%), HER2-enriched (HR-/HER2+, 4%), and triple-negative (HR-/HER2-, 12%) [104,105]. The majority of breast cancers (84%) express estrogen and progesterone receptors, indicating the essential role of estrogen in breast cancer development. Table 2 shows the effects of AMPK-activating biguanides on different types of breast cancer.
Table 2.
The effect of AMPK-activating biguanides on different types of breast cancers
|
AMPK and aromatase in human ER+ breast cancer
Estradiol, produced in breast tissue of postmenopausal women from androgen precursors [106], has proliferative and possibly genotoxic effects on breast epithelial cells [107,108]. When the ovaries cease to produce estrogens in postmenopausal women, local aromatase expression in the breast preadipocytes (fibroblasts) and subcutaneous adipose tissue produce estrogens that drive ER+ breast cancer development [109]. Both AMPK and aromatase are expressed in breast preadipocytes, indicating a potentially close relationship. Human aromatase gene expression is regulated by the transcription factors cAMP-responsive element (CRE)-binding protein (CREB) and/or CCAAT-enhancer-binding protein-β (C/EBPβ) [110,111]. In human preadipocytes, AMPK activation decreases aromatase expression via decreased transcriptional activity of CREB [5]. AMPK activation also downregulates C/EBPβ expression in mouse preadipocytes [112] (Figure 2). Thus, the pAMPK pathway negatively regulates aromatase expression and estrogen formation in breast preadipocytes. Since aromatase inhibitors are widely used in postmenopausal women with breast cancer, there is a potential for synergism between an aromatase inhibitor and metformin; this possibility is under exploration in a Korean neoadjuvant trial [24]. In a preoperative trial of metformin in breast cancer patients, significant upregulation of pAMPK and downregulation of pAkt were demonstrated compared to controls [113,114]. Tamoxifen is the primary adjuvant therapy for premenopausal women with ER+ breast cancer, and the synergistic effects of metformin and tamoxifen have also been considered in a subgroup analysis of the ongoing MA32 adjuvant metformin trial [115] (Table 3).
AMPK in human HER2+ breast cancers
There are four different members of the family of EGFRs (or ErbBs): ErbB1/EGFR, ErbB2/HER2/neu, ErbB3/HER3, and ErbB4/HER4 [116]. The HER2 isoforms are important factors that promote cell proliferation when energy supplies are adequate. Overexpression of the HER2 oncogene can be found in both luminal B and HER2-enriched breast cancers [117,118].
ErbB2/HER2 in energy utilization
The activated ErbB2/HER2 receptor exerts proliferative roles via three major distinct pathways: Ras/Raf/MAPK, JAK/Stat, and PI3K/AKT/mTOR [117]. ErbB2/HER2 activation promotes synthesis of glycolytic intermediates used in synthesis of lipid, protein, and glycogen in order to maintain cell proliferation. Importantly, the PI3K/AKT/mTOR pathway is involved in energy regulation of cancer cells, and is opposed by the actions of AMPK. The AMPK pathway is not activated under normal conditions, when nutrient and energy supply is adequate for proliferating cancer cells. However, when energy is lacking, AMPK is activated by a high AMP/ATP ratio to promote energy (ATP) production. Meanwhile AMPK activation opposes the actions of PI3K/AKT/mTOR and inhibits the biosynthesis of macromolecules needed for cell proliferation. Thus, energy production is promoted at the expense of growth [117,119,120].
Combined anti-HER2 and biguanides treatment
Anti-HER2 treatment has been used over the last decade to enhance survival rates in both initial and metastatic stages of HER2+ breast cancers [121]. The HER2 inhibitor trastuzumab is the currently approved treatment for the HER2 subtype of breast carcinoma; however, clinical practice shows that resistance develops within 1 year after start of the treatment [122]. HER2 inhibitor use is also associated with severe side effects of mitochondrial dysfunction, leading to cardiomyopathy in cancer patients [123,124]. The underlying reason for this side effect is that ErbB2/HER2 is crucial for normal cardiac development and functioning [125,126]. In order to overcome the serious cardiac side effects and lack of sustained efficacy of anti-HER2 treatment, the simultaneous inhibition of HER2 and activation of AMPK by biguanides has been suggested based on the demonstrated cardioprotective effects of the biguanides [127].
Another HER2 inhibitor is lapatinib, which can block the ErbB2/HER2 pathway in treatment of HER2 breast cancer [127]. Lapatinib acts only in HER2-overexpressing breast cancer tissues, and not in cardiac tissue where HER2 is essential for appropriate functioning [127,128]. As a result, the incidence of cardiomyopathy is lower with lapatinib compared to trastuzumab [3,124,127]. However, as with trastuzumab, resistance may also be a problem, as has been demonstrated at the molecular level by increased Akt and p70S6 kinase 1. Again, biguanides can be added to the lapatinib treatment regimen to downregulate the PI3K/Akt/mTOR pathway and prevent lapatinib resistance [117,118,129].
AMPK in human TNBCs
TNBCs are a very heterogeneous group of cancers and are usually associated with cytokeratin 5/6 and EGFR-positive staining. Since AMPK activation targets and suppresses Akt/mTOR, it has positive effects in all TNBCs. In addition, AMPK activation suppresses EGFR expression; phosphorylation of MAPK, Src, and STAT3; and expression of cyclin D1 and cyclin E [115,130]. The ongoing MA32 adjuvant metformin trial includes patients with TNBC to test the effectiveness of biguanides in this group. A formal subset analyses of TNBC patients will further evaluate the anti-tumor effects of metformin in TNBC breast cancer cell lines.
Conclusions
AMPK activation decreases systemic glucose and insulin levels and at the same time directly inhibits mTOR, a central component in regulation of cell growth, reducing the proliferation of all breast cancer subtypes. AMPK-activating metformin and phenformin are expected to be novel therapeutics for breast cancer prevention and treatment via energy stress. However, the opposing actions of biguanides have yet to be resolved. In the review by Zadra et al. [1], AMPK is described as a conditional tumor suppressor or a contextual oncogene. Clinical studies cited therein reveal that the expression levels of pAMPK or acetyl CoA carboxylase 1 measured in tumors are generally associated with no difference or a favorable outcome, but in a few studies, higher levels of AMPK are associated with higher tumor grade or lower overall survival. Cells deficient in LKB1/AMPK are unable to restore ATP levels and are more susceptible to cell death. In the context of metabolic stress, AMPK inhibitors rather than activators may work well with compounds that cause metabolic stress. Observational trials of patients with diabetes generally show some positive benefit of metformin but case-control trials have not yet been concluded. To date, there is no evidence from clinical trials that treatment with metformin has a negative effect on cancer incidence in any setting. Survival outcomes with metformin therapy have been studied in patients with diabetes and breast cancer. In one study, Peeters et al. [131] found no difference in breast cancer-specific survival with metformin treatment, whereas another study He et al. [132] found a significantly lengthened survival (HR 0.52) among patients treated with metformin. More definitive results await the conclusion of case-control trials. Assuming positive results of these clinical trials, future studies will be needed to determine metformin dose and tissue availability, phenformin side effects, and interaction with the existing treatments for different types of breast cancers.
Acknowledgements
This work was supported by Avon Foundation for Women and Lynn Sage Cancer Research Foundation.
Disclosure of conflict of interest
None.
References
- 1.Zadra G, Batista JL, Loda M. Dissecting the Dual Role of AMPK in Cancer: From Experimental to Human Studies. Mol Cancer Res. 2015;13:1059–1072. doi: 10.1158/1541-7786.MCR-15-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle. 2009;8:88–96. doi: 10.4161/cc.8.1.7499. [DOI] [PubMed] [Google Scholar]
- 4.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown KA, McInnes KJ, Hunger NI, Oakhill JS, Steinberg GR, Simpson ER. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res. 2009;69:5392–5399. doi: 10.1158/0008-5472.CAN-09-0108. [DOI] [PubMed] [Google Scholar]
- 6.Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villena JA, Viollet B, Andreelli F, Kahn A, Vaulont S, Sul HS. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-alpha2 subunit. Diabetes. 2004;53:2242–2249. doi: 10.2337/diabetes.53.9.2242. [DOI] [PubMed] [Google Scholar]
- 9.Birk JB, Wojtaszewski JF. Predominant alpha2/beta2/gamma3 AMPK activation during exercise in human skeletal muscle. J Physiol. 2006;577:1021–1032. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5’-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528:221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Agustin O, Hernandez-Morante JJ, Martinez-Plata E, Sanchez de Medina F, Garaulet M. Differences in AMPK expression between subcutaneous and visceral adipose tissue in morbid obesity. Regul Pept. 2010;163:31–36. doi: 10.1016/j.regpep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Nakano Y, Itabashi K, Sakurai M, Aizawa M, Dobashi K, Mizuno K. Accumulation of subcutaneous fat, but not visceral fat, is a predictor of adiponectin levels in preterm infants at term-equivalent age. Early Hum Dev. 2014;90:213–217. doi: 10.1016/j.earlhumdev.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5’-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 14.Oakhill JS, Chen ZP, Scott JW, Steel R, Castelli LA, Ling N, Macaulay SL, Kemp BE. beta-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK) Proc Natl Acad Sci U S A. 2010;107:19237–19241. doi: 10.1073/pnas.1009705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross FA, Jensen TE, Hardie DG. Differential regulation by AMP and ADP of AMPK complexes containing different gamma subunit isoforms. Biochem J. 2016;473:189–199. doi: 10.1042/BJ20150910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751–760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JM, Seo WY, Song KH, Chanda D, Kim YD, Kim DK, Lee MW, Ryu D, Kim YH, Noh JR, Lee CH, Chiang JY, Koo SH, Choi HS. AMPK-dependent repression of hepatic gluconeogenesis via disruption of CREB. CRTC2 complex by orphan nuclear receptor small heterodimer partner. J Biol Chem. 2010;285:32182–32191. doi: 10.1074/jbc.M110.134890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S, Levine AJ. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67:3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 22.Auciello FR, Ross FA, Ikematsu N, Hardie DG. Oxidative stress activates AMPK in cultured cells primarily by increasing cellular AMP and/or ADP. FEBS Lett. 2014;588:3361–6. doi: 10.1016/j.febslet.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown KA, Samarajeewa NU, Simpson ER. Endocrine-related cancers and the role of AMPK. Mol Cell Endocrinol. 2013;366:170–179. doi: 10.1016/j.mce.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Thompson AM. Molecular pathways: preclinical models and clinical trials with metformin in breast cancer. Clin Cancer Res. 2014;20:2508–2515. doi: 10.1158/1078-0432.CCR-13-0354. [DOI] [PubMed] [Google Scholar]
- 25.Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, Lage R, Fernandez-Mallo D, Martinez-Sanchez N, Ruiz-Pino F, Liu J, Morgan DA, Pinilla L, Gallego R, Saha AK, Kalsbeek A, Fliers E, Bisschop PH, Dieguez C, Nogueiras R, Rahmouni K, Tena-Sempere M, Lopez M. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morad V, Abrahamsson A, Dabrosin C. Estradiol affects extracellular leptin:adiponectin ratio in human breast tissue in vivo. J Clin Endocrinol Metab. 2014;99:3460–3467. doi: 10.1210/jc.2014-1129. [DOI] [PubMed] [Google Scholar]
- 27.Brown KA, McInnes KJ, Takagi K, Ono K, Hunger NI, Wang L, Sasano H, Simpson ER. LKB1 expression is inhibited by estradiol-17beta in MCF-7 cells. J Steroid Biochem Mol Biol. 2011;127:439–443. doi: 10.1016/j.jsbmb.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 30.McInnes KJ, Corbould A, Simpson ER, Jones ME. Regulation of adenosine 5’,monophosphate-activated protein kinase and lipogenesis by androgens contributes to visceral obesity in an estrogen-deficient state. Endocrinology. 2006;147:5907–5913. doi: 10.1210/en.2006-0879. [DOI] [PubMed] [Google Scholar]
- 31.Kanaya N, Vonderfecht S, Chen S. Androgen (dihydrotestosterone)-mediated regulation of food intake and obesity in female mice. J Steroid Biochem Mol Biol. 2013;138:100–106. doi: 10.1016/j.jsbmb.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 33.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardie DG. New roles for the LKB1-->AMPK pathway. Curr Opin Cell Biol. 2005;17:167–173. doi: 10.1016/j.ceb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signalling. J Mol Endocrinol. 2010;44:87–97. doi: 10.1677/JME-09-0063. [DOI] [PubMed] [Google Scholar]
- 36.de Queiroz EA, Akamine EH, de Carvalho MH, Sampaio SC, Fortes ZB. Metformin reduces the Walker-256 tumor development in obese-MSG rats via AMPK and FOXO3a. Life Sci. 2015;121:78–87. doi: 10.1016/j.lfs.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 37.Taliaferro-Smith L, Nagalingam A, Zhong D, Zhou W, Saxena NK, Sharma D. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene. 2009;28:2621–2633. doi: 10.1038/onc.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mauro L, Pellegrino M, Giordano F, Ricchio E, Rizza P, De Amicis F, Catalano S, Bonofiglio D, Panno ML, Ando S. Estrogen receptor-alpha drives adiponectin effects on cyclin D1 expression in breast cancer cells. FASEB J. 2015;29:2150–2160. doi: 10.1096/fj.14-262808. [DOI] [PubMed] [Google Scholar]
- 39.Shrestha A, Nepal S, Kim MJ, Chang JH, Kim SH, Jeong GS, Jeong CH, Park GH, Jung S, Lim J, Cho E, Lee S, Park PH. Critical Role of AMPK/FoxO3A Axis in Globular Adiponectin-Induced Cell Cycle Arrest and Apoptosis in Cancer Cells. J Cell Physiol. 2016;231:357–369. doi: 10.1002/jcp.25080. [DOI] [PubMed] [Google Scholar]
- 40.Park SY, Kim MH, Ahn JH, Lee SJ, Lee JH, Eum WS, Choi SY, Kwon HY. The Stimulatory Effect of Essential Fatty Acids on Glucose Uptake Involves Both Akt and AMPK Activation in C2C12 Skeletal Muscle Cells. Korean J Physiol Pharmacol. 2014;18:255–261. doi: 10.4196/kjpp.2014.18.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellingson WJ, Chesser DG, Winder WW. Effects of 3-phosphoglycerate and other metabolites on the activation of AMP-activated protein kinase by LKB1-STRAD-MO25. Am J Physiol Endocrinol Metab. 2007;292:E400–407. doi: 10.1152/ajpendo.00322.2006. [DOI] [PubMed] [Google Scholar]
- 42.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 43.Aw DK, Sinha RA, Xie SY, Yen PM. Differential AMPK phosphorylation by glucagon and metformin regulates insulin signaling in human hepatic cells. Biochem Biophys Res Commun. 2014;447:569–573. doi: 10.1016/j.bbrc.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 44.Taylor EB, Hurst D, Greenwood LJ, Lamb JD, Cline TD, Sudweeks SN, Winder WW. Endurance training increases LKB1 and MO25 protein but not AMP-activated protein kinase kinase activity in skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287:E1082–1089. doi: 10.1152/ajpendo.00179.2004. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Weindruch R, Saupe KW. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am J Physiol Endocrinol Metab. 2004;287:E1032–1037. doi: 10.1152/ajpendo.00172.2004. [DOI] [PubMed] [Google Scholar]
- 46.Guh JH, Chang WL, Yang J, Lee SL, Wei S, Wang D, Kulp SK, Chen CS. Development of novel adenosine monophosphate-activated protein kinase activators. J Med Chem. 2010;53:2552–2561. doi: 10.1021/jm901773d. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Bang S, Chen Y, Ahima RS, Kim SF. Convergence of IPMK and LKB1-AMPK signaling pathways on metformin action. Mol Endocrinol. 2014;28:1186–1193. doi: 10.1210/me.2014-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348:607–614. [PMC free article] [PubMed] [Google Scholar]
- 49.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 50.He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA, Radovick S, Wondisford FE. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pernicova I, Korbonits M. Metformin-mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143–56. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 52.Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS, Shaw RJ. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dykens JA, Jamieson J, Marroquin L, Nadanaciva S, Billis PA, Will Y. Biguanide-induced mitochondrial dysfunction yields increased lactate production and cytotoxicity of aerobically-poised HepG2 cells and human hepatocytes in vitro. Toxicol Appl Pharmacol. 2008;233:203–210. doi: 10.1016/j.taap.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 54.Lega IC, Shah PS, Margel D, Beyene J, Rochon PA, Lipscombe LL. The Effect of Metformin on Mortality Following Cancer among Patients with Diabetes. Cancer Epidemiol Biomarkers Prev. 2014;23:1974–84. doi: 10.1158/1055-9965.EPI-14-0327. [DOI] [PubMed] [Google Scholar]
- 55.Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 56.Wairagu PM, Phan AN, Kim MK, Han J, Kim HW, Choi JW, Kim KW, Cha SK, Park KH, Jeong Y. Insulin priming effect on estradiol-induced breast cancer metabolism and growth. Cancer Biol Ther. 2015;16:484–492. doi: 10.1080/15384047.2015.1016660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rice S, Pellat L, Ahmetaga A, Bano G, Mason HD, Whitehead SA. Dual effect of metformin on growth inhibition and oestradiol production in breast cancer cells. Int J Mol Med. 2015;35:1088–1094. doi: 10.3892/ijmm.2015.2108. [DOI] [PubMed] [Google Scholar]
- 58.Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fried LE, Arbiser JL. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid Redox Signal. 2009;11:1139–1148. doi: 10.1089/ars.2009.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagalingam A, Arbiser JL, Bonner MY, Saxena NK, Sharma D. Honokiol activates AMP-activated protein kinase in breast cancer cells via an LKB1-dependent pathway and inhibits breast carcinogenesis. Breast Cancer Res. 2012;14:R35. doi: 10.1186/bcr3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen D, Pamu S, Cui Q, Chan TH, Dou QP. Novel epigallocatechin gallate (EGCG) analogs activate AMP-activated protein kinase pathway and target cancer stem cells. Bioorg Med Chem. 2012;20:3031–3037. doi: 10.1016/j.bmc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo LS, Li HX, Li CY, Zhang SY, Chen J, Wang QL, Gao JM, Liang JQ, Gao MT, Wu YJ. Synergistic antitumor activity of vitamin D3 combined with metformin in human breast carcinoma MDA-MB-231 cells involves m-TOR related signaling pathways. Pharmazie. 2015;70:117–122. [PubMed] [Google Scholar]
- 63.Deshmukh RR, Dou QP. Proteasome inhibitors induce AMPK activation via CaMKKbeta in human breast cancer cells. Breast Cancer Res Treat. 2015;153:79–88. doi: 10.1007/s10549-015-3512-2. [DOI] [PubMed] [Google Scholar]
- 64.Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007;282:32549–32560. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vingtdeux V, Chandakkar P, Zhao H, Davies P, Marambaud P. Small-molecule activators of AMP-activated protein kinase (AMPK), RSVA314 and RSVA405, inhibit adipogenesis. Mol Med. 2011;17:1022–1030. doi: 10.2119/molmed.2011.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abdulrahman RM, Boon MR, Sips HC, Guigas B, Rensen PC, Smit JW, Hovens GC. Impact of Metformin and Compound C on NIS Expression and Iodine Uptake In Vitro and In Vivo: A Role for CRE in AMPK Modulation of Thyroid Function. Thyroid. 2014;24:78–87. doi: 10.1089/thy.2013.0041. [DOI] [PubMed] [Google Scholar]
- 67.Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5’-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 68.Fox MM, Phoenix KN, Kopsiaftis SG, Claffey KP. AMP-Activated Protein Kinase alpha 2 Isoform Suppression in Primary Breast Cancer Alters AMPK Growth Control and Apoptotic Signaling. Genes Cancer. 2013;4:3–14. doi: 10.1177/1947601913486346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- 70.Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab. 2012;23:83–89. doi: 10.1016/j.tem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 2008;68:5492–5499. doi: 10.1158/0008-5472.CAN-07-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown KA, McInnes KJ, Hunger NI, Oakhill JS, Steinberg GR, Simpson ER. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res. 2009;69:5392–5399. doi: 10.1158/0008-5472.CAN-09-0108. [DOI] [PubMed] [Google Scholar]
- 73.Quentin T, Kitz J, Steinmetz M, Poppe A, Bar K, Kratzner R. Different expression of the catalytic alpha subunits of the AMP activated protein kinase--an immunohistochemical study in human tissue. Histol Histopathol. 2011;26:589–596. doi: 10.14670/HH-26.589. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y, Storr SJ, Johnson K, Green AR, Rakha EA, Ellis IO, Morgan DA, Martin SG. Involvement of metformin and AMPK in the radioresponse and prognosis of luminal versus basal-like breast cancer treated with radiotherapy. Oncotarget. 2014;5:12936–12949. doi: 10.18632/oncotarget.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hadad SM, Baker L, Quinlan PR, Robertson KE, Bray SE, Thomson G, Kellock D, Jordan LB, Purdie CA, Hardie DG, Fleming S, Thompson AM. Histological evaluation of AMPK signalling in primary breast cancer. BMC Cancer. 2009;9:307. doi: 10.1186/1471-2407-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 77.Hadad SM, Fleming S, Thompson AM. Targeting AMPK: a new therapeutic opportunity in breast cancer. Crit Rev Oncol Hematol. 2008;67:1–7. doi: 10.1016/j.critrevonc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 78.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 79.Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5’-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287:562–567. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- 80.Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, Mamer OA, Avizonis D, DeBerardinis RJ, Siegel PM, Jones RG. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chou CC, Lee KH, Lai IL, Wang D, Mo X, Kulp SK, Shapiro CL, Chen CS. AMPK reverses the mesenchymal phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a signaling axis. Cancer Res. 2014;74:4783–4795. doi: 10.1158/0008-5472.CAN-14-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gollavilli PN, Kanugula AK, Koyyada R, Karnewar S, Neeli PK, Kotamraju S. AMPK inhibits MTDH expression via GSK3beta and SIRT1 activation: potential role in triple negative breast cancer cell proliferation. FEBS J. 2015;282:3971–3985. doi: 10.1111/febs.13391. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka Y, Yano H, Ogasawara S, Yoshioka S, Imamura H, Okamoto K, Tsuneoka M. Mild Glucose Starvation Induces KDM2A-Mediated H3K36me2 Demethylation through AMPK To Reduce rRNA Transcription and Cell Proliferation. Mol Cell Biol. 2015;35:4170–4184. doi: 10.1128/MCB.00579-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown KA, Hunger NI, Docanto M, Simpson ER. Metformin inhibits aromatase expression in human breast adipose stromal cells via stimulation of AMP-activated protein kinase. Breast Cancer Res Treat. 2010;123:591–596. doi: 10.1007/s10549-010-0834-y. [DOI] [PubMed] [Google Scholar]
- 85.Rodriguez M, Potter DA. CYP1A1 regulates breast cancer proliferation and survival. Mol Cancer Res. 2013;11:780–792. doi: 10.1158/1541-7786.MCR-12-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhuang Y, Miskimins WK. Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal. 2008;3:18. doi: 10.1186/1750-2187-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phoenix KN, Vumbaca F, Fox MM, Evans R, Claffey KP. Dietary energy availability affects primary and metastatic breast cancer and metformin efficacy. Breast Cancer Res Treat. 2010;123:333–344. doi: 10.1007/s10549-009-0647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Z, Ren L, Liu C, Xia T, Zha X, Wang S. Phenformin Induces Cell Cycle Change, Apoptosis, and Mesenchymal-Epithelial Transition and Regulates the AMPK/mTOR/p70s6k and MAPK/ERK Pathways in Breast Cancer Cells. PLoS One. 2015;10:e0131207. doi: 10.1371/journal.pone.0131207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jhaveri TZ, Woo J, Shang X, Park BH, Gabrielson E. AMP-activated kinase (AMPK) regulates activity of HER2 and EGFR in breast cancer. Oncotarget. 2015;6:14754–14765. doi: 10.18632/oncotarget.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fan C, Wang Y, Liu Z, Sun Y, Wang X, Wei G, Wei J. Metformin exerts anticancer effects through the inhibition of the Sonic hedgehog signaling pathway in breast cancer. Int J Mol Med. 2015;36:204–214. doi: 10.3892/ijmm.2015.2217. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.El-Masry OS, Brown BL, Dobson PR. Effects of activation of AMPK on human breast cancer cell lines with different genetic backgrounds. Oncol Lett. 2012;3:224–228. doi: 10.3892/ol.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Youn SH, Lee JS, Lee MS, Cha EY, Thuong PT, Kim JR, Chang ES. Anticancer properties of pomolic acid-induced AMP-activated protein kinase activation in MCF7 human breast cancer cells. Biol Pharm Bull. 2012;35:105–110. doi: 10.1248/bpb.35.105. [DOI] [PubMed] [Google Scholar]
- 93.Li P, Zhao M, Parris AB, Feng X, Yang X. p53 is required for metformin-induced growth inhibition, senescence and apoptosis in breast cancer cells. Biochem Biophys Res Commun. 2015;464:1267–1274. doi: 10.1016/j.bbrc.2015.07.117. [DOI] [PubMed] [Google Scholar]
- 94.Toyama EQ, Herzig S, Courchet J, Lewis TL Jr, Loson OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, Shaw RJ. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science. 2016;351:275–281. doi: 10.1126/science.aab4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Appleyard MV, Murray KE, Coates PJ, Wullschleger S, Bray SE, Kernohan NM, Fleming S, Alessi DR, Thompson AM. Phenformin as prophylaxis and therapy in breast cancer xenografts. Br J Cancer. 2012;106:1117–1122. doi: 10.1038/bjc.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jalava P, Kuopio T, Juntti-Patinen L, Kotkansalo T, Kronqvist P, Collan Y. Ki67 immunohistochemistry: a valuable marker in prognostication but with a risk of misclassification: proliferation subgroups formed based on Ki67 immunoreactivity and standardized mitotic index. Histopathology. 2006;48:674–682. doi: 10.1111/j.1365-2559.2006.02402.x. [DOI] [PubMed] [Google Scholar]
- 97.Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 99.Domenech E, Maestre C, Esteban-Martinez L, Partida D, Pascual R, Fernandez-Miranda G, Seco E, Campos-Olivas R, Perez M, Megias D, Allen K, Lopez M, Saha AK, Velasco G, Rial E, Mendez R, Boya P, Salazar-Roa M, Malumbres M. AMPK and PFKFB3 mediate glycolysis and survival in response to mitophagy during mitotic arrest. Nat Cell Biol. 2015;17:1304–1316. doi: 10.1038/ncb3231. [DOI] [PubMed] [Google Scholar]
- 100.Wu D, Cederbaum AI. Sodium salicylate increases CYP2E1 levels and enhances arachidonic acid toxicity in HepG2 cells and cultured rat hepatocytes. Mol Pharmacol. 2001;59:795–805. doi: 10.1124/mol.59.4.795. [DOI] [PubMed] [Google Scholar]
- 101.von Eyss B, Jaenicke LA, Kortlever RM, Royla N, Wiese KE, Letschert S, McDuffus LA, Sauer M, Rosenwald A, Evan GI, Kempa S, Eilers M. A MYC-Driven Change in Mitochondrial Dynamics Limits YAP/TAZ Function in Mammary Epithelial Cells and Breast Cancer. Cancer Cell. 2015;28:743–757. doi: 10.1016/j.ccell.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 102.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 104.Anderson WF, Rosenberg PS, Katki HA. Tracking and evaluating molecular tumor markers with cancer registry data: HER2 and breast cancer. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, Boscoe FP, Cronin KA, Lake A, Noone AM, Henley SJ, Eheman CR, Anderson RN, Penberthy L. Annual Report to the Nation on the Status of Cancer, 1975-2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab. 2012;23:83–89. doi: 10.1016/j.tem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Santen R, Cavalieri E, Rogan E, Russo J, Guttenplan J, Ingle J, Yue W. Estrogen mediation of breast tumor formation involves estrogen receptor-dependent, as well as independent, genotoxic effects. Ann N Y Acad Sci. 2009;1155:132–140. doi: 10.1111/j.1749-6632.2008.03685.x. [DOI] [PubMed] [Google Scholar]
- 108.Bulun SE, Lin Z, Zhao H, Lu M, Amin S, Reierstad S, Chen D. Regulation of aromatase expression in breast cancer tissue. Ann N Y Acad Sci. 2009;1155:121–131. doi: 10.1111/j.1749-6632.2009.03705.x. [DOI] [PubMed] [Google Scholar]
- 109.Chetrite GS, Cortes-Prieto J, Philippe JC, Wright F, Pasqualini JR. Comparison of estrogen concentrations, estrone sulfatase and aromatase activities in normal, and in cancerous, human breast tissues. J Steroid Biochem Mol Biol. 2000;72:23–27. doi: 10.1016/s0960-0760(00)00040-6. [DOI] [PubMed] [Google Scholar]
- 110.Chen D, Reierstad S, Lu M, Lin Z, Ishikawa H, Bulun SE. Regulation of breast cancer-associated aromatase promoters. Cancer Lett. 2009;273:15–27. doi: 10.1016/j.canlet.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 111.Deb S, Zhou J, Amin SA, Imir AG, Yilmaz MB, Lin Z, Bulun SE. A novel role of sodium butyrate in the regulation of cancer-associated aromatase promoters I. 3 and II by disrupting a transcriptional complex in breast adipose fibroblasts. J Biol Chem. 2006;281:2585–2597. doi: 10.1074/jbc.M508498200. [DOI] [PubMed] [Google Scholar]
- 112.He Y, Li Y, Zhao T, Wang Y, Sun C. Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through LKB1/AMPK pathway. PLoS One. 2013;8:e70135. doi: 10.1371/journal.pone.0070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hadad SM, Coates P, Jordan LB, Dowling RJ, Chang MC, Done SJ, Purdie CA, Goodwin PJ, Stambolic V, Moulder-Thompson S, Thompson AM. Evidence for biological effects of metformin in operable breast cancer: biomarker analysis in a pre-operative window of opportunity randomized trial. Breast Cancer Res Treat. 2015;150:149–155. doi: 10.1007/s10549-015-3307-5. [DOI] [PubMed] [Google Scholar]
- 114.Dowling RJ, Niraula S, Chang MC, Done SJ, Ennis M, McCready DR, Leong WL, Escallon JM, Reedijk M, Goodwin PJ, Stambolic V. Changes in insulin receptor signaling underlie neoadjuvant metformin administration in breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res. 2015;17:32. doi: 10.1186/s13058-015-0540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goodwin PJ, Stambolic V, Lemieux J, Chen BE, Parulekar WR, Gelmon KA, Hershman DL, Hobday TJ, Ligibel JA, Mayer IA, Pritchard KI, Whelan TJ, Rastogi P, Shepherd LE. Evaluation of metformin in early breast cancer: a modification of the traditional paradigm for clinical testing of anti-cancer agents. Breast Cancer Res Treat. 2011;126:215–220. doi: 10.1007/s10549-010-1224-1. [DOI] [PubMed] [Google Scholar]
- 116.Ferguson KM. Structure-based view of epidermal growth factor receptor regulation. Annu Rev Biophys. 2008;37:353–373. doi: 10.1146/annurev.biophys.37.032807.125829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eroles P, Bosch A, Perez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat Rev. 2012;38:698–707. doi: 10.1016/j.ctrv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 118.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provinciali M, Re F, Franceschi C. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–693. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 119.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 120.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 121.Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch C, Cameron D, Mano M, Pedrini JL, Veronesi A, Mendiola C, Pluzanska A, Semiglazov V, Vrdoljak E, Eckart MJ, Shen Z, Skiadopoulos G, Procter M, Pritchard KI, Piccart-Gebhart MJ, Bell R Herceptin Adjuvant (HERA) Trial Study Team. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–244. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 122.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 123.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7:332–344. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 124.Spector NL, Yarden Y, Smith B, Lyass L, Trusk P, Pry K, Hill JE, Xia W, Seger R, Bacus SS. Activation of AMP-activated protein kinase by human EGF receptor 2/EGF receptor tyrosine kinase inhibitor protects cardiac cells. Proc Natl Acad Sci U S A. 2007;104:10607–10612. doi: 10.1073/pnas.0701286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hubner N, Chien KR, Birchmeier C, Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002;99:8880–8885. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 127.Shell SA, Lyass L, Trusk PB, Pry KJ, Wappel RL, Bacus SS. Activation of AMPK is necessary for killing cancer cells and sparing cardiac cells. Cell Cycle. 2008;7:1769–1775. doi: 10.4161/cc.7.12.6016. [DOI] [PubMed] [Google Scholar]
- 128.Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Durand JB, Force T. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 129.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 130.Deng XS, Wang S, Deng A, Liu B, Edgerton SM, Lind SE, Wahdan-Alaswad R, Thor AD. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Cell Cycle. 2012;11:367–376. doi: 10.4161/cc.11.2.18813. [DOI] [PubMed] [Google Scholar]
- 131.Peeters PJ, Bazelier MT, Vestergaard P, Leufkens HG, Schmidt MK, de Vries F, De Bruin ML. Use of metformin and survival of diabetic women with breast cancer. Curr Drug Saf. 2013;8:357–363. doi: 10.2174/15680266113136660069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann Oncol. 2012;23:1771–1780. doi: 10.1093/annonc/mdr534. [DOI] [PMC free article] [PubMed] [Google Scholar]


