Abstract
Advanced pancreatic cancer is one of the most lethal malignant human diseases lacking effective treatment. Its extremely low survival rate necessitates development of novel therapeutic approach. Human neural stem cells (NSCs) are known to have tumor-tropic effect. We genetically engineered them to express rabbit carboxyl esterase (F3.CE), which activates prodrug CPT-11(irinotecan) into potent metabolite SN-38. We found significant inhibition of the growth of BxPC3 human pancreatic cancer cell line in vitro by F3.CE in presence of CPT-11. Apoptosis was also markedly increased in BxPC3 cells treated with F3.CE and CPT-11. The ligand VEGF and receptor VEGF-1(Flt1) were identified to be the relevant tumor-tropic chemoattractant. We confirmed in vivo that in mice injected with BxPC3 on their skin, there was significant reduction of tumor size in those treated with both F3.CE and BxPC3 adjacent to the cancer mass. Administration of F3.CE in conjunction with CPT-11 could be a new possibility as an effective treatment regimen for patients suffering from advanced pancreatic cancer.
Keywords: CPT-11, carboxyl esterase (CE), gene therapy, human neural stem cell, pancreatic cancer
INTRODUCTION
Pancreatic cancer is one of the most lethal human cancers and continues to be a major health problem. Conventional therapeutic approaches, such as surgery, radiation, chemotherapy, or combinations of these modalities, have little impact on the course of pancreatic cancer [1]. Patients with locally advanced pancreatic cancer have a median survival time of 8–12 months, and patients with distant metastases have significantly worse outcomes with a median survival time of 3– 6 months [2–4]. The aggressive nature and disappointing treatment results of advanced pancreatic cancer requires novel approach.
The recent discovery of the inherent tumor-tropic properties of neural stem cells (NSCs) [5] has led to the development of enzyme-prodrug gene therapy approach for malignant tumors in the brain including gliomas and medulloblastomas [6–9]. NSCs could be transduced with therapeutic genes in high efficiency, and rapidly expand to numbers required for therapeutic applications. We have previously generated a clonally derived immortalized human NSC line via a retroviral vector encoded with v-myc gene, and the HB1.F3 (F3) human NSCs were utilized in stem cell-based therapy in animal models of human neurological disorders [10–13].
The use of therapeutic NSCs is highly attractive because delivery vehicles can disseminate therapeutic gene products to invasive cancer cells. In previous studies, we have transduced F3 human NSCs with cytosine deaminase (CD) which converts the prodrug 5-fluorocytosine (5-FC) to 5-fluoruracil (5-FU). Transplantation of F3.CD NSCs and administration of 5-FC in mice bearing brain tumors including glioma, medulloblastoma and brain metastases of breast cancer and lung cancer resulted in significant inhibition of tumor growth [6–8, 14–25].
More recently, we have transduced F3 human NSCs with carboxylesterase, an enzyme that hydrolyzes chemotherapy agent CPT-11 (Irinotecan) to SN-38 (7-Ethyl-10-hydroxy-camptothecin), which is 1000 times more potent than CPT-11. F3.CE NSCs was administered in mice bearing brain tumors and other solid cancers including neuroblastoma, melanoma and ovarian cancer, then administration of CPT-11 was followed. There was significant inhibition of cancer growth in cancer bearing animals. We established the proof of concept that various cancers including brain tumors and other solid cancers can be effectively targeted using this approach [19, 26–30]. In the present study, we evaluated the therapeutic efficacy of F3 human NSCs encoding carboxylesterase administered in combination with prodrug CPT-11 to nude mice bearing pancreatic cancer.
RESULTS
F3.CE human NSC line expressing rabbit carboxylesterase (CE)
Expression of CE gene in the F3.CE cells was analyzed by reverse transcription-PCR. CE transcript was detected in F3.CE cells but not in parental F3 cells (Figure 1). To confirm the enzyme activity of CE, F3.CE cells were exposed to CPT-11 at concentrations of 0.05-5 μM for 48 hr. The rate of survival in F3.CE cells was reduced considerably by 48 hr exposure to prodrug CPT-11 at concentrations of <5 μM (Figure 2). When co-culture of BxPC3 pancreatic cancer cells and F3.CE cells were exposed to 1 μM CPT-11 for 48 hr, less than 20% of BxPC3 cancer cells survived, indicating that F3.CE cells processed prodrug CPT-11 efficiently into cytotoxic SN-38 (Figure 2).
Figure 1. Establishment of HB1.F3 human neural stem cells expressing the carboxylesterase (CE) gene.
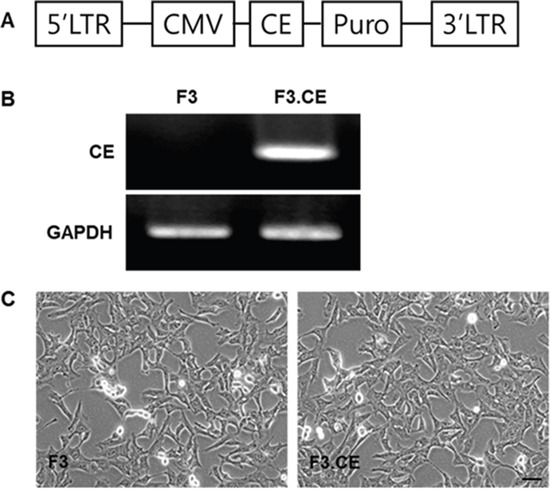
Expression of CE transcript was confirmed by RT-PCR, detecting CE transcripts in F3.CE, but not in F3 cells. A. Vector map of pLPCX.CE. B. Successful transduction of CE was confirmed by reverse transcription-PCR (237 bp). GAPDH = control. C. Phase contrast microscopy of F3 and F3.CE human NSCs. F3 : nerual stem cells, CE : carboxyl esterase, RT-PCR : reverse transcription-polymerase chain reaction
Figure 2. Suicide effect of F3.CE/CPT-11 system.
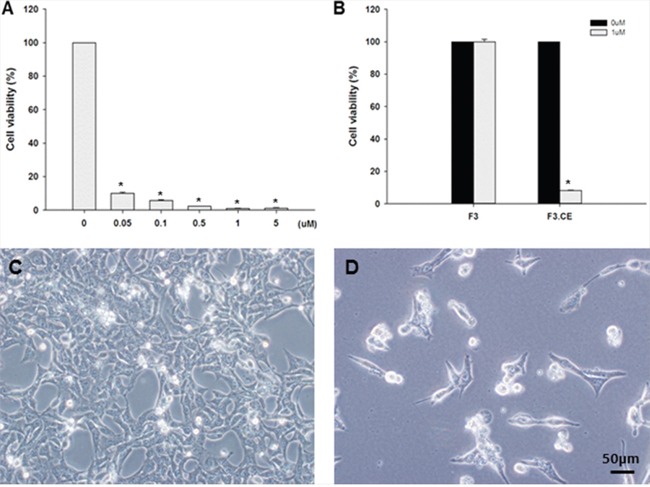
A. 1×104 F3.CE cells were cultured with various concentration of CPT-11 at 37°C for 48 hr and the survival was determined. CPT-11 at concentration of 1 μM for 48 hrs killed >98% of F3.CE cells. B. BXPC3 human pancreatic cancer cells were incubated with 1 μM CPT-11 (3.75 mg/kg) for 48 hr with or without F3.CE cells. C., D. BXPC3 cancer cells co-incubated with or without F3.CE cells for 48 hr.
Assessment of apoptosis of BxPC3 cells treated with F3 or F3.CE
Cell viability of BxPC3 was measured with propidium iodine (PI)-labeled dead cells using Muse cell count and viability kit. Viable cells significantly decreased with F3.CE cells in the presence of 1uM CPT-11 (Figure 3A).
Figure 3. Assessment of apoptosis by BxPC3 cells were treated with F3 or F3.CE and CPT-11.
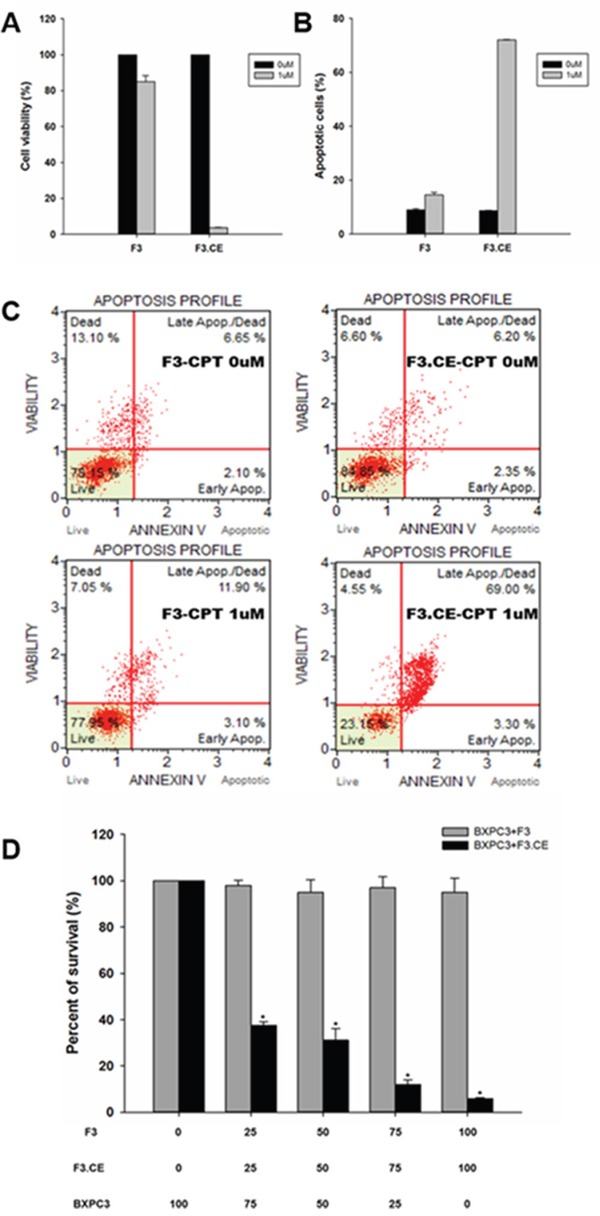
BXPC3 cells were treated with or without F3.CE cells at the indicated doses for 48 hrs. A. Cell viability was measured with propidium iodine (PI). Significantly less percentage of BXPC3 cells were viable when treated with 1 μM CPT-11 and F3.CE, compared to F3. B., C. annexin V-based apoptosis assay was performed as described in Materials and Methods. BXPC3 cells treated with CPT-11 and F3.CE showed significantly increased ratio of apoptotic cells. D. Bystander effect of the CE produced by the F3.CE cells was confirmed using a co-culture system of F3 or F3.CE cells and BXPC3 pancreas adenocarcinoma cells. BXPC3 cells with F3 or F3.CE cells were seeded in 96-well plates (total 1 × 104 cells per well, BXPC3 cells: F3 or F3.CE cells = 100:0, 75:25, 50:50, 25:75, or 0:100). After 48 hrs in co-culture, cells were treated with 1.0 μM/mL CPT-11 for 48 hrs and cell survival was determined (each group, n = 3) (p< 0.05).
Annexin V-based apoptosis assay showed significant increase in the ratio of apoptotic cells in BxPC3 cells treated with F3.CE and CPT-11 (1 uM) at the same time. This assay solely measures apoptotic cells only, without counting necrotic cells. This phenomenon was not observed with F3 and CPT-11(1 uM), or with CPT-11(0 uM) (p<0.05) (Figure 3B and 3C).
In vitro bystander effects on pancreatic cancer cells
Next, in vitro bystander effects of F3.CE cells on BxPC3 pancreatic cancer cells were determined using F3.CE - BxPC3 co-culture system and conditioned medium derived from F3 or F3.CE cells. In the co-culture experiment, application of 1 μM CPT-11 to BxPC3 pancreatic cancer cells had little effects on the survival until 48 hrs after the treatment. Toxic effects of CPT -11 (1 μM) was not observed when BxPC3 cancer cells were co-cultured with parental F3 cells (Figure 3D). In contrast, the survival of BxPC3 cancer cells co-cultured with F3.CE cells (cancer cells: F3.CE cells = 75:25, 50:50, or 25:75) was significantly reduced by 48 hr after exposure to 1 μM CPT-11 (P < 0.05, Figure 3D). Without CPT-11, co-culture with F3 or F3.CE had no effect on the survival of BxPC3 cancer cells (data not shown).
In vivo therapeutic efficacy of F3.CE cells in cancer bearing mice
Timeline for the establishment of pancreas adenocarcinoma animal model and subsequent treatment using F3.CE cells and CPT-11 is shown on (Figure 4). In histologic study performed at 3 weeks after the last CPT-11 injection, cancer bearing animals treated with F3.CE cells and CPT-11 showed a significant reduction in cancer volume (Figure 5). The in vivo therapeutic efficacy of F3.CE cells against pancreas cancer was determined by tumor volume measurement. We measure and trace the tumor volumes from 2 weeks to end point at 8 weeks (Figure 5B). When final tumor volumes were determined 3 weeks after the last CPT-11 injection, the F3.CE + CPT-11 group mice showed significantly reduced tumor volumes (mean ± S.E. = 55.1 ± 15.8 mm3) compared with the sham control (2324.9 ± 662.8 mm3, p=0.001), F3.CE only group (2137.6 ± 377.5 mm3, p=0.001), and CPT-11 only group (1302.6 ± 168.6 mm3, p=0.001), respectively. There was 97.6 % reduction in tumor volume in F3.CE + CPT-11 group compared with the sham control group. There was 44% reduction in tumor volume in CPT-11 only group animals indicating that CPT-11 also acts as anticancer therapeutic. F3.CE cells encoding rabbit CE enzyme could convert chemotherapeutic agent CPT-11 into its more potent form, SN-38 at the site of the cancer and induced significantly additive tumor-killing activity.
Figure 4. Timeline for the establishment of pancreas adenocarcinoma animal model and subsequent treatment using F3.CE cells and CPT-11.
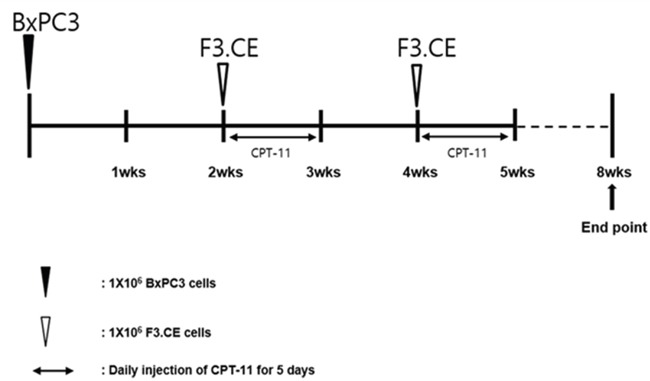
Human pancreas adenocarcinoma cells (1 × 106 cells in 10 μL PBS) was injected into the subcutaneous dorsa of mice in the proximal midline. 6-week old SCID mice (n=7 each). At 14 and 28 days after tumor cell implantation, F3.CE cells (1 × 106 cells in 100 μL PBS) were injected subcutaneously at four sites, 1 mm distant from the tumor. At 15~19 and 29~33 days after tumor cell implantation, CPT-11 (3.75 mg/kg) was injected into peritoneum once a day. Eight weeks from tumor implantation, the mice were sacrificed and the tumor mass measurement was performed.
Figure 5. Treatment with F3.CE cells and CPT-11 has a significant therapeutic effect in vivo.
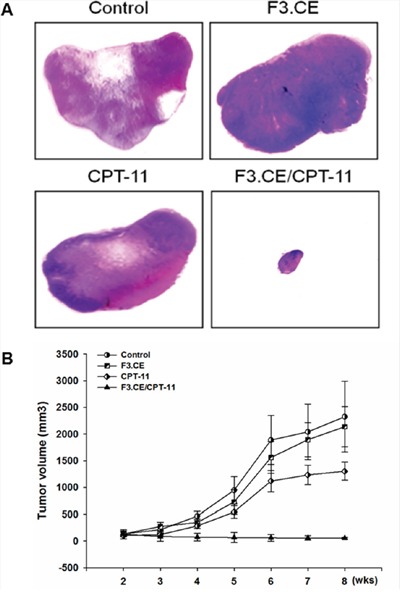
A. The representative images of skin slices for each group. Tumor bearing mice treated with F3.CE and CPT-11 showed significantly smaller tumor volumes compared with other groups including sham control group, tumor bearing animals transplanted with F3.CE cell but without CPT-11 treatment or animals injected with CPT-11 but without F3.CE cell transplantation (H&E stain). B. Tumor bearing mice treated with F3.CE and CPT -11 showed significantly smaller tumor volumes compared with other groups including sham control group, tumor bearing animals transplanted with F3.CE cell but without CPT-11 treatment or animals injected with CPT-11 but without F3.CE cell transplantation.
DISCUSSION
In the present study, we observed that human pancreatic cancer cell line BxPC3 treated with F3.NSCs expressing rabbit carboxylesterase (F3.CE) and application of prodrug CPT-11 showed marked growth inhibition and increased apoptosis in vitro. We also confirmed that transplantation of F3.CE and CPT-11 in immune-incompetent mice bearing BxPC3 human pancreatic cancer cells markedly inhibited cancer growth.
The NSCs were engineered to express rabbit carboxylesterase (CE). The enzyme activates the prodrug CPT-11 to the active drug SN-38, which has 1,000-fold more potent topoisomerase I inhibitory effect. In turn, SN-38 preferentially kills dividing cancer cells specifically at the tumor sites [30]. We have previously reported that F3.CE cells migrate selectively to tumor sites and they have a therapeutic effect on disseminated neuroblastoma [26, 30], melanoma [28], ovarian cancer [19], breast cancer brain metastasis [17] and medulloblastoma [29] upon administration of prodrug CPT-11. Recently we also have reported that the NSCs expressing the cytosine deaminase (CD). NSCs expressing CD and interferon-β selectively migrated toward cancer mass in nude mice bearing the PANC-1 human pancreatic cancer. Following administration of prodrug 5-fluorocytosine (5-FC), marked inhibition of cancer growth was observed [33].
Multimodal treatment for pancreatic cancer including surgical resection, radiation and chemotherapy has substantially improved the survival rate in patients with pancreatic cancer, however, it remains incurable in large proportion of patients. Therefore, there is profound need for both effective and less-toxic therapy for patients suffering from pancreatic cancer. The gene therapy approach targeting pancreatic cancer should fulfill this requirement.
Stem cell-based gene therapy using neural stem cells (NSCs) has received much attention as an innovative cancer therapy [11]. Previously we have adopted CE/CPT-11 enzyme/prodrug gene therapy approach for treatment of gliomas and disseminated neurobastoma animal models [19, 26–30] involving the conversion of a prodrug CPT-11 into SN-38 [30, 34]. CPT-11 (Irinotecan) is known to induce severe toxicities (diarrhea, neutropenia) that limit its clinical use [34]. The complex pharmacokinetics of CPT-11 and the involvement of several enzymes other than UGT (i.e., carboxylestherases, CYP450 isoforms), and transmembrane transporters (ABCB1, ABCC1, ABCG2, SLCO1B1) make it difficult to identify patients with an optimal sensitivity and specificity [35]. However, if our approach of activating CPT-11 with the designated enzyme inside of the tumor using NSCs could be used clinically, it might strengthen the efficacy and safety of the chemotherapy.
In conclusion, the present results demonstrate for the first time that the F3.CE cells successfully exerted therapeutic efficacy on BxPC3 pancreatic adenocarcinoma cells upon administration of CPT-11.
MATERIALS AND METHODS
Cell culture
HB1.F3 (F3), a stably immortalized human NSC cell line, was derived from human fetal telencephalon at 15 weeks of gestation by introducing a retroviral vector encoding v-myc [31, 32]. F3 and F3 cells overexpressing rabbit CE gene (F3.CE) cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mmol/l L-glutamine, 100 units/mL penicillin and 100 μg/mL streptomycin (DMEM-10% FBS) (Sigma-Aldrich, St. Louis, MO). BxPC3 human pancreas adenocarcinoma cell line was obtained from Dr. Yun-Hee Kim in the National Cancer Center (South Korea) and maintained in DMEM-10% FBS.
Generation of F3.CE human NSC line
The clonal F3.CE human NSC line was derived from the parental F3 NSC line. An expression plasmid encoding rabbit CE was constructed using the retroviral pLPCXpuro (Clontech, Palo Alto, CA) as previously described [30]. Successful transduction of the F3.CE cells was confirmed by reverse transcription–PCR (Figure 1) using the primer pair shown in Table 1. To confirm the activity of CE in F3.CE cells, the cytotoxic effect of CPT-11 on F3 or F3.CE cells was analyzed using a cell viability assay. F3 or F3.CE cells (3 × 104/well) were plated in 6-well plates. Twenty-four hrs after seeding, 0.5-5 μM of CPT-11 (Hanmi Pharma, Seoul, Korea) was applied for 48 hrs, and the status of the cells was analyzed using a microscope. Cell viability was performed using Muse™ Cell Analyzer (Millipore, Billerica, MA) following manufacturer's instruction. Briefly, after the indicated treatments, the cells were detached and washed with PBS and incubated with Muse cell count and viability solution for 5min. After staining, the cells were processed in muse apparatus.
Table 1. Sequence of PCR primers.
| Gene | Sequence | Size (bp) |
|---|---|---|
| SCF | Sense 5'-ACTTGGATTCTCACTTGCATTT-3' Antisense 5'-CTTTCTCAGGACTTAATGTTGAAG-3' |
505 |
| c-kit | Sense 5'-GCCCACAATAGATTGGTATTT-3' Antisense 5'-AGCATCTTTACAGCGACAGTC-3' |
332 |
| SDF-1 | Sense 5'-ATGAACGCCAAGGTCGTGGTC-3' Antisense 5'-GGCTGTTGTGCTTACTTGTTT-3' |
200 |
| CXCR4 | Sense 5'-CTCTCCAAAGGAAAGCGAGGTGGACAT-3' Antisense 5'-AGACTGTACACTGTAGGTGCTGAAATCA-3' |
733 |
| VEGF | Sense 5'-AAGCCATCCTGTGTGCCCCTGATG-3' Antisense 5'-GCTCCTTCCTCCTGCCCGGCTCAC-3' |
541 |
| VEGFR1 | Sense 5'-GCAAGGTGTGACTTTTGTTC-3' Antisense 5'-AGGATTTCTTCCCCTGTGTA-3' |
512 |
| VEGFR2 | Sense 5'-ACGCTGACATGTACGGTCTAT-3' Antisense 5'-GCCAAGCTTGTACCATGTGCG-3' |
438 |
| GAPDH | Sense 5'-CATGACCACAGTCCATGCCATCACT-3' Antisense 5'-TGAGGTCCACCACCCTGTTGCTGTA-3' |
450 |
In vitro “bystander effect” experiments
BxPC3 human pancreas adenocarcinoma cells were plated in 6-well plates with F3 or F3.CE cells (BxPC3 cells:F3 or F3.CE cells = 100:0, 75:25, 50:50, 25:75, or 0:100). BxPC3 and F3 or F3.CE cells were maintained in DMEM-10%FBS. After 24 hrs of cell growth, 1.0 μM CPT-11 was added to the mixed cell cultures and 48 hrs later, cell viability was determined utilizing Muse™ Cell Analyzer as described above.
Apoptosis assay
Annexin V & Dead Cell Assay was performed utilizing Muse™ Cell Analyzer following manufacturer's instruction. Briefly, after the indicated treatments, the cells were incubated with Annexin V and Dead Cell Reagent (7-AAD) for 20min and the events for dead, late apoptotic, early apoptotic, and live cells were counted.
Pancreas cancer animal model
Animal experiments in this study have been reviewed and approved by the Animal Care and Use Committee of Chung-Ang University (IRB: 11-0086). Six-week-old male BALB/c nude mice were purchased from Saeronbio Inc. (Kyunggi, Korea). Mice were caged in a barrier care facility, and fed with animal chow and water ad libitum. Mice were randomized into four groups, 7 mice each. The control group was injected with BxPC3 human pancreatic adenocarcinoma cells only. The second group received BxPC3 cancer cells and prodrug CPT-11. The third group received BxPC3 cancer cells and F3.CE human NSCs, while the fourth group receiving BxPC3 cancer cells and F3.CE human NSCs was treated with CPT-11. The fourth group was administered with F3.CE and CPT-11 regimen according to the time line (Figure 4).
Animals were anesthetized via intraperitoneal injection of Zoletil before all surgical procedures and were observed until fully recovered. Before tumor cell injection, mice were shaved and the dorsal skin cleaned with ethanol. Human pancreas cancer cells (BxPC3, 1 × 106 cells in 100 μL PBS) were injected into the subcutaneous dorsa of mice in the proximal midline of 6-week old SCID mice (n=7). At 14 and 28 days after cancer cell implantation, F3.CE cells (2.5 × 105 cells in 25 μL PBS) were injected subcutaneously at four sites (i.e. 1 × 106 cells in 100 μL PBS in total), 1 mm distant from the cancer. At 15~19 and 29~33 days after cancer cell implantation, CPT-11 (3.75 mg/kg) was injected into peritoneum once a day. Eight weeks after cancer implantation, the mice were sacrificed in accordance with institutional guidelines, and the removed cancer tissues were fixed in 4% paraformaldehyde and the cancer mass measurement was performed.
Immunohistochemistry
Representative cancer tissues (three from each group) were paraffin-embedded. Sections (5 μm) were stained with hematoxylin-eosin (H&E) to evaluate tumor size.
Statistics analysis
All experiments is repeated three times. Two-way ANOVA tests were used to evaluate differences in all experiments between groups. P values < 0.05 were considered statistically significant. Analyses were performed using SigmaPlot version 12; (Systat software Inc., San Jose, CA, USA).
Acknowledgments
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No.PJ0117922016)” Rural Development Administration, Republic of Korea.
Footnotes
CONFLICTS OF INTEREST
The authors disclose no potential conflicts of interest
REFERENCES
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Huguet F, Girard N, Guerche CS, Hennequin C, Mornex F, Azria D. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J clin oncol. 2009;27:2269–2277. doi: 10.1200/JCO.2008.19.7921. [DOI] [PubMed] [Google Scholar]
- 3.Shaib YH, Davila JA, El-Serag HB. The epidemiology of pancreatic cancer in the United States: changes below the surface. Aliment Pharmacol Ther. 2006;24:87–94. doi: 10.1111/j.1365-2036.2006.02961.x. [DOI] [PubMed] [Google Scholar]
- 4.Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–1326. doi: 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt NO, Przylecki W, Yang W, Ziu M, Teng Y, Kim SU, Black PM, Aboody KS, Carroll RS. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005;7:623–629. doi: 10.1593/neo.04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimato S, Natsume A, Takeuchi H, Wakabayashi T, Fujii M, Ito M, Ito S, Park IH, Bang JH, Kim SU, Yoshida J. Human neural stem cells target and deliver therapeutic gene to experimental leptomeningeal medulloblastoma. Gene ther. 2007;14:1132–1142. doi: 10.1038/sj.gt.3302932. [DOI] [PubMed] [Google Scholar]
- 7.Kim SK, Kim SU, Park IH, Bang JH, Aboody KS, Wang KC, Cho BK, Kim M, Menon LG, Black PM, Carroll RS. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin cancer res. 2006;12:5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- 8.Kendall SE, Najbauer J, Johnston HF, Metz MZ, Li S, Bowers M, Garcia E, Kim SU, Barish ME, Aboody KS, Glackin CA. Neural stem cell targeting of glioma is dependent on phosphoinositide 3-kinase signaling. Stem Cells. 2008;26:1575–1586. doi: 10.1634/stemcells.2007-0887. [DOI] [PubMed] [Google Scholar]
- 9.Kim SK, Cargioli TG, Machluf M, Yang W, Sun Y, Al-Hashem R, Kim SU, Black PM, Carroll RS. PEX-producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clin cancer res. 2005;11:5965–5970. doi: 10.1158/1078-0432.CCR-05-0371. [DOI] [PubMed] [Google Scholar]
- 10.Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology. 2004;24:159–171. doi: 10.1111/j.1440-1789.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim SU. Neural stem cell-based gene therapy for brain tumors. Stem cell rev. 2011;7:130–140. doi: 10.1007/s12015-010-9154-1. [DOI] [PubMed] [Google Scholar]
- 12.Kim SU, Lee HJ, Kim YB. Neural stem cell-based treatment for neurodegenerative diseases. Neuropathology. 2013;33:491–504. doi: 10.1111/neup.12020. [DOI] [PubMed] [Google Scholar]
- 13.Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J neurosci res. 2009;87:2183–2200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y, Hamada H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Kim Y, Jo MY, Kim HS, Jin Y, Kim SU, Jin J, Joo KM, Nam DH. Combined treatment of tumor-tropic human neural stem cells containing the CD suicide gene effectively targets brain tumors provoking a mild immune response. Oncol rep. 2011;25:63–68. [PubMed] [Google Scholar]
- 16.Zhao D, Najbauer J, Annala AJ, Garcia E, Metz MZ, Gutova M, Polewski MD, Gilchrist M, Glackin CA, Kim SU, Aboody KS. Human neural stem cell tropism to metastatic breast cancer. Stem Cells. 2012;30:314–325. doi: 10.1002/stem.784. [DOI] [PubMed] [Google Scholar]
- 17.Joo KM, Park IH, Shin JY, Jin J, Kang BG, Kim MH, Lee SJ, Jo MY, Kim SU, Nam DH. Human neural stem cells can target and deliver therapeutic genes to breast cancer brain metastases. Mol ther. 2009;17:570–575. doi: 10.1038/mt.2008.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito S, Natsume A, Shimato S, Ohno M, Kato T, Chansakul P, Wakabayashi T, Kim SU. Human neural stem cells transduced with IFN-beta and cytosine deaminase genes intensify bystander effect in experimental glioma. Cancer gene ther. 2010;17:299–306. doi: 10.1038/cgt.2009.80. [DOI] [PubMed] [Google Scholar]
- 19.Kim KY, Kim SU, Leung PC, Jeung EB, Choi KC. Influence of the prodrugs 5-fluorocytosine and CPT-11 on ovarian cancer cells using genetically engineered stem cells: tumor-tropic potential and inhibition of ovarian cancer cell growth. Cancer sci. 2010;101:955–962. doi: 10.1111/j.1349-7006.2009.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thu MS, Najbauer J, Kendall SE, Harutyunyan I, Sangalang N, Gutova M, Metz MZ, Garcia E, Frank RT, Kim SU, Moats RA, Aboody KS. Iron labeling and pre-clinical MRI visualization of therapeutic human neural stem cells in a murine glioma model. PLoS one. 2009;4:e7218. doi: 10.1371/journal.pone.0007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao D, Najbauer J, Garcia E, Metz MZ, Gutova M, Glackin CA, Kim SU, Aboody KS. Neural stem cell tropism to glioma: critical role of tumor hypoxia. Mol cancer res. 2008;6:1819–1829. doi: 10.1158/1541-7786.MCR-08-0146. [DOI] [PubMed] [Google Scholar]
- 22.Lin D, Najbauer J, Salvaterra PM, Mamelak AN, Barish ME, Garcia E, Metz MZ, Kendall SE, Bowers M, Kateb B, Kim SU, Johnson M, Aboody KS. Novel method for visualizing and modeling the spatial distribution of neural stem cells within intracranial glioma. NeuroImage. 2007;37:S18–26. doi: 10.1016/j.neuroimage.2007.03.076. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Lee JE, Kim SU, Cho KG. Stereological analysis on migration of human neural stem cells in the brain of rats bearing glioma. Neurosurgery. 2010;66:333–342. doi: 10.1227/01.NEU.0000363720.07070.A8. [DOI] [PubMed] [Google Scholar]
- 24.Lee DH, Ahn Y, Kim SU, Wang KC, Cho BK, Phi JH, Park IH, Black PM, Carroll RS, Lee J, Kim SK. Targeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cells. Clin cancer res. 2009;15:4925–4934. doi: 10.1158/1078-0432.CCR-08-3076. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Kim JY, Kim SU, Cho KG. Therapeutic effect of genetically modified human neural stem cells encoding cytosine deaminase on experimental glioma. Biochem biophys res commun. 2012;417:534–540. doi: 10.1016/j.bbrc.2011.11.155. [DOI] [PubMed] [Google Scholar]
- 26.Aboody KS, Bush RA, Garcia E, Metz MZ, Najbauer J, Justus KA, Phelps DA, Remack JS, Yoon KJ, Gillespie S, Kim SU, Glackin CA, Potter PM, Danks MK. Development of a tumor-selective approach to treat metastatic cancer. PLoS one. 2006;1:e23. doi: 10.1371/journal.pone.0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seol HJ, Jin J, Seong DH, Joo KM, Kang W, Yang H, Kim J, Shin CS, Kim Y, Kim KH, Kong DS, Lee JI, Aboody KS, et al. Genetically engineered human neural stem cells with rabbit carboxyl esterase can target brain metastasis from breast cancer. Cancer lett. 2011;311:152–159. doi: 10.1016/j.canlet.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Gutova M, Najbauer J, Chen MY, Potter PM, Kim SU, Aboody KS. Therapeutic targeting of melanoma cells using neural stem cells expressing carboxylesterase, a CPT-11 activating enzyme. Curr stem cell res ther. 2010;5:273–276. doi: 10.2174/157488810791824421. [DOI] [PubMed] [Google Scholar]
- 29.Lim SH, Choi SA, Lee JY, Wang KC, Phi JH, Lee DH, Song SH, Song JH, Jin X, Kim H, Lee HJ, Lim I, Kim SU, Kim SK. Therapeutic targeting of subdural medulloblastomas using human neural stem cells expressing carboxylesterase. Cancer gene ther. 2011;18:817–824. doi: 10.1038/cgt.2011.52. [DOI] [PubMed] [Google Scholar]
- 30.Danks MK, Yoon KJ, Bush RA, Remack JS, Wierdl M, Tsurkan L, Kim SU, Garcia E, Metz MZ, Najbauer J, Potter PM, Aboody KS. Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer res. 2007;67:22–25. doi: 10.1158/0008-5472.CAN-06-3607. [DOI] [PubMed] [Google Scholar]
- 31.Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, Ko Y, Jeong SW, Kim SU. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25:1204–1212. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- 32.Kim SU, Nagai A, Nakagawa E, Choi HB, Bang JH, Lee HJ, Lee MA, Lee YB, Park IH. Production and characterization of immortal human neural stem cell line with multipotent differentiation property. Methods Mol Biol. 2008;438:103–121. doi: 10.1007/978-1-59745-133-8_10. [DOI] [PubMed] [Google Scholar]
- 33.Kim DJ, Yi BR, Lee HR, Kim SU, Choi KC. Pancreatic tumor mass in a xenograft mouse model is decreased by treatment with therapeutic stem cells following introduction of therapeutic genes. Oncol rep. 2013;30:1129–1136. doi: 10.3892/or.2013.2564. [DOI] [PubMed] [Google Scholar]
- 34.Potter PM, Pawlik CA, Morton CL, Naeve CW, Danks MK. Isolation and partial characterization of a cDNA encoding a rabbit liver carboxylesterase that activates the prodrug irinotecan (CPT-11) Cancer res. 1998;58:2646–2651. [PubMed] [Google Scholar]
- 35.Di Paolo A, Bocci G, Polillo M, Del Re M, Di Desidero T, Lastella M, Danesi R. Pharmacokinetic and pharmacogenetic predictive markers of irinotecan activity and toxicity. Curr Drug Metab. 2011;12:932–943. doi: 10.2174/138920011798062283. [DOI] [PubMed] [Google Scholar]


