Abstract
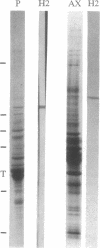
One of our monoclonal antibodies against the heavy chain of bovine kinesin (H2) also recognized the heavy chain of squid kinesin. The immunofluorescence pattern of H2 in axoplasm was similar to that seen in mammalian cells with antibodies specific for kinesin light and heavy chains, indicating that squid kinesin is also concentrated on membrane-bounded organelles. Although kinesin is assumed to be a motor for translocation of membrane-bounded organelles in fast axonal transport, direct evidence has been lacking. Perfusion of axoplasm with purified H2 at 0.1-0.4 mg/ml resulted in a profound inhibition of both the rates and number of organelles moving in anterograde and retrograde directions in the interior of the axoplasm, and comparable inhibition was noted in bidirectional movement along individual microtubules at the periphery. Maximal inhibition developed over 30-60 min. Perfusion with higher concentrations of H2 (greater than 1 mg of IgG per ml) were less effective, whereas perfusion with 0.04 mg of H2 per ml resulted in minimal inhibition. Movement of membrane-bounded organelles after perfusion with comparable levels of irrelevant mouse IgG (0.04 to greater than 1 mg/ml) were not distinguishable from perfusion with buffer controls. Inhibition of fast axonal transport by an antibody specific for kinesin provides direct evidence that kinesin is involved in the translocation of membrane-bounded organelles in axons. Moreover, the inhibition of bidirectional axonal transport by H2 raises the possibility that kinesin may play some role in both anterograde and retrograde axonal transport.
Full text
PDF

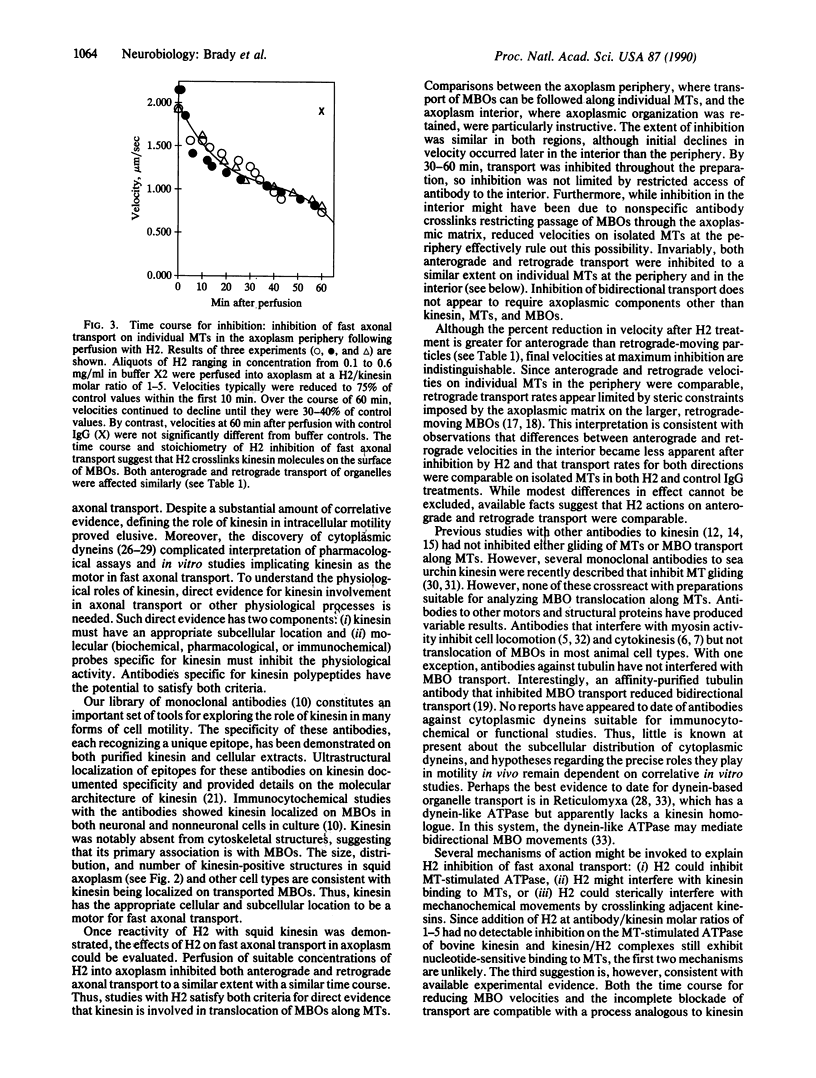

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom G. S., Wagner M. C., Pfister K. K., Brady S. T. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry. 1988 May 3;27(9):3409–3416. doi: 10.1021/bi00409a043. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brady S. T. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985 Sep 5;317(6032):73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Lasek R. J., Allen R. D. Fast axonal transport in extruded axoplasm from squid giant axon. Science. 1982 Dec 10;218(4577):1129–1131. doi: 10.1126/science.6183745. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Lasek R. J., Allen R. D. Video microscopy of fast axonal transport in extruded axoplasm: a new model for study of molecular mechanisms. Cell Motil. 1985;5(2):81–101. doi: 10.1002/cm.970050203. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Lasek R. J., Allen R. D., Yin H. L., Stossel T. P. Gelsolin inhibition of fast axonal transport indicates a requirement for actin microfilaments. Nature. 1984 Jul 5;310(5972):56–58. doi: 10.1038/310056a0. [DOI] [PubMed] [Google Scholar]
- Euteneuer U., Koonce M. P., Pfister K. K., Schliwa M. An ATPase with properties expected for the organelle motor of the giant amoeba, Reticulomyxa. Nature. 1988 Mar 10;332(6160):176–178. doi: 10.1038/332176a0. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Pfister K. K., Yorifuji H., Wagner M. C., Brady S. T., Bloom G. S. Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration. Cell. 1989 Mar 10;56(5):867–878. doi: 10.1016/0092-8674(89)90691-0. [DOI] [PubMed] [Google Scholar]
- Höner B., Citi S., Kendrick-Jones J., Jockusch B. M. Modulation of cellular morphology and locomotory activity by antibodies against myosin. J Cell Biol. 1988 Dec;107(6 Pt 1):2181–2189. doi: 10.1083/jcb.107.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingold A. L., Cohn S. A., Scholey J. M. Inhibition of kinesin-driven microtubule motility by monoclonal antibodies to kinesin heavy chains. J Cell Biol. 1988 Dec;107(6 Pt 2):2657–2667. doi: 10.1083/jcb.107.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Kiehart D. P., Mabuchi I., Inoué S. Evidence that myosin does not contribute to force production in chromosome movement. J Cell Biol. 1982 Jul;94(1):165–178. doi: 10.1083/jcb.94.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Gelfand V. I. Bovine brain kinesin is a microtubule-activated ATPase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8530–8534. doi: 10.1073/pnas.83.22.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasek R. J., Brady S. T. Attachment of transported vesicles to microtubules in axoplasm is facilitated by AMP-PNP. Nature. 1985 Aug 15;316(6029):645–647. doi: 10.1038/316645a0. [DOI] [PubMed] [Google Scholar]
- Leslie R. J., Hird R. B., Wilson L., McIntosh J. R., Scholey J. M. Kinesin is associated with a nonmicrotubule component of sea urchin mitotic spindles. Proc Natl Acad Sci U S A. 1987 May;84(9):2771–2775. doi: 10.1073/pnas.84.9.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye R. J., Porter M. E., Scholey J. M., McIntosh J. R. Identification of a microtubule-based cytoplasmic motor in the nematode C. elegans. Cell. 1987 Oct 23;51(2):309–318. doi: 10.1016/0092-8674(87)90157-7. [DOI] [PubMed] [Google Scholar]
- Mabuchi I., Okuno M. The effect of myosin antibody on the division of starfish blastomeres. J Cell Biol. 1977 Jul;74(1):251–263. doi: 10.1083/jcb.74.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Lasek R. J. Cross-bridges mediate anterograde and retrograde vesicle transport along microtubules in squid axoplasm. J Cell Biol. 1985 Dec;101(6):2181–2193. doi: 10.1083/jcb.101.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. R., Lasek R. J. Monomer-polymer equilibria in the axon: direct measurement of tubulin and actin as polymer and monomer in axoplasm. J Cell Biol. 1984 Jun;98(6):2064–2076. doi: 10.1083/jcb.98.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murofushi H., Ikai A., Okuhara K., Kotani S., Aizawa H., Kumakura K., Sakai H. Purification and characterization of kinesin from bovine adrenal medulla. J Biol Chem. 1988 Sep 5;263(25):12744–12750. [PubMed] [Google Scholar]
- Neighbors B. W., Williams R. C., Jr, McIntosh J. R. Localization of kinesin in cultured cells. J Cell Biol. 1988 Apr;106(4):1193–1204. doi: 10.1083/jcb.106.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Shpetner H. S., Vallee R. B. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol. 1987 Sep;105(3):1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Vallee R. B. Retrograde transport by the microtubule-associated protein MAP 1C. Nature. 1987 Nov 12;330(6144):181–183. doi: 10.1038/330181a0. [DOI] [PubMed] [Google Scholar]
- Pfister K. K., Wagner M. C., Bloom G. S., Brady S. T. Modification of the microtubule-binding and ATPase activities of kinesin by N-ethylmaleimide (NEM) suggests a role for sulfhydryls in fast axonal transport. Biochemistry. 1989 Nov 14;28(23):9006–9012. doi: 10.1021/bi00449a008. [DOI] [PubMed] [Google Scholar]
- Pfister K. K., Wagner M. C., Stenoien D. L., Brady S. T., Bloom G. S. Monoclonal antibodies to kinesin heavy and light chains stain vesicle-like structures, but not microtubules, in cultured cells. J Cell Biol. 1989 Apr;108(4):1453–1463. doi: 10.1083/jcb.108.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M. E., Scholey J. M., Stemple D. L., Vigers G. P., Vale R. D., Sheetz M. P., McIntosh J. R. Characterization of the microtubule movement produced by sea urchin egg kinesin. J Biol Chem. 1987 Feb 25;262(6):2794–2802. [PubMed] [Google Scholar]
- Scholey J. M., Heuser J., Yang J. T., Goldstein L. S. Identification of globular mechanochemical heads of kinesin. Nature. 1989 Mar 23;338(6213):355–357. doi: 10.1038/338355a0. [DOI] [PubMed] [Google Scholar]
- Scholey J. M., Porter M. E., Grissom P. M., McIntosh J. R. Identification of kinesin in sea urchin eggs, and evidence for its localization in the mitotic spindle. Nature. 1985 Dec 5;318(6045):483–486. doi: 10.1038/318483a0. [DOI] [PubMed] [Google Scholar]
- Sinard J. H., Pollard T. D. Microinjection into Acanthamoeba castellanii of monoclonal antibodies to myosin-II slows but does not stop cell locomotion. Cell Motil Cytoskeleton. 1989;12(1):42–52. doi: 10.1002/cm.970120106. [DOI] [PubMed] [Google Scholar]
- Vale R. D. Intracellular transport using microtubule-based motors. Annu Rev Cell Biol. 1987;3:347–378. doi: 10.1146/annurev.cb.03.110187.002023. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Mitchison T., Steuer E., Reese T. S., Sheetz M. P. Different axoplasmic proteins generate movement in opposite directions along microtubules in vitro. Cell. 1985 Dec;43(3 Pt 2):623–632. doi: 10.1016/0092-8674(85)90234-x. [DOI] [PubMed] [Google Scholar]
- Wagner M. C., Pfister K. K., Bloom G. S., Brady S. T. Copurification of kinesin polypeptides with microtubule-stimulated Mg-ATPase activity and kinetic analysis of enzymatic properties. Cell Motil Cytoskeleton. 1989;12(4):195–215. doi: 10.1002/cm.970120403. [DOI] [PubMed] [Google Scholar]