Abstract
A survey is presented of the various technical and scientific challenges that had to be met during the 10-year period before the first successful live birth after IVF and embryo transfer was achieved, and the approaches used to meet these challenges is discussed. Records dated from January 1969 to July 1978 indicate that a minimum of 282 women were involved in 495 cycles scheduled for laparoscopic oocyte recovery, of which 457 cycles (92%) proceeded to attempted egg collection. A total of 1361 eggs were recovered over 388 cycles, of which 1237 (91%) are recorded as having been inseminated in 331 (85%) of these cycles. Approximately 221 embryos were described in 165 (43%) of the 388 cycles. A total of 112 embryo transfers were attempted, which resulted in five clinical pregnancies with two live births. This paper discusses the ways in which hormonal stimulation of follicle growth to the pre-ovulatory stage was varied, and the endocrine monitoring of these variations in blood, urine and follicular fluid, as well as their influence on egg recovery and fertilization rates. Variations in media composition and preparation are also described. It is concluded that, whilst driven by scientific reasoning, the approach adopted in trying to achieve successful IVF was empirical rather than evidence-driven.
Keywords: blastocyst development, early pregnancy, human embryo culture, luteal phase abnormalities, natural cycles, pregnancy losses, stimulated follicular maturation
Introduction
Louise Joy Brown was born on 25 July 1978, an event that has had a major impact on science, medicine and society (Franklin, 2013). In the two preceding papers, we reported on our analysis of research notes spanning 1969 to 1978 covering the period when Edwards, Steptoe and Purdy were working in Oldham and Cambridge towards this achievement (Elder and Johnson, 2015a), and on the numbers of treatment cycles involved and their outcomes (Elder and Johnson, 2015b). In this paper, we consider the evidence relating to variations in key aspects of the procedures that Edwards, Steptoe and Purdy attempted in order to overcome the numerous technical, scientific, practical and logistical challenges they faced. An overview of some of the many problems that had to be addressed and overcome (Edwards and Steptoe, 1974) is presented in Table 1. We present a historical perspective on the steps taken to resolve these issues. The data for this report are taken primarily from the two sets of notebooks, L0–L9 and H1-H4, H7-H9, as described in Elder and Johnson (2015a), supplemented with additional material from the Edwards’ archive, which are referenced as RGE1, 2 etc., followed by the date.
Table 1.
Some of challenges that had to be overcome before the first successful live birth following IVF and embryo transfer was achieved.
| Challenge |
|---|
| Technical aspects of follicle aspiration (‘new suction gadget’) |
| Ovulation induction |
| Timing of laparoscopy |
| Ovarian stimulation |
| Cycle monitoring |
| Oocyte culture |
| Sperm preparation |
| Insemination procedure: medium, timing |
| Culture for embryo cleavage: medium, assessment |
| Technical aspects of embryo transfer, including route of transfer, medium and timing |
| Luteal support |
Results and discussion
Laparoscopic oocyte recovery procedure: follicle aspiration, ovulation induction and timing
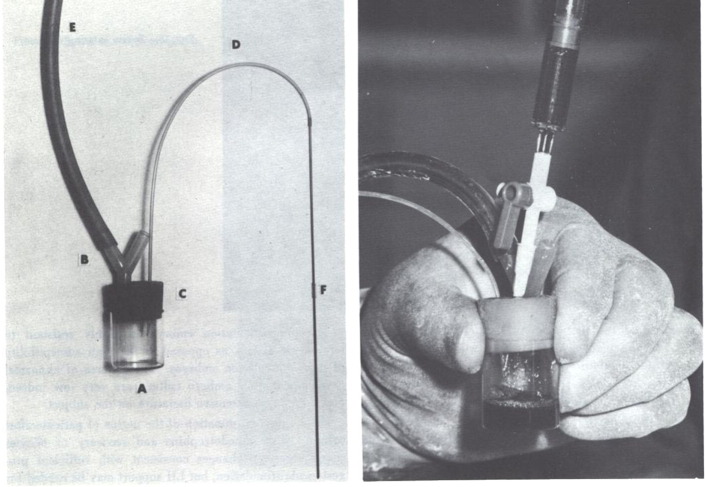
Having realized that in-vitro matured oocytes were not competent to develop as embryos, preliminary attempts at laparoscopic oocyte recovery (LOR) may have already commenced late in 1968, as mentioned in Edwards and Steptoe’s 1980 account in their volume A Matter of Life (Edwards and Steptoe, 1980, pp.80–81) and reported in Edwards et al. (1969, p.635; published on 15 February, submitted December, 1968). During 1969 the main emphasis was on improving the timing and technique of laparoscopy and recovery of eggs after triggering oocyte maturation. Follicles were initially aspirated using a syringe and needle, but this method was found to be unsatisfactory (Steptoe and Edwards, 1970). In September 1969 a “new suction gadget – boiled” was introduced (Figure 1), which had a bypass valve that allowed the assistant to control suction, with the result that clearer follicular fluids could be collected. This ‘gadget’ was then used with variations in suction pressure of 1, 4, 5, 8, 10, 12 cm Hg, before establishing an ‘optimum’ pressure of no greater than 12 cm Hg, 'since higher pressures may damage the oocytes' (Edwards et al., 1980a, Edwards et al., 1980b, Steptoe and Edwards, 1970).
Figure 1.
Photograph of “suction device”: a simple aspirator for withdrawing the contents of human follicles. A vacuum applied at the wide arm (E) is directed through the needle when required by simply blocking the open Y-arm (B) in the aspirator. Oocytes and follicular fluid are withdrawn through the needle (F) and its lead (D) into the collecting chamber (A). The collecting chamber can be easily removed from the neoprene bung (C) and replaced (Edwards and Steptoe, 1975).
Initially, human chorionic gonadotrophin (HCG; Pregnyl, Organon) was administered in order to induce final follicular maturation when an adequate concentration of urinary oestrogens was detected in 24-hour samples (> 75 μg/day), usually on day 11 or 12 of the cycle. Although guided by the timing of oocyte maturation in vitro (Edwards, 1965), scheduling the laparoscopy was determined mainly by the team’s availability and their access to the operating theatre: “HCG was given at an arbitrary time, selected for our own purposes and not because the patient had been fully primed by the HMG” (Steptoe and Edwards, 1970). The dataset for 1969 records that HCG was administered 29–31 hours prior to laparoscopy (See Table 2 and Suppl. Table in Elder and Johnson, 2015b).
Table 2.
Stimulation and monitoring of treatment cycles by year.
| Year | Cycles (n) (stimulated or monitored) | Stimulation protocol (Pn x doses) | IU of HCG (Hours between HCG and LOR) [Day of LOR] | Urine monitoring | Serum monitoring | Cancelled LORa (n) | LOR converted to ITI/AIbor outcome unrecorded (n) | LOR with documented egg recovery (n) (% of stimulated cycles started) | Eggs (n) | Mean no. of eggs/cycle (range) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1969 | 58 | P2 x 1 or 2; then from July P3/P2 on alternate days; then P3 x 4 or x 3 | 3000–12,000; 5000 from Sept (28.75–29.50) [D10–12] | 24-h TE, alternate days from D6 – D8 + occasional PD | NR | None | None | 48 (83) | 136 | 2.3 (1–8) |
| 1970 | 62 | P4 x 3 | 5000 (Jan–Feb: 29.5–30.0; Mar–Dec: 32.0–33.5) [D10–D12] | 24-h TE + PD, every 2 days + luteal phase in some case. Results plotted on graphs | NR | None | 3 | 55 (89) | 274 | 5.0 (1–12) |
| 1971 | 41 | P4 x 3 | 5000 (33.5–36.0) | 24-h urines: TE, PD, every 3 – 4 days approx + PT in March/April | NR | None | 1 | 36 (88) | 175 | 4.9 (1–12) |
| 1972 | 41 | P4 x 3, P3 x 4, P5 x 3, P5, P2 P2 | 5000, 8000 in Sept (31.0–33.5) | 24-h urines – TE, PD every 2 days + post--Lap | July: occasional Lap-day TE, Prl; April–June: Blood groups, both partners | 1 | None | 39 (95) | 187 | 4.8 (1–11) |
| 1973 | 44 | P3 x 4, P2 x 4 (18 cases) CL/Pregnyl (17 cases); NC/Pregnyl (9 cases) | 5000 (3) 8000 (15) 5000 (2) 10,000 (2) 8000 (13) 5000 (32.5–33.0) | 24-h urines: TE, PD every 2 days, + post--LapCL cases – daily from March onwards, LH and FSH from March | Occasional single TE, FSH, LH, Prl (not all cases, not all tests) | 6 | 12 | 25 (57) | 86 | 3.4 (1–8) |
| 1974 | 37 + 3C | NC + Pregnyl (25 cases); NC + MRC LH (2 cases); P3 x 4 or P4 x 3 (11 cases) | 5000 (32.5–33.0) | Daily 24-h urines –TE, PD, LH | Occasional single TE, FSH, LH, Prl (not all cases, not all tests) | 2 | None | 28 + 2c (75) | 85 + 4c | 2.9 (1–6) |
| 1975 | 36 | NC + Pregnyl (6 cases) P3 x 4 or P4 x 3 (30 cases) | 5000 (NR) | Daily 24-h urines: TE, PD, LH – + PT from August | HCG from Lap day in Sept. Occasional luteal + Prl, LH from Sept. | 2 | None | 33 (92) | 117 | 3.5 (1–7) |
| 1976 | 55 + 1c | P4 x 4, P5 x 3, P5 x 4, P5, P2, P2 | 5000, 3000 (7 cases) (32 where recorded) | Daily 24-h TE, PD, LH, + luteal phase | From Sept: E, FSH, P, Prl, 1–6 samples per cycle (not all cases, not all tests) | 4 | 3 ovaries not accessible (P212, 224, 232) | 44 + 1c (80) | 147 + 2C | 3.3 (1–9) |
| 1977 | 43 | P5 x 3 + Parlodel; CL/HMG (2 ), NC/Hi-Gonavis (3 ) | 5000 2 cases MRC LH (32–33) | 24-h urines: TE, PD, LH + luteal phase. From Nov: Hi-Gonavis | Every 2–3 days: E, P, FSH, LH, HCG, Prl | 10 | None | 28 (65) | 97 | 3.5 (1–9) |
| 1978 | 78 + 2c | NC/Hi-Gonavis (78) [+ P5 x 4 in 2 cases for sterilization] | 5000 | Daily 24-h E, PD, LH; 3-h LH from D5 – D7 until surge detected | E, P, LH, FSH, Prl every 2 days + post-ET | 13 | 2 ovaries not accessible (P232, 213) | 52 + 2c (68) | 57 + 4c | 1.2 (1–2) |
| Total | 495 + 6c | – | – | – | – | 38 | 22 | 388 + 5c (78) | 1371 | 3.6 (1–12) |
AI = artificial insemination; CL = Clomid; D = day; E = estrogen; ET = embryo transfer; FSH = follicle-stimulating hormone; HCG = human chorionic gonadotrophin; HMG = human menopausal gonadotropin; ITI = intra-tubal insemination; Lap = laparoscopy; LOR = laparoscopic oocyte retrieval; NC = natural cycle ; NR = not recorded; TE = total estrogen; Pn = number of Pergonal ampoules; P = progesterone; PD = pregnandiol ; Prl = prolactin; PT = pregnanetriol.
See Table 3 (Elder and Johnson, 2015b).
See Table 4 (Elder and Johnson, 2015b).
Cases for laparoscopic sterilization (see Table 1, Elder and Johnson, 2015b).
Doses of HCG were initially related to the dose of gonadotropin administered for inducing follicular maturation, and varied from 3000 to 12,000 IU (Table 1; Steptoe and Edwards, 1970), 5000 IU being adopted from September 1969 onwards, when “a regimen of three injections of HMG between days 2 and 9, and 5000 IU of HCG on days 9–11 of the menstrual cycle gave the best response” (Steptoe and Edwards, 1970). The interval between HCG and egg collection was also varied, and the duration of this interval was compared with the presence of corpora lutea to confirm whether or not ovulation had already taken place at the time of laparoscopy. The notebooks record that laparoscopy was carried out on days 10–12, with an initial interval between HCG and laparoscopy of 28.75–29.50 h, increased to 29.50–30.00 h from mid-January 1970, and then to 32.0–33.5 h in mid-March 1970 (See Table 2 and Suppl. Table in Elder and Johnson, 2015b). This interval was adjusted for natural cycles during 1978, when the timing of the laparoscopy was set at 15–35 h after detecting the LH surge (Edwards et al., 1980a, p.748 and Table IV). Eggs recovered are described as ‘fixed’ prior to October 1969, and are presumably those that were studied cytologically and/or chromosomally with many not fully mature at recovery (Elder and Johnson, 2015b, Steptoe and Edwards, 1970).
Follicle stimulation regimes
Two important initial goals were to aspirate oocytes from their follicles just before ovulation was expected, and to have more than one pre-ovulatory oocyte for aspiration. Injection of the gonadotrophic hormones, human menopausal gonadotrophin (HMG) and HCG, was felt to be necessary in order to control the menstrual cycle and regulate follicle growth, oocyte maturation and ovulation (Edwards et al., 1980a, Edwards et al., 1980b, Steptoe and Edwards, 1970). Table 2 shows the variations over the years in the stimulation regime used to induce follicular maturation, described in Steptoe and Edwards (1970) as “a priming dose of HMG, followed by an ovulatory dose of HCG”. In the absence of any knowledge about the potential ovarian response, and in order to avoid the risk of ovarian hyperstimulation syndrome, low doses were used initially. In 1969 injections of two ampoules of HMG (Pergonal, G.D Searle and Co.) were given once or twice only, starting early in the menstrual cycle (although not always on the same cycle day, “because of the limited times when laparoscopy could be done” (Steptoe and Edwards, 1970). By the end of the year this had been increased to three or four ampoules given in three doses on alternate days, usually days 6, 8 and 10 of the cycle. Thus, from July 1969, the dose was increased to three alternating with two ampoules, and then to three or four doses of three ampoules. The data comparing stimulation regime, follicular size, number of oocytes retrieved and oocyte classification was described in detail in Steptoe and Edwards (1970, Tables I, II, III, IV, and V); that paper also mentions that improvements seen were “probably due to improved technique”. It was further observed that “lower doses of gonadotropin seemed to interfere with the cycle and extend the interval to menstruation, whereas higher doses in repeated injections appeared to dominate the natural cycle, and impose an induced cycle”. However, they also speculated that ”increasing doses of HMG could cause excessive secretion of oestrogen and perhaps induce premature release of LH and early ovulation”. During 1970, the dose was again increased to four ampoules, and then again during 1972 to five ampoules, three or four doses administered on alternate days (Table 2; confirmed in Edwards et al., 1972, published in September).
Having observed that high levels of oestrogen in the follicular phase were unfavourable for the luteal phase endometrium (Edwards et al., 1980b), attempts were made at the beginning of 1973 to modulate the effects of high oestrogen levels by replacing Pergonal stimulation with either clomiphene-stimulated cycles or natural cycles + HCG to induce follicular maturation. Between January and June of that year, 17 patients were stimulated with 50 mg twice daily of clomiphene citrate (Table 2; Clomid; supplier name not found, but is recorded as being from Merrill-National Laboratories in Steptoe et al., 1986); for six of these patients (L361–366, P 114, 128, 110, 129, 120, 130), cycles were converted to timed intercourse without laparoscopy, presumably because urinary oestrogens failed to reach adequate concentrations. Five of the other 10 Clomid cycles [P121(1), 127, 368, 371, 121(2)] resulted in no eggs being retrieved after laparoscopy, and the other five [P38(3), 125(1), 114(2), 110(2), 131(1)] had only one or two eggs. Non-induced (natural) cycles (NC) are mentioned in December 1973 (Table 2) with the addition of HCG or Pregnyl (Organon) to induce follicular maturation: in six of the nine cycles in December, no eggs were retrieved after laparoscopy. During 1974, NC + Pregnyl or LH was used, in 25 and two cases respectively; of the 40 scheduled cycles (Table 2), two were cancelled and in only three did oocyte retrieval fail. We speculate that this improvement, which continued in the six cases in 1975, may have been due to experience gained with the repeated monitoring of cycles and improving skill in follicular oocyte aspiration that was carried out during this period (see section on monitoring, below).
Pergonal-stimulated cycles were also continued during 1973/74 (Table 2), and only Pergonal-stimulated cycles were used from mid-1975 until October 1977, three or four doses of five ampoules on alternate days in the majority of cases; occasionally five ampoules were given as a first dose, followed by two doses of two ampoules each ('5,2,2' regime). Many patients were found to have high serum levels of prolactin (Prl) after Pergonal/HCG administration (Edwards et al., 1980b), and, between February and July 1977, 1.25–2.50 mg bromocryptine (Parlodel) daily was added from the mid-follicular phase and throughout the luteal phase. Two cycles were stimulated with clomiphene/Pergonal in the second half of 1977. Then, apart from two patients for laparoscopic sterilization who were stimulated with Pergonal, all subsequent cases in the final series of 81 cases from November 1977 to July 1978 were carried out in natural cycles with detailed monitoring to detect the LH surge (see below).
The effect of these changes in stimulation regime on the number of eggs retrieved can be seen in Table 2. The percentage of cycles with successful egg recovery increased from 83% to 95% by 1972, with a maximum of 11 or 12 eggs in some cycles. The percentage of successful LOR dropped during 1973 and the first half of 1974, with the use of clomiphene and natural cycles, and then increased again with re-introduction of HMG stimulation in 1975 (92%, maximum seven eggs) and 1976 (80%, maximum nine eggs). These various changes were attempts to retain control over ovulation whilst not prejudicing the luteal endometrium against implantation. However, the failure to establish an ongoing pregnancy prompted an alternative approach towards the end of 1977, avoiding the use of exogenous hormones altogether and thereby the accompanying adverse effects on oestrogen levels and the luteal phase. This re-introduction of natural cycles in parallel with more detailed endocrine monitoring meant a reduction in the numbers of successful LOR and eggs to 65–68%, but with better overall success in retrieving competent preovulatory oocytes for successful IVF (Edwards et al., 1980b).
Endocrine monitoring: urines
See Table 2. In 1969, urinary total oestrogen (TE) in 24-hour samples was estimated only once at first, and then on alternate days after initiation of HMG therapy to assess follicular maturation: “we were unable to do a detailed analysis of urinary steroids for each day of the treatment cycle” (Steptoe and Edwards, 1970). Pregnanediol (PD), a product of progesterone (P) metabolism, was also measured in the last sample collected in some cases. From 1970 onwards, both TE and PD were measured in all follicular-phase samples, and occasionally throughout the luteal phase, in the latter cases as a means to assess the luteal state. Estimation of a second metabolic product of progesterone, Pregnanetriol (PT), which was routinely assayed in at-risk pregnancies during the 1960s, was added in March/April of 1973, but was discontinued soon thereafter. LH and FSH were assayed from March 1973, and, from 1974 onwards, the 24-hour urine TE, PD and LH assays were carried out daily from just before or just after the first injection of HMG, as well as daily after laparoscopy, and then every second or third day towards the end of the luteal phase, so as to monitor the effect of follicular phase stimulation and egg retrieval on the luteal phase. Urinary HCG was also monitored after embryo transfer, in order to detect potential implantation of an embryo (‘biochemical pregnancy’).
Monitoring of the follicular phase in order to detect the onset of the LH surge in natural cycles was introduced in November 1977, using samples of urine collected every three hours (RGE1, 1978). Every third or fourth sample was tested to provide a baseline concentration, and as soon as an elevation was detected, several successive samples were tested in order to confirm whether the surge had begun. LH was monitored via the Hi-Gonavis Kit (Mochida Pharmaceuticals). Collection of 3-hourly urines was discontinued after laparoscopy, and the luteal phase was monitored via daily 24-hour urinary TE, PD and LH assays.
Endocrine monitoring: serum
See Table 2. By the beginning of 1970, it was acknowledged that, for later stages of the work, a correct hormonal background would be required for tubal transport and implantation before a pregnancy could be established (Steptoe and Edwards, 1970). Only urine data was used initially, rather than more valuable plasma data, because the latter “would have imposed repeated trauma on our patients” (Edwards and Steptoe, 1974, p.30). The first records of serum monitoring appear in July 1972, with occasional assays for TE/FSH/LH/Prl. Occasional blood sampling was continued during 1973 and 1974; from September 1975, serum HCG was monitored after laparoscopy, and luteal Prl/LH tested in occasional samples. Blood samples were taken more regularly during 1976: combinations of TE, FSH, P, Prl were tested in one to six samples per cycle before and/or after LOR (not all tests, not all cycles). In 1977 and 1978 blood samples were taken every 2 or 3 days during natural cycles and analysed for TE, P, FSH, LH, Prl, with the addition of HCG testing post-embryo transfer. Between April and June 1972, blood groups are recorded for each partner, possibly reflecting Edwards’ interests in immunological causes of infertility (Edwards, 1968a, Edwards, 1968b, Edwards, 1972, Edwards, 1973, Gardner et al., 1973).
Endocrine monitoring: follicular fluid
Samples of fluid from individual aspirated follicles (FF) were collected throughout, from September 1969 to February 1978. Some FF samples were used for culture of oocytes in 1969; steroid assays (TE, P) were used to gain information about the health and developmental stage of the follicles in response to gonadotropin stimulation, and whether follicles were atretic or pre-ovulatory (Edwards et al., 1980b, Steptoe and Edwards, 1970). This information, in combination with the results of detailed endocrine monitoring and outcome of IVF, formed the basis for the numerous papers published between 1972 and 1978 (Edwards, 1973, Edwards, 1974, Edwards and Steptoe, 1975, Edwards et al., 1972, Edwards et al., 1977, Edwards et al., 1980a, Edwards et al., 1980b, Fowler et al., 1977, Fowler et al., 1978a, Fowler et al., 1978b, Fowler et al., 1978c). Thus, in Edwards et al. (1972, September), observations on follicles from 68 patients were described. Records show that both FF and eggs were collected from 27 patients in 1969, 55 patients in 1970, 39 patients in 1971, and 36 patients to the end of July 1972, a total of 157. Given that the stimulation regime used in this paper was only settled on in 1970, this leaves a maximum of 130 patients, and since data from only those patients with three or more follicles were reported, this number reduces to (33 + 32 + 26 =) 91. Moreover, only one-third of FF samples were described as being ‘clean’ of blood, so it seems likely that the 68 patients in that study were drawn from this group of 91. In that paper, FF from six follicles taken from ‘some’ naturally cycling patients are described in addition; these may be the three patients described in August and September of 1971 (L290/P100, 291/P101 and 301/P106; Suppl. data 2 in Elder and Johnson, 2015b), but we have found little information on these cases in the notebooks. In a paper published 2 years later, Edwards and Steptoe (1974) describe the effects of increasing the interval between the first HMG and HCG injections and conclude that ovulation prior to laparoscopy did not occur if the duration of follicular stimulation before HCG injection did not exceed 9 days. Some of these data are tabulated and summarized towards the end of notebook L0, and show that egg recovery is lowest when the period from first HMG to HCG injection was extended to 9 or 10 days.
Combined endocrine monitoring
Notebooks H3 and H4 reveal that a major effort was invested in endocrine monitoring alone, separate from LOR cycles, during the periods April 1973 – 10 February 1974 (H3) and 19 February 1974 – 22 June 1976 (H4). Thus, daily 24-hour urine samples were collected from approximately day 5 or 6 of the cycle, continuing for several days into the luteal phase, and were assayed for TE, PD and LH. Occasional blood samples were tested for TE, PD, Prl and FSH. There are no records of LOR for parts of this period (e.g. 8 August to 6 December 1973, and 9 June 1974 to 16 March 1975), and so it seems that this period was used to gain additional information about the endocrinology of individual patient menstrual cycles. A total of 96 patients were monitored in 152 cycles of three different types, many of the patients monitored through several cycles (see Table 3): (i) Clomid with timed intercourse (CTI): 16 patients, 18 cycles; (ii) natural cycles with Pregnyl (NC + HCG): 12 patients, 17 cycles; (iii) natural cycles (NC): 90 patients, 117 cycles. Most natural cycles were monitored between October 1973 and August 1974 (108 cycles), the remaining nine being spread between February and December 1975. Some of the monitored Clomid and NC + HCG cycles led to laparoscopy attempts in that cycle, and the LOR details for these are included in the data for 1973 and 1974 (Table 2). Twenty-seven of the 96 patients monitored through 35 cycles during this period have no record of any laparoscopy, previously, contemporaneously or subsequently (and so are not represented in Suppl. Table, Elder and Johnson, 2015b or elsewhere in these papers other than in Table 3). The remaining 69 patients have records of one or more LOR cycles, some of which were carried out during 1973–1975, or before or after this period.
Table 3.
Monitored cycles for 96 patients (L358 – 472) between April 1973 and December 1975. (a) All 96 patients; (b) A subset of 27 of the 96 patients with no record of laparoscopic oocyte recovery (Oct 1973 – Aug 1974 + 1 Feb 1975).(a)
| Type of cycle | Date | Patients (n) | Cycles (n) |
|---|---|---|---|
| CTI | April – July 1973 | 16a | 18 |
| NC + HCG | July 1973 – Dec 1975 | 12b | 17 |
| NC | Oct 1973 – Aug 1974, and Feb – Dec 1975 | 90c | 117 |
| All types in toto | April 1973 – Dec 1975 | 96d | 152 |
CTI = Clomid + timed intercourseLOR = laparoscopic oocyte recoveryNC + HCG = natural cycle with Pregnyl to induce ovulationNC = natural cycle only.
Five patients had only one CTI cycle, and no further monitoring or treatment.
Seven patients also in CTI group.
Eleven patients also included in CTI group.
Sixty-nine of these patients were also prepared for LOR either at the same time, or in previous or subsequent cycles.
One patient had 1 CTI + 1 NC; the other patient had 1 CTI.
One patient had 1 NC + HCG + two NC; the other patient had 2 NC + HCG.
Luteal support
As early as 1970, Steptoe and Edwards (1970) had drawn attention to possible problems of using ovulation induction and follicle aspiration for subsequent embryo transfer and implantation. A shortened luteal phase was noticed after gonadotropin administration, and this was investigated by measuring urinary levels of oestrogen and pregnanediol, whether or not embryo transfer had been carried out (Edwards and Steptoe, 1974, Edwards et al., 1980b). A significant inverse correlation was found between follicular oestrogen levels and the duration of the luteal phase, reflecting a situation similar to that reported in the rabbit to that reported in the rabbit (Beier, 1974). The notebooks record that between March and August 1977, early secretory phase endometrial biopsies were examined histologically for several patients, confirming that the histology of the uterus was abnormal after gonadotropin stimulation, with shortening of the luteal phase, as reported in Edwards et al. (1980a), p.743: “…endometrial biopsies taken from patients who did not have an embryo implanted indicated that the histology of the uterus was abnormal...” The additional material from the Edwards’ archive contains records of approximately 150 endometrial biopsies taken between 1969 and 1977, including day-2 as well as secretory-phase biopsies (RGE2, 1976).
Attempts were therefore made to correct luteal deficiency, first by modifying stimulation in an attempt to lower oestrogen levels (see section on stimulation, above), and then by providing exogenous luteal support. Luteal administration of Pregnyl is first mentioned in October 1972, three or four injections of up to 5000 IU. Both Pregnyl and intramuscular progesterone are mentioned as luteal support during 1973–74, with the addition of Primolut depot (hydroxyprogesterone hexanoate) in July 1975, along with occasional mention of Ritodrine, Indocid, and Ethinyl Estradiol supplementation (Table 4). Bromocriptine (Parlodel) was given in the follicular and luteal phase to a number of patients in 1977, and three patients were also given luteal Clomiphene in an attempt to reduce high oestrogen levels. However, with the exception of those patients given Primolut, none showed any sign of early pregnancy. The administration of Primolut depot did delay the onset of menstruation (Edwards and Steptoe, 1974), and eight samples described as “uterine casts/decidual tissue/products of conception” were sent for histological examination, seven between June and October 1976, and one in July 1977. Handwritten histology reports relating to endometrial biopsies for three of these eight patients were found (RGE2, 1976): all three indicate “decidual changes in stroma; no embryonic or trophectoderm tissue”.
Table 4.
Luteal-phase support.
| Year | LOR with documented egg recoverya (n) (% of stimulated cycles started) | LOR in which ET recorded (% of LOR with eggs recovered) | Luteal support | Pregnancies recorded (n) (% per ET; % per LOR with eggs recovered) |
|---|---|---|---|---|
| 1969 | 48 (83) | 0 | NR | None |
| 1970 | 55 (89) | 0 | NR | None |
| 1971 | 36 (88) | 1 (3) | NR | None |
| 1972 | 39 (95) | 5 (13; + 1 GIFT) | Pregnyl, 5000/3000 IU Sept 1972 (1 case only) | None |
| 1973 | 25 (57) | 7 (28; + 5 GIFT) | Pregnyl 1500/3000/1000 + Progesterone i.m. (11/73) | None |
| 1974 | 28 (75) | 9 (32; + 1 GIFT??) | Ritodrine, Progesterone, Pregnyl, Indocid | None |
| 1975 | 33 (92) | 11 (33) | Primolut from June, then Primolut + Indocid + EE Dec: Progesterone + Pregnyl | 1 ectopic (9; 3) |
| 1976 | 44 (80) | 27 (61) | Indocid, Primolut, Progesterone/EE or combinations; Pregnyl in Sept, Parlodel in Dec | 1 × biochemical, 9 possible EPL (37; 23) |
| 1977 | 28 (65) | 21 (75) [19 SC; 2 NC] | Parlodel, EE, Pregnyl, Clomid (none for NC) Progesterone i.m., then PV (by tampon) | 1 × ?EPL 1 × live birth (10; 7) |
| 1978 | 52 (68) | 31 (60) | NR | 2 × miscarriage 1 × live birth (10; 6) |
| Total | 388 (78) | 112 (29) [+ 6 (or 7?) GIFT = 30] | 5 clinical (4; 1) [+ 11 possible pre-clinical = 10; 3] |
EPL = early pregnancy loss; ET = embryo transfer; GIFT = gamete intrafallopian transfer; LOR = laparoscopic oocyte retrieval; NC = natural cycle; NR = not recorded; EE = Ethinyl Estradiol.
Excluding cases for laparoscopic sterilization (no ET and no luteal support).
Sperm preparation and insemination
See Table 5. Details of sperm preparation and insemination are recorded from October 1969 (L186). Seminal plasma was removed from the semen sample by washing twice, ‘with gentle centrifugation’ (Edwards et al., 1970, Edwards et al., 1980a, Edwards et al., 1980b), and spermatozoa were suspended to a final concentration between 8 × 105 ml− 1 and 2 × 106 ml− 1, depending on the quality of the sample. ‘Barry’s B’ medium was used initially, with modifications to NaCl and KCl concentrations, based upon Na+/K+ analysis of follicular fluid. In August 1970, sperm wash medium is noted as ‘Tyr B 7.5 g/L NaCl, 0.39 g/L KCl, pH 7.55 290 mOsm’. Streptomycin and BSA (0.36%) were added in 1971; pH and osmolarity are recorded for every batch of medium made, pH 7.55–7.6, 290–296 mOsm. During 1975, Earle’s BSS was introduced for sperm washing (Earles BSS + 0.1 g% glucose and 0.36 g% BSA + 0.11 g% Pyr + Penicillin), and this formula continued to be used through 1976 and 1977. Sperm quality details are almost all written in Edwards’ hand, with no indication of sperm count numbers, and only ‘sketchy’ descriptions, usually restricted to better-than-average samples, e.g., "sperm excellent", "sperm going like a rocket" (see Suppl. Table, Column W, in Elder and Johnson, 2015b).
Table 5.
Culture conditions, 1969–1978.
| Year | LOR with documented egg recoverya (n) (% of stimulated cycles started) | Eggs retrieved (n) | Medium 1: flushing, egg wash, sperm wash, fertilization | Medium 2: cleavage/embryo transfer | Cycles with insemination (n) (% of LOR with egg recovery) [eggs inseminated (n), % of eggs recovered] | Cycles with embryos reported (n) (% of LOR with egg inseminated) [bTotal embryos (n), % of eggs inseminated] |
|---|---|---|---|---|---|---|
| 1969 | 48 (83) | 136 | No records until Sept; M199, Barry’s B + BSA or HSA; FF: 10%, 20%, 50% | Minitube culture, 5% CO2 in air; M199, Hank’s+ FCS, BSA, HSA, 2.5%; | 7 (15) [27, 20] | 1 (14) [1, 4] |
| 1970 | 55 (89) | 274 | Microdrop culture, 5% CO2 60% FF/Barry’s B, ‘special B’ (high pH), + Na/K Whittingham’s/Weymouth’s 1:1 , 15% FCS TyrB + 0.36% BSA, 1%BSA; + Pyr, lactate, bicarb; Tyr B minus extra bicarb + Heparin 10 IU/ml; Whittingham’s + 20% FCS | Whittinghams + incr. Na/K; Ham’s F10 + 0.36 g% BSA, or 20%, 40% FCS M199 + pyr, lact, bicarb, BSA M199 + 10 % FCS + 5%PO4 + 100iuml bicarb; Ham’s F12 + 20%FCS or 0.72% BSA + 1x L-Glutamine Reduced O2 in May; Aug: 20% human serum | 51 (93) [272, 99] | 25 (49) [36, 13; + 3 × 2PN] |
| 1971 | 36 (88) | 175 | Tyr + Na/K for sperm and fertilization, + 0.36% BSA; Tyr for eggs:minus extra bicarb, + PO4 + Hep for flushing Streptomycin added for sperm | Ham’s F10, F12, + 20%FCS (inactivated), + 5%, 10%, 20% HS 5% CO2 in air, 5 % O2/5 % CO2/90 % N2 from September; Oldham vs Cambridge water compared | 36 (100) [175, 100] | 13 (36) [18, 10 + 1 × 2?PN] |
| 1972 | 39 (95) | 187 | No phenol red in Tyrode’s; TyrB ± bicarb, BSA/FCS/HS + Pyr, Penicillin Stock solutions made. Autoclaved BDH water; 5 % O2/5 % CO2/90 % N2 | Ham’s F10 + L-Glutamine + 20 % FCS + 5 % HS; Sept: Ham’s F10 + 20% maternal serum collected at beginning of laparoscopy | 35 (90) [179, 96] | 10 (29) [15, 8; + 6 × 2PN & 1 × Syngamy] |
| 1973 | 25 (57) | 86 | New batches Pyr, BSA, BDH water in glass or plastic bottles, SVT for different paraffins & sera, FCS tested with HeLa & GC cultures | As previously – serum inactivated/not inact., Maternal serum, Paternal serum; F10 minus bicarb for ET | 20 (80) [81, 95] | 8 (40) [8, 10; 2 × 2PN, 3 × ?2PN] |
| 1974 | 28 (76) | 85 | As previously, SVT for different sources of HSA; extra Ca & Mg added in June | As previously, new F10 for ET: no BSA or bicarb | 28 (100) [81, 95] | 10 (36) [15,18; + 1 × 2PN] |
| 1975 | 33 (92) | 117 | As before, extra Ca, Mg, P; McCoy’s modified w/bicarb September: EBSS + glucose, BSA, Pyr, Pen. Several SVTs for different batches of media, sera, maternal serum Nov: Tyrode compared with Hoppit’s by SVT Dec: non-autoclaved water, EBSS + Pyr, 0.21% bicarb, BSA, HS, | F10 reinforced: + Ca, Mg, Glutamine, bicarb. No bicarb for ET. Laparoscopy plasma, inactivated serum. All tested with SVT | 33 (100) [117, 100] | 15 (46) [17, 15; + 1 × 2PN] |
| 1976 | 44 (80) | 147 | Earle’s for flushing, Fert, Sperm wash + Pyr, Pen, ± bicarb | Ham’s F10 + bicarb, glutamine, Pen. FF assays, GC cultures, ‘serum new method’, inactivated + not inactivated; F10 for cleavage and ET. All checked for osmolarity | 44 (100) [149, 100] | 29 (66) [42, 29] |
| 1977 | 28 (65) | 97 | As 1976, Earles Fert & Hep Earle’s Alb, + 0.36% BSA. Osmometer checked with new standards: all media adjusted to 279–273 | As 1976, F10 ‘serum new method; FF assays, GC cultures, serum not inactivated | 27 (96) [95, 98] | 22 (82) [20 SC; 2 NC][35, 37] |
| 1978 | 52 (67) | 57 | As 1977 | As 1977; FF assays, GC cultures | 50 (96) [60, 98] | 34 (68) [34, 57] |
| Total | 388 (78) | 1361 | 331 (85) [1237/1361, 91] | 165 (51) [221/1237; 18] |
ET = embryo transfer; FCS = fetal calf serum; FF = follicular fluid; LOR = laparoscopic oocyte recovery; NC = natural cycle; SC = stimulated cycle
Cases for laparoscopic sterilization not included
In 1970, Edwards et al. (1970) reported that at the end of the laparoscopy procedure, “the oocytes were suspended in droplets consisting of fluid from their own follicle (when available) and the medium being tested for fertilization. After incubation for 1–4 h at 37° C the oocytes were washed through two changes of the medium under test before being placed in the suspensions of spermatozoa. Preovulatory oocytes would be ready for fertilization … by 3–4 h after collection. Many oocytes were obviously not preovulatory, and therefore unsuitable for fertilization, but all were placed in the fertilization droplets in order to simplify our procedure” (Edwards et al, 1970, p.1371). The records then show that oocytes were inseminated soon after the end of the laparoscopy (see Suppl. Table, Column Q, in Elder and Johnson, 2015b), washed two or three times in the medium being tested for fertilization, and then transferred into suspensions of spermatozoa in 0.05 ml microdrops under oil (Brinster, 1963), at a concentration of 105 to 106 ml− 1, depending on the subjective assessment of sample motility (Edwards et al., 1970). Time intervals between HCG administration and insemination are recorded from 1971–1975, varying from 32.4 to 34.5 h after HCG. The cultures were examined 3 h later, and the concentrations of spermatozoa were sometimes reduced after cumulus dispersal was observed, 4–6 h after insemination.
Culture media
See Table 5. The notebooks reveal a major investment of time and effort in trying to identify optimal conditions for in-vitro culture. Their first successful attempts to achieve fertilization and cleavage were described in Edwards et al. (1970), which records the different types of media and culture conditions that were tried: based on analysis of follicular fluid electrolytes, Bavister’s medium was modified by reducing NaCl and increasing KCl concentrations. Also tried were Whittingham’s (unmodified), Waymouth’s and Ham’s F10 with extra bicarbonate to raise the pH to 7.5–7.6, supplemented with bovine serum albumin, with pyruvate added to Waymouth’s. During this period, the embryos were assessed for development/timing of cleavage divisions, and analysed for cell number/mitoses after it was clear that development had ceased, approximately 48 h after the last division was observed. Although results of fertilization and cleavage using the different media are reported in Edwards et al. (1970), this paper also states “firm conclusions about the value of different media obviously cannot be drawn when so few ova were available”.
The notebooks reveal that during 1969, oocytes were cultured in follicular fluid diluted 1:1 or 1:2 with Tyrode B. M199 and Hank’s are also mentioned, as well as addition of bicarbonate and bovine or human serum albumin. TE was measured in the follicular fluid of eggs that were inseminated. Five inseminated eggs were transferred to live rabbit oviducts in December, but no embryo development was seen after recovering the eggs the following day. In March 1970, three fertilized eggs were transferred to live rabbit oviducts, with the same result, eggs recovered as "1-cell”. "Minitubes" were used initially for embryo culture, microdroplets under oil being mentioned first in January 1970. An atmosphere of 5% CO2 in air was obtained by placing culture dishes inside a gas-filled desiccator, with various gas mixtures for culture being tried: "reduced O2", 5 % CO2, 5 % O2/5 % CO2/90 % N2. Notebook H0 contains flame photometry and osmolarity records during 1970, with mention of Tyrode B, Tyrode C, Ham’s F10 and F12, M16, M199, Whittingham’s, Whitten, Weymouth, Hank’s-with or without pyruvate, lactate, bicarbonate, heparin, citrate, NaCl, KCl, as well as fetal calf serum (FCS), or bovine serum albumin (BSA) in concentrations varying from 0.5 to 50% (see Edwards et al., 1970). Maternal serum (MS) was introduced for some cases in August 1970, and continued in use in varying concentrations thereafter. Paternal serum was also added for a few cases in 1972–73, as well as seminal plasma for two cases in 1974. The osmolarity of human FF samples is also recorded, and all batches of media were checked for pH and osmolarity, because of the idea that FF might be involved in sperm capacitation (see Edwards, 1974, p. 211). Whenever possible, the eggs from a single patient were divided between two or three different types of media and/or serum concentrations in an attempt to compare different culture media, type and concentration of sera. Heparin was added to media used for flushing follicles, and Penicillin added to egg wash media. By November 1970, a system apparently evolved (see Edwards et al., 1970) whereby different types of media were used for each stage, with varying amounts of BSA, HSA, FCS and/or MS:
Fertilization: TyrB + NaCl + KCL, pH 7.55, 295 mOsm + BSA
Sperm wash: same, pH 7.6
Egg wash: minus extra bicarb, + PO4 buffer, + 100 IU/l Pen, pH 7.3, 290 mOsm
Flushing: as egg wash, + 10 IU/ml Heparin, pH 7.3 290 mOsm
Cleavage: Ham’s F10, F12, + 20 % FCS or 0.72 % BSA + 1 × L-glutamine
Between 1971 and 1975, Tyr B was also then supplemented with NaCl, KCl, MgCl2, CaCl2, PO4, glucose, pyruvate, in varying concentrations. Ham’s F10 was supplemented with varying concentrations of L-glutamine, as well as extra Ca and Mg. From 1974, bicarbonate was removed from media for embryo transfer. Media pH was measured as being between 7.3 and 7.6, and osmolarity was gradually reduced, from a range of 293–310 in 1972 to 285–287 (1973), 280–283 (1974) and 279–284 thereafter. Although the rationale behind these various changes is not recorded in the data available to us, the modifications were evidently aimed towards achieving the osmolarity found in FF (Edwards, 1974), presumptively providing optimal conditions for fertilization and embryo culture. Further correspondence in archive material (RGE3, undated) contains several handwritten undated sheets with media recipes, including one note that indicates consultation with J Paul (Dr John Paul, Beatson Institute, Glasgow), the recognized authority on tissue culture at that time. This collection of papers also contains correspondence during 1970–71 with Flow Laboratories about the exact composition of F10/F12 and glutamine supplementation, with Hoechst regarding the purity of BSA, with BDH regarding the composition of their gelatin preparation, and with Millipore regarding the wetting agent used in their filters (RGE4, 1970). All of this correspondence emphasized their concern about possible toxicity in the choice of materials used for culture of human tissue cells.
During 1972 and 1973, comparisons were made between media made with Oldham versus Cambridge water and media made with BDH water when made up in chromic-acid washed glass versus plastic flasks. Different sources of BSA and FCS were compared, as well as different methods of preparing maternal serum (inactivated versus not inactivated). Sodium and potassium levels in serum and follicular fluid were measured by flame photometry, and the osmolarity of 15 different samples of maternal serum was checked. Stock salt solutions were made up for media preparation, with different ‘recipes’ for the various types of media. ‘Heavy’ and ‘light’ paraffin were tested in sperm viability assays, and different concentrations of FCS were tested for their effects on cell count and morphology in HeLa and granulosa cell cultures.
Earle’s Balanced Salt Solution (EBSS) was introduced in September 1975, and numerous tests and comparisons were made using different batches of serum and paraffin, as well as differing concentrations of bicarbonate, all with osmolarity/pH checks and sperm viability assays. By June 1976, recipes for five different media are listed, and these remained (more or less) constant, with new batches made every 2–3 weeks until the records end in July 1978 (Suppl. Table). Varying concentrations of FCS, HSA and/or maternal serum were also added to the media before use, settling on 7.5% for sperm/egg wash and fertilization, and 15% human serum for ‘cleavage media’ by 1977.
Table 5 indicates key changes in the culture system in relation to the number of cases with successful culture of embryos/embryo transfer. The first sign of successful fertilization is described in November 1969 (L190/P9), and cleaved embryos were first seen in January 1970 (L200/P50), after collecting eight eggs: sperm samples were washed in ‘Barry’s B’, then ‘some of Barry’s Special B’ added 2 h later. The eggs were split into two groups, four transferred to Whittingham’s, and four kept in ‘Barry’s B’; 4-cell embryos were observed in both media, and were fixed in formalin. The first blastocysts are described in August 1970 (L247/P42), after transferring inseminated eggs to Ham’s F10 containing 20% inactivated human serum. As described in Steptoe et al. (1971) and in Edwards and Steptoe (1980), these blastocysts were fixed and stained for chromosomal analysis. The first embryo transfer procedure was attempted 16 months later in December 1971 (L303/P75). The first pregnancy, in June 1975 (Steptoe and Edwards, 1976), followed their twenty-fifth embryo transfer procedure, 42 months after the first embryo transfer (L446/P38).
Embryo freezing
During the summer of 1977, preliminary attempts were made to cryopreserve a few embryos in the hope of bypassing the adverse effects of stimulation on the luteal phase uterus by transferring frozen-thawed embryos in a subsequent natural cycle. However, the thawed embryos did not continue to develop in vitro (Edwards and Steptoe, 1977), a failure that contributed to their next crucial decision: to avoid the use of drugs completely and to attempt to collect single pre-ovulatory eggs in natural cycles monitored for the LH surge (Edwards and Steptoe, 1980, p.132-135).
Embryo transfer
The first attempt at embryo transfer was in December 1971 (L303/P75), a 16-cell embryo 80 hours post-oocyte retrieval. Although they recognized that evidence from bovine and laboratory animals suggested that reimplantation via the cervix could result in a low rate of implantation, this route was chosen because “Passage through the cervix was adopted as the method giving least trouble to the mother; a fine catheter of 2.0 mm diameter was passed through the cervical canal in the uterine cavity, and a smaller cannula containing the embryo was threaded through it." (Edwards and Steptoe, 1974, p.32). This paper recommends that trans-cervical embryo transfer should be fully tested in patients, in order to avoid a further surgical procedure to gain access to the uterine cavity. Nothing in the notes mentions the details of trans-cervical transfers, although Edwards et al. (1980a) state that: “A bi-valve speculum was passed with the patient in a modified lithotomy position … no instruments were attached to the cervix … the loaded cannula was passed through the cervix so that its end would lie in the body of the uterus about 1 cm from the fundus …”. As indicated in Table 4, injections of Ritodrine (Philps-Duphass) or indomethacin were later given as a precaution against uterine contractions after trans-cervical transfer. Of the 112 embryo transfer procedures carried out between 1971 and 1978, 106 were trans-cervical, four via laparoscopy (L412/P158, April 1974, L604/P217, L607/P54, L608/P55, September 1976), one via intraperitoneal surgery (L430/P144, May 1974) and one via mini-laparotomy (L704/P275, April 1978). A total of six or seven gamete intra-fallopian transfer (GIFT) procedures are also recorded between 1972 and 1974 (Elder and Johnson, 2015b).
It was widely believed at the time that that embryos had to be transferred when they were at the 8- to 16-cell stage, a belief based on two lines of evidence. The first came from observations made on embryos flushed from the uteri of naturally mated women, suggesting that embryos moved into the uterus at these stages (Croxatto et al., 1972), and the second from observations that earlier transfers were lethal for the embryos of animals (Marston et al., 1977, Polge, 2000). Accordingly, the first five embryo transfers carried out in 1972 were of embryos at the 8- to 16-cell stage; however, during 1973, six of the seven embryo transfers were of 5- to 6-cell-stage embryos on day 3, and one embryo transfer of 8- to 12-cells on day 4. Between 1974 and 1976 embryos were transferred mainly at the morula/blastocyst stage, on day 4/5, and in 1977–78 embryos were transferred at 8- to 16-cell stage, on the evening of day2/morning of day3, or 8- to 16-cell morula stage on day 4.
Genetic analysis of embryos
Evidence of Edwards’ continuing interest in developmental genetics (Johnson, 2011) and in preimplantation genetic diagnosis (PGD) in particular (Edwards and Gardner, 1968), are found in 1970 with references in the notes to “Y bodies”: Lap248 (blastocyst – negative) and Lap249 (12–16 cell – negative), and in 1971: Lap278 (early blastocyst – “at least one Y body – photo”) and Lap279 (early blastocyst –“no Y fluorescence”). The Y body had been described by Pearson and Bobrow (1970) using quinacrine fluorescent dye to stain interphase nuclei from males. References to Y bodies start with the development of the first blastocysts, because Melander (1962) had shown that sex chromatin could not be detected in pre-blastocyst stage embryos, the nuclei at earlier stages being too heterochromatic. Presumptively that might also have prevented attempts at visualisation of the Y body? References to Y bodies cease after embryo transfer becomes the norm, reflecting the requirement to fix and stain the embryos for their detection, which was no longer possible.
General discussion
Table 6 summarises the data for each of the pregnancies that are recorded: 11 potential pre-clinical pregnancies, one ectopic, two miscarriages and two live births. Table 7 outlines a timeline of the critical events described here. Four features stand out in this description of how Edwards, Purdy and Steptoe attempted to move from IVF to a term pregnancy. First, the scale of the problem, as summarized in Table 1, was daunting. So much had to be worked out from scratch.
Table 6.
Summary of data for all potential pregnancies recorded.
| Date (ET number) | L number/P number | Age of patient (years) | Stimulation protocol | Culture media | Embryo details | Luteal support | Outcome (date) |
|---|---|---|---|---|---|---|---|
| June 1975 (25) | L446/P38 | 35 | P4 x 3 | Tyr B, Tyr B reinforced, F10 reinforced, 15% HS | Morula, D4 | Primolut depot + oral Primolut | Cornual ectopic |
| June 1976 (38) | L484/P202 | 27 | P5 x 3 | F10 + extra Ca/Mg, Earles/7.5% HS, then F10 15% HS | 2 blastocysts | Pregny/Indocid/Progesterone, + Primolut depot | ?Early pregnancy loss (16 Jul) |
| June 1976 (41) | L488/P204 | 25 | P5 x 3 | F10/7.5% HS, then F10 15% HS | 1 morula, 1 blastocyst | Pregny/Indocid Primolut depot | ?Early pregnancy loss (16 Jul) |
| June 1976 (43) | L491/P185 | 34 | P5 x 3 | “Serum new method”, Earles/7.5% HS, then F10 15% HS | 32-cell, D5 | Pregnyl/Indocid/Progesterone | Passed tissue. ?Foetal tissue present. |
| June 1976 (45) | L494/P188 | 27 | P5 x 3 | Earles/7.5% HS, then F10 15% HS | Early blastocyst, D5 | Pregny/Indocid Progesterone | Curettage (10 Sept). ?Retained products |
| July 1976 (48) | L498/P211 | 31 | P5 x 4 | Earles/7.5% HS, then F10 15% HS | Morula, D4 | Pregnyl/Primolut | ?Passed material (17 Jul) |
| July 1976 (49) | L500/P131 | 30 | P5 x 3 | Earles/7.5% HS, then F10 15% HS | Blastocyst, D5 | Pregny/Primolut/EE | ?Decidual cast (19 Aug) |
| July 1976 (50) | L501/P213 | 27 | P5 x 3 | Earles/7.5% HS, then F10 15% HS | Blastocyst, D5 | Pregny/Primolut/EE | ?Large piece of material (24 Aug) |
| Sept 1976 (54) | L607/P28 | 40 | P5 x 4 | Inact. serum, FF; F10/15% HS | Morula/blastocyst, via lap, D4 | Pregnyl/Primolut | Uterine casts/decidua/POC, histology negative |
| Sept 1976 (55) | L608/P207 | NR | P5 x 3 | Inact. serum,FF; Earle’s 7.5% HS, then F10, 10%, then 15% HS | 2 morula/early blastocysts, via lap | Pregnyl/Primolut | Uterine casts/decidua/POC, histology negative |
| Sept 1976 (56) | L612/P199 | 35 | P5 x 4 | Inact. serum, FF F10 15% HS | 8-cell, D3 | Pregnyl/Primolut No Indocid | Biochemical (HCG = 57), histology negative |
| July 1977 (74) | L659/P257 | 36 | P5 x 3 | Earles/7.5% HS, then F10 15% HS | 2 embryos, D2 | Primolut/Parlodel | Tissue (5 Aug): decidua – no chorionic villi |
| Nov 1977 (81) | 666/P264 | 31 | NC | Earles/7.5% HS, then F10 15% HS | 8-cell, D2 evening | None | Live birth (Jul 1978) |
| Jan 1978 (82) | 673/P267 | 37 | NC | Earles/7.5% HS, then F10 15% HS | 8-cell, D2 evening | None | 9/10-week gestational sac; ERPOC (Mar) |
| May 1978 (97) | 712/P247 | 31 | NC | Earles/7.5% HS, then F10 15% HS | 8-cell, D2 evening | None | Live birth (Jan 1979) |
| July 1978 (109) | 738/P265 | 31 | NC | 12–16-cell, D3 | None | Miscarried (26 Nov) |
D = day; EE = ethinyl estradiol; ERPOC = evacuation of retained products of conception; ET = embryo transfer; HCG = human chorionic gonatotrophin; lap = laparoscopy; POC = products of conception; Pn = number of ampoules of Pergonal.
Table 7.
Timeline of the critical events in dataset.
| Event | Date | No. in series/Patient number | L number |
|---|---|---|---|
| “New suction gadget” | September 1969 | 43/P35 | 179 |
| Insemination recorded | October 1969 | 50/P42 | 186 |
| Fertilization recorded | November 1969 | 54/P9 | 190 |
| “Excellent” embryos (8-cell, 4-cell) | January 1970 | 64/P50 | 200 |
| First blastocysts | August and September 1970 | 111/P42, | 247 |
| 112/P38, | 248 | ||
| 117/P49 | 253 | ||
| First embryo transfer | December 1971 | 169/P75 | 303 |
| Daily urinary monitoring | March 1972 | 190/P71 | 324 |
| First GIFT | 26 April 1972 | 193/P114 | 327 |
| Luteal support introduced | October 1972 | 211/P107 | 345 |
| Additional cycles monitored before LOR | April 1973 to December 1975 | N/A | L358–472, + 26 patients without L number |
| Regular sperm viability testing for media, serum, oil etc | From July 1973 | N/A | N/A |
| Clomiphene or NC/HCG cycles | January to June 1973, December 1973 | 217–237/P246–P256 | 351–371 380–390 |
| First pregnancy | June 1975 | 312/P38 | 446 |
| Serum monitoring introduced | September 1976 | 377/P28(9) | 607 |
| Hi-Gonavis for LH monitoring | November 1977 | 438/P263 | 665 |
| First live birth | ET July 1977 | 439/P264 | 666 |
ET = embryo transfer; GIFT = gamete intrafallopian transfer; HCG = human chorionic gonadotrophin; LOR = laparascopic oocyte retrieval; N/A = not applicable; NC = natural cycle.
Second, the transparency of the three workers is striking: they published in minute detail the ways in which they were attempting to overcome each problem, to the extent that there is remarkably little information from the notebooks that is not available already in the public domain. What is also notable about this body of work is its thoroughness, solidly based on an informed reading of the contemporary literature, as exemplified by the comprehensive account by Edwards (1974) of follicular fluid properties. In this regard, the approach taken was ethically sound, a theme to which we return in the next paper.
Third, the data reveal the extraordinary amount of work involved in achieving the two live births: it is exhausting to read how meticulously all the media were prepared and tested; likewise, the hormonal assays required collection of multiple samples of fluids followed by assay procedures and recording and analysis of results. Much of this work was done by Purdy, whose role in the whole enterprise is reassessed in Johnson and Elder (2015b). Some of the research undertaken was truly heroic – especially the 24-hour monitoring of 3-hourly urine samples for LH when natural cycles were being used: all these had to be done on site, as rapid feedback was required. The original Hi-Gonavis kit based on haemagglutination was very unreliable (M. Macnamee, personal communication), which may explain why some natural cycle LH surges (11/78) were missed. It is less clear to what extent the other hormonal assays were also done in-house; however, the technology for any kind of endocrine assays in those days was poor relative to today, giving less reproducible results. A series of 39 hormone reports from GD Searle Scientific Services (dated between 10 and 11 December 1969 and 9–10 March 1970; RGE5, 1969) indicates that assays for urinary TE and P were sent off initially to be done commercially (confirmed in Edwards et al., 1972), but lab results found in the Edwards’ archive show that during 1975 assays were carried out at ODGH (RGE6, 1975). The analysis of aspirated follicle fluids (FF) for steroid content (TE, P) was certainly done in-house by radioimmunoassay (although initially by a protein binding assay for progestins). These in-house assays were done in Cambridge eventually, but initially in collaboration with Abrahams in Los Angeles and with Fotherby at the Hammersmith Hospital, London (Abrahams et al., 1970, Edwards et al., 1972), and were used to gain information about the health and development of the follicles in response to gonadotropin stimulation. This information, in combination with the results of detailed endocrine monitoring of urine (and later blood) and the outcome of IVF, formed the basis for the numerous papers published between 1972 and 1980 (Edwards, 1973, Edwards, 1974, Edwards and Steptoe, 1975, Edwards et al., 1972, Edwards et al., 1977, Edwards et al., 1980a, Edwards et al., 1980b, Fowler et al., 1977, Fowler et al., 1978a, Fowler et al., 1978b, Fowler et al., 1978c, YoungLai et al., 1972).
Fourth, whilst our account describes their attempts at a rationally derived and methodically scientific approach to overcome the many problems encountered, it is difficult to conclude that any specific variation in treatment tried had any impact on whether a sustainable pregnancy was achieved. This conclusion contrasts with the ways in which the earlier events in the sequence of problems (such as optimizing the stimulation regime to maximize egg recovery) were solved methodically and rationally. Such a contrasting conclusion is not surprising, as, whilst the earliest challenges in the sequence have direct and measurable outcomes, the ultimate challenge of a live birth after embryo transfer involved several factors, as outlined and discussed in Edwards et al., 1980a, p.752-754), in particular: “attention must obviously be given to the methods used for placing embryos, and to the uterine physiology at the moment of implantation … the placement of embryos is not necessarily the weakest point in our technique. The embryos implanted only when all of the sequences had been successfully accomplished – aspiration, fertilization and cleavage included…”. He there identified the important features in the sequence, i.e. (i) transfer technique, (ii) timing and numbers of embryos transferred, (iii) embryo quality, and (iv) endometrial receptivity. The third and fourth of these factors remain problematic today, and then, as now, there were strong reasons to believe that achieving the first might prejudice the second. Given that the challenge of post-transfer failure occupied the three researchers for 7 of the 9 years between achieving IVF and Louise Brown’s birth, the approach to each of the four factors, identified above as potential influences on the outcome, will be discussed briefly.
-
(i)
Transfer technique: In the early 1970s, non-surgical cervical transfer approaches in animals generally were under-developed; indeed it was only some 40 years later that such a technique was described for the mouse (Green et al., 2009). Moreover what was known was unpromising, as this route had been proving problematic in cattle for some years, as Edwards was well aware given that most of the research had been pioneered by Rowson and Moor in Cambridge at the Institute for Animal Reproduction (Polge, 2000). Eventually highly successful, it took years of painstaking experiments to perfect a simple reliable technique. It remains unclear today why such a (now) routine procedure should have proved so difficult to master, but one suggestion is that operator skills are the most important variable in achieving high pregnancy rates in cattle (Schneider et al., 1980, Seidel et al., 1975). It seems likely that Steptoe did most of the transfers, although Edwards evidently did some himself (see Johnson and Elder, 2015b), but this information is not recorded in the notes. Most of these transfers were trans-cervical, with only 6/112 using Steptoe’s surgical skills. Following the ectopic pregnancy in 1974, Steptoe insisted that all women on the programme who had damaged tubes should have their cornua sealed during the exploratory laparoscopy that each underwent before being accepted definitively on the programme (NF and JW, pp.5 and 30–31; in Suppl. Material 1 in Johnson and Elder, 2015a): a consequence of his fear of the possible consequences following transfer of embryos high in the uterine fundus.
-
(ii)
Timing and numbers of embryos transferred: whether or not Edwards’ belief that the time of day was critical for embryo transfer is correct, what seems likely is that success might have come earlier had they been in a position to transfer more than a maximum of two embryos. However, rarely was this possibility available to them, although on two occasions in 1977, three embryos were transferred (L637 and L650). Edwards had contemplated the consequences of multiple pregnancies back in 1965 (Edwards, 1965), and was firmly of the view that it was better to err on the side of caution, again reflecting his concern for the patient’s welfare.
-
(iii)
Embryo quality: this leads us to embryo quality assessment, which then, as now, was achieved largely by qualitative appearance and the division rate and synchrony of embryonic blastomeres. More significant than embryo quality, however, was quantity. Thus, some of the changes to stimulatory regimes had adverse effects on oocyte recovery or fertilisation rates (see Table 2, Table 5), and with recourse to natural cycles, only a single embryo was available. Ultimately, the embryos that were being produced then were probably no more the cause of their problems than they are today.
-
(iv)
Endometrial receptivity: from 1973, a major effort was directed at either trying to reduce the adverse impact of the ovarian stimulation injections on the luteal-phase uterus or circumventing it in some way. Notably, during the summer of 1977, preliminary attempts were made to cryopreserve a few embryos in the hope of bypassing the adverse effects of stimulation on the luteal-phase uterus by transferring frozen-thawed embryos in a subsequent natural cycle. This suggestion is, to our knowledge, the first time this possibility was ever raised, and it remains pertinent to discussions in clinical IVF some 38 years later (Cohen and Alikani, 2013a). However, the failure of this approach contributed to their next crucial decision taken in November 1977: to avoid the use of drugs completely and to attempt to collect single pre-ovulatory eggs in natural cycles monitored for the LH surge (Edwards and Steptoe, 1980, p.132-135) – no mean task at that time, as the shocked staff realised when told of the decision (NF, p.38, in Johnson and Elder, 2015a, Suppl. Material 1). By the time that the decision to use monitored natural cycles was implemented, 437 LOR (84% of total) and 79 embryo transfers (71% of total) had already been performed.
In conclusion, it is important to re-emphasize just how little was known at the time about the areas of study in which they were engaged, and how limited was their access to helpful technology. What shines through is their persistence in the face of constant failure. Whilst in no sense could their approach be described as a double-blind controlled trial, it was an empirical study, albeit soundly based on a thorough reading and understanding of the literature – and as such does not differ fundamentally from most comparable research even today (Cohen and Alikani, 2013b). The persistence of both their entire team and their patients was based on trying to climb this steep ‘learning curve’ by adapting and reacting to what was being learned. In the next paper, we enquire more closely into the roles played by their patients in this process.
The following are the supplementary related to this article.
Media compositions settled on by June 1976.
Acknowledgements
The research was supported by grants from the Wellcome Trust (088708, 094985 and 100606), which otherwise had no involvement in the research or its publication.
Biography

Kay Elder joined Bourn Hall in 1984 as Clinical Assistant to Patrick Steptoe, directing the Out-Patient Department from 1985 to 1987. Her scientific background as a research scientist at Imperial Cancer Research Fund prior to a medical degree at Cambridge University naturally led her to Bob Edwards and the IVF laboratory, where she worked as a senior embryologist from 1987. A programme of Continuing Education for IVF doctors, scientists and nurses at Bourn Hall was established in 1989, which she directed for 16 years. During this period she also helped to set up and run two Master’s degree programmes in Clinical Embryology, and she continues to mentor and tutor postgraduate students of Clinical Embryology at the University of Leeds. In her current role as Senior Research Scientist at Bourn Hall she co-ordinates research collaborations with the MRC Laboratory of Molecular Biology in Cambridge and the MRC National Institute for Medical Research in Mill Hill.
Contributor Information
Kay Elder, Email: kay.elder@bourn-hall.com.
Martin H. Johnson, Email: mhj21@cam.ac.uk.
References
- Abrahams G.E., Odell W.D., Edwards R.G., Purdy J.M. Solid-phase radioimmunoassay of estrogens in biological fluids. In: Diczfausy E., A., editors. Steroid assay by protein binding. Karolinska Institute; Stockholm: 1970. [Google Scholar]
- Beier H. Oviductal and uterine fluids. J. Reprod. Fertil. 1974;37:221–237. doi: 10.1530/jrf.0.0370221. [DOI] [PubMed] [Google Scholar]
- Brinster R.L. A method for in vitro culture of mouse ova from two-cell to blastocyst. Exp. Cell Res. 1963;32:205–208. doi: 10.1016/0014-4827(63)90093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Alikani M. The time has come to radically rethink assisted reproduction. Reprod. BioMed. Online. 2013;27:323–324. doi: 10.1016/j.rbmo.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Cohen J., Alikani M. Evidence-based medicine and its application in clinical preimplantation embryology. Reprod. BioMed. Online. 2013;27:547–561. doi: 10.1016/j.rbmo.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Croxatto H.B., Diaz S., Fuentealba B., Croxatto H.D., Carrillo D., Fabres C. Studies on the duration of egg transport in the human oviduct. I. The time interval between ovulation and egg recovery from the uterus in normal women. Fertil. Steril. 1972;23:447–458. doi: 10.1016/s0015-0282(16)39069-0. [DOI] [PubMed] [Google Scholar]
- Edwards R.G. Maturation in vitro of human ovarian oocytes. Lancet. 1965;ii:926. doi: 10.1016/s0140-6736(65)92903-x. [DOI] [PubMed] [Google Scholar]
- Edwards R.G. Immunological aspects of infertility. Proc. R Soc. Med. 1968;62:25–26. [PMC free article] [PubMed] [Google Scholar]
- Edwards R.G. Transmission of antibodies across membranes of the reproductive tracts. In: Edwards R.G., editor. Proc. Symp. Int. Coordination Committee for the Immunology of Reproduction, Geneva. International Planned Parenthood Federation; London: 1968. pp. 28–48. (Immunology and Reproduction). [Google Scholar]
- Edwards R.G. Immunological influences. In: Austin C.R., Short R.V., editors. Reproductive Patterns. Vol. 4. Cambridge University Press; Cambridge: 1972. pp. 94–127. (Reproduction in Mammals). [Google Scholar]
- Edwards R.G. Current concepts of the growth of ovarian follicles. Int. Planned Parenthood Fed. Med. Bull. 1973;7(4) [PubMed] [Google Scholar]
- Edwards R.G. Follicular fluid. J. Reprod. Fertil. 1974;37:189–219. doi: 10.1530/jrf.0.0370189. [DOI] [PubMed] [Google Scholar]
- Edwards R.G., Gardner R.L. Control of the sex ratio at full term in the rabbit by transferring sexed blastocysts. Nature. 1968;218:346–348. doi: 10.1038/218346a0. [DOI] [PubMed] [Google Scholar]
- Edwards R.G., Steptoe P.C. Control of human ovulation, fertilization and implantation. Proc. Roy. Soc. Med. 1974;67:932–936. [PMC free article] [PubMed] [Google Scholar]
- Edwards R.G., Steptoe P.C. Induction of follicular growth, ovulation and luteinization in the human ovary. J. Reprod. Fertil. 1975;22:121–163. (Supplement) [PubMed] [Google Scholar]
- Edwards R.G., Steptoe P.C. The Freezing of Mammalian Embryos. Vol. 52. Elsevier, Excerpta Medica; North Holland: 1977. The relevance of the frozen storage of human embryos; pp. 235–250. (Ciba Foundation Symposium). [DOI] [PubMed] [Google Scholar]
- Edwards R.G., Steptoe P.C. The Story of IVF - a Medical Breakthrough; Hutchinson, London: 1980. A Matter of Life. [Google Scholar]
- Edwards R.G., Bavister B.D., Steptoe P.C. Early stages of fertilization in vitro of human oocytes matured in vitro. Nature. 1969;221:632–635. doi: 10.1038/221632a0. [DOI] [PubMed] [Google Scholar]
- Edwards R.G., Steptoe P.C., Purdy J.M. Fertilization and cleavage in vitro of preovulatory human oocytes. Nature. 1970;227:1307–1309. doi: 10.1038/2271307a0. [DOI] [PubMed] [Google Scholar]
- Edwards R.G., Steptoe P.C., Abraham G.E., Walters E., Purdy J.M., Fotherby K. Steroid assays and preovulatory follicular development in human ovaries primed with gonadotrophins. Lancet. 1972;ii:611–615. doi: 10.1016/s0140-6736(72)93013-9. [DOI] [PubMed] [Google Scholar]
- Edwards R.G., Fowler R.E., Gore-Langton R.E., Gosden R.G., Jones E.C., Readhead C., Steptoe P.C. Normal and abnormal follicular growth in mouse, rat and human ovaries. J. Reprod. Fertil. 1977;51:237–263. doi: 10.1530/jrf.0.0510237. [DOI] [PubMed] [Google Scholar]
- Edwards R.G., Steptoe P.C., Purdy J.M. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J. Obstet. Gynaecol. 1980;87:737–756. doi: 10.1111/j.1471-0528.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- Edwards R.G., Steptoe P.C., Fowler R.E., Baillie J. Observations on preovulatory human oocytes and their aspirates. Br. J. Obstet. Gynaecol. 1980;87:769–779. doi: 10.1111/j.1471-0528.1980.tb04612.x. [DOI] [PubMed] [Google Scholar]
- Elder K., Johnson M.H. The Oldham Notebooks: an analysis of the development of IVF 1969–1978. I. Introduction, Materials and Methods. Reprod. BioMed. Soc. 2015;1:3–8. doi: 10.1016/j.rbms.2015.04.001. (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder K., Johnson M.H. The Oldham Notebooks: an analysis of the development of IVF 1969-1978. II. The treatment cycles and their outcomes. Reprod. BioMed. Soc. 2015;1:9–18. doi: 10.1016/j.rbms.2015.04.003. (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler R.E., Chan S.T.H., Walters D.E., Edwards R.G., Steptoe P.C. Steroidogenesis in human follicles approaching ovulation as judged from assays of follicular fluid. J. Endocrinol. 1977;72:259–271. doi: 10.1677/joe.0.0720259. [DOI] [PubMed] [Google Scholar]
- Fowler R.E., Edwards R.G., Walters D.E., Chan S.T.H., Steptoe P.C. Steroidogenesis in preovulatory follicles of patients given human menopausal and chorionic gonadotraphins as judged by the radioimmunoassay of steroids in follicular fluid. J. Endocrinol. 1978;77:161–169. doi: 10.1677/joe.0.0770161. [DOI] [PubMed] [Google Scholar]
- Fowler R.E., Fox N.L., Edwards R.G., Walters D.E., Steptoe P.C. Steroidogenesis by cultured granulosa cells aspirated from human follicles using pregnenolone and androgens as precursors. J. Endocrinol. 1978;77:171–183. doi: 10.1677/joe.0.0770171. [DOI] [PubMed] [Google Scholar]
- Fowler R.E., Fox N.L., Edwards R.G., Steptoe P.C. Steroid production from 17*-hydroxypregnenolone and dehydroepiandrosterone by human granulosa cells in vitro. J. Reprod. Fertil. 1978;54:109–117. doi: 10.1530/jrf.0.0540109. [DOI] [PubMed] [Google Scholar]
- Franklin S. Conception through a looking glass: the paradox of IVF. Reprod. BioMed. Online. 2013;27:747–755. doi: 10.1016/j.rbmo.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Gardner R.L., Johnson M.H., Edwards R.G. Are H-2 antigens expressed in the preimplantation blastocyst? In: Bratanov K., Edwards R.G., Vulchanov V.H., Dikov V., Somlev B., editors. Bulgarian Academy of Sciences Press; Sofia: 1973. pp. 480–486. (Immunology of Reproduction). [Google Scholar]
- Green M., Bass S., Spear B. A device for the simple and rapid transcervical transfer of mouse embryos eliminates the need for surgery and potential post-operative complications. Biotechniques. 2009;7:919–924. doi: 10.2144/000113257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.H. Robert Edwards: the path to IVF. Reprod. BioMed. Online. 2011;23:245–262. doi: 10.1016/j.rbmo.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.H., Elder K. The Oldham Notebooks: an analysis of the development of IVF 1969-1978. IV. Ethical aspects. Reprod. BioMed. Soc. 2015;1:34–45. doi: 10.1016/j.rbms.2015.04.002. (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.H., Elder K. The Oldham Notebooks: an analysis of the development of IVF 1969-1978. V. The role of Jean Purdy reassessed. Reprod. BioMed. Soc. 2015:46–57. doi: 10.1016/j.rbms.2015.04.005. (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston J.H., Penn R., Sivelle P.C. Successful autotransfer of tubal eggs in the rhesus monkey (Macaca mulatta) J. Reprod. Fertil. 1977;49:175–176. doi: 10.1530/jrf.0.0490175. [DOI] [PubMed] [Google Scholar]
- Melander Y. Chromosomal behaviour during the origin of sex chromatin in the rabbit. Hereditas. 1962;48:645–661. [Google Scholar]
- Pearson P.L., Bobrow M. Technique for identifying Y chromosomes in human interphase nuclei. Nature. 1970;226:78–80. doi: 10.1038/226078a0. [DOI] [PubMed] [Google Scholar]
- Polge C. Lionel Edward Aston Rowson, O.B.E. 28 May 1914–26 July 1989. Biogr. Membr. Fell. R Soc. 2000:483–497. [Google Scholar]
- RGE1 HiGonavis results for L670–675, January 1978. Also, but undated, “Data for Fig. 24” – medication, TE, PD, HCG, oral Primolut, Depot primolut, Ethinyl Estradiol. Patient Matter. 1978 Folder 9. [Google Scholar]
- RGE2 Hand-written notes on the histopathology reports on 3 1976 pre-clinical pregnancies. Patient Matter. 1976 Folder 11. [Google Scholar]
- RGE3, undated. Various media formulas/recipes including “Japanese Nosh” (JP’s hand, undated), Tyrode, Barry’s, Tyr B + a scrap of paper re communication with John Paul: “J Paul: 10% variation not much difference” (undated). Data/Journals/Papers Folder 5.
- RGE4 Correspondence: with Flow Laboratories on 21st November 1970 - a query re adding glutamine and a reply on 23rd November 1970 suggesting that they add 200 mM glutamine to F10 and F12, 0.5 ml/100 mls; with BDH on 3rd December 1970 for information re gelatin powder; with Millipore on 10th December 1970 re frothing/XS wetting agents, plus a reply 1st February 1971 - wetting-agent-free filters sent, further enquiry on 9th March 1971; from JP with Hoechst on 16th December 1970 saying that use of disc gel electrophoresis had demonstrated an extra line in the albumin batch and asking what it was plus a reply on 15th January 1971 saying was 100% pure albumin, and a request on 8th March 1971 requesting information re batch of Albumin A602, which was sent for analysis. Data/Journals/Papers. 1970 Folder 5. [Google Scholar]
- RGE5 . Loose sheets found in note book L0. 1969. 39 hormone reports from G.D. Searle Scientific Services (dated between 10-11/12/1969 and 9-10/3/1970. [Google Scholar]
- RGE6 ODGH results for 1975 in an envelope with a note “please file in patient notes” – someone apparently failed to do so. Patient Matter. 1975 Folder 11. [Google Scholar]
- Schneider H.J., Castleberry R.S., Griffin J.L. Commercial aspects of bovine embryo transfer. Theriogenology. 1980;13:73–85. doi: 10.1016/0093-691x(80)90016-3. [DOI] [PubMed] [Google Scholar]
- Seidel G.E., Bowen J.M., Homan N.R., Okun N.E. Fertility of heifers with sham embryo transfer through cervix. Vet. Rec. 1975;97:307–308. doi: 10.1136/vr.97.16.307. [DOI] [PubMed] [Google Scholar]
- Steptoe P.C., Edwards R.G. Laparoscopic recovery of preovulatory human oocytes after priming of ovaries with gonadotrophins. Lancet. 1970;i:683–689. doi: 10.1016/s0140-6736(70)90923-2. [DOI] [PubMed] [Google Scholar]
- Steptoe P.C., Edwards R.G. Reimplantation of a human embryo with subsequent tubal pregnancy. Lancet. 1976;i:880–882. doi: 10.1016/s0140-6736(76)92096-1. [DOI] [PubMed] [Google Scholar]
- Steptoe P.C., Edwards R.G., Purdy J.M. Human blastocysts grown in culture. Nature. 1971;229:132–133. doi: 10.1038/229132a0. [DOI] [PubMed] [Google Scholar]
- Steptoe P.C., Edwards R.G., Walters D.E. Observations on 767 clinical pregnancies and 500 births afterhuman in-vitro fertilization. Hum. Reprod. 1986;1:89–94. doi: 10.1093/oxfordjournals.humrep.a136366. [DOI] [PubMed] [Google Scholar]
- YoungLai E.V., Edwards R.G., Steptoe P.C. The enzymatic activity of aspirates of pre-ovulatory human follicles. Can. J. Biochem. 1972;50:233–236. doi: 10.1139/o72-032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Media compositions settled on by June 1976.