Abstract
Currently, 5.4 million Americans suffer from AD, and these numbers are expected to increase up to 16 million by 2050. Despite tremendous research efforts, we still do not have drugs or agents that can delay, or prevent AD and its progression, and we still do not have early detectable biomarkers for AD. Multiple cellular changes have been implicated in AD, including synaptic damage, mitochondrial damage, production and accumulation of Aβ and phosphorylated tau, inflammatory response, deficits in neurotransmitters, deregulation of the cell cycle, and hormonal imbalance. Research into AD has revealed that miRNAs are involved in each of these cellular changes and interfere with gene regulation and translation. Recent discoveries in molecular biology have also revealed that microRNAs play a major role in post-translational regulation of gene expression. The purpose of this article is to review research that has assessed neuroprotective and neurodegenerative characteristics of microRNAs in brain samples from AD transgenic mouse models and patients with AD.
Keywords: microRNAs, Alzheimer’s disease, Phosphorylated tau, Synaptic damage, Mitochondrial dysfunction
Introduction
Alzheimer’s disease (AD) is an age-related, multifactorial, progressive neurodegenerative disease, characterized by memory loss, multiple cognitive impairments, and changes in personality and behavior. Currently, 5.4 million Americans suffer from AD, and this number is expected to increase up to 16 million by 2050. With a growing aging population not only in the United States but worldwide, AD has become a major health concern. AD has had a huge economic impact, with dementia health care costs alone reaching an estimated total of $818 billion worldwide in 2015 (World Alzheimer Report 2015). Despite extensive research into AD, we still do not have drugs or agents that can delay or prevent AD progression, and we do not have detectable biomarkers for early AD diagnosis.
AD is associated with synaptic loss, mitochondrial dysfunction, amyloid beta (Aβ) production and accumulation, inflammatory responses, phosphorylated tau formation and accumulation, cell cycle deregulation, impaired cholinesterase transmission, deficits in neurotransmission, calcium dyshemeostatis and hormonal imbalance, neuronal loss, and an accumulation of senile plaques and neurofibrillary tangles in learning and memory regions of the brain [1–4]. Synaptic pathology and mitochondrial damage have been identified as early events in AD pathogenesis [5]. An accumulation of Aβ and mislocalization of phosphorylated tau in synapses cause synaptic starvation and degeneration, and cognitive decline in AD patients [6]. The precise cause underlying AD pathogenesis are not completely known or understood.
Anatomical studies of AD brains and the brains from several lines of AD transgenic mice have revealed that AD begins in brain regions that are responsible for learning and memory, including layer 2 of the entorhinal cortex. The AD process spreads to the hippocampus, temporal cortex, frontoparietal cortex and, finally, to subcortical nuclei [4,7].
AD occurs in two forms, early-onset familial and late-onset sporadic. Early-onset AD is caused by genetic mutations in 3 loci: the amyloid precursor protein (APP), Presenilin 1 (PS1), and Presenilin 2 (PS2). However, genetic mutations in these 3 loci are responsible for only a small proportion (1–2%) of all AD cases [1–3]. The remaining AD cases are late-onset sporadic AD, but unlike early-onset AD, the cause of late-onset AD is not known. However, several risk factors have been identified in late-onset AD, including apolipoprotein E (ApoE) genotype 4 and genetic polymorphisms in sortilin-related receptor 1, clusterin, complement component receptor 1, CD2AP, CD33, EPHA1, and MS4A4/MS4A6E genes [1]. Multiple risk factors, including lifestyle, diet, environmental factors such as stress, vascular diseases, depression, stroke, hypertension, traumatic brain injury, and type 2 diabetes are other contributing factors to late-onset AD [8].
In early-onset AD, the pathological cellular changes occur early in a person’s life (typically less than 30 years old) because of genetic mutation(s), whereas in late-onset AD, cellular and pathological changes occur later in life due to aging processes that take time to induce AD [1,2]. At cellular and pathological levels, there are no marked differences between early-onset AD and late-onset AD.
Research into AD has revealed that micro RNAs (miRNAs) play a large role in the regulation of genes, protein expressions, and phenotypic changes in human diseases. Well-studied miRNAs in human diseases include cancer [9,10], cardiovascular diseases [11], hypertension [12], nephropathy [13], stroke [14], and neurodegenerative diseases such as schizophrenia [15], Huntington’s [16], Parkinson’s [17], and AD [18–20].
miRNAs regulate genes that are responsible for Aβ production and phosphorylated tau, including APP, PS1, and PS2 [Table 1]. miRNAs also regulate cellular changes via the ApoE4 genotype and other polymorphisms, including sortilin-related receptor 1, clusterin, complement component receptor 1, CD2AP, CD33, EPHA1, and MS4A4/MS4A6E genes that are involved in AD pathogenesis [21].
Table 1.
Neuroprotective and neurodegenerative miRNAs in Alzheimer’s diseases
| A. Neuroprotective miRNAs in Alzheimer’s disease | ||||
|---|---|---|---|---|
| miRNAs | Status in AD | Target gene | Effect on target genes | Reference |
| miR-29a/b-1 cluster | Brain | BACE1/β-secretase | Upregulation | Hébert et al., 2008 [89] |
| miR-101 | - | APP | Downregulation | Vilardo et al., 2010 [90] |
| miR-124 | ↓ brain | BACE1 | Downregulation | Fang et al., 2012 [41] |
| miR-132/212 | ↓ brain | PTEN, FOXO3a and P300 | Downregulation | Wong et al., 2013 [91] |
| miR-34 | - | tau | Downregulation | Dikson et al., 2013 [92] |
| miR-193b | ↓ hippocampus | APP | Downregulation | Liu et al., 2014 [93] |
| miR-188-3p | ↓ brain | BACE1 | Downregulation | Zhang et al., 2014 [49] |
| miR-339-5p | ↓ brain | BACE1 | Downregulation | Long et al., 2014 [50] |
| miR-212/132 & miR23a/b | ↓ frontalcortex | sirt1 | Upregulation | Weinberg et al., 2015 [94] |
| miR-219 | ↓ brain | tau | Downregulation | Santa-Maria et al., 2015 [95] |
| miR-16 | ↓ neuronal cells | APP | Downregulation | Zhang et al., 2015 [96] |
| Mir-29c | ↓ peripheral blood | BACE1 | Downregulation | Yang et al., 2015 [48] |
| miR-135b | ↓ peripheral blood | BACE1 | Downregulation | Zhang et al., 2016 [97] |
| miR-1229-3p | - | SORL1 | Downregulation | Ghanbari et al., 2016 [98] |
| miR-15/107 family | ↓ brain | CDK5R1 | Downregulation | Moncini et al., 2016 [99] |
| miR-603 | ↑ hippocampus | LRPAP1 | Downregulation | Zhang et al., 2016 [100] |
| miR-29 | ↓ brain | hBACE1 | Downregulation | Pereira et al., 2016 [101] |
| B. Neurodegenerative miRNAs in Alzheimer’s disease | ||||
|---|---|---|---|---|
| miR-26b | ↑ brain cortex | Rb1 | Upregulation | Absalon et al., 2013 [102] |
| miR-30a-5p | - | BDNF | Downregulation | Croce et al., 2013 [103] |
| miR-206 | ↑ brain | BDNF | Downregulation | Tian et al., 2014 [104] |
| miR-125 | ↑ brain | DUSP6, PPP1CA & Bcl-W | Downregulation | Banzhaf-Strathmann et al., 2014 [105] |
| miR-33 | - | ABCA1 | Downregulation | Kim et al., 2015 [67] |
| miR-34a | ↑ brain | VAMP2, SYT1, HCN1, NR2A, GLUR1,NDUFC2, | Downregulation | Sarkar et al., 2016 [106] |
| miR-126 | ↑ brain | IRS-1 and PIK3R2 | Downregulation | Kim et al., 2016 [107] |
This article reviews research on protective and toxic miRNAs in AD. Also reviewed is research that focuses on miRNAs that may be responsible for early-onset familial AD and late-onset sporadic AD.
Biogenesis of microRNAs
miRNAs are a large family of conserved small (20–22 nucleotides), non-coding RNAs. miRNAs play a central role in the post-transcriptional regulation of gene expression [20,22]. In mammals, miRNAs are believed to control about 50% of all protein-coding genes [23]. At present, over 2000 miRNAs have been identified (see details at www.mirbase.org). One-third of these miRNAs are found in the coding part of genes, and the remaining are in the intronic regions.
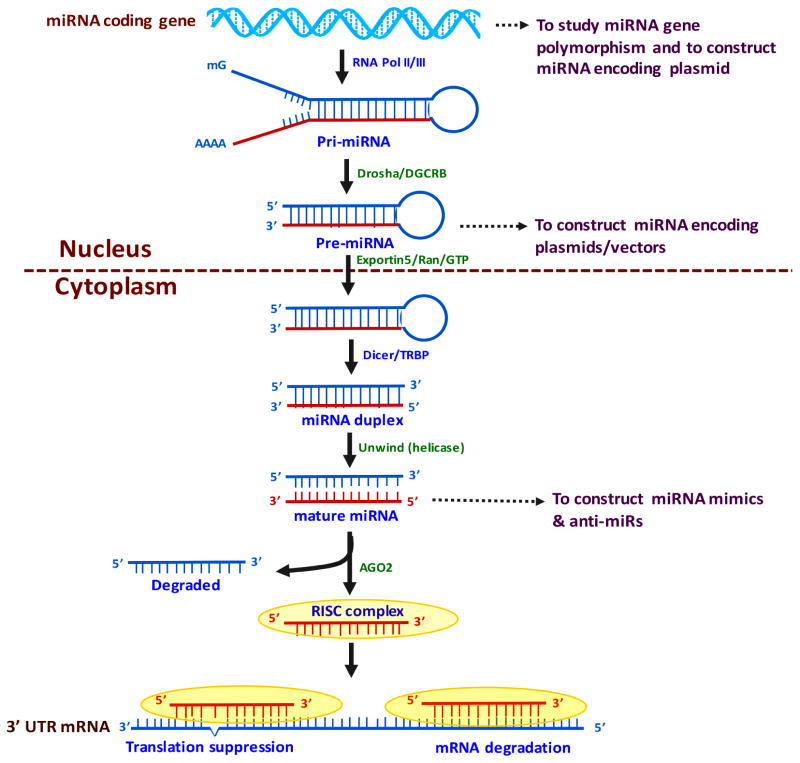
As shown in Figure 1, miRNA biogenesis is initiated in the nucleus with transcription of primary miRNA transcripts from miRNA coding genes. Pri-miRNAs are converted to hair-pin loop pre-miRNAs by enzymatic digestion with Drosha and DGCR8 proteins. Pre-miRNAs are transported to the cytoplasm by Exportin-5/Ran/GTP proteins where they are again digested by the cytoplasmic proteins Dicer and TRBP, resulting in the generation of the miRNA duplex. Duplex structure is unwinded by helicase, resulting in the generation of mature miRNA strands. Mature miRNAs form the RISC complex with the Ago2 protein and target the 3′UTR of mRNA, and they modulate gene activity either by translation suppression or mRNA cleavage [24,25]. miRNAs can be found as a group or a family, harboring the same initial sequence and targeting a single gene. In some cases, a single miRNA can affect a large number of genes that are involved in the regulation of multiple cellular events and pathways [26].
Figure 1. Hypothesized miRNA biogenesis pathway.
miRNA biogenesis initiates in the nucleus with transcription of primary miRNA transcripts from miRNA coding genes by RNA Polymerase II or III. pri-miRNA converted to hair-pin loop pre-miRNA by enzymatic digestion with Drosha and DGCR8 proteins. Pre-miRNA is transported to the cytoplasm by Exportin-5/Ran/GTP proteins and is digested by the cytoplasmic proteins Dicer/TRBP and generates miRNA duplex. The duplex structure is unwound by helicase, and a mature miRNA strand is generated. Mature miRNAs form the RISC complex with the Ago2 protein. The mature miRNAs target the 3′UTR of mRNA and modulate gene activity either by suppressing mRNA cleavage or the translation.
Research has revealed that miRNAs are differentially expressed in different cell types and tissues in mammals, including humans. miRNAs are believed to alter multiple cellular processes, including development, cell proliferation, replicative senescence, and aging [27,28]. Over 70% of reported miRNAs are expressed in the human brain. miRNAs found in the human brain include: miR-9, miR-7, miR-128, miR-125 a-b, miR-23, miR-132, miR-137, and miR-139. A recent deep RNA sequencing analysis has revealed a large number of miRNAs that are brain-specific, including: miR-134, miR-135, let-7g, miR-101, miR-181a-b, miR-191, miR-124, miR-let-7c, let-7a, miR-29a, and miR-107 [29,30]. Most of these miRNAs are responsible for synaptic functions, neurotransmitter release, synapse formation and neurite outgrowth. Expression levels of several miRNAs are altered in a diseased state, such as AD.
The AD brain and microRNAs
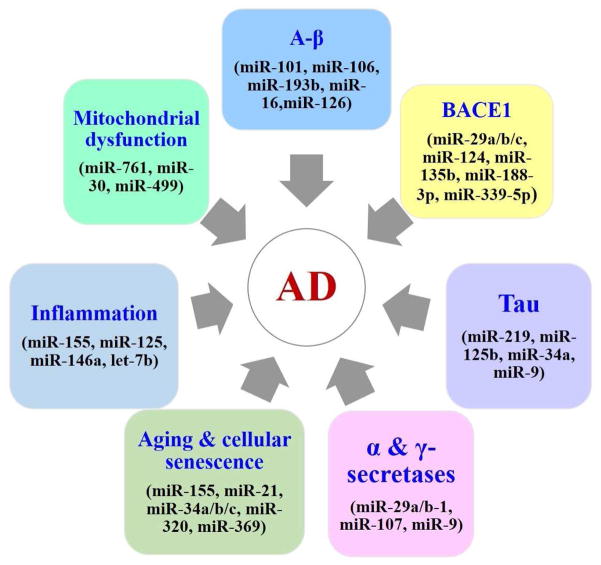
The progressive loss of synapses and neurons, reduced volume of the hippocampus, and reduction in size and weight are typical features of the AD brain. Similar to brains from humans with AD, brains from AD mice have most of these same features. The loss of synapses and synaptic damage correlate the closest with cognitive decline and memory loss in AD patients and AD mice [5]. Studies revealed a reduction in miRNA expression in the AD brain, which in turn appears to correlate with a reduction and in Aβ production and reduced phosphorylated tau (Table 1). In contrast, several miRNAs are known to increase levels of Aβ, phosphorylated tau, and inflammation not only in the brains of humans with AD but also in the brains of mice with AD (Figure 2). Interestingly, the brains from Dicer knockout mice exhibited similar features found in the brains from humans and mice with AD, such as reduced brain size, enlarged ventricles, inflammation of brain, loss of synaptic branching and connectivity, and spine length [31–33]. Dicer knockout mice also showed oxidative stress, phosphorylated tau, and memory loss, and reduced levels of a large number of miRNAs [31–33], conditions also found in the brains of humans and mice with AD. The similarities between the brains of humans with AD and Dicer knockout mice suggest that Dicer may play a large role in memory and cognition and that a progressive loss of Dicer may be linked to reduced learning and memory in persons with AD. Research is needed to investigate whether Dicer is linked to cognitive decline in AD.
Figure 2. AD risk factors and associated miRNAs.
Deregulation of miRNAs expression modulates the expression of certain key AD related genes and promote disease progression. Beside miRNAs also involved in several cellular process that are critical for AD.
Amyloid beta and microRNAs
Aβ production and Aβ deposits in the brains of humans with AD are accompanied by alterations in the levels of many miRNAs from distinct miRNA classes. These miRNAs may be involved in AD pathogenesis, in particular the generation of Aβ [18,34–37]. Several miRNA classes, such as miR-101 and miR-106, target APP, resulting in an elevated generation of Aβ [18,36,38,39]. Interestingly, several nucleotide polymorphisms associated with AD are located in the miRNA-binding region of the APP mRNA, which can modulate protein expressions [40].
Another class of miRNAs that are downregulated in AD is the neuron-specific miR-124 [41]. The downregulated miR-124 leads to an over-expression of its targeted mRNA, polypyrimidine-tract binding protein 1 (a pre-RNA splicing regulator), in turn leading to the altered splicing of APP [41]. miR-124 also targets the Aβ cleaving enzyme 1 BACE1, and the downregulation of miR-124 promotes the transformation of APP into Aβ, probably by activating BACE1 [42]. It is not known whether there are reduced levels of other miRNAs in the brains from humans and mice with AD. Reduced levels of miR-9, miR-29a, miR-29b, and miR-107 may result in elevated BACE1 expression and an over-production of Aβ known to characterize brains from humans and mice with AD [37,38,].
Studies in mutant AD mice suggest that miR-298 and miR-328 have similar roles [44]. Loss of miR-9, miR-29a, and miR-29b in AD, together with the loss of miR-137 and miR-181c disinhibits serine palmitoyltransferase, the rate-limiting enzyme of ceramide synthesis, leading to the mislocation of BACE1 in lipid rafts and augmenting the excessive processing of APP into Aβ [43].
Increasing evidence suggests that miRNAs affect Aβ production. As shown in Table 1, several miRNAs increase Aβ levels and others reduce Aβ production. On the other hand, Aβ itself reciprocally impacts the production of miRNAs, including miR-9, miR-106b, and let7 [43,45,46]. Despite evidence in support of a role for reduced levels of miRNAs in AD, it remains unclear whether reduced miRNAs play a primary role in AD induction.
Kim and colleagues (2014) studied the effects of elevated levels of miRNA126 in dopamine neuronal cell survival in models of Parkinson’s disease [47]. They showed that elevated levels of miR-126 increase the vulnerability of neurons to ubiquitous toxicity that is mediated by staurosporine or Aβ42. The neuroprotective factors IGF-1, nerve growth factor, brain-derived neurotrophic factor, and soluble APP α could diminish, but not abrogate, the toxic effects of miR-126. MiR-126 overexpressing neurons from a Tg6799 familial mouse model of AD exhibited an increase in Aβ42 toxicity, but surprisingly, both Aβ42 and miR-126 promoted neurite sprouting. Pathway analysis revealed that the overexpression of miR-126 downregulated elements in the GF/PI3K/AKT and ERK signaling cascades, including AKT, GSK-3β, and ERK; the phosphorylation of tau; and the miR-126 targets IRS-1 and PIK3R2. In this same study, the inhibition of miR-126 was found to be neuroprotective against both STS and Aβ42 toxicity. Although the Kim study focused on Parkinson’s disease, it provides evidence for a novel miR-126 mechanism in a neurodegenerative disease that is capable of regulating GF/PI3K signaling in neurons, suggesting that miR-126 may be an important mechanistic link between metabolic dysfunction and neurotoxicity in another neurodegenerative disease, namely AD.
BACE1 and microRNAs
Altered levels of miRNAs increase the production of Aβ and BACE1 activity. Yang and colleagues (2015) studied the expression levels of the miR-29 family in peripheral blood samples from patients with AD and age-matched controls [48]. They found a comparatively marked decrease in miR-29c expression and a significant increase in BACE1 expression in the samples from the AD patients. Correlation analysis revealed that miR-29c expression negatively correlated with the protein expression of BACE1 in the samples from the AD patients. Yang and colleagues also investigated the role of miR-29 on hippocampal neurons in vitro and in vivo. They found miR-29c upregulation promoted learning and memory behaviors in SAMP8 mice by increasing the activity of the protein kinase A/cAMP response element-binding protein, which is involved in neuroprotection, suggesting that miR-29c may be a possible therapeutic target against AD [48].
Zhang and colleagues (2014) studied the role of miR-188-3p that targets BACE1 in humans and mice with AD [49]. They found miR-188-3p to be significantly downregulated in the brains of AD humans and the AD TG mice, an APP mouse model of AD. The downregulated miR-188-3p was restored by the inhibition of MAGL. Overexpression of miR-188-3p in the hippocampus of the TG mice reduced BACE1, Aβ, and neuroinflammation, and prevented deterioration in hippocampal basal synaptic transmission, long-term potentiation, spatial learning, and memory. Loss of miR-188-3p function correlated with 2-AG-induced suppression of BACE1. Moreover, miR-188-3p expression was upregulated by 2-AG or peroxisome proliferator-activated receptor-γ (PPARγ) agonists and suppressed by PPARγ antagonism or NF-κB activation. Reduction of Aβ and neuroinflammation by MAGL inhibition was occluded by PPARγ antagonism. In addition, BACE1 suppression by 2-AG and PPARγ activation was eliminated by the knockdown of NF-κB. The Zhang study revealed a novel molecular mechanism underlying improved synaptic and cognitive function in TG mice by 2-AG signaling – a mechanism that appears to upregulate miR-188-3p expression through the PPARγ and NF-κB signaling pathways, resulting in suppression of BACE1 expression and the consequent formation of Aβ [49].
Long and colleagues (2014) identified miR-339-5p, a known miRNA, as a key contributor to the regulatory network [50]. Two distinct miR-339-5p target sites were predicted in the BACE1 3′-UTR by in silico analyses, and both were found to be linked to BACE1. Co-transfection of miR-339-5p with a BACE1 3′-UTR reporter construct resulted in significant reduction in reporter expression [50]. Mutation of both target sites eliminated this effect. Delivery of the miR-339-5p mimic also significantly inhibited expression of the BACE1 protein in human glioblastoma cells and human primary brain cultures. Delivery of target protectors designed against the miR-339-5p BACE1 3′-UTR target sites in primary human brain cultures significantly elevated BACE1 expression. In addition, miR-339-5p levels were significantly reduced in brain specimens from AD patients compared to those from age-matched controls. Therefore, miR-339-5p appears to regulate BACE1 expression in human brain cells and to be dysregulated in at least a subset of AD patients, warranting the study of miR-339-5p as a novel drug target for patients with AD [50].
Using AD mouse models, Boissonneault and colleagues studied the roles of miR-298 and miR-328 in Aβ production in mice With AD [44]. They observed a loss in correlation between BACE1 mRNA and protein levels in the hippocampus of the AD mouse model. These findings prompted an investigation of the regulatory role of the BACE1 3′-UTR element in AD progression and the possible involvement of specific miRNAs in cultured neuronal cells and fibroblastic cells from humans with AD. Using such experimental approaches, these researchers demonstrated that miR-298 and miR-328 recognize specific binding sites in the 3′-UTR of the BACE1 mRNA and exert regulatory effects on ACE1 protein expression in cultured neuronal cells [44]. These results may point to a molecular basis underlying BACE1 deregulation in AD and may offer new perspectives on AD.
Galimberti et al. (2014) studied the profiles of circulating miRNAs in serum and cerebrospinal fluids (CSF) from humans with AD and correlated them with profiles of AD patients [51]. Using a two-step analysis – micro-array analysis followed by validation via real-time PCR – they found miR-23a to be down-regulated in the serum from 22 AD patients compared to 18 non-inflammatory controls (NINDCs), 8 inflammatory neurological controls (INDCs), and 10 patients with frontotemporal dementia. Significant down-regulation of miR-125b and of miR-26b was also confirmed in the CSF from AD patients. These researchers found that cell-free miR-125b serum levels from AD patients are less than levels from INDCs and NINDCs with an accuracy of 82%.
Alexandrov and colleagues (2013) studied the effects of miRNA-34a on TREM2 mRNA 3′-untranslated region of TREM2 and found that miRNA-34a significantly down-regulated TREM2 expression [52]. Mutations in TREM2 are known to cause rare, autosomal recessive forms of early onset dementia that present with and without bone cysts and fractures Aluminum-induced miRNA-34a up-regulation and TREM2 down-regulation were effectively quenched with the natural phenolic compound and the NF-kB inhibitor CAPE (2-phenylethyl-(2E)-3-(3,4-dihydroxyphenyl) acrylate; caffeic-acid phenethyl ester). These results suggest that an epigenetic mechanism in AD involving an aluminum-triggered, NF-kB-sensitive, miRNA-34a-mediated down-regulation of TREM2 expression may impair phagocytic responses that may ultimately contribute to an accumulation and aggregation of the Aβ42 peptide, amyloidogenesis, and inflammatory degeneration in the AD brain [52].
Alexandrov et al. (2012) analyzed the relative amount of Aβ and miRNA in CSF from the neocortices of patients with AD and of age-matched controls, in short post-mortem intervals (PMI <2.1 hr) [53]. They found a decreased but nonsignificant abundance of Aβ42 in the CSF and extracellular fluid of AD patients. The most abundant nucleic acids in the CSF and ECF from AD patients were miRNAs. This result led to additional studies of the speciation and inducibility. Fluorescent miRNA-array-based analysis indicated significant increases in miRNA-9, miRNA-125b, miRNA-146a, and miRNA-155 in the CSF and ECF of AD patients [53]. Primary human neuronal-glial cell co-cultures stressed with AD-derived ECF also displayed an up-regulation of these four miRNAs, an effect that was quenched using the anti-NF-κB agent caffeic acid phenethyl ester. Increases in miRNAs were confirmed independently, using a highly sensitive LED-Northern dot blot assay. Several of these NF-κB-sensitive miRNAs are known to be up-regulated in AD brain and are associated with the progressive spreading of inflammatory neurodegeneration. Results from these confirmation studies indicated that miRNA-9, miRNA-125b, miRNA-146a, and miRNA-155 are CSF- and ECF-abundant. NF-κB-sensitive pro-inflammatory miRNAs, and their enrichment in circulating CSF and ECF, suggest that miRNAs may be involved in the modulation or proliferation of miRNA-triggered pathogenic signaling throughout the central nervous system [53].
Using SAMP8 mice and BALb/c mice, Liu et al. (2012) examined the post-transcriptional regulation mechanism of APP mediated by micro ribonucleic acids [54]. They found miR-16 to be one of the post-transcriptional regulators of APP in the SAMP8 mice. Overexpression of miR-16, both in vitro and in vivo, led to reduced APP expression. Further, miR-16 and APP displayed complementary expression patterns in the SAMP8 mice and BALb/c embryos. Taken together, these findings indicate that an abnormally low expression of miR-16 could lead to an accumulation of APP in AD mice and that APP may be a target for miR-16 [54].
Pogue and colleagues (2009) studied the effects of complement factor H on metal-sulfate-stressed human brain cells when miR146a was modulated [55]. They found an NF-kappaB-sensitive, miRNA-146a-mediated modulation of CFH gene expression in AD neurons that may contribute to inflammatory responses in aluminum-stressed HN cells. This finding underscores the potential of just a nanomolar of aluminum that may be necessary to drive genotoxic mechanisms characteristic of neurodegenerative disease processes.
Alpha secretase and microRNAs
Aβ secretase is an enzyme in AD that cleaves the amyloid domain of APP and reduces the production of the Aβ peptide in neurons. There are numerous miRNAs that are involved in the increased activity of alpha secretase in neurons, and there are other miRNAs responsible for reduced alpha secretase activity, the latter group of which results in an increase in alpha secretase production and a cascade of cellular changes in AD progression.
Interestingly, the loss of miR-107 disinhibits the alpha-secretase ADAM10 and favors the non-amyloidogenic pathway of APP processing. Compensatory effects from this disinhibitation shunts the cleavage of APP away from the generation of Aβ plaque toward the generation of soluble APP [56].
Cerebrospinal fluids and microRNAs
Using open-array technology, Denk and colleagues (2015) studied the CSF of AD patients (n = 22) and controls (n = 28) to profile the expression of 1178 different miRNAs [57]. Using a Cq of 34 as cut-off, they identified positive signals from 441 miRNAs, but could not identify positive signals from 729 other miRNAs indicating that at least 37% of all miRNAs in the body are present in the brain. They found 74 down-regulated miRNAs and 74 up-regulated miRNAs with a 1.5 fold change threshold. By applying the new explorative “measure of relevance” method, they identified 6 reliable and 9 informative biomarkers. Confirmatory MANCOVA revealed reliable miR-100, miR-146a, and miR-1274a as differentially expressed in AD, an analysis that reached Bonferroni-corrected significance. MANCOVA also confirmed the differential expression of informative miR-103, miR-375, miR-505, miR-708, miR-4467, miR-219, miR-296, miR-766, and miR-3622b-3p. Discrimination analysis using a combination of miR-100, miR-103, and miR-375 detected AD in the CSF by positively classifying controls and AD patients with 96.4% and 95.5% accuracy, respectively. Using the ingenuity database, Denk et al. identified a set of AD-associated genes that these miRNAs targeted [57]. These targets included genes involved in the regulation of tau and amyloid pathways in AD, such as MAPT, BACE1, and mTOR.
Gamma secretase complex and microRNAs
γ-secretases are a group of widely expressed, intramembrane-cleaving proteases involved in many physiological processes associated with AD. Mutations in PS1 and PS2 lead to increased γ-secretase activity, which has been associated with the formation of Aβ in AD. In addition to PS1 and PS2, two other subunits – nicastrin and anterior-pharynx defective-1 – were identified as essential co-factors. These four γ-secretase enzymes together generate an active and stable complex that cleaves APP at the end of the Aβ domain in APP. Inhibition of these enzymes redirects the amyloidogenic pathway towards the non-amyloidogenic pathway by reducing Aβ production. Similar to miRNAs that activate BACE1, several MiRNAs are believed to be involved in the increased production of γ-secretase.
Loss of presenilin function has been proposed to underlie memory impairment and neurodegeneration in AD pathogenesis [58]. Using brain tissue from the PS1 knockout mouse, Krichevsky and colleagues (2003) studied the miRNA profiles [59]. They found that the down-regulation of miR-9 coincides with neurodegeneration in PS1 knockout mice. Other studies using zebrafish and mice found miR-9 to be an important regulator of neurogenesis [60,61]. Based on these results, miR-9, which is downregulated in the AD brain, may actively participate in maintaining neurons and in sustaining Aβ production. Further research is needed to determine the role of γ-secretase-linked miRNAs in AD.
Tau and microRNAs
The detrimental effects of miRNA changes in AD neurons might not be restricted to Aβ formation. For example, the loss of miR-15a favors the hyperphosphorylation of tau by disinhibiting ERK1 [62]. Increased levels of miR-128 lead to decreased activity of Bcl2-associated athanogene, leading to a reduction in the removal of sarkosyl-insoluble tau, a reduction that is known to favor the formation of toxic tau inclusions [63]. Based on studies in an AD TG mouse model, putative increases in levels of miR-34a were found to suppress Bcl2 expression, which exacerbated neuronal loss by the recruitment of caspase-3 and apoptosis [64]. Increases in miR-206, in the temporal cortex of AD brains, led to the suppression of BNDF, which contributed to compromises in morphological and functional synaptic plasticity [65].
Li and colleagues (2011) examined miRNA-146a levels in several human primary brain and retinal cell lines from the neocortex and hippocampus of patients in early-, moderate-, and late-stage AD, and of 5 different TG mouse models of AD (Tg2576, TgCRND8, PSAPP, 3xTg-AD, and 5xFAD) [66]. Inducible expression of miRNA-146a was significantly up-regulated in a primary co-culture of human neuronal-glial cells that were stressed using interleukin1-beta. This up-regulation was quenched using specific NF-κB inhibitors that include curcumin. Expression of miRNA-146a correlated with senile plaque density and synaptic pathology in the Tg2576 and 5xFAD TG mouse models [66].
ApoE4 and microRNAs
The ApoE4 genotype is a major risk factor for late-onset sporadic AD. Alterations in microRNAs and cognitive decline are expected in humans with the ApoE4 genotype–mainly because the ApoE4 status increases the production of Aβ [1]. Several lines of evidence support this notion. However, there are no published studies that have linked miRNAs and the ApoE4 genotype in AD patients.
Kim et al. (2014) studied miR-33 and its relationship to ABCA1 and Aβ levels in the brain [67]. Overexpression of miR-33 impaired cellular cholesterol efflux and dramatically increased extracellular Aβ levels by promoting the secretion of Aβ and impairing the clearance of Aβ in neurons. In contrast, genetic deletion of mir-33 in mice dramatically increased ABCA1 levels and ApoE lipidation, but decreased endogenous Aβ levels in the cortex. Most importantly, pharmacological inhibition of miR-33 via antisense oligonucleotide specifically in the brain markedly decreased Aβ levels in the cortex of APP/PS1 mice, suggesting that miR33 is a potential therapeutic strategy for AD. Additional research is needed to determine how ApoE4-linked miRNAs alter cellular changes in the AD brain, such as altering the production and accumulation of Aβ, the phosphorylation of tau, and the triggering of synaptic damage. Further research is needed to understand precise links between miRNAs and ApoE4 genotype association with Aβ levels in patients with AD and mouse models of AD.
Inflammation and microRNAs
Inflammatory responses are strongly associated with altered miRNA expressions in the AD brain. miRNA-155 is involved in diverse physiological and pathological mechanisms, such as inflammation and immunity. Recent studies indicate that miR-155 regulates T-cell functions during inflammation. Song and Lee (2015) investigated the role of miRNA-155 in AD, finding miRNA-155 to be a multifunctional miRNA in AD pathogenesis, with a distinct expression profile and links to T-cell functions [68].
In addition, in studies of miR-125b and miR-146 in the human AD brain, levels of these miRNA were found to be elevated, which might aggravate neuroinflammation [69,70] and reduce complement factor H, which is associated with the neuronal release of mR-146a and miR-155 and inflammatory spreading in the AD brain [70,71]. Altered miR-106b levels impact the expression of transforming growth factor beta178.
In investigating the significance of miRNA release in the AD brain, Lehmann et al. (2012) focused on the miRNA let-7b [72]. They found that, following its release, let-7b activates the toll-like receptor 7, resulting in neuronal degeneration. They also found that loss of miR-29a disrupts the activity of another target gene, neuronal navigator 3, a protein that is involved in axonal guidance and is enriched in degenerating pyramidal neurons in AD [73].
Mitochondrial microRNAs and AD
Dysfunction of mitochondria and oxidative stress have been found to be involved in neurodegenerative diseases, including AD. Mitochondria are cytoplasmic organelles, and control/regulate cell survival and cell death. Mitochondria perform several key cellular functions, including ATP production, regulation of intracellular calcium, apoptotic cell death, sites of free radical production, and scavenging and activation of caspase family of proteases. Mitochondria are synthesized in cell soma, travel along axons and dendrites, and supply ATP for several synaptic functions, including synapse formation and outgrowth, neurotransmitter release and vesicle fusion. Mitochondria move from cell soma to nerve terminals via kinesin-based anterograde fashion and travel back to cell soma via dynein-based retrograde manner.
The human mitochondria carries 37 polypeptide genes in a 16.5 kb circular genome. The DNA of mitochondria has 2 strands: an outer strand enriched with guanine (heavy strand) and an inner strand enriched with cytosine (light strand). It also has a non-coding segment comprised of a displacement loop, a region of 1121 base pairs.
Studies have identified several mitochondrial miRNAs in the human mitochondria [74–81]. More recently, Shinde and Bhandra (2015) have identified six pr-miRNAs and miRNAs from mitochondria genome, indicating miRNAs in the mitochondrial genome [82]. Berray et al. (2011) identified 169 miRNAs in the mitochondrial genome that are believed to regulate polypeptide genes in the mitochondrial genome, oxidative phosphorylation, and ATP synthesis [81].
miRNAs regulate mitochondrial structure. A recent study of miR-761 found that it is responsible for the downregulation of the mitochondrial fission factor (Mff) and the suppression of mitochondrial fission machinery [83]. In studies of miR-30, Goud and Hua (2015) found miR-30a, -30b, and -30d to be highly expressed in myocardial cells that are exposed to hydrogen peroxide [84]. The gene p53, a target of the miR-30 family, promotes Drp1 transcription while triggering apoptosis. Goud and Hua concluded that miR-30 regulates mitochondrial fission and apoptosis via the targeting of P53 and Drp1 [84].
miR-30 and miR499 have been found to be involved in regulating mitochondrial dynamics via Drp1 through p53 and calcineurin [85,86]. Li et al. (2010) found that miR-30 family members inhibit mitochondrial fission and target p53, which is known to induce mitochondrial fission by transcriptionally upregulating Drp1 expression. miR-30 inhibits mitochondrial fission by suppressing the expression of p53 and its downstream target Drp1 [85]. Regarding miR499, Wang and colleagues (2011) found that it directly targets α and β isoforms of the calcineurin and inhibits cardiomyocyte apoptosis by suppressing calcineurin-mediated dephosphorylation of Drp1, which in turn decreases the translocation of Drp1 to mitochondria and Drp1-mediated activation of mitochondrial fission. Findings from these studies revealed that Drp1 is regulated by p53 [86,87]. However, there are no published studies characterizing the role of miRNAs in mitochondrial dynamics either for fission activity or fusion in the AD. Additional research is needed to understand the role of miRNAs, particularly mitochondrial miRNAs, in mitochondrial dynamics and mitochondrial biogenesis in the disease process of AD.
Aging, cellular senescence, and microRNAs
With aging the number one risk factor for AD and other neurodegenerative diseases, it is important to assess miRNAs that affect aging and cellular senescence in order to determine the roles of miRNAs in aging. Multiple tissues, including skin, skeletal muscle, and the brain, are the best indicators of aging and senescence. Skin, as the largest organ in the human body, performs important functions and protects internal organs from external aggression and environmental factors, heat, trauma and microbial pathogens. Culturing skin, skeletal muscle, and brain cells, and studying them for mRNA and miRNAs, may yield new insights into the role of miRNAs in aging.
Increasing evidence suggests that miRNAs are largely implicated in aging and cellular changes associated with aging. Some of these cellular changes are: telomerase shortening (miR155, miR138, miR34abc), inflammaging (miR155, miR21, miR146a), oxidative stress and mitochondrial dysfunction (miR34a, miR335, miR146a, miR145), DNA damage response (miR34abc, miR192, miR194, miR215, miR421, miR24, miR504, miR125b, miR106b, miR21, miR210, miR373), protein misfolding (miR320, miR1, miR26b, miR106b, miR301b), stem cell impairment (miR369, miR371, miR29c, miR499, let7, miR290, miR291, miR292, miR293, miR294, miR295) and altered nutrient sensing (miR17, miR19b, miR126, miR190b, miR496, miR20a, miR106a, miR1, miR320, miR206) [88]. Altered miRNA expression is associated with aging, which in turn implicates AD. Further research is needed to better understand the involvement of age-related miRNAs in AD pathogenesis and in other neurodegenerative diseases.
Circulatory microRNAs as peripheral biomarkers for AD
A research field of peripheral miRNAs is rapidly emerging in the study of neurodegenerative diseases. Recent molecular biology discoveries (Kumar and Reddy 2016) have revealed a notable number of miRNAs in peripheral tissues, such as serum, plasma, ECF and CSF [20]. The levels of miRNAs in these fluids change with age, body physiology, diet, and disease state.
Recently, Kumar and Reddy (2016) assessed miRNAs in the blood and CSF from patients with AD in order to inform the development of miRNAs as possible peripheral biomarkers for AD [20]. They found a small number of changes in miRNAs associated with cognition in non-demented healthy humans and in humans with mild cognitive impairment and with AD.
Kumar and Reddy hypothesize that miRNA analysis of a blood sample should be able to diagnose whether a person has or will have cognitive impairment due to AD. In addition to a blood-based miRNA analysis, imaging of the brain should provide data on synaptic and neuronal connectivity, hippocampal volume and brain size that should be able to indicate the onset of AD. These two types of tests in conjunction should provide an accurate diagnosis of AD-related dementia.
Conclusions and future directions
AD continues to be a growing health concern that affects millions of persons worldwide. Although progress that has been made in AD research in terms of understanding the molecular basis of early onset familial AD and late-onset sporadic AD, we still do not have drugs or agents that can delay or prevent AD progression, and we still have not identified early detectable biomarkers for AD. A major breakthrough in developing such biomarkers is the discovery that miRNAs connect missing link between cellular changes and disease progression.
There are many questions about miRNAs in the AD brain that need to be answered, including which specific miRNAs are involved in cellular changes associated with AD pathogenesis and progression; which specific miRNAs are involved in cellular changes associated with other neurodegenerative diseases and aging; whether AD can be prevented, delayed, or stopped via strategic alterations of miRNA expression; and whether and how blood and imaging tests can be developed that focus on identifying miRNAs changes that correspond with AD onset and progression. Comprehensive epidemiological-based miRNA studies are urgently needed to inform the development of miRNA-based diagnostic tools.
Highlights.
Multiple cellular changes have been implicated in Alzheimer’s disease.
MicroRNAs interfere with gene regulation and translation.
Differential expression of MicroRNAs modulate genes related to Alzheimer’s disease.
MicroRNAs affect the levels of amyloid beta, phosphorylated tau and synaptic damage.
MicroRNAs affect aging, cellular senescence, inflammation and mitochondrial dysfunction in Alzheimer’s disease.
Acknowledgments
Work presented in this article is supported by NIH grants – AG042178 and AG47812 and Garrison Family Foundation (to PHR). Present work is also supported by Alzheimer’s Association New Investigator Research Grant and Center of Excellence For Translational Neuroscience and Therapeutics (to APR)
Abbreviations
- AD
Alzheimer’s Disease
- miRNA
microRNA
- Aβ
Amyloid β
- APP
Amyloid Precursor Protein
- PS1
Presenilin 1
- PS2
Presenilin 2
- ApoE
Apolipoprotein E
- CD2AP
Cluster of Differentiation 2 Associated Protein
- mRNA
messenger RNA
- BACE1
Beta-site Amyloid precursor protein Cleaving Enzyme 1
- IGF1
Insulin-like growth factor 1
- GSK-3β
Glycogen synthase kinase 3
- PI3K
Phosphatidylinositol-3-kinase
- GF
Growth Factor
- ERK
extracellular signal-regulated kinase
- IRS1
Insulin receptor substrate 1
- PIK3R2
Phosphoinositide-3-Kinase Regulatory Subunit 2
- TG
Transgenic
- MAGL
Monoacylglycerol lipase
- PPARγ
peroxisome proliferator-activated receptor γ
- NF-κB
Nuclear Factor Kappa B Subunit
- INDCs
Inflammatory Neurological Controls
- NINDCs
Non-inflammatory controls
- TREM2
Triggering Receptor Expressed On Myeloid Cells 2
- CAPE
Caffeic-Acid Phenethyl Ester
- CSF
Cerebrospinal Fluid
- ECF
Extra Cellular Fluid
- SAMP8
Senescence Accelerated Mouse Prone 8
- ADAM10
A Disintegrin and metalloproteinase domain-containing protein 10
- MANCOVA
Multivariate analysis of covariance
- MAPT
Microtubule Associated Protein Tau
- mTOR
Mechanistic Target of Rapamycin
- ABCA1
Adenosine Triphosphate Binding; Cassette Subfamily A Member 1
- Drp1
Dynamin-related protein 1
- PTEN
Phosphatase and Tensin Homolog
- FOXO3a
Forkhead Box O3
- Sirt1
silent mating type information regulation 2 homolog
- SORL1
Sortilin-Related Receptor 1
- CDK5R1
Cyclin-Dependent Kinase 5, Regulatory Subunit 1
- LRPAP1
Low Density Lipoprotein Receptor Related Protein Associated Protein 1
- Rb1
Retinoblastoma 1
- DUSP6
Dual Specificity Phosphatase 6
- PPP1CA
Protein Phosphatase 1 Catalytic Subunit Alpha
- VAMP2
Vesicle Associated Membrane Protein 2
- SYT1
Synaptotagmin 1
- HCN1
Hyperpolarization-activated Cyclic Nucleotide-gated channel 1
- NR2A
N-methyl-D-aspartate receptor subunit 2A
- GluR1
Glutamate Receptor 1
- NDUFC2
Nicotinamide Adenine Dinucleotide Ubiquinone Oxidoreductase subunit C2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Selkoe DJ. Alzheimer’s Disease: Genes, Proteins, and Therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;5:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-β in Alzheimer’s disease. Nat Rev Neuro. 2007;8:449–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 4.Reddy PH, Manczaka M, Maoa P, Calkinsa MJ, Reddy AP, Shirendeba U. Amyloid-β and mitochondria in aging and Alzheimer’s disease: Implications for synaptic damage and cognitive decline. J Alz Dis. 2010;20:S499–S512. doi: 10.3233/JAD-2010-100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P, Manczak M. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta. 2012;1811:639–649. doi: 10.1016/j.bbadis.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy PH. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer’s disease. Brain Res. 2011;1415:136–148. doi: 10.1016/j.brainres.2011.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy PH, McWeeney S. Mapping cellular transcriptosomes in autopsied Alzheimer’s disease subjects and relevant animal models. Neurobiol Aging. 2006;27:1060–1077. doi: 10.1016/j.neurobiolaging.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Mao P, Reddy PH. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: implications for early intervention and therapeutics. Biochim Biophys Acta. 2011;1812:1359–1370. doi: 10.1016/j.bbadis.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolas FE, Lopez-Martinez AF. MicroRNAs in human diseases. Recent Pat DNA Gene Seq. 2010;4:142–154. doi: 10.2174/187221510794751659. [DOI] [PubMed] [Google Scholar]
- 10.Bao B, Ali S, Kong D, Sarkar SH, Wang Z, Banerjee S, Aboukameel A, Padhye S, Philip PA, Sarkar FH. Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS One. 2011;6:e17850. doi: 10.1371/journal.pone.0017850. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Quiat D, Olson EN. MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J Clin Invest. 2013;123:11–18. doi: 10.1172/JCI62876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei C, Henderson H, Spradley C, Li L, Kim IK, Kumar S, Hong N, Arroliga AC, Gupta S. Circulating miRNAs as potential marker for pulmonary hypertension. PLoS One. 2013;8:e64396. doi: 10.1371/journal.pone.0064396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papagregoriou G, Erguler K, Dweep H, Voskarides K, Koupepidou P, Athanasiou Y, Pierides A, Gretz N, Felekkis KN, Deltas C. A miR-1207-5p binding site polymorphism abolishes regulation of HBEGF and is associated with disease severity in CFHR5 nephropathy. PLoS One. 2012;7:e31021. doi: 10.1371/journal.pone.0031021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koutsis G, Siasos G, Spengos K. The emerging role of microRNA in stroke. Curr Top Med Chem. 2013;13:1573–1588. doi: 10.2174/15680266113139990106. [DOI] [PubMed] [Google Scholar]
- 15.Beveridgea NJ, Cairnsa MJ. MicroRNA dysregulation in schizophrenia. Neurobiology of Disease. 2012;46:263–271. doi: 10.1016/j.nbd.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Kocerha J, Liu Y, Willoughby D, Chidamparam K, Benito J, Nelson K, Xu Y, Chi T, Engelhardt H, Moran S, Yang SH, Li SH, Li XJ, Larkin K, Neumann A, Banta H, Yang JJ, Chan AW. Longitudinal transcriptomic dysregulation in the peripheral blood of transgenic Huntington’s disease monkeys. BMC Neurosci. 2013;14:88. doi: 10.1186/1471-2202-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mouradian MM. MicroRNAs in Parkinson’s disease. Neurobiol Dis. 2012;46:279–284. doi: 10.1016/j.nbd.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 18.Delay C, Hebert SS. MicroRNAs and Alzheimer’s disease mouse models: Current Insights and Future Research Avenues. Int J Alz Dis. 2011;2011:894938. doi: 10.4061/2011/894938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicolas G, Wallon D, Goupil C, Richard AC, Pottier C, Dorval V, Sarov-Rivière M, Riant F, Hervé D, Amouyel P, Guerchet M, Ndamba-Bandzouzi B, Mbelesso P, Dartigues JF, Lambert JC, Preux PM, Frebourg T, Campion D, Hannequin D, Tournier-Lasserve E, Hébert SS, Rovelet-Lecrux A. Mutation in the 3′untranslated region of APP as a genetic determinant of cerebral amyloid angiopathy. Eur J Hum Genet. 2016;24:92–98. doi: 10.1038/ejhg.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Reddy PH. Are circulating microRNAs peripheral biomarkers for Alzheimer’s disease? Biochim Biophys Acta. 2016;1862:1617–1627. doi: 10.1016/j.bbadis.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long JM, Lahiri DK. Current drug targets for modulating Alzheimer’s amyloid precursor protein: role of specific micro-RNA species. Curr Med Chem. 2011;18:3314–3321. doi: 10.2174/092986711796504592. [DOI] [PubMed] [Google Scholar]
- 23.Maffioletti E, Tardito D, Gennarelli M, Bocchio-Chiavetto L. Micro spies from the brain to the periphery: new clues from studies on microRNAs in neuropsychiatric disorders. Front Cell Neurosci. 2014;8:75. doi: 10.3389/fncel.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters L, Meister G. Argonaute proteins: mediators of RNA silencing. Mol Cell. 2007;26:611–623. doi: 10.1016/j.molcel.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Eulalio A, Huntzinger E, Nishihara T, Rehwinkel J, Fauser M, Izaurralde E. Deadenylation is a widespread effect of miRNA regulation. RNA. 2009;15:21–32. doi: 10.1261/rna.1399509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartel DP. MicroRNAs: Genomics, Biogenesis, Mechanism and Function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 28.Hooten NN, Fitzpatrick M, Wood WH, De S, Ejiogu N, Zhang Y, Mattison JA, Becker KG, Zonderman AB, Evans MK. Age-related changes in microRNA levels in serum. Aging. 2013;5:725–740. doi: 10.18632/aging.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adlakha YK, Saini N. Brain microRNAs and insights into biological functions and therapeutic potential of brain enriched miRNA-128. Mol Cancer. 2014;13:33. doi: 10.1186/1476-4598-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao NY, Hu HY, Yan Z, Xu Y, Hu H, Menzel C, Li N, Chen W, Khaitovich P. Comprehensive survey of human brain microRNA by deep sequencing. BMC Genomics. 2010;11:4. doi: 10.1186/1471-2164-11-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hebert LE, Bienias JL, Aggarwal NT, Wilson RS, Bennett DA, Shah RC, Evans DA. Change in risk of Alzheimer disease over time. Neurology. 2010;75:786–791. doi: 10.1212/WNL.0b013e3181f0754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, Ullian EM. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28:4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawase-Koga Y, Low R, Otaegi G, Pollock A, Deng H, Eisenhaber F, Maurer-Stroh S, Sun T. RNAase-III enzyme Dicer maintains signaling pathways for differentiation and survival in mouse cortical neural stem cells. J Cell Sci. 2010;123:586–594. doi: 10.1242/jcs.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Junn E, Mouradian MM. MicroRNAs in neurodegenerative diseases and their therapeutic potential. Pharmacol Ther. 2012;133:142–150. doi: 10.1016/j.pharmthera.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunez-Iglesias J, Liu CC, Morgan TE, Finch CE, Zhou XJ. Joint genome-wide profiling of miRNA and mRNA expression in Alzheimer’s disease cortex reveals altered miRNA regulation. PLoS One. 2010;5:e8898. doi: 10.1371/journal.pone.0008898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schonrock N, Gotz J. Decoding the non-coding RNAs in Alzheimer’s disease. Cell Mol Life Sci. 2012;69:3543–3559. doi: 10.1007/s00018-012-1125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang WX, Huang Q, Hu Y, Stromberg AJ, Nelson PT. Patterns of microRNA expression in normal and early Alzheimer’s disease human temporal cortex: white matter versus gray matter. Acta Neuropathol. 2011;121:193–205. doi: 10.1007/s00401-010-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hébert SS, Horré K, Nicolaï L, Bergmans B, Papadopoulou AS, Delacourte A, Strooper BD. MicroRNA regulation of Alzheimer’s Amyloid precursor protein expression. Neurobiol Dis. 2009;33:422–428. doi: 10.1016/j.nbd.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Patel N, Hoang D, Miller N, Ansaloni S, Huang Q, Rogers JT, Lee JC, Saunders AJ. MicroRNAs can regulate human APP levels. Mol Neurodegener. 2008;3:10. doi: 10.1186/1750-1326-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delay C, Calon F, Mathews P, Hébert SS. Alzheimer-specific variants in the 3′UTR of Amyloid precursor protein affect microRNA function. Mol Neurodegener. 2011;6:70. doi: 10.1186/1750-1326-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith P, Hashimi AA, Girard J, Delay C, Hébert SS. In vivo regulation of amyloid precursor protein neuronal splicing by microRNAs. J Neurochem. 2011;116:240–247. doi: 10.1111/j.1471-4159.2010.07097.x. [DOI] [PubMed] [Google Scholar]
- 42.Fang M, Wang J, Zhang X, Geng Y, Hu Z, Rudd JA, Ling S, Chen W, Han S. The miR-124 regulates the expression of BACE1/β-secretase correlated with cell death in Alzheimer’s disease. Toxicology Letters. 2012;209:94–105. doi: 10.1016/j.toxlet.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 43.Schonrock N, Humphreys DT, Preiss T, Götz J. Target gene repression mediated by miRNAs miR-181c and miR-9 both of which are down-regulated by amyloid-β. J Mol Neurosci. 2012;46:324–335. doi: 10.1007/s12031-011-9587-2. [DOI] [PubMed] [Google Scholar]
- 44.Boissonneault V, Plante I, Rivest S, Provost P. MicroRNA-298 and microRNA-328 regulate expression of mouse beta-amyloid precursor protein-converting enzyme 1. J Biol Chem. 2009;284:1971–1981. doi: 10.1074/jbc.M807530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Liu J, Zong Y, Xu Y, Deng W, Zhu H, Liu Y, Ma C, Huang L, Zhang L, Qin C. miR-106b aberrantly expressed in a double transgenic mouse model for Alzheimer’s disease targets TGF-β type II receptor. Brain Res. 2010;1357:166–1674. doi: 10.1016/j.brainres.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 46.Schonrock N, Ke YD, Humphreys D, Staufenbiel M, Ittner LM, Preiss T, Götz J. Neuronal microRNA deregulation in response to Alzheimer’s disease amyloid-beta. PLoS One. 2010;5:e11070. doi: 10.1371/journal.pone.0011070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim W, Lee Y, McKenna ND, Yi M, Simunovic F, Wang Y, Kong B, Rooney RJ, Seo H, Stephens RM, Sonntag KC. miR-126 contributes to Parkinson’s disease by dysregulating the insulin-like growth factor/phosphoinositide 3-kinase signaling. Neurobiol Aging. 2014;35:1712–1721. doi: 10.1016/j.neurobiolaging.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang G, Song Y, Zhou X, Deng Y, Liu T, Weng G, Yu D, Pan S. MicroRNA-29c targets βsite amyloid precursor protein-cleaving enzyme 1 and has a neuroprotective role in vitro and in vivo. Mol Med Rep. 2015;12:3081–3088. doi: 10.3892/mmr.2015.3728. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Hu M, Teng Z, Tang YP, Chen C. Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregulation of microRNA-188-3p in a mouse model of Alzheimer’s disease. J Neurosci. 2014;34:14919–14933. doi: 10.1523/JNEUROSCI.1165-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Long JM, Ray B, Lahiri DK. MicroRNA-339-5p down-regulates protein expression of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in human primary brain cultures and is reduced in brain tissue specimens of Alzheimer disease subjects. J Biol Chem. 2014;289:5184–5198. doi: 10.1074/jbc.M113.518241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galimberti D, Villa C, Fenoglio C, Serpente M, Ghezzi L, Cioffi SMG, Arighi A, Fumagalli G, Scarpini E. Circulating miRNAs as potential biomarkers in Alzheimer’s disease. J Alz Dis. 2014;42:1261–1267. doi: 10.3233/JAD-140756. [DOI] [PubMed] [Google Scholar]
- 52.Alexandrov PN, Zhao Y, Jones BM, Bhattacharjee S, Lukiw WJ. Expression of the phagocytosis-essential protein TREM2 is down-regulated by an aluminum-induced miRNA-34a in a murine microglial cell line. J Inorg Biochem. 2013;128:267–269. doi: 10.1016/j.jinorgbio.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alexandrov PN, Dua P, Hill JM, Bhattacharjee S, Zhao Y, Lukiw WJ. microRNA (miRNA) speciation in Alzheimer’s disease (AD) cerebrospinal fluid (CSF) and extracellular fluid (ECF) Int J Biochem Mol Biol. 2012;3:365–373. [PMC free article] [PubMed] [Google Scholar]
- 54.Liu W, Liu C, Zhu J, Shu P, Yin B, Gong Y, Qiang B, Yuan J, Peng X. MicroRNA-16 targets amyloid precursor protein to potentially modulate Alzheimer’s-associated pathogenesis in SAMP8 mice. Neurobiol Aging. 2012;33:522–534. doi: 10.1016/j.neurobiolaging.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 55.Pogue AI, Li YY, Cui JG, Zhao Y, Kruck TP, Percy ME, Tarr MA, Lukiw WJ. Characterization of an NF-kappaB-regulated, miRNA-146a-mediated down-regulation of complement factor H (CFH) in metal-sulfate-stressed human brain cells. J Inorg Biochem. 2009;103:1591–1595. doi: 10.1016/j.jinorgbio.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Augustin R, Endres K, Reinhardt S, Kuhn PH, Lichtenthaler SF, Hansen J, Wurst W, Trümbach D. Computational identification and experimental validation of microRNAs binding to the Alzheimer-related gene ADAM10. BMC Med Genet. 2012;17:13–35. doi: 10.1186/1471-2350-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denk J, Boelmans K, Siegismund C, Lassner D, Arlt S, Jahn H. MicroRNA profiling of CSF reveals potential biomarkers to detect Alzheimer’s disease. PloS One. 2015;10:e0126423. doi: 10.1371/journal.pone.0126423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen J, Kelleher RJ. The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. PNAS. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9:1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jing L, Jya Y, Lu J, Han R, Li J, Wang S, Peng T, Jia Y. MicroRNA-9 promotes differentiation of mouse bone mesenchymal stem cells into neurons by Notch signaling. Neuro Report. 2011;22:206–211. doi: 10.1097/WNR.0b013e328344a666. [DOI] [PubMed] [Google Scholar]
- 61.Bonev B, Pisco A, Papalopulu N. MicroRNA-9 reveals regional diversity of neural progenitors along the anteriorposterior axis. Developmental Cell. 2011;20:19–32. doi: 10.1016/j.devcel.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hébert SS, Papadopoulou AS, Smith P, Galas MC, Planel E, Silahtaroglu AN, Sergeant N, Buée L, Strooper BD. Genetic ablation of Dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Hum Mol Genet. 2010;19:3959–3969. doi: 10.1093/hmg/ddq311. [DOI] [PubMed] [Google Scholar]
- 63.Carrettiero DC, Hernandez I, Neveu P, Papagiannakopoulos T, Kosik KS. The cochaperone BAG2 sweeps paired helical filament-insoluble tau from the microtubule. J Neurosci. 2009;29:2151–2161. doi: 10.1523/JNEUROSCI.4660-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, Liu P, Zhu H, Xu Y, Ma C, Dai X, Huang L, Liu Y, Zhang L, Qin C. miR-34a, a microRNA up-regulated in a double transgenic mouse model of Alzheimer’s disease, inhibits bcl2 translation. Brain Res Bull. 2009;80:268–273. doi: 10.1016/j.brainresbull.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Lee ST, Chu K, Jung KH, Kim JH, Huh JY, Yoon H, Park DK, Lim JY, Kim JM, Jeon D, Ryu H, Lee SK, Kim M, Roh JK. miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Ann Neurol. 2012;72:269–277. doi: 10.1002/ana.23588. [DOI] [PubMed] [Google Scholar]
- 66.Li YY, Cui JG, Hill JM, Bhattacharjee S, Zhao Y, Lukiw WJ. Increased expression of miRNA-146a in Alzheimer’s disease transgenic mouse models. Neurosci Lett. 2011;487:94–98. doi: 10.1016/j.neulet.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim J, Yoon H, Horie T, Burchett JM, Restivo JL, Rotllan N, Ramírez CM, Verghese PB, Ihara M, Hoe HS, Esau C, Fernández-Hernando C, Holtzman DM, Cirrito JR, Ono K, Kim J. microRNA-33 Regulates ApoE Lipidation and Amyloid-β Metabolism in the Brain. J Neurosci. 2015;35:14717–14726. doi: 10.1523/JNEUROSCI.2053-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song J, Lee JE. miR-155 is involved in Alzheimer’s disease by regulating T lymphocyte function. Front Aging Neurosci. 2015;7:61. doi: 10.3389/fnagi.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z, Gu X, Fang Y, Xiang J, Chen Z. microRNA expression profiles in human colorectal cancers with brain metastases. Oncol Lett. 2012;2:346–350. doi: 10.3892/ol.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lukiw WJ, Alexandrov PN. Regulation of complement factor H (CFH) by multiple miRNAs in Alzheimer’s disease (AD) brain. Mol Neurobiol. 2012;46:11–19. doi: 10.1007/s12035-012-8234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Redis RS, Calin S, Yang Y, You MJ, Calin GA. Cell-to-cell miRNA transfer: from body homeostasis to therapy. Pharmacol Ther. 2012;136:169–174. doi: 10.1016/j.pharmthera.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lehmann SM, Krüger C, Park B, Derkow K, Rosenberger K, Baumgart J, Trimbuch T, Eom G, Hinz M, Kaul D, Habbel P, Kälin R, Franzoni E, Rybak A, Nguyen D, Veh R, Ninnemann O, Peters O, Nitsch R, Heppner FL, Golenbock D, Schott E, Ploegh HL, Wulczyn FG, Lehnardt S. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci. 2012;15:827–835. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- 73.Shioya M, Obayashi S, Tabunoki H, Arima K, Saito Y, Ishida T, Satoh J. Aberrant microRNA expression in the brains of neurodegenerative diseases: miR-29a decreased in Alzheimer disease brains targets neurone navigator 3. Neuropathol Appl Neurobiol. 2010;36:320–330. doi: 10.1111/j.1365-2990.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- 74.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metabolism. 2009;10:273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bian Z, Li L-M, Tang R, Hou DX, Chen X, Zhang CY, Zen K. Identification of mouse liver mitochondria-associated miRNAs and their potential biological functions. Cell Research. 2010;20:1076–1078. doi: 10.1038/cr.2010.119. [DOI] [PubMed] [Google Scholar]
- 76.Huang L, Mollet S, Souquere S, Roy FL, Ernoult-Lange M, Pierron G, Dautry F, Weil D. Mitochondria associate with P-bodies and modulate microRNA-mediated RNA interference. J Bio Chem. 2011;286:24219–24230. doi: 10.1074/jbc.M111.240259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Favaro E, Ramachandran A, McCormick R, Gee H, Blancher C, Crosby M, Devlin C, Blick C, Buffa F, Li JL, Vojnovic B, das Neves RP, Glazer P, Iborra F, Ivan M, Ragoussis J, Harris AL. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PLoS One. 2010;5:e10345. doi: 10.1371/journal.pone.0010345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kren BT, Wong PY, Sarver A, Zhang X, Zeng Y, Steer CJ. MicroRNAs identified in highly purified liver-derived mitochondria may play a role in apoptosis. RNA Bio. 2009;6:65–72. doi: 10.4161/rna.6.1.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Z, Li Y, Zhang H, Huang P, Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene. 2010;29:4362–4368. doi: 10.1038/onc.2010.193. [DOI] [PubMed] [Google Scholar]
- 80.Lung B, Zemann A, Madej MJ, Schuelke M, Techritz S, Ruf S, Bock R, Hüttenhofer A. Identification of small non-coding RNAs from mitochondria and chloroplasts. Nucleic Acids Research. 2006;34:3842–3852. doi: 10.1093/nar/gkl448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barrey E, Saint-Auret G, Bonnamy B, Damas D, Boyer O, Gidrol X. Pre-microRNA and mature microRNA in human mitochondria. PLoS One. 2011;6:e20220. doi: 10.1371/journal.pone.0020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shinde S, Bhadra U. A Complex Genome-MicroRNA Interplay in Human Mitochondria. BioMed Research International. 2015;2015:206382. doi: 10.1155/2015/206382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Long B, Wang K, Li N, Murtaza I, Xiao J-Y, Fan Y-Y, Liu C-Y, Li W-H, Cheng Z, Li P. miR-761 regulates the mitochondrial network by targeting mitochondrial fission factor. Free Radic Biol Med. 2013;65:371–379. doi: 10.1016/j.freeradbiomed.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goud MR, Hua ZA. Role of microRNA in the regulation of mitochondrial functions. Sci Lett. 2015;3:83–88. [Google Scholar]
- 85.Li J, Donath S, Li Y, Qin D, Prabhakar BS, Li P. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet. 2010;6:e1000795. doi: 10.1371/journal.pgen.1000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, Li YR, Li PF. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 87.Kandimalla R, Reddy PH. Multiple faces of dynamin-related protein 1 and its role in Alzheimer’s disease pathogenesis. Biochim Biophy Acta. 2016;1862:814–828. doi: 10.1016/j.bbadis.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harries LW. MicroRNAs as Mediators of the Ageing Process. Genes. 2014;5:656–670. doi: 10.3390/genes5030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hebert SS, Horre K, Nicolaï L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, Strooper BD. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/β-secretase expression. PNAS. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vilardo E, Barbato C, Ciotti M, Cogoni C, Ruberti F. MicroRNA-101 regulates amyloid precursor protein expression in hippocampal neurons. J Biol Chem. 2010;285:18344–18351. doi: 10.1074/jbc.M110.112664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong HA, Veremeyko T, Patel N, Lemere CA, Walsh DM, Esau C, Vanderburg C, Krichevsky AM. De-repression of FOXO3a death axis by microRNA-132 and −212 causes neuronal apoptosis in Alzheimer’s disease. Human Molecular Genetics. 2013;22:3077–3092. doi: 10.1093/hmg/ddt164. [DOI] [PubMed] [Google Scholar]
- 92.Dickson JR, Kruse C, Montagna DR, Finsen B, Wolfe MS. Alternative polyadenylation and miR-34 family members regulate tau expression. J Neurochem. 2013;127:739–749. doi: 10.1111/jnc.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu CG, Song J, Zhang YQ, Wang PC. MicroRNA-193b is a regulator of amyloid precursor protein in the blood and cerebrospinal fluid derived exosomal microRNA-193b is a biomarker of Alzheimer’s disease. Mol Med Report. 2014;10:2395–2400. doi: 10.3892/mmr.2014.2484. [DOI] [PubMed] [Google Scholar]
- 94.Weinberg RB, Mufson EJ, Counts SE. Evidence for a neuroprotective microRNA pathway in amnestic mild cognitive impairment. Front Neurosci. 2015;9:430. doi: 10.3389/fnins.2015.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santa-Maria I, Alaniz ME, Renwick N, Cela C, Fulga TA, Vactor DV, Tuschl T, Clark LN, Shelanski ML, McCabe BD, Crary JF. Dysregulation of microRNA-219 promotes neurodegeneration through post-transcriptional regulation of tau. J Clin Invest. 2015;125:681–686. doi: 10.1172/JCI78421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang B, Chen CF, Wang AH, Lin QF. MiR-16 regulates cell death in Alzheimer’s disease by targeting amyloid precursor protein. Eur Rev Med Pharmacol Sci. 2015;19:4020–4027. [PubMed] [Google Scholar]
- 97.Zhang Y, Xing H, Guo S, Zheng Z, Wang H, Xu D. MicroRNA-135b has a neuroprotective role via targeting of β-site APP-cleaving enzyme 1. Exp Therap Med. 2016;12:809–814. doi: 10.3892/etm.2016.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghanbari M, Ikram MA, de Looper HW, Hofman A, Erkeland SJ, Franco OH, Dehghan A. Genome-wide identification of microRNA-related variants associated with risk of Alzheimer’s disease. Sci Rep. 2016;6:28387. doi: 10.1038/srep28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moncini S, Lunghi M, Valmadre A, Grasso M, Vescovo VD, Riva P, Denti MA, Venturin M. The miR-15/107 family of microRNA genes regulates CDK5R1/p35 with implications for Alzheimer’s disease pathogenesis. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0002-4. [DOI] [PubMed] [Google Scholar]
- 100.Zhang C, Lu J, Liu B, Cui Q, Wang Y. Primate-specific miR-603 is implicated in the risk and pathogenesis of Alzheimer’s disease. Aging. 2016;8:272–290. doi: 10.18632/aging.100887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pereira PA, Tomás JF, Queiroz JA, Figueiras AR, Sousa F. Recombinant pre-miR-29b for Alzheimer’s disease therapeutics. Sci Rep. 2016;6:19946. doi: 10.1038/srep19946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Absalon S, Kochanek DM, Raghavan V, Krichevsky AM. MiR-26b, upregulated in Alzheimer’s disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons. J Neurosci. 2013;33:14645–14659. doi: 10.1523/JNEUROSCI.1327-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Croce N, Gelfo F, Ciotti MT, Federici G, Caltagirone C, Bernardini S, Angelucci F. NPY modulates miR-30a-5p and BDNF in opposite direction in an in vitro model of Alzheimer disease: a possible role in neuroprotection? Mol Cell Biochem. 2013;376:189–195. doi: 10.1007/s11010-013-1567-0. [DOI] [PubMed] [Google Scholar]
- 104.Tian N, Cao Z, Zhang Y. MiR-206 decreases brain-derived neurotrophic factor levels in a transgenic mouse model of Alzheimer’s disease. Neurosci Bull. 2014;30:191–197. doi: 10.1007/s12264-013-1419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Banzhaf-Strathmann J, Benito E, May S, Arzberger T, Tahirovic S, Kretzschmar H, Fischer A, Edbauer D. MicroRNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer’s disease. EMBO J. 2014;33:1667–1680. doi: 10.15252/embj.201387576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sarkar S, Jun S, Rellick S, Quintana DD, Cavendish JZ, Simpkins JW. Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state Network activity. Brain Research. 2016;1646:139–151. doi: 10.1016/j.brainres.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim W, Noh H, Lee Y, Jeon J, Shanmugavadivu A, McPhie DL, Kim KS, Cohen BM, Seo H, Sonntag KC. MiR-126 regulates growth factor activities and vulnerability to toxic insult in neurons. Mol Neurobiol. 2016;53:95–108. doi: 10.1007/s12035-014-8989-x. [DOI] [PMC free article] [PubMed] [Google Scholar]