Abstract
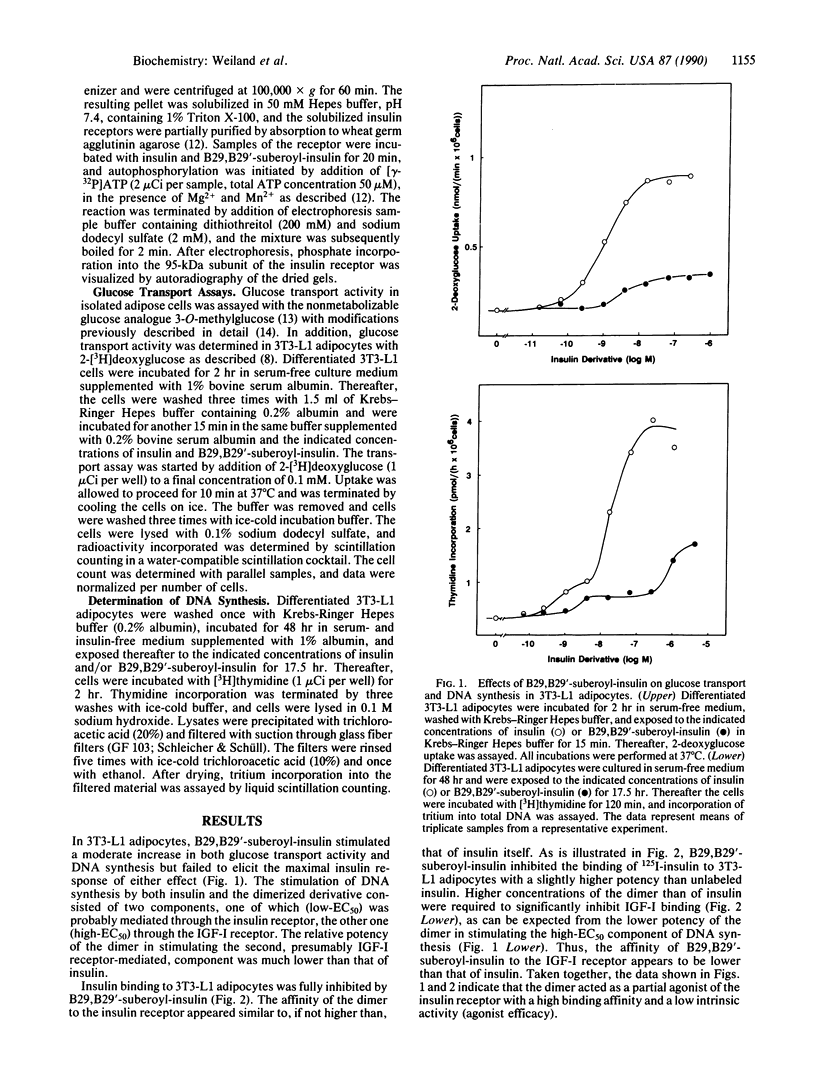
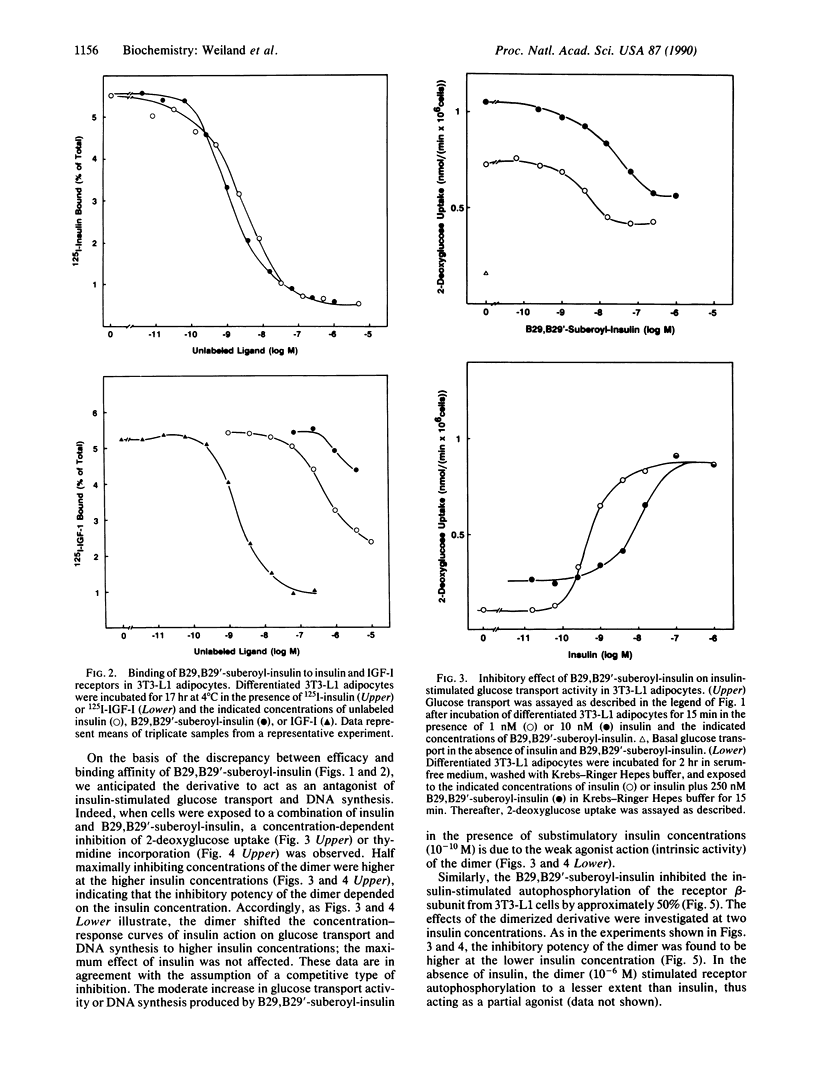
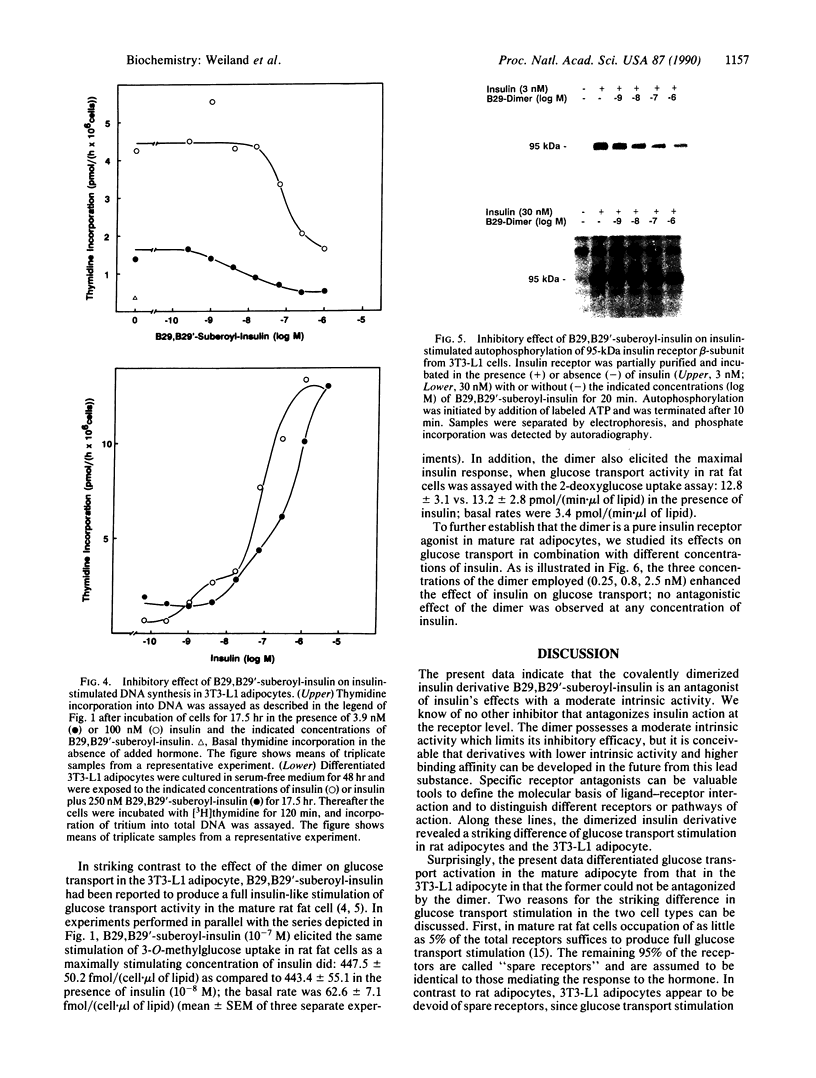
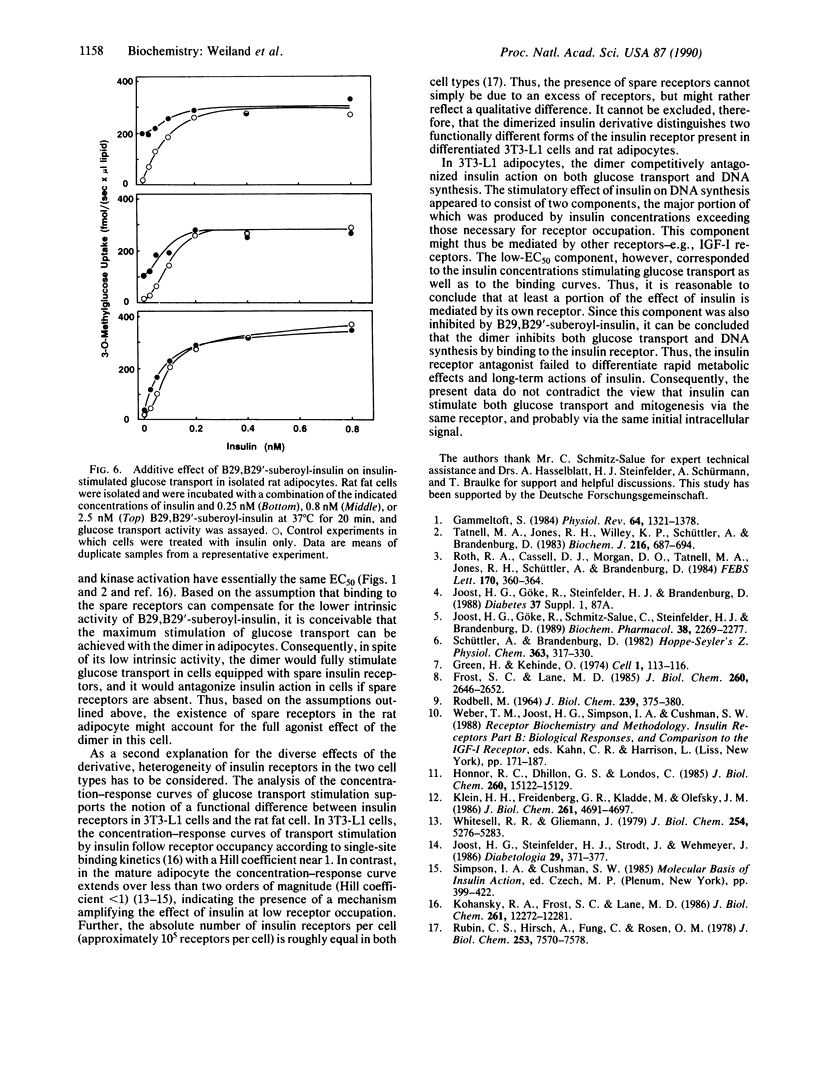
In the present study we describe the antagonistic effects of the covalently dimerized insulin derivative B29,B29'-suberoyl-insulin on insulin receptors in 3T3-L1 mouse cells. In differentiated 3T3-L1 adipocytes, the derivative fully inhibits binding of 125I-labeled insulin to its receptor with about the same affinity as unlabeled insulin. In contrast, the dimerized derivative only partially (approximately 20%) mimics insulin's effects on glucose transport and DNA synthesis in the absence of insulin. In the presence of insulin, the agent competitively inhibits insulin-stimulated DNA synthesis ([3H]thymidine incorporation into total DNA), glucose transport activity (2-deoxyglucose uptake rate), and insulin receptor tyrosine kinase activity. In rat adipocytes, in contrast, the dimerized derivative stimulates glucose transport (initial 3-O-methylglucose as well as 2-deoxyglucose uptake rates) to the same extent as insulin does, and it fails to inhibit the effect of insulin. The data indicate that the dimerized insulin derivative B29,B29'-suberoyl-insulin is an insulin receptor antagonist (partial agonist) which retains a moderate intrinsic activity. The effects of this agent reveal a striking difference in insulin receptor-mediated stimulation of glucose transport between 3T3-L1 fatty fibroblasts and the mature rat adipocyte.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frost S. C., Lane M. D. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J Biol Chem. 1985 Mar 10;260(5):2646–2652. [PubMed] [Google Scholar]
- Gammeltoft S. Insulin receptors: binding kinetics and structure-function relationship of insulin. Physiol Rev. 1984 Oct;64(4):1321–1378. doi: 10.1152/physrev.1984.64.4.1321. [DOI] [PubMed] [Google Scholar]
- Honnor R. C., Dhillon G. S., Londos C. cAMP-dependent protein kinase and lipolysis in rat adipocytes. I. Cell preparation, manipulation, and predictability in behavior. J Biol Chem. 1985 Dec 5;260(28):15122–15129. [PubMed] [Google Scholar]
- Joost H. G., Göke R., Schmitz-Salue C., Steinfelder H. J., Brandenburg D. Quantitative dissociation of glucose transport stimulation and insulin receptor tyrosine kinase activation in isolated adipocytes with a covalent insulin dimer (B29,B29'-suberoyl-insulin). Biochem Pharmacol. 1989 Jul 15;38(14):2269–2277. doi: 10.1016/0006-2952(89)90465-6. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Steinfelder H. J., Strodt J., Wehmeyer J. Modulation of glucose transport in hamster adipocytes by insulin and by beta- and alpha 2-adrenoceptor agonists. Diabetologia. 1986 Jun;29(6):371–377. doi: 10.1007/BF00903347. [DOI] [PubMed] [Google Scholar]
- Klein H. H., Freidenberg G. R., Kladde M., Olefsky J. M. Insulin activation of insulin receptor tyrosine kinase in intact rat adipocytes. An in vitro system to measure histone kinase activity of insulin receptors activated in vivo. J Biol Chem. 1986 Apr 5;261(10):4691–4697. [PubMed] [Google Scholar]
- Kohanski R. A., Frost S. C., Lane M. D. Insulin-dependent phosphorylation of the insulin receptor-protein kinase and activation of glucose transport in 3T3-L1 adipocytes. J Biol Chem. 1986 Sep 15;261(26):12272–12281. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J., Morgan D. O., Tatnell M. A., Jones R. H., Schüttler A., Brandenburg D. Effects of covalently linked insulin dimers on receptor kinase activity and receptor down regulation. FEBS Lett. 1984 May 21;170(2):360–364. doi: 10.1016/0014-5793(84)81344-7. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Schüttler A., Brandenburg D. Preparation and properties of covalently linked insulin dimers. Hoppe Seylers Z Physiol Chem. 1982 Mar;363(3):317–330. doi: 10.1515/bchm2.1982.363.1.317. [DOI] [PubMed] [Google Scholar]
- Tatnell M. A., Jones R. H., Willey K. P., Schüttler A., Brandenburg D. Evidence concerning the mechanism of insulin-receptor interaction and the structure of the insulin receptor from biological properties of covalently linked insulin dimers. Biochem J. 1983 Dec 15;216(3):687–694. doi: 10.1042/bj2160687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell R. R., Gliemann J. Kinetic parameters of transport of 3-O-methylglucose and glucose in adipocytes. J Biol Chem. 1979 Jun 25;254(12):5276–5283. [PubMed] [Google Scholar]




