Summary
Interleukin‐33 (IL‐33) induces T helper type 2 (Th2) cytokine production and eosinophilia independently of acquired immunity, leading to innate immunity‐mediated allergic inflammation. Allergy‐related innate myeloid cells such as eosinophils, basophils and mast cells express the IL‐33 receptor (IL‐33R), but it is still unknown how IL‐33 regulates allergic inflammation involving these cells and their progenitors. Here, we revealed that the functional IL‐33R was expressed on eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs). In the presence of IL‐33, these progenitors did not expand, but produced a high amount of Th2 and pro‐inflammatory cytokines such as IL‐9, IL‐13, IL‐1β and IL‐6. The amount of cytokines produced by these progenitors was greater than that by mature cells. In vivo, IL‐33 stimulated the expansion of EoPs, but it was dependent upon the elevated serum IL‐5 that is presumably derived from type 2 innate lymphoid cells that express functional IL‐33R. These data collectively suggest that EoPs, BaPs and MCPs are not only the sources of allergy‐related granulocytes, but can also be sources of allergy‐related cytokines in IL‐33‐induced inflammation. Because such progenitors can differentiate into mature granulocytes at the site of inflammation, they are potential therapeutic targets in IL‐33‐related allergic diseases.
Keywords: basophils, cytokines, eosinophils, haematopoiesis, mast cells
Introduction
Interleukin‐33 (IL‐33), a member of the IL‐1 family cytokines, is involved in the pathogenesis of allergic diseases.1 Various experimental models have demonstrated the critical roles of IL‐33 in the induction of allergic inflammation such as asthma,2 allergic rhinitis3 and peanut allergy.4 In humans, increased expression of IL‐33 was observed in pathological specimens isolated from patients with asthma5 and allergic conjunctivitis.6 Furthermore, the IL‐33 and IL‐33 receptor (IL‐33R) genes were identified as disease‐susceptibility genes for asthma.7, 8
IL‐33 induces allergic inflammation through T helper type 2 (Th2) cytokine production and eosinophilia. In mice treated with IL‐33, the serum concentration of Th2 cytokines was elevated.9 A wide variety of cells, including eosinophils,10 basophils,11 mast cells,9, 12 dendritic cells,13 Th2 cells,9 natural killer (NK) cells,14, 15 NKT cells,14, 15 B1 B cells16 and recently identified type 2 innate lymphoid cells (ILC2s),17, 18 express the IL‐33R, and it was reported that almost all of these cells have the potential to produce Th2 cytokines in vitro in response to IL‐33. Following IL‐33‐induced Th2 cytokine production, the number of eosinophils in the spleen and the peripheral blood increased in IL‐33‐treated mice, which was accompanied by the elevation of serum IL‐5.9 IL‐33‐induced eosinophilia did not occur in IL‐5−/− mice, indicating that this response is dependent upon IL‐5.19
It is of note that IL‐33 induces allergic inflammation independently of acquired immunity. In a parasite infection model, Th2 cytokine production was induced before the establishment of parasite‐specific acquired immunity,20, 21 suggesting the existence of an alternative Th2 pathway that does not depend on acquired immunity. Because IL‐33 is released from epithelial and endothelial cells in response to protease allergens, lipopolysaccharide, or tissue injury, and directly stimulates IL‐33R‐expressing cells, including innate immune cells, to produce Th2 cytokines, IL‐33‐induced Th2 inflammation does not require acquired immunity.22 Moreover, in vivo administration of IL‐33 induces asthmatic airway inflammation in RAG‐2−/− mice23 that lack mature T cells and B cells. Among IL‐33‐responsive cells, eosinophils, basophils and mast cells, as well as ILC2s, can differentiate without RAG gene expression and constitutively express IL‐33R, suggesting that these myeloid cells may play critical roles in IL‐33‐induced innate‐type allergic inflammation, similar to ILC2s.
Recently, it has been indicated that not only mature myeloid cells but also myeloid progenitors may be involved in the pathogenesis of allergic inflammation. It was reported that CD34+ haematopoietic progenitors secrete Th2 cytokines in response to IL‐33.24 Myeloid progenitors exist systemically,24, 25, 26 and notably the number of myeloid progenitors correlates with the disease activity of asthma.27, 28 These results indicate that myeloid progenitors might act as effector cells in allergic inflammation. However, it is not yet clarified which myeloid progenitors express IL‐33R and how IL‐33 regulates allergic inflammation involving mature myeloid cells and myeloid progenitors.
In this study, we reveal that the functional IL‐33R was expressed on eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs) in the haematopoietic stem/progenitor fraction. IL‐33 induced Th2 and pro‐inflammatory cytokine production by these progenitors. The level of cytokine production of these progenitors was greater than that of mature cells. In vivo, IL‐33 induced specific expansion of EoPs via IL‐5, presumably produced by ILC2s. Collectively, these data suggest that EoPs, BaPs and MCPs not only act as the sources of mature granulocytes, but can also secrete a high amount of allergy‐related cytokines in IL‐33‐induced inflammation.
Materials and methods
Mice
The C57BL/6J mice used in this experiment were purchased from SLC Japan, Inc., Shizuoka, Japan. All mice used in this study were bred and maintained in the Centre of Biomedical Research, Research Centre for Human Disease Modelling, Graduate School of Medical Sciences, Kyushu University. All animal experiments in this study were approved by the Animal Care and Use Committee, Kyushu University.
Antibodies, cell labelling and sorting
Haematopoietic stem/progenitor cells, mature leucocytes and ILC2s were isolated as previously described17, 18, 29, 30, 31, 32 with some modification. The fractions of haematopoietic cells used in this study are listed in the Supplementary material (Table S1). Antibodies used in this experiment are as follows: CD3 (17A2), CD4 (GK1.5), CD8 (53‐6.7), CD11c (N418), CD19 (6D5), CD34 (RAM34), CD43 (S7), B220 (RA3‐6B2), IL‐5Rα (CD125, T21), IL‐7Rα (CD127, A7R34), Sca‐1 (D7), c‐Kit (2B8), FcγRII/III (93), FcεRIα (MAR‐1), Gr‐1 (RB6‐8C5), CCR3 (83101), DX5 (DX5), NK1.1 (PK136) and IgM (II/41). These antibodies were purchased from BD Biosciences (San Jose, CA), BioLegend (San Diego, CA), Affymetrix (Santa Clara, CA) or R&D Systems (Minneapolis, MN). For the analysis of ST2 expression, cells were stained with biotinylated anti‐mouse ST2 antibody (DJ8, MD Bioproducts, St Paul, MN), followed by staining with phycoerythrin/Cy7‐conjugated streptavidin (BioLegend). Dead cells were excluded by propidium iodide staining. Cells were analysed and double‐sorted with a FACSAria III (BD Biosciences). The purity of double‐sorted cells was over 98%. Flowjo software version 7.5.2. (Tree Star Inc., Ashland, OR) was used for the analysis of flow cytometric data.
Cell culture
Cells were cultured in Iscove's modified Dulbecco's medium supplemented with 20% fetal bovine serum (StemCell Technologies, Vancouver, BC, Canada), 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies, Carlsbad, CA). Cell cultures were performed in the presence of various cytokine combinations, such as stem cell factor (1 ng/ml), IL‐3 (1 or 2 ng/ml), IL‐5 (2 ng/ml), IL‐11 (1 ng/ml), granulocyte–macrophage colony‐stimulating factor (GM‐CSF; 1 ng/ml), thrombopoietin (1 ng/ml), human erythropoietin (0·5 U/ml) and IL‐33 (50 ng/ml). These cytokines were purchased from R&D Systems. All cell cultures were conducted at 37° in a humidified incubator under 5% CO2.
In vivo administration of IL‐33 and anti‐IL‐5 antibody
Phosphate‐buffered saline or 0·5 μg of IL‐33 was injected intraperitoneally into mice for seven consecutive days. Rat IgG1 or anti‐mouse IL‐5 antibody (BioLegend; 50 μg per mouse) was simultaneously injected. Mice were killed after 24 hr of the last injection, and specimens, including the bone marrow, spleen, mesenteric lymph node, peripheral blood and peritoneal lavage fluid, were recovered. White blood cells in the bone marrow (per hindleg), spleen, peritoneal cavity and peripheral blood were counted with Celltac α (Nihon Kohden, Shinjuku, Tokyo, Japan). Serum samples were recovered by the centrifugation (600 g, 10 min) of peripheral blood.
Multiplex cytokine analysis
The cytokine concentration in the culture supernatants or serum samples was determined with BioPlex Pro Mouse Cytokine Grp I Panel 23‐Plex (Bio‐Rad, Hercules, CA). Multiplex cytokine analysis was conducted with LABScan 100 (Luminex, Austin, TX), and the data were analysed with bio‐plex manager software ver 6.1 (Bio‐Rad). Ten thousand myeloid progenitor cells were cultured in the presence of IL‐3 (1 ng/ml) with or without IL‐33 (50 ng/ml). The culture supernatants were recovered on day 3.
RNA extraction and quantitative real‐time PCR
Total RNA was extracted with TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA) following the manufacturer's protocol, and subjected to reverse transcription with a Quantitect Reverse Transcription Kit (Qiagen, Hilden, Germany). Quantitative real‐time PCR was conducted with a 7500 Real Time PCR System (Applied Biosystems, Foster, CA). TaqMan probe of Il5 (IL‐5: Mm00439646_m1) was purchased from Life Technologies. 18S rRNA was used as an internal control. The sequence of 18S rRNA TaqMan probe is described as follows: the forward primer is 5′‐AGTCCCTGCCCTTTGTACACA‐3′, the reverse primer is 5′‐GATCCGAGGGCCTCACTAAAC‐3′, and the probe is 5′‐(FAM)‐CGCCCGTCGCTACTACCGATTGG‐(TAMRA)‐3′. The relative expression of Il5 was calculated by the ΔΔCt method.
Microarray analysis
Total mRNA purified from myeloid progenitors by TRIzol Reagent was amplified and biotinylated with the TotalPrep RNA Amplification Kit (Illumina, San Diego, CA). Biotinylated cRNA was hybridized to the bead chip at 58° for 16 hr in a humidified chamber. Hybridized chips were stained with Cy3‐conjugated streptavidin and scanned with a BeadArray Reader (Illumina). The microarray data were analysed with genespring GX software ver13.0 (Agilent Technologies, Santa Clara, CA). Gene sets used in this study were downloaded from the Molecular Signature Database of GSEA (Broad Institute, Cambridge, MA) and the standard name is shown as follows: KEGG_CYTOKINE_CYTOKINE_RECEPTOR_INTERACTION (cytokines, chemokines and the receptors), KEGG_CELL_ADHESION_MOLECULES_CAMS (adhesion molecules), KEGG_CELL_CYCLE (cell cycle), KEGG_APOPTOSIS (apoptosis). The microarray data are shown in the Gene Expression Omnibus (GEO). The GEO accession number is GSE76385.
Statistical analysis
Student's t‐test was used for the evaluation of statistical significance. A P‐value of P < 0·05 was considered to indicate a statistically significant difference.
Results
IL‐33R is expressed on EoPs, BaPs, MCPs and MEPs in haematopoietic stem/progenitor cells
IL‐33R is composed of a heterodimer of ST2 and IL‐1 receptor accessory protein (IL‐1RAcP).1, 33 To investigate when the expression of IL‐33R occurs during myeloid development, we first analysed ST2 expression on myeloid cells, including progenitors. Consistent with previous reports,9, 10, 11, 12 mature eosinophils, basophils and mast cells expressed ST2 (Fig. 1). Their lineage‐committed progenitor cells, EoPs, BaPs and MCPs, also expressed ST2 (Fig. 1). Granulocyte/monocyte progenitors, the progenitors that can differentiate into EoPs, BaPs and MCPs, did not express ST2 (Fig. 1), indicating that ST2 expression occurs after commitment to the eosinophil, basophil and mast cell lineages.
Figure 1.
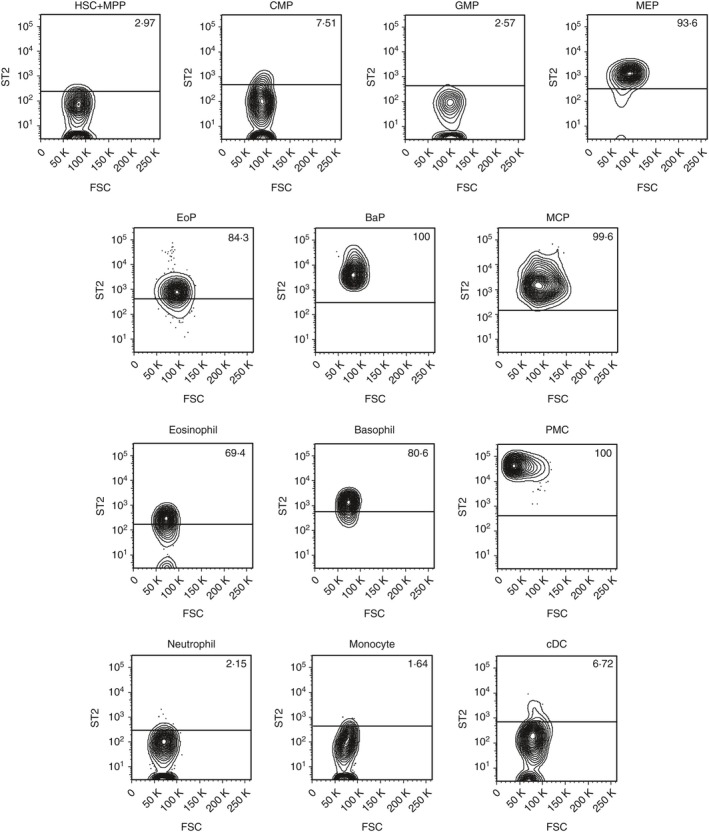
ST2 is expressed on EoPs, BaPs, MCPs and MEPs in mouse haematopoietic stem/progenitor cells. ST2 expression on mouse haematopoietic stem cells and myeloid lineage cells. Representative data of three independent experiments are shown. HSC, haematopoietic stem cell; MPP, multipotent progenitor; CMP, common myeloid progenitor; GMP, granulocyte/monocyte progenitor; MEP, megakaryocyte/erythrocyte progenitor; EoP, eosinophil progenitor; BaP, basophil progenitor; MCP, mast cell progenitor; PMC, peritoneal mast cell; cDC, conventional dendritic cell.
Megakaryocyte/erythrocyte progenitors (MEPs) also expressed ST2 (Fig. 1). There was no homogeneous expression of ST2 in lymphoid lineage cells in the steady state (see Supplementary material, Fig. S1a), though the possibility remains that a sub‐fraction of each population might express ST2. Outside haematopoietic tissues, ILC2s purified from the mesenteric lymph node expressed ST2 (see Supplementary material, Fig. S1b) as previously reported.17, 18
All of these ST2‐expressing cells also expressed IL‐1RAcP mRNA, the other component of the IL‐33R (see Supplementary material, Fig. S2), suggesting that they possess a functional IL‐33R.
IL‐33 induces the gene expression of cytokines and chemokines associated with allergic inflammation in EoPs, BaPs and MCPs
To clarify the effect of IL‐33 on myeloid progenitors, we evaluated their gene expression profiles after IL‐33 stimulation in vitro (Fig. 2). To this aim, EoPs, BaPs and MCPs were doubly sorted with a purity of over 98%. The representative plots of EoPs, BaPs and MCPs are shown in the Supplementary material (Fig. S3). EoPs, BaPs and MCPs showed immature morphology and differentiated into eosinophils, basophils and mast cells, respectively (see Supplementary material, Fig. S4a).
Figure 2.
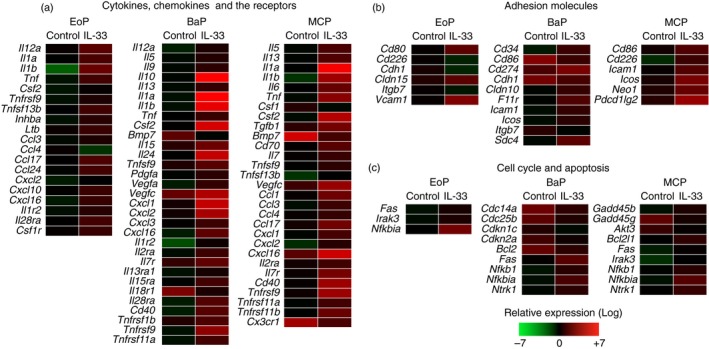
Interleukin‐33 (IL‐33) induces the expression of genes related to cytokines and chemokines in eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs). A total of 104 cells of EoPs, BaPs or MCPs were cultured in the presence of IL‐3 (1 ng/ml) with or without IL‐33 (50 ng/ml) for 48 hr. The gene expression of (a) cytokines, chemokines and their receptors, (b) adhesion molecules, (c) cell cycle and apoptosis that showed over twofold change are shown. The scale of gene expression signals is shown in the figure.
The purified myeloid progenitors were cultured with or without IL‐33, and then microarray analysis was conducted. Genes that showed over two‐fold change in the expression level were selected for analysis. Among these genes, we focused on genes that were related to effector functions (cytokines, chemokines and adhesion molecules), cell cycle and apoptosis listed in the gene sets as described in the Materials and methods.
Among cytokine genes (Fig. 2a), BaPs and MCPs expressed Th2 cytokines such as Il9 (IL‐9), Il10 (IL‐10) and Il13 (IL‐13), and to a lesser extent so did EoPs (see the raw data shown in GEO as described in the Materials and methods). Interestingly, these myeloid progenitors also expressed a series of pro‐inflammatory cytokines including Il1a (IL‐1α), Il1b (IL‐1β), Il6 (IL‐6), Tnf (tumour necrosis factor‐α) and Csf2 (GM‐CSF). In contrast, neither Th1 cytokines such as Ifng (interferon‐γ) and Il12b (IL‐12p40) nor Th17 cytokines such as Il17a (IL‐17A) and Il17f (IL‐17F) were expressed by these progenitors (see the raw data shown in GEO as described in the Materials and methods). Among the Th2 cytokines, the expression of Il4 (IL‐4) was not induced in these myeloid progenitors (see Supplementary material, Fig. S5a).
As for chemoattractant genes, these myeloid progenitors expressed multiple chemokines including Ccl3 (CCL3), Ccl17 (CCL17), Cxcl1 (CXCL1), Cxcl2 (CXCL2) and Cxcl16 (CXCL16) (Fig. 2a). These chemokines mediate the migration of inflammatory leucocytes including T cells, monocytes, neutrophils and eosinophils that play an important role in the pathogenesis of allergic diseases.34, 35 The gene expression of inflammatory mediators released from allergy‐related granulocytes such as major basic protein, eosinophil cationic proteins (eosinophil‐associated ribonucleases) and histamine decarboxylase was not induced (see the raw data shown in GEO as described in the Materials and methods).
In terms of cell‐cycle‐related and apoptosis‐related genes (Fig. 2c), pro‐apoptotic genes such as Fas (Fas) or Nfkbia (IκBα) were up‐regulated in these myeloid progenitors after IL‐33 stimulation. In contrast, the gene expression of Cdkn1c (p57) and Cdkn2a (p16), the negative regulators of the cell cycle, was down‐regulated in BaPs stimulated with IL‐33. In MCPs, the expression of positive regulators of cell survival such as Bcl2l1 (Bcl‐xL) was induced, while the expression of Akt3 (Akt3), another positive regulator, was suppressed.
Because MEPs also expressed functional IL‐33R (Fig. 1 and see Supplementary material, Fig. S2a), we examined the effect of IL‐33 on MEPs. IL‐33 induced the expression of only a few genes related to cytokine receptors, adhesion molecules, cell cycle or apoptosis (see Supplementary material, Fig. S6a).
EoPs, BaPs and MCPs produce a higher amount of Th2 and pro‐inflammatory cytokines than mature cells in response to IL‐33
Based on our microarray data (Fig. 2a), we next investigated the cytokine production potential of these myeloid progenitors. IL‐33 induced Th2 cytokine expression, including IL‐9, IL‐10 and IL‐13, by EoPs, BaPs and MCPs both at the mRNA level (see Supplementary material, Fig. S7) and the protein level (Fig. 3). IL‐5 mRNA was up‐regulated in MCPs (see Supplementary material, Fig. S7), but IL‐5 protein production was not significantly induced (Fig. 3c). IL‐33 did not induce the production of IL‐4 in EoPs or BaPs (see Supplementary material, Fig. S5b). A slight increase of IL‐4 production in MCPs in response to IL‐33 was observed, but the amount was < 30 pg/ml (see Supplementary material, Fig. S5b). The pro‐inflammatory cytokines IL‐1α, IL‐1β, IL‐6, TNF‐α and GM‐CSF were also produced by EoPs, BaPs and MCPs (Figs 3 and see Supplementary material, Fig. S7). Surprisingly, for most of these cytokines, myeloid progenitors produced a greater amount of cytokines than mature cells (Fig. 3). These data suggest that lineage‐committed myeloid progenitors might possess sufficient potential to induce and exacerbate both Th2‐type and non‐Th2‐type inflammation in response to IL‐33.
Figure 3.
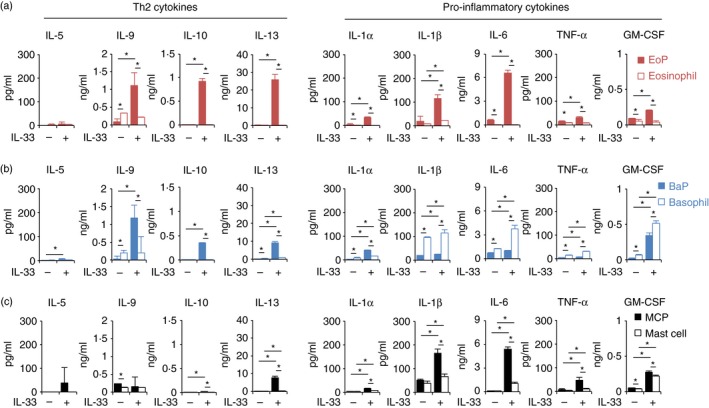
Eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs) possess a greater potential for cytokine production than mature cells in response to interleukin‐33 (IL‐33). A total of 104 cells of (a) EoPs, (b) BaPs or (c) MCPs were cultured in the presence of IL‐3 (1 ng/ml) with or without IL‐33 (50 ng/ml). The culture supernatants were recovered on day 3 and the cytokine concentrations were determined by multiplex cytokine analysis. Data are shown as mean ± standard deviation of three independent experiments. *P < 0·05.
IL‐33 does not induce the proliferation of EoPs, BaPs or MCPs in vitro
Next, we examined the effect of IL‐33 on the proliferation of EoPs, BaPs and MCPs. IL‐33 did not induce the proliferation of EoPs, but IL‐5 did (Fig. 4a). Also, IL‐33 did not promote the proliferation of BaPs or MCPs, although IL‐3 did (Fig. 4b,c). Hence, IL‐33 does not possess the potential to expand these myeloid progenitors in vitro.
Figure 4.

Interleukin‐33 (IL‐33) does not induce the proliferation of eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs) in vitro. A total of 104 cells of (a) EoPs, (b) BaPs or (c) MCPs were cultured with or without IL‐3 (2 ng/ml) or IL‐5 (2 ng/ml) or IL‐33 (50 ng/ml). The cell numbers were counted on day 2 and day 4 microscopically. Data are shown as the mean ± standard deviation of three independent experiments. *P < 0·05.
To evaluate whether IL‐33 could induce the maturation of EoPs, BaPs and MCPs, we conducted a short‐term culture and investigated cell morphology. These myeloid progenitors retained immature morphology after 3 days of culture (see Supplementary material, Fig. S4b left) compared with mature cells (see Supplementary material, Fig. S4a right). IL‐33 did not change the immature morphology (see Supplementary material, Fig. S4b right), indicating that IL‐33 does not induce the maturation of EoPs, BaPs or MCPs.
In terms of MEPs, IL‐33 did not affect the proliferation of MEPs (see Supplementary material, Fig. S6b) or the erythrocyte/megakaryocyte lineage determination downstream of MEPs (see Supplementary material, Fig. S6c).
IL‐33 induces the expansion of EoPs followed by eosinophilia in vivo
As for IL‐33‐induced eosinophilia, it has been shown that IL‐33 increases the number of eosinophils in the periphery.9 However, it still remains unclear which cells are the initial targets of IL‐33, resulting in eosinophilia. To clarify this, we injected IL‐33 into mice for seven consecutive days and analysed the number of both mature myeloid cells and myeloid progenitors. Consistent with a previous report,9 eosinophils in the spleen dramatically expanded by 20‐fold in response to IL‐33 treatment (Fig. 5a). In addition, a slight increase of monocytes, neutrophils and basophils was also observed (Fig. 5a). There was no significant difference in the number of peritoneal mast cells in IL‐33‐injected mice (Fig. 5b).
Figure 5.
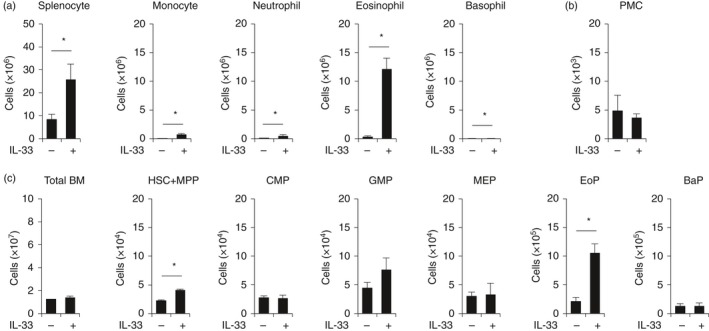
Interleukin‐33 (IL‐33) induces the specific expansion of eosinophil progenitors (EoPs) in vivo. IL‐33 (0·5 μg/mouse) was injected into mice on seven consecutive days. Total cell number of each cell population in the (a) spleen, (b) peritoneal cavity and (c) bone marrow was determined. Data are shown as the mean ± standard deviation (n = 3). *P < 0·05.
In terms of myeloid progenitors in the bone marrow, a five‐fold expansion of EoPs was observed in mice treated with IL‐33 (Fig. 5c). In contrast, there was no significant difference in the number of common myeloid progenitors, granulocyte/monocyte progenitors, MEPs or BaPs (Fig. 5c). These data suggest that in vivo administration of IL‐33 specifically induced the expansion of EoPs, resulting in eosinophil lineage‐specific expansion.
IL‐5 is indispensable for IL‐33‐induced EoP expansion in vivo
Because IL‐33 did not directly induce the proliferation of EoPs (Fig. 4a), we thought that the specific expansion of EoPs in IL‐33‐treated mice was induced indirectly by other cytokines. Therefore, we next evaluated the serum concentration of cytokines related to eosinophil differentiation in IL‐33‐treated mice. The level of serum IL‐5 was specifically increased (Fig. 6a). Intraperitoneal administration of anti‐IL‐5 antibodies abolished the IL‐33‐induced expansion of EoPs and eosinophils (Fig. 6b), suggesting that the proliferation of EoPs is mediated by IL‐5. This IL‐5 dependency is the same as that observed for mature eosinophils.9, 19 To clarify the origin of serum IL‐5, we purified IL‐33R‐expressing cells from IL‐33‐treated mice and evaluated the expression level of IL‐5 mRNA. The significant expression of IL‐5 mRNA was observed in ILC2s (Fig. 6c). Collectively, IL‐33 induces specific expansion of EoPs through IL‐5 presumably produced by ILC2s, leading to eosinophilic inflammation in vivo.
Figure 6.
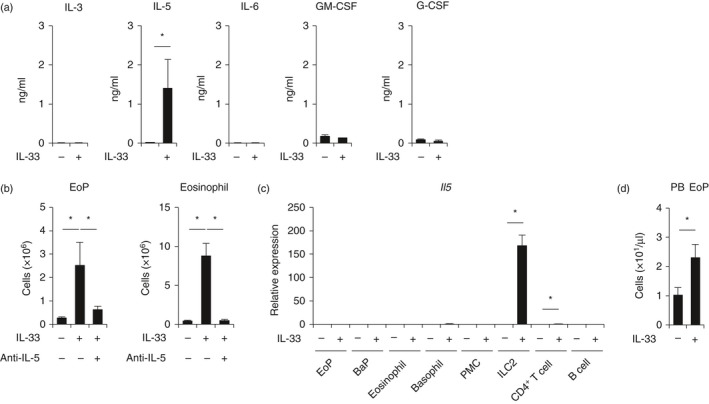
Interleukin‐33 (IL‐33)‐induced expansion of eosinophil progenitors (EoPs) and eosinophils is dependent on IL‐5. (a) Serum concentration of cytokines in IL‐33‐treated mice. (b) The number of EoPs in the bone marrow or eosinophils in the spleen of IL‐33‐treated mice with or without anti‐IL‐5 antibody injection. (c) IL‐5 expression of IL‐33‐responsive cells in IL‐33‐treated mice. (d) The number of EoPs in peripheral blood. Data are shown as the mean ± standard deviation (n = 3). *P < 0·05.
To evaluate whether IL‐33 possesses an additive effect on IL‐5‐induced expansion of EoPs, we cultured EoPs in the presence of IL‐5 and/or IL‐33. IL‐33 tended to suppress the proliferation of EoPs induced by IL‐5 (see Supplementary material, Fig. S8), indicating that IL‐33 does not show a synergistic effect on IL‐5‐induced proliferation of EoPs.
IL‐33 promotes EoP emigration into the peripheral blood
In allergic inflammation, it was demonstrated that EoPs emigrate from bone marrow, home to the peripheral tissues, and differentiate into mature eosinophils locally, which is called ‘in situ haematopoiesis’.26 To investigate whether the expanded EoPs egress from bone marrow, we analysed the number of EoPs in the peripheral blood. As shown in Fig. 6(d), the absolute number of EoPs in the peripheral blood of IL‐33‐treated mice was increased. Given that myeloid progenitors including EoPs express allergy‐related chemokine receptors such as CCR3 and CCR5 (see Supplementary material, Fig. S9), EoPs could migrate from bone marrow to peripheral tissues for in situ haematopoiesis also in IL‐33‐induced inflammation.
Discussion
In this study, it was demonstrated that EoPs, BaPs and MCPs might act as effector cells in IL‐33‐induced inflammation. Previously, haematopoietic progenitors were shown to exist throughout the body.24, 25, 26 CD34+ haematopoietic progenitors purified from umbilical cord blood produced Th2 cytokines in response to IL‐33.24 A correlation between the number of myeloid progenitors at the inflammatory site and the disease activity of asthma27, 28 was also demonstrated. Furthermore, low‐dose corticosteroid treatment, which reduced the number of mature eosinophils in the peripheral blood, did not suppress the allergen‐induced expansion of EoPs in the bone marrow,36 indicating that these progenitors are relatively resistant to corticosteroid treatment. Collectively, these data suggest that myeloid progenitors are also involved in the pathogenesis of allergic diseases. However, it was not yet clarified which fraction of myeloid progenitors responds to IL‐33. Here, we demonstrated that EoPs, BaPs and MCPs express a functional IL‐33R (Fig. 1 and see Supplementary material, Fig. S2) and produce Th2 cytokines in response to IL‐33 (Fig. 3). The Th2 cytokine production potential of these progenitors was greater than that of mature cells (Fig. 3). These data suggest that EoPs, BaPs and MCPs are not only the sources of mature granulocytes, but also produce a large amount of Th2 cytokines in allergic diseases. Given that myeloid progenitors develop earlier than mature myeloid cells and localize to peripheral tissues, these myeloid progenitors could initiate allergic inflammation in response to IL‐33, even though they are rare populations.
In addition to Th2‐type diseases, it has been demonstrated that IL‐33 is also involved in the pathogenesis of pro‐inflammatory diseases such as rheumatoid arthritis and inflammatory bowel diseases. The concentration of IL‐33 in the serum and synovial fluid from patients with rheumatoid arthritis was significantly increased and correlated with disease activity.37 Blocking IL‐33 signalling by the administration of anti‐ST2 antibodies or soluble ST2‐Fc fusion protein attenuated disease severity in collagen‐induced arthritis.38, 39 A correlation between the serum levels of IL‐33 and disease activity in patients with ulcerative colitis was also reported.40 A part of these pro‐inflammatory effects are attributed to mature mast cells.41 In our hands, EoPs, BaPs and MCPs produced a variety of pro‐inflammatory cytokines, including IL‐1β, IL‐6 and TNF‐α (Fig. 3), which play critical roles in the pathogenesis of rheumatoid arthritis and inflammatory bowel diseases. These myeloid progenitors might also be involved in the inflammation of pro‐inflammatory diseases such as rheumatoid arthritis and inflammatory bowel diseases.
As regards eosinopoiesis, it has been demonstrated that IL‐33 induces eosinophilia in an IL‐5‐dependent manner.9, 19 Furthermore, it was reported that c‐Kit+ progenitor cells in the bone marrow can differentiate into eosinophils in vitro in the presence of IL‐33 alone.42 However, it is not fully understood which cells are the precise targets of IL‐33 and how IL‐33 regulates eosinopoiesis. Here, we showed that IL‐33‐induced eosinophilia specifically begins at the EoP stage in vivo (Fig. 5c). The expansion of EoPs was induced by IL‐5 (Fig. 6a,b), not by IL‐33 directly (Fig. 4a). IL‐33 did not show an additive effect on IL‐5‐induced expansion of EoPs (see Supplementary material, Fig. S8). The major source of IL‐5 in IL‐33‐induced eosinophilia is presumably ILC2s (Fig. 6c) that express functional IL‐33R (see Supplementary material, Figs S1b and S2b). The expanded EoPs, which expressed CCR3 and CCR5 (see Supplementary material, Fig. S9), egressed from the bone marrow into the peripheral blood (Fig. 6d), and that should contribute to ‘in situ haematopoiesis’ at the local site of inflammation.
In this study, we could not evaluate the degree of the contribution of myeloid progenitors to allergic inflammation. Because myeloid progenitors are detected in the inflammatory site of patients with allergies,24, 27 we thought that EoPs, BaPs and MCPs could be involved in the inflammation of allergic diseases. To evaluate this, a novel marker that can be used for the specific depletion of EoPs, BaPs or MCPs needs to be identified.
In summary, EoPs, BaPs and MCPs expressed functional IL‐33Rs. IL‐33 induced not only eosinophilia through IL‐5‐dependent EoP expansion but also the production of Th2 and pro‐inflammatory cytokines by EoPs, BaPs and MCPs. These myeloid progenitors, which are the sources of both allergy‐related granulocytes and effector cytokines, are therefore potential therapeutic targets in IL‐33‐related allergic diseases.
Disclosures
We received financial support from the Japan Society for the Promotion of Science (Grant‐in‐Aid for Scientific Research (C)), AstraZeneca (AstraZeneca R&D Research Grant) and Takeda Science Foundation (Takeda Science Foundation, Research Grant for Medical Science). We have no conflict of interests to disclose.
Supporting information
Appendix S1. Methods.
Table S1. Fraction of haematopoietic cells used in this study.
Figure S1. Type 2 innate lymphoid cells (ILC2s) constitutively express ST2 in mouse lymphoid lineage cells.
Figure S2. ST2‐expressing cells also express Interleukin‐1 receptor accessory protein (IL‐1RAcP).
Figure S3. Representative plots of eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs).
Figure S4. Eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs) differentiate into eosinophils, basophils and mast cells, respectively.
Figure S5. Interleukin‐33 (IL‐33) does not affect the proliferation of megakaryocyte/erythrocyte progenitors (MEPs) or the erythrocyte/megakaryocyte lineage determination downstream of MEPs.
Figure S6. Interleukin‐33 (IL‐33) induces the gene expression of T helper type 2 (Th2) and pro‐inflammatory cytokines in eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs).
Figure S7. Interleukin‐33 (IL‐33) does not induce IL‐4 production in eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs).
Figure S8. Interleukin‐33 (IL‐33) does not show an additive effect on IL‐5‐induced proliferation of eosinophil progenitors (EoPs).
Figure S9. Eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs) express allergy‐related chemokine receptors.
Acknowledgements
Hirofumi Tsuzuki, Kohta Miyawaki, Ayako Takaki, Shun‐ichiro Ota and Yuri Ota performed the experiments. Hirofumi Tsuzuki, Yojiro Arinobu, Kohta Miyawaki, Hiroki Mitoma, Mitsuteru Akahoshi, Yasuo Mori, Hiromi Iwasaki, Hiroaki Niiro, Hiroshi Tsukamoto and Koichi Akashi designed this study. Hirofumi Tsuzuki, Yojiro Arinobu and Koichi Akashi wrote this paper.
Author Contributions
This study was supported by Grant‐in‐Aid for Scientific Research (C) (research number: 23591463), AstraZeneca R&D Research Grant (no research number) and Takeda Science Foundation, Research Grant for Medical Science (no research number). We thank Masatsugu Oka, Yuki Iihoshi and Kanako Matsuo (Seiko co., ltd.) for their cooperation.
Contributor Information
Yojiro Arinobu, Email: yarinobu@cancer.med.kyushu-u.ac.jp.
Koichi Akashi, Email: akashi@med.kyushu-u.ac.jp.
References
- 1. Liew FY, Pitman NI, McInnes IB. Disease‐associated functions of IL‐33: the new kid in the IL‐1 family. Nat Rev Immunol 2010; 10:103–10. [DOI] [PubMed] [Google Scholar]
- 2. Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A et al IL‐33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci USA 2010; 107:18581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haenuki Y, Matsushita K, Futatsugi‐Yumikura S, Ishii KJ, Kawagoe T, Imoto Y et al A critical role of IL‐33 in experimental allergic rhinitis. J Allergy Clin Immunol 2012; 130:184–94. [DOI] [PubMed] [Google Scholar]
- 4. Chu DK, Llop‐Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE et al IL‐33, but not thymic stromal lymphopoietin or IL‐25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol 2013; 131:187–200. [DOI] [PubMed] [Google Scholar]
- 5. Préfontaine D, Lajoie‐Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ et al Increased expression of IL‐33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol 2009; 183:5094–103. [DOI] [PubMed] [Google Scholar]
- 6. Matsuda A, Okayama Y, Terai N, Yokoi N, Ebihara N, Tanioka H et al The role of interleukin‐33 in chronic allergic conjunctivitis. Invest Ophthalmol Vis Sci 2009; 50:4646–52. [DOI] [PubMed] [Google Scholar]
- 7. Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM et al Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet 2009; 41:342–7. [DOI] [PubMed] [Google Scholar]
- 8. Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S et al A large‐scale, consortium‐based genomewide association study of asthma. N Engl J Med 2010; 363:1211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK et al IL‐33, an interleukin‐1‐like cytokine that signals via the IL‐1 receptor‐related protein ST2 and induces T helper type 2‐associated cytokines. Immunity 2005; 23:479–90. [DOI] [PubMed] [Google Scholar]
- 10. Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL‐1 family cytokine, IL‐33, potently activates human eosinophils. J Allergy Clin Immunol 2008; 121:1484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suzukawa M, Likura M, Koketsu R, Nagase H, Tamura C, Komiya A et al An IL‐1 cytokine member, IL‐33, induces human basophil activation via its ST2 receptor. J Immunol 2008; 181:5981–9. [DOI] [PubMed] [Google Scholar]
- 12. Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M et al IL‐33 induces IL‐13 production by mouse mast cells independently of IgE‐FcεRI signals. J Leukoc Biol 2007; 82:1481–90. [DOI] [PubMed] [Google Scholar]
- 13. Rank MA, Kobayashi T, Kozaki H, Barternes KR, Squillace DL, Kita H. IL‐33‐activated dendritic cells induce an atypical TH2‐type response. J Allergy Clin Immunol 2009; 123:1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smithgall MD, Comeau MR, Yoon B‐RP, Kaufman D, Armitage R, Smith DE. IL‐33 amplifies both Th1‐ and Th2‐type responses through its activity on human basophils, allergen‐reactive Th2 cells, iNKT and NK cells. Int Immunol 2008; 20:1019–30. [DOI] [PubMed] [Google Scholar]
- 15. Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S et al The pro‐Th2 cytokine IL‐33 directly interacts with invariant NKT and NK cells to induce IFN‐γ production. Eur J Immunol 2009; 39:1046–55. [DOI] [PubMed] [Google Scholar]
- 16. Komai‐Koma M, Gilchrist DS, McKenzie ANJ, Goodyear CS, Xu D, Liew FY. IL‐33 activates B1 cells and exacerbates contact sensitivity. J Immunol 2011; 186:2584–91. [DOI] [PubMed] [Google Scholar]
- 17. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H et al Innate production of TH2 cytokines by adipose tissue‐associated c‐Kit+ Sca‐1+ lymphoid cells. Nature 2009; 463:540–4. [DOI] [PubMed] [Google Scholar]
- 18. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA et al Nuocytes represent a new innate effector leukocyte that mediates type‐2 immunity. Nature 2010; 464:1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dyer KD, Percopo CM, Rosenberg HF. IL‐33 promotes eosinophilia in vivo and antagonizes IL‐5‐dependent eosinophil hematopoiesis ex vivo . Immunol Lett 2013; 150:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL‐33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol 2008; 180:2443–9. [DOI] [PubMed] [Google Scholar]
- 21. Yasuda K, Muto T, Kawagoe T, Matsumoto M, Sasaki Y, Matsushita K et al Contribution of IL‐33‐activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode‐infected mice. Proc Natl Acad Sci USA 2012; 109:3451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakae S, Morita H, Ohno T, Arae K, Matsumoto K, Saito H. Role of interleukin‐33 in innate‐type immune cells in allergy. Allergol Int 2013; 62:13–20. [DOI] [PubMed] [Google Scholar]
- 23. Kondo Y, Yoshimoto T, Yasuda K, Futatsugi‐Yumikura S, Morimoto M, Hayashi N et al Administration of IL‐33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol 2008; 20:791–800. [DOI] [PubMed] [Google Scholar]
- 24. Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M et al CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol 2009; 123:472–8. [DOI] [PubMed] [Google Scholar]
- 25. Massberg S, Schaerli P, Knezevic‐Maramica I, Köllnberger M, Tubo N, Moseman EA et al Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 2007; 131:994–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Radinger M, Loetvall J. Eosinophil progenitors in allergy and asthma – do they matter? Pharmacol Ther 2009; 121:174–84. [DOI] [PubMed] [Google Scholar]
- 27. Robinson DS, Damia R, Zeibecoglou K, Molet S, North J, Yamada T et al CD34+/interleukin‐5Rα messenger RNA+ cells in the bronchial mucosa in asthma: potential airway eosinophil progenitors. Am J Respir Cell Mol Biol 1999; 20:9–13. [DOI] [PubMed] [Google Scholar]
- 28. Inman MD, Ellis R, Wattie J, Denburg JA, O'Byrne PM. Allergen‐induced increase in airway responsiveness, airway eosinophilia, and bone‐marrow eosinophil progenitors in mice. Am J Respir Cell Mol Biol 1999; 21:473–9. [DOI] [PubMed] [Google Scholar]
- 29. Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 1997; 91:661–72. [DOI] [PubMed] [Google Scholar]
- 30. Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000; 404:193–7. [DOI] [PubMed] [Google Scholar]
- 31. Iwasaki H, Mizuno S‐I, Mayfield R, Shigematsu H, Arinobu Y, Seed B et al Identification of eosinophil lineage‐committed progenitors in the murine bone marrow. J Exp Med 2005; 201:1891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arinobu Y, Iwasaki H, Gurish MF, Mizuno S‐I, Shigematsu H, Ozawa H et al Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci USA 2005; 102:18105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL‐1 receptor accessory protein and ST2 comprise the IL‐33 receptor complex. J Immunol 2007; 179:2551–5. [DOI] [PubMed] [Google Scholar]
- 34. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol 2015; 16:45–56. [DOI] [PubMed] [Google Scholar]
- 35. Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol 2015; 135:299–310. [DOI] [PubMed] [Google Scholar]
- 36. Wood LJ, Sehmi R, Gauvreau GM, Watson RM, Foley R, Denburg JA et al An inhaled corticosteroid, budesonide, reduces baseline but not allergen‐induced increases in bone marrow inflammatory cell progenitors in asthmatic subjects. Am J Respir Crit Care Med 1999; 159:1457–63. [DOI] [PubMed] [Google Scholar]
- 37. Matsuyama Y, Okazaki H, Tamemoto H, Kimura H, Kamata Y, Nagatani K et al Increased levels of interleukin 33 in sera and synovial fluid from patients with active rheumatoid arthritis. J Rheumatol 2010; 37:18–25. [DOI] [PubMed] [Google Scholar]
- 38. Palmer G, Talabot‐Ayer D, Lamacchia C, Toy D, Seemayer CA, Viatte S et al Inhibition of interleukin‐33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum 2009; 60:738–49. [DOI] [PubMed] [Google Scholar]
- 39. Leung BP, Xu D, Culshaw S, McInnes IB, Liew FY. A novel therapy of murine collagen‐induced arthritis with soluble T1/ST2. J Immunol 2004; 173:145–50. [DOI] [PubMed] [Google Scholar]
- 40. Pastorelli L, Garg RR, Hoang SB, Spina L, Mattioli B, Scarpa M et al Epithelial‐derived IL‐33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci USA 2010; 107:8017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu D, Jiang H‐R, Kewin P, Li Y, Mu R, Fraser AR et al IL‐33 exacerbates antigen‐induced arthritis by activating mast cells. Proc Natl Acad Sci USA 2008; 105:10913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stolarski B, Kurowska‐Stolarska M, Kewin P, Xu D, Liew FY. IL‐33 exacerbates eosinophil‐mediated airway inflammation. J Immunol 2010; 185:3472–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Methods.
Table S1. Fraction of haematopoietic cells used in this study.
Figure S1. Type 2 innate lymphoid cells (ILC2s) constitutively express ST2 in mouse lymphoid lineage cells.
Figure S2. ST2‐expressing cells also express Interleukin‐1 receptor accessory protein (IL‐1RAcP).
Figure S3. Representative plots of eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs).
Figure S4. Eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs) differentiate into eosinophils, basophils and mast cells, respectively.
Figure S5. Interleukin‐33 (IL‐33) does not affect the proliferation of megakaryocyte/erythrocyte progenitors (MEPs) or the erythrocyte/megakaryocyte lineage determination downstream of MEPs.
Figure S6. Interleukin‐33 (IL‐33) induces the gene expression of T helper type 2 (Th2) and pro‐inflammatory cytokines in eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs).
Figure S7. Interleukin‐33 (IL‐33) does not induce IL‐4 production in eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs).
Figure S8. Interleukin‐33 (IL‐33) does not show an additive effect on IL‐5‐induced proliferation of eosinophil progenitors (EoPs).
Figure S9. Eosinophil progenitors (EoPs), basophil progenitors (BaPs) and mast cell progenitors (MCPs) express allergy‐related chemokine receptors.


