Abstract
The bio-preservation potential of Lactococcus garvieae lies in its capacity to inhibit the growth of staphylococci, especially Staphylococcus aureus, in dairy products and in vitro. In vitro, inhibition is modulated by the level of aeration, owing to hydrogen peroxide (H2O2) production by L. garvieae under aeration. The S. aureus response to this inhibition has already been studied. However, the molecular mechanisms of L. garvieae underlying the antagonism against S. aureus have never been explored. This study provides evidence of the presence of another extracellular inhibition effector in vitro. This effector was neither a protein, nor a lipid, nor a polysaccharide, nor related to an L-threonine deficiency. To better understand the H2O2-related inhibition mechanism at the transcriptome level and to identify other mechanisms potentially involved, we used RNA sequencing to determine the transcriptome response of L. garvieae to different aeration levels and to the presence or absence of S. aureus. The L. garvieae transcriptome differed radically between different aeration levels mainly in biological processes related to fundamental functions and nutritional adaptation. The transcriptomic response of L. garvieae to aeration level differed according to the presence or absence of S. aureus. The higher concentration of H2O2 with high aeration was not associated with a higher expression of L. garvieae H2O2-synthesis genes (pox, sodA, and spxA1) but rather with a repression of L. garvieae H2O2-degradation genes (trxB1, ahpC, ahpF, and gpx). We showed that L. garvieae displayed an original, previously undiscovered, H2O2 production regulation mechanism among bacteria. In addition to the key factor H2O2, the involvement of another extracellular effector in the antagonism against S. aureus was shown. Future studies should explore the relation between H2O2-metabolism, H2O2-producing LAB and the pathogen they inhibit. The nature of the other extracellular effector should also be determined.
Keywords: Lactococcus garvieae, Staphylococcus aureus, hydrogen peroxide, transcriptome, growth inhibition, anti-pathogenic interactions
Introduction
Interest in Lactic Acid Bacteria (LAB) as bio-preservation agents against foodborne pathogens has been growing for the last 15 years (Ito et al., 2003; Batdorj et al., 2007; Charlier et al., 2009; Adesokan et al., 2010). Considering their beneficial properties, the dairy industry could find it useful to employ Lactococcus garvieae strains as starter or adjunct cultures, provided the strains are safe (Fernández et al., 2010). Aquatic strains of L. garvieae are frequently referenced as fish pathogens (Eldar and Ghittino, 1999; Vendrell et al., 2006) but other strains are regarded as opportunistic pathogens for humans (Aguado-Urda et al., 2011; Ortiz et al., 2014). Despite its ubiquity in foods such as milk (Devriese et al., 1999; Villani et al., 2001; Callon et al., 2007) and dairy products of various origins (Flórez and Mayo, 2006; Fortina et al., 2007; Alomar et al., 2008b; El-Baradei et al., 2008; Jokovic et al., 2008; Alegría et al., 2009; Sip et al., 2009; Monfredini et al., 2012; Pangallo et al., 2014; Morandi et al., 2015), to our knowledge, L. garvieae has never been involved in a foodborne disease outbreak. L. garvieae is of interest for bio-preservation owing to its ability to inhibit the growth of staphylococci, particularly Staphylococcus aureus, as has been observed in milk, in cheese and in vitro (Alomar et al., 2008a,b; Delbes-Paus et al., 2010; Delpech et al., 2015). The transcriptome response of S. aureus to this inhibition has already been explored (Delpech et al., 2015). It is associated with a repression of the stress response (especially H2O2 response) and of cell division genes and with modulation of the expression of virulence genes (particularly agrA, hld, and enterotoxin-encoding genes) genes. However, the molecular mechanisms of L. garvieae underlying the antagonism against S. aureus have never been explored.
Since L. garvieae produces low amounts of acetic and lactic acid in milk (Alomar et al., 2008a,b; Nouaille et al., 2009; Sip et al., 2009; Delbes-Paus et al., 2010; Delpech et al., 2015; Morandi et al., 2015), its antagonism against S. aureus is not associated with acidification. It is also unlikely to be associated with nutritional competition (Alomar et al., 2008a). With high aeration, S. aureus inhibition is mainly associated with hydrogen peroxide (H2O2) production by L. garvieae, as already observed in vitro (Delbes-Paus et al., 2010; Delpech et al., 2015). With low aeration, the inhibition is weaker and H2O2 is not detected. The addition of catalase (an H2O2-degrading enzyme) partly suppressed the inhibition (Delbes-Paus et al., 2010). Delbes-Paus et al. (2010) therefore suggested that at least one other molecule from L. garvieae must be involved in the residual inhibition of S. aureus in the absence of H2O2. The role and nature of this molecule have not yet been determined.
In Gram-positive bacteria, the aeration level and the presence of H2O2 generally strongly affect the expression of H2O2 metabolism genes. The expression of major H2O2 degradation genes (ahpC, ahpF, gshR, gpo, trxA, and trxB) and their related proteins is strongly induced in L. lactis (Pedersen et al., 2008) under aeration and in Bacillus subtilis in the presence of H2O2 (Mostertz et al., 2004). As regards the major H2O2 synthesis genes, sodA is generally regulated in the same way as H2O2-degradation genes while the pyruvate oxidase (pox) gene is not affected by these parameters. Biological functions of L. lactis related to aerobiosis, i.e., O2 response, menaquinone metabolism and stress response, are variably affected by the presence of S. aureus (Nouaille et al., 2009). However, little is known about the H2O2-metabolism of L. garvieae as an anti-pathogenic process.
In this study, we aimed to characterize the antagonism of L. garvieae against S. aureus in vitro in greater depth. Firstly, we investigated the presence of potential antagonist molecules in H2O2-free L. garvieae supernatants and their impact on S. aureus growth. Secondly, the transcriptome of L. garvieae with different aeration levels and in different biotic environments (absence or presence of S. aureus) was determined through RNA sequencing, an accurate and efficient method for revealing bacterial transcriptome profiles (Pinto et al., 2011). The resulting data led us to a better understanding of L. garvieae hydrogen peroxide metabolism and to some initial hypotheses on the nature of a new inhibition effector.
Materials and Methods
Strains and Culture Conditions
Lactococcus garvieae N201 and S. aureus SA15, isolated from raw milk, were obtained from the INRA UR545 collection (Alomar et al., 2008a). Both strains were aerobically grown in Brain-Heart Infusion broth (“BHI”, Biokar Diagnostic, Pantin, France) for 20 h, at 30°C for L. garvieae and at 37°C for S. aureus. They were then inoculated separately or in co-culture at 106 cells.mL-1 for S. aureus and 107 cells.mL-1 for L. garvieae into BHI buffered at pH = 7 with phosphate buffer KH2PO4, 3H2O/K2HPO4 at 0.1 mol.L-1 (KH2PO4, 3H2O, Riedel-de-Haen, Honeywell GmbH, Seelze, Germany; K2HPO4, Merck KGaA, Darmstadt, Germany) previously equilibrated at 30°C. Pure cultures and co-cultures of both strains were performed with either a high or a low aeration depending on the experiment. Low aeration cultures were set in static, fully filled and sealed, 50-mL Nunc EZ Flip conical centrifuge tubes (Sigma–Aldrich, St. Louis, MO, USA). High aeration was obtained by a mechanical shaking at 150 rpm on 50-mL cultures in 250-mL Erlenmeyers. All cultures were incubated at 30°C for 24 h in an Infors HT Minitron (Infors AG, Bottmingen, Switzerland). The cultivable cell counts were determined after plating for each sampling time as described by Delpech et al. (2015).
Impact of H2O2-Free Co-culture Supernatants on S. aureus Growth
The potential presence of other antistaphylococcal molecules in L. garvieae N201 and S. aureus SA15 co-culture supernatant and their impact on S. aureus planktonic growth were investigated. After adding catalase at 400 U.mL-1, a pure culture of S. aureus SA15, a co-culture of S. aureus SA15 and L. garvieae N201 and plain BHI were incubated with low aeration as described above. After 6 h of incubation, 40 mL of each tube were centrifuged at 9,600 × g for 10 min at 4°C and supernatants stored at 4°C until utilization. Four milliliters of the remaining S. aureus culture were centrifuged at 7,500 × g for 10 min. The cell pellet was resuspended at a concentration of ∼106 cells.mL-1 in 20 mL of fresh 2X-concentrated BHI buffered at pH = 7. The supernatants were filtered through a cellulose acetate membrane (pore size 0.45 μm; GVS S.p.A., Zola Predosa, Italy). They were either treated with proteinase K (AMRESCO LLC, Solon, OH, USA, ref: 0706-100MG) and pronase E (Merck KGaA, ref: 537088), i.e., with two proteases, or treated with lipase (Sigma–Aldrich, ref: L3126-100G), or treated with α-amylase (Sigma–Aldrich, ref: A3176-500KU), or not treated at all. Each enzyme was added at 0.2 mg.mL-1 from stock solutions at 10 mg.mL-1 prepared in 200 mM of phosphate buffer at pH = 7. Treatment with proteases consisted of a first incubation of the supernatants with proteinase K at 50°C for 2 h 15 and a second incubation with pronase E at 37°C for 2 h 15. Treatments with lipase and α-amylase consisted of an incubation of the supernatants with the enzyme at 37°C for 5 h. After each incubation step, the enzyme was inactivated by heating (95°C during 10 min) and all supernatants were filtered again through a cellulose acetate membrane (pore size 0.45 μm; GVS S.p.A.). Prepared supernatants and S. aureus culture in 2X-BHI were then distributed at 1:1 volume ratio (total volume = 1.5 mL) in a CytoOne 24-well cell plate covered with a lid (ref: CC7672-7524; STARLAB, Hambourg, Germany). The cell plate was incubated for 25 h in a SAFAS Xenius XC spectrophotometer (SAFAS Monaco, Monaco) and thermostated at 30°C with a Julabo CryoStat (JULABO Gmbh, Seelbach, Germany). S. aureus growth in each well was determined by measuring the OD600 every 15 min for 1 h and then every 30 min for 24 h. Before each OD600 measurement, the cell plate was shaken at 5 Hz for 20 s with an orbital diameter of 6 mm. The whole experimental design was repeated three times. To identify sample means that were significantly different from each other, statistical analyses were performed on values at 9, 12, 15, 18, 21, and 24 h, using Statistica software (StatSoft) with a single-factor analysis of variance (ANOVA) followed by a Newman–Keuls post hoc test.
Sample Preparation for RNA Analysis
Pure cultures and co-cultures of L. garvieae N201 and S. aureus SA15 were grown with high or low aeration, as specified above. After 3, 6, 9, and 24 h of incubation, 40 mL of each culture were centrifuged at 9,600 × g for 10 min at 4°C. Hydrogen peroxide concentrations and pH values were determined on the supernatants by enzymatic reaction and spectrophotometry as described by Delbes-Paus et al. (2010). The cell pellets were immediately frozen in an ethanol bath and stored at -80°C. Extraction of total RNA from the frozen cell pellets was performed as described by Delpech et al. (2015). For each sample of total RNA obtained with one culture condition, a first part of the aliquot was used for RNA sequencing after rRNA depletion and a second part of the aliquot was used for RT-qPCR analyses. The whole experimental design was repeated three times.
Determination of L. garvieae Transcriptome Changes by RNA Sequencing
Ribosomal RNAs were removed from the total RNA (2 × 5 μg of RNA by sample) using a RiboZero Magnetic Kit for Gram Positive Bacteria (Illumina Inc., San Diego, CA, USA) according to the manufacturer’s instructions. The quality and concentration of RNA in each sample were assessed using a RNA 6000 pico kit (Agilent Technologies, Santa Clara, CA, USA). The following steps were performed by the MGX Platform (Montpellier GenomiX, CNRS, Montpellier, France) using Illumina kits and devices (Illumina Inc.): construction of the mRNA library using a TruSeq Stranded mRNA Sample Preparation Kit; cluster generation with the cBot system using a Cluster Generation Kit; hybridization of the sequencing primer on the flow-cell; 50-bp single-read sequencing using a HiSeq 2000 device with SBS technology; informatic pretreatments, i.e., image analysis with the HiSeq Control Software and Real-Time Analysis component, base-calling with the RTA software and demultiplexing with CASAVA (Illumina). The RNAseq data are available from NCBI GEO datasets under the accession number GSE74030.
The quality scores across all bases of all reads and the N (non-attributed bases) content across all bases were determined for each condition with the FastQC software from the Babraham Institute1. Both analyses showed good quality values (data not shown). The reads were next aligned simultaneously on reference genomes, i.e., L. garvieae N201 (unpublished, GOLD project ID = Gp0034836 and NCBI BioProject ID = 184287) and S. aureus MW2 (RefSeq number = NC_003923.1), using the BWA package (Li and Durbin, 2009) with a seed of 32 bases and a maximum of two mismatches tolerated on the seed. Using the Samtools suite (Li et al., 2009) we excluded from further analyses those reads with low alignment quality scores (MAPQ index < 20). Reads which mapped on multiple sites (between 0.3 and 3.2% depending on the sample) and reads which did not map on any site considering the stringency applied (between 8.7 and 15.5% depending on the sample) were excluded from further analysis (see Supplementary Table 1). Reads overlapping genes were counted with the HTSeq Count software in Union mode (Anders et al., 2015). Differentially expressed genes were identified using the Bioconductor R2 packages EdgeR, DESeq and DESeq2 (Anders and Huber, 2010; Robinson et al., 2010; Love et al., 2014). Genes with less than 15 reads (from the three biological replicates of two compared samples) were filtered and thus removed from the analysis. Data were normalized using the Relative Log Expression (RLE) normalization factor for EdgeR and the DESeq normalization factor for DESeq and DESeq2. Gene expression changes with adjusted p-value of less than 0.05 (by the FDR method from Benjamini–Hochberg) were declared differentially expressed. Differentially expressed genes highlighted by at least one of the three packages were considered for further investigation.
Genes were sorted into two lists: one of genes differentially expressed in both pure culture and co-culture and one of genes differentially expressed only in pure culture or only in co-culture. These lists were separately subjected to a Blast2Go analysis (Conesa et al., 2005). Blast2Go analysis consisted first of a BlastX3 on the nr database with 20 hits and a maximum E-value of 1.0E-15. The resulting data were enriched with an InterPro scan analysis (Zdobnov and Apweiler, 2001). Blast hits of each sequence were then mapped with Gene Ontology terms (Gene Ontology Consortium, 2008) annotated with a maximum E-value of 1.0E-6, a cut-off of 55 and a GO weight of 5. The number of genes involved in each Biological Process GO category was calculated from combined graphs with no filter and a score alpha of 0.6. Since more than 450 different biological processes were identified for each category of genes, we excluded biological processes involving less than nine genes. When several biological processes involved the same genes and were associated with comparable functions, we considered only the one most relevant to our scientific hypotheses.
For a deeper analysis of S. aureus direct effect on L. garvieae gene expression regardless of aeration level, data mining was undertaken using the regression functions in Microsoft excel. Expression values were systematically plotted for each pair of samples (co-culture versus pure culture), a standard residual value was determined from the regression analysis for each gene across multiple pair wise comparisons and the mean value was determined. This mean standard residual value was used to rank the genes and identify the strongest gene expression differences. A standard residual cut-off of 5 was used as the threshold for significance to account for false discovery using a Bonferroni correction and approximating the standard residual to an equivalent p-value for the number of comparisons.
Determination of Gene Expression by RT-qPCR
Total RNA was retro-transcribed using a High Capacity cDNA Reverse Transcription kit (Invitrogen, Life Technologies, Carlsbad, CA, USA) following the supplier’s instructions. Ct of genes of interest (see Supplementary Table 2) were determined by RT-qPCR as described by Delpech et al. (2015). All primers were designed using PrimerExpress® software (Applied Biosystems®, Life Technologies). By comparison with the Ct of the tufB reference gene stable in our conditions (data not shown), expression of a gene of interest (“goi”) was calculated using the formula introduced by Pfaffl (2001).
The influence of two experimental factors was studied: the presence of S. aureus (with high or low aeration, gene expression in co-culture divided by gene expression in pure culture) and aeration level (in presence or absence of S. aureus, gene expression in shaken condition divided by gene expression in static condition). Statistical analyses were performed using Statistica software (StatSoft, Inc., StatSoft France, Maisons-Alfort, France) by single-factor ANOVA followed by a Newman–Keuls post hoc test.
Results
Effect of Enzyme-Treated Co-culture Supernatants on S. aureus Planktonic Growth
Since the inhibition of S. aureus may not be related solely to hydrogen peroxide, we looked for another possible anti-staphylococcal molecule produced by L. garvieae in the culture supernatants. We monitored S. aureus growth (OD600) over 24 h in either non-inoculated BHI or in supernatants prepared from H2O2-free cultures (presence of catalase) with low aeration (either pure culture of S. aureus or co-culture of L. garvieae and S. aureus). In order to avoid any nutritional competition, BHI at a final concentration of 1X was added to each culture.
Growth of S. aureus in supernatant from S. aureus pure culture was comparable to that in plain BHI (data not shown). During the stationary phase, the OD600 measured in S. aureus cultures in supernatant from co-culture was lower than that in supernatant from S. aureus pure culture (see Data Sheet 1). This inhibition was still observed when these supernatants were treated with proteases, lipase or α-amylase, the main macromolecule-degrading enzymes. pH values remained between 6.9 and 7.1 in all cultures (data not shown).
Determination of Aeration and S. aureus Effects on L. garvieae Transcriptome by RNA Sequencing
To identify the best conditions for studying the transcriptome of L. garvieae N201 with respect to its capacity to inhibit S. aureus SA15, we followed the growth of the two bacteria in pure cultures and co-cultures in BHI for 24 h under high or low aeration conditions (Table 1).
Table 1.
Cell counts and H2O2 concentration over 24 h in cultures of Lactococcus garvieae N201 and Staphylococcus aureus SA15 with high or low aeration levels.
| Aeration level | Culture |
L. garvieae cell concentration (log [CFU.mL-1]) |
S. aureus cell concentration (log [CFU.mL-1]) |
Hydrogen peroxide concentration (mM) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 3 h | 6 h | 9 h | 24 h | 0 h | 3 h | 6 h | 9 h | 24 h | 0 h | 3 h | 6 h | 9 h | 24 h | ||
| High | N201 | 6.6a | 7.3a | 8.4a | 9.0a | 8.6a | NT | NT | NT | NT | NT | ND | NT | 0.5a | 1.7a | 1.5a |
| SA15 | NT | NT | NT | NT | NT | 5.6a | 5.2ab | 6.1a | 7.4a | 9.0a | ND | NT | ND | ND | ND | |
| N201 + SA15 | 6.5a | 7.4a | 8.5a | 8.6a | 8.7a | 5.6a | 4.8a | 3.9b | 3.4b | 4.5b | ND | NT | 0.5a | 1.6a | 1.5a | |
| Low | N201 | 6.6a | 7.4a | 8.4a | 8.7a | 8.9a | NT | NT | NT | NT | NT | ND | NT | ND | ND | ND |
| SA15 | NT | NT | NT | NT | NT | 5.6a | 6.1c | 7.0a | 7.3a | 8.0c | ND | NT | ND | ND | ND | |
| N201 + SA15 | 6.5a | 7.3a | 8.8a | 8.8a | 8.9a | 5.6a | 5.6bc | 6.2a | 6.5a | 6.2d | ND | NT | ND | ND | ND | |
In the same column, letters (a, b, and c) indicate homogeneous statistical groupings (p-value < 0.05 by Newman–Keuls method). ND, non-detectable values (below the spectrometry detection limit). NT, not tested.
The growth of L. garvieae was not affected by S. aureus. The growth of S. aureus was inhibited by L. garvieae at both aeration levels. With high aeration, maximal inhibition was observed from 9 to 24 h (difference with pure culture of 4 and 4.5 log CFU/ml, respectively, at 9 and 24 h). At 9 h, H2O2 concentration reached a peak concomitant with the lowest S. aureus concentration. With low aeration, inhibition was weaker than with high aeration and was observed later, at 24 h. Concomitantly, H2O2 was not detected in these cultures. pH values remained between 6.9 and 7.1 in all cultures (data not shown).
Considering these data, we analyzed the L. garvieae transcriptome in pure culture and in co-culture with S. aureus after 9 h of incubation with high and the low aerations. RNA sequencing generated a number of reads per sample, ranging from 12,365,133 to 15,670,131 depending on the sample after the initial quality filter (see Supplementary Table 1). Between 83.1 and 91.0% of these reads mapped correctly onto the reference genomes. Considering the direct effect of S. aureus on L. garvieae gene expression, analysis of the mapped reads using the Bioconductor R packages failed to identify expression differences (data not shown). Additional regression analyses highlighted 39 genes differentially expressed in presence of S. aureus, of which 4 genes under low aeration and 35 genes under high aeration conditions (see Supplementary Table 3). The expression of ∼18% of L. garvieae genes, i.e., 358 genes, differed between the two aeration levels (see Supplementary Table 4). Among these 358 genes, the expression of 181 genes differed regardless of the presence or absence of S. aureus (i.e., similarly in pure culture and in co-culture). The expression of 177 L. garvieae genes responded differently to different aeration levels, depending on the presence or absence of S. aureus: 88 gene expressions differed only in pure culture and 89 gene expressions differed only in co-culture.
Effect of Aeration Level on L. garvieae Biological Processes
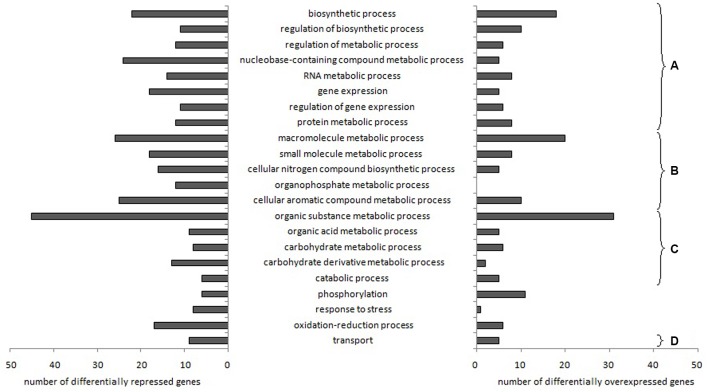
After RNA sequencing data treatments, 22 biological processes (named according to Gene Ontology termes) related to L. garvieae genes differentially expressed depending on aeration level in both pure culture and co-culture were identified using Blast2Go (Figure 1). Changes in gene expression are shown in Supplementary Table 4.
FIGURE 1.
Lactococcus garvieae biological processes involving genes differentially expressed depending on aeration level in both pure culture and co-culture. Bars represent the number of genes significantly overexpressed (left) or repressed (right) with high aeration compared to low aeration, according to at least one of the three R packages used (EdgeR, DESeq, and DESeq2). After the Blast2Go analysis and data filtering, biological processes were manually sorted into several categories: (A) processes related to fundamental growth function, i.e., global cellular, metabolic and biosynthetic processes, (post-)transcriptional and (post-)translational functions, (B) processes potentially associated with fundamental growth functions but also potentially associated with other metabolisms, (C) nutrition-related processes and (D) transport processes.
Most of the biological processes affected were related to fundamental growth functions (Figures 1A,B) and nutrition (Figure 1C). With high aeration, two genes (ilvA, LCGN_1922) related to “threonine metabolism” were repressed while two genes (LCGN_1919, LCGN_1920) were over-expressed.
Fourteen genes related to “transport” processes were differently expressed depending on aeration level both in pure culture and co-culture, including genes involved in the transport of metals (lead, cadmium, zinc, copper and/or mercury) and vitamins (riboflavin and folate).
Genes and GO biological processes related to O2 and H2O2 metabolism were also affected. “Oxidation-reduction process” and “response to stress” biological processes involved more repressed genes (17 and 8, respectively) than over-expressed genes (6 and 1, respectively). Three genes related to H2O2 metabolism (ahpF, pox, and spxA1) and one gene related to O2 consumption (lox) were repressed with the high aeration level. Several stress response genes (hrcA, groES, groEL, dnaK, dnaJ, grpE, clpB, and five genes belonging to the universal stress protein family) were repressed. Genes related to peroxide resistance (ohrA and ohrR) were strongly over-expressed under high aeration conditions. Electron Transport Chain (ETC) genes (cydB, menH, and ubiE) were over-expressed.
Modulation by S. aureus of L. garvieae Response to Aeration
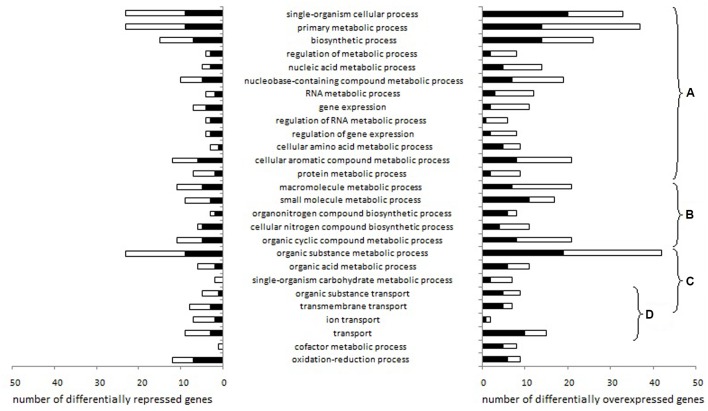
We identified 27 Gene Ontology biological processes related to genes differentially expressed depending on aeration level either in pure culture or in co-culture (Figure 2).
FIGURE 2.
Lactococcus garvieae biological processes involving genes differentially expressed depending on aeration level exclusively in pure culture or exclusively in co-culture. Stacked bars represent the number of genes significantly overexpressed (left) or repressed (right) with high aeration compared to low aeration in pure culture only (black bars) or in co-culture only (white bars), according to at least one of the three R packages used (EdgeR, DESeq, and DESeq2). After the Blast2Go analysis and data filtering, biological processes were manually sorted into several categories: (A) processes related to fundamental growth function, i.e., global cellular, metabolic and biosynthetic processes, (post-)transcriptional and (post-)translational functions, (B) processes potentially associated with fundamental growth functions but also potentially associated with other metabolisms, (C) nutrition-related processes and (D) transport processes.
Changes in gene expressions are shown in Supplementary Table 4. Most of the biological processes affected were related to fundamental growth functions (Figures 2A,B) or nutrition (Figure 2C). Under high aeration conditions, four lysine metabolism genes (LCGN_0575, LCGN_0576, LCGN_0577, LCGN_0578) and two threonine metabolism genes (LCGN_0576 and LCGN_0577) were over-expressed exclusively in pure culture while four galactose metabolism genes were over-expressed exclusively in co-culture (LCGN_1809, LCGN_1810, LCGN_1811, and LCGN_1812). Eight genes involved in pyruvate and carbohydrate metabolism and the citrate cycle (glmS, glmM, galE, PTS-Man-EIIC and EIID pdhA, pdhB, DLAT) were repressed by S. aureus. With low aeration, the enolase encoding gene was repressed by S. aureus.
Four biological processes related to transport functions were affected (Figure 2D): “transport,” “transmembrane transport,” “organic substance transport” and “ion transport.” In pure culture, genes involved in the transport of cobalt/zinc/cadmium (LCGN_1867) and an unspecified monosaccharide (LCGN_0332) were over-expressed with high aeration while a gene involved in copper transport (LCGN_1427) was repressed. Two genes of the fur regulon (ferrous iron transport) were over-expressed with high aeration, one of them in pure culture (feoA) and one in co-culture (feoB).
With low aeration, the H2O2 synthesis gene sodA was up-regulated by S. aureus. The H2O2 degradation gene ahpC was repressed by high aeration in co-culture but not in pure culture. In pure culture, two genes related to O2 consumption, noxE and LCGN_0208, were repressed and six genes related to ETC, cydA, cydC, menB, menC, menD, and LCGN_0364, were over-expressed.
Impact of Aeration on the Expression of L. garvieae Genes Potentially Involved in the Antagonism against S. aureus
To complete the RNA-seq data obtained only at 9 h, we determined the expression of ten genes from the cultures previously used for RNA-seq analyses, at 6, 9, and 24 h by RT-qPCR. These genes, potentially involved in the antagonism mechanisms, were involved in O2 consumption (noxE and lox), H2O2 synthesis (pox and sodA), H2O2 degradation (ahpC, ahpF, gpx, and trxB1) and resistance to other peroxides (ohrA and ohrR).
The presence of S. aureus induced only slight changes in their expression (data not shown). With high aeration, ahpC and pox were over-expressed 2.4-fold and 2.0-fold in the presence of S. aureus at 6 h. With low aeration, S. aureus induced a 3.1-fold over-expression of trxB1 at 9 h and a 3.3-fold repression of gpx at 24 h.
Conversely, RT-qPCR results showed major differences in H2O2-metabolism gene expression between the two aeration levels (Table 2). The expression of ohrA was strongly induced under high aeration conditions in both pure culture and co-culture at 9 h. While RNA sequencing identified trxA2 as up-regulated with high aeration in both pure culture and co-culture at 9 h (Supplementary Table 4), RT-qPCR identified other H2O2-degradation genes (ahpC, ahpF, gpx, and trxB1) as repressed at 6, 9, or 24 h. The lactate mono-oxygenase gene lox seemed slightly repressed with high aeration but this modification was significant only at 9 h in co-culture. In pure culture with high aeration, the expression of noxE reached a peak at 6 h when it was 11.0 times higher than with low aeration. With low aeration, noxE expression gradually increased over time. No significant difference in the expression of H2O2-synthesis genes (pox and sodA) according to aeration level was observed.
Table 2.
Effect of high aeration level on the expression of L. garvieae genes in pure culture and co-culture, as determined by RT-qPCR.
| In pure culturea |
In co-culturea |
||||||
|---|---|---|---|---|---|---|---|
| Gene | 6 h | 9 h | 24 h | 6 h | 9 h | 24 h | |
| Degradation of H 2O2 | ahpC | 0.6∗ | 0.6∗ | 0.4∗∗ | |||
| ahpF | 0.3∗∗ | NT | 0.4∗ | NT | |||
| gpx | 0.2∗ | 0.4∗ | |||||
| trxB1 | 0.2∗∗ | 0.2∗∗ | |||||
| Degradation of other peroxides | ohrA | 16.7∗∗ | NT | 7.0∗ | 20.2∗ | NT | |
| O2 consumption/H2O synthesis | noxE | 11.0∗∗ | 0.4∗ | 0.1∗ | |||
| lox | NT | 0.4∗∗ | NT | ||||
aIndicated values, at 6, 9, or 24 h in pure culture or in co-culture, corresponds to the ratios of gene expressions with high aeration to gene expressions with low aeration. Only ratios that are significant according to the Newman–Keuls test are shown (∗p-value < 0.1, ∗∗p-value < 0.05). NT, not tested.
Discussion
This study aimed to improve understanding of the mechanisms underlying the antagonism of L. garvieae against S. aureus, especially as regards H2O2-related pathways.
The high aeration level and the resulting high H2O2 concentration were associated with stronger inhibition of S. aureus, confirming previous observations under the same conditions (Alomar et al., 2008a; Delbes-Paus et al., 2010; Delpech et al., 2015). It was already known that this inhibition is associated with a modulation of the expression of S. aureus virulence genes and a repression of the H2O2 response, stress response and cell division genes of S. aureus by L. garvieae and with high aeration (Delpech et al., 2015). However, the transcriptome adaptation of L. garvieae to prevailing aeration conditions during this interaction had not been explored until this study. It is known that difference in aeration is associated with drastic modifications of the L. lactis transcriptome (Pedersen et al., 2008; Dijkstra et al., 2014) and consequent metabolic adaptations (Jensen et al., 2001; Nordkvist et al., 2003; Pedersen et al., 2008; Dijkstra et al., 2014; Larsen et al., 2016a,b). In accordance with these findings, we found that different aeration levels were associated with significant differences in the L. garvieae transcriptome in biological processes related to fundamental growth functions, nutritional and metabolic adaptations and transport functions. Moreover, L. garvieae genes involved in fundamental growth functions were slightly repressed by S. aureus under both aeration levels suggesting an impact of S. aureus on L. garvieae metabolism. While L. garvieae is a catalase-negative bacterium, the main S. aureus enzyme involved in H2O2 dismutation is the O2-forming catalase KatA (Cosgrove et al., 2007). The S. aureus catalase may affect the transcriptomic response of L. garvieae to different aeration conditions by modulating O2 and H2O2 concentrations. It is known that the katA gene of S. aureus SA15 is repressed by L. garvieae in high aeration conditions but not in low aeration conditions (Delpech et al., 2015). This may explain why the presence of S. aureus (via its catalase production) modulated the L. garvieae transcriptome response under different aeration conditions. Indeed, with low aeration, the L. garvieae H2O2 synthesis gene sodA expression was slightly higher in presence of S. aureus. With high aeration, the main enzymes involved in O2 consumption of L. lactis (NADH-oxidase NoxE and ETC enzymes (Tachon et al., 2010), were repressed only in L. garvieae pure culture. The expression of the L. garvieae noxE reached a peak at 6 h (beginning of exponential growth) and then decreased until 24 h. This suggests that L. garvieae NoxE is the main enzyme responsible for O2 consumption at the beginning of exponential growth with high aeration, as observed for L. lactis NoxE (Lopez de Felipe and Hugenholtz, 2001; Tachon et al., 2010). The induction of most of the L. garvieae ETC genes (cydA, cydB, cydC, menB, menC, menD, menH, and LCGN_0364) under high aeration conditions at 9 h (beginning of stationary phase) suggested that ETC may be involved in O2 consumption by L. garvieae during the stationary phase, as already shown with L. lactis (Tachon et al., 2009, 2010).
Previous studies have showed that hydrogen peroxide production by L. garvieae depends on aeration level and plays a key role in S. aureus inhibition. As regards S. aureus, Delpech et al. (2015) suggested that the stronger inhibition with a high aeration level may be caused by the higher concentration of H2O2 associated with the L. garvieae-induced repression of S. aureus genes involved in the H2O2 response (katA and sodA at 6 and 9 h) and cell division (mraZ, mraW and potentially the dcw cluster). As regards L. garvieae, our RNA-sequencing and RT-qPCR analyses showed an overexpression of the H2O2-degradation genes ahpC, ahpF, gpx, and trxB1 under low aeration conditions compared to high aeration, while the expression of H2O2 synthesis genes pox and sodA remained stable. The expression of the main H2O2 degradation genes of Gram-positive bacteria is generally induced more under aeration and in the presence of H2O2 (Mostertz et al., 2004; Pedersen et al., 2008). Also, H2O2 metabolism may be very different in L. garvieae compared to other Gram-positive bacteria. Although the difference in H2O2 concentration between the two aeration levels was probably primarily conditioned by the availability of O2, our transcriptome results suggest that it was also associated with a control of H2O2 degradation by L. garvieae rather than with a control of H2O2 synthesis. Since the AhpCF peroxy-redoxin system was repressed, the OhrAR system was probably essential for the resistance of L. garvieae to ROS (other than H2O2) under the high aeration conditions. The widespread organic hydroperoxide detoxifying system ohrAR, known to be over-expressed under high O2 conditions (Mongkolsuk et al., 1998; Fuangthong et al., 2001; Chuchue et al., 2006; Oh et al., 2007; Atichartpongkul et al., 2010; da Silva Neto et al., 2012; Clair et al., 2013), was consistently induced in L. garvieae at the high aeration level.
The fact that S. aureus population levels were lower in the stationary phase in H2O2-free supernatant from a co-culture of S. aureus and L. garvieae revealed the presence of a new molecule involved in this inhibition. This effector is extracellular, is produced by L. garvieae during its exponential growth phase and can reduce the population level of S. aureus during its stationary growth phase. In view of the results of our enzymatic treatments on supernatants, the inhibitory gap during the stationary phase was probably caused neither by hydrogen peroxide, nor by a protein, nor by a lipid, nor by a polysaccharide. The only putative bacteriocin identified in the L. garvieae N201 genome was homologous to garvieaecin Q (GarQ, data not shown), a class IId bacteriocin (Tosukhowong et al., 2012). Class IId bacteriocins are generally sensitive to protease treatments as stringent as the one we used in this study (Kuo et al., 2013; Song et al., 2014), suggesting that garvieacin Q is unlikely to be the effector we are seeking. This effector may instead be related to several genes identified by RNA seq as being regulated by aeration level, such as genes involved in metal homeostasis (e.g., siderophores (Hannauer et al., 2015), chemical and ionic equilibrium (Doyle et al., 1975; Chudobova et al., 2015), transport of vitamin-related compounds (Schlievert et al., 2013) and export of unknown proteins. For example, the differential expression of ferrous ion transport encoding genes has already been observed in L. lactis under oxidative stress in milk (Larsen et al., 2016b). It may also be related to signaling molecules (stress, quorum sensing). It is known that L. garvieae can modify the expression of several S. aureus genes involved in environment-sensing systems like the agr system, CodY or two-component systems SaeRS and SrrAB (Delpech et al., 2015).
RNA-seq revealed variations in the expression of the codY gene (involved in nutritional adaptation (Guédon et al., 2001; Ercan et al., 2015), and of several nutritional-related metabolisms (lysine, threonine, mannitol, aspartate, ribose, fructose, and galactose). This suggests that L. garvieae adapts its nutritional behavior to the prevailing aeration level and the presence or absence of S. aureus. In a rich medium like BHI, there should be little nutritional competition. It is known that L. garvieae can consume all the L-threonine in micro-filtered milk in less than 3 h (Alomar et al., 2008b) and that S. aureus growth could be inhibited by L-threonine depletion (Pohl et al., 2009). However, we showed that the antagonism of L. garvieae against S. aureus was not associated with nutritional competition for L-threonine in micro-filtered milk (see Supplementary Table 5).
Conclusion
RNA sequencing analyses revealed a L. garvieae transcriptome adaptation to aeration level. This adaptation differed depending on the presence or absence of S. aureus. Our findings show that the control of autogenic H2O2 levels by L. garvieae was probably carried out by H2O2 degradation genes rather than H2O2 synthesis genes. Our study also leads us to suggest that an unidentified effector was involved in the inhibition of S. aureus in the stationary phase. The potential inhibitory role of metals, siderophores and signal molecules (e.g., stress signal, quorum sensing) generated by L. garvieae should be investigated. In order to promote the use of H2O2-producing bacteria as bio-preservation agents, future studies should explore the relation between H2O2-metabolism, H2O2-producing LAB and the pathogen they inhibit.
Author Contributions
PD carried out all the experiments, excluding preparation of cDNA libraries and RNA sequencing, and drafted the manuscript helped by CD and SB. ER, ED, and GB analyzed RNA sequencing data. SN performed the RNA sequencing (preparation of libraries and the sequencing itself). GG, M-CM, CD and SB conceived the study. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Adrien Nivoliez for his help in determining the quality of our RNAs in the Probionov laboratory, Magali Cordaillat-Simmons, David Tropel, Laurent Rios, Monique Zagorec, Yves Le Loir, Pierre Renault, Hélène Falentin, and Christophe Chassard for their helpful advice and Maryline Bornes and Harriet Coleman for English proofreading.
Funding. PD received a Ph.D. fellowship from the FEDER (Fond Européen de Développement Economique et Régional) and the Conseil Régional of the Auvergne region in the framework of the Pharmabiotic Research Institute (PRI) cluster.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2017.00359/full#supplementary-material
References
- Adesokan I. A., Ekanola Y. A., Okanlawon B. M. (2010). Influence of cultural conditions on hydrogen peroxide production by lactic acid bacteria isolated from some Nigerian traditional fermented foods. Afr. J. Microbiol. Res. 4 1991–1996. [Google Scholar]
- Aguado-Urda M., López-Campos G. H., Blanco M. M., Fernández-Garayzábal J. F., Cutuli M. T., Aspiroz C., et al. (2011). Genome sequence of Lactococcus garvieae 21881, isolated in a case of human septicemia. J. Bacteriol. 193 4033–4034. 10.1128/JB.05090-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegría A., Alvarez-Martín P., Sacristán N., Fernández E., Delgado S., Mayo B. (2009). Diversity and evolution of the microbial populations during manufacture and ripening of Casín, a traditional Spanish, starter-free cheese made from cow’s milk. Int. J. Food Microbiol. 136 44–51. 10.1016/j.ijfoodmicro.2009.09.023 [DOI] [PubMed] [Google Scholar]
- Alomar J., Lebert A., Montel M.-C. (2008a). Effect of temperature and pH on growth of Staphylococcus aureus in co-culture with Lactococcus garvieae. Curr. Microbiol. 56 408–412. 10.1007/s00284-007-9079-3 [DOI] [PubMed] [Google Scholar]
- Alomar J., Loubiere P., Delbes C., Nouaille S., Montel M.-C. (2008b). Effect of Lactococcus garvieae, Lactococcus lactis and Enterococcus faecalis on the behaviour of Staphylococcus aureus in microfiltered milk. Food Microbiol. 25 502–508. 10.1016/j.fm.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Anders S., Huber W. (2010). Differential expression analysis for sequence count data. Genome Biol. 11:R106 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S., Pyl P. T., Huber W. (2015). HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31 166–169. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atichartpongkul S., Fuangthong M., Vattanaviboon P., Mongkolsuk S. (2010). Analyses of the regulatory mechanism and physiological roles of Pseudomonas aeruginosa OhrR, a transcription regulator and a sensor of organic hydroperoxides. J. Bacteriol. 192 2093–2101. 10.1128/JB.01510-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batdorj B., Trinetta V., Dalgalarrondo M., Prévost H., Dousset X., Ivanova I., et al. (2007). Isolation, taxonomic identification and hydrogen peroxide production by Lactobacillus delbrueckii subsp. lactis T31, isolated from Mongolian yoghurt: inhibitory activity on food-borne pathogens. J. Appl. Microbiol. 103 584–593. 10.1111/j.1365-2672.2007.03279.x [DOI] [PubMed] [Google Scholar]
- Callon C., Duthoit F., Delbès C., Ferrand M., Le Frileux Y., De Crémoux R., et al. (2007). Stability of microbial communities in goat milk during a lactation year: molecular approaches. Syst. Appl. Microbiol. 30 547–560. 10.1016/j.syapm.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Charlier C., Cretenet M., Even S., Le Loir Y. (2009). Interactions between Staphylococcus aureus and lactic acid bacteria: an old story with new perspectives. Int. J. Food Microbiol. 131 30–39. 10.1016/j.ijfoodmicro.2008.06.032 [DOI] [PubMed] [Google Scholar]
- Chuchue T., Tanboon W., Prapagdee B., Dubbs J. M., Vattanaviboon P., Mongkolsuk S. (2006). ohrR and ohr are the primary sensor/regulator and protective genes against organic hydroperoxide stress in Agrobacterium tumefaciens. J. Bacteriol. 188 842–851. 10.1128/JB.188.3.842-851.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudobova D., Dostalova S., Ruttkay-Nedecky B., Guran R., Rodrigo M. A. M., Tmejova K., et al. (2015). The effect of metal ions on Staphylococcus aureus revealed by biochemical and mass spectrometric analyses. Microbiol. Res. 170 147–156. 10.1016/j.micres.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Clair G., Lorphelin A., Armengaud J., Duport C. (2013). OhrRA functions as a redox-responsive system controlling toxinogenesis in Bacillus cereus. J Proteomics 94 527–539. 10.1016/j.jprot.2013.10.024 [DOI] [PubMed] [Google Scholar]
- Conesa A., Götz S., García-Gómez J. M., Terol J., Talón M., Robles M. (2005). Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21 3674–3676. 10.1093/bioinformatics/bti610 [DOI] [PubMed] [Google Scholar]
- Cosgrove K., Coutts G., Jonsson I.-M., Tarkowski A., Kokai-Kun J. F., Mond J. J., et al. (2007). Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J. Bacteriol. 189 1025–1035. 10.1128/JB.01524-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Neto J. F., Negretto C. C., Netto L. E. S. (2012). Analysis of the organic hydroperoxide response of chromobacterium violaceum reveals that OhrR is a cys-based redox sensor regulated by thioredoxin. PLoS ONE 7:e47090 10.1371/journal.pone.0047090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbes-Paus C., Dorchies G., Chaabna Z., Callon C., Montel M.-C. (2010). Contribution of hydrogen peroxide to the inhibition of Staphylococcus aureus by Lactococcus garvieae in interaction with raw milk microbial community. Food Microbiol. 27 924–932. 10.1016/j.fm.2010.05.031 [DOI] [PubMed] [Google Scholar]
- Delpech P., Bornes S., Alaterre E., Bonnet M., Gagne G., Montel M.-C., et al. (2015). Staphylococcus aureus transcriptomic response to inhibition by H2O2-producing Lactococcus garvieae. Food Microbiol. 51 163–170. 10.1016/j.fm.2015.05.014 [DOI] [PubMed] [Google Scholar]
- Devriese L. A., Hommez J., Laevens H., Pot B., Vandamme P., Haesebrouck F. (1999). Identification of aesculin-hydrolyzing streptococci, lactococci, aerococci and enterococci from subclinical intramammary infections in dairy cows. Vet. Microbiol. 70 87–94. 10.1016/S0378-1135(99)00124-8 [DOI] [PubMed] [Google Scholar]
- Dijkstra A. R., Alkema W., Starrenburg M. J., Hugenholtz J., van Hijum S. A., Bron P. A. (2014). Fermentation-induced variation in heat and oxidative stress phenotypes of Lactococcus lactis MG1363 reveals transcriptome signatures for robustness. Microb. Cell Fact. 13 148 10.1186/s12934-014-0148-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J. J., Marshall R. T., Pfander W. H. (1975). Effects of cadmium on the growth and uptake of cadmium by microorganisms. Appl. Microbiol. 29 562–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Baradei G., Delacroix-Buchet A., Ogier J. C. (2008). Bacterial biodiversity of traditional Zabady fermented milk. Int. J. Food Microbiol. 121 295–301. 10.1016/j.ijfoodmicro.2007.11.014 [DOI] [PubMed] [Google Scholar]
- Eldar A., Ghittino C. (1999). Lactococcus garvieae and Streptococcus iniae infections in rainbow trout Oncorhynchus mykiss: similar, but different diseases. Dis. Aquat. Org. 36 227–231. 10.3354/dao036227 [DOI] [PubMed] [Google Scholar]
- Ercan O., Wels M., Smid E. J., Kleerebezem M. (2015). Genome-wide transcriptional responses to carbon starvation in nongrowing Lactococcus lactis. Appl. Environ. Microbiol. 81 2554–2561. 10.1128/AEM.03748-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández E., Alegría Á., Delgado S., Mayo B. (2010). Phenotypic, genetic and technological characterization of Lactococcus garvieae strains isolated from a raw milk cheese. Int. Dairy J. 20 142–148. 10.1016/j.idairyj.2009.11.004 [DOI] [Google Scholar]
- Flórez A. B., Mayo B. (2006). Microbial diversity and succession during the manufacture and ripening of traditional, Spanish, blue-veined Cabrales cheese, as determined by PCR-DGGE. Int. J. Food Microbiol. 110 165–171. 10.1016/j.ijfoodmicro.2006.04.016 [DOI] [PubMed] [Google Scholar]
- Fortina M. G., Ricci G., Foschino R., Picozzi C., Dolci P., Zeppa G., et al. (2007). Phenotypic typing, technological properties and safety aspects of Lactococcus garvieae strains from dairy environments. J. Appl. Microbiol. 103 445–453. 10.1111/j.1365-2672.2006.03265.x [DOI] [PubMed] [Google Scholar]
- Fuangthong M., Atichartpongkul S., Mongkolsuk S., Helmann J. D. (2001). OhrR is a repressor of ohrA, a key organic hydroperoxide resistance determinant in Bacillus subtilis. J. Bacteriol. 183 4134–4141. 10.1128/JB.183.14.4134-4141.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gene Ontology Consortium (2008). The gene ontology project in 2008. Nucleic Acids Res. 36 D440–D444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guédon E., Serror P., Ehrlich S. D., Renault P., Delorme C. (2001). Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol. Microbiol. 40 1227–1239. 10.1046/j.1365-2958.2001.02470.x [DOI] [PubMed] [Google Scholar]
- Hannauer M., Sheldon J. R., Heinrichs D. E. (2015). Involvement of major facilitator superfamily proteins SfaA and SbnD in staphyloferrin secretion in Staphylococcus aureus. FEBS Lett. 589 730–737. 10.1016/j.febslet.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Ito A., Sato Y., Kudo S., Sato S., Nakajima H., Toba T. (2003). The screening of hydrogen peroxide-producing lactic acid bacteria and their application to inactivating psychrotrophic food-borne pathogens. Curr. Microbiol. 47 0231–236. 10.1007/s00284-002-3993-1 [DOI] [PubMed] [Google Scholar]
- Jensen N. B. S., Melchiorsen C. R., Jokumsen K. V., Villadsen J. (2001). Metabolic behavior of Lactococcus lactis MG1363 in microaerobic continuous cultivation at a low dilution rate. Appl. Environ. Microbiol. 67 2677–2682. 10.1128/AEM.67.6.2677-2682.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokovic N., Nikolic M., Begovic J., Jovcic B., Savic D., Topisirovic L. (2008). A survey of the lactic acid bacteria isolated from Serbian artisanal dairy product kajmak. Int. J. Food Microbiol. 127 305–311. 10.1016/j.ijfoodmicro.2008.07.026 [DOI] [PubMed] [Google Scholar]
- Kuo Y.-C., Liu C.-F., Lin J.-F., Li A.-C., Lo T.-C., Lin T.-H. (2013). Characterization of putative class II bacteriocins identified from a non-bacteriocin-producing strain Lactobacillus casei ATCC 334. Appl. Microbiol. Biotechnol. 97 237–246. 10.1007/s00253-012-4149-2 [DOI] [PubMed] [Google Scholar]
- Larsen N., Brøsted Werner B., Jespersen L. (2016a). Transcriptional responses in Lactococcus lactis subsp. cremoris to the changes in oxygen and redox potential during milk acidification. Lett. Appl. Microbiol. 63 117–123. 10.1111/lam.12596 [DOI] [PubMed] [Google Scholar]
- Larsen N., Moslehi-Jenabian S., Werner B. B., Jensen M. L., Garrigues C., Vogensen F. K., et al. (2016b). Transcriptome analysis of Lactococcus lactis subsp. lactis during milk acidification as affected by dissolved oxygen and the redox potential. Int. J. Food Microbiol. 226 5–12. 10.1016/j.ijfoodmicro.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Li H., Durbin R. (2009). Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics 25 1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., et al. (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25 2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Felipe F., Hugenholtz J. (2001). Purification and characterisation of the water forming NADH-oxidase from Lactococcus lactis. Int. Dairy J. 11 37–44. 10.1016/S0958-6946(01)00031-0 [DOI] [Google Scholar]
- Love M. I., Huber W., Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15 550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfredini L., Settanni L., Poznanski E., Cavazza A., Franciosi E. (2012). The spatial distribution of bacteria in Grana-cheese during ripening. Syst. Appl. Microbiol. 35 54–63. 10.1016/j.syapm.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Mongkolsuk S., Praituan W., Loprasert S., Fuangthong M., Chamnongpol S. (1998). Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestris pv. phaseoli. J. Bacteriol. 180 2636–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi S., Silvetti T., Miranda Lopez J. M., Brasca M. (2015). Antimicrobial activity, antibiotic resistance and the safety of lactic acid bacteria in raw milk valtellina casera cheese. J. Food Saf. 35 193–205. 10.1111/jfs.12171 [DOI] [Google Scholar]
- Mostertz J., Scharf C., Hecker M., Homuth G. (2004). Transcriptome and proteome analysis of Bacillus subtilis gene expression in response to superoxide and peroxide stress. Microbiology 150 497–512. 10.1099/mic.0.26665-0 [DOI] [PubMed] [Google Scholar]
- Nordkvist M., Jensen N. B. S., Villadsen J. (2003). Glucose metabolism in Lactococcus lactis MG1363 under different aeration conditions: requirement of acetate to sustain growth under microaerobic conditions. Appl. Environ. Microbiol. 69 3462–3468. 10.1128/AEM.69.6.3462-3468.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouaille S., Even S., Charlier C., Le Loir Y., Cocaign-Bousquet M., Loubière P. (2009). Transcriptomic response of Lactococcus lactis in mixed culture with Staphylococcus aureus. Appl. Environ. Microbiol. 75 4473–4482. 10.1128/AEM.02653-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.-Y., Shin J.-H., Roe J.-H. (2007). Dual role of OhrR as a repressor and an activator in response to organic hydroperoxides in Streptomyces coelicolor. J. Bacteriol. 189 6284–6292. 10.1128/JB.00632-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz C., López J., Del Amo E., Sevilla T., García P. E., San Román J. A. (2014). Lactococcus garvieae infective endocarditis: report of 2 cases and review of the literature. Rev. Esp. Cardiol. 67 776–778. 10.1016/j.recesp.2014.04.010 [DOI] [PubMed] [Google Scholar]
- Pangallo D., Saková N., Koreòová J., Puškárová A., Kraková L., Valík L., et al. (2014). Microbial diversity and dynamics during the production of May bryndza cheese. Int. J. Food Microbiol. 170 38–43. 10.1016/j.ijfoodmicro.2013.10.015 [DOI] [PubMed] [Google Scholar]
- Pedersen M. B., Garrigues C., Tuphile K., Brun C., Vido K., Bennedsen M., et al. (2008). Impact of aeration and heme-activated respiration on Lactococcus lactis gene expression: identification of a heme-responsive operon. J. Bacteriol. 190 4903–4911. 10.1128/JB.00447-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A. C., Melo-Barbosa H. P., Miyoshi A., Silva A., Azevedo V. (2011). Application of RNA-seq to reveal the transcript profile in bacteria. Genet. Mol. Res. 10 1707–1718. 10.4238/vol10-3gmr1554 [DOI] [PubMed] [Google Scholar]
- Pohl K., Francois P., Stenz L., Schlink F., Geiger T., Herbert S., et al. (2009). CodY in Staphylococcus aureus: a regulatory link between metabolism and virulence gene expression. J. Bacteriol. 191 2953–2963. 10.1128/JB.01492-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D., McCarthy D. J., Smyth G. K. (2010). edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26 139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert P. M., Merriman J. A., Salgado-Pabón W., Mueller E. A., Spaulding A. R., Vu B. G., et al. (2013). Menaquinone analogs inhibit growth of bacterial pathogens. Antimicrob. Agents Chemother. 57 5432–5437. 10.1128/AAC.01279-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sip A., Wieckowicz M., Olejnik-Schmidt A., Gardo A., Gorlas R., Grajek W. (2009). Occurrence of lactic acid bacteria with activity against Listeria in Polish regional cheeses produced in the Tatrzan’Sko-Beskidzki District. Acta Sci. Pol. 8 27–44. [Google Scholar]
- Song D.-F., Zhu M.-Y., Gu Q. (2014). Purification and characterization of Plantaricin ZJ5 a new bacteriocin produced by Lactobacillus plantarum ZJ5. PLoS ONE 9:e105549 10.1371/journal.pone.0105549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachon S., Brandsma J. B., Yvon M. (2010). NoxE NADH oxidase and the electron transport chain are responsible for the ability of Lactococcus lactis to decrease the redox potential of milk. Appl. Environ. Microbiol. 76 1311–1319. 10.1128/AEM.02120-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachon S., Michelon D., Chambellon E., Cantonnet M., Mezange C., Henno L., et al. (2009). Experimental conditions affect the site of tetrazolium violet reduction in the electron transport chain of Lactococcus lactis. Microbiology 155 2941–2948. 10.1099/mic.0.029678-0 [DOI] [PubMed] [Google Scholar]
- Tosukhowong A., Zendo T., Visessanguan W., Roytrakul S., Pumpuang L., Jaresitthikunchai J., et al. (2012). ieacin Q, a novel class II bacteriocin from Lactococcus garvieae BCC 43578. Appl. Environ. Microbiol. 78 1619–1623. 10.1128/AEM.06891-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell D., Balcázar J. L., Ruiz-Zarzuela I., de Blas I., Gironés O., Múzquiz J. L. (2006). Lactococcus garvieae in fish: a review. Comp. Immunol. Microbiol. Infect. Dis. 29 177–198. 10.1016/j.cimid.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Villani F., Aponte M., Blaiotta G., Mauriello G., Pepe O., Moschetti G. (2001). Detection and characterization of a bacteriocin, garviecin L1-5 produced by Lactococcus garvieae isolated from raw cow’s milk. J. Appl. Microbiol. 90 430–439. 10.1046/j.1365-2672.2001.01261.x [DOI] [PubMed] [Google Scholar]
- Zdobnov E. M., Apweiler R. (2001). InterProScan–an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17 847–848. 10.1093/bioinformatics/17.9.847 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.