Abstract
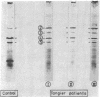
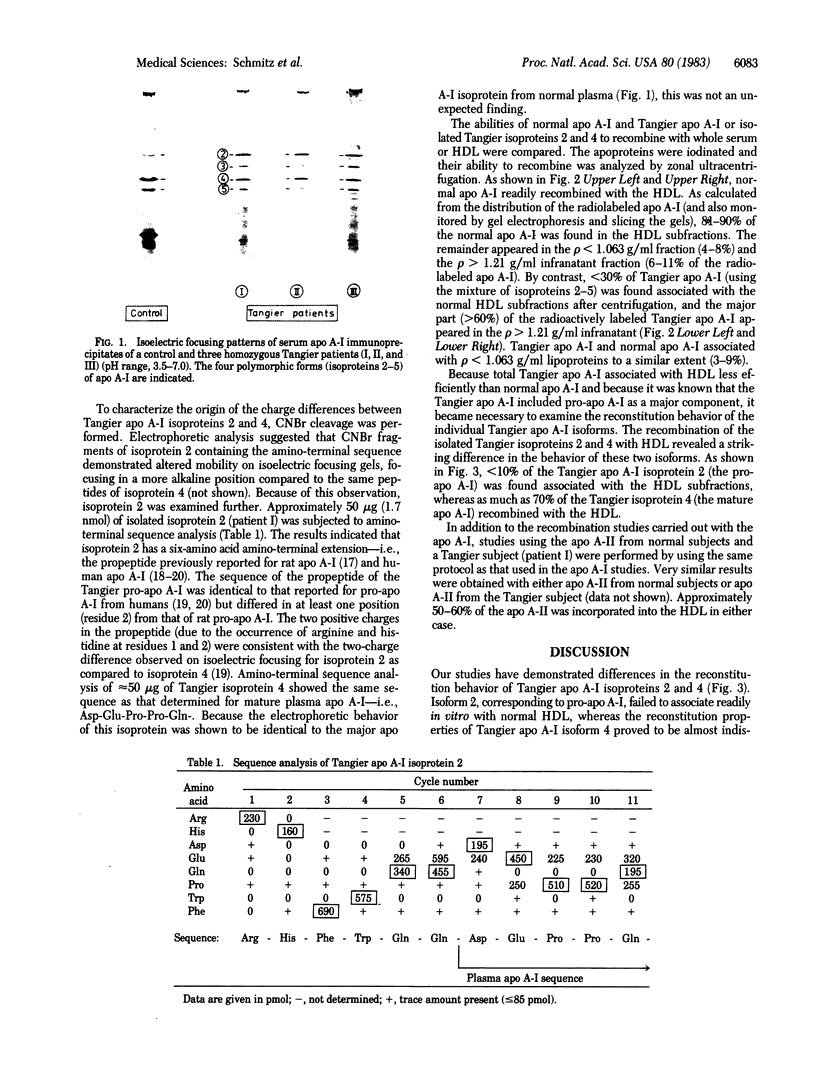
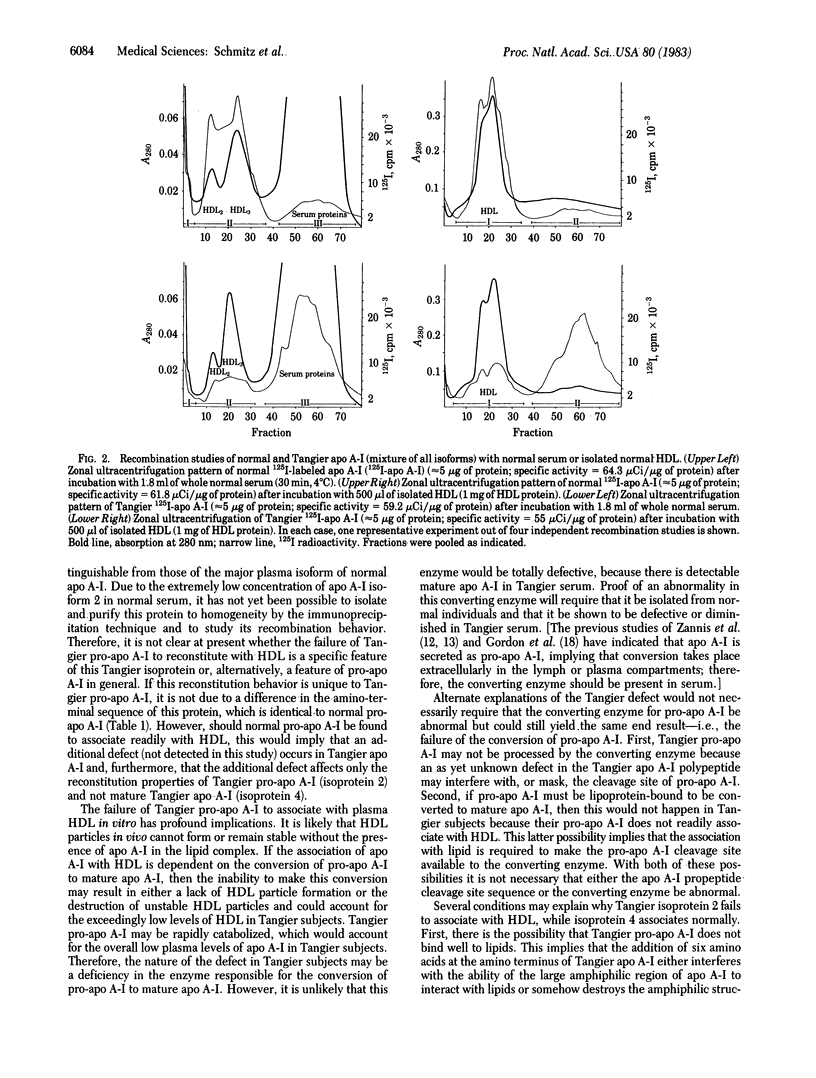
Isoforms of apolipoprotein A-I (apo A-I) from subjects with Tangier disease were characterized, and their ability to recombine with normal high density lipoproteins (HDL) was studied. In contrast to normal serum, in which isoprotein 4 is the dominant species [79 +/- 1.8% (mean +/- SD)], the Tangier serum contained much less total apo A-I (approximately equal to 1% of that in normal serum), and isoproteins 2 and 4 were present in roughly equivalent amounts (35.3 +/- 2.5% and 42.7 +/- 3.6%, respectively). The Tangier isoprotein 2 was shown to correspond to pro-apo A-I, having a six-amino acid amino-terminal extension with the sequence: Arg-His-Phe-Trp-Gln-Gln-. The Tangier isoprotein 4 had the same amino-terminal sequence as normal circulating plasma apo A-I. Its association with normal HDL (70%) was similar to the association of normal apo A-I with HDL (80-90%) in recombination experiments. In marked contrast to this behavior, very little (less than 10%) of Tangier isoprotein 2 (pro-apo A-I) associated with HDL in recombination experiments. These results suggest that the underlying defect in Tangier disease may be a faulty conversion of pro-apo A-I to mature apo A-I, either due to a defect in the converting enzyme activity or to a further specific structural defect in Tangier apo A-I. The failure of Tangier pro-apo A-I to associate with HDL may be at least partially responsible for the HDL deficiency in Tangier subjects.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assmann G., Capurso A., Smootz E., Wellner U. Apoprotein A metabolism in Tangier disease. Atherosclerosis. 1978 Aug;30(4):321–332. doi: 10.1016/0021-9150(78)90125-9. [DOI] [PubMed] [Google Scholar]
- Assmann G., Herbert P. N., Fredrickson D. S., Forte T. Isolation and characterization of an abnormal high density lipoprotein in Tangier Diesase. J Clin Invest. 1977 Jul;60(1):242–252. doi: 10.1172/JCI108761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann G., Schriewer H., Funke H. Zur Richtigkeit der HDL-Cholesterin- und HDL-Apolipoprotein A-I-Bestimmung nach Phosphorwolframsäure/MgCl2-Präzipitation Apolipoprotein B-haltiger Lipoproteine. J Clin Chem Clin Biochem. 1981 May;19(5):273–278. [PubMed] [Google Scholar]
- Assmann G., Simantke O., Schaefer H. E., Smootz E. Characterization of high density lipoproteins in patients heterozygous for Tangier disease. J Clin Invest. 1977 Nov;60(5):1025–1035. doi: 10.1172/JCI108853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann G., Smootz E., Adler K., Capurso A., Oette K. The lipoprotein abnormality in Tangier disease: quantitation of A apoproteins. J Clin Invest. 1977 Mar;59(3):565–575. doi: 10.1172/JCI108672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann G., Smootz E. High density lipoprotein infusion and partial plasma exchange in Tangier disease. Eur J Clin Invest. 1978 Jun;8(3):131–135. doi: 10.1111/j.1365-2362.1978.tb00825.x. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer H. B., Jr, Fairwell T., Meng M., Kay L., Ronan R. Human proapoA-ITangier: isolation of proapoA-ITangier and amino acid sequence of the propeptide. Biochem Biophys Res Commun. 1983 Jun 29;113(3):934–940. doi: 10.1016/0006-291x(83)91088-4. [DOI] [PubMed] [Google Scholar]
- FREDRICKSON D. S. THE INHERITANCE OF HIGH DENSITY LIPOPROTEIN DEFICIENCY (TANGIER DISEASE). J Clin Invest. 1964 Feb;43:228–236. doi: 10.1172/JCI104907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrans V. J., Fredrickson D. S. The pathology of Tangier disease. A light and electron microscopic study. Am J Pathol. 1975 Jan;78(1):101–158. [PMC free article] [PubMed] [Google Scholar]
- Glickman R. M., Green P. H., Lees R. S., Tall A. Apoprotein A-I synthesis in normal intestinal mucosa and in Tangier disease. N Engl J Med. 1978 Dec 28;299(26):1424–1427. doi: 10.1056/NEJM197812282992602. [DOI] [PubMed] [Google Scholar]
- Gordon J. I., Sims H. F., Lentz S. R., Edelstein C., Scanu A. M., Strauss A. W. Proteolytic processing of human preproapolipoprotein A-I. A proposed defect in the conversion of pro A-I to A-I in Tangier's disease. J Biol Chem. 1983 Mar 25;258(6):4037–4044. [PubMed] [Google Scholar]
- Gordon J. I., Smith D. P., Andy R., Alpers D. H., Schonfeld G., Strauss A. W. The primary translation product of rat intestinal apolipoprotein A-I mRNA is an unusual preproprotein. J Biol Chem. 1982 Jan 25;257(2):971–978. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Law S. W., Gray G., Brewer H. B., Jr cDNA cloning of human apoA-I: amino acid sequence of preproapoA-I. Biochem Biophys Res Commun. 1983 Apr 15;112(1):257–264. doi: 10.1016/0006-291x(83)91824-7. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Pagnan A., Havel R. J., Kane J. P., Kotite L. Characterization of human very low density lipoproteins containing two electrophoretic populations: double pre-beta lipoproteinemia and primary dysbetalipoproteinemia. J Lipid Res. 1977 Sep;18(5):613–622. [PubMed] [Google Scholar]
- Patsch J. R., Sailer S., Kostner G., Sandhofer F., Holasek A., Braunsteiner H. Separation of the main lipoprotein density classes from human plasma by rate-zonal ultracentrifugation. J Lipid Res. 1974 Jul;15(4):356–366. [PubMed] [Google Scholar]
- Scanu A. M., Edelstein C. Solubility in aqueous solutions of ethanol of the small molecular weight peptides of the serum very low density and high density lipoproteins: relevance to the recovery problem during delipidation of serum lipoproteins. Anal Biochem. 1971 Dec;44(2):576–588. doi: 10.1016/0003-2697(71)90247-8. [DOI] [PubMed] [Google Scholar]
- Schaefer E. J., Blum C. B., Levy R. I., Jenkins L. L., Alaupovic P., Foster D. M., Brewer H. B., Jr Metabolism of high-density lipoprotein apolipoproteins in Tangier disease. N Engl J Med. 1978 Oct 26;299(17):905–910. doi: 10.1056/NEJM197810262991701. [DOI] [PubMed] [Google Scholar]
- Schaefer E. J., Eisenberg S., Levy R. I. Lipoprotein apoprotein metabolism. J Lipid Res. 1978 Aug;19(6):667–687. [PubMed] [Google Scholar]
- Schmitz G., Ilsemann K., Melnik B., Assmann G. Isoproteins of human apolipoprotein A-II: isolation and characterization. J Lipid Res. 1983 Aug;24(8):1021–1029. [PubMed] [Google Scholar]
- Weisgraber K. H., Rall S. C., Jr, Mahley R. W. Human E apoprotein heterogeneity. Cysteine-arginine interchanges in the amino acid sequence of the apo-E isoforms. J Biol Chem. 1981 Sep 10;256(17):9077–9083. [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L., Katz A. J. Isoproteins of human apolipoprotein A-I demonstrated in plasma and intestinal organ culture. J Biol Chem. 1980 Sep 25;255(18):8612–8617. [PubMed] [Google Scholar]
- Zannis V. I., Karathanasis S. K., Keutmann H. T., Goldberger G., Breslow J. L. Intracellular and extracellular processing of human apolipoprotein A-I: secreted apolipoprotein A-I isoprotein 2 is a propeptide. Proc Natl Acad Sci U S A. 1983 May;80(9):2574–2578. doi: 10.1073/pnas.80.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannis V. I., Lees A. M., Lees R. S., Breslow J. L. Abnormal apoprotein A-I isoprotein composition in patients with Tangier disease. J Biol Chem. 1982 May 10;257(9):4978–4986. [PubMed] [Google Scholar]