Summary
The ocular surface is constantly exposed to environmental irritants, allergens and pathogens, against which it can mount a prompt immune response to preserve its integrity. But to avoid unnecessary inflammation, the ocular surface's mucosal immune system must also discriminate between harmless and potentially dangerous antigens, a seemingly complicated task. Despite its unique features, the ocular surface is a mucosal lining, and as such, it shares some homeostatic and pathophysiological mechanisms with other mucosal surfaces. The purpose of this review is to explore the mucosal homeostatic immune function of the ocular surface in both the healthy and diseased states, with a special focus on mucosal immunology concepts. The information discussed in this review has been retrieved by PubMed searches for literature published from January 1981 to October 2016.
Keywords: allergic conjunctivitis, conjunctival inflammation, dry eye, mucosal tolerance, ocular mucosal immune system, ocular surface
Abbreviations
- DC
dendritic cell
- DED
dry eye disease
- IBD
irritable bowel disease
- IL
interleukin
- MAPK
mitogen‐activated protein kinase
- NF‐κB
nuclear factor‐κB
- NK
natural killer
- OS
ocular surface
- OVA
ovalbumin
- TGF
transforming growth factor
- Th2
T helper type 2
- Treg
regulatory T
- TSLP
thymic stromal lymphopoeitin
- VEGFR
vascular endothelial growth factor receptor
Introduction
The ocular surface (OS), which comprises the cornea, the limbus, the conjunctiva and the tear film, contributes the largest portion of the eye's optical power, so playing a key role in the visual system. A sharp image on the retina can only be formed if light rays refract first through a clear, smooth corneal surface, and the latter depends on the protection afforded by its mucosal environment. To this aim, the surrounding limbus and the conjunctiva are armed with a fullly fledged immune system because the OS is highly exposed to pathogens. Inflammation is, however, a double‐edged sword, and as a proof of this, irreversible sight‐threatening damage of the cornea and the conjunctiva is the end stage of many OS disorders when left untreated.1 In addition to the inherent complexity of the cornea's immune privilege,2 which is not the focus of this review, there are many unique aspects to the immune physiology of the OS, all of which have been extensively reviewed elsewhere: the tear film3 and the mucin layer/glycocalyx,4 the epithelial layer5 with its goblet cells,6 and the conjunctiva‐associated lymphoid tissue.7 But the OS could also be thought of as a specialized mucosal lining, and as such, it shares some homeostatic and pathological mechanisms with other mucosae. The purpose of this review is to explore the mucosal homeostatic immune function of the OS in both the healthy and diseased states.
Immune homeostasis in mucosal linings
From an immunological viewpoint, all mucosal linings are faced with a dilemma: whether to disregard or to mount an inflammatory response to the wide array of foreign antigens to which they are exposed. Mucosal surfaces can react vigorously because they are endowed with potent immune systems, but inflammation comes at a cost. Therefore, they strive for an uninflamed physiological state to allow for proper organ function, and to do so mucosal linings are armed with active mechanisms that keep the immune response at bay. Hence, lack of inflammation in the basal state does not imply the absence of an immune response, but the supremacy of the aforementioned regulatory mechanisms. The latter are collectively referred to as mucosal immune tolerance, and in a strict sense they are a form of peripheral immune tolerance with both innate and adaptive immune components. Mucosal immune tolerance was first observed in the gastrointestinal tract, and it could be defined as “a state of local and systemic immune unresponsiveness that is induced after an innocuous antigen is delivered through a mucosal surface”.8
The mucosal epithelial lining, the actual barrier with the environment, is the main innate constituent of mucosal tolerance, as it plays a crucial role in the decision process of which type of immune response (regulatory or pro‐inflammatory) to conduct.9 Intestinal epithelial cells are in close proximity to commensal bacteria, yet they do not initiate an inflammatory reaction aimed towards bacterial elimination.10 However, intestinal epithelial cells do keep commensal bacteria in check by secreting a thick mucus layer and antimicrobial peptides.10 Airway epithelial cells are constantly exposed to harmless airborne antigens, and the way they respond to these proteins determines a healthy state or allergic disease.11 The molecular and cellular mechanisms vary greatly from organ to organ, but there is a common governing principle in mucosal immunity: the epithelial lining initiates and orchestrates the immune response. In the gut, epithelial cells secrete soluble factors such as transforming growth factor‐β (TGF‐β), interleukin‐10 (IL‐10), retinoic acid, prostaglandin E2 and thymic stromal lymphopoeitin, all of which influence dendritic cells (DCs).12 Most of these factors have also been detected in the airway epithelium, where they can be modulated directly by some allergens that trigger signalling through apical receptors.13
At any rate, the adaptive immune response that typifies mucosal tolerance is initiated by antigen‐presenting cells, namely DCs, under the conditioning influence of epithelial cells. There are a few subtypes of epithelial and stromal DCs in each mucosal site, some of which seem more involved in pathogenic immune responses and others in mucosal tolerance.11, 14 Recently, it has become clear that steady‐state migration of DCs to the draining lymph nodes, a key step in establishing mucosal tolerance,15 is not simply a default program for these cells but a tightly regulated process in which nuclear factor‐κB (NF‐κB) signalling is deeply involved.16 However, the homeostatic upstream signals that activate this intracellular pathway in mucosal DCs remain to be identified. At least in the gut, there is evidence that luminal mucin delivers homeostatic signals to epithelial cells and DCs through its carbohydrate residues,17 and that passage of mucus‐lined antigen from goblet cells to DCs leads to tolerogenic conditioning of the latter.18
Actual mucosal tolerance towards a specific antigen is carried out by regulatory T (Treg) cells, which inhibit innate immune cells, DCs and effector B and T lymphocytes that ultimately would drive mucosal inflammation.19 There are several subtypes of Treg cells, some of which originate in the thymus as a result of T‐cell ontogeny (central or natural Treg cells), and others that develop in the peripheral lymphoid organs from naive precursors after contacting tolerogenic DCs (peripheral or inducible Treg cells). Natural and most inducible Treg cells are CD4+ Foxp3+ CD25+, and other inducible Treg cells are CD4+ Foxp3– (Tr1 cells). There are also CD8+ Treg cells. The contribution of these subtypes to mucosal homeostasis varies from organ to organ,20 and they also differ in how they suppress inflammation.19
The OS immune system
The OS comprises both innate and adaptive immune mechanisms that aid in maintaining its integrity (Fig. 1). Tear film clearance and regular blinking continuously remove antigens and microbes away from the OS,3 whereas the glycocalyx and the tight junctions in the apical cell layers of the conjunctival and corneal epithelia serve as a formidable physical barrier with immunomodulatory properties.4, 21, 22 In addition, tears favour the protective, tonic activation of stress response‐associated transcription factors NF‐κB and activator protein 1 (AP‐1) in these cells,23 which influence mucosal immune responses.24, 25 The epithelial layer itself is not a mere barricade, but an active component of the immune system: corneal and conjunctival epithelial cells secrete microbicidal and immunomodulatory peptides and cytokines and can respond to pathogen‐ and danger‐associated molecular patterns through their functional Toll‐ and NOD‐like receptor signalling system.26, 27, 28, 29 Moreover, the OS epithelium expresses membrane receptors that modulate DC and lymphocyte function.28, 30, 31, 32 On the one hand, corneal epithelial cells constitutively express programmed death‐ligand 1, which deters lymphocytic infiltration by reducing chemokine secretion,33 and also secrete vascular endothelial growth factor receptor 3 and pigment epithelium derived factor, which prevent neovessel growth and may also have an immunomodulatory role.34 In addition, conjunctival goblet cells specifically modulate DCs through secretion and extracellular activation of TGF‐β 2 in a thrombospondin‐1‐dependent fashion.32 On the other hand, in the context of allergic disease, corneal and conjunctival keratinocytes produce thymic stromal lymphopoeitin and favour a T helper type 2 (Th2) response.30, 31 By contrast, these epithelial cells, when exposed to desiccating conditions, secrete chemokines CCL20, CXCL9, CXCL10 and CXCL11, which preferentially recruit Th1/Th17 effector T cells from the circulation, and express membrane ligands that activate resident natural killer (NK) cells and favour interferon‐γ release.35, 36 Hence, differential ‘sensing’ of environmental challenges by the OS epithelium sets the stage for rather dissimilar immune responses.
Figure 1.
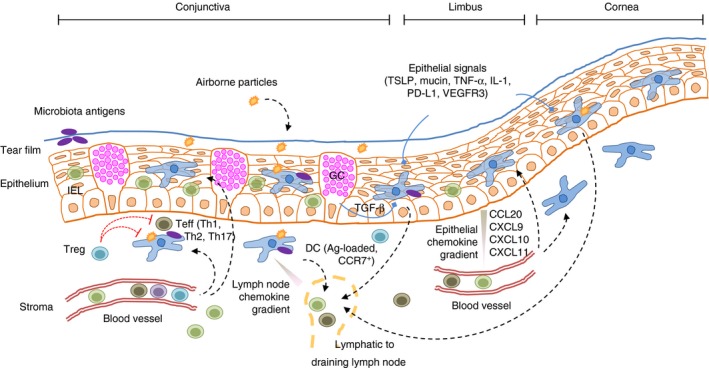
Some of the most relevant cell types and interactions in the ocular surface mucosal system. Airborne and microbiota‐derived antigens (Ag) reach the tear film, the outermost layer of the ocular surface. Conjunctival and corneal keratinocytes and conjunctival goblet cells (GC) make up the epithelial barrier, and within their confines also reside epithelial dendritic cells (DC) and intraepithelial lymphocytes (IEL). There is extensive crosstalk between all these cell types through soluble factors and membrane receptors: transforming growth factor‐β (TGF‐β), thymic stromal lymphoeitin (TSLP), tumour necrosis factor‐α (TNF‐α), interleukin‐1 (IL‐1), programmed cell death‐ligand 1 (PD‐L1) and vascular endothelial growth factor receptor 3, among others. Keratinocytes also secrete chemokines CCL20 and CXCL9‐11, which attract blood‐borne lymphocytes and DCs. Among lymphocytes, T cells play a pivotal role in mucosal tolerance, and there are regulatory (Treg) and effector (Teff) T cells. The former suppress inflammation, whereas the latter favour it.
In a strict sense, OS DCs initiate adaptive immune responses, either by priming naive T cells in the draining lymph node or by activating effector T cells in situ.37 DCs are most abundant in the conjunctiva of humans and mice,38, 39 and their density decreases from the limbus towards the central cornea under normal conditions.40, 41, 42 All major non‐lymphoid tissue‐resident DC populations (CD11b+, CD103+ and plasmacytoid) have been identified in the normal murine conjunctiva, and these populations change under inflammatory conditions.41, 42 The DC subtypes play different roles in maintaining homeostasis and inducing inflammation in other mucosal sites, and this also applies to the OS.41 Conjunctival CD11b+ DCs are more abundant, increase after allergic challenge and apparently induce more potent secondary allergic inflammation than CD103+ DCs.41 The CD103+ and plasmacytoid DCs might be more important for immune homeostasis, as observed in other mucosal sites,43 but specific studies for conjunctival DCs are lacking. The cornea, on the other hand, is also endowed with most, if not all, major DC subtypes.40, 44, 45 However, some of these DCs display features not found in other mucosal sites, such as no MHC II expression in the basal state.46 As in other mucosal sites, the normal conjunctiva bears a sizable lymphocyte population. Intraepithelial T cells are mostly CD8+, but NK and γδ T cells are also abundant.47 By contrast, CD4+ and CD8+ T‐cell numbers are more balanced in the lamina propria.48 Remarkably, the DC and T‐cell subtypes that carry out immune tolerance at other mucosal sites are well characterized, but little is known about tolerogenic DCs and Treg cells in the OS. There is at least evidence for a homeostatic role of CD8+ Treg cells and CD4+ CD25+ Foxp3+ Treg cells in dry eye disease (DED),49, 50 but it remains to be established whether these are natural or inducible Treg cells.
Finally, it should be noted that the OS is subject to a highly contrasting circadian rhythm because sleep‐associated eyelid closure markedly reduces oxygen exchange and the secretion and clearance of tears.51 There is increased complement activation in the tear film during the first hours of sleep, which is later accompanied by a significant influx of neutrophils.52 Among other changes,51 hypoxia increases Toll‐like receptor expression in conjunctival epithelial cells,27 and blinking induces physiological corneal epithelial cell exfoliation, which drops to a minimum during sleep.53 In other words, a physiological but pro‐inflammatory shift in the OS takes place daily in the closed eye.51, 54, 55
Evidence for mucosal immune tolerance at the OS
Mucosal immune tolerance can be operationally defined and characterized by the immune system's ability to actively suppress systemic immunization against a specific antigen if such an antigen is administered beforehand through a mucosal surface.8 In other words, it is a form of mucosally induced peripheral tolerance that goes beyond the apparent local unresponsiveness to a given antigen, as it implicates an active, antigen‐specific regulatory immune response with systemic implications. The existence of mucosal tolerance at the OS was first reported in 1994,56 and the underlying immune mechanisms were addressed a few years later by Egan et al.,57 who developed a murine model that involved the ocular instillation of a harmless antigen [ovalbumin (OVA)] and allowed tracking of the specific immune response. They showed that repeated ocular instillation of a low dose of OVA or single administration of a higher dose was sufficient to prevent subsequent systemic immunization with the same antigen and a strong adjuvant. Consistently, a similar experimental set up had been employed more than a decade earlier to characterize oral tolerance.58
Moreover, Egan et al.57 observed that after ocular instillation, OVA peptide‐bearing antigen‐presenting cells could be detected in the submandibular lymph node, but not in the conjunctiva or in other lymph nodes, so determining the anatomical site for T‐cell priming during tolerance induction in the OS. The submandibular lymph node is also crucial for tolerance induction in the closely linked nasal mucosa.59 In fact, both mucosal surfaces are connected through the nasolacrimal ducts, through which tears secreted onto the OS drain into the nasal cavity. Therefore, it could be argued that systemic tolerance after ocular instillation is an artefact induced by passive antigen drainage to the nasal mucosa and subsequent nasal tolerance induction. However, Chentoufi et al. later showed that both mucosal surfaces can independently respond to antigen after surgical nasolacrimal duct closure in rabbits.60 More importantly, immune responses that originated in either site differed in the T‐cell migration pattern, highlighting that each mucosal site imprints its own phenotype to the immune response despite their common draining lymph node.60
Migration of antigen‐loaded DCs from the mucosal lining is a prerequisite for tolerance induction, and these cells rely on CCR7 expression to follow a chemokine gradient stemming from the lymph nodes.15, 61 CCR7 also plays a key role in immunogenic DC migration from the OS,62 and it is likely to be involved in tolerance induction as well. Although there is no conclusive experimental evidence, antigens in the tear film are most likely sampled in the conjunctiva. The conjunctival epithelium is permeable to large molecules,63 and its population of goblet cells might aid the delivery of antigen to lamina propria DCs, as shown in the gut.18 Moreover, healthy corneal epithelium seems to be quite impermeable to soluble proteins.64 At any rate, migration of antigen‐loaded DCs to the submandibular lymph node in mice peaks at 24 hr after single ocular instillation of antigen, and there it induces vigorous T‐cell proliferation that peaks on day 3.57 Remarkably, the OVA‐specific T‐cell adoptive transfer system that was used in those experiments suggested that some ocular antigen‐loaded DCs might be migrating and activating T cells in distant lymph nodes. Consistently, other reports around that time suggested that antigen‐loaded DCs could reach distal secondary lymphoid organs, such as the spleen, and activate T cells.65, 66 More recent work, however, has apparently settled this issue in favour of initial T‐cell priming at the local lymph node and rapid migration of activated T cells to other lymphoid organs, where they continue to proliferate.61, 67 Of note, if the corneal epithelial barrier is mechanically removed in mice, topically applied antigen rapidly reaches the lymphatic system and even the spleen, but this probably represents a pathological situation and not the physiological setting.64
Regarding the T cells responsible for conjunctival tolerance, Egan et al.57 suggested that ocular instillation of antigen in mice leads to the development of anergic T cells. However, they did not perform the required experiments to properly identify Treg cell function,68 and others have shown that oral tolerance induction after administration of a high antigen dose is indeed due to T‐cell anergy,69 but low antigen dose is conducive to specific T‐cell suppression. More recently, we corroborated that low doses of antigen instilled onto the OS lead to the generation of antigen‐specific Treg cells.70 These Treg cells can be found in the lymph nodes and spleen after tolerance induction and readily suppress antigen‐specific effector T‐cell proliferation after transfer to naive recipients.71, 72 Some of these cells are CD4+ ICOS+ Foxp3– and secrete IL‐10,70 so resembling the Tr1 Treg cells that are known to mediate mucosal tolerance in the nasal and bronchial surfaces.20, 73
In summary, there is evidence from mouse studies that a mucosal adaptive immune response continuously takes place at the OS (Fig. 2). This process begins with corneal and conjunctival DCs picking up local antigens, which could originate from the environment, from the microbiota or even ocular autoantigens. In the basal state, the OS microenvironment imprints a tolerogenic profile on the DCs that migrate to the lymph nodes. Once there, DCs encounter naive T cells to which they present antigens and induce a Treg cell phenotype. These Treg cells can then home to the OS, where they become specifically activated by their cognate antigen and exert their regulatory effect, so contributing to the non‐inflammatory milieu and local homeostasis.
Figure 2.
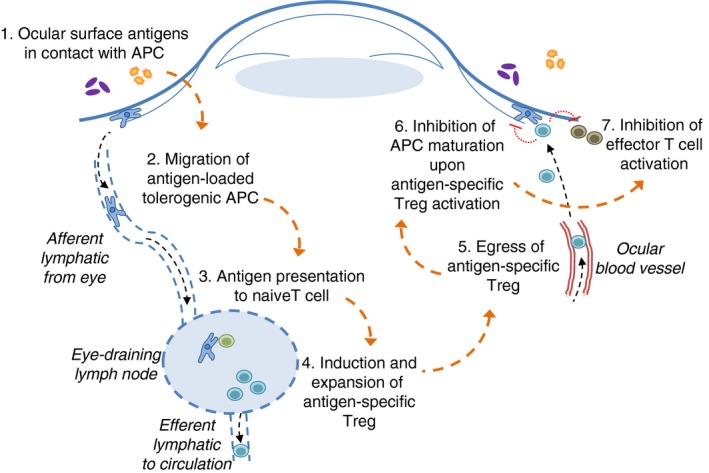
Ocular surface mucosal immune tolerance at work. (1) Airborne and microbiota‐derived antigens (Ag) reach the ocular surface, where they are eventually taken up by antigen‐presenting cells (APC). (2) In the basal state, still uncharacterized factors condition these APC to a tolerogenic profile, and also trigger their lymphatic migration to the draining lymph node. (3) Once there, APC present ocular surface‐derived antigens to circulating naive T cells, (4) which upon specific recognition expand and become regulatory T (Treg) cells because of the tolerogenic profile that was previously imprinted on the APC at the ocular surface. Some Treg cells leave the lymph node and eventually reach the bloodstream, where they circulate indefinitely until they detect specific signals during their transit through ocular surface blood vessels. (5) After traversing the vessel wall, Treg cells come in contact with ocular surface APC that present the same antigen that they first encountered in the lymph node. (6) After this activation step, Treg cells deliver inhibitory signals to APC and to effector T cells, (7) which effectively suppress the local inflammatory response, thus promoting further tolerance induction to new antigens.
Mucosal immune tolerance in OS disease
For immune‐mediated OS disease to develop, the mucosal homeostatic mechanisms must be overcome by the inflammatory stimuli that elicit the disease in the first place. Depending on the disorder, inflammation may be initiated by chemical or physical agents that damage the OS, by danger‐ and pathogen‐associated molecular patterns that drive the innate response, by self and/or non‐self antigens targeted by an adaptive immune response, or by a combination of them.
Dry eye disease
A few years ago, Stern et al. proposed that DED could be thought of as an autoimmune mucosal disease of the OS,74 or in other words, as a localized autoimmune process that arises after OS immune tolerance is disrupted, probably by tear hyperosmolarity and/or microbial stimuli. The concept was initially supported by mouse studies showing that the disease phenotype could be transferred to T‐cell‐deficient recipients by the CD4+ T cells from affected donors.75, 76 These pathogenic CD4+ T cells readily migrate to the cornea, conjunctiva and local lymph nodes and are of a Th1/Th17 profile.47, 75, 76, 77 As administration of exogenous antigen was not required to induce disease, it followed that the CD4+ T cells involved must be specific for some OS autoantigen. In fact, DED‐specific autoreactive antibodies have been shown to contribute to OS damage in mice.78 However, it should be noted that the OS is also exposed to potential antigens from the environment and the local microbiota, and so the specificity of the pathogenic CD4+ T cells could also include non‐self antigens from these sources.
From a pathophysiology perspective, DED and inflammatory bowel disease (IBD) have striking common points. IBD is characterized by a Th1/Th17 T‐cell response localized to the intestinal mucosa and can also be modelled in mice by adoptive transfer of naive CD4+ T cells to T‐cell‐deficient recipients.79 Moreover, autoantibodies are readily detected in patients with IBD,80 and therefore a strictly autoimmune aetiology also seemed likely at first. However, it was later found that germ‐free mice do not develop intestinal inflammation in the adoptive transfer and other colitis models, which revealed the crucial role that the gut microbiota plays in IBD pathogenesis.81 In addition, among the many IBD susceptibility genes isolated so far, those related to bacterial recognition and mucosal barrier function have the strongest association with disease.82 Because of all these findings, IBD is currently thought of as a disruption of mucosal tolerance towards the gut microbiota, which is elicited by still unidentified environmental factors in genetically susceptible individuals.83
We have recently shown that mucosal tolerance to an exogenous antigen is indeed disrupted in two different murine models of DED, but this phenomenon is detectable only after continuous desiccating stress is exerted on the OS for 3 days.71, 72 Remarkably, prevention of this change in the mucosal immune response by topical NF‐κB inhibitors was associated with reduced corneal damage under the same desiccating stress conditions, a finding that highlights its pathogenic role.71, 72 The role of challenging environmental conditions initiating mucosal inflammation at the OS is well established. Tear hyperosmolarity resulting from increased evaporation is frequently observed in patients with DED,84 and desiccating stress readily induces OS inflammation after 90 min in healthy subjects.85 The aforementioned timing of mucosal tolerance breakdown in murine DED models is in line with the early events that take place at the OS epithelium under desiccating stress: expression of activating NK cell receptor ligands and secretion of Th1 chemokines36 in the first 6 hr and the consequent burst of IFN‐γ released by conjunctival NK cells that peaks in the first 3 days.86 Within OS epithelial cells, hyperosmolar stress readily activates the mitogen‐activated protein kinase (MAPK) pathway, which in turn up‐regulates many pro‐inflammatory genes and leads to increased expression of several pro‐inflammatory cytokines87 and matrix metalloproteinases.88 Matrix metalloproteinase‐9 plays a crucial role in the disruption of the OS epithelial barrier, a hallmark finding of DED, and its secretion is further promoted by the IL‐17 stemming from the adaptive immune response that later ensues.4, 89 Moreover, interferon‐γ secreted by NK and T cells favours goblet cell loss,90 another key feature of DED, and these cells contribute significantly to the protective mucin layer of the OS and exert an immunomodulatory influence on DCs.32 Loss of the epithelial barrier probably accounts for the exacerbated inflammatory response to bacterial lipopolysaccharide that has been reported in DED.28 The ocular microbiota that could serve as a source of these microbial by‐products, however, still remains uncharacterized and subject to much debate.91, 92
All of these reports integrate into an alternative, non‐autoimmune hypothesis for DED pathogenesis (Fig. 3): that harsh environmental conditions on the cornea and conjunctiva of susceptible individuals lead to a series of pro‐inflammatory changes in the epithelial lining, which eventually disrupt the epithelial barrier and mucosal tolerance to the abundant exogenous antigens available at the OS, so setting the stage for the pathogenic Th1/Th17 adaptive immune response that potentiates corneal damage.
Figure 3.
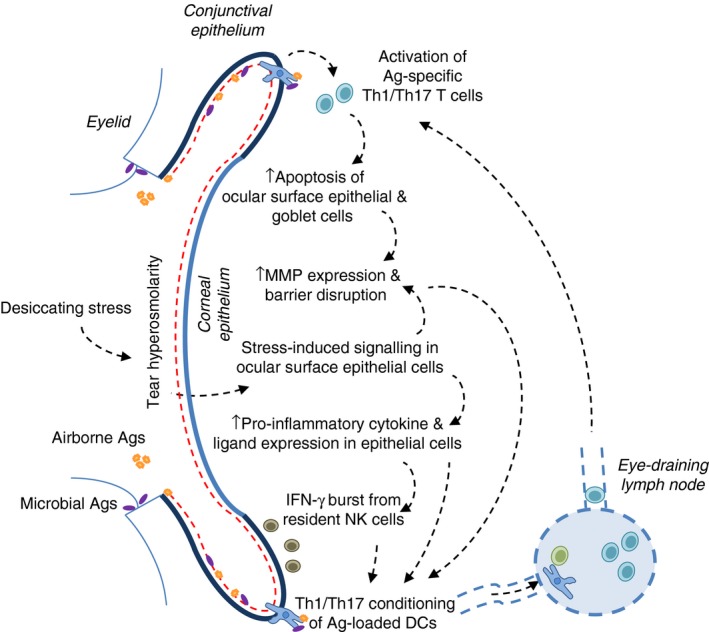
Alternative non‐autoimmune hypothesis to dry eye disease pathogenesis. Desiccating stress (a common denomination for harsh environmental conditions) and tear film abnormalities lead to tear hyperosmolarity acting on ocular surface epithelial cells, which in response activate specific signalling pathways. As a result, there is an increase in pro‐inflammatory mediators and subsequent epithelial barrier disruption, which in turn promotes the T heper type 1 (Th1)/Th17 conditioning of dendritic cells that capture microbial and airborne antigens that reach the ocular surface. Upon migration to the lymph node, these dendritic cells initiate an effector Th1/Th17 T‐cell response that further fuels this vicious cycle at the ocular surface by contributing to epithelial cell damage.
Ocular allergy
The four clinical forms of ocular allergy (allergic conjunctivitis, vernal keratoconjunctivitis, atopic keratoconjunctivitis and giant papillary conjunctivitis) differ in the contribution of IgE‐mediated and non‐IgE‐mediated immune reactions in their pathogenesis,93 but they share the hallmark Th2 adaptive immune response to an otherwise harmless antigen that typifies allergic disease. In other words, an allergic reaction at a mucosal surface is in fact a clinical example of the breakdown of mucosal tolerance to one or more specific antigens. The breach of tolerance that a mucosal allergic response actually represents has been studied most extensively in the context of oral tolerance and food allergy,8 but nonetheless still applies to other mucosal sites. In the context of ocular allergy, the most relevant aspects of mucosal tolerance are the pathogenic mechanisms that cause its disruption and the therapeutic opportunities offered by its manipulation.
The incidence of ocular allergy has been on the rise for the past two decades in both developed and developing nations, with some reports suggesting a prevalence of up to 20% of the population.94 Increased exposure to air pollution is partially responsible for this phenomenon,95 and several pathogenic mechanisms have been outlined.96 Diesel exhaust particles increase oxidative stress and induce a pro‐inflammatory response in corneal and conjunctival epithelial cells, with release of IL‐6 and changes in mucin expression.97, 98, 99 Cigarette smoke has similar effects on the OS epithelium,100 and at least for the airways, it prevents the induction of mucosal tolerance to aerosolized protein.101 DCs migrating from the lungs of smoke‐exposed mice exhibit an immunogenic phenotype,101 which facilitates Th2 sensitization.102 The OS mucosal response has not been analysed under comparable conditions, but given the evidence that a pro‐inflammatory response does take place at the OS epithelium,97, 98, 99, 100 it is plausible to consider a similar disruptive effect on the local mucosal response. In this regard, it is interesting to consider the evidence of increased DED incidence and severity in smokers,103, 104 an OS disease with an entirely different immunopathology but that shares the kick‐start of mucosal tolerance disruption in its pathogenesis.71, 72
The therapeutic aspect of OS mucosal tolerance has been explored for vaccination purposes105 and for treating uveitis,56 but not specifically for ocular allergy. Another form of mucosal tolerance, oral tolerance (generation of inducible Treg cells after antigen ingestion), was shown to be effective in mice with allergic conjunctivitis. Such an approach involved transgenic rice seeds engineered to express pollen allergens,106 an ingenious way of obtaining the large amounts of antigen required for oral tolerance induction. As Treg cells induced at a particular mucosa are expected to preferentially home to that mucosal site, it would be expected that Treg cells induced after ocular instillation readily suppressed antigen‐specific ocular allergy. This is indeed the case for OVA‐induced allergic conjunctivitis in mice,107 and similar strategies might be successful for treating ocular and extraocular allergy in patients.108, 109
Eyedrop preservative toxicity
Toxicity from eye drop preservative toxicity is frequently observed in glaucoma patients under long‐term medical treatment.110 Benzalkonium chloride, a commonly used preservative, induces epithelial cell death, pro‐inflammatory cytokine secretion and inflammatory infiltration of the OS.111, 112 In addition, this preservative readily disrupts conjunctival immune tolerance in mice, in part by conditioning DCs migrating from the OS to the lymph nodes, which in turn induce effector T cells instead of tolerogenic Treg cells.70 This observation could explain the increased incidence of allergic reactions and DED in preservative‐exposed patients, and the concept was demonstrated in a murine model of allergic conjunctivitis.107 In brief, physiological OS immune tolerance to harmless antigen protects from subsequent allergic conjunctivitis even if mice are actively immunized with a potent adjuvant such as alum. However, if tolerance is affected by simultaneous instillation of the antigen and the preservative, mice develop full‐blown allergic reactions. Remarkably, benzalkonium chloride‐induced NF‐κB activation in the OS epithelium appears to be a key event in the pathophysiology of this model, as topical co‐delivery of NF‐κB inhibitors completely prevents the subsequent allergic reaction. These findings are consistent with the widely described role of the epithelial NF‐κB pathway in mucosal tolerance.9, 25 It is still unclear how the preservative leads to increased NF‐κB activation in ocular epithelial cells, although its reported effect on the Wnt/β‐catenin intracellular pathway in corneal epithelial cells could be a link.113 In any case, the iatrogenic nature of eyedrop preservative toxicity provides a unique therapeutic opportunity, for the event that incites the breakdown in mucosal homeostasis is known.
Therapeutic manipulation of ocular mucosal tolerance
In the same way that current models of extraocular mucosal immunology can improve our understanding of OS pathophysiology, therapeutic strategies for extraocular mucosal disorders might apply to the eye. As was mentioned earlier for ocular allergy, antigen‐specific mucosal immunotherapy looks promising.108, 109 In the context of ocular mucosal tolerance, there is also the prospect of modulating epithelial activation and specifically targeting DC conditioning, effector T cells and Treg cells.
Within corneal and conjunctival cells, there are two major signalling pathways that have been studied extensively: MAPK87, 114, 115, 116, 117 and NF‐κB.70, 71, 72, 107, 118 Both have significant impact on the mucosal immune outcome, as they control the extent of epithelial cell activation and the subsequent programming of local DCs. Activation of MAPK in OS epithelial cells leads to secretion of pro‐inflammatory factors IL‐1β, IL‐8, tumour necrosis factor‐α and metalloproteinase‐9,117 and the activation of the NF‐κB pathway induces a comparable array of pro‐inflammatory mediators in corneal epithelial cells.119 Remarkably, two antibiotics that are in clinical use for DED and meibomian gland dysfunction, doxycycline and azithromycin, are known to inhibit the activation of MAPK116 and NF‐κB,119 respectively, in these cells, and these intracellular effects might account for their clinical efficacy at improving DED signs.120 In line with this, NF‐κB inhibitors delivered topically to the OS can improve disease outcome measures in animal models of DED, allergy, preservative toxicity and corneal burn.70, 71, 72, 107, 121
Imprinting of OS DCs with a tolerogenic profile is probably an additional effect of topical NF‐κB inhibitors,70, 71, 72, 107, 121 as this pathway plays a pivotal role in DC maturation as well.16 There are additional molecular targets specific for DCs, such as topical blockade of CCR7, which prevents their migration to the lymph node in DED.62 However, CCR7 is also responsible for DC migration under homeostatic conditions in other mucosal surfaces,15 so there might be disadvantages to such an approach. Given the importance that retinoic acid and TGF‐β have for the induction of tolerogenic DCs and Treg cells in the gut, it is readily apparent how the local delivery of either of these mediators improved disease score in mouse models of IBD.122 However, these findings might not translate to OS disease, where TGF‐β seems to exert both anti‐ and pro‐inflammatory effects.32, 116, 123 On the other hand, Treg‐based immunotherapy is being explored for several mucosal disorders,124, 125 and in vitro‐expanded CD25+ Foxp3+ Treg cells can suppress OS inflammation in murine DED.50 Regarding effector T cells, they are known to rely on their CCR6 receptor, which binds the CCL20 chemokine produced by corneal and conjunctival epithelial cells, to home to the OS.35, 126 Hence, the strategy of blocking CCL20 by topical instillation of anti‐CCL20 antibody to ameliorate murine DED is intriguing,35 and is akin to successful approaches explored in other mucosal surfaces.127
Finally, the ocular microbiome has become a hot research topic. Whether there is a stable microbiota in every OS is still subject to debate, but evidence is accumulating on the changes in microbial diversity in the context of eye disease.91, 92, 128, 129 As the microbiota in other mucosal surfaces is known to have potent immunoregulatory functions, its therapeutic manipulation could be of benefit for OS disease. Tear levels of secretory IgA are reduced in germ‐free mice, and this protein is known to promote mucosal tolerance by promoting IL‐10 production and modulating DCs.128 However, it is unclear whether this effect on IgA levels is exerted by the ocular microbiome or by commensals elsewhere. Intriguingly, intestinal microbial imbalance worsens DED in mice, and consistently, intestinal bacterial diversity is reduced in patients with DED.129 Moreover, increased prevalence of a single bacterial species on the ocular surface and increased specific systemic IgG titres are associated with the development of chronic OS inflammation in another mouse model of DED,128 a finding that supports the non‐autoimmune hypothesis for DED detailed above.
Conclusions
The ocular mucosal immune system shares many features with other mucosal surfaces, among others, the ability to mount an immunomodulatory adaptive response to the diverse harmless antigens that reach its confines. Hence, ocular mucosal tolerance is a crucial homeostatic mechanism, and at the same time, its disruption is perhaps the tipping point that skews the balance towards disease. Here we summarized the published evidence on such mechanism (or lack thereof) in the basal state and in several clinical entities. By doing so from a general mucosal immunology viewpoint, we established similarities and differences with the gut and the airways. As there is still much to learn about ocular mucosal immune pathophysiology, it should be of advantage to apply to its study the immunological models that have already been established for other mucosal sites.
Disclosures
The authors have no competing interests.
Acknowledgements
Agencia Nacional de Promoción Científica y Tecnológica (PICT 2013‐1436 and PICT 2015‐0971), Pan‐American Association of Ophthalmology, Fundación Alberto J. Roemmers, Fundación Allende.
The authors have no commercial or proprietary interest in any concept or product described in this article.
References
- 1. Sheng X‐L, Li H‐P, Liu Q‐X, Rong W‐N, Du W‐Z, Ma L et al Prevalence and associated factors of corneal blindness in Ningxia in northwest China. Int J Ophthalmol 2014; 7:557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Niederkorn JY, Larkin DFP. Immune privilege of corneal allografts. Ocul Immunol Inflamm 2010; 18:162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDermott AM. Antimicrobial compounds in tears. Exp Eye Res 2013; 117:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mantelli F, Mauris J, Argüeso P. The ocular surface epithelial barrier and other mechanisms of mucosal protection: from allergy to infectious diseases. Curr Opin Allergy Clin Immunol 2013; 13:563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ueta M, Kinoshita S. Ocular surface inflammation is regulated by innate immunity. Prog Retin Eye Res 2012; 31:551–75. [DOI] [PubMed] [Google Scholar]
- 6. McCauley HA, Guasch G. Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol Med 2015; 21:492–503. [DOI] [PubMed] [Google Scholar]
- 7. Agnifili L, Mastropasqua R, Fasanella V, Di Staso S, Mastropasqua A, Brescia L et al In vivo confocal microscopy of conjunctiva‐associated lymphoid tissue in healthy humans. Invest Ophthalmol Vis Sci 2014; 55:5254–62. [DOI] [PubMed] [Google Scholar]
- 8. Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol 2012; 5:232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the “epimmunome”. Nat Immunol 2010; 11:656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goto Y, Ivanov II. Intestinal epithelial cells as mediators of the commensal–host immune crosstalk. Immunol Cell Biol 2013; 91:204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Papazian D, Hansen S, Würtzen PA. Airway responses towards allergens – from the airway epithelium to T cells. Clin Exp Allergy 2015; 45:1268–87. [DOI] [PubMed] [Google Scholar]
- 12. Rescigno M. Dendritic cell–epithelial cell crosstalk in the gut. Immunol Rev 2014; 260:118–28. [DOI] [PubMed] [Google Scholar]
- 13. Lambrecht BN, Hammad H. Dendritic cell and epithelial cell interactions at the origin of murine asthma. Ann Am Thorac Soc 2014; 11(Suppl. 5):S236–43. [DOI] [PubMed] [Google Scholar]
- 14. Aliberti J. Immunity and tolerance induced by intestinal mucosal dendritic cells. Mediators Inflamm 2016; 2016:3104727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hintzen G, Ohl L, del Rio M‐L, Rodriguez‐Barbosa J‐I, Pabst O, Kocks JR et al Induction of tolerance to innocuous inhaled antigen relies on a CCR7‐dependent dendritic cell‐mediated antigen transport to the bronchial lymph node. J Immunol 2006; 177:7346–54. [DOI] [PubMed] [Google Scholar]
- 16. Baratin M, Foray C, Demaria O, Habbeddine M, Pollet E, Maurizio J et al Homeostatic NF‐κB signaling in steady‐state migratory dendritic cells regulates immune homeostasis and tolerance. Immunity 2015; 42:627–39. [DOI] [PubMed] [Google Scholar]
- 17. Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K et al Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013; 342:447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA et al Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012; 483:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arce‐Sillas A, Álvarez‐Luquín DD, Tamaya‐Domínguez B, Gomez‐Fuentes S, Trejo‐García A, Melo‐Salas M et al Regulatory T cells: molecular actions on effector cells in immune regulation. J Immunol Res 2016; 2016:1720827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang H, Kong H, Zeng X, Guo L, Sun X, He S. Subsets of regulatory T cells and their roles in allergy. J Transl Med 2014; 12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Menon BB, Kaiser‐Marko C, Spurr‐Michaud S, Tisdale AS, Gipson IK. Suppression of Toll‐like receptor‐mediated innate immune responses at the ocular surface by the membrane‐associated mucins MUC1 and MUC16. Mucosal Immunol 2015; 8:1000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bauskar A, Mack WJ, Mauris J, Argüeso P, Heur M, Nagel BA et al Clusterin seals the ocular surface barrier in mouse dry eye. PLoS ONE 2015; 10:e0138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mun JJ, Tam C, Evans DJ, Fleiszig SMJ. Modulation of epithelial immunity by mucosal fluid. Sci Rep 2011; 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wullaert A, Bonnet MC, Pasparakis M. NF‐κB in the regulation of epithelial homeostasis and inflammation. Cell Res 2011; 21:146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pasparakis M. Role of NF‐κB in epithelial biology. Immunol Rev 2012; 246:346–58. [DOI] [PubMed] [Google Scholar]
- 26. Redfern RL, Reins RY, McDermott AM. Toll‐like receptor activation modulates antimicrobial peptide expression by ocular surface cells. Exp Eye Res 2011; 92:209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li J, Setiawan M, Wu H, Beuerman RW, Zhao P. Regulation of Toll‐like receptor expression in human conjunctival epithelial cells. Mediators Inflamm 2014; 2014:493596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simmons KT, Xiao Y, Pflugfelder SC, de Paiva CS. Inflammatory response to lipopolysaccharide on the ocular surface in a murine dry eye model. Invest Ophthalmol Vis Sci 2016; 57:2443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGilligan VE, Gregory‐Ksander MS, Li D, Moore JE, Hodges RR, Gilmore MS et al Staphylococcus aureus activates the NLRP3 inflammasome in human and rat conjunctival goblet cells. PLoS ONE 2013; 8:e74010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zheng X, Ma P, de Paiva CS, Cunningham MA, Hwang CS, Pflugfelder SC et al TSLP and downstream molecules in experimental mouse allergic conjunctivitis. Invest Ophthalmol Vis Sci 2010; 51:3076–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deng R, Su Z, Lu F, Zhang L, Lin J, Zhang X et al A potential link between bacterial pathogens and allergic conjunctivitis by dendritic cells. Exp Eye Res 2014; 120:118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Contreras‐Ruiz L, Masli S. Immunomodulatory cross‐talk between conjunctival goblet cells and dendritic cells. PLoS ONE 2015; 10:e0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El Annan J, Goyal S, Zhang Q, Freeman GJ, Sharpe AH, Dana R. Regulation of T‐cell chemotaxis by programmed death‐ligand 1 (PD‐L1) in dry eye‐associated corneal inflammation. Investig Opthalmology Vis Sci 2010; 51:3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferrari G, Hajrasouliha AR, Sadrai Z, Ueno H, Chauhan SK, Dana R. Nerves and neovessels inhibit each other in the cornea. Invest Ophthalmol Vis Sci 2013; 54:813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dohlman TH, Chauhan SK, Kodati S, Hua J, Chen Y, Omoto M et al The CCR6/CCL20 axis mediates Th17 cell migration to the ocular surface in dry eye disease. Invest Ophthalmol Vis Sci 2013; 54:4081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coursey TG, Bohat R, Barbosa FL, Pflugfelder SC, de Paiva CS. Desiccating stress‐induced chemokine expression in the epithelium is dependent on upregulation of NKG2D/RAE‐1 and release of IFN‐γ in experimental dry eye. J Immunol 2014; 193:5264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Forrester JV, Xu H, Kuffová L, Dick AD, McMenamin PG. Dendritic cell physiology and function in the eye. Immunol Rev 2010; 234:282–304. [DOI] [PubMed] [Google Scholar]
- 38. Rodrigues MM, Rowden G, Hackett J, Bakos I. Langerhans cells in the normal conjunctiva and peripheral cornea of selected species. Invest Ophthalmol Vis Sci 1981; 21:759–65. [PubMed] [Google Scholar]
- 39. Efron N, Al‐Dossari M, Pritchard N. In vivo confocal microscopy of the bulbar conjunctiva. Clin Experiment Ophthalmol 2009; 37:335–44. [DOI] [PubMed] [Google Scholar]
- 40. Hattori T, Chauhan SK, Lee H, Ueno H, Dana R, Kaplan DH et al Characterization of Langerin‐expressing dendritic cell subsets in the normal cornea. Invest Ophthalmol Vis Sci 2011; 52:4598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khandelwal P, Blanco‐Mezquita T, Emami P, Lee HS, Reyes NJ, Mathew R et al Ocular mucosal CD11b+ and CD103+ mouse dendritic cells under normal conditions and in allergic immune responses. . Kovats S, editor. PLoS ONE 2013; 8:e64193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohbayashi M, Manzouri B, Flynn T, Toda M, Ikeda Y, Nakamura T et al Dynamic changes in conjunctival dendritic cell numbers, anatomical position and phenotype during experimental allergic conjunctivitis. Exp Mol Pathol 2007; 83:216–23. [DOI] [PubMed] [Google Scholar]
- 43. Nakano H, Free ME, Whitehead GS, Maruoka S, Wilson RH, Nakano K et al Pulmonary CD103+ dendritic cells prime Th2 responses to inhaled allergens. Mucosal Immunol 2012; 5:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yamagami S, Yokoo S, Usui T, Yamagami H, Amano S, Ebihara N. Distinct populations of dendritic cells in the normal human donor corneal epithelium. Invest Ophthalmol Vis Sci 2005; 46:4489–94. [DOI] [PubMed] [Google Scholar]
- 45. Lee EJ, Rosenbaum JT, Planck SR. Epifluorescence intravital microscopy of murine corneal dendritic cells. Invest Ophthalmol Vis Sci 2010; 51:2101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hamrah P, Zhang Q, Liu Y, Dana MR. Novel characterization of MHC class II‐negative population of resident corneal Langerhans cell‐type dendritic cells. Invest Ophthalmol Vis Sci 2002; 43:639–46. [PubMed] [Google Scholar]
- 47. Zhang X, Volpe EA, Gandhi NB, Schaumburg CS, Siemasko KF, Pangelinan SB et al NK cells promote Th‐17 mediated corneal barrier disruption in dry eye. PLoS ONE 2012; 7:e36822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hingorani M, Metz D, Lightman SL. Characterisation of the normal conjunctival leukocyte population. Exp Eye Res 1997; 64:905–12. [DOI] [PubMed] [Google Scholar]
- 49. Zhang X, Schaumburg CS, Coursey TG, Siemasko KF, Volpe EA, Gandhi NB et al CD8+ cells regulate the T helper‐17 response in an experimental murine model of Sjögren syndrome. Mucosal Immunol 2014; 7:417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Siemasko KF, Gao J, Calder VL, Hanna R, Calonge M, Pflugfelder SC et al In vitro expanded CD4+CD25+Foxp3+ regulatory T cells maintain a normal phenotype and suppress immune‐mediated ocular surface inflammation. Invest Ophthalmol Vis Sci 2008; 49:5434–40. [DOI] [PubMed] [Google Scholar]
- 51. Sack RA, Beaton A, Sathe S, Morris C, Willcox M, Bogart B. Towards a closed eye model of the pre‐ocular tear layer. Prog Retin Eye Res 2000; 19:649–68. [DOI] [PubMed] [Google Scholar]
- 52. Tan KO, Sack RA, Holden BA, Swarbrick HA. Temporal sequence of changes in tear film composition during sleep. Curr Eye Res 1993; 12:1001–7. [DOI] [PubMed] [Google Scholar]
- 53. Yamamoto K, Ladage PM, Ren DH, Li L, Petroll WM, Jester JV et al Effect of eyelid closure and overnight contact lens wear on viability of surface epithelial cells in rabbit cornea. Cornea 2002; 21:85–90. [DOI] [PubMed] [Google Scholar]
- 54. Prabhasawat P, Tseng SC. Frequent association of delayed tear clearance in ocular irritation. Br J Ophthalmol 1998; 82:666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de Paiva CS, Pflugfelder SC. Tear clearance implications for ocular surface health. Exp Eye Res 2004; 78:395–7. [DOI] [PubMed] [Google Scholar]
- 56. Dua HS, Donoso LA, Laibson PR. Conjunctival instillation of retinal antigens induces tolerance. Does it invoke mucosal tolerance mediated via conjunctiva associated lymphoid tissues (CALT)? Ocul Immunol Inflamm 1994; 2:29–36. [DOI] [PubMed] [Google Scholar]
- 57. Egan RM, Yorkey C, Black R, Loh WK, Stevens JL, Storozynsky E et al In vivo behavior of peptide‐specific T cells during mucosal tolerance induction: antigen introduced through the mucosa of the conjunctiva elicits prolonged antigen‐specific T cell priming followed by anergy. J Immunol 2000; 164:4543–50. [DOI] [PubMed] [Google Scholar]
- 58. Mowat AM, Strobel S, Drummond HE, Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology 1982; 45:105–13. [PMC free article] [PubMed] [Google Scholar]
- 59. Wolvers DA, Coenen‐de Roo CJ, Mebius RE, van der Cammen MJ, Tirion F, Miltenburg AM et al Intranasally induced immunological tolerance is determined by characteristics of the draining lymph nodes: studies with OVA and human cartilage gp‐39. J Immunol 1999; 162:1994–8. [PubMed] [Google Scholar]
- 60. Chentoufi AA, Dasgupta G, Nesburn AB, Bettahi I, Binder NR, Choudhury ZS et al Nasolacrimal duct closure modulates ocular mucosal and systemic CD4+ T‐cell responses induced following topical ocular or intranasal immunization. Clin Vaccine Immunol 2010; 17:342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G et al Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med 2006; 203:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kodati S, Chauhan SK, Chen Y, Dohlman TH, Karimian P, Saban D et al CCR7 is critical for the induction and maintenance of Th17 immunity in dry eye disease. Invest Ophthalmol Vis Sci 2014; 55:5871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hämäläinen KM, Kananen K, Auriola S, Kontturi K, Urtti A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci 1997; 38:627–34. [PubMed] [Google Scholar]
- 64. Dang Z, Kuffová L, Liu L, Forrester JV. Soluble antigen traffics rapidly and selectively from the corneal surface to the eye draining lymph node and activates T cells when codelivered with CpG oligonucleotides. J Leukoc Biol 2014; 95:431–40. [DOI] [PubMed] [Google Scholar]
- 65. Smith KM, Davidson JM, Garside P. T‐cell activation occurs simultaneously in local and peripheral lymphoid tissue following oral administration of a range of doses of immunogenic or tolerogenic antigen although tolerized T cells display a defect in cell division. Immunology 2002; 106:144–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gütgemann I, Fahrer AM, Altman JD, Davis MM, Chien YH. Induction of rapid T cell activation and tolerance by systemic presentation of an orally administered antigen. Immunity 1998; 8:667–73. [DOI] [PubMed] [Google Scholar]
- 67. Ciabattini A, Pettini E, Fiorino F, Prota G, Pozzi G, Medaglini D. Distribution of primed T cells and antigen‐loaded antigen presenting cells following intranasal immunization in mice. Zimmer J, editor. PLoS ONE 2011; 6:e19346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Azimi M, Aslani S, Mortezagholi S, Salek A, Javan MR, Rezaiemanesh A et al Identification, isolation, and functional assay of regulatory T cells. Immunol Invest 2016; 45:584–602. [DOI] [PubMed] [Google Scholar]
- 69. Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci USA 1994; 91:6688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Galletti JG, Gabelloni ML, Morande PE, Sabbione F, Vermeulen ME, Trevani AS et al Benzalkonium chloride breaks down conjunctival immunological tolerance in a murine model. Mucosal Immunol 2013; 6:24–34. [DOI] [PubMed] [Google Scholar]
- 71. Guzmán M, Keitelman I, Sabbione F, Trevani AS, Giordano MN, Galletti JG. Desiccating stress‐induced disruption of ocular surface immune tolerance drives dry eye disease. Clin Exp Immunol 2016; 184:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guzmán M, Keitelman I, Sabbione F, Trevani AS, Giordano MN, Galletti JG. Mucosal tolerance disruption favors disease progression in an extraorbital lacrimal gland excision model of murine dry eye. Exp Eye Res 2016; 151:19–22. [DOI] [PubMed] [Google Scholar]
- 73. Pellerin L, Jenks JA, Bégin P, Bacchetta R, Nadeau KC. Regulatory T cells and their roles in immune dysregulation and allergy. Immunol Res 2014; 58:358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol 2013; 32:19–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Niederkorn JY, Stern ME, Pflugfelder SC, De Paiva CS, Corrales RM, Gao J et al Desiccating stress induces T cell‐mediated Sjögren's syndrome‐like lacrimal keratoconjunctivitis. J Immunol 2006; 176:3950–7. [DOI] [PubMed] [Google Scholar]
- 76. Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR et al Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol 2009; 182:1247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen Y, Chauhan SK, Lee HS, Saban DR, Dana R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol 2014; 7:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stern ME, Schaumburg CS, Siemasko KF, Gao J, Wheeler LA, Grupe DA et al Autoantibodies contribute to the immunopathogenesis of experimental dry eye disease. Invest Ophthalmol Vis Sci 2012; 53:2062–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ostanin DV, Bao J, Koboziev I, Gray L, Robinson‐Jackson SA, Kosloski‐Davidson M et al T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol 2009; 296:G135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Landers CJ, Cohavy O, Misra R, Yang H, Lin Y, Braun J et al Selected loss of tolerance evidenced by Crohn's disease‐associated immune responses to auto‐ and microbial antigens. Gastroenterology 2002; 123:689–99. [DOI] [PubMed] [Google Scholar]
- 81. Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E et al Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin‐10‐deficient mice. Infect Immun 1998; 66:5224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY et al Host‐microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012; 491:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol 2015; 37:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Baudouin C, Aragona P, Messmer EM, Tomlinson A, Calonge M, Boboridis KG et al Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting. Ocul Surf 2013; 11:246–58. [DOI] [PubMed] [Google Scholar]
- 85. Alex A, Edwards A, Hays JD, Kerkstra M, Shih A, de Paiva CS et al Factors predicting the ocular surface response to desiccating environmental stress. Invest Ophthalmol Vis Sci 2013; 54:3325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen Y, Chauhan SK, Saban DR, Sadrai Z, Okanobo A, Dana R. Interferon‐γ‐secreting NK cells promote induction of dry eye disease. J Leukoc Biol 2011; 89:965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li D‐Q, Luo L, Chen Z, Kim H‐S, Song XJ, Pflugfelder SC. JNK and ERK MAP kinases mediate induction of IL‐1β, TNF‐α and IL‐8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res 2006; 82:588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li D‐Q, Chen Z, Song XJ, Luo L, Pflugfelder SC. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci 2004; 45:4302–11. [DOI] [PubMed] [Google Scholar]
- 89. Pflugfelder SC, Farley W, Luo L, Chen LZ, de Paiva CS, Olmos LC et al Matrix metalloproteinase‐9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol 2005; 166:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhang X, Chen W, De Paiva CS, Corrales RM, Volpe EA, McClellan AJ et al Interferon‐γ exacerbates dry eye‐induced apoptosis in conjunctiva through dual apoptotic pathways. Investig Opthalmology Vis Sci 2011; 52:6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Willcox MDP. Characterization of the normal microbiota of the ocular surface. Exp Eye Res 2013; 117:99–105. [DOI] [PubMed] [Google Scholar]
- 92. Zegans ME, Van Gelder RN. Considerations in understanding the ocular surface microbiome. Am J Ophthalmol 2014; 158:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Reyes NJ, Saban DR. T helper subsets in allergic eye disease. Curr Opin Allergy Clin Immunol 2014; 14:477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gomes PJ. Trends in prevalence and treatment of ocular allergy. Curr Opin Allergy Clin Immunol 2014; 14:451–6. [DOI] [PubMed] [Google Scholar]
- 95. Berra M, Galperín G, Dawidowski L, Tau J, Márquez I, Berra A. Impact of wildfire smoke in Buenos Aires, Argentina, on ocular surface. Arq Bras Oftalmol 2015; 78:110–4. [DOI] [PubMed] [Google Scholar]
- 96. Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet 2014; 383:1581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tau J, Novaes P, Matsuda M, Tasat DR, Saldiva PH, Berra A. Diesel exhaust particles selectively induce both proinflammatory cytokines and mucin production in cornea and conjunctiva human cell lines. Invest Ophthalmol Vis Sci 2013; 54:4759–65. [DOI] [PubMed] [Google Scholar]
- 98. Fujishima H, Satake Y, Okada N, Kawashima S, Matsumoto K, Saito H et al Effects of diesel exhaust particles on primary cultured healthy human conjunctival epithelium. Ann Allergy Asthma Immunol 2013; 110:39–43. [DOI] [PubMed] [Google Scholar]
- 99. Lasagni Vitar RM, Tau J, Reides CG, Berra A, Ferreira SM, Llesuy SF. Evaluation of oxidative stress markers in human conjunctival epithelial cells exposed to diesel exhaust particles (DEP). Invest Ophthalmol Vis Sci 2015; 56:7058–66. [DOI] [PubMed] [Google Scholar]
- 100. Rummenie VT, Matsumoto Y, Dogru M, Wang Y, Hu Y, Ward SK et al Tear cytokine and ocular surface alterations following brief passive cigarette smoke exposure. Cytokine 2008; 43:200–8. [DOI] [PubMed] [Google Scholar]
- 101. Robays LJ, Lanckacker EA, Moerloose KB, Maes T, Bracke KR, Brusselle GG et al Concomitant inhalation of cigarette smoke and aerosolized protein activates airway dendritic cells and induces allergic airway inflammation in a TLR‐independent way. J Immunol 2009; 183:2758–66. [DOI] [PubMed] [Google Scholar]
- 102. Moerloose KB, Robays LJ, Maes T, Brusselle GG, Tournoy KG, Joos GF. Cigarette smoke exposure facilitates allergic sensitization in mice. Respir Res 2006; 7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Matsumoto Y, Dogru M, Goto E, Sasaki Y, Inoue H, Saito I et al Alterations of the tear film and ocular surface health in chronic smokers. Eye (Lond) 2008; 22:961–8. [DOI] [PubMed] [Google Scholar]
- 104. Thomas J, Jacob GP, Abraham L, Noushad B. The effect of smoking on the ocular surface and the precorneal tear film. Australas Med J 2012; 5:221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Seo KY, Han SJ, Cha H‐R, Seo S‐U, Song J‐H, Chung S‐H et al Eye mucosa: an efficient vaccine delivery route for inducing protective immunity. J Immunol 2010; 185:3610–9. [DOI] [PubMed] [Google Scholar]
- 106. Fukuda K, Ishida W, Harada Y, Wakasa Y, Takagi H, Takaiwa F et al Prevention of allergic conjunctivitis in mice by a rice‐based edible vaccine containing modified Japanese cedar pollen allergens. Br J Ophthalmol 2015; 99:705–9. [DOI] [PubMed] [Google Scholar]
- 107. Guzmán M, Sabbione F, Gabelloni ML, Vanzulli S, Trevani AS, Giordano MN et al Restoring conjunctival tolerance by topical nuclear factor‐κB inhibitors reduces preservative‐facilitated allergic conjunctivitis in mice. Invest Ophthalmol Vis Sci 2014; 55:6116–26. [DOI] [PubMed] [Google Scholar]
- 108. Ye Y‐L, Chuang Y‐H, Chiang B‐L. Strategies of mucosal immunotherapy for allergic diseases. Cell Mol Immunol 2011; 8:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nye M, Rudner S, Bielory L. Emerging therapies in allergic conjunctivitis and dry eye syndrome. Expert Opin Pharmacother 2013; 14:1449–65. [DOI] [PubMed] [Google Scholar]
- 110. Baudouin C, Labbe A, Liang H, Pauly A, Brignole‐Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res 2010; 29:312–34. [DOI] [PubMed] [Google Scholar]
- 111. De Saint Jean M. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci 1999; 40:619–30. [PubMed] [Google Scholar]
- 112. Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea 2009; 28:1023–7. [DOI] [PubMed] [Google Scholar]
- 113. Zhou Y. Modulation of the canonical Wnt pathway by benzalkonium chloride in corneal epithelium. Exp Eye Res 2011; 93:355–62. [DOI] [PubMed] [Google Scholar]
- 114. Di Girolamo N, Coroneo MT, Wakefield D. UVB‐elicited induction of MMP‐1 expression in human ocular surface epithelial cells is mediated through the ERK1/2 MAPK‐dependent pathway. Invest Ophthalmol Vis Sci 2003; 44:4705–14. [DOI] [PubMed] [Google Scholar]
- 115. Luo L, Li D‐Q, Doshi A, Farley WJ, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP‐9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci 2004; 45:4293–301. [DOI] [PubMed] [Google Scholar]
- 116. Kim H‐S, Luo L, Pflugfelder SC, Li D‐Q. Doxycycline inhibits TGF‐β1‐induced MMP‐9 via Smad and MAPK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci 2005; 46:840–8. [DOI] [PubMed] [Google Scholar]
- 117. Luo L, Li D‐Q, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens 2005; 31:186–93. [DOI] [PubMed] [Google Scholar]
- 118. Lan W, Petznick A, Heryati S, Rifada M, Tong L. Nuclear factor‐κB: central regulator in ocular surface inflammation and diseases. Ocul Surf 2012; 10:137–48. [DOI] [PubMed] [Google Scholar]
- 119. Li D‐Q, Zhou N, Zhang L, Ma P, Pflugfelder SC. Suppressive effects of azithromycin on zymosan‐induced production of proinflammatory mediators by human corneal epithelial cells. Invest Ophthalmol Vis Sci 2010; 51:5623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kashkouli MB, Fazel AJ, Kiavash V, Nojomi M, Ghiasian L. Oral azithromycin versus doxycycline in meibomian gland dysfunction: a randomised double‐masked open‐label clinical trial. Br J Ophthalmol 2015; 99:199–204. [DOI] [PubMed] [Google Scholar]
- 121. Saika S, Miyamoto T, Yamanaka O, Kato T, Ohnishi Y, Flanders KC et al Therapeutic effect of topical administration of SN50, an inhibitor of nuclear factor‐κB, in treatment of corneal alkali burns in mice. Am J Pathol 2005; 166:1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Conway TF, Hammer L, Furtado S, Mathiowitz E, Nicoletti F, Mangano K et al Oral delivery of particulate transforming growth factor β1 and all‐trans retinoic acid reduces gut inflammation in murine models of inflammatory bowel disease. J Crohns Colitis 2015; 9:647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. De Paiva CS, Volpe EA, Gandhi NB, Zhang X, Zheng X, Pitcher JD et al Disruption of TGF‐β signaling improves ocular surface epithelial disease in experimental autoimmune keratoconjunctivitis sicca. PLoS ONE 2011; 6:e29017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Desreumaux P, Foussat A, Allez M, Beaugerie L, Hébuterne X, Bouhnik Y et al Safety and efficacy of antigen‐specific regulatory T‐cell therapy for patients with refractory Crohn's disease. Gastroenterology 2012; 143:1207–17‐2. [DOI] [PubMed] [Google Scholar]
- 125. Navarro S, Lazzari A, Kanda A, Fleury S, Dombrowicz D, Glaichenhaus N et al Bystander immunotherapy as a strategy to control allergen‐driven airway inflammation. Mucosal Immunol 2015; 8:841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Coursey TG, Gandhi NB, Volpe EA, Pflugfelder SC, de Paiva CS. Chemokine receptors CCR6 and CXCR3 are necessary for CD4+ T cell mediated ocular surface disease in experimental dry eye disease. PLoS ONE 2013; 8:e78508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Daubeuf F, Jung F, Douglas GJ, Chevalier E, Frossard N. Protective effect of a Protein Epitope Mimetic CCR10 antagonist, POL7085, in a model of allergic eosinophilic airway inflammation. Respir Res 2015; 16:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kugadas A, Gadjeva M. Impact of microbiome on ocular health. Ocul Surf 2016; 14:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. de Paiva CS, Jones DB, Stern ME, Bian F, Moore QL, Corbiere S et al Altered mucosal microbiome diversity and disease severity in Sjögren syndrome. Sci Rep 2016; 6:23561. [DOI] [PMC free article] [PubMed] [Google Scholar]


