Abstract
Introduction
The aim of this systematic review was to document efficacy, safety and quality of evidence of analgesic interventions after total knee arthroplasty (TKA).
Methods
This PRISMA-compliant and PROSPERO-registered review includes all-language randomized controlled trials of medication-based analgesic interventions after TKA. Bias was evaluated according to Cochrane methodology. Outcomes were opioid consumption (primary), pain scores at rest and during mobilization, adverse events, and length of stay. Interventions investigated in three or more trials were meta-analysed. Outcomes were evaluated using forest plots, Grading of Recommendations Assessment, Development and Evaluation (GRADE), L’Abbe Plots and trial sequential analysis.
Results
The included 113 trials, investigating 37 different analgesic interventions, were characterized by unclear/high risk of bias, low assay sensitivity and considerable differences in pain assessment tools, basic analgesic regimens, and reporting of adverse events. In meta-analyses single and continuous femoral nerve block (FNB), intrathecal morphine, local infiltration analgesia, intraarticular injection of local anaesthetics, non-steroidal anti-inflammatory drugs, and gabapentinoids demonstrated significant analgesic effects. The 24-hour morphine-sparing effects ranged from 4.2 mg (CI: 1.3, 7.2; intraarticular local anaesthetics), to 16.6 mg (CI: 11.2, 22; single FNB). Pain relieving effects at rest at 6 hours ranged from 4 mm (CI: -10, 2; gabapentinoids), to 19 mm (CI: 8, 31; single FNB), and at 24 hours from 3 mm (CI: -2, 8; gabapentinoids), to 16 mm (CI: 8, 23; continuous FNB). GRADE-rated quality of evidence was generally low.
Conclusion
A low quality of evidence, small sample sizes and heterogeneity of trial designs prohibit designation of an optimal procedure-specific analgesic regimen after TKA.
Introduction
The primary goals of postoperative analgesic treatment are to reduce pain, opioid requirements and consequently opioid-related adverse events, in order to optimize rehabilitation. Enhancing these outcomes has potential beneficial influence on patient morbidity and satisfaction, the degree of required postoperative care, as well as economic perspectives. Total knee arthroplasty (TKA) is a frequently performed orthopedic procedure followed by moderate to severe pain. Therefore, an efficient postoperative analgesic treatment based on sound evidence from the published literature is important for this procedure [1]. Recent research on postoperative pain after total hip arthroplasty suggest, however, that it may be difficult to allow a designation of a “best proven intervention” from the available scientific evidence [2], and it is reasonable to believe that this applies for TKA as well.
The hypothesis of this review was, that no globally recognized, best proven, gold standard analgesic treatment or intervention exists for TKA. The aim of this systematic review of all randomized, controlled clinical trials (RCTs) considering postoperative pain treatment after TKA is therefore to document the evidence for postoperative analgesic interventions after TKA.
Materials and methods
The review meets requirements of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [3]. Registration in the PROSPERO International prospective register of systematic reviews was completed on April 23, 2014, prior to initiation of the study (registration number: CRD42014014940). Updated searches were carried out on June 17, 2016, and September 19, 2016, and registered in the protocol as amendments.
Our methods are similar to those reported in a recent review of postoperative pain treatment after total hip arthroplasty (THA) published by our research group [2]. As the two reviews are associated the methods and results sections are reported in a similar way to secure uniformity.
Literature search
Trials were sought in Pubmed, Embase and The Cochrane Library according to S1 Appendix. The last search date was September 9, 2016. The PROSPECT database [4] and reference lists were screened for eligible trials as well.
Inclusion criteria
Inclusion criteria were randomized controlled trials of unilateral total knee arthroplasty that compared postoperative analgesic outcomes of a perioperative analgesic intervention against placebo in a control group. Basic analgesic regimens and rescue analgesics had to be administered under equal conditions in the intervention and control groups. Trials where different rescue analgesics were administered, e.g. morphine and acetaminophen p.n., were included for qualitative analyses, but not meta-analyses. We only included trials with interventions initiated in the immediate perioperative period that reported either opioid-sparing effect, pain at rest or pain during mobilization. Trials concerning knee fractures, trials including patients less than 18 years, and data published in summary clinical trials, editorials, letters, and comments were excluded.
Outcomes
The primary outcome was 0–24 hours postoperative cumulated opioid consumption.
Secondary outcomes were pain both at rest and on mobilization at 6 and 24 hours postoperatively, opioid related and intervention associated adverse events, and length of hospital stay (LOS).
Data extraction
We extracted the following data: Trial sample size; basic analgesic regimen (i.e. analgesics administered to both intervention- and control group as a fixed regimen); rescue analgesics and 24 hour cumulated dose; pain score at rest and during movement at 6 ± 2 hours and 24 ± 4 hours postoperatively; opioid-related adverse events (postoperative nausea or vomiting (PONV), sedation, dizziness, pruritus, urinary retention, constipation and respiratory depression); intervention-associated adverse events as reported; LOS; and documented and predefined discharge criteria.
Assay sensitivity (a trials ability to detect an absolute difference between groups if there is one) was deemed low if a control group demonstrated a pain score on a visual analogue scale (VAS 0–100 mm) below 30 mm and/or a 0–24 hour cumulated i.v. morphine consumption below 15 mg.
Data extraction and bias evaluation was carried out by two authors independently. Disagreements were solved during meetings with all authors.
Missing data
For trials with unclear bias domains or missing information regarding primary outcomes, the corresponding author was contacted by email and if unresponsive, another inquiry was sent two weeks later. We used open questions as "Please describe all measures taken to secure random sequence allocation" to avoid false confirmation on suggested measures.
Bias assessment
We used the Cochrane bias assessment tool [5] to evaluate the following domains: Random sequence allocation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other potential threats to validity (including conflict of interest). Domains were rated as low, high, or unclear risk of bias. If all domains were low the summarized risk of bias was rated low; if one or more domains were high the summarized risk was rated high; and if one or more domains were unclear with no high risk domains, the summarized risk was rated unclear.
In addition, we evaluated trial sample size as a contributor to bias. A cumulated trial sample size of < 50 patients was rated as high risk of bias, 50–199 as moderate risk of bias, 200–499 as low risk of bias, and > 499 as very low risk of bias based on Dechartres et al. [6].
Data analysis
Handling of data
Meta-analyses were carried out in Review Manager 5® [7] whenever three or more trials regarding a specific intervention reported a 0–24 hour opioid consumption. Opioids were converted to i.v. morphine equivalents according to S2 Appendix. Pain scores, side effects and LOS were analyzed when reported in three ore more trials. Visual analogue scale (VAS 0–10) and Numerical Rating Scale 0–10 (NRS 0–10), were converted to VAS 0–100. Median and interquartile range (IQR)/range was converted to mean and standard deviation according to The Cochrane Handbook 7.7.3.5 [8], or Hozo et al [9], as appropriate. For results presented only as mean, a standard deviation was calculated from the p-value according to The Cochrane Handbook 7.7.3.3 [8], and we used the conservative approach p = 0.05 if the p-value was expressed as p < 0.05. Some trials had more than one intervention group. In these cases we either merged intervention groups or split the control group, according to The Cochrane Handbook 7.7.a [8].
Forest plots were calculated with a 95% confidence interval (CI) mean difference for continuous data and risk ratio (RR) with a 95% CI for dichotomous data. Random effects model was used whenever I^2 was above 30%. For I^2 between 0 and 30% fixed and random effects models were compared and the most conservative approach (the model with the widest 95% CI) was used to take into account the heterogeneity of included trials. P-values of less than 0.05 were considered statistically significant.
Heterogeneity
L’Abbé plots were conducted for each meta-analysed intervention to describe the degree of heterogeneity for morphine consumption and pain scores [10].
Strength of evidence
In meta-analyses, low information size (number of patients included) and repeated significance testing increase the risk of type I and II errors (false positive and false negative results, respectively). This risk can be reduced by performing trial sequential analysis (TSA) [11]. A forest plot describes whether the tested intervention reaches significance through the classic p<0.05, whereas TSA accounts for interim analyses and the heterogeneity of the trials as well. In TSA, the normal stationary threshold for significance with a Z-score at 1.96 for p = 0.05 is penalized if the included trials demonstrates a high degree of heterogeneity. An intervention with a high degree of heterogeneity requires a higher information size to reach the threshold for significance compared to a forest plot analysis. This is calculated as the a priori estimated information size (APIS).
TSA was performed for morphine consumption and pain scores, for all interventions that were included in meta-analyses.
We used Trial Sequential Analysis Viewer 0.9 Beta (The Copenhagen Trial Unit (CTU)) and followed the CTU guidelines (an alpha-value of 0.05 and a beta-value of 0.9) [12]. The sensitivity to detect a mean difference was set to 10 mg i.v. morphine equivalents/24 hours and 15 mm on a VAS 0–100 mm scale [13, 14].
Summary of findings
Quality of evidence was assessed with The Grading of Recommendations Assessment, Development and Evaluation (GRADE). Five factors were evaluated for each outcome: Study limitations; publication bias; indirectness of evidence; inconsistent results; and imprecision (evaluation based on results in TSA) [15].
Outcome effects and quality of evidence were summarized according to GRADE using GRADEpro 3.6.
Results
Retrieved trials
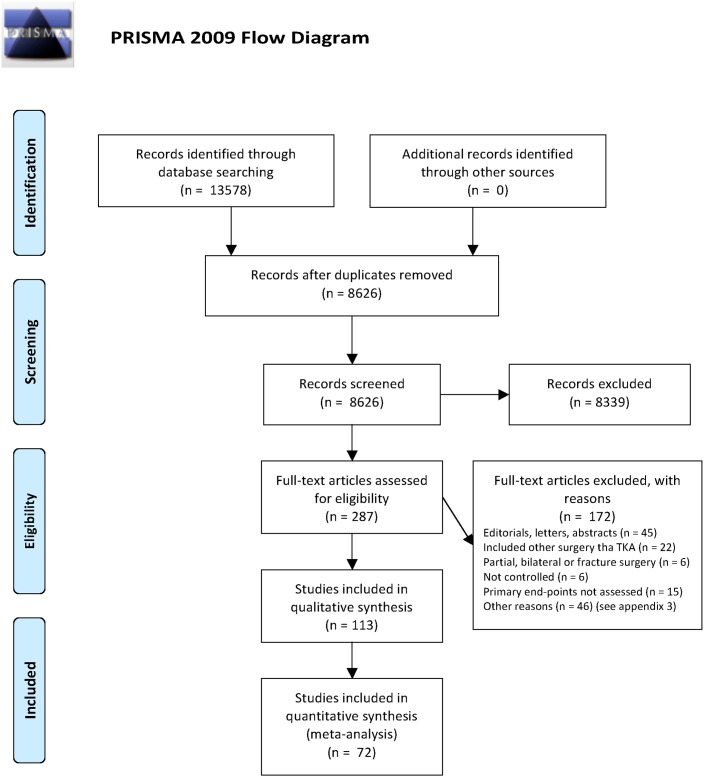
Search on Pubmed, EMBASE and The Cochrane Library identified 5126, 5806 and 2646 citations, respectively. The first author removed 4952 duplicates. Two authors assessed the remaining 8626 citations individually, compared results and consequently 287 trials were downloaded in full-text, of which 22 were written in a non-English language. We managed to acquire 285 trials of which 172 met one or more exclusion criteria (S3 Appendix).
Thus, 113 randomized placebo-controlled trials concerning postoperative analgesic interventions after TKA were included for review (Fig 1 PRISMA flowchart). The total number of patients was 8407.
Fig 1. Flow chart of trial selection.
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097. For more information, visit www.prisma-statement.org.
The included trials comprised 37 different treatment interventions. Interventions that qualified for meta-analyses, were single injection femoral nerve block (FNB), continuous FNB, intrathecal morphine, local infiltration analgesia (LIA), intraarticular injection with local anaesthetics, non-steroidal anti-inflammatory drugs (NSAIDs)/COX-2-inhibitors, and gabapentinoids.
Of all trials 36, 10, 3, and 1 had two, three, four and five separate intervention groups, respectively.
The follow-up period in the included trials was: 1 day in 20 trials, 2 days in 36 trials, 3 days in 16 trials, 4–7 days in 8 trials, ≥2 weeks in 22 trials, and unclear in 11 trials.
Detailed study information from the included trials is summarized in Table 1.
Table 1. Study information.
| Author | Trial sample size intervention and control groups. Risk of trial sample size bias | Treatment intervention Group 1 | Treatment intervention Group 2 | Treatment intervention Control group | Basic analgesic regimen all groups | Type of supplemental analgesic | Assessment of pain scores | Length of stay assessment | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rest 6 h 24 h | Movement 6h 24 h | |||||||||||
| Allen, H. W. (1998) [16] | 12/12/12 | High (<50 patients) | FNB bupivacaine 0.25% 30 mL with epinephrine. Sham sciatic block | FNB and sciatic nerve block 30 mL 0.25% bupivacaine / epinephrine each | Sham sciatic and femoral nerve blocks | Ketorolac 15/30 mg i.v. x 4 (depending on age/weight). | PCA i.v. morphine | Yes | Yes | No | Yes | No |
| Chan, E. Y. (2013) [17] | 69/66 | Moderate (50–199 patients) | FNB bupivacaine 0.25% 20 mL with epinephrine | Continuous FNB, but different baseline treatment. Not controllable. | No block | None 0–24 h | PCA i.v. morphine | Yes | Yes | No | Yes | Yes |
| Chan, M. H. (2012) [18] | 20/21/20/21 | Moderate | FNB bupivacaine 0.375% 0.4 mL/kg with epinephrine after spinal anesthesia but before surgical procedure | FNB bupivacaine 0.375% 0.4 mL/kg with epinephrine after completion of surgical procedure | Group 3: Saline after spinal anesthesia but before the surgical procedure. Group 4:Saline after surgical procedure | None (author info) | PCA i.v. morphine | Yes | Yes | Yes | Yes | No |
| Good, R. P. (2007) [19] | 22/20 | High | FNB bupivacaine 0.5% 40 mL with epinephrine preoperative | - | Matching saline | - | PCA i.v. morphine | No | No | No | No | No |
| Hunt, K. J. (2009) [20] | 33/24 | Moderate | FNB bupivacaine 0.5% 10–15 mL | - | Matching saline | - | PCA i.v. morphine | No | No | No | No | No |
| Jeong, M. S. (2011) [21] | 43/33 | Moderate | FNB bupivacaine 0.5% 20 mL and lidocaine 1% 10 mL | - | No block | Periarticular injection of bupivacaine 40 mg / ketorolac 2 mg / morphine 8 mg / epinephrine. Oral celecoxib 200 mg x 2 | PCA i.v. fentanyl / ketorolac / zofran and i.v. tramadol | Yes | Yes | No | No | No |
| Kardash, K. (2007) [22] | 19/20/20 | Moderate | FNB bupivacaine 0.5% 20 mL with epinephrine | Obturator nerve block bupivacaine 0.5% 20 mL with epinephrine | Sham block: no injection but bandage over the inguinal area | Oral acetaminophen 650 mg x 4. Oral celecoxib 100 mg x 2 | PCA i.v. fentanyl and i.m. Ketorolac if VAS > 60 | No | Yes | No | Yes | Yes |
| Lee, A. R. (2011) [23] | 38/40 | Moderate | FNB bolus levobupivacaine 0.25% 30 mL with epinephrine administered in the PACU | - | No block | - | PCEA ropivacaine / fentanyl and i.v. me-peridine if VAS > 50 | Yes | No | Yes | No | No |
| Ng, H. P. (2001) [24] | 12/12/12/12 | High | 3-in-1 FNB ropivacaine 0.25% 30 mL | Group 2: 3-in-1 FNB ropivacaine 0.5% 30 mL. Group 3: 3-in-1 FNB bupivacaine 0.25% 30 mL | Matching saline | None 0–24 h | PCA i.v. morphine | Yes | Yes | Yes | Yes | Yes |
| Ozen, M. (2006) [25] | 15/15 | High | 3-in-1 FNB ropivacaine 0.375% 40 mL before general anaesthesia | - | No block | - | PCA i.v. morphine | Yes | Yes | No | No | No |
| Sahin, L. (2014) [26] | 51/53 | Moderate | FNB bupivacaine 0.5% 40 mL with epinephrine | - | Matching saline | Oral ibuprofen 600 mg x 3 | PCA i.v. morphine | Yes | Yes | Yes | Yes | No |
| Tugay, N. (2006) [27] | 8/7/8 | High | FNB bupivacaine 0.25% 40 mL at the start intraoperative | FNB bupivacaine 0.25% 40 mL at the end intraoperative | No block | - | PCA i.v. morphine | Yes | Yes | No | No | Yes |
| Wang, H. (2002) [28] | 15/15 | High | FNB bupivacaine 0.25% 40 mL with epinephrine intraoperative | - | Matching saline | - | PCA i.v. morphine | No | Yes | No | Yes | Yes |
| YaDeau, J. T. (2005) [29] | 41/39 | Moderate | FNB bupivacaine 0.375% 30 mL with epinephrine intraoperative | - | No block | None 0–24 h | PCEA bupivacaine / hydromorphone | No | No | No | No | No |
| Hirst, G. C. (1996) [30] | 11/11/11 | High | Single FNB bupivacaine 0.5% 20 mL with epinephrine | FNB bupivacaine 0.5% 20 mL with epinephrine. Continuous bupivacaine 0.125% 6 mL/h for 48 h | Mock FNB and sham continuous infusion | - | PCA i.v. morphine | No | Yes | No | Yes | No |
| Edwards, N. D. and E. M. Wright (1992) [31] | 19/18 | High | 3-in-1 FNB bupivacaine 0.25% 30 mL and continuous bupivacaine 0.125% 6mL/h for 24 h | - | No block | - | I.m. papaveretum | Yes | Yes | No | No | No |
| Ganapathy, S. (1999) [32] | 20/20/22 | Moderate | FNB bupivacaine 0.1% 30 mL bolus and continuous 10 mL/h for 48 h | FNB bupivacaine 0.2% 30 mL bolus and continuous 10 mL/h for 48 h | Matching saline | Rectal indomethacin 100 mg x 2 | PCA i.v. morphine | No | No | No | No | No |
| Kaloul, I. (2004) [33] | 20/20/20 | Moderate | FNB ropivacaine 0.5% with epinephrine 30 mL and continuous ropivacaine 0.2% 12 mL/h for 48 h | Psoas compartment nerve block ropivacaine 0.5% with epinephrine 30 mL and continuous ropivacaine 0.2% 12 mL/h for 48 h | No block | Rectal indomethacin 100 mg x 2 | PCA i.v. morphine | Yes | Yes | No | No | No |
| Park, C. K. (2010) [34] | 20/20/20/20 | Moderate | FNB bupivacaine 0.25% 20 mL with epinephrine and continuous bupivacaine 0.125% 2 mL/h | FNB bupivacaine 0.25% 20 mL with epinephrine and continuous bupivacaine 0.125%: Group 2: 4 mL/h. Group 3: 6 mL/h | FNB bupivacaine 0.25% 20 mL with epinephrine. No continuous FNB | - | PCA i.v. morphine / ketorolac | No | No | No | Yes | No |
| Seet, E. (2006) [35] | 17/18/20 | Moderate | Continuous 3-in-1 FNB ropivacaine 0.15% 10 mL/h for 24 h and then 5 mL/h for the next 24 h | Continuous 3-in-1 FNB ropivacaine 0.2% 10 mL/h for 24 h and then 5 mL/h for the next 24 h | No block | Oral acetaminophen 1 g x 4. Oral rofecoxib 50 mg x 1 | PCA i.v. morphine | Yes | Yes | Yes | Yes | Yes |
| Serpell, M. G. (1991) [36] | 13/16 | High | Continuous lumbar plexus block bupivacaine 0.5% with epinephrine. Bolus 0.3 mL/kg at 6–8 h intervals for 48 h | - | No block | - | PCA i.v. morphine. i.m. morphine, oral paracetamol and NSAIDs p.n. | No | Yes | No | No | No |
| Watson, M. W. (2005) [37] | 16/16 | High | Lumbar plexus block levo-bupivacaine 0.1% 15 mL and continuous 10 mL/h for 48 h | - | Matching saline | Sciatic nerve block levobupivacaine 0.5% 15 mL. Lumbar blexus block levobupivacaine 0.5% 25 mL. Oral codeine 16 mg/acetaminophen 1 g x 4. Oral diclofenac 50 mg x 3 | PCA i.v. morphine | Yes | Yes | Yes | Yes | Yes |
| Wyatt, M. C. (2015) [38] | 42/42 | Moderate | FNB bupivacaine 0.25% 15 mL and continuous bupivacaine 0.125% 0–10 mL/h for 48 h | - | FNB bupivacaine 0.25% 15 mL. Matching saline for continuous FNB | Oral acetaminophen 1 g x 4 | I.v. morphine equivalents | Yes | Yes | No | No | Yes |
| Olive, D. J. (2015) [39] | 26/28/27 | Moderate | FNB ropivacaine 0.75% 20 mL with epinephrine and continuous 8–14 mL/h until POD 2 morning. Intrathecal morphine 0.175 mg | FNB ropivacaine 0.75% 20 mL with epinephrine and continuous 8–14 mL/h until POD 2 morning. No intrathecal morphine | Intrathecal morphine 0.175 mg. No FNB | Oral acetaminophen 1 g x 4. Oral celecoxib 200 mg x 2 | PCA i.v. morphine | Yes | Yes | No | No | No |
| Barrington, JW. (2016) [40] | 41/38 | Moderate | Intrathecal morphine 0.2–0.25 mg adjuncts to spinal anesthesia with bupivacaine 0.75% 9 mg | - | Spinal anesthesia with bupivacaine 0.75% 9 mg | I.v. acetaminophen 1 g on induction. Oral Celebrex 200 mg x 1. Preoperative oral oxycontin 20 mg. I.v. dexamethasone 10 mg on induction. Periarticular ropivacaine 0.5% 50 mL, ketorolac 30 mg and epinephrine | Opioids calculated as morphine equivalents (total consumption throughout the study) | Yes | Yes | No | No | Yes |
| Cole, P. J. (2000) [41] | 18/18 | High | Spinal morphine 0.3 mg adjunct to bupivacaine | - | Placebo adjunct to bupivacaine | Oral diclofenac 50 mg x 3 | PCA i.v. morphine | No | No | Yes | Yes | No |
| Hur, M. J. (2007) [42] | 16/18/20 | Moderate | Intrathecal morphine 50 microgram | Intrathecal morphine 100 microgram | No intrathecal treatment | Not described | PCEA levobupi-vacaine / fentanyl and oral ketorolac if VAS > 30 | Yes | Yes | Yes | Yes | No |
| Jacobson, L. (1989) [43] | 7/7/7/7/7 | High | Intrathecal diamorphine 0.25 mg adjunct to spinal bupivacaine 0.5% 15 mg | Adjunct to spinal bupivacaine 0.5% 15 mg: Intrathecal diamorphine. Group 2: 0.75 mg. Group 3: 1.5 mg. Group 4: 2.5 mg | Spinal bupivacaine 0.5% 15 mg | - | I.m. morphine p.n. | Yes | Yes | No | No | No |
| Kunopart, M. (2014) [44] | 15/15/15/15 | Moderate | Intrathecal morphine sulfate 0.1 mg adjuncts to spinal anesthesia with bupivacaine 0.5% 15 mg | Adjunct to spinal anesthesia with bupivacaine 0.5% 15 mg: Intrathecal morphine. Group 2: 0.2 mg. Group 3: 0.3 mg | Spinal bupivacaine 0.5% 15 mg | - | PCA i.v. morphine | Yes | Yes | No | No | No |
| Lauretti, G. R. (2013) [45] | 19/20/19/18 | Moderate | Intrathecal morphine 0.2 mg and bupivacaine 15 mg | Group 2: Intrathecal ketorolac 2 mg adjunct to bupivacaine 15 mg. Group 3: Intrathecal morphine 0.2 mg and ketorolac 2 mg adjunct to bupivacaine 15 mg | Intrathecal bupivacaine 15 mg | - | I.v. ketoprofen p.n. and tramadol i.v. | No | No | No | Yes | No |
| Park, C. K. (2009) [46] | 20/20/20/20/ 20 | Moderate | Intrathecal 0.05 mg morphine adjunct to bupivacaine 0.5% 6–13 mg with epinephrine | Group 2: Intrathecal morphine 0.1 mg. Group 3: Intrathecal morphine 0.15 mg adjunct to bupivacaine 0.5% 6–13 mg with epinephrine | Intrathecal bupivacaine 0.5% 6–13 mg with epinephrine | 3-in-1 femoral nerve block bupivacaine 0.25% / epinephrine 20 mL and continuous bupivacaine 0.125% 2 mL/h. I.m. diclofenac 90 mg x 2 | PCA continuous FNB, i.m. diclofenac and i.m. butorphanol if VAS > 50 | Yes | Yes | Yes | Yes | No |
| Tan, P. H. (2001) [47] | 20/20/20 | Moderate | Intrathecal morphine 0.3 mg and bupivacaine 0.5% 3 mL | Intrathecal bupivacaine 0.5% 3 mL and neostigmine 50 microgram | Intrathecal bupvacaine 0.5% 3 mL and saline | - | I.m. diclofenac p.n. if VAS > 4i | Yes | Yes | No | No | No |
| Busch, C. A. (2006) [48] | 32/32 | Moderate | LIA ropivacaine 400 mg, ketorolac 30 mg, epimorphine 5 mg and epinephrine | - | No injection | No description | PCA i.v. morphine | No | No | Yes | Yes | Yes |
| Chinachoti, T. (2012) [49] | 50/49 | Moderate | Intraoperative LIA bupivacaine 0.25% 20 ml | - | Placebo | Oral acetaminophen 1 g x 4. I.v. parecoxib 40 mg x 2. I.v. etoricoxib 120 mg x 1. Femoral nerve block bupivacaine 0.25% 20 mL | PCA i.v. morphine | Yes | Yes | No | No | No |
| Choi, H. G. (2006) [50] | 20/20 | High | LIA bupivacaine 150 mg and morphine 10 mg | - | Matching saline | Continuous epidural infusion of bupivacaine 150 mg / morphine 5 mg 0–48 h | I.m. diclofenac p.n. | Yes | Yes | No | No | No |
| Essving, P. (2010) [51] | 24/23 | High | LIA ropivacaine 400 mg, ketorolac 30 mg and epinephrine 0.5 mg during operation. At 21 h: intraarticular ropivacaine 200 mg, ketorolac 30 mg and epinephrine 0.1 mg during operation. | - | No LIA during surgery. At 21 h: saline injection intraarticularly | Oral acetaminophen 1 g x 4 | PCA i.v. morphine mg | Yes | Yes | Yes | Yes | Yes |
| Fu, P. (2009) [52] | 40/40 | Moderate | LIA bupivacaine 30 mg, morphine 5 mg and betamethasone 1 mL | - | Saline | Celecoxib 200 mg | PCA i.v. morphine mg | Yes | Yes | No | Yes | No |
| Kazak Bengisun, Z. (2010) [53] | 20/20/20 | Moderate | Intraoperative LIA bupivacaine 200 mg and epinephrine 0.5 mg in 150 mL. And 120 mg bupivacaine with epinephrine bolus at 10 and 22 h | Intraoperative LIA levobupivacaine 200 mg and epinephrine 0.5 mg in 150 mL. And 120 mg levobupivacaine with epinephrine bolus at 10 and 22 h | Matching saline | - | PCA tramadol and diclofenac if VAS > 50 | Yes | Yes | Yes | Yes | Yes |
| Kim, T. W. (2015) [54] | 43/43/42/43/43/42 | Low (200–499 patients) | LIA ropivacaine 180 mg | LIA ropivacaine 180 mg and morphine 5 mg | Matching saline | I.v. ketorolac 30 mg x 3. Fentanyl patch 25 mikrogram / third day | PCA i.v. fentanyl or i.m pethidine | Yes | Yes | No | No | No |
| Leownorasate, M. (2014) [55] | 21/21 | High | LIA levopubivacaine 100 mg, diclofenac 75 mg, morphine 5 mg and epinephrine | - | No injection | None (author info) | PCA i.v. morphine | Yes | Yes | No | No | Yes |
| Lu, H. H. (2014) [56] | 15/15 | High | LIA ropivacaine 300 mg, morphine 5 mg and epinephrine 10 microgram | - | Matching saline | Epidural lidocaine 0.1% 5 mL/h. Oral celebrex 200 mg x 2 or if oral intake not possible pericoxib i.v. 40 mg x 2 | - | Yes | Yes | Yes | Yes | No |
| Milani, P. (2015) [57] | 32/30 | Moderate | LIA ropivacaine 1% 20 mL | - | Matching saline | Etoricoxib 90 mg x 1. Oxycodone/naloxone 20/10 mg x 2 | I.m. ketorolac 10 p.n. if VAS > 4 | Yes | No | No | No | No |
| Niemelainen, M. (2014) [58] | 27/29 | High | LIA levopubivacaine 150 mg, ketorolac 30 mg and epinephrine 0.5 mg | - | Matching saline | Oral acetaminophen 1 g x 4. Oral meloxicam 15 mg x 1 (2 h after surgery) | PCA i.v. oxycodone | No | No | No | No | No |
| Ong, J. C. (2010) [59] | 16/21/17 | Moderate | Continuous LIA bupivacaine 0.25% 4 mL | LIA bupivacaine 100 mg, morphine 10 mg and ketorolac 1 mL. Continuous LIA bupivacaine 0.25% 4 mL | No injection | None (author info) | PCA i.v. morphine | Yes | Yes | No | No | Yes |
| Vaishya, R. (2016) [60] | 40/40 | Moderate | LIA bupivacaine 0.25% 20 mL, morphine 15 mg, ketorolac 30 mg, epinephrine. Total dose 75 mL | - | Placebo | I.v. paracetamol 1 g x 3. I.v. diclofenac 75 mg x 2 | PCA i.v. Morphine | Yes | Yes | Yes | Yes | Yes |
| Vendittoli, P. A. (2006) [61] | 22/20 | High | LIA ropivacaine 275 mg, ketorolac 30 mg and epinephrine continued by ropivacaine 125 mg at wound closure | - | No injection | Acetaminophen 500 mg x 4. Celecoxib 200 mg x 2 | PCA i.v. morphine | No | No | No | No | Yes |
| Yuenyongviwat, V. (2012) [62] | 30/30 | Moderate | LIA bupivacaine 0.25% 20 mL before wound closure | - | Matching saline | Oral acetaminophen 1 g x 4. Oral meloxicam 7.5 mg x 2 | PCA i.v. morphine | No | No | No | No | Yes |
| Zhang, J. (2007) [63] | 30/30 | Moderate | LIA bupivacaine 0.5% 30 mL, morphine 10 mg and epinephrine | - | No injection | I.v. lornoxicam 0.3 mg/h for 48 h | I.m. morphine p.n. PCA tramadol 500 mg | Yes | Yes | Yes | Yes | No |
| Zhang, S. (2011) [64] | 27/27/26 | Moderate | Single-injection LIA ropivacaine 300 mg and ketorolac 30 mg. Continuous saline | Single-injection LIA ropivacaine 300 mg and ketorolac 30 mg. Continuous ropivacaine 8 mg/h and ketorolac 1.25 mg/h for 48 h | Matching saline | Oral celecoxib 200 mg x 2 | PCA i.v. morphine | Yes | Yes | Yes | Yes | No |
| Nakai, T. (2013) [65] | 21/19/20 | Moderate | Intraarticular injection bupivacaine 0.5% 20 mL, morphine 10 mg and epinephrine 0.3 mg | LIA ropivacaine 0.75% 30 mL, morphine 10 mg, betamethasone 4 mg and epinephrine 0.25 mg | No injection | No description | Suppository diclofenac | Yes | Yes | No | No | No |
| Badner, N. H. (1996) [66] | 28/27/27 | Moderate | Intraarticular injection bupivacaine 0.5% 30 mL with epinephrine before skin incision. Intraarticular saline after wound closure. | Intraarticular saline before skin incision. Intraarticular bupivacaine 0.5% 30 mL with epinephrine after wound closure. | Intraarticular saline before skin incision. Intraarticular saline after wound closure. | No description | PCA i.v. morphine | No | Yes | No | No | No |
| Browne, C. (2004) [67] | 30/30 | Moderate | Intraarticular injection bupivacaine 0.5% 20 mL with epinephrine after closure. | - | Intraarticular injection saline 20 mL with epinephrine after closure. | No description | Opioids calculated as morphine equivalents | No | No | No | No | No |
| Mauerhan, D. R. (1997) [68] | 26/24/28/27 | Moderate | Intraarticular injection morphine 5 mg | Intraarticular injection: Group 2: Bupivacaine 50 mg. Group 3: Bupivacaine 50 mg and morphine 5 mg | Matching saline | None (author info) | PCA i.v. morphine or meperidine | Yes | Yes | No | No | No |
| Rosen, A. S. (2010) [69] | 24/24 | High | Intraarticular ropivacaine 0.2% 100 mL after closure | - | Matching saline | - | PCA i.v. morphine and other narcotics converted to i.v. morphine equivalents | Yes | Yes | No | No | No |
| Safa, B. (2014) [70] | 33/32/35 | Moderate | Sciatic nerve block with ropivacaine 0.5% 20 mL preoperatively. Posterior capsule injection of saline at the end of surgery. | Sciatic nerve block with saline. Posterior capsule injection with ropivacaine 0.2% 20 mL at the end of surgery. | Sciatic nerve block with saline. Posterior capsule injection with saline at the end of surgery. | FNB ropivacaine 0.5% 20 mL. Oral acetaminophen 1 g x 4. Oral celecoxib 200 mg x 2. Oral gabapentin 200 mg x 3 | PCA i.v. hydromorphone | Yes | Yes | Yes | Yes | Yes |
| Shen, S. J. (2015) [71] | 20/16 | High | Intraarticular bupivacaine 0.5% 60 mL | - | Matching saline | I.v. parecoxib 40 mg preoperative. None postoperative | I.m. meperidine p.n. | Yes | Yes | No | No | No |
| Feng, Y. (2004) [72] | 15/15 | High | Oral rofecoxib 25 mg 1 h prior to surgery | - | Matching placebo | Not described | PCEA morphine / bupivacaine / droperidol | No | No | No | No | No |
| Huang, Y. M. (2008) [73] | 40/40 | Moderate | Oral celecoxib 400 mg 1 h preoperative and 200 mg x 2 daily | - | No capsules | - | PCA i.v. morphine | Yes | Yes | No | Yes | No |
| Hubbard, R. C. (2003) [74] | 61/65/63 | Moderate | I.v. parecoxib 20 mg x 2 | I.v. parecoxib 40 mg x 2 | Matching placebo | - | PCA i.v. morphine | Yes | Yes | No | No | No |
| Inan, N. (2007) [75] | 20/20 | High | I.v. lornoxicam 16 mg before surgery and 8 mg x 2 daily | - | Matching saline | - | PCA i.v. morphine | Yes | Yes | No | No | No |
| Rawal, N. (2013) [76] | 222/230/223/98 | Very low (>499 patients) | Oral etoricoxib 90 mg x 1 and matching placebo x 2 | Group 2: Oral etoricoxib 120 mg x 1 and matching placebo x 2. Group 3: Oral ibuprofen 600 mg x 3 | Matching placebo | - | PCA i.v. morphine | No | No | No | No | No |
| Sarridou, D. G. (2015) [77] | 45/45 | Moderate | I.v. parecoxib 40 mg x 2 | - | Matching placebo | FNB ropivacaine 0.75% 20 mL and continuous 0.2% 10 mL/h | PCA i.v. morphine | Yes | Yes | No | No | No |
| Silvanto, M. (2002) [78] | 24/24/16 | Moderate | I.v. diclofenac 75 mg in PACU and oral diclofenac 50 mg x 3 daily | I.v. ketoprofen 100 mg in PACU and oral keotoprofen 100 mg x 3 daily | Matching placebo | - | PCA i.v. oxycodone | Yes | No | No | No | No |
| Zhu, Y. (2014) [79] | 50/50 | Moderate | I.v. parecoxib 40 mg x 2 | - | Matching saline | Periarticular injection morphine 4 mg / ropivacaine 35 mg / epinephrine. Postoperative not described | I.v. morphine p.n. if VAS > 40 | Yes | Yes | No | Yes | No |
| Zhu, YZ. (2016) [80] | 60/62 | Moderate | I.v. parecoxib 40 mg x 2 | - | Placebo | No description | PCA fentanyl | Yes | Yes | No | No | Yes |
| Niruthisard, S. (2013) [81] | 25/22/22/25 | Moderate | Oral pregabalin 150 mg and placebo the morning of surgery | At the morning of surgery: Group 2: Oral celecoxib 400 mg and placebo. Group 3: Oral pregabalin 150 mg and celecoxib 400 mg | Matching placebo | - | PCA i.v. morphine | Yes | Yes | Yes | Yes | No |
| Clarke, H.A. (2009) [82] | 7/8/7/7/7 | High | Oral gabapentin 600 mg preoperative and placebo postoperative | Group 2: Oral gabapentin 600 mg preoperative and 100 mg postoperative. Group 3: Oral gabapentin 600 mg preoperative and 200 mg postoperative. Group 4: Oral gabapentin 600 mg preoperative and 300 mg postoperative | Matching placebo | Femoral and sciatic nerve block ropivacaine 0.5% 20 mL each preoperative. Oral celecoxib 200 mg x 2 | PCA i.v. morphine | No | Yes | No | No | No |
| Clarke, H. A. (2014) [83] | 88/77 | Moderate | Oral gabapentin 600 mg 2 h before surgery and 200 mg x 3 postoperative | - | Matching placebo | Femoral and sciatic nerve block ropivacaine 0.5% 20 mL each preoperative. Oral celecoxib 200 mg x 2 | PCA i.v. morphine | No | No | No | Yes | No |
| Lee, J. K. (2014) [84] | 21/20 | High | Oral pregabalin 150 mg 1 h before operation | - | None | Periarticular injection bupivacaine 0.5% 10 mL / morphine 5 mg / epinephrine / methylprednisolone 1 mL. Oral celecoxib 200 mg x 2 | PCA i.v. fentanyl and i.m. tramadol if VAS > 40 | Yes | Yes | Yes | Yes | No |
| Lunn, T. H. (2015) [85] | 91/92/91 | Low | Oral gabapentin cumulated 1300 mg daily divided in 4 doses | Oral gabapentin cumulated 900 mg daily divided in 3 doses + 1 placebo | Matching placebo | LIA ropivacaine 0.2% with epinephrine. Oral slow release acetaminophen 2 g x 2. Oral celecoxib 200 mg x 2 | Oral morphine equivalents | Yes | Yes | Yes | Yes | Yes |
| Paul, J. E. (2013) [86] | 52/49 | Moderate | Oral gabapentin 600 mg preoperatively and 200 mg x 3 postoperative | - | Matching placebo | Oral acetaminophen 1 g x 4. Oral ketorolac 15 mg x 4 | PCA i.v. morphine | No | No | No | No | Yes |
| YaDeau, J. T. (2015) [87] | 28/29/29/29 | Moderate | Oral pregabalin 50 mg two capsules before operation and 1 capsule x 2 postoperative | Oral pregabalin two capsules before operation and 1 capsule x 2 postoperative. Group 2: 100 mg capsules. Group 3: 150 mg capsules | Matching placebo | FNB bupivacaine 0.25% 30 mL / epinephrine. Oral dexamethasone 6 mg preoperative. Oral meloxicam 7.5–15 mg | PCEA bupivacaine / hydromorphine. Oral oxycodone-paracetamol 5 mg / 325 mg p.n. | No | Yes | No | No | Yes |
| Andersen, H. L. (2013) [88] | 20/20 | High | Saphenous nerve block ropivacaine 0.75% 15 mL administered 3 times: in the PACU, 8:00 PM and 8:00 AM | - | Matching saline | LIA single-dose 100mL ropivacaine 2 mg/mL and epinephrine. Dexamethasone 8 mg preoperative. Acetaminophen 1 g x 4. Oral extended release morphine 10 mg x 2 | PCA i.v. morphine and FNB boluses of ropivacaine | No | Yes | No | No | Yes |
| Jenstrup, M. T. (2012) [89] | 34/37 | Moderate | Adductor canal block ropivacaine 0.75% 30 ml immediately postoperative and additional 15 ml at 6, 12 and 18 h postoperatively. | - | Matching saline | Oral acetaminophen 1 g x 4. Oral ibuprofen 400 mg x 4 | PCA i.v. morphine | Yes | Yes | Yes | Yes | No |
| Krishnan, SH. (2016) [90] | 48/49 | Moderate | Adductor canal block bupivacaine 30 mL 0.25% and buprenorphine 0.2 mg | - | Adductor canal block bupivacaine 30 mL 0.25% | No description | Oral hydrocodone equivalents | No | No | No | No | No |
| Shah, N. A. (2015) [91] | 46/39 | Moderate | Adductor canal block ropivacaine 0.75% 30 mL and continuous ropivacaine 0.25% 30 mL every 4 h 0–24 h | - | Adductor canal block ropivacaine 0.75% 30 mL and continuous matching saline | Intraarticular infiltration sensorcaine 0.25% 20 mL. Oral acetaminophen 500 mg x 4. I.v. diclofenac 75 mg x 3 | I.m. tramadol 50 mg for breakthrough pain | Yes | Yes | No | No | Yes |
| Casati, A. (2005) [92] | 20/19/19 | Moderate | FNB ropivacaine 0.75% 25 mL and clonidine 1 microgram/kg. Continuous ropivacaine 0.2% | FNB bolus ropivacaine 0.75% 25 mL and clonidine 1 microgram/kg. Continuous ropivacaine 0.2% and clonidine 1 microgram/mL | FNB ropivacaine 0.75% 25 mL. Continuous ropivacaine 0.2% | Sciatic nerve block 0.75% 15 mL. Ketoprofen 10 mg i.v. x 3 | PCA continuous FNB and i.v. fentanyl | Yes | Yes | Yes | Yes | No |
| Ekmekci, P. (2010) [93] | 20/20/20 | Moderate | Continuous FNB ropivacaine 0.2% and tramadol 1 mg/mL 0.1 mL/h for 48 h | Continuous FNB with ropivacaine 0.2% and tramadol 2 mg/mL 0.1 mL/h for 48 h | Continuous FNB with ropivacaine 0.2% 0.1 mL/h for 48 h | FNB ropivacaine 0.5% 0.3 mL/kg. I.m. meperidine 25 mg preoperative | I.m. diclofenac if VAS > 40 | Yes | Yes | No | No | No |
| Elmawgoud, A. A. (2008) [94] | 20/20/20 | Moderate | FNB ropivacaine 0.2% 30 mL with fentanyl 4 mikrog/mL and continuous 6 mL/h for 24 h | FNB ropivacaine 0.2% 30 mL and magnesium sulphate 50 mg/mL and continuous 6 mL/h for 24 h | FNB ropivacaine 0.2% 30 mL and continuous 6 mL/h for 24 h | - | PCA i.v. morphine | Yes | Yes | No | No | No |
| Kosel, J. (2015) [95] | 28/20 | High | FNB bupivacaine 0.25% with epinephrine 0.5 mL/kg and buprenorphine 0.3 mg | - | FNB bupivacaine 0.25% with epinephrine 0.5 mL/kg | None | I.m. morphine p.n., tramadol p.n., acet-aminophen p.n. and ketoprophene p.n. | No | Yes | No | No | Yes |
| McNamee, D. A. (2001) [96] | 25/25/24 | Moderate | Combined femoral and sciatic block with bupivacaine 2 mg/kg divided equally between femoral and sciatic nerves | Combined femoral and sciatic block with ropivacaine 2 mg/kg divided equally between femoral and sciatic nerves | No peripheral nerve block but area prepared and dressing applied to the appropriate sites | - | PCA i.v. morphine | Yes | Yes | No | No | No |
| Abdallah, F. W. (2014) [97] | 17/18/18 | Moderate | Proximal sciatic nerve block at infragluteal level of 2:1 bupivacaine 0.5% and lidocaine 2% 30 mL with epinephrine. Distal sham 1 mL saline | Distal sciatic nerve block at popliteal level of 2:1 bupivacaine 0.5% and lidocaine 2%30 mL with epinephrine. Proximal sham 1 mL | Sham injection 1 mL each location | Continuous FNB ropivacaine 0.2% bolus with epinephrine 20 mL and infusion at 5 mL/h. Acetaminophen 1 g x 4. Celecoxib 200 mg x 2. Oxycodone-controlled release 10 mg x 3 | PCA continuous FNB i.v. fentanyl p.n., oral oxycodone p.n., PCA i.v. hydro-morphine if NRS > 60 | Yes | Yes | Yes | Yes | No |
| Cappelleri, G. (2011) [98] | 19/18 | High | Continuous sciatic nerve block levobupivacaine 0.06% 0.1 mL/kg. Continuous lumbar plexus block levobupivacaine 0.125% 8 mL/h | - | Continuous sciatic nerve block saline 0.1 mL/kg. Continuous lumbar plexus block levobupivacaine 0.125% 8 mL/h | I.v. ketorolac 30 mg x 3 | PCA i.v. morphine | Yes | Yes | Yes | Yes | No |
| Martinez Navas, A. and M. Echevarria Moreno (2006) [99] | 7/10 | High | Sciatic nerve block ropivacaine 0.5% 20 mL. Continuous ropivacaine 0.2% 5 mL/h | - | Sciatic nerve block ropivacaine 0.5% 20 mL | FNB Ropivacaine 0.2% 0,4 ml/kg and continuous 5 ml/h + PCA boluses. I.v. acetaminophen 1 g x 4. I.m. diclofenac 50 mg x 2 | S.c. morphine-chloride p.n. | Yes | Yes | Yes | Yes | No |
| Sato, K. (2014) [100] | 30/30 | Moderate | Sciatic nerve block ropivacaine 0.2% 20 mL. Continuous ropivacaine 0.2% 5 mL/h | - | Continuous sciatic nerve block ropivacaine 0.2% 20 mL. Continuous saline | FNB ropivacaine 0.5% 20 mL and continuous ropivacaine 0.2% 5 mL/h. Oral loxoprofen 60 mg x 3 | PCA i.v. morphine | Yes | Yes | No | No | Yes |
| McNamee, D. A. (2002) [101] | 24/27 | Moderate | Obturator nerve block ropivacaine 0.75% 5 mL. Femoral and sciatic nerve block ropivacaine 0.75%15 mL to each nerve | - | Femoral and sciatic nerve block ropivacaine 0.75% 15 mL to each nerve | Ranitidine 150 mg 1 h preoperative. None postoperative | PCA i.v. morphine | No | No | Yes | Yes | No |
| Runge, C. (2016) [102] | 23/26 | High | Obturator nerve block bupivacaine 46 mg, clonidine 0.0375 mg, dexamethasone 2 mg, epinephrine | - | No block | Femoral triangle block bupivacaine 46 mg, clonidine 0.0375 mg, dexamethasone 2 mg, epinephrine | PCA i.v. morphine | Yes | Yes | Yes | Yes | Yes |
| Frassanito, L. (2009) [103] | 22/22 | High | Single lumbar plexus block ropivacaine 0.6% 30 mL. Single sciatic block ropivacaine 0.6% 15 mL. Continuous lumbar plexus infusion of ropivacaine 0.2% 10 mL/h for 48 h | - | Single lumbar plexus block ropivacaine 0.6% 30 mL. Single sciatic nerve block ropivacaine 0.6% 15 mL | Fentanyl i.v. 50 mikrog preoperative. I.v. acetaminophen 1 g x 4 | I.v. tramadol p.n. if VAS > 40 mm | Yes | Yes | No | No | No |
| Badner, N. H. (1997) [104] | 25/26/24 | Moderate | Intraarticular bupivacaine 0.5% 30 mL and morphine 1 mg with epinephrine | Intraarticular saline 30 mL and morphine 1 mg with epinephrine | Intraarticular bupivacaine 0.5% 30 mL with epinephrine and 1 mL saline | No description | PCA i.v. morphine | Yes | Yes | No | No | No |
| Garcia, J. B. (2010) [105] | 25/25 | Moderate | Intraarticular 10 mg morphine in 20 mL | - | Matching saline | - | S.c. morphine p.n. | Yes | Yes | No | No | No |
| Guara Sobrinho, H. (2012) [106] | 19/17/20 | Moderate | Intraarticular ketamine 0.25 mg/kg in 20 mL just before complete closure of the skin | Intraarticular ketamine 0.5 mg/kg in 20 mL | Intraarticular saline 20 mL | - | I.v. morphine pn | Yes | Yes | No | No | No |
| Schotanus, M. G. (2015) [107] | 25/25 | Moderate | Intracapsular LIA ropivacaine 2% 150 mL. 100 mL of these with epinephrine 0.01% | - | Intracapsular LIA ropivacaine 2% 150 mL | Oral acetaminophen 1 g x 2. Oral etoricoxib 90 mg x 1. Oral gabapentin 300 mg x 1 | Tramadol p.n. | Yes | Yes | No | No | Yes |
| Ali, A. (2015) [108] | 97/95 | Moderate | Continuous intraarticular infusion of ropivacaine 15 mg/h for 48 h | - | Matching saline | Periarticular injection ropivacaine 300 mg / ketorolac 30 mg / epinephrine. Oral acetaminophen 1 g x 4. Oral diclofenac 25 mg x 3. Patch buprenorphine 10 mikrogram/h | Oxycodone p.n. | Yes | Yes | No | No | Yes |
| Gomez-Cardero, P. and E. C. Rodriguez-Merchan (2010) [109] | 25/25 | Moderate | Continuous intraarticular infusion of ropivacaine 0.2% 5 mL/h, cumulated 300 mL | - | Matching saline | Oral acetaminophen 1 g x 4. I.v. ketorolac 10 mg x 3 | I.v. morphine or s.c. pethidine p.n. | No | Yes | No | No | Yes |
| Williams, D. (2013) [110] | 24/25 | High | Continuous intraarticular infusion of bupivacaine 0.5% 2 mL/h for 48 h | - | Matching saline | Oral acetaminophen 650 mg x 6. I.v. ketorolac 15 mg x 4. Gabapentin 300 mg x 2. Oxycodone 10 mg x 2 | PCA i.v. morphine | Yes | Yes | No | No | Yes |
| Andersen, K. V. (2013) [111] | 30/30 | Moderate | Intraoperative LIA ropivacaine 300 mg and ketorolac 30 mg. Postoperative intraarticular ropivacaine 100 mg and ketorolac 15 mg every 6h | - | Intraoperative LIA ropivacaine 300 mg and saline. Postoperative intraarticular ropivacaine 100 mg and ketorolac 15 mg every 6h | Oral acetaminophen 1 g x 4 | PCA i.v. morphine | Yes | Yes | Yes | Yes | Yes |
| Sean, V. W. (2011) [112] | 50/50 | Moderate | Periarticular bupivacaine 0.5% 0.5 mL/kg with epinephrine half in deep tissue and half in skin at closure. In deep tissue triamcinolone acetonide (corticosteroid) 40 mg was added | - | Periarticular bupivacaine 0.5% 0.5 mL/kg with epinephrine half in deep tissue and half in skin at closure | Oral naproxen, unclear dose | PCA i.v. morphine | Yes | Yes | No | No | Yes |
| Tsukada, S. (2016) [113] | 38/37 | Moderate | Periarticular ropivacaine 300 mg with morphine 8 mg, ketoprofen 50 mg, epinephrine and methylprednisolone 40 mg | - | Periarticular ropivacaine 300 mg with morphine 8 mg, ketoprofen 50 mg, epinephrine | I.v. Flurbiprofen 50 mg four h after spinal anaesthesia. | Suppository diclofenac | Yes | Yes | No | No | No |
| Yue, D. B. (2013) [114] | 36/36 | Moderate | Periarticular ropivacaine 0.75% 30 mL with epinephrine and betamethasone 1 mL | - | Periarticular ropivacaine 0.75% 30 mL with epinephrine | Oral celecoxib 200 mg regularly | PCA i.v. morphine | Yes | No | No | No | No |
| Axelsson, K. (2005) [115] | 15/15/15 | High | Low dose: Epidural initiated in the PACU: ropivacaine 1.25 mg/mL + morphine 0.02 mg/mL, 8 mL/h | High dose: Epidural initiated in the PACU: ropivacaine 2 mg/mL + morphine 0.02 mg/mL, 8 mL/h | Matching saline | Oral acetaminophen 1 g preoperative. I.m. oxycodon 0.1 mg/kg preoperative. No description of postoperative | PCA i.v. morphine | Yes | Yes | No | Yes | No |
| Daabiss, M. A. and A. Kandil (2013) [116] | 40/40/40 | Moderate | Epidural bolus bupivacaine 0.5% 1 mL and magnesium sulphate 50 mg. And intraoperative epidural infusion magnesium sulphate 10 mg/h | Epidural bolus bupivacaine 0.5% 1 mL and midazolam 0.05 mg/kg. And intraoperative epidural saline infusion | Epidural bolus bupivacaine 0.5% 1 mL and saline. And intraoperative epidural saline infusion | - | PCEA fentanyl and i.m. pethidine if VAS > 30 | Yes | Yes | No | No | No |
| Hendolin, H. (1996) [117] | 10/10/10/11 | High | I.m. morphine 0.14 mg/kg 1 h preoperative. Epidural morphine 4 mg at 0 h and 3 mg at 10 h postoperative | Group 2: I.m. saline 1 h preoperative. Epidural morphine 4 mg at 0 h and 3 mg at 10 h postoperative. Group 3: I.m. morphine 0.14 mg/kg 1 h preoperative. Epidural saline 0 and 10 h postoperative | Matching i.m. and epidural saline | None (author info) | PCA i.v. fentanyl | Yes | Yes | No | No | No |
| Abrisham, S. M. (2014) [118] | 20/20 | High | Transdermal fentanyl patch 4.2 mg/patch | - | Placebo patches | None | PCA i.v. morphine | Yes | Yes | No | No | No |
| Sathitkarnmanee, T. (2014) [119] | 20/20 | High | Transdermal fentanyl patch 50 microgram/h constituted 10–12 h before surgery | - | Placebo patch | None (author info) | PCA i.v. morphine | Yes | Yes | Yes | No | No |
| Stiller, C. O. (2007) [120] | 22/28 | Moderate | I.v. tramadol 100 mg x 4 | - | Matching saline | Oral acetaminophen 1 g x 4 | PCA i.v. morphine | Yes | No | No | No | No |
| Aveline, C. (2009) [121] | 24/25/24 | Moderate | I.v. nefopam 0.2 mg/kg bolus after anaesthetic induction and 0.12 mg/kg/h until the end of surgery followed by 0.06 mg/kg/h until POD2 | I.v. ketamine 0.2 mg/kg bolus after anaesthetic induction and 0.12 mg/kg/h until the end of surgery followed by 0.06 mg/kg/h until POD2 | Placebo | None | PCA i.v. morphine | Yes | Yes | Yes | Yes | Yes |
| Adam, F. (2005) [122] | 20/20 | High | Ketamine 0.5 mg/kg bolus followed by 0.003 mg/kg/min during surgery and 0.0015 mg/kg/min after surgery | - | Placebo | FNB ropivacaine 0.75% 0.3 mL/kg and continuous ropivacaine 0.2% 0.1 mL/kg/h | PCA i.v. morphine | Yes | Yes | No | No | Yes |
| Cengiz, P. (2014) [123] | 30/30 | Moderate | Intraoperative i.v. ketamine 6 microgram/kg/minute until closure | - | Placebo | I.v. acetaminophen 1 g x 3 | PCA i.v. morphine | Yes | Yes | No | No | No |
| Casey, G. (2006) [124] | 20/20 | High | Oral nimodipine 90 mg 1 h preoperative and 30 mg x 4 postoperative | - | Placebo | Oral acetaminophen x 4 unknown dose | PCA i.v. morphine | Yes | Yes | Yes | Yes | No |
| Chan IA. (2016) [125] | 20/20 | High | I.v. dexmedetomidine 0.5 microg/kg bolus and 0.5 microg/kg/h infusion during surgery | - | Placebo | None | PCA i.v. morphine | Yes | Yes | No | No | No |
| Ho, K. Y. (2010) [126] | 23/24 | High | Oral duloxetine 60 mg 2 h before surgery and the morning of POD1 | - | Placebo | Acetaminophen 1 g x 4 | PCA i.v. morphine | Yes | Yes | Yes | Yes | No |
| Lunn, T. H. (2011) [127] | 24/24 | High | I.v. solu-medrol 125 mg just before spinal anesthesia | - | Matching saline | Oral slow-release acetaminophen 2 g x 2. Oral celecoxib 200 mg x 2. Oral gabapentin 300 mg morning + 600 mg evening | I.v. sufentanil and oral oxycodone p.n. | Yes | Yes | Yes | Yes | Yes |
| Frassanito, L. (2015) [128] | 20/20 | High | I.v. magnesium 40 mg/kg bolus and 10 mg/kg/h during surgery | - | Placebo | I.v. fentanyl 50 microgram preoperative. I.v. acetaminophen 1 g x 4. I.v. ketorolac 30 mg x 2 | PCA i.v. morphine | No | No | No | No | No |
FNB: femoral nerve block. LIA: local infiltration analgesia. PACU: postanaesthetic care unit. PCA: patient controlled analgesia. POD1: postoperative day 1. PONV: postoperative nausea and vomiting.
Risk of bias in included trials
105 trials contained at least one unclear domain (a total of 350 unclear domains). We contacted the corresponding authors by email. Email addresses were either irretrievable or permanently out of use in 22 trials. Corresponding authors for the remaining 83 trials were contacted. Forty authors replied regarding 119 unclear domains and 74 were resolved (5 high and 69 low). Forty-four domains remained unclear.
The summarized risk of bias was low in 18 trials, unclear in 65 and high in 30 (Fig 2). Further, the trial sample size bias was high in 41 trials, moderate in 69, and low or lower in three.
Fig 2. Risk of bias in included studies.
Green plus is low risk, yellow question mark is unclear risk, and red minus is high risk of bias. Slanted lines indicate that the trial is part of both surrounding subgroups. * Indicates that information regarding the bias domain has been reevaluated after obtaining an elaboration from the corresponding author of the trial.
Supplemental and basic analgesic regimens
Sixty trials administered i.v. morphine patient-controlled analgesia (PCA) as rescue medication, and reported a 0–24 hours cumulated consumption, while the remaining 53 trials administered i.v./i.m. fentanyl, oxycodone, hydromorphone, meperidine, papaveretum (a mixture of morphine, papaverine, and codeine), sufentanil or NSAIDs; patient-controlled continuous FNB; or epidural local anaesthetics/opioids. Eighty-nine trials reported cumulated opioid consumption over 20–72 hours postoperatively, seven of these also administered a second non-opioid rescue analgesic. In five trials included in meta-analyses, other types of opioids were converted to i.v. morphine equivalents. Postoperative 0–24 hours morphine consumption in the control groups for trials included in the meta-analyses ranged from 5.5–116 mg with a corrected mean of 33.1 mg per patient.
A supplemental opioid with no underlying basic analgesic regimen, was administered in 37 trials. Sixty-three trials administered a basic analgesic regimen in addition to supplemental rescue analgesics; seven trials administered acetaminophen, 13 trials NSAIDs, 12 trials acetaminophen + NSAID, seven trials local injection + other analgesics, 15 trials nerve blocks + other analgesics, and 11 trials administered different combinations of analgesics (Table 1).
Pain scores
Pain score was reported as VAS 0–100 in 42 trials; as VAS 0–10 in 52 trials; and as either numerical rating scale 0–10 (NRS 0–10), WOMAC pain scale 0–10, or verbal pain scale (VPS) 0–3 in 18 trials (S4 Appendix). After conversion to VAS 0–100 mm equivalents values in control groups ranged from 0–80 mm and 0–82 mm at rest and during mobilization, respectively. Mean pain scores in control groups for trials included in the meta-analyses were 38 mm at 6 hours rest, 33 mm at 24 hours rest, 50 mm at 6 hour movement, and 53 mm at 24 hours movement.
Pain scores at rest at 6 hours postoperatively were reported in 84 trials, and at 24 hours postoperatively in 89 trials. Pain during mobilization was reported in 33 trials at 6 hours postoperatively, and in 42 trials at 24 hours postoperatively (S4 Appendix).
Other outcomes
Ninety trials reported PONV, 24 sedation, 16 dizziness, and 43 pruritus (S4 Appendix).
LOS was reported in 36 trials of which 15 described clearly predefined discharge criteria. No trials before 2001 reported LOS. Of the 36 trials six demonstrated a statistically significant reduction in LOS.
Nineteen trials demonstrated low assay sensitivity for pain score (i.e. pain scores below 30 mm in control groups at 6 or 24h postoperatively). Thirteen trials demonstrated low assay sensitivity for morphine consumption (i.e. no morphine consumption above 15 mg i.v. morphine equivalents 0–24 hours postoperatively in control groups).
Results related to specific interventions
Seven meta-analyses were carried out. Forest plots for primary and secondary outcomes are presented in Figs 3–5 and S5–S11 Appendices, L’Abbé plots and TSA are presented in S12–S25 Appendices.
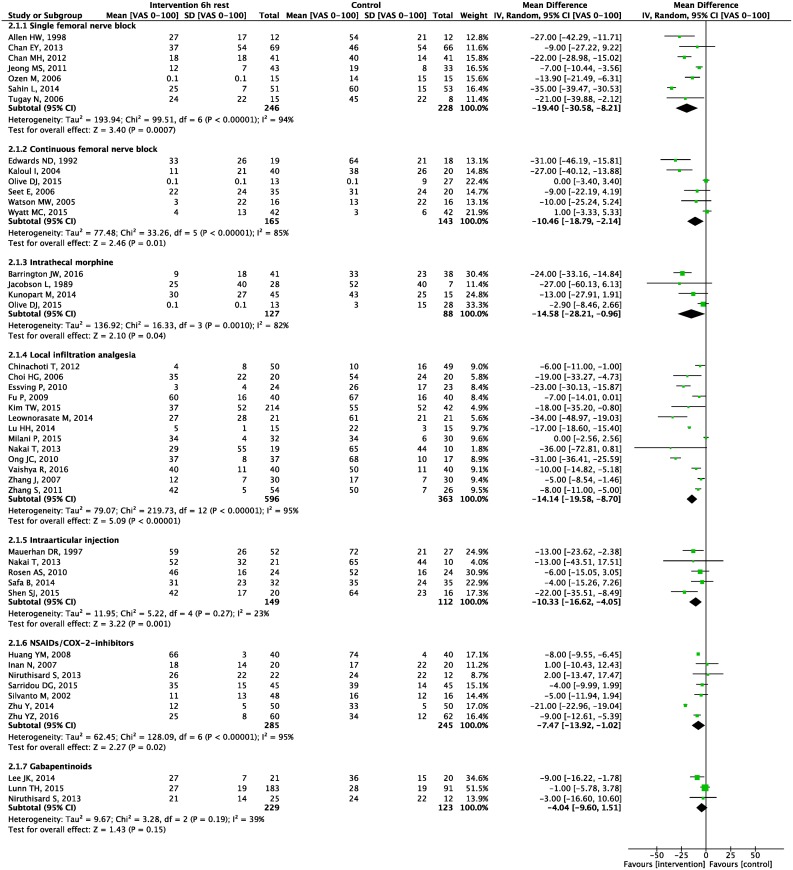
Fig 3. 0–24 hour morphine consumption.
Forest plot displaying mean difference in 0–24 hour morphine consumption for each meta-analyzed intervention. Green squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
Fig 5. 24 hours pain scores.
Forest plot displaying mean difference in pain scores 24 hours postoperative at rest for each meta-analyzed intervention. Green squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
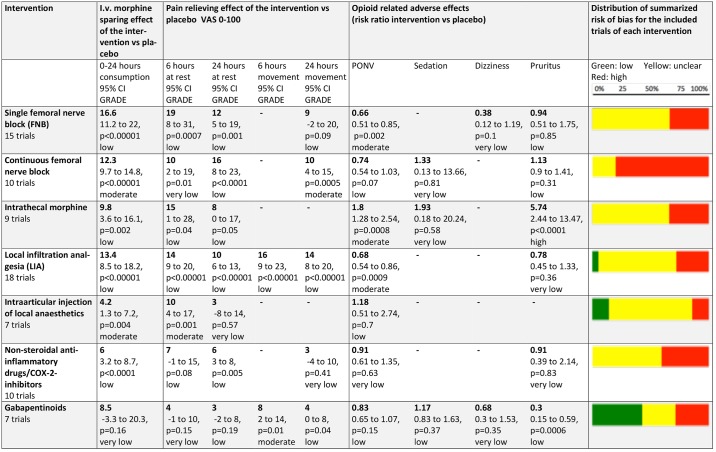
Fig 6 presents a summary of all the meta-analysed subgroups regarding outcomes, GRADE-rated quality of evidence and the estimated risk of bias of the included trials.
Fig 6. Efficacy, quality of evidence and risk of bias.
A summary of each meta-analyzed intervention regarding the effect on each outcome (opioid sparing effect in i.v. morphine equivalents mg, pain scores, and side effects), the GRADE-rated quality of evidence for each outcome and the estimated risk of bias of the included trials. The bold numbers are mean reductions for the relevant outcome, below each bold number is the 95% confidence interval, the p-value and the quality of evidence. Below each intervention the number of trials investigating the specific intervention is depicted. The colored bars to the right depict the distribution of summarized risk of bias for the included trials. Not all trials investigated all relevant outcomes. GRADE: The Grading of Recommandations Assessment, Development and Evaluation.
Single injection femoral nerve block
Fifteen trials tested single FNB as an intervention [16–30]. Four of these trials tested the intervention in addition to a basic analgesic regimen.
The summarized risk of bias was low in zero, unclear in 10, and high in five trials (Fig 2), and the trial sample size implicated a high risk of bias in seven trials, and a moderate risk in eight trials. L’Abbé plots demonstrated a lower degree of heterogeneity for pain score at rest and moderate degrees for morphine consumption and pain during movement (S12 Appendix).
Meta-analyses demonstrated a statistically significant 0–24 hour postoperative morphine sparing effect of 16.6 mg (95% CI: 11 to 22; p<0.00001) (Fig 3), and a reduction in postoperative pain scores at 6 hours at rest of 19 mm (8 to 31; P = 0.0007), at 24 hours at rest of 12 mm (5 to 19; P = 0.001), and at 24 hours during movement of 9 mm (-2 to 20; P = 0.09) (Figs 4 and 5, S6 Appendix).
Fig 4. 6 hours pain scores.
Forest plot displaying mean difference in pain scores 6 hours postoperative at rest for each meta-analyzed intervention. Green squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
In TSA, reductions in both morphine consumption and pain scores at rest at 6 and 24 hours were above the threshold for significance. Morphine consumption and 24 hours pain score at rest reached APIS concluding that single FNB has a positive effect on these outcomes (S13 Appendix).
In meta-analyses, RR for nausea and vomiting was 0.66 (0.51 to 0.85; P = 0.002), for dizziness 0.38 (0.12 to 1.19; P = 0.1) and for pruritus 0.94 (0.51 to 1.75; P = 0.85) (S7, S9 and S10 Appendices).
Urinary retention was registered in four trials [17, 19, 21, 22], deep venous thrombosis (DVT) in two [17, 21], soreness/pain in the back in two [18, 23], hypotension in one [25], numbness around the knee in one [16], and infection around the site of injection in one [26]. No significant differences between active and control groups were reported.
Quality of evidence (GRADE) was moderate for PONV; low for the opioid sparing effect and pain scores and pruritus; and very low for dizziness. Results are summarized in Table 2.
Table 2. Summarized outcomes in Grading of Recommendations Assessment, Development and Evaluation (GRADE) for each major intervention.
| Table 2 summary of findings: | |||||
|---|---|---|---|---|---|
| Single femoral nerve block compared to Placebo or no intervention for pain after TKA | |||||
| Patient or population: pain after TKA. Setting: The immediate postoperative period. Intervention: Single femoral nerve block. Comparison: Placebo or no intervention | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with Placebo or no intervention | Risk with Single femoral nerve block | ||||
| Morphine consumption assessed with: 0–24 hour postoperative | The mean morphine consumption was 32.2 mg | The mean morphine consumption in the intervention group was 16.6 mg lower (11.2 lower to 22 lower) | - | 439 (7 RCTs) | ⨁⨁◯◯ LOW a,b |
| Pain score 6 h postoperative at rest assessed with: VAS 0–100 | The mean pain score 6 h postoperative at rest was 43 mm | The mean pain score 6 h postoperative at rest in the intervention group was 19 mm lower (8 lower to 31 lower) | - | 474 (7 RCTs) | ⨁⨁◯◯ LOW a,b |
| Pain score 24 h postoperative at rest assessed with: VAS 0–100 | The mean pain score 24 h postoperative at rest was 29 mm | The mean pain score 24 h postoperative at rest in the intervention group was 12 mm lower (5 lower to 19 lower) | - | 520 (9 RCTs) | ⨁⨁◯◯ LOW a,b |
| Pain score 24 h postoperative at movement assessed with: VAS 0–100 | The mean pain score 24 h postoperative at movement was 52 mm | The mean pain score 24 h postoperative at movement in the intervention group was 9 mm lower (20 lower to 2 higher) | - | 391 (6 RCTs) | ⨁⨁◯◯ LOW a,b,c,d |
| Postoperative nausea and vomiting (PONV) assessed with: Number of events | 279 per 1,000 | 184 per 1,000 (142 to 237) | RR 0.66 (0.51 to 0.85) | 706 (11 RCTs) | ⨁⨁⨁◯ MODERATE a |
| Dizziness assessed with: Number of events | 234 per 1,000 | 89 per 1,000 (28 to 278) | RR 0.38 (0.12 to 1.19) | 340 (4 RCTs) | ⨁◯◯◯ VERY LOW a,b,d,e |
| Pruritus assessed with: Number of events | 113 per 1,000 | 106 per 1,000 (57 to 197) | RR 0.94 (0.51 to 1.75) | 464 (6 RCTs) | ⨁⨁◯◯ LOW a,d |
| Length of stay (LOS) | The mean length of stay was 5.5 days | The mean length of stay in the intervention group was 0.3 days lower (0.9 lower to 0.3 higher) | - | 332 (5 RCTs) | ⨁◯◯◯ VERY LOW a,b,d,e |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence: High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
a. There were studies of unclear and high summarized risk of bias.
b. There was heterogeneity as noted by I^2.
c. The intervention did not reach either threshold for significance or a priori estimated information size in trial sequential analysis.
d. 95% confidence interval includes 'no effect'.
e. There were less than 400 participants in total.
Various local anaesthetics +/- epinephrine were administered in the trials (Table 1). The evidence did not provide information about optimal drug-, and dose-regimens.
Continuous femoral nerve block
Ten trials tested continuous FNB as an intervention [30–39]. Six of these trials tested the intervention in addition to a basic analgesic regimen.
The summarized risk of bias was low in zero, unclear in two, and high in eight trials (Fig 2), and the trial sample size implicated a high risk of bias in four trials and a moderate risk in six. L’Abbé plots demonstrated homogeneity for morphine consumption and pain scores (S14 Appendix).
Meta-analyses demonstrated a statistically significant 0–24 hour postoperative morphine sparing effect of 12.3 mg (95% CI: 9.7 to 14.8; P<0.00001) (Fig 3), and a reduction in pain scores at rest at 6 hours postoperatively of 10 mm (2 to 19; P = 0.01), at 24 hours at rest of 16 mm (8 to 23; P<0.00001) and at 24 hours during movement of 10 mm (4 to 15; P = 0.0005) (Figs 4 and 5, S6 Appendix).
In TSA, reductions in both morphine consumption and pain scores at rest at 6 hours and 24 hours, and pain scores during movement at 24 hours, were above the threshold for significance and reached APIS, concluding that continuous femoral nerve block has a positive effect on these outcomes (S15 Appendix).
In meta-analyses, RR for nausea and vomiting was 0.74 (0.54 to 1.03, P = 0.07), for sedation 1.33 (0.13 to 13.66; P = 0.81) and for pruritus 1.13 (0.9 to 1.41; P = 0.31) (S7, S8 and S10 Appendices). One study demonstrated a significant increase in obturator motor blockade at 6 hours postoperatively [33]. Urinary retention was registered in one trial [35], cardiac events in one [38] and hypotension in two [35, 37]. No significant differences between active and control groups were reported.
Quality of evidence (GRADE) was moderate for reduction in morphine consumption and pain score at 24 hours during movement; low for 24 hours pain score at rest, PONV and pruritus; and very low for 6 hours pain score at rest and sedation. Results are summarized in Table 3.
Table 3. Summarized outcomes in Grading of Recommendations Assessment, Development and Evaluation (GRADE) for each major intervention.
| Table 3 summary of findings: | |||||
|---|---|---|---|---|---|
| Continuous femoral nerve block compared to Placebo or no intervention for pain after TKA | |||||
| Patient or population: pain after TKA. Setting: The immediate postoperative setting. Intervention: Continuous femoral nerve block. Comparison: Placebo or no intervention | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with Placebo or no intervention | Risk with Continuous femoral nerve block | ||||
| Morphine consumption assessed with: 0–24 hour postoperative | The mean morphine consumption was 20.5 mg | The mean morphine consumption in the intervention group was 12.3 mg lower (9.7 lower to 14.8 lower) | - | 264 (6 RCTs) | ⨁⨁⨁◯ MODERATE a |
| Pain score 6 h postoperative at rest assessed with: VAS 0–100 | The mean pain score 6 h postoperative at rest was 20 mm | The mean pain score 6 h postoperative at rest in the intervention group was 10 mm lower (2 lower to 19 lower) | - | 308 (6 RCTs) | ⨁◯◯◯ VERY LOW a,b,c,d |
| Pain score 24 h postoperative at rest assessed with: VAS 0–100 | The mean pain score 24 h postoperative at rest was 33 mm | The mean pain score 24 h postoperative at rest in the intervention group was 16 mm lower (8 lower to 23 lower) | - | 404 (8 RCTs) | ⨁⨁◯◯ LOW a,b,e |
| Pain score 24 h postoperative at movement assessed with: VAS 0–100 | The mean pain score 24 h postoperative at movement was 64 mm | The mean pain score 24 h postoperative at movement in the intervention group was 10 mm lower (4 lower to 15 lower) | - | 183 (4 RCTs) | ⨁⨁⨁◯ MODERATE a |
| Postoperative nausea and vomiting (PONV) assessed with: Number of events | 580 per 1,000 | 429 per 1,000 (313 to 597) | RR 0.74 (0.54 to 1.03) | 220 (5 RCTs) | ⨁⨁◯◯ LOW a,c,f |
| Sedation assessed with: Number of events | 286 per 1,000 | 380 per 1,000 (37 to 1,000) | RR 1.33 (0.13 to 13.66) | 167 (3 RCTs) | ⨁◯◯◯ VERY LOW a,b,c,f |
| Pruritus assessed with: Number of events | 507 per 1,000 | 573 per 1,000 (457 to 716) | RR 1.13 (0.90 to 1.41) | 175 (3 RCTs) | ⨁⨁◯◯ LOW a,c,f |
| Length of stay (LOS) | The mean length of stay was 6.4 days | The mean length of stay in the intervention group was 0.3 days lower (0.8 lower to 0.2 higher) | - | 171 (3 RCTs) | ⨁⨁◯◯ LOW a,c,f |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence: High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
a. There were studies of unclear and high summarized risk of bias.
b. There was heterogeneity as noted by I^2.
c. There were less than 400 participants in total.
d. The intervention did not reach either threshold for significance or a priori estimated information size in trial sequential analysis.
e. Low assay sensitivity in Seet et al explains the heterogeneity.
f. 95% confidence interval includes 'no effect'.
Various local anaesthetics +/- epinephrine were administered in all trials (Table 1). The evidence did not allow designation of optimal drug-, and dose-regimens.
Intrathecal morphine adjunct to local anaesthetics
Nine trials tested intrathecal morphine as an intervention [39–47]. Four of these trials tested the intervention in addition to a basic analgesic regimen.
The summarized risk of bias was low in zero, unclear in six, and high in three trials (Fig 2), and the trial sample size implicated a high risk of bias in two trials and a moderate risk in seven. L’Abbé plots demonstrated moderate degrees of heterogeneity for morphine consumption and pain scores (S16 Appendix).
Meta-analyses demonstrated a statistically significant 0–24 hour postoperative morphine sparing effect of 9.8 mg (95% CI: 3.6 to 16.1, P = 0.002) (Fig 3), a reduction in pain scores at rest at 6 hours postoperatively of 15 mm (1 to 28, P = 0.04), and at 24 hours at rest of 8 mm (0 to 17; P = 0.05) (Figs 4 and 5).
In TSA, morphine consumption reached the threshold for significance but not APIS. Pain score at 24 hours rest reached the boundary for futility and APIS concluding that there is no reason for further investigation of this outcome (S17 Appendix).
In meta-analyses, RR for nausea and vomiting was 1.8 (1.28 to 2.54; P = 0.0008), for sedation 1.93 (0.18 to 20.24; P = 0.58) and for pruritus 5.74 (2.44 to 13.47; P<0.0001) (S7, S8 and S10 Appendices). Hypoxemia was registered in one trial [41], respiratory depression in two [41, 47], urinary retention in three [42, 43, 45] and anxiety in one [47]. No significant differences between active and control groups were reported.
Quality of evidence (GRADE) was high for the increase in pruritus; moderate for increase in PONV; low for opioid sparing effect and reduction in pain score at 6 and 24 hours at rest; and very low for the increase in sedation. Results are summarized in Table 4.
Table 4. Summarized outcomes in Grading of Recommendations Assessment, Development and Evaluation (GRADE) for each major intervention.
| Table 4 summary of findings: | |||||
|---|---|---|---|---|---|
| Intrathecal morphine compared to Placebo or no intervention for pain after TKA | |||||
| Patient or population: pain after TKA. Setting: The immediate postoperative setting. Intervention: Intrathecal morphine. Comparison: Placebo or no intervention. | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with Placebo or no intervention | Risk with Intrathecal morphine | ||||
| Morphine consumption assessed with: 0–24 hour postoperative | The mean morphine consumption was 23.6 mg | The mean morphine consumption in the intervention group was 9.8 mg lower (3.6 lower to 16.1 lower) | - | 172 (4 RCTs) | ⨁⨁◯◯ LOW a,b |
| Pain score 6 h postoperative at rest assessed with: VAS 0–100 | The mean pain score 6 h postoperative at rest was 27 mm | The mean pain score 6 h postoperative at rest in the intervention group was 15 mm lower (28 lower to 1 lower) | - | 215 (4 RCTs) | ⨁⨁◯◯ LOW a,b,c,d,e |
| Pain score 24 h postoperative at rest assessed with: VAS 0–100 | The mean pain score 24 h postoperative at rest was 28 mm | The mean pain score 24 h postoperative at rest in the intervention group was 8 mm lower (17 lower to 0) | - | 176 (4 RCTs) | ⨁⨁◯◯ LOW a,b,e |
| Postoperative nausea and vomiting (PONV) assessed with: Number of events | 165 per 1,000 | 297 per 1,000 (209 to 419) | RR 1.80 (1.27 to 2.54) | 496 (9 RCTs) | ⨁⨁⨁◯ MODERATE a |
| Sedation assessed with: Number of events | 18 per 1,000 | 35 per 1,000 (3 to 368) | RR 1.93 (0.18 to 20.24) | 214 (3 RCTs) | ⨁◯◯◯ VERY LOW a,b,e,f |
| Pruritus assessed with: Number of events | 67 per 1,000 | 383 per 1,000 (163 to 898) | RR 5.74 (2.44 to 13.47) | 494 (9 RCTs) | ⨁⨁⨁⨁ HIGH a,b, h |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence: High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
a. There were studies of unclear and high summarized risk of bias.
b. There was heterogeneity as noted by I^2.
c. Low assay sensitivity in Olive et al explains the heterogeneity.
d. The intervention did not reach either threshold for significance or a priori estimated information size in trial sequential analysis.
e. 95% confidence interval includes 'no effect'.
f. There were less than 400 participants in total.
h. RR above five.
Diamorphine was administered in one trial and morphine in the others. Due to heterogeneity amongst trials there were no dose-response relationship and the evidence did not provide information regarding optimal dosages.
Local Infiltration Analgesia (LIA)
Eighteen trials tested LIA as an intervention [48–65]. Thirteen of these trials tested the intervention in addition to a basic analgesic regimen.
The summarized risk of bias was low in one, unclear in 12, and high in five trials (Fig 2), and the trial sample size implicated a high risk of bias in six trials, a moderate risk in 11 and a low risk in one. L’Abbé plots demonstrated low degrees of heterogeneity for morphine consumption and pain scores at rest. Moderate degrees were present for pain scores during movement (S18 Appendix).
Meta-analyses demonstrated a statistically significant 0–24 hour postoperative morphine sparing effect of 13.4 mg (95% CI: 8.5 to 18.2; P<0.00001) (Fig 3), and a reduction in pain scores at rest at 6 hours postoperatively of 14 mm (9 to 20; P<0.00001), at 24 hours rest of 10 mm (6 to 13; P<0.00001), at 6 hours during movement of 16 mm (9 to 23; P<0.00001) and at 24 hours during movement of 14 mm (8 to 20; P<0.00001) (Figs 4 and 5, S5 and S6 Appendices).
In TSA, threshold for significance and APIS were reached for all outcomes, concluding that LIA has a positive effect on these outcomes (S19 Appendix).
In meta-analyses RR for nausea and vomiting was 0.68 (0.54 to 0.86; P = 0.0009) and for pruritus 0.78 (0.45 to 1.33; P = 0.36) (S7 and S10 Appendices). One study demonstrated a significant reduction in blood loss [55] and one demonstrated a significant increase in skin blisters due to cannula [59]. Hypotension was registered in one trial [50], respiratory distress/depression in two [50, 64], headache in one [50], positive cultures from the catheter tips in one [51], rash in one [52], urinary retention in seven [52, 54, 59–62, 64], DVT in four [52, 61, 63, 64], incision complications in three [52, 54, 55], cardiac or CNS events in two [55, 63], slight numbness in one [56] and constipation in one [62]. No significant differences between active and control groups were reported.
Quality of evidence (GRADE) was moderate for PONV; low for the opioid sparing effect and reduction in pain scores, and very low for pruritus. Results are summarized in Table 5.
Table 5. Summarized outcomes in Grading of Recommendations Assessment, Development and Evaluation (GRADE) for each major intervention.
| Table 5 summary of findings: | |||||
|---|---|---|---|---|---|
| Local infiltration analgesia compared to Placebo or no intervention for pain after TKA | |||||
| Patient or population: pain after TKA. Setting: The immediate postoperative setting. Intervention: Local infiltration analgesia. Comparison: Placebo or no intervention | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with Placebo or no intervention | Risk with Local infiltration analgesia | ||||
| Morphine consumption assessed with: 0–24 hour postoperative | The mean morphine consumption was 38.9 mg | The mean morphine consumption in the intervention group was 13.4 mg lower (8.5 lower to 18.2 lower) | - | 960 (12 RCTs) | ⨁⨁◯◯ LOW a,b |
| Pain score 6 h postoperative at rest assessed with: VAS 0–100 | The mean pain score 6 h postoperative at rest was 42 mm | The mean pain score 6 h postoperative at rest in the intervention group was 14 mm lower (9 lower to 20 lower) | - | 959 (13 RCTs) | ⨁⨁◯◯ LOW a,b |
| Pain score 24 h postoperative at rest assessed with: VAS 0–100 | The mean pain score 24 h postoperative at rest was 36 mm | The mean pain score 24 h postoperative at rest in the intervention group was 10 mm lower (6 lower to 13 lower) | - | 939 (13 RCTs) | ⨁⨁◯◯ LOW a,b |
| Pain score 6 h postoperative at movement assessed with: VAS 0–100 | The mean pain score 6 h postoperative at movement was 50 mm | The mean pain score 6 h postoperative at movement in the intervention group was 16 mm lower (9 lower to 23 lower) | - | 361 (6 RCTs) | ⨁⨁◯◯ LOW a,b |
| Pain score 24 h postoperative at movement assessed with: VAS 0–100 | The mean pain score 24 h postoperative at movement was 59 mm | The mean pain score 24 h postoperative at movement in the intervention group was 14 mm lower (8 lower to 20 lower) | - | 377 (6 RCTs) | ⨁⨁◯◯ LOW a,b |
| Postoperative nausea and vomiting (PONV) assessed with: Number of events | 273 per 1,000 | 186 per 1,000 (147 to 235) | RR 0.68 (0.54 to 0.86) | 795 (10 RCTs) | ⨁⨁⨁◯ MODERATE a |
| Pruritus assessed with: Number of events | 314 per 1,000 | 245 per 1,000 (141 to 417) | RR 0.78 (0.45 to 1.33) | 368 (6 RCTs) | ⨁◯◯◯ VERY LOW a,b,c,d |
| Length of stay (LOS) | The mean length of stay was 5.6 days | The mean length of stay in the intervention group was 1 days lower (1.9 lower to 0.2 lower) | - | 449 (8 RCTs) | ⨁⨁◯◯ LOW a,b |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence: High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
a. There were studies of unclear and high summarized risk of bias.
b. There was heterogeneity as noted by I^2.
c. There were less than 400 participants in total.
d. 95% confidence interval includes 'no effect'.
Trials were too heterogeneous (administration of different combinations of local anaesthetics, morphine, NSAIDs, steroids and epinephrine, Table 1) to provide information about optimal drug-, and dose-regimens.
Intraarticular injection of local anaesthetics
Seven trials tested intraarticular injection of local anaesthetics as an intervention [65–71]. Two of these trials tested the intervention in addition to a basic analgesic regimen.
The summarized risk of bias was low in one, unclear in five, and high in one trial (Fig 2), and the trial sample size implicated a high risk of bias in two trials and a moderate risk in five. L’Abbé plots demonstrated homogeneity for morphine consumption and pain scores at rest at 6 hours and higher degrees of heterogeneity at 24 hours (S20 Appendix).
Meta-analyses demonstrated a statistically significant 0–24 hour postoperative morphine sparing effect of 4.2 mg (95% CI: 1.3 to 7.2; P = 0.004) (Fig 3), and a reduction in pain scores at rest at 6 hours postoperatively of 10 mm (4 to 17; 0.001) and at 24 hours at rest of 3 mm (-8 to 14; P = 0.57) (Figs 4 and 5).
In TSA, reductions in both morphine consumption and pain scores at rest at 6 hours were above the threshold for significance and reached APIS concluding that intraarticular injection has a positive effect on these outcomes (S21 Appendix).
In meta-analyses RR for nausea and vomiting was 1.18 (0.51 to 2.74; P = 0.70) (S7 Appendix). Respiratory depression was registered in two trials [67, 69], sinus tachycardia in one [67], DVT in one [69], wound healing complications in one [69]. No significant differences between active and control groups were reported.
Quality of evidence (GRADE) was moderate for opioid sparing effect and 6 hours pain score; low for increase in PONV; and very low for 24 hours pain score. Results are summarized in Table 6.
Table 6. Summarized outcomes in Grading of Recommendations Assessment, Development and Evaluation (GRADE) for each major intervention.
| Table 6 summary of findings: | |||||
|---|---|---|---|---|---|
| Intraarticular injection compared to Placebo or no intervention for pain after TKA | |||||
| Patient or population: pain after TKA. Setting: The immediate postoperative setting. Intervention: Intraarticular injection. Comparison: Placebo or no intervention | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with Placebo or no intervention | Risk with Intraarticular injection | ||||
| Morphine consumption assessed with: 0–24 hour postoperative | The mean morphine consumption was 39.7 mg | The mean morphine consumption in the intervention group was 4.2 mg lower (1.3 lower to 7.2 lower) | - | 372 (6 RCTs) | ⨁⨁⨁◯ MODERATE a,b |
| Pain score 6 h postoperative at rest assessed with: VAS 0–100 | The mean pain score 6 h postoperative at rest was 54 mm | The mean pain score 6 h postoperative at rest in the intervention group was 10 mm lower (4 lower to 17 lower) | - | 261 (5 RCTs) | ⨁⨁⨁◯ MODERATE c |
| Pain score 24 h postoperative at rest assessed with: VAS 0–100 | The mean pain score 24 h postoperative at rest was 47 mm | The mean pain score 24 h postoperative at rest in the intervention group was 3 mm lower (14 lower to 8 higher) | - | 261 (5 RCTs) | ⨁◯◯◯ VERY LOW a,c,d,e |
| Postoperative nausea and vomiting (PONV) assessed with: Number of events | 219 per 1,000 | 258 per 1,000 (112 to 599) | RR 1.18 (0.51 to 2.74) | 139 (3 RCTs) | ⨁⨁◯◯ LOW c,e,f |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence: High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
a. There was heterogeneity as noted by I^2.
b. Low assay sensitivity for Browne et al and Safa et al explains heterogeneity however confidence intervals for separate trials are not convincing.
c. There were studies of unclear and high summarized risk of bias.
d. The intervention did not reach either threshold for significance or a priori estimated information size in trial sequential analysis.
e. 95% confidence interval includes 'no effect'.
f. There were less than 400 participants in total.
Trials were too heterogeneous (administration of different combinations of local anaesthetics, morphine, steroids and epinephrine, Table 1) to provide information about optimal drug-, and dose-regimens.
NSAIDs/COX-2-inhibitors
Ten trials tested NSAIDs/COX-2-inhibitors as an intervention [72–81]. Two of these trials tested the intervention in addition to a basic analgesic regimen.
The summarized risk of bias was low in zero, unclear in six, and high in four trials (Fig 2) and the trial sample size implicated a high risk of bias in two trials, a moderate risk in seven and a very low risk in one. L’Abbé plots demonstrated low degrees of heterogeneity for morphine consumption and moderate degrees for pain scores (S22 Appendix).
Meta-analyses demonstrated a statistically significant 0–24 hour postoperative morphine sparing effect of 6 mg (95% CI: 3.2 to 8.7; P<0.0001) (Fig 3), and a reduction in pain scores at rest at 6 hours postoperatively of 7 mm (1 to 14; P = 0.02), at 24 hours at rest of 5 mm (3 to 8; P<0.0001), and at 24 hours during movement of 3 mm (-4 to 10; P = 0.41) (Figs 4 and 5, S6 Appendix).
In TSA, threshold for significance and APIS were reached for morphine consumption and pain at 6 and 24 hours rest concluding that NSAIDs and COX-2-inhibitors have a positive effect on these outcomes. The reduction in pain scores during movement at 24 hours reached the threshold for futility and APIS (S23 Appendix).
In meta-analyses RR for nausea and vomiting was 0.91 (0.61 to 1.35 P = 0.63) and for pruritus 0.91 (0.39 to 2.14; P = 0.83) (S7 and S10 Appendices).
Bleeding was registered in one trial [73], hypo/hypertension in one [74], anemia in two [74, 76], urinary retention in three [74, 76, 78], dry mouth in one [75], gastric pain in one [78] and constipation, hyperhidrosis, pyrexia, headache and confusion in one [76]. No significant differences between active and control groups were reported.
Quality of evidence (GRADE) was low for the opioid sparing effect and reduction in pain score at 6 and 24 hours at rest, and very low for remaining outcomes. Results are summarized in Table 7.
Table 7. Summarized outcomes in Grading of Recommendations Assessment, Development and Evaluation (GRADE) for each major intervention.
| Table 7 summary of findings: | |||||
|---|---|---|---|---|---|
| NSAIDs or COX-2-inhibitors compared to Placebo or no intervention for pain after TKA | |||||
| Patient or population: pain after TKA. Setting: The immediate postoperative setting. Intervention: NSAIDs or COX-2-inhibitors. Comparison: Placebo or no intervention | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with Placebo or no intervention | Risk with NSAIDs or COX-2-inhibitors | ||||
| Morphine consumption assessed with: 0–24 hour postoperative | The mean morphine consumption was 24.8 mg | The mean morphine consumption in the intervention group was 6 mg lower (3.2 lower to 8.7 lower) | - | 533 (6 RCTs) | ⨁⨁◯◯ LOW a,b |
| Pain score 6 h postoperative at rest assessed with: VAS 0–100 | The mean pain score 6 h postoperative at rest was 23 mm | The mean pain score 6 h postoperative at rest in the intervention group was 7 mm lower (15 lower to 1 higher) | - | 408 (6 RCTs) | ⨁⨁◯◯ LOW a,b,c |
| Pain score 24 h postoperative at rest assessed with: VAS 0–100 | The mean pain score 24 h postoperative at rest was 28 mm | The mean pain score 24 h postoperative at rest in the intervention group was 6 mm lower (3 lower to 8 lower) | - | 408 (6 RCTs) | ⨁⨁◯◯ LOW a,b |
| Pain score 24 h postoperative at movement assessed with: VAS 0–100 | The mean pain score 24 h postoperative at movement was 46 mm | The mean pain score 24 h postoperative at movement in the intervention group was 3 mm lower (10 lower to 4 higher) | - | 214 (3 RCTs) | ⨁◯◯◯ VERY LOW a,b,c |
| Postoperative nausea and vomiting (PONV) assessed with: Number of events | 234 per 1,000 | 213 per 1,000 (143 to 316) | RR 0.91 (0.61 to 1.35) | 1218 (7 RCTs) | ⨁◯◯◯ VERY LOW a,b,c |
| Pruritus assessed with: Number of events | 115 per 1,000 | 104 per 1,000 (45 to 245) | RR 0.91 (0.39 to 2.14) | 849 (3 RCTs) | ⨁◯◯◯ VERY LOW a,b,c |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence:High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
a. There were studies of unclear and high summarized risk of bias.
b. There was heterogeneity as noted by I^2.
c. 95% confidence interval includes 'no effect'.
Trials were too heterogeneous (time of administration, specific drugs, oral/i.v. administration) to provide information about optimal drug-, and dose-regimens.
Gabapentinoids
Seven trials tested gabapentinoids as an intervention [81–87]. Six of these trials tested the intervention in addition to a basic analgesic regimen.
The summarized risk of bias was low in three, unclear in two, and high in two trials (Fig 2) and the trial sample size implicated a high risk of bias in two trials, a moderate risk in four and a low risk in one. L’Abbé plots demonstrated moderate degrees heterogeneity for morphine consumption and pain scores (S24 Appendix).
Meta-analyses demonstrated non-significant reductions for 0–24 hour postoperative morphine sparing effect of 8.5 mg (95% CI: -3.3 to 20.3; P = 0.16) (Fig 3), and pain scores at rest at 6 hours postoperatively of 4 mm (-1 to 10; P = 0.15) and at 24 hours at rest of 3 mm (-2 to 8; P = 0.19). Significant reductions in pain scores at 6 and 24 hours during movement of 8 mm (2 to 14; P = 0.01) and 4 mm (0 to 8; P = 0.04), respectively, were demonstrated (Figs 4 and 5, S5 and S6 Appendices).
In TSA, threshold for significance and APIS were reached for pain at 6 and 24 hours during movement concluding that gabapentinoids have a positive effect on these outcomes. Threshold for futility and APIS were reached for pain at rest at 6 and 24 hours concluding that further testing of these outcomes is futile (S25 Appendix).
In meta-analyses RR for nausea and vomiting was 0.83 (0.65 to 1.07, P = 0.15), for sedation 1.17 (0.83 to 1.63, P = 0.37), for dizziness 0.68 (0.3 to 1.53, P = 0.35) and for pruritus 0.3 (0.15 to 0.59; P = 0.0006) (S7–S10 Appendices).
One study reported an accumulation of undesirable reactions due to the study drug in the intervention groups; lapse of memory function, impaired balance, hypotension, diplopia, sedation, dizziness and fatigue [85].
Quality of evidence (GRADE) was moderate for pain score at 6 hours at movement; low for pain scores at 24 hours at rest and during movement, PONV, sedation and pruritus; and very low for opioid sparing effect, reduction in pain score at 6 hours at rest and dizziness. Results are summarized in Table 8.
Table 8. Summarized outcomes in Grading of Recommendations Assessment, Development and Evaluation (GRADE) for each major intervention.
| Table 8 summary of findings: | |||||
|---|---|---|---|---|---|
| Gabapentinoids compared to Placebo or no intervention for pain after TKA | |||||
| Patient or population: pain after TKA. Setting: The immediate postoperative setting. Intervention: Gabapentinoids. Comparison: Placebo or no intervention | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with Placebo or no intervention | Risk with Gabapentinoids | ||||
| Morphine consumption assessed with: 0–24 hour postoperative | The mean morphine consumption was 45.6 mg | The mean morphine consumption in the intervention group was 8.5 mg lower (20.3 lower to 3.3 higher) | - | 238(3 RCTs) | ⨁◯◯◯ VERY LOW a,b,c,d |
| Pain score 6 h postoperative at rest assessed with: VAS 0–100 | The mean pain score 6 h postoperative at rest was 29 mm | The mean pain score 6 h postoperative at rest in the intervention group was 4 mm lower (10 lower to 1 higher) | - | 352 (3 RCTs) | ⨁◯◯◯ VERY LOW a,b,d |
| Pain score 24 h postoperative at rest assessed with: VAS 0–100 | The mean pain score 24 h postoperative at rest was 29 mm | The mean pain score 24 h postoperative at rest in the intervention group was 3 mm lower (8 lower to 2 higher) | - | 388 (4 RCTs) | ⨁⨁◯◯ LOW a,d |
| Pain score 6 h postoperative at movement assessed with: VAS 0–100 | The mean pain score 6 h postoperative at movement was 45 mm | The mean pain score 6 h postoperative at movement in the intervention group was 8 mm lower (2 lower to 14 lower) | - | 352 (3 RCTs) | ⨁⨁⨁◯ MODERATE a |
| Pain score 24 h postoperative at movement assessed with: VAS 0–100 | The mean pain score 24 h postoperative at movement was 51 mm | The mean pain score 24 h postoperative at movement in the intervention group was 4 mm lower (0.1 lower to 8 lower) | - | 517 (4 RCTs) | ⨁⨁◯◯ LOW a,d |
| Postoperative nausea and vomiting (PONV) assessed with: Number of events | 374 per 1,000 | 311 per 1,000 (243 to 401) | RR 0.83 (0.65 to 1.07) | 497 (6 RCTs) | ⨁⨁◯◯ LOW a,d |
| Sedation assessed with: Number of events | 267 per 1,000 | 312 per 1,000 (221 to 435) | RR 1.17 (0.83 to 1.63) | 305 (4 RCTs) | ⨁⨁◯◯ LOW a,d,e |
| Dizziness assessed with: Number of events | 417 per 1,000 | 283 per 1,000 (125 to 638) | RR 0.68 (0.30 to 1.53) | 179 (3 RCTs) | ⨁◯◯◯ VERY LOW a,b,d,e |
| Pruritus assessed with: Number of events | 282 per 1,000 | 84 per 1,000 (42 to 166) | RR 0.30 (0.15 to 0.59) | 382 (5 RCTs) | ⨁⨁◯◯ LOW a,b,e,f |
| Length of stay (LOS) | The mean length of stay was 2.9 days | The mean length of stay in the intervention group was 0.1 days higher (0.7 lower to 0.9 higher) | - | 490 (3 RCTs) | ⨁⨁◯◯ LOW b,d |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence: High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
a. There were studies of unclear and high summarized risk of bias.
b. There was heterogeneity as noted by I^2.
c. The intervention did not reach either threshold for significance or a priori estimated information size in trial sequential analysis.
d. 95% confidence interval includes 'no effect'.
e. There were less than 400 participants in total.
f. RR below 0.5.
Trials were too heterogeneous (time of administration and specific drugs) to provide information about optimal drug-, and dose-regimens.
Qualitative analyses
Forty-one trials investigated other interventions: Adductor canal block [88–91]; clonidine, tramadol, fentanyl, magnesium or buprenorphine added to FNB [92–95]; single and continuous sciatic plexus nerve block [96–100]; obturator nerve block [101, 102]; continuous lumbar plexus block [103]; morphine, ketamine or epinephrine added to intraarticular injections of local anaesthetics [104–107]; continuous intraarticular injection of local anaesthetics [108–110]; ketorolac added to periarticular injection of local anaesthetic [111]; steroids added to LIA [112–114]; epidural analgesia with ropivacaine and morphine [115]; magnesium, midazolam or morphine added to epidural bupivacaine/ropivacaine [116, 117]; fentanyl patch [118, 119]; i.v. tramadol [120]; i.v. nefopam [121]; i.v. ketamine [121–123]; p.o. nimodipine [124]; i.v. dexmedetomidine [125]; i.v. duloxetine [126]; i.v. methyl-prednisolone [127]; and i.v. magnesium [128].
Twenty-four interventions were administered together with a basic analgesic regimen (S4 Appendix).
The risk of bias was low in 12 trials, unclear in 25, and high in four.
Nine trials did not demonstrate a significant effect on opioid consumption and/or pain scores: Clonidine, tramadol or buprenorphine added to FNB [92, 93, 95]; continuous lumbar plexus block [103]; morphine, ketamine or epinephrine added to intraarticular local anaesthetics [104, 106, 107]; betamethasone added to periarticular local anaesthetics [114]; and i.v. magnesium [128]. The remaining trials demonstrated statistically significant analgesic effects. Four trials demonstrated a statistically significant effect on opioid-related adverse events: Sciatic nerve block a reduction in PONV [97, 98], dexmedetomidine a reduction in PONV and pruritus [125], and epidural analgesia with ropivacaine and morphine an increase in pruritus [115] (Table 9).
Table 9. Qualitative analysis of other interventions.
Treatment effects and adverse events are presented as results in control group → intervention group. FNB: femoral nerve block. PCA: patient controlled analgesia. LIA: local infiltration analgesia. PACU: postoperative care unit.
| Author | Treatment intervention | Treatment effect on analgesic consumption | Highest pain level in a control group (VAS 0–100) (assay sensitivity) | Treatment effect on pain scores at 6 h postoperative | Treatment effect on pain scores at 24 h postoperative | Length of hospital stay in days | PONV, events registration time | Sedation, events registration time | Dizziness, events registration time | Pruritus, events registration time |
|---|---|---|---|---|---|---|---|---|---|---|
| Andersen, H. L. (2013) [88] | Saphenous nerve block ropivacaine 0.75% 15 mL administered 3 times: in the PACU, 8:00 PM and 8:00 AM | PCA i.v. morphine day of surgery 36 → 23 mg, p = 0.28 NS Total number of FNB catheter ropivacaine boluses 2 → 0 NS | 40 mm at 6 h rest | 40 → 20 mm at rest (8pm POD0) p<0.05 | 30 → 20 mm at rest (8pm POD0) NS p>0.05 | 3.1 → 3.1 NS | 3/20 → 2/20 POD1 NS | - | - | - |
| Jenstrup, M. T. (2012) [89] | Adductor canal block ropivacaine 0.75% 30 ml immediately postoperative and additional 15 ml at 6, 12 and 18 h postoperatively. | PCA i.v. morphine 0–24 h 56 → 40 mg p = 0.006 | 70 mm at 6 h movement | 42 → 38 mm at rest NS p>0.05. 70 → 66 mm at movement p = 0.01 | 21 → 13 mm at rest p = 0.01. 58 → 39 mm at movement p = 0.01 | - | 19/37 → 8/34 0–24 h NS | - | - | - |
| Krishnan, SH. (2016) [90] | Adductor canal block bupivacaine 30 mL 0.25% ± buprenorphine 0.2 mg | Oral hydrocodone equivalents 0–24 h, 35.8 → 25.3 mg, NS p = 0.0076 | - | - | - | - | 9/49 → 13/48 0–24 h NS p = 0.305 | - | - | 1/49 → 1/48 0–24 h NS p = 0.747 |
| Shah, N. A. (2015) [91] | Adductor canal block ropivacaine 0.75% 30 mL ± continuous ropivacaine 0.25% 30 mL every 4 h 0–24 h | I.m. tramadol 0–24 h 5 → 0 mg NS p>0.05 | 27 mm at 24 h rest | 27 → 21 mm at rest p<0.001 | 27 → 21 mm at rest p<0.001 | 3.2 → 3.1 NS p = 0.157 | 1/39 → 1/46 0–48 h NS | - | - | - |
| Casati, A. (2005) [92] | FNB ropivacaine 0.75% 25 mL ± clonidine 1 microgram/kg (group 1). Continuous ropivacaine 0.2% ± clonidine 1 microgram/mL (group 2) | PCA continuous FNB POD1: 170 → 169 (group 1) and 164 mL (group 2) p = 0.51 NS. Patients requiring i.v. fentanyl. 14 → 10 (both groups) NS | 30 mm at 24 h movement | 12 → 18 (group 1) and 26 mm (group 2) at rest NS p>0.05. 19 → 26 (group 1) and 26 mm (group 2) at movement NS p>0.05 | 15 → 26 (group 1) and 26 mm (group 2) at rest NS p>0.05. 30 → 38 (group 1) and 37 mm (group 2) at movement NS p>0.05 | - | 4/19 → 6/20 and 5/19 0–48 h NS | - | - | - |
| Ekmekci, P. (2010) [93] | Continuous FNB ropivacaine 0.2% ± tramadol 1–2 mg/mL 0.1 mL/h for 48 h | I.m. diclofenac 0–24 h NS | 21 mm at 6 h rest | 21 → 16 (group 1) and 18 mm (group 2) at rest NS p>0.05 | 17 → 14 (group 1) and 6 mm (group 2) at rest NS p>0.05 | - | - | - | - | - |
| Elmawgoud, A. A. (2008) [94] | FNB ropivacaine 0.2% 30 mL ± fentanyl 4 mikrog/mL (group 1) ± magnesium (group 2) | PCA i.v. morphine 0–24 h 33.3 → 17.3 (group 1) and 16.5 mg (group 2) p<0.05 | 50 mm at 24 h rest | 30 → 20 mm at rest (both groups) p<0.05 | 50 → 30 (group 1) and 20 mm (group 2) at rest p<0.05 | - | - | - | - | - |
| Kosel, J. (2015) [95] | FNB bupivacaine 0.25% with epinephrine 0.5 mL/kg ± buprenorphine 0.3 mg | I.m. morphine 0–24 h 12.5 → 10 mg NS p = 0.4 | 40 mm at 24 h rest | - | 40 → 36 mm at rest NS p = 0.57 | 10.3 → 10.7 NS p = 0.61 | - | - | - | - |
| McNamee, D. A. (2001) [96] | Combined femoral and sciatic block with bupivacaine (group 1) / ropivacaine (group 2) 2 mg/kg divided equally between femoral and sciatic nerves | PCA i.v. morphine 0–24 h 36 → 14 (group 1) and 19 mg (group 2) p<0.05 | 29 mm at 24 h movement | 15 → 0 (group 1) and 5 mm (group 2) at movement p<0.05 (group 1) p>0.05 (group 2) | 29 → 17 (group 1) and 29 mm (group 2) at movement p<0.05 (group 1) p>0.05 (group 2) | - | - | - | - | - |
| Abdallah, F. W. (2014) [97] | Proximal (group 1) / distal (group 2) sciatic nerve block of 2:1 bupivacaine 0.5% and lidocaine 2% 30 mL with epinephrine | Oral morphine equivalents 0–24 h 167 → 131 (group 1) and 128 mg (group 2) p = 0.01 | 62 mm at 6 and 24 h movement | 48 → 28 (group 1) and 15 mm (group 2) at rest 53 → 28 (group 1) and 35 mm (group 2) at movement NS p>0.05 | 35 → 35 (group 1) and 40 mm (group 2) at rest 63 → 60 (group 1) and 58 mm (group 2) at movement NS p>0.05 | - | 13/18 → 5/17 and 4/18 0–24 h p = 0.005 | - | - | - |
| Cappelleri, G. (2011) [98] | Continuous lumbar plexus block levobupivacaine 0.125% 8 mL/h ± Continuous sciatic nerve block levobupivacaine 0.06% 0.1mL/kg | PCA i.v. morphine 0–24 h 4.6 → 2.3 mg p<0.01 | 29 mm at 24 h movement | 14 → 15 mm at rest NS p>0.05. 10 → 10 mm at movement NS p>0.05 | 21 → 8 mm at rest NS p>0.05. 29 → 10 mm at movement p<0.05 | - | 7/18 → 1/19 0–48 h p = 0.013 | - | - | 1/18 → 0/19 0–48 h NS |
| Martinez Navas, A. and M. Echevarria Moreno (2006) [99] | Sciatic nerve block ropivacaine 0.5% 20 mL ± Continuous ropivacaine 0.2% 5 mL/h | S.c. morphine 0–24 h NS | 52 mm at 24 h movement | 17 → 9 mm at rest. 21 → 11 mm at movement NS p>0.05 | 25 → 9 mm at rest NS p>0.05. 52 → 11 mm at movement p<0.05 | - | 0/10 → 0/7 Unclear NS | - | - | - |
| Sato, K. (2014) [100] | Sciatic nerve block ropivacaine 0.2% 20 mL± Continuous ropivacaine 0.2% 5 mL/h | PCA i.v. morphine 0–24 h 7.3 → 4 mg NS p = 0.057 | 32 mm at 24 h rest | 22 → 19 mm at rest NS p>0.05 | 32 → 12 mm at rest p<0.05 | 23 → 25 NS | 6/30 → 5/30 0–48 h NS | - | - | - |
| McNamee, D. A. (2002) [101] | Obturator nerve block ropivacaine 0.75% 5 mL Femoral and sciatic nerve block ro-pivacaine 0.75%15 mL each nerve | PCA i.v. morphine 0–24 h 45 → 32 mg p<0.05 | 25 mm at 24 h movement | 7 → 5 mm at movement NS p>0.05 | 25 → 28 mm at movement NS p>0.05 | - | - | - | - | - |
| Runge, C. (2016) [102] | Obturator nerve block bupivacaine 46 mg, clonidine 0.0375 mg, dexamethasone 2 mg, epinephrine | PCA i.v. morphine 0–24 h 20 → 2 mg p = 0.0007 | 40 mm at 6 h rest | 20 → 5 mm at rest p>0.05. 40 → 15 mm at movement p<0.025 | 19 → 0 mm at rest. 30 → 18 mm at movement p<0.025 | NS | NS | - | - | - |
| Frassanito, L. (2009) [103] | Single lumbar plexus block ropivacaine 0.6% 30 mL. Single sciatic block ropivacaine 0.6% 15 mL ± Continuous lumbar plexus infusion of ropivacaine 0.2% 10 mL/h for 48 h | I.v. tramadol 0–72 h 236 → 185 mg NS p = 0.06 | 50 mm at 24h rest | 0 → 5 mm at rest (unclear time for registration) NS p>0.05 | 50 → 38 mm at rest (unclear time for registration) NS p>0.05 | - | 3/22 → 1/22 0–48 h NS | - | - | - |
| Badner, N. H. (1997) [104] | Intraarticular injection ± bupivacaine 0.5% 30 mL (group 1) ± morphine 1 mg (group 2) with epinephrine | PCA i.v. morphine 0–24 h 68 → 58 (group 1) and 55 mg (group 2) NS p>0.05 | 63 mm at 6 h rest | 63 → 60 (group 1) and 55 mm (group 2) at rest NS p>0.05 | 52 → 51 (group 1) and 45 mm (group 2) at rest NS p>0.05 | - | - | - | - | - |
| Garcia, J. B. (2010) [105] | Intraarticular 10 mg morphine in 20 mL | S.c. morphine 0–24 h 20.6 → 12.2 mg p = 0.0001 | 80 mm at 6 h rest | 80 → 50 mm at rest p = 0.006 | 20 → 20 mm at rest NS p>0.05 | - | 7/25 → 7/25 0–24 h NS | 4/25 → 4/25 0–24 h NS | - | - |
| Guara Sobrinho, H. (2012) [106] | Intraarticular ketamine 0.25 (group 1) / 0.5 (group 2) mg/kg in 20 mL | I.v. morphine 0–24 h 14.5 → 14.9 (group 1) and 14 mg (group 2) NS p = 0.52 | 55 mm at 6 h rest | 55 → 48 (group 1) and 58 mm (group 2) at rest NS p = 0.68 | 32 → 31 (group 1) and 31 mm (group 2) at rest NS p = 0.76 | - | 7/20 → 8/19 and 6/17 0–24 h NS | 4/20 → 1/19 and 4/17 0–24 h NS | 1/20 → 3/19 and 5/17 0–24 h NS | - |
| Schotanus, M. G. (2015) [107] | Intracapsular LIA ropivacaine 2% 150 mL ± epinephrine 0.01% | - | 34 mm at 24 h rest | 26 → 28 mm at rest NS p>0.05 | 34 → 29 mm at rest NS p>0.05 | - | - | - | - | - |
| Ali, A. (2015) [108] | Continuous intraarticular infusion of ropivacaine 15 mg/h for 48 h | Oxycodone 0–72 h 25 → 20 mg NS p = 0.06 | 40 mm at 24 h rest | 21 → 14 mm at rest (8pm) p = 0.05 | 40 → 33 mm at rest (day 1 at 12 noon) p = 0.02 | 4.1 → 4.1 NS p = 0.8 | NS | - | - | - |
| Gomez-Cardero, P. and E. C. Rodriguez-Merchan (2010) [109] | Continuous intraarticular infusion of ropivacaine 0.2% 5 mL/h, cumulated 300 mL | Patients requiring supplementary analgesia 38 → 14% p<0.05 | 57 mm at 24 h rest | - | 57 → 38 mm at rest p<0.001 | 7.3 → 5.7 p<0.001 | - | - | - | - |
| Williams, D. (2013) [110] | Continuous intraarticular infusion of bupivacaine 0.5% 2 mL/h for 48 h | PCA i.v. morphine 0–24 h 19.2 → 13.8 mg NS p = 0.58 | 31 mm at 6 h rest | 31 → 24 mm at rest NS p = 0.0428 | 21 → 17 mm at rest NS p = 0.0386 | 3.9 → 4.7 NS p = 0.155 | 3/25 → 1/24 NS | 12/25 → 9/24 0–24 h NS | - | 0/25 → 1/24 0–24 h NS |
| Andersen, K. V. (2013) [111] | Intraoperative LIA ropivacaine 300 mg ± ketorolac 30 mg. Postoperative intraarticular ropivacaine 100 mg and ketorolac 15 mg every 6h | PCA i.v. morphine 0–24 h 25 → 5 mg p<0.0001 | 64 mm at 24 h movement | 29 → 6 mm at rest (2–6 h mean values) p = 0.0003 29 → 19 mm at rest p<0.002 | 40 → 12 mm at rest (6–24 h mean values) p = 0.0001 64 → 29 mm at rest p<0.0001 | 3 (3–3) → 3 (2–3) median interquartile range p = 0.02 | NS | - | - | NS |
| Sean, V. W. (2011) [112] | Periarticular bupivacaine 0.5% 0.5 mL/kg with epinephrine ± Deep tissue triamcinolone acetonide (corticosteroid) 40 mg | PCA i.v. morphine 0–24 h 1.3 → 0.9 mg p = 0.03 | 18 mm at 6 h rest | 18 → 17 mm at rest NS p>0.05 | 14 → 12 mm at rest NS p>0.05 | 6.8 → 5.2 p = 0.02 | - | - | - | - |
| Tsukada, S. (2016) [113] | Periarticular ropivacaine 300 mg with morphine 8 mg, ketoprofen 50 mg, epinephrine ± methylprednisolone 40 mg | Diclofenac consumption on the night of surgery NS | 26 mm at 24 h rest | 3 → 2 mm at rest NS p = 0.8 | 26 → 17 mm at rest NS p = 0.057 | - | 1/37 → 2/38 night of surgery NS p = 0.57 | - | - | 0/37 → 1/38 night of surgery NS p = 0.31 |
| Yue, D. B. (2013) [114] | Periarticular ropivacaine 0.75% 30 mL with epinephrine ± betamethasone 1 mL | PCA i.v. morphine day 1 13.5 → 13 mg NS p>0.05 | 82 mm at 24 h movement | 43 → 41 mm at rest NS p>0.05 | 63 → 62 mm at rest NS p>0.05. 82 → 83 mm at movement NS p>0.05 | - | - | - | - | - |
| Axelsson, K. (2005) [115] | Epidural initiated in the PACU: ropivacaine 1.25 (group 1) / 2 (group 2) mg/mL + morphine 0.02 mg/mL, 8 mL/h. | PCA i.v. morphine 0–24 h 45.8 → 21.8 (group 1) and 8.2 mg (group 2) p<0.001 and p<0.0001 | 59 mm at 24 h movement | 33 → 35 (group 1) and 4 mm (group 2) at rest 58 → 46 (group 1) and 9 mm (group 2) at movement p>0.05 (group 1) p<0.01 (group 2) | 21 → 16 (group 1) and 9 mm (group 2) at rest p>0.05 59 → 42 (group 1) and 22 mm (group 2) at movement p>0.05 (group 1) p<0.01 (group 2) | - | 3/15 → 4/15 and 2/15 Unclear NS | - | 1/15 → 3/15 and 1/15 Unclear NS | 1/15 → 8/15 and 8/15 Unclear p<0.05 |
| Daabiss, M. A. and A. Kandil (2013) [116] | Epidural bolus bupivacaine 0.5% 1 mL ± magnesium sulphate 50 mg and infusion 10 mg/h (group 1) / ± midazolam 0.05 mg/kg (group 2) | PCEA fentanyl 0–24 h 321 → 220 (group 1) and 256 microgram (group 2) p<0.05 I.m. pethidine 0–24 h 92 → 53 (group 1) and 70 mg (group 2) p<0.05 | 5 mm at 6 and 24 h rest | 5 → 3 (group 1) and 4 mm (group 2) at rest NS p>0.05 | 5 → 5 (group 1) and 5 mm (group 2) at rest NS p>0.05 | - | 3/40 → 1/40 and 2/40 0–24 h NS | 0/40 → 0/40 and 2/40 0.24 h NS | - | - |
| Hendolin, H. (1996) [117] | Group 1: I.m. morphine 0.14 mg/kg 1 h preoperative. Epidural morphine 4 mg at 0 h and 3 mg at 10 h postoperative. Group 2: Epidural morphine 4 mg at 0 h and 3 mg at 10 h postoperative. Group 3: I.m. morphine 0.14 mg/kg 1 h preoperative | PCA i.v. fentanyl 0–20 h 0.82 → 0.46 (group 1), 0.39 (group 2) and 0.79 mg/kg (group 3) p<0.01 (group 1 and 2), p>0.05 (group 3) | 48 mm at 6 h rest | 48 → 26 (group 1), 27 (group 2) and 25 mm (group 3) at rest p<0.001 | 23 → 17 (group 1), 23 mm (group 2) and 35 mm (group 3) at rest NS p>0.05 | - | 5/11 → 6/10, 6/10 and 4/10 0–20 h NS | NS | - | 0/11 → 3/10, 5/10 and 2/10 0–20 h NS |
| Abrisham, S. M. (2014) [118] | Transdermal fentanyl patch 4.2 mg/patch | PCA i.v. morphine 0–72 h 40 → 33 mg p = 0.01 | 67 mm at 6 h rest | 67 → 54 mm at rest p = 0.035 | 57 → 37 mm at rest p = 0.002 | - | 9/20 → 5/20 Unclear NS | - | - | 2/20 → 4/20 Unclear NS |
| Sathitkarnmanee, T. (2014) [119] | Transdermal fentanyl patch 50 microgram/h constituted 10–12 h before surgery | PCA i.v. morphine 0–24 h 24.9 → 15.4 mg p = 0.001 | 64 mm at 6 h movement | 46 → 27 mm at rest (mean score 0–48 h) p = 0.002 64 → 44 mm at movement (mean score 0–48 h) p = 0.002 | - | - | - | - | - | - |
| Stiller, C. O. (2007) [120] | I.v. tramadol 100 mg x 4 | PCA i.v. morphine 0–24 h 72 → 51 mg p<0.05 | 63 mm at 6 h rest | 60 → 63 mm at rest NS p>0.05 | - | - | 15/32 → 11/31 0–24 h NS | 13/32 → 9/31 0-24h NS | - | - |
| Aveline, C. (2009) [121] | I.v. nefopam (group 1) / ketamine (group 2) 0.2 mg/kg after induction, 0.12 mg/kg/h during surgery followed by 0.06 mg/kg/h until POD2 | PCA i.v. morphine 0–24 h 56.8 → 39.3 (group 1) and 39.2 mg (group 2) p<0.0001 | 60 mm at 6 h movement | 41 → 38 (group 1) and 34 mm (group 2) at rest 60 → 58 (group 1) and 56 mm (group 2) at movement NS p>0.05 | 36 → 37 (group 1) and 23 mm (group 2) at rest p<0.005 (group 2) 55 → 47 (group 1) and 50 mm (group 2) at movement NS p>0.05 | 14.1 → 13 (group 1) and 12 (group 2) NS | 9/24 → 7/24 and 4/25 0–48 h NS | 0–48 h NS | - | - |
| Adam, F. (2005) [122] | Ketamine 0.5 mg/kg bolus followed by 0.003 mg/kg/min during surgery and 0.0015 mg/kg/min after surgery | PCA i.v. morphine 0–48 h 69 → 45 mg p<0.02 | 33 mm at 24 h rest | 28 → 26 mm at rest NS p>0.05 | 33 → 29 mm at rest NS p>0.05 | 11 → 11 NS | 3/20 → 2/20 Unclear NS | 0/20 → 0/20 Unclear NS | - | - |
| Cengiz, P. (2014) [123] | Intraoperative i.v. ketamine 6 microgram/kg/minute until closure | PCA i.v. morphine 0–24 h 85.2 → 47 mg p<0.001 | 21 mm at 6 h rest | 21 → 9 mm at rest p<0.001 | 6 → 2 mm at rest p<0.001 | - | 5/30 → 1/30 0–24 h NS | - | - | - |
| Casey, G. (2006) [124] | Oral nimodipine 90 mg 1 h preoperative and 30 mg x 4 postoperative | PCA i.v. morphine 0–24 h 45 → 62 mg p = 0.02 | 57 mm at 6h rest | 50 → 47 mm at rest 57 → 58 mm at movement NS p>0.05 | 34 → 23 mm at rest 51 → 45 mm at movement NS p>0.05 | - | 8/20 → 7/20 Unclear NS | - | - | - |
| Chan IA. (2016) [125] | I.v. dexmedetomidine 0.5 microg/kg bolus and 0.5 microg/kg/h infusion during surgery | PCA i.v. morphine 0–24 h 61.2 → 29.2 mg NS p<0.001 | 50 mm at 24 h rest | 50 → 44 mm at rest NS p = 0.41 | 50 → 48 mm at rest NS p = 0.79 | - | 7/20 → 1/20 0–24 h p = 0.005 | - | - | 6/20 → 1/20 0–24 h p = 0.015 |
| Ho, K. Y. (2010) [126] | Oral duloxetine 60 mg 2 h before surgery and the morning of POD1 | PCA i.v. morphine 0–24 h 19.8 → 12.9 mg p = 0.039 | 50 mm at 24 h movement | 10 → 10 mm at rest 25 → 20 mm at movement NS p>0.05 | 10 → 10 mm at rest 50 → 20 mm at movement NS p>0.05 | - | 5/24 → 3/23 Unclear NS | 3/24 → 0/23 Unclear NS | 3/24 → 2/23 Unclear NS | 1/24 → 0/23 Unclear NS |
| Lunn, T. H. (2011) [127] | I.v. solumedrol 125 mg just before spinal anesthesia | Patients requiring sufentanil 0–24 h 10 → 2 p<0.05. Oral oxycodone 0–24 h, 20 → 10 mg p<0.05 | 70 mm at 24 h movement | 34 → 17 mm at rest 65 → 16 mm at movement p<0.01 | 47 → 19 mm at rest 70 → 27 mm at movement p<0.01 | 2 → 2 NS | 4/24 → 2/24 0–24 h NS | - | - | - |
| Frassanito, L. (2015) [128] | I.v. magnesium 40 mg/kg bolus and 10 mg/kg/h during surgery | PCA i.v. morphine 0–24 h, 14.4 → 13.9 mg NS p = 0.4 | 30 mm at 24 h rest | 0 → 0 (average of rest and movement) NS p>0.05 | 30 → 25 (average of rest and movement) NS p>0.05 | - | 3/20 → 6/20 0–24 h NS | - | - | 2/20 → 4/20 0–24 h NS |
Discussion
We have reviewed randomized controlled trials regarding postoperative analgesia after TKA, and have demonstrated analgesic effects in meta-analyses for single injection- and continuous FNB, intrathecal morphine, LIA, intraarticular injection of local anaesthetics, NSAIDs/COX-2 inhibitors, and gabapentinoids; and furthermore in stand-alone trials for a number of different interventions, according to the PRISMA checklist (S26 Appendix). By conducting meta-analyses we have enhanced the evidence to the highest level possible with the present trials. While this sounds promising, the quality of evidence throughout the included data is discouragingly low due to uncertain or high risk of bias, low sample size in trials and meta-analysis interventions, heterogeneous results, and low assay sensitivity. These findings are similar to the results in our recent systematic review on pain management after THA [2]. Consequently, we have demonstrated that no optimal strategy for postoperative pain treatment after TKA exist in the literature.
The accepted level of pain varies in the analyzed material. In some trials no basic analgesic regimens were provided and high pain scores were accepted, whereas in other trials acetaminophen, NSAIDs, gabapentin, and even FNB were administered as adjuncts to the intervention. In these trials both intervention- and control groups tended to have lower pain scores. These differences lead to a considerable variance in assay sensitivity amongst trials. The wide variation in trial-setup may be accounted for by cultural or tradition based differences in the approach to analgesic treatment, e.g. the propensity to apply invasive procedures or the general pain threshold.
Interpretation of meta-analysed interventions
For oral treatments we analyzed two subgroups: NSAIDs/COX-2-inhibitors and gabapentinoids.
The included trials in the NSAID subgroup were characterized by low assay sensitivity, which contributes to a low absolute effect. However, the intervention provided a small but statistically significant effect on pain scores and morphine consumption. The adverse event profile of NSAIDs is controversial and short follow up periods in randomized pain trials in general may be problematic for the detection hereof (17). However, the meta-analyses did not demonstrate an increased risk of adverse effects which is supported by similar results in the review regarding THA [2].
The evidence regarding gabapentinoids was even less convincing, with insignificant results partially due to a low number of included trials.
Four meta-analysed interventions investigated procedure specific local anaesthetic interventions: single FNB, continuous FNB, LIA, and intraarticular injection of local anaesthetics. When reviewing the outcomes, intraarticular injection tended to be inferior compared to the other interventions.
Single FNB performed slightly better in two out of three primary outcomes compared to continuous FNB. The strength of evidence in TSA was generally high for both interventions. Continuous FNB is a more invasive, time consuming and for the patient cumbersome procedure due to the postoperative catheterization.
Single FNB and LIA provided equally satisfying analgesia. Both procedures demonstrated a relevant reduction in morphine consumption, pain scores and PONV. A recent systematic review of trials comparing LIA to FNB after TKA reported a small insignificant difference in analgesic effect favoring LIA [129]. The current evidence does not allow designation of a superior intervention amongst the two, but the well-known risk of motor blockade with FNB may render this method less attractive [130]. It should be noted that different combinations of drugs and dosages were administered for both FNB and LIA. Pinpointing optimal analgesics regimens for FNB and LIA are imperative for designation of a superior intervention.
The meta-analysis of intrathecal morphine demonstrated some analgesic effect on morphine consumption and pain scores, but a rather large increase in morphine related adverse events.
Strengths and limitations
The large amounts of data in this review were manually typed with more than 12.000 separate boxes in Excel®, creating a major potential for typing errors. To minimize this risk data were analyzed and registered by two independent authors with prior data extraction experience, and subsequently compared.
In a considerable number of trials data were presented as medians and range/IQR, likely because of a skewed distribution. Treating data as normally distributed by converting to mean/SD was necessary, but nonetheless a limitation.
About half of the corresponding authors replied our emails regarding bias. This resolved 74 unclear domains and altered the total number of trials with low summarized risk of bias from six to 15. This is still only 13% of all trials. For trials included in meta-analyses this proportion was 7%, which is problematic as the quality of the meta-analyses is partially limited by the quality of the trials. The majority of trials had an unclear summarized risk of bias. We believe that in most of these trials, relatively few and easily attainable measures would be required to improve this risk from unclear to low, especially if authors had access to a standardized postoperative pain trial protocol that took into account the pitfalls leading to high or unclear risk of bias.
Opioid consumption and pain intensity are associated, hence both outcomes require assessment. Whether opioid consumption and pain should be calculated as absolute or relative differences between treatment and control groups, or as the number of patients with a predefined level of pain, is controversial. In this review we chose to report effects as absolute (mean) differences, which may be arguable.
The majority of included interventions each provided acceptable levels of analgesia, however it is probably reasonable to keep postoperative analgesic treatment to a limited number of interventions. Each additional intervention added to the standard postoperative analgesic regimen may increase the risk of adverse effects or events [131]. Regarding invasive procedures we must consider the risk of inducing severe adverse effects and the time consumption by qualified personnel such as doctors. Furthermore, we know little about the effect of combining different analgesic interventions after TKA [132]. Thus, the absolute analgesic effect may decline for each additional analgesic, because different interventions may affect the same analgesic pathways, and because the analgesic potential is probably lower when pain levels are already reduced by other analgesics.
In conclusion, no gold treatment for pain treatment after TKA exists in the literature. The GRADE rated recommendations varied from very low to moderate (except for one high) for the different interventions. High or unclear risk of bias, heterogeneity of trial designs, and the small trial sample sizes, are challenges in designation of a best proven optimal postoperative analgesic regimen for TKA. A way to overcome these challenges may be to establish standard research guidelines regarding postoperative pain management, and focus on conducting high quality upscale trials.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
Green squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
(PDF)
Green squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
(PDF)
Blue squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
(PDF)
Blue squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
(PDF)
Blue squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
(PDF)
Blue squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
(PDF)
Green squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
(PDF)
Higher degrees of heterogeneity were demonstrated for pain at 6 and 24 hours rest and 24 hours movement. The size of the ball resembles the number of included patients in that trial and it is standardized across the different plots.
(PDF)
A priori estimated information sizes (APIS) (333, 730, 321 and 560 patients, respectively) were based on an alpha-value of 0.05 and a beta-value of 0.9. The sensitivity to detect a mean difference for opioid consumption was predefined as 10 mg morphine equivalents 0–24 h postoperative and for pain scores a mean difference of 15 mm (VAS 0–100 mm). The blue line depicts the cumulative Z-score of the meta-analysis. The outer red lines illustrate the sequential z-score threshold for significance. The inner red lines illustrate the area of futility. The burgundy lines represent a stationary Z-score at 1.96 corresponding to p = 0.05. Threshold for significance was reached for morphine consumption and pain at 6 and 24 hours rest. Morphine consumption and pain score at 24 hours rest reached APIS, concluding that the intervention has an effect on these outcomes.
(PDF)
Homogeneity was demonstrated for morphine consumption and pain scores. The size of the ball resembles the number of included patients in that trial and it is standardized across the different plots.
(PDF)
A priori estimated information sizes (APIS) (47, 268, 272 and 69 patients, respectively) were based on an alpha-value of 0.05 and a beta-value of 0.9. Threshold for significance and APIS were reached for morphine consumption and all pain scores concluding that continuous femoral nerve block has a positive effect on these outcomes. For further elaboration see S13 Appendix.
(PDF)
Moderate degrees of heterogeneity were demonstrated for morphine consumption and pain scores. The size of the ball resembles the number of included patients in that trial and it is standardized across the different plots.
(PDF)
A priori estimated information sizes (APIS) (183, 490 and 151 patients, respectively) were based on an alpha-value of 0.05 and a beta-value of 0.9. Morphine consumption reached the threshold for significance but not APIS. Pain score at 24 hours rest reached the boundary for futility and APIS concluding that there is no reason for further investigation of this outcome. For further elaboration see S13 Appendix.
(PDF)
Lower degrees of heterogeneity were demonstrated for morphine consumption and pain scores at rest. Moderate degrees were present for pain scores at movement. The size of the ball resembles the number of included patients in that trial and it is standardized across the different plots.
(PDF)
A priori estimated information sizes (APIS) (635, 349, 149, 217 and 174 patients, respectively) were based on an alpha-value of 0.05 and a beta-value of 0.9. Threshold for significance and APIS were reached for all end-points, concluding that LIA has a positive effect on these outcomes. For further elaboration see S13 Appendix.
(PDF)
Homogeneity was demonstrated for morphine consumption and pain at 6. Pain scores at 24 hours at rest were heterogeneous. The size of the ball resembles the number of included patients in that trial and it is standardized across the different plots.
(PDF)
A priori estimated information sizes (APIS) (88, 127 and 394 patients, respectively) were based on an alpha-value of 0.05 and a beta-value of 0.9. Threshold for significance and APIS were reached for morphine consumption and pain at 6 hours rest concluding that intraarticular injection has a positive effect on these outcomes. For further elaboration see S13 Appendix.
(PDF)
Lower degrees of heterogeneity were demonstrated for morphine consumption and moderate degrees of heterogeneity for pain scores. The size of the ball resembles the number of included patients in that trial and it is standardized across the different plots.
(PDF)
A priori estimated information sizes (APIS) (115, 270, 32 and 166 patients, respectively) were based on an alpha-value of 0.05 and a beta-value of 0.9. Threshold for significance and APIS were reached for morphine consumption and pain at 6 and 24 hours rest concluding that NSAIDs and COX-2-inhibitors has a positive effect on these outcomes. Morphine consumption and pain at 24 hours rest reached APIS with the first trial. Threshold for futility and APIS were reached for pain at 24 h movement concluding that further testing of this end-point is futile. For further elaboration see S13 Appendix.
(PDF)
Moderate degrees of heterogeneity was demonstrated for morphine consumption and pain at 24 hours rest. The size of the ball resembles the number of included patients in that trial and it is standardized across the different plots.
(PDF)
A priori estimated information sizes (APIS) (905, 132, 104, 147 and 108 patients, respectively) were based on an alpha-value of 0.05 and a beta-value of 0.9. Threshold for significance and APIS were reached for pain at 6 and 24 hours at movement concluding that gabapentinoids have a positive effect on these outcomes. Threshold for futility and APIS were reached for pain at 6 and 24 h at rest concluding that further testing of these end-points is futile. For further elaboration see S13 Appendix.
(PDF)
(DOC)
Acknowledgments
Thanks to Chenxi Huang M.D. for translation of Chinese articles. No other than above-mentioned authors contributed to the article.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003;362(9399):1921–8. 10.1016/S0140-6736(03)14966-5 [DOI] [PubMed] [Google Scholar]
- 2.Hojer Karlsen AP, Geisler A, Petersen PL, Mathiesen O, Dahl JB. Postoperative pain treatment after total hip arthroplasty: a systematic review. Pain. 2015;156(1):8–30. 10.1016/j.pain.0000000000000003 [DOI] [PubMed] [Google Scholar]
- 3.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology. 2009;62(10):1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 4.Choice Pharma. PROSPECT [cited 2014 16 Jan]. http://www.postoppain.org/frameset.htm.
- 5.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. Bmj. 2013;346:f2304 10.1136/bmj.f2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Informatics and Knowledge Management Department. http://tech.cochrane.org Denmark: The Cochrane Collaboration; November 2012 [January 29, 2014]. http://tech.cochrane.org/revman.
- 8.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]: The Cochrane Collaboration, 2009; [cited 2014 March 14]. http://www.cochrane-handbook.org.
- 9.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC medical research methodology. 2005;5:13 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song F. Exploring heterogeneity in meta-analysis: is the L'Abbe plot useful? Journal of clinical epidemiology. 1999;52(8):725–30. [DOI] [PubMed] [Google Scholar]
- 11.Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of clinical epidemiology. 2008;61(1):64–75. 10.1016/j.jclinepi.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 12.Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA): Copenhagen Trial Unit, Center for Clinical Intervention Research, Department 3344 Rigshospitalet, Denmark.
- 13.Kelly AM. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain? Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 1998;5(11):1086–90. [DOI] [PubMed] [Google Scholar]
- 14.Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Annals of emergency medicine. 1996;27(4):485–9. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ, et al. What is "quality of evidence" and why is it important to clinicians? Bmj. 2008;336(7651):995–8. 10.1136/bmj.39490.551019.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen HW, Liu SS, Ware PD, Nairn CS, Owens BD. Peripheral nerve blocks improve analgesia after total knee replacement surgery. Anesthesia and analgesia. 1998;87(1):93–7. [DOI] [PubMed] [Google Scholar]
- 17.Chan EY, Fransen M, Sathappan S, Chua NH, Chan YH, Chua N. Comparing the analgesia effects of single-injection and continuous femoral nerve blocks with patient controlled analgesia after total knee arthroplasty. The Journal of arthroplasty. 2013;28(4):608–13. 10.1016/j.arth.2012.06.039 [DOI] [PubMed] [Google Scholar]
- 18.Chan MH, Chen WH, Tung YW, Liu K, Tan PH, Chia YY. Single-injection femoral nerve block lacks preemptive effect on postoperative pain and morphine consumption in total knee arthroplasty. Acta anaesthesiologica Taiwanica: official journal of the Taiwan Society of Anesthesiologists. 2012;50(2):54–8. [DOI] [PubMed] [Google Scholar]
- 19.Good RP, Snedden MH, Schieber FC, Polachek A. Effects of a preoperative femoral nerve block on pain management and rehabilitation after total knee arthroplasty. American journal of orthopedics (Belle Mead, NJ). 2007;36(10):554–7. [PubMed] [Google Scholar]
- 20.Hunt KJ, Bourne MH, Mariani EM. Single-injection femoral and sciatic nerve blocks for pain control after total knee arthroplasty. The Journal of arthroplasty. 2009;24(4):533–8. 10.1016/j.arth.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 21.Jeong MS, Song EK, Seon JK, Byun JW, Lee KJ, Jung YW. Effectiveness of Pain Relief for Femoral Nerve Block in Multimodal Pain Control Protocols in Total Knee Arthroplasty. Journal of the Korean Orthopaedic Association [Internet]. 2011; 46(3):[237–43 pp.]. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/775/CN-01044775/frame.html. [Google Scholar]
- 22.Kardash K, Hickey D, Tessler MJ, Payne S, Zukor D, Velly AM. Obturator versus femoral nerve block for analgesia after total knee arthroplasty. Anesthesia and analgesia. 2007;105(3):853–8. 10.1213/01.ane.0000278158.36843.f7 [DOI] [PubMed] [Google Scholar]
- 23.Lee AR, Choi DH, Ko JS, Choi SJ, Hahm TS, Kim GH, et al. Effect of combined single-injection femoral nerve block and patient-controlled epidural analgesia in patients undergoing total knee replacement. Yonsei medical journal. 2011;52(1):145–50. 10.3349/ymj.2011.52.1.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng HP, Cheong KF, Lim A, Lim J, Puhaindran ME. Intraoperative single-shot "3-in-1" femoral nerve block with ropivacaine 0.25%, ropivacaine 0.5% or bupivacaine 0.25% provides comparable 48-hr analgesia after unilateral total knee replacement. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 2001;48(11):1102–8. [DOI] [PubMed] [Google Scholar]
- 25.Ozen M, Inan N, Tumer F, Uyar A, Baltaci B. The effect of 3-in-1 femoral nerve block with ropivacaine 0.375% on postoperative morphine consumption in elderly patients after total knee replacement surgery. Agri: Agri (Algoloji) Dernegi'nin Yayin organidir = The journal of the Turkish Society of Algology. 2006;18(4):44–50. [PubMed] [Google Scholar]
- 26.Sahin L, Korkmaz HF, Sahin M, Atalan G. Ultrasound-guided single-injection femoral nerve block provides effective analgesia after total knee arthroplasty up to 48 hours. Agri: Agri (Algoloji) Dernegi'nin Yayin organidir = The journal of the Turkish Society of Algology. 2014;26(3):113–8. [DOI] [PubMed] [Google Scholar]
- 27.Tugay N, Saricaoglu F, Satilmis T, Alpar U, Akarcali I, Citaker S, et al. Single-injection femoral nerve block. Effects on the independence level in functional activities in the early postoperative period in patients with total knee arthroplasty. Neurosciences (Riyadh, Saudi Arabia). 2006;11(3):175–9. [PubMed] [Google Scholar]
- 28.Wang H, Boctor B, Verner J. The effect of single-injection femoral nerve block on rehabilitation and length of hospital stay after total knee replacement. Regional anesthesia and pain medicine. 2002;27(2):139–44. [DOI] [PubMed] [Google Scholar]
- 29.YaDeau JT, Cahill JB, Zawadsky MW, Sharrock NE, Bottner F, Morelli CM, et al. The effects of femoral nerve blockade in conjunction with epidural analgesia after total knee arthroplasty. Anesthesia and analgesia. 2005;101(3):891–5, table of contents. 10.1213/01.ANE.0000159150.79908.21 [DOI] [PubMed] [Google Scholar]
- 30.Hirst GC, Lang SA, Dust WN, Cassidy JD, Yip RW. Femoral nerve block. Single injection versus continuous infusion for total knee arthroplasty. Regional anesthesia. 1996;21(4):292–7. [PubMed] [Google Scholar]
- 31.Edwards ND, Wright EM. Continuous low-dose 3-in-1 nerve blockade for postoperative pain relief after total knee replacement. Anesthesia and analgesia. 1992;75(2):265–7. [DOI] [PubMed] [Google Scholar]
- 32.Ganapathy S, Wasserman RA, Watson JT, Bennett J, Armstrong KP, Stockall CA, et al. Modified continuous femoral three-in-one block for postoperative pain after total knee arthroplasty. Anesthesia and analgesia. 1999;89(5):1197–202. [PubMed] [Google Scholar]
- 33.Kaloul I, Guay J, Cote C, Fallaha M. The posterior lumbar plexus (psoas compartment) block and the three-in-one femoral nerve block provide similar postoperative analgesia after total knee replacement. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 2004;51(1):45–51. 10.1007/BF03018546 [DOI] [PubMed] [Google Scholar]
- 34.Park CK, Cho CK, Lee GG, Lee JH. Optimizing dose infusion of 0.125% bupivacaine for continuous femoral nerve block after total knee replacement. Korean journal of anesthesiology. 2010;58(5):468–76. 10.4097/kjae.2010.58.5.468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seet E, Leong WL, Yeo AS, Fook-Chong S. Effectiveness of 3-in-1 continuous femoral block of differing concentrations compared to patient controlled intravenous morphine for post total knee arthroplasty analgesia and knee rehabilitation. Anaesthesia and intensive care. 2006;34(1):25–30. [DOI] [PubMed] [Google Scholar]
- 36.Serpell MG, Millar FA, Thomson MF. Comparison of lumbar plexus block versus conventional opioid analgesia after total knee replacement. Anaesthesia [Internet]. 1991; 46(4):[275–7 pp.]. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/092/CN-00075092/frame.html. [DOI] [PubMed] [Google Scholar]
- 37.Watson MW, Mitra D, McLintock TC, Grant SA. Continuous versus single-injection lumbar plexus blocks: comparison of the effects on morphine use and early recovery after total knee arthroplasty. Regional anesthesia and pain medicine. 2005;30(6):541–7. 10.1016/j.rapm.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 38.Wyatt MC, Wright T, Locker J, Stout K, Chapple C, Theis JC. Femoral nerve infusion after primary total knee arthroplasty: a prospective, double-blind, randomised and placebo-controlled trial. Bone & joint research. 2015;4(2):11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olive DJ, Barrington MJ, Simone SA, Kluger R. A randomised controlled trial comparing three analgesia regimens following total knee joint replacement: continuous femoral nerve block, intrathecal morphine or both. Anaesthesia and intensive care. 2015;43(4):454–60. [DOI] [PubMed] [Google Scholar]
- 40.Barrington JW, Emerson RH, Lovald ST, Lombardi AV, Berend KR. No Difference in Early Analgesia Between Liposomal Bupivacaine Injection and Intrathecal Morphine After TKA. Clinical orthopaedics and related research. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cole PJ, Craske DA, Wheatley RG. Efficacy and respiratory effects of low-dose spinal morphine for postoperative analgesia following knee arthroplasty. British journal of anaesthesia. 2000;85(2):233–7. [DOI] [PubMed] [Google Scholar]
- 42.Hur MJ, Kim YJ, Baik H, Kim JH. Effect of Intrathecal Morphine for Total Knee Replacement Arthroplasty Elderly Patients. Korean journal of anesthesiology [Internet]. 2007; 52(2):[172–8 pp.]. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/644/CN-01044644/frame.html. [Google Scholar]
- 43.Jacobson L, Kokri MS, Pridie AK. Intrathecal diamorphine: a dose-response study. Annals of the Royal College of Surgeons of England. 1989;71(5):289–92. [PMC free article] [PubMed] [Google Scholar]
- 44.Kunopart M, Chanthong P, Thongpolswat N, Intiyanaravut T, Pethuahong C. Effects of single shot femoral nerve block combined with intrathecal morphine for postoperative analgesia: a randomized, controlled, dose-ranging study after total knee arthroplasty. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2014;97(2):195–202. [PubMed] [Google Scholar]
- 45.Lauretti GR, Righeti CC, Mattos AL. Intrathecal ketorolac enhances intrathecal morphine analgesia following total knee arthroplasty. Journal of anaesthesiology, clinical pharmacology. 2013;29(4):503–8. 10.4103/0970-9185.119155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park CK, Cho CK, Lee JH, Shin HH. Optimizing the dose of intrathecal morphine when combined with continuous 3-in-1 nerve block after total knee replacement. Korean journal of anesthesiology [Internet]. 2009; 57(1):[69–77 pp.]. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/189/CN-01046189/frame.html. [DOI] [PubMed] [Google Scholar]
- 47.Tan PH, Chia YY, Lo Y, Liu K, Yang LC, Lee TH. Intrathecal bupivacaine with morphine or neostigmine for postoperative analgesia after total knee replacement surgery. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 2001;48(6):551–6. 10.1007/BF03016831 [DOI] [PubMed] [Google Scholar]
- 48.Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, Bourne RB, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. The Journal of bone and joint surgery American volume. 2006;88(5):959–63. 10.2106/JBJS.E.00344 [DOI] [PubMed] [Google Scholar]
- 49.Chinachoti T, Lungnateetape A, Raksakietisak M. Periarticular infiltration of 0.25% bupivacaine on top of femoral nerve block and intrathecal morphine improves quality of pain control after total knee arthroplasty: a randomized double-blind placebo controlled clinical trial. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2012;95(12):1536–42. [PubMed] [Google Scholar]
- 50.Choi HG, Kim SG, Kwon SB, Kim JS, Kwon HU, Kang PS. The Analgesic Effect of Postoperative Combined Epidural, Soft Tissue, and Intra-articular Injection of Morphine and Bupivacaine in Patients undergoing Total Knee Arthroplasty. Korean journal of anesthesiology [Internet]. 2006; 50(5):[546–51 pp.]. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/328/CN-01044328/frame.html. [Google Scholar]
- 51.Essving P, Axelsson K, Kjellberg J, Wallgren O, Gupta A, Lundin A. Reduced morphine consumption and pain intensity with local infiltration analgesia (LIA) following total knee arthroplasty. Acta orthopaedica. 2010;81(3):354–60. 10.3109/17453674.2010.487241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu P, Wu Y, Wu H, Li X, Qian Q, Zhu Y. Efficacy of intra-articular cocktail analgesic injection in total knee arthroplasty—a randomized controlled trial. The Knee. 2009;16(4):280–4. 10.1016/j.knee.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 53.Kazak Bengisun Z, Aysu Salviz E, Darcin K, Suer H, Ates Y. Intraarticular levobupivacaine or bupivacaine administration decreases pain scores and provides a better recovery after total knee arthroplasty. Journal of anesthesia. 2010;24(5):694–9. 10.1007/s00540-010-0970-x [DOI] [PubMed] [Google Scholar]
- 54.Kim TW, Park SJ, Lim SH, Seong SC, Lee S, Lee MC. Which analgesic mixture is appropriate for periarticular injection after total knee arthroplasty? Prospective, randomized, double-blind study. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2014. [DOI] [PubMed] [Google Scholar]
- 55.Leownorasate M, Ruangsillapanu N. Post-op pain and blood loss in total knee arthroplasty: an RCT using periarticular injection with diclofenac-based multimodal drugs. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2014;97(12):1332–7. [PubMed] [Google Scholar]
- 56.Lu HH, Li GF, Bai L, Sun JX, Jiang Z, Yin F. Continuous analgesia of local infiltration after total knee arthroplasty. [Chinese]. Chinese Journal of Tissue Engineering Research. 2014;18(4):529–34. 10.3969/j.issn.2095-4344. [DOI] [Google Scholar]
- 57.Milani P, Castelli P, Sola M, Invernizzi M, Massazza G, Cisari C. Multimodal Analgesia in Total Knee Arthroplasty: A Randomized, Double-Blind, Controlled Trial on Additional Efficacy of Periarticular Anesthesia. The Journal of arthroplasty. 2015;30(11):2038–42. 10.1016/j.arth.2015.05.035 [DOI] [PubMed] [Google Scholar]
- 58.Niemelainen M, Kalliovalkama J, Aho AJ, Moilanen T, Eskelinen A. Single periarticular local infiltration analgesia reduces opiate consumption until 48 hours after total knee arthroplasty. Acta orthopaedica. 2014:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ong JC, Chin PL, Fook-Chong SM, Tang A, Yang KY, Tay BK. Continuous infiltration of local anaesthetic following total knee arthroplasty. Journal of orthopaedic surgery (Hong Kong). 2010;18(2):203–7. [DOI] [PubMed] [Google Scholar]
- 60.Vaishya R, Wani AM, Vijay V. Local Infiltration Analgesia reduces pain and hospital stay after primary TKA: randomized controlled double blind trial. Acta orthopaedica Belgica. 2015;81(4):720–9. [PubMed] [Google Scholar]
- 61.Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin MC, et al. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. The Journal of bone and joint surgery American volume. 2006;88(2):282–9. 10.2106/JBJS.E.00173 [DOI] [PubMed] [Google Scholar]
- 62.Yuenyongviwat V, Pornrattanamaneewong C, Chinachoti T, Chareancholvanich K. Periarticular injection with bupivacaine for postoperative pain control in total knee replacement: a prospective randomized double-blind controlled trial. Advances in orthopedics. 2012;2012:107309 10.1155/2012/107309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J, Jiang Y, Shao JJ, Shen H, Wang Q, Zhang XL. Effect of periarticular multimodal drug injection on pain after total knee arthroplasty. [Chinese]. Journal of Clinical Rehabilitative Tissue Engineering Research. 2007;11(43):8678–82. [Google Scholar]
- 64.Zhang S, Wang F, Lu ZD, Li YP, Zhang L, Jin QH. Effect of single-injection versus continuous local infiltration analgesia after total knee arthroplasty: a randomized, double-blind, placebo-controlled study. The Journal of international medical research. 2011;39(4):1369–80. 10.1177/147323001103900423 [DOI] [PubMed] [Google Scholar]
- 65.Nakai T, Tamaki M, Nakamura T, Nakai T, Onishi A, Hashimoto K. Controlling pain after total knee arthroplasty using a multimodal protocol with local periarticular injections. Journal of orthopaedics. 2013;10(2):92–4. 10.1016/j.jor.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Badner NH, Bourne RB, Rorabeck CH, MacDonald SJ, Doyle JA. Intra-articular injection of bupivacaine in knee-replacement operations. Results of use for analgesia and for preemptive blockade. The Journal of bone and joint surgery American volume. 1996;78(5):734–8. [DOI] [PubMed] [Google Scholar]
- 67.Browne C, Copp S, Reden L, Pulido P, Colwell C Jr. Bupivacaine bolus injection versus placebo for pain management following total knee arthroplasty. The Journal of arthroplasty. 2004;19(3):377–80. [DOI] [PubMed] [Google Scholar]
- 68.Mauerhan DR, Campbell M, Miller JS, Mokris JG, Gregory A, Kiebzak GM. Intra-articular morphine and/or bupivacaine in the management of pain after total knee arthroplasty. The Journal of arthroplasty. 1997;12(5):546–52. [DOI] [PubMed] [Google Scholar]
- 69.Rosen AS, Colwell CW Jr., Pulido PA, Chaffee TL, Copp SN. A randomized controlled trial of intraarticular ropivacaine for pain management immediately following total knee arthroplasty. HSS journal: the musculoskeletal journal of Hospital for Special Surgery. 2010;6(2):155–9. 10.1007/s11420-010-9155-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Safa B, Gollish J, Haslam L, McCartney CJ. Comparing the effects of single shot sciatic nerve block versus posterior capsule local anesthetic infiltration on analgesia and functional outcome after total knee arthroplasty: a prospective, randomized, double-blinded, controlled trial. The Journal of arthroplasty. 2014;29(6):1149–53. 10.1016/j.arth.2013.11.020 [DOI] [PubMed] [Google Scholar]
- 71.Shen SJ, Peng PY, Chen HP, Lin JR, Lee MS, Yu HP. Analgesic Effects of Intra-Articular Bupivacaine/Intravenous Parecoxib Combination Therapy versus Intravenous Parecoxib Monotherapy in Patients Receiving Total Knee Arthroplasty: A Randomized, Double-Blind Trial. BioMed research international. 2015;2015:450805 10.1155/2015/450805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng Y, Ju H, Yang BX, An HY, Zhou YY. Postoperative analgesic and anti-inflammatory effects of rofecoxib after total knee replacement. [Chinese]. Zhonghua wai ke za zhi [Chinese journal of surgery]. 2004;42(10):617–21. [PubMed] [Google Scholar]
- 73.Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC musculoskeletal disorders. 2008;9:77 10.1186/1471-2474-9-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hubbard RC, Naumann TM, Traylor L, Dhadda S. Parecoxib sodium has opioid-sparing effects in patients undergoing total knee arthroplasty under spinal anaesthesia. British journal of anaesthesia. 2003;90(2):166–72. [DOI] [PubMed] [Google Scholar]
- 75.Inan N, Ozcan N, Takmaz SA, Ozcan A, Erdogan I, Baltaci B. Efficacy of lornoxicam in postoperative analgesia after total knee replacement surgery. Agri: Agri (Algoloji) Dernegi'nin Yayin organidir = The journal of the Turkish Society of Algology. 2007;19(2):38–45. [PubMed] [Google Scholar]
- 76.Rawal N, Viscusi E, Peloso PM, Minkowitz HS, Chen L, Shah S, et al. Evaluation of etoricoxib in patients undergoing total knee replacement surgery in a double-blind, randomized controlled trial. BMC musculoskeletal disorders. 2013;14:300 10.1186/1471-2474-14-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarridou DG, Chalmouki G, Braoudaki M, Koutsoupaki A, Mela A, Vadalouka A. Intravenous parecoxib and continuous femoral block for postoperative analgesia after total knee arthroplasty. A randomized, double-blind, prospective trial. Pain physician. 2015;18(3):267–76. [PubMed] [Google Scholar]
- 78.Silvanto M, Lappi M, Rosenberg PH. Comparison of the opioid-sparing efficacy of diclofenac and ketoprofen for 3 days after knee arthroplasty. Acta anaesthesiologica Scandinavica. 2002;46(3):322–8. [DOI] [PubMed] [Google Scholar]
- 79.Zhu Y, Wang S, Wu H, Wu Y. Effect of perioperative parecoxib on postoperative pain and local inflammation factors PGE2 and IL-6 for total knee arthroplasty: a randomized, double-blind, placebo-controlled study. European journal of orthopaedic surgery & traumatology: orthopedie traumatologie. 2014;24(3):395–401. [DOI] [PubMed] [Google Scholar]
- 80.Zhu YZ, Yao R, Zhang Z, Xu H, Wang LW. Parecoxib prevents early postoperative cognitive dysfunction in elderly patients undergoing total knee arthroplasty: A double-blind, randomized clinical consort study. Medicine. 2016;95(28):e4082 10.1097/MD.0000000000004082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Niruthisard S, Earsakul A, Bunburaphong P, Chinda P, Anutinmanee R, Prapreuttham S, et al. Preoperative pregabalin and/or celecoxib for pain management after total knee arthroplasty under intrathecal morphine: A randomized controlled trial. Asian Biomedicine. 2013;7(4):578–85. 10.5372/1905-7415.0704.215. [DOI] [Google Scholar]
- 82.Clarke HA, Pereira S, Kennedy D, Gilron I, Katz J, Gollish J, et al. Gabapentin decreases morphine consumption and improves functional recovery following total knee arthroplasty. Pain research & management: the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur. 2009;14(3):217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clarke HA, Katz J, McCartney CJ, Stratford P, Kennedy D, Page MG, et al. Perioperative gabapentin reduces 24 h opioid consumption and improves in-hospital rehabilitation but not post-discharge outcomes after total knee arthroplasty with peripheral nerve block. British journal of anaesthesia. 2014;113(5):855–64. 10.1093/bja/aeu202 [DOI] [PubMed] [Google Scholar]
- 84.Lee JK, Chung KS, Choi CH. The Effect of a Single Dose of Preemptive Pregabalin Administered With COX-2 Inhibitor: A Trial in Total Knee Arthroplasty. The Journal of arthroplasty. 2014. [DOI] [PubMed] [Google Scholar]
- 85.Lunn TH, Husted H, Laursen MB, Hansen LT, Kehlet H. Analgesic and sedative effects of perioperative gabapentin in total knee arthroplasty: a randomized, double-blind, placebo-controlled dose-finding study. Pain. 2015;156(12):2438–48. 10.1097/j.pain.0000000000000309 [DOI] [PubMed] [Google Scholar]
- 86.Paul JE, Nantha-Aree M, Buckley N, Cheng J, Thabane L, Tidy A, et al. Gabapentin does not improve multimodal analgesia outcomes for total knee arthroplasty: a randomized controlled trial. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 2013;60(5):423–31. 10.1007/s12630-013-9902-1 [DOI] [PubMed] [Google Scholar]
- 87.YaDeau JT, Lin Y, Mayman DJ, Goytizolo EA, Alexiades MM, Padgett DE, et al. Pregabalin and pain after total knee arthroplasty: a double-blind, randomized, placebo-controlled, multidose trial. British journal of anaesthesia. 2015;115(2):285–93. 10.1093/bja/aev217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andersen HL, Gyrn J, Moller L, Christensen B, Zaric D. Continuous saphenous nerve block as supplement to single-dose local infiltration analgesia for postoperative pain management after total knee arthroplasty. Regional anesthesia and pain medicine. 2013;38(2):106–11. 10.1097/AAP.0b013e31827900a9 [DOI] [PubMed] [Google Scholar]
- 89.Jenstrup MT, Jaeger P, Lund J, Fomsgaard JS, Bache S, Mathiesen O, et al. Effects of adductor-canal-blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta anaesthesiologica Scandinavica. 2012;56(3):357–64. 10.1111/j.1399-6576.2011.02621.x [DOI] [PubMed] [Google Scholar]
- 90.Krishnan SH, Gilbert LA, Ghoddoussi F, Applefield DJ, Kassab SS, Ellis TA 2nd. Addition of buprenorphine to local anesthetic in adductor canal blocks after total knee arthroplasty improves postoperative pain relief: a randomized controlled trial. Journal of clinical anesthesia. 2016;33:432–7. 10.1016/j.jclinane.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 91.Shah NA, Jain NP, Panchal KA. Adductor Canal Blockade Following Total Knee Arthroplasty-Continuous or Single Shot Technique? Role in Postoperative Analgesia, Ambulation Ability and Early Functional Recovery: A Randomized Controlled Trial. The Journal of arthroplasty. 2015;30(8):1476–81. 10.1016/j.arth.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 92.Casati A, Vinciguerra F, Cappelleri G, Aldegheri G, Fanelli G, Putzu M, et al. Adding clonidine to the induction bolus and postoperative infusion during continuous femoral nerve block delays recovery of motor function after total knee arthroplasty. Anesthesia and analgesia. 2005;100(3):866–72, table of contents. 10.1213/01.ANE.0000143952.75985.8F [DOI] [PubMed] [Google Scholar]
- 93.Ekmekci P, Yilmaz AA, Ozgencil E, Hasdogan M, Akan O, Okten F. The effect of tramadol adjunct to ropivacaine in continuous femoral nerve block in patients undergoing total knee arthroplasty for postoperative pain treatment. [Turkish]. Anestezi Dergisi. 2010;18(4):194–200. [Google Scholar]
- 94.EA A., Badawy A, Elkassem SA, Rashwan D. Effect of addition of magnesium sulphate and fentanyl to ropivacaine continuous femoral nerve block in patients undergoing elective total knee replacement. Journal of Medical Sciences. 2008;8(4):395–9. 10.3923/jms.2008.395.399. [DOI] [Google Scholar]
- 95.Kosel J, Bobik P, Siemiatkowski A. Buprenorphine added to bupivacaine prolongs femoral nerve block duration and improves analgesia in patients undergoing primary total knee arthroplasty-a randomised prospective double-blind study. The Journal of arthroplasty. 2015;30(2):320–4. 10.1016/j.arth.2014.07.016 [DOI] [PubMed] [Google Scholar]
- 96.McNamee DA, Convery PN, Milligan KR. Total knee replacement: a comparison of ropivacaine and bupivacaine in combined femoral and sciatic block. Acta anaesthesiologica Scandinavica. 2001;45(4):477–81. [DOI] [PubMed] [Google Scholar]
- 97.Abdallah FW, Chan VW, Gandhi R, Koshkin A, Abbas S, Brull R. The analgesic effects of proximal, distal, or no sciatic nerve block on posterior knee pain after total knee arthroplasty: a double-blind placebo-controlled randomized trial. Anesthesiology. 2014;121(6):1302–10. 10.1097/ALN.0000000000000406 [DOI] [PubMed] [Google Scholar]
- 98.Cappelleri G, Ghisi D, Fanelli A, Albertin A, Somalvico F, Aldegheri G. Does continuous sciatic nerve block improve postoperative analgesia and early rehabilitation after total knee arthroplasty? A prospective, randomized, double-blinded study. Regional anesthesia and pain medicine. 2011;36(5):489–92. 10.1097/AAP.0b013e3182286a2b [DOI] [PubMed] [Google Scholar]
- 99.Martinez Navas A, Echevarria Moreno M. [Continuous versus single-dose sciatic nerve block to complement a femoral block after total knee replacement surgery: a randomized clinical trial]. Revista espanola de anestesiologia y reanimacion. 2006;53(4):214–9. [PubMed] [Google Scholar]
- 100.Sato K, Adachi T, Shirai N, Naoi N. Continuous versus single-injection sciatic nerve block added to continuous femoral nerve block for analgesia after total knee arthroplasty: a prospective, randomized, double-blind study. Regional anesthesia and pain medicine. 2014;39(3):225–9. 10.1097/AAP.0000000000000076 [DOI] [PubMed] [Google Scholar]
- 101.McNamee DA, Parks L, Milligan KR. Post-operative analgesia following total knee replacement: an evaluation of the addition of an obturator nerve block to combined femoral and sciatic nerve block. Acta anaesthesiologica Scandinavica. 2002;46(1):95–9. [DOI] [PubMed] [Google Scholar]
- 102.Runge C, Borglum J, Jensen JM, Kobborg T, Pedersen A, Sandberg J, et al. The Analgesic Effect of Obturator Nerve Block Added to a Femoral Triangle Block After Total Knee Arthroplasty: A Randomized Controlled Trial. Regional anesthesia and pain medicine. 2016;41(4):445–51. 10.1097/AAP.0000000000000406 [DOI] [PubMed] [Google Scholar]
- 103.Frassanito L, Vergari A, Messina A, Caputo C, Gunnella B. Anaesthesia for total knee arthroplasty: Efficacy of single-injection or continuous lumbar plexus associated with sciatic nerve blocks. European Journal of Anaesthesiology. 2009;26:116. [PubMed] [Google Scholar]
- 104.Badner NH, Bourne RB, Rorabeck CH, Doyle JA. Addition of morphine to intra-articular bupivacaine does not improve analgesia following knee joint replacement. Regional anesthesia [Internet]. 1997; 22(4):[347–50 pp.]. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/587/CN-00141587/frame.html. [DOI] [PubMed] [Google Scholar]
- 105.Garcia JB, Barbosa Neto JO, Vasconcelos JW, Ferro LS, Silva RC. Analgesic efficacy of the intra-articular administration of high doses of morphine in patients undergoing total knee arthroplasty. Revista brasileira de anestesiologia. 2010;60(1):1–12. [DOI] [PubMed] [Google Scholar]
- 106.Guara Sobrinho H, Garcia JB, Vasconcelos JW, Sousa JC, Ferro LS. Analgesic efficacy of the intra-articular administration of S(+)- ketamine in patients undergoing total knee arthroplasty. Revista brasileira de anestesiologia. 2012;62(5):665–75. 10.1016/S0034-7094(12)70165-4 [DOI] [PubMed] [Google Scholar]
- 107.Schotanus MG, Bemelmans YF, van der Kuy PH, Jansen J, Kort NP. No advantage of adrenaline in the local infiltration analgesia mixture during total knee arthroplasty. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2015. [DOI] [PubMed] [Google Scholar]
- 108.Ali A, Sundberg M, Hansson U, Malmvik J, Flivik G. Doubtful effect of continuous intraarticular analgesia after total knee arthroplasty: a randomized double-blind study of 200 patients. Acta orthopaedica. 2015;86(3):373–7. 10.3109/17453674.2014.991629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gomez-Cardero P, Rodriguez-Merchan EC. Postoperative analgesia in TKA: ropivacaine continuous intraarticular infusion. Clinical orthopaedics and related research. 2010;468(5):1242–7. 10.1007/s11999-009-1202-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Williams D, Petruccelli D, Paul J, Piccirillo L, Winemaker M, de Beer J. Continuous infusion of bupivacaine following total knee arthroplasty: a randomized control trial pilot study. The Journal of arthroplasty. 2013;28(3):479–84. 10.1016/j.arth.2012.07.016 [DOI] [PubMed] [Google Scholar]
- 111.Andersen KV, Nikolajsen L, Haraldsted V, Odgaard A, Soballe K. Local infiltration analgesia for total knee arthroplasty: should ketorolac be added? British journal of anaesthesia. 2013;111(2):242–8. 10.1093/bja/aet030 [DOI] [PubMed] [Google Scholar]
- 112.Sean VW, Chin PL, Chia SL, Yang KY, Lo NN, Yeo SJ. Single-dose periarticular steroid infiltration for pain management in total knee arthroplasty: a prospective, double-blind, randomised controlled trial. Singapore medical journal. 2011;52(1):19–23. [PubMed] [Google Scholar]
- 113.Tsukada S, Wakui M, Hoshino A. The impact of including corticosteroid in a periarticular injection for pain control after total knee arthroplasty: a double-blind randomised controlled trial. The bone & joint journal. 2016;98-b(2):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yue DB, Wang BL, Liu KP, Guo WS. Efficacy of multimodal cocktail periarticular injection with or without steroid in total knee arthroplasty. Chinese medical journal. 2013;126(20):3851–5. [PubMed] [Google Scholar]
- 115.Axelsson K, Johanzon E, Essving P, Weckstrom J, Ekback G. Postoperative extradural analgesia with morphine and ropivacaine. A double-blind comparison between placebo and ropivacaine 10 mg/h or 16 mg/h. Acta anaesthesiologica Scandinavica. 2005;49(8):1191–9. 10.1111/j.1399-6576.2005.00715.x [DOI] [PubMed] [Google Scholar]
- 116.Daabiss MA, Kandil A. Evaluation of the effect of magnesium vs. midazolam as adjunct to epidural bupivacaine in patients undergoing total knee replacement. British Journal of Medical Practitioners. 2013;6(2). [Google Scholar]
- 117.Hendolin H, Nuutinen L, Kokki H, Tuomisto L. Does morphine premedication influence the pain and consumption of postoperative analgesics after total knee arthroplasty? Acta anaesthesiologica Scandinavica. 1996;40(1):81–5. [DOI] [PubMed] [Google Scholar]
- 118.Abrisham SM, Ghahramani R, Heiranizadeh N, Kermani-Alghoraishi M, Ayatollahi V, Pahlavanhosseini H. Reduced morphine consumption and pain severity with transdermal fentanyl patches following total knee arthroplasty. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2014;22(7):1580–4. [DOI] [PubMed] [Google Scholar]
- 119.Sathitkarnmanee T, Tribuddharat S, Noiphitak K, Theerapongpakdee S, Pongjanyakul S, Huntula Y, et al. Transdermal fentanyl patch for postoperative analgesia in total knee arthroplasty: a randomized double-blind controlled trial. Journal of pain research. 2014;7:449–54. 10.2147/JPR.S66741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stiller CO, Lundblad H, Weidenhielm L, Tullberg T, Grantinger B, Lafolie P, et al. The addition of tramadol to morphine via patient-controlled analgesia does not lead to better post-operative pain relief after total knee arthroplasty. Acta anaesthesiologica Scandinavica. 2007;51(3):322–30. 10.1111/j.1399-6576.2006.01191.x [DOI] [PubMed] [Google Scholar]
- 121.Aveline C, Gautier JF, Vautier P, Cognet F, Hetet HL, Attali JY, et al. Postoperative analgesia and early rehabilitation after total knee replacement: a comparison of continuous low-dose intravenous ketamine versus nefopam. European journal of pain (London, England). 2009;13(6):613–9. [DOI] [PubMed] [Google Scholar]
- 122.Adam F, Chauvin M, Du Manoir B, Langlois M, Sessler DI, Fletcher D. Small-dose ketamine infusion improves postoperative analgesia and rehabilitation after total knee arthroplasty. Anesthesia and analgesia. 2005;100(2):475–80. 10.1213/01.ANE.0000142117.82241.DC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cengiz P, Gokcinar D, Karabeyoglu I, Topcu H, Cicek GS, Gogus N. Intraoperative low-dose ketamine infusion reduces acute postoperative pain following total knee replacement surgery: a prospective, randomized double-blind placebo-controlled trial. Journal of the College of Physicians and Surgeons—Pakistan: JCPSP. 2014;24(5):299–303. doi: 04.2014/JCPSP.299303 [PubMed] [Google Scholar]
- 124.Casey G, Nortcliffe SA, Sharpe P, Buggy DJ. Perioperative nimodipine and postoperative analgesia. Anesthesia and analgesia. 2006;102(2):504–8. 10.1213/01.ane.0000194448.37407.6a [DOI] [PubMed] [Google Scholar]
- 125.Chan IA, Maslany JG, Gorman KJ, O'Brien JM, McKay WP. Dexmedetomidine during total knee arthroplasty performed under spinal anesthesia decreases opioid use: a randomized-controlled trial. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 2016;63(5):569–76. 10.1007/s12630-016-0597-y [DOI] [PubMed] [Google Scholar]
- 126.Ho KY, Tay W, Yeo MC, Liu H, Yeo SJ, Chia SL, et al. Duloxetine reduces morphine requirements after knee replacement surgery. British journal of anaesthesia. 2010;105(3):371–6. 10.1093/bja/aeq158 [DOI] [PubMed] [Google Scholar]
- 127.Lunn TH, Kristensen BB, Andersen LO, Husted H, Otte KS, Gaarn-Larsen L, et al. Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial. British journal of anaesthesia. 2011;106(2):230–8. 10.1093/bja/aeq333 [DOI] [PubMed] [Google Scholar]
- 128.Frassanito L, Messina A, Vergari A, Colombo D, Chierichini A, Della Corte F, et al. Intravenous infusion of magnesium sulfate and postoperative analgesia in total knee arthroplasty. Minerva anestesiologica. 2015;81(11):1184–91. [PubMed] [Google Scholar]
- 129.Albrecht E, Guyen O, Jacot-Guillarmod A, Kirkham KR. The analgesic efficacy of local infiltration analgesia vs femoral nerve block after total knee arthroplasty: a systematic review and meta-analysis. British journal of anaesthesia. 2016;116(5):597–609. 10.1093/bja/aew099 [DOI] [PubMed] [Google Scholar]
- 130.Kandasami M, Kinninmonth AW, Sarungi M, Baines J, Scott NB. Femoral nerve block for total knee replacement—a word of caution. The Knee. 2009;16(2):98–100. 10.1016/j.knee.2008.10.007 [DOI] [PubMed] [Google Scholar]
- 131.Mathiesen O, Wetterslev J, Kontinen VK, Pommergaard HC, Nikolajsen L, Rosenberg J, et al. Adverse effects of perioperative paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta anaesthesiologica Scandinavica. 2014;58(10):1182–98. 10.1111/aas.12380 [DOI] [PubMed] [Google Scholar]
- 132.Dahl JB, Nielsen RV, Wetterslev J, Nikolajsen L, Hamunen K, Kontinen VK, et al. Post-operative analgesic effects of paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta anaesthesiologica Scandinavica. 2014;58(10):1165–81. 10.1111/aas.12382 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
Green squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
(PDF)
Green squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
(PDF)
Blue squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
(PDF)
Blue squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
(PDF)
Blue squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
(PDF)
Blue squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
(PDF)
Green squares with horizontal lines represent mean differences and 95% confidence intervals for each trial. Black tiles represent the mean difference of each intervention.
(PDF)
Higher degrees of heterogeneity were demonstrated for pain at 6 and 24 hours rest and 24 hours movement. The size of the ball resembles the number of included patients in that trial and it is standardized across the different plots.
(PDF)
A priori estimated information sizes (APIS) (333, 730, 321 and 560 patients, respectively) were based on an alpha-value of 0.05 and a beta-value of 0.9. The sensitivity to detect a mean difference for opioid consumption was predefined as 10 mg morphine equivalents 0–24 h postoperative and for pain scores a mean difference of 15 mm (VAS 0–100 mm). The blue line depicts the cumulative Z-score of the meta-analysis. The outer red lines illustrate the sequential z-score threshold for significance. The inner red lines illustrate the area of futility. The burgundy lines represent a stationary Z-score at 1.96 corresponding to p = 0.05. Threshold for significance was reached for morphine consumption and pain at 6 and 24 hours rest. Morphine consumption and pain score at 24 hours rest reached APIS, concluding that the intervention has an effect on these outcomes.
(PDF)
Homogeneity was demonstrated for morphine consumption and pain scores. The size of the ball resembles the number of included patients in that trial and it is standardized across the different plots.
(PDF)
A priori estimated information sizes (APIS) (47, 268, 272 and 69 patients, respectively) were based on an alpha-value of 0.05 and a beta-value of 0.9. Threshold for significance and APIS were reached for morphine consumption and all pain scores concluding that continuous femoral nerve block has a positive effect on these outcomes. For further elaboration see S13 Appendix.
(PDF)
Moderate degrees of heterogeneity were demonstrated for morphine consumption and pain scores. The size of the ball resembles the number of included patients in that trial and it is standardized across the different plots.
(PDF)
A priori estimated information sizes (APIS) (183, 490 and 151 patients, respectively) were based on an alpha-value of 0.05 and a beta-value of 0.9. Morphine consumption reached the threshold for significance but not APIS. Pain score at 24 hours rest reached the boundary for futility and APIS concluding that there is no reason for further investigation of this outcome. For further elaboration see S13 Appendix.
(PDF)
Lower degrees of heterogeneity were demonstrated for morphine consumption and pain scores at rest. Moderate degrees were present for pain scores at movement. The size of the ball resembles the number of included patients in that trial and it is standardized across the different plots.
(PDF)
A priori estimated information sizes (APIS) (635, 349, 149, 217 and 174 patients, respectively) were based on an alpha-value of 0.05 and a beta-value of 0.9. Threshold for significance and APIS were reached for all end-points, concluding that LIA has a positive effect on these outcomes. For further elaboration see S13 Appendix.
(PDF)
Homogeneity was demonstrated for morphine consumption and pain at 6. Pain scores at 24 hours at rest were heterogeneous. The size of the ball resembles the number of included patients in that trial and it is standardized across the different plots.
(PDF)
A priori estimated information sizes (APIS) (88, 127 and 394 patients, respectively) were based on an alpha-value of 0.05 and a beta-value of 0.9. Threshold for significance and APIS were reached for morphine consumption and pain at 6 hours rest concluding that intraarticular injection has a positive effect on these outcomes. For further elaboration see S13 Appendix.
(PDF)
Lower degrees of heterogeneity were demonstrated for morphine consumption and moderate degrees of heterogeneity for pain scores. The size of the ball resembles the number of included patients in that trial and it is standardized across the different plots.
(PDF)
A priori estimated information sizes (APIS) (115, 270, 32 and 166 patients, respectively) were based on an alpha-value of 0.05 and a beta-value of 0.9. Threshold for significance and APIS were reached for morphine consumption and pain at 6 and 24 hours rest concluding that NSAIDs and COX-2-inhibitors has a positive effect on these outcomes. Morphine consumption and pain at 24 hours rest reached APIS with the first trial. Threshold for futility and APIS were reached for pain at 24 h movement concluding that further testing of this end-point is futile. For further elaboration see S13 Appendix.
(PDF)
Moderate degrees of heterogeneity was demonstrated for morphine consumption and pain at 24 hours rest. The size of the ball resembles the number of included patients in that trial and it is standardized across the different plots.
(PDF)
A priori estimated information sizes (APIS) (905, 132, 104, 147 and 108 patients, respectively) were based on an alpha-value of 0.05 and a beta-value of 0.9. Threshold for significance and APIS were reached for pain at 6 and 24 hours at movement concluding that gabapentinoids have a positive effect on these outcomes. Threshold for futility and APIS were reached for pain at 6 and 24 h at rest concluding that further testing of these end-points is futile. For further elaboration see S13 Appendix.
(PDF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.